Abstract
OBJECTIVE
To assess sex-specific differences in early brain structure and function of preterm infants after red blood cell (RBC) transfusions.
STUDY design
A single-center subset of infants with a birth weight <1000 g and gestational age 22-29 weeks were enrolled from the NICHD Neonatal Research Network Transfusion of Prematures (TOP) Trial. Hemoglobin concentration obtained directly prior to each transfusion (ptHb) was obtained longitudinally throughout each infant’s neonatal intensive care unit stay and used as a marker of degree of anemia (n=97). Measures of regional brain volumes using magnetic resonance imaging (MRI) were obtained at ~40 weeks postmenstrual age or at hospital discharge, if earlier (n=29). Measures of brain function were obtained at 12 months’ corrected age using the Bayley Scales of Infant & Toddler Development, 3rd Edition (Bayley-III) (n=34).
RESULTS
PtHb positively correlated with neonatal cerebral white matter volume in males (B=+0.283, p=0.006), but not females (B=−0.099, p=0.713), resulting in a significant sex interaction (p=0.010). Bayley-III gross motor scores and a pooled mean score were significantly lower in association with higher ptHb in females (gross motor score: B=−3.758, p=0. 013; pooled mean score: B=−1.225, p=0.030), but not males (gross motor score: B=+1.758, p=0.167; pooled mean score: B=+0.621, p = 0.359). Higher ptHb was associated with descriptively lower performance on multiple Bayley-III subscales in females, but not males.
CONCLUSION
This study demonstrates sex-specific associations between an early marker of anemia and RBC transfusion status (i.e. ptHb) with both neonatal white matter volumes, and early cognitive function at 12-months-old, in preterm infants.
Keywords: premature, premature infant, brain, neurodevelopment, neurocognitive outcome, brain outcome, sex difference, anemia
Despite the increase in survival of preterm infants over the last several decades, preterm infants remain at risk for several short-term and long-term morbidities, including deficits in motor, sensory, cognitive, and behavioral domains 1–7. Therefore, understanding early brain developmental outcomes may be critically important in determining early interventions for these infants. One possible etiology of altered neurodevelopment in the first weeks of life, as well as an early marker of risk that can be targeted for intervention, is the incidence of neonatal anemia, and its treatment: red blood cell (RBC) transfusion. Until recently8, it was not clear whether minimizing anemia and maintaining a higher, but normal, hemoglobin (Hb) level through transfusions, or tolerating anemia and minimizing transfusions, was better for the developing brain, as several studies have had variable results 9–12.
the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Transfusion of Prematures (TOP) trial published the findings that the primary outcome of death or neurodevelopmental impairment (NDI) was not different in extremely low birth weight (ELBW) infants randomized to either a low- or high- hemoglobin threshold for RBC transfusions13. However, most neurodevelopmental outcomes for premature birth are far less severe than NDI, such as deficits in learning, attention, and behavioral issues. Moreover, these deficits are common, occurring in 50-70% of children born with very low birth weight 14, 15, and can have lifelong, significant impacts on functioning. Another important consideration in evaluations of transfusion status on neurodevelopmental outcomes is exploration of sex-specific outcomes. Sex differences are critical biological variables in investigations of brain structure and function 16–18. we have reported on sex-specific findings of prematurity 19 as well as in the context of the impact of transfusion status on brain structure and function outcomes 20–23.
The current study evaluates the impact of transfusion status on optimal brain structure and function in a subset of neonates from the TOP trial who participated at the University of Iowa. Neonatal pre-transfusion hemoglobin (ptHb) levels were used to predict brain structure from term-equivalent MRI scans (~ 40 weeks’ gestational age) and functional outcomes at 12 months utilizing the Bayley Scales of Infant & Toddler Development, 3rd Edition (Bayley-III). All analyses evaluated sex-specific differences.
METHODS
The current study was carried out at the University of Iowa Stead Family Children’s Hospital. Infants were enrolled in the multicenter NICHD Neonatal Research Network Transfusion of Prematures (TOP) Trial, a large two-armed randomized clinical trial comparing liberal and restrictive transfusion guidelines based on hemoglobin threshold in extremely low birth weight infants24. Infants fulfilled the following inclusion criteria: birth weight ≤1000 g and gestational age ≥22 weeks but <29 completed weeks, and <48 hours of age. Exclusion criteria included: considered nonviable by attending neonatologist; cyanotic congenital heart disease; parental opposition to transfusion of blood; parents with hemoglobinopathy or congenital anemia; fetal transfusion; twin-to-twin transfusion syndrome; isoimmune hemolytic disease; severe acute hemorrhage, acute shock, sepsis with coagulopathy, or need for perioperative transfusion; prior blood transfusion on clinical grounds beyond the first 6 hours of life. From the 124 eligible infants, two infants received no RBC transfusions, one infant passed away prior to the end of the study, and 24 infants had no longitudinal hemoglobin measurements (see [Figure 1; available at www.jpeds.com] for flow diagram of participants). There were a total of 29 infants that were included in the included in final neonatal brain structure analyses and 34 infants that were included in final 12-month-old cognitive function analyses. 9 infants were included in both brain structure & cognitive function analyses.
Figure 1. CONSORT Flow Diagram of Study Participants.
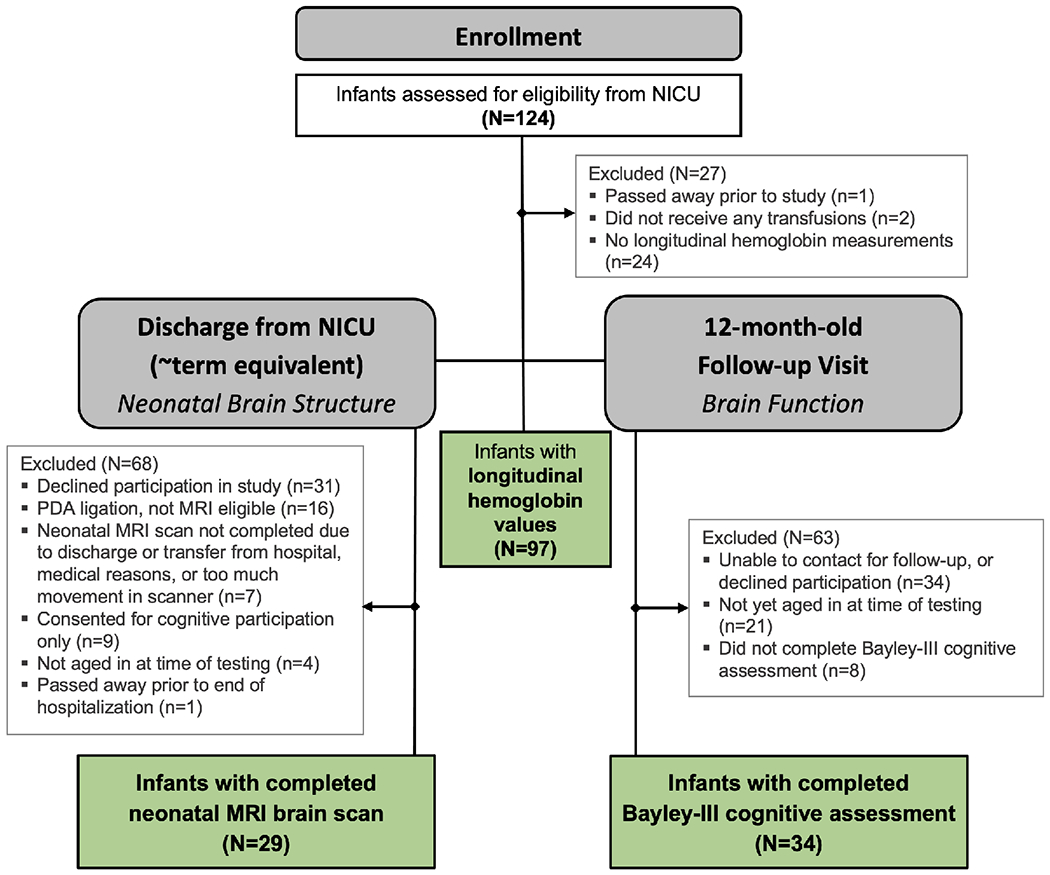
29 infants were included in final neonatal brain structure analyses;
34 infants were included in final 12-month-old cognitive function analyses;
9 infants were included in both brain structure & cognitive function analyses.
A standard transfusion volume of 15 ml/kg was given according to criteria determined by the TOP trial protocol 25. All RBCs transfused were tested and screened according to the policies of the hospital’s blood bank. Blood samples used in this study were left-over from clinically-indicated blood draws obtained by venous, arterial, or prewarmed heel capillary sampling, or from indwelling umbilical catheters, if present. Adequate clinically-indicated blood samples for all infants were obtained longitudinally throughout the hospital stay according to unit policy, at least weekly for the first month, and were not timed in any prescribed way relative to the blood transfusions. Clinical blood samples were stored at 4 degrees Celsius and processed (plasma was separated) within 4 hours of sample collection time. Plasma samples were subsequently stored at −80 degrees Celsius until analysis. All of the parents in this study agreed to have their infants’ scavenged blood assessed for cytokines and biomarkers. Hemoglobin obtained directly prior to each transfusion – termed the pre-transfusion Hb (ptHb) – was used as a marker of the degree of anemia prior to transfusion in each infant. Lower ptHb is therefore associated with lower transfusion threshold strategies (e.g. waiting for an infant’s hemoglobin to drop to the lower limit of normal range before transfusing the infant), whereas higher ptHb is generally associated with higher transfusion thresholds (e.g. transfusing infants at the upper limit of normal hemoglobin range).
Brain Structure
All infants underwent an unsedated MRI scan of their brain at approximately term equivalence (~40 weeks’ postmenstrual age) or shortly before discharge from the hospital, if earlier. An established protocol was used, in which infants were fed, then rocked to sleep by their mothers. Once asleep, they were carefully transferred to the MRI scanner. All procedures were approved by the local institutional review board, and parents signed a consent form prior to participation. Parents were compensated monetarily for the participation of their infant. Two 3T Siemens MRI scanners (TIM Trio and Skyra) were used in this study.
Structural Measures of Interest
The following regional volumes were extracted from the label maps for each neonate: white matter (WM), cerebral gray matter (GM), unmyelinated cerebral white matter (UWM), venous blood, cerebellar GM, cerebellar WM, basal ganglia, thalamus, hippocampus, and cerebrospinal fluid (CSF). Additional composite brain variables were created manually, including total intracranial volume (ICV), a sum of all regional volumes; and cerebellar tissue, a composite of cerebellar GM and WM.
Cognitive Function
The Bayley Scales of Infant Development, 3rd Edition (Bayley-III), were used to evaluate early cognitive function in these infants. Infants in the current study returned for a follow-up visit at approximately 12 months’ corrected age to undergo Bayley-III, including the sub-scale assessments of cognition, fine motor, gross motor, receptive language, and expressive language function.
Statistical Analyses
Mean ptHb concentrations, averaged throughout the hospital stay, were used to evaluate the relationship of transfusion status with measures of brain structure from MRI scans at term equivalent, and function at 12 months-corrected age.
SAS software (version 9.4, Cary, NC) was used to evaluate the association of mean ptHb with neonatal brain structural measures by robust linear regressions. All tissue-specific brain areas (WM, GM, and UWM) were reported as ratios to total ICV, as this accounts for the global decrement in volume by performing comparisons on the proportional measure. In order to reduce the influence of outliers in this small sample as well as account for wide leverage points in the data, robust linear regression models with MM-estimations were used in these analyses. All analyses controlled for birth weight. Each infant’s Bayley-III scale scores were assembled into a 5-entry vector for use as the dependent variable in repeated-measures analysis of variance (ANOVA), accounting for correlation among the scales. The effect of the primary independent variable, ptHb, was allowed to vary by sex and among scales. The ANOVA model also included covariate effects of gestational age, birth weight, and chronological age. Sex- and scale-specific estimates of the linear trend vs. ptHb, with 95% confidence limits, were calculated from the fitted model, adjusting to mean covariate values. Pooled sex-specific trends were obtained by averaging over the five scales and adjusting to a common scale mean. Robust regression identified and down-weighted 15 extreme datapoints using the Talworth criterion with 90% efficiency. P<0.05 was taken as the threshold for statistical significance.
RESULTS
Infants in this study were enrolled as part of the multicenter NICHD Neonatal Research TOP Trial (NCT01702805)24. Enrollment dates for the entire multi-site clinical trial were between October 8, 2012 and January 2020. The infants included in this study were enrolled from a single-center site between February 6, 2013 and February 18, 2017. (Table i; available at www.jpeds.com) shows the demographics of all infants with hemoglobin measurements during the hospitalization (97 infants), infants with Bayley-III cognitive assessments at 12 months (34 infants), and infants with neonatal MRI brain imaging scans (29 infants). There were no significant differences between males and females in birthweight (BW), gestational age (GA), chronological or corrected age at follow-up cognitive assessment, or incidence of intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL). (Table II; available at www.jpeds.com) shows all measures of hemoglobin obtained longitudinally throughout the hospital stay. There were no significant differences in any hemoglobin measures between sexes.
Table 1.
Demographics of Infant Participants
| All Infant Participants With Hemoglobin Measurements during NICU Hospitalization (N=97) | Infant Participants with Neonatal MRI Brain Imaging [at ~Term-Equivalence] (N=29) | Infant Participants with Bayley-III Cognitive Assessment at 12-months-old (N=34) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Male (N=43) | Female (N=54) | All Infants (N=97) | p / M=F * | Male (N=19) | Female (N=10) | All Infants (N=29) | p / M=F * | Male (N=18) | Female (N=16) | All Infants (N=34) | p / M=F * | |
| Birth Weight (g) | ||||||||||||
| Mean (SD) | 725 (163) | 696 (174) | 709 (169) | p = 0.400 | 765 (153) | 798 (218) | 776 (175) | p = 0.673 | 734 (176) | 658 (192) | 698 (185) | p = 0.237 |
| Median [Min, Max] | 747 [279, 1010] | 687 [379, 997] | 712 [279, 1010] | 785 [394, 1010] | 896 [412, 988] | 801 [394, 1010] | 765 [394, 1010] | 633 [395, 984] | 694 [394, 1010] | |||
| Gestational Age (wks) | ||||||||||||
| Mean (SD) | 25.4 (1.61) | 25.3 (1.69) | 25.4 (1.65) | p = 0.629 | 25.5 (1.25) | 26.2 (1.06) | 25.8 (1.21) | p = 0.137 | 25.3 (1.54) | 24.8 (1.42) | 25.1 (1.49) | p = 0.296 |
| Median [Min, Max] | 25.7 [22.4, 28.4] | 25.5 [22.3, 28.4] | 25.7 [22.3, 28.4] | 25.9 [23.1, 28.6] | 26.1 [24.0, 28.1] | 26.0 [23.1, 28.6] | 25.8 [22.4, 27.4] | 25.0 [22.4, 26.9] | 25.4 [22.4, 27.4] | |||
| 12-Month Chronological Age (months) | ||||||||||||
| Mean (SD) | 16.3 (1.49) | 16.0 (0.758) | 16.1 (1.15) | p = 0.447 | 16.7 (2.03) | 15.9 (1.43) | 16.4 (1.83) | p = 0.377 | 15.8 (0.825) | 15.8 (0.476) | 15.8 (0.669) | p = 0.784 |
| Median [Min, Max] | 15.8 [14.4, 20.3] | 15.9 [14.9, 18.4] | 15.9 [14.4, 20.3] | 15.7 [14.4, 20.3] | 15.3 [14.9, 18.4] | 15.6 [14.4, 20.3] | 15.6 [14.4, 17.8] | 15.8 [15.0, 16.6] | 15.7 [14.4, 17.8] | |||
| 12-Month Corrected Age (months) | ||||||||||||
| Mean (SD) | 12.5 (1.36) | 12.3 (0.826) | 12.4 (1.10) | p = 0.508 | 13.0 (1.75) | 12.3 (1.48) | 12.8 (1.64) | p = 0.449 | 12.1 (0.877) | 12.0 (0.464) | 12.1 (0.697) | p = 0.864 |
| Median [Min, Max] | 12.0 [10.6, 15.8] | 12.1 [11.3, 14.9] | 12.1 [10.6, 15.8] | 12.2 [10.6, 15.8] | 11.7 [11.3, 14.9] | 12.1 [10.6, 15.8] | 11.9 [10.6, 14.3] | 12.1 [11.3, 13.0] | 11.9 [10.6, 14.3] | |||
| Intraventricular Hemorrhage (IVH), Germinal Matrix Hemorrhage (GMH), and Periventricular Leukomalacia (PVL) Grading | p = 0.890 | p = 0.161 | p = 0.492 | |||||||||
| Normal (No IVH/PVL) | 31 (72.1%) | 43 (79.6%) | 74 (76.3%) | 13 (68.4%) | 8 (80.0%) | 21 (72.4%) | 13 (72.2%) | 14 (87.5%) | 27 (79.4%) | |||
| Grade I IVH or GMH | 6 (14.0%) | 4 (7.4%) | 10 (10.3%) | 3 (15.8%) | 2 (20.0%) | 5 (17.2%) | 3 (16.7%) | 1 (6.2%) | 4 (11.8%) | |||
| Grade II IVH or GMH | 3 (7.0%) | 2 (3.7%) | 5 (5.2%) | 1 (5.3%) | 0 (0%) | 1 (3.4%) | 1 (5.6%) | 0 (0%) | 1 (2.9%) | |||
| Grade III IVH or GMH | 3 (7.0%) | 1 (1.9%) | 4 (4.1%) | 1 (5.3%) | 0 (0%) | 1 (3.4%) | 1 (5.6%) | 1 (6.2%) | 2 (5.9%) | |||
| Grade IV IVH or GMH -- periventricular hemorrhagic infarction | 0 (0%) | 3 (5.6%) | 3 (3.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| PVL Present | 0 (0%) | 1 (1.9%) | 1 (1.0%) | 1 (5.3%) | 0 (0%) | 1 (3.4%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
Independent Samples Student T-test for equal mean between males & females.
Table 2.
Measures of Hemoglobin in the Neonatal Intensive Care Unit (Bayley-III Participants Only)*
| Males (N=17) | Females (N=13) | Total (N=30) | p / M=F * | |
|---|---|---|---|---|
| Pre-transfusion Hemoglobin(ptHgb) | 0.764 | |||
| Mean (SD) | 10.4 (0.907) | 10.3 (1.40) | 10.4 (1.13) | |
| Median [Min, Max] | 10.7 [9.12, 12.1] | 10.1 [7.70, 12.3] | 10.4 [7.70, 12.3] | |
| Lowest Hemoglobin | ||||
| Mean (SD) | 8.69 (0.978) | 8.83 (1.23) | 8.75 (1.09) | 0.730 |
| Median [Min, Max] | 8.60 [6.80, 10.3] | 9.30 [6.60, 10.7] | 8.70 [6.60, 10.7] | |
| Highest Hemoglobin | 0.770 | |||
| Mean (SD) | 16.9 (1.70) | 16.8 (1.44) | 16.8 (1.56) | |
| Median [Min, Max] | 16.7 [14.5, 20.3] | 16.3 [15.2, 20.1] | 16.5 [14.5, 20.3] | |
| Average Hemoglobin (throughout hospital stay) | ||||
| Mean (SD) | 12.4 (0.944) | 12.4 (0.755) | 12.4 (0.847) | 0.974 |
| Median [Min, Max] | 12.8 [10.6, 13.8] | 12.7 [11.4, 13.4] | 12.7 [10.6, 13.8] | |
| Number of Transfusions | ||||
| Mean (SD) | 7.00 (4.03) | 6.69 (3.75) | 6.87 (3.85) | 0.831 |
| Median [Min, Max] | 6.00 [2.00, 17.0] | 6.00 [1.00, 15.0] | 6.00 [1.00, 17.0] |
Independent Samples Student T-test for equal mean between males & females; Four Bayley-III participants had missing hemoglobin values and are excluded from this analysis
Neonatal Brain Imaging & Baseline Cognitive Performance
(Table III; available at www.jpeds.com) and (Table IV; available at www.jpeds.com) demonstrate the average neonatal brain imaging variables and Bayley-III scores on all five subscales, respectively, by sex. There were no significant differences in neonatal brain structure or baseline Bayley-III cognitive performance between males and females in this small sample.
Table 3.
Neonatal MRI Brain Variables
| Male (N=19) | Female (N=10) | All Infants (N=29) | p / M=F * | |
|---|---|---|---|---|
| Volume (in cubic centimeters, cc, x 103) a | ||||
|
| ||||
| Total Intracranial Volume (ICV) | ||||
| Mean (SD) | 466(66.3) | 465 (112) | 466 (82.9) | p = 0.994 |
| Median [Min, Max] | 452 [363, 586] | 439 [324, 650] | 450 [324, 650] | |
|
| ||||
| [Brain Tissue Type] Volume % Total ICV b | ||||
|
| ||||
| White Matter | ||||
| Mean (SD) | 3.18 (0.324) | 3.12 (0.325) | 3.16 (0.320) | p = 0.653 |
| Median [Min, Max] | 3.13 [2.61, 3.97] | 3.20 [2.52, 3.55] | 3.19 [2.52, 3.97] | |
| Gray Matter | ||||
| Mean(SD) | 33.09 (5.61) | 32.05 (5.42) | 32.73 (5.47) | p = 0.636 |
| Median [Min, Max] | 34.28 [21.74, 42.10] | 29.63 [26.05, 42.79] | 32.73 [21.74, 42.79] | |
| Unmyelinated White Matter | ||||
| Mean (SD) | 35.36 (5.70) | 35.21 (6.54) | 35.31 (5.89) | p = 0.949 |
| Median [Min, Max] | 34.56 [23.99, 45.92] | 35.44 [26.92, 45.39] | 35.20 [23.99, 45.92] | |
| Cerebellar Tissue | ||||
| Mean (SD) | 4.41 (0.602) | 4.41 (0.700) | 4.41 (0.625) | p = 0.984 |
| Median [Min, Max] | 4.44 [3.35, 5.52] | 4.27 [3.61, 5.64] | 4.39 [3.35, 5.64] | |
Independent Samples Student T-test for equal mean between males & females.
Volumes expressed in cubic centimeters (cc’s) multiplied by 103
Volume (in cc’s) of specific brain tissue type to ICV Ratios, expressed as % of total ICV; this accounts for the global decrement in volume, by performing comparisons on the proportional measure
Table 4.
Bayley-III Cognitive Assessment Subscale Scores
| Male (N=18) | Female (N=16) | All Infants (N=34) | p / M=F * | |
|---|---|---|---|---|
| Cognitive Raw Score (Corrected Age) | ||||
| Mean (SD) | 39.8 (10.3) | 38.4 (5.01) | 39.2 (8.18) | p = 0.614 |
| Median [Min, Max] | 41.0 [4.00, 53.0] | 37.5 [31.0, 48.0] | 40.0 [4.00, 53.0] | |
| Receptive Communication Raw Score (Corrected Age) | ||||
| Mean (SD) | 13.7 (2.78) | 14.0 (1.79) | 13.8 (2.32) | p = 0.719 |
| Median [Min, Max] | 14.0 [8.00, 19.0] | 14.0 [10.0, 17.0] | 14.0 [8.00, 19.0] | |
| Expressive Communication Raw Score (Corrected Age) | ||||
| Mean (SD) | 14.7 (4.06) | 13.7 (4.19) | 14.2 (4.09) | p = 0.495 |
| Median [Min, Max] | 15.0 [4.00, 22.0] | 12.5 [7.00, 22.0] | 15.0 [4.00, 22.0] | |
| Fine Motor Raw Score (Corrected Age) | ||||
| Mean (SD) | 26.2 (6.54) | 26.4 (1.90) | 26.3 (4.87) | p = 0.895 |
| Median [Min, Max] | 27.0 [2.00, 32.0] | 27.0 [21.0, 30.0] | 27.0 [2.00, 32.0] | |
| Gross Motor Raw Score (Corrected Age) | ||||
| Mean (SD) | 38.9 (7.24) | 31.6 (13.3) | 35.6 (10.9) | p = 0.078 |
| Median [Min, Max] | 40.0 [17.0, 48.0] | 36.0 [5.00, 45.0] | 39.0 [5.00, 48.0] |
Independent Samples Student T-test for equal mean between males & females.
Pre-transfusion Hb Prediction of Brain Structure at Term Equivalent
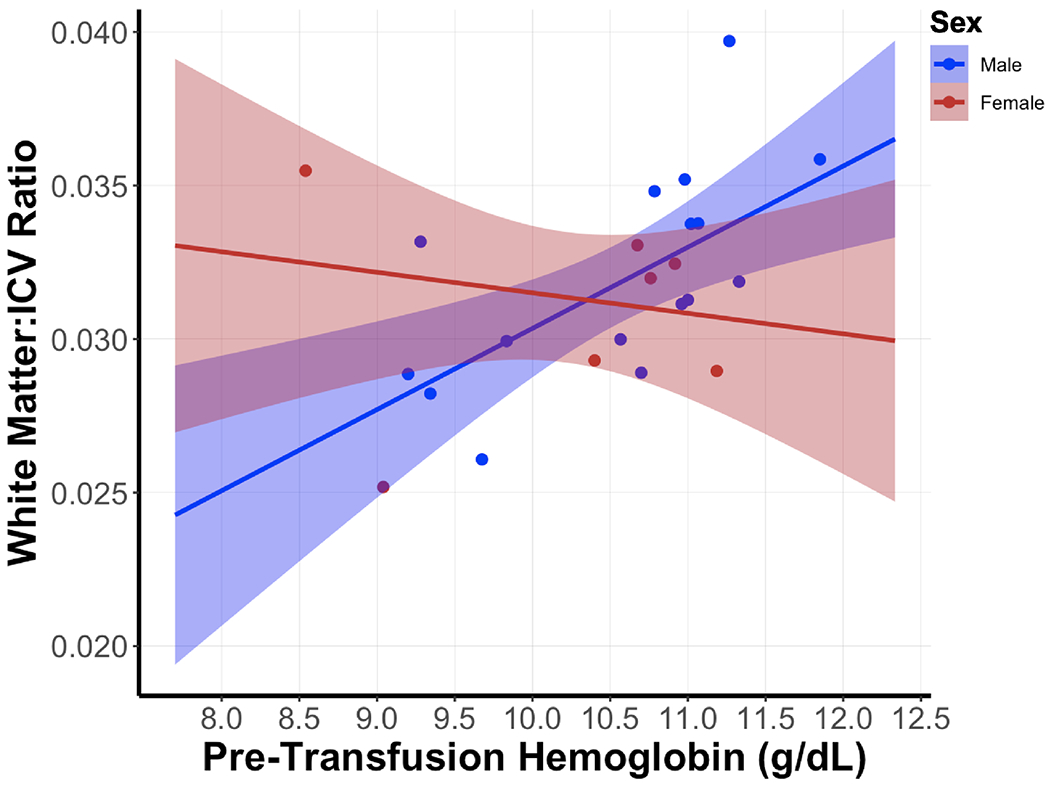
Table V shows the results of the regression analyses of ptHb with neonatal MRI brain variables. There were no significant associations between ptHb and total intracranial volume, cerebellar tissue, GM, or UWM in males or females. PtHb was significantly positively associated with WM in all infant participants (B = +0.268, F = 19.00, p < 0.001); that is, the higher ptHb, the greater the white matter volume. However, this was exclusively driven by the males, as ptHb was positively correlated with white matter volume in males (B = +0.283, F = 10.90, p = 0.006). For females, there was no relationship of ptHb with white matter volume (B = −0.099, F = 0.14, p = 0.713), resulting in a significant sex interaction (F = 8.26, p=0.010). Figure 2 shows this regression model visually, demonstrating that there is a significant difference in white matter volumes in association with ptHb between males and females. A post-hoc sensitivity analysis, in which the same regression analyses and sex interactions were performed after excluding infants diagnosed with any grade of IVH or PVL, was unable to be performed due to small sample numbers.
Table 5.
Robust Regression Analysis of Covariance of Pre-Transfusion Hgb (PtHb) with Neonatal MRI Brain Variables*
| Overall | Male (N=16) | Female (N=7) | Sex Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B† | SE | F | p | B† | SE | F | p | B† | SE | F | p | (p / M=F) | |
| Volume (cc’s) | |||||||||||||
| Total Intracranial Volume (ICV) | −32489.61 | 22216.83 | 2.14 | 0.159 | −37182.84 | 23263.43 | 2.55 | 0.134 | 5829.96 | 88614.84 | 0.00 | 0.951 | 0.764 |
| [Brain Tissue Type] Volume (expressed as % of ICV) | |||||||||||||
| White Matter (WM) | 0.268 | 0.062 | 19.00 | <0.001 | 0.283 | 0.086 | 10.90 | 0.006 | −0.099 | 0.267 | 0.14 | 0.713 | 0.010 |
| Gray Matter (GM) | −0.009 | 1.382 | 0.00 | 0.995 | −0.433 | 1.503 | 0.08 | 0.778 | 1.204 | 3.426 | 0.12 | 0.743 | 0.497 |
| Unmyelinated White Matter (UWM) | −0.745 | 1.612 | 0.21 | 0.649 | 0.399 | 1.816 | 0.05 | 0.829 | −3.851 | 5.093 | 0.57 | 0.492 | 0.239 |
| Cerebellum | 0.066 | 0.158 | 0.18 | 0.680 | 0.114 | 0.191 | 0.36 | 0.560 | −0.076 | 0.512 | 0.02 | 0.890 | 0.632 |
Controlling for birth weight; Six participants had missing hemoglobin values and are excluded from this analysis
B, Beta coefficient estimate, slope of relationship between predictor (pre-transfusion hemoglobin, PtHb) and outcome variable (e.g. brain area volume); SE, Standard Error of Beta Coefficient
Figure 2. Regression Model of White Matter Volume (expressed as ratio to total intracranial volume [ICV]) with Mean Pre-Transfusion Hemoglobin Level (g/dL).
Pre-transfusion Hb Prediction of Cognitive Function at 12 months Corrected Age
Table VI shows the results of the multivariate analyses of ptHb with all five Bayley-III subscales, as well as a pooled trend estimate. In every case the estimated trends for male and females went in opposite directions. Figure 3 displays this data visually by sex. Elevations of ptHb were associated with descriptively lower performance on four out of the five Bayley-III subscales in females, including cognitive, expressive language, and fine and gross motor function. Of the five individual subscales, only gross motor function showed a significant sex interaction with ptHb (B = +5.516, SE = 1.864, p = 0.007): that is, an increase in ptHb was associated with lower scores on Bayley-III gross motor function in females only (−3.758 Bayley-III score for every 1 g/dL increase in ptHb; SE = 1.395, p = 0.013), but not males (B = +1.758, SE = 1.232, p = 0.167). The overall mean Bayley score also showed significant sex-specific associations with ptHb levels (B = +1.846, SE = 0.851, p = 0.041): females had lower mean Bayley scores with higher ptHb (B = −1.225, SE = 0.528, p = 0.030), but males’ mean Bayley scores showed no association with ptHb levels (B = +0.621, SE = 0.664, p = 0.359). A post-hoc sensitivity analysis was subsequently conducted, in which the same regression analyses and sex interactions were performed after excluding infants diagnosed with any grade of IVH or PVL. This analysis did not change the results of any cognitive sub-scale or composite scores regression or sex interaction significance.
Table 6.
Pooled Regression Analysis of Covariance of Pre-Transfusion Hgb with Five Bayley Subscales*
| Male (N=17) | Female (N=13) | Sex Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B† | SE | t | p | B† | SE | t | p | Difference** | SE | t | (p / M=F) | |
| Cognitive | 0.959 | 1.167 | 0.82 | 0.420 | −1.264 | 0.837 | −1.51 | 0.145 | 2.223 | 1.436 | 1.55 | 0.135 |
| Expressive Language | 0.360 | 0.972 | 0.37 | 0.714 | −1.252 | 0.725 | −1.73 | 0.098 | 1.613 | 1.212 | 1.33 | 0.196 |
| Receptive Language | −0.428 | 0.759 | −0.56 | 0.578 | 0.264 | 0.537 | 0.49 | 0.628 | −0.692 | 0.938 | −0.74 | 0.468 |
| Fine Motor | 0.455 | 0.683 | 0.67 | 0.512 | −0.115 | 0.502 | −0.23 | 0.822 | 0.569 | 0.846 | 0.67 | 0.508 |
| Gross Motor | 1.758 | 1.232 | 1.43 | 0.167 | −3.758 | 1.395 | −2.69 | 0.013 | 5.516 | 1.864 | 2.96 | 0.007 |
| Mean | 0.621 | 0.664 | 0.94 | 0.359 | −1.225 | 0.528 | −2.32 | 0.030 | 1.846 | 0.851 | 2.17 | 0.041 |
Controlling for birth weight, GA, and chronological age at cognitive assessment; Four participants had missing hemoglobin values and are excluded from this analysis
B, Beta coefficient estimate, fitted slope of relationship between predictor (ptHb) and outcome variable (Bayley subscale raw score or mean)
SE, Standard Error of Beta Coefficient
Difference in Beta coefficients between males and females
Figure 3. Pooled Regression Models of Cognitive Measures at 12 months old with Mean Pre-Transfusion Hemoglobin Level (g/dL).
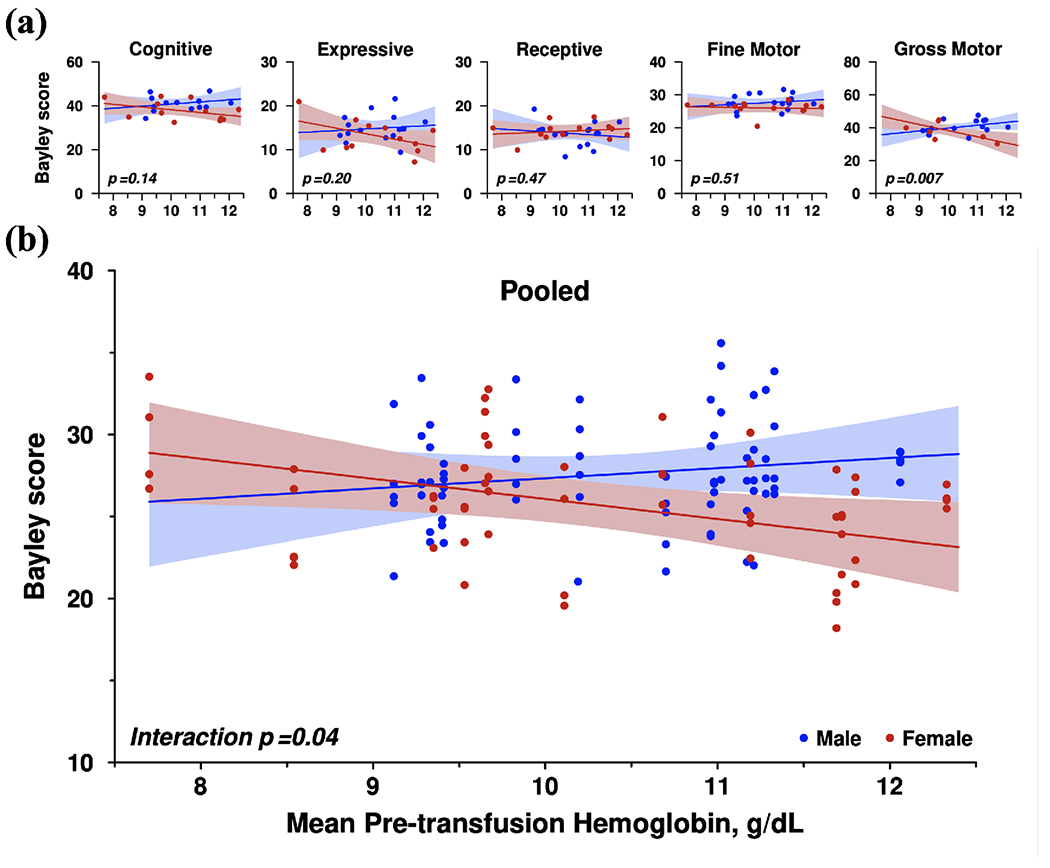
- Regression Models of Five Individual Subscales (cognitive, expressive language, receptive language, fine motor, gross motor) of Bayley Scales of Infant & Toddler Development, 3rd edition (Bayley-III) with Mean Pre-transfusion Hemoglobin Level
- Pooled Regression Model of Bayley Scores with Mean Pre-transfusion Hemoglobin Level; Adjusted to common scale mean
DISCUSSION
The current study demonstrates the sex-specific differences in both brain structure at term-equivalent age and brain function at 12 months of age in response to hemoglobin level in the preterm infant. Neonatal cerebral white matter volume was directly related to hemoglobin level in males only, in whom higher ptHb was associated with higher white matter volumes, while in females there was no such association. A converse pattern was found in the 12-month developmental scores, in which higher ptHb predicted lower (worse) Bayley-III gross motor and overall mean scores in females only, while in males there was no such association. Taken together, these results suggest that males with the lowest hemoglobin levels would have the worst outcomes, while females with the highest hemoglobin levels may have the worst outcomes. This pattern of sex-specific findings as a function of pre-transfusion hemoglobin (ptHb) level is shown in (Figure 4; available at www.jpeds.com).
Figure 4. Sex-Specific Outcomes Based on Hemoglobin Levels.
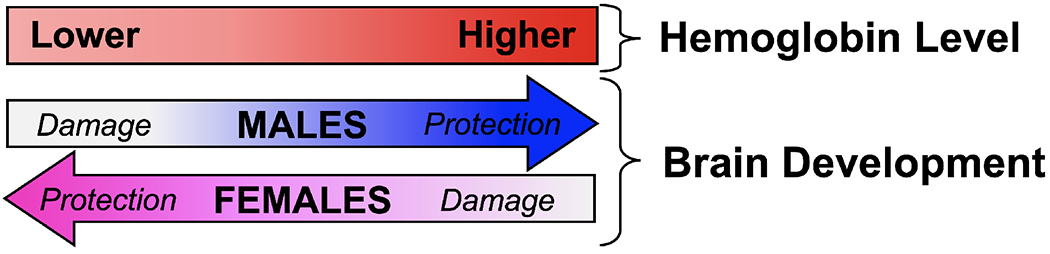
Hypothesis of Sex-Specific Effect of Hemoglobin Level on Brain Development
The current findings from short term outcomes in infancy are supportive of our findings evaluating long-term outcomes of infants assigned to higher or lower hemoglobin thresholds for transfusion, where females in the higher hemoglobin threshold group had the worst outcomes in regard to both brain structure (volume of white matter) and brain function (cognitive skills) 20–22. Also, in support of this sex-specific hypothesis is a study in which cognitive outcomes were studied in premature infants who were randomized to delayed cord clamping (DCC) or early cord clamping (ECC). These two groups can be considered parallel to our groups in which DCC would be similar to a higher threshold group and ECC would be analogous to a lower threshold group. At 12-month follow-up, scores on the Ages and Stages Questionnaire were 12 points lower in the females in the DCC compared with females in the ECC group while males, in the ECC group had scores that were 5 points lower than the boys in the DCC group, resulting in a significant sex by group interaction 26. This is the same pattern of poorest outcome in higher threshold females and lower threshold males found in both our short term outcomes (current study) and long-term outcomes studies of infants randomized to higher or lower hemoglobin thresholds for transfusion.
Among the important factors to consider in evaluations of transfusion status on neurodevelopmental outcomes are methodology of analysis and exploration of sex-specific outcomes. In regard to methodology, instead of defining binary categories of liberal versus restrictive, and death/NDI versus no death/NDI, a more powerful approach is identification of continuous measures of transfusion status and neurodevelopmental outcomes rather than categorical variables. That is, the continuous measures used in this study provided a more granular approach to outcomes by identifying measures that relate to the variability in transfusion status and predict the spectrum of functional outcomes. Secondly, sex differences are critical biological variables in investigations of brain structure and function 16–18. As such, we have reported on sex-specific findings of prematurity 19 as well as in the context of the impact of transfusion status on brain structure and function outcomes 20–23. We found that females have worse brain structural and functional outcomes with liberal transfusion strategies OBJ. Given the pattern of sex-specific outcomes, it is likely that there are sex-specific mechanisms that underlie the different patterns of response to RBC transfusion. (Figure 5; available at www.jpeds.com) features a hypothesis in which higher transfusion thresholds in females may lead to an inflammatory process affecting brain development, whereas in males with lower transfusion thresholds, anemia is the driver for impaired brain development.
Figure 5. Potential Sex-Specific Mechanisms Underlying Neurodevelopmental Outcomes of Transfusion.
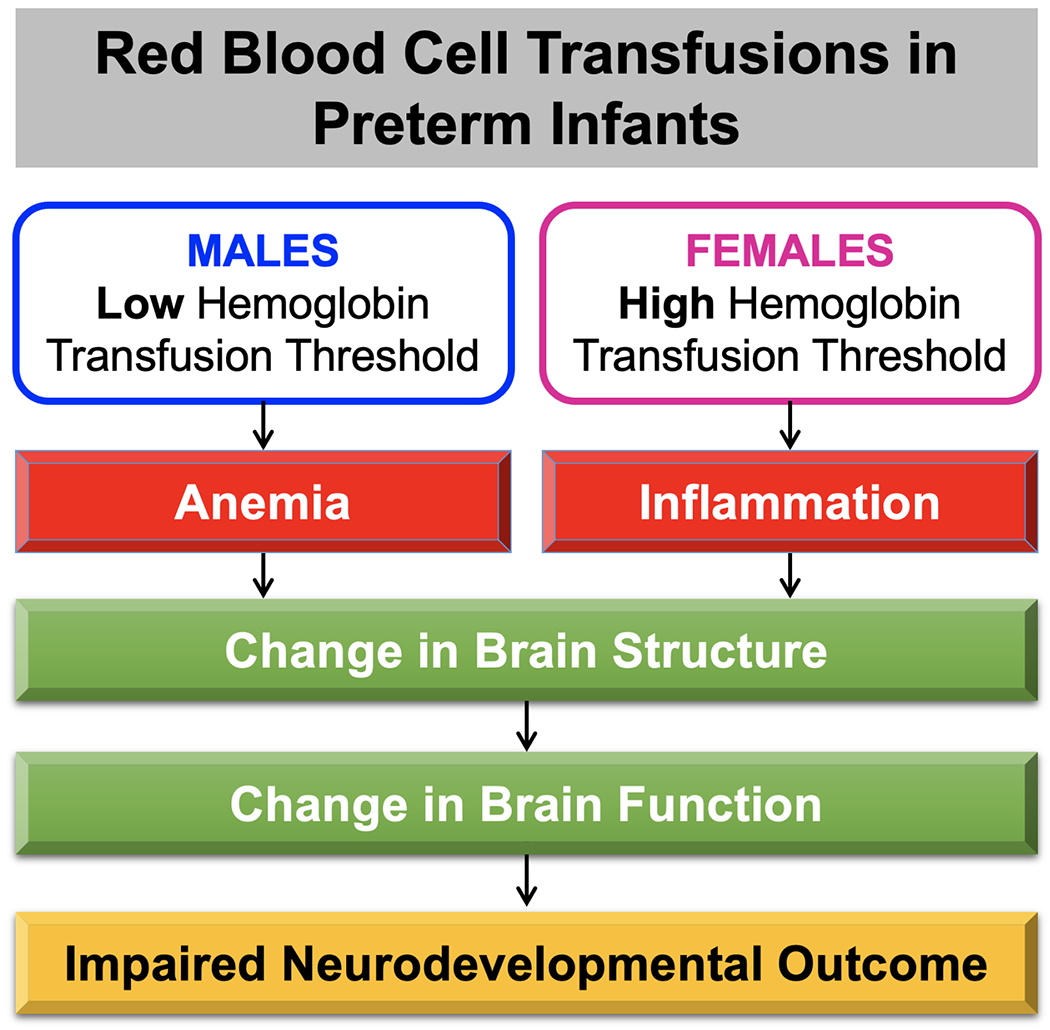
Schematic representation of potential mechanisms of brain injury, in which females with liberal transfusions leads to an inflammatory process affecting brain development, whereas males with restricted transfusions points to anemia as the driver for impaired brain development.
In regard to females and cerebral white matter maldevelopment, two major pathologic hallmarks of injury are activation of microglia and depletion of “premyelinating” oligodendrocytes 27. Premyelinating oligodendrocytes are highly vulnerable to injury by excitotoxicity, free radicals, and proinflammatory cytokines. Perinatal inflammation has been proposed as a risk to white matter in particular and a potential precursor to later neurodevelopmental deficits. , pro-inflammatory cytokine production and endothelial activation after RBC transfusion, often referred to as transfusion-related immunomodulation, has been proposed to underlie several neonatal morbidities 28, 29. Two potential factors are that white matter in females is more vulnerable to inflammation, or that inflammatory processes are more robust in females. Our study evaluating the same cohort of TOP infants as described here found a sex-specific difference in the neonatal inflammatory response to RBC transfusion, showing a rise in monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and interferon gamma-induced protein 10 (IP-10), all of which were limited to females 30. Further, elevations of MCP-1, which has been shown to be associated with brain injury in premature infants 31, were associated with lower Bayley-III cognitive, language, and motor scores at 12 months in these preterm infants. These findings, in conjunction with the current findings, lend support to the notion that the mechanism by which female premature neonates are more vulnerable to abnormal brain development is via inflammation.
A potential distinctive mechanism for abnormal brain development after transfusion in males is anemia, which results in an overall lack of vital substrates such as oxygen and iron. Iron deficiency has been shown to be vital for brain development, and even initial iron deficiency that is later corrected can have long-term impacts 32. Moreover, studies have shown that male infants have lower iron stores than females 33, 34. In addition, an animal model of chronic hypoxia showed sex-specific deficits in which male brain development was highly vulnerable whereas females were mostly unaffected 35. These findings support the theory that for males, factors driving poor outcomes in those with restricted transfusions may be due to anemia. A limitation of the current study is that we are unable to answer the question of whether or not anemia itself is associated with the observed deficits in brain structure and function, as most extremely low birth weight infants will receive at least one RBC transfusion during their neonatal intensive care hospital stay.
One significant limitation of the current neurocognitive and brain structural associations with ptHb in this study were the small sample sizes in regression analyses for a number of reasons: marked loss to follow-up at 12 months for Bayley assessment, inherent difficulty in imaging neonates without sedation, and the exclusionary criterion for participation in brain imaging in this study at an institution with surgical clips used for patent ductus arteriosus (PDA) closures. Therefore, there is the potential for selection bias introduced by this low follow-up rate. In order to reduce the influence of outliers in this small sample, as well as account for wide leverage points in the data, robust linear regression models with MM-estimations were used in these analyses to account for the small sample sizes. It is important to note that the significant interactions demonstrating structural and functional outcomes (i.e. white matter volume or Bayley-III score) by sex, especially in the small sample size of this study, add weight to the hypothesis that the associations observed are truly sex-specific. Larger sample sizes may provide further evidence of these associations by allowing for identification of sex-specific differences in brain structural drivers of cognitive outcomes, or structure-function correlations. However, it should also be highlighted that there were no significant differences between participants and non-participants (for both the brain structural and cognitive outcome arms of the study) in all demographic characteristics, including gestational age, birth weight, age at testing, presence of IVH/PVL, or measures of hemoglobin in this sample. These are all potential confounders in brain outcome studies, but with no differences demonstrated between infants that were included in the follow-up studies and those that were not, there may be increased confidence that the results of this study are generalizable to other preterm infants. Another limitation in this study was that there were more males (N=19) than females (N=10) with high-quality MRI scans. Although females had descriptively lower WM volumes in association with increased ptHb in this study, there remains the possibility that the increased structural WM volume observed in the males is the norm for all premature infants, rather than a specific neurodevelopmental sequela for the preterm males in the higher threshold group in this study. A third limitation to the current study includes the fact that although the pre-transfusion hemoglobin is a readily available and easily measured serum marker in the clinical setting, the actual effects of hemoglobin on the brain are likely multifactorial. However, an averaged value of ptHb throughout the hospitalization may be a better continuous marker of the overall oxygen-deficient state, or lack thereof, of the infant over several weeks to months during a critical time in development it may also represent the sustained result of increased transfusions, e.g. a mounted inflammatory response, rather than a transient response to another biologic process in the infant.
This study investigated the potential association of average ptHb, which was obtained as a longitudinal measurement throughout the hospitalization, with structural brain volumes in the neonatal period in preterm infants, and cognitive functioning at 12 months old. Our results show sex-specific findings of the poorest outcomes in males managed with lower hemoglobin thresholds for transfusion and females with higher transfusion thresholds. Possible mechanisms for these findings were explored. Future investigations will be necessary to validate the sex-specific differences we observed in this subset by studying larger numbers of subjects, as well as to elucidate the mechanisms of adverse brain outcomes, both in the neonatal period and later in development.
Supplementary Material
Acknowledgments
This design and conduct of this research study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript were made possible by National Institute for Heart, Lung, and Blood (NHLBI) Parent Project Grant #5P01HL046925-22, Sub-Project ID 5779, “IMMUNOLOGIC AND NEURODEVELOPMENTAL CONSEQUENCES OF NEONATAL ANEMIA AND THROMBOCYTOPENIA AND THEIR TREATMENTS – Project 4”, and National Institute for Mental Health (NIMH) Program Grant #5T32MH019113-26 “Iowa Neuroscience Specialty Program in Research Education (INSPIRE) Program Grant.” The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. [DOI] [PubMed] [Google Scholar]
- [2].Orchinik LJ, Taylor HG, Espy KA, Minich N, Klein N, Sheffield T, et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. Journal of the International Neuropsychological Society : JINS 2011;17:1067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arpino C, Compagnone E, Montanaro ML, Cacciatore D, De Luca A, Cerulli A, et al. Preterm birth and neurodevelopmental outcome: a review. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2010;26:1139–49. [DOI] [PubMed] [Google Scholar]
- [4].Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. [DOI] [PubMed] [Google Scholar]
- [5].Allen MC. Neurodevelopmental outcomes of preterm infants. Current opinion in neurology. 2008;21:123–8. [DOI] [PubMed] [Google Scholar]
- [6].Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA : the journal of the American Medical Association. 2003;289:3264–72. [DOI] [PubMed] [Google Scholar]
- [7].Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA : the journal of the American Medical Association 2002;288:728–37. [DOI] [PubMed] [Google Scholar]
- [8].Bell EF. Red cell transfusion thresholds for preterm infants: finally some answers. Archives of disease in childhood Fetal and neonatal edition. 2021. [DOI] [PubMed] [Google Scholar]
- [9].Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. Jama. 2016;315:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang YC, Chan OW, Chiang MC, Yang PH, Chu SM, Hsu JF, et al. Red Blood Cell Transfusion and Clinical Outcomes in Extremely Low Birth Weight Preterm Infants. Pediatrics and neonatology. 2017;58:216–22. [DOI] [PubMed] [Google Scholar]
- [11].Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. The Journal of pediatrics. 2006;149:301–7. [DOI] [PubMed] [Google Scholar]
- [13].Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383:2639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aylward GP. Update on neurodevelopmental outcomes of infants born prematurely. Journal of developmental and behavioral pediatrics : JDBP 2014;35:392–3. [DOI] [PubMed] [Google Scholar]
- [15].Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ, Victorian Infant Collaborative Study G. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013; 131:e1053–61. [DOI] [PubMed] [Google Scholar]
- [16].Cahill L Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. [DOI] [PubMed] [Google Scholar]
- [17].Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benavides A, Metzger A, Tereschenko A, Conrad A, Bell EF, Spencer J, et al. Sex-specific alterations in preterm brain. Pediatric research. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Benavides A, Conrad AL, Brumbaugh JE, Magnotta V, Bell EF, Nopoulos P. Long-term outcome of brain structure in female preterm infants: possible associations of liberal versus restrictive red blood cell transfusions. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Archives of pediatrics & adolescent medicine. 2011;165:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McCoy TE, Conrad AL, Richman LC, Brumbaugh JE, Magnotta VA, Bell EF, et al. The relationship between brain structure and cognition in transfused preterm children at school age. Developmental neuropsychology. 2014;39:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2011;17:347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Transfusion of Prematures Trial (TOP). 2000 Feb 29 ed. Bethesda (MD): National Library of Medicine (US); 2012. [Google Scholar]
- [25].Kim RE, Nopoulos P, Paulsen J, Johnson H. Efficient and Extensible Workflow: Reliable Whole Brain Segmentation for Large-Scale, Multi-center Longitudinal Human MRI Analysis Using High Performance/Throughput Computing Resources. In: Oyarzun L, et al. , editor. Clinical Image-Based Procedures Translational Research in Medical Imaging CLIP 2015 Lecture Notes in Computer Science. Cham: Springer; 2016. [Google Scholar]
- [26].Andersson O, Domellof M, Andersson D, Hellstrom-Westas L. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA pediatrics. 2014;168:547–54. [DOI] [PubMed] [Google Scholar]
- [27].Kaindl AM, Favrais G, Gressens P. Molecular mechanisms involved in injury to the preterm brain. Journal of child neurology. 2009;24:1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kinjo T, Ohga S, Ochiai M, Honjo S, Tanaka T, Takahata Y, et al. Serum chemokine levels and developmental outcome in preterm infants. Early human development. 2011;87:439–43. [DOI] [PubMed] [Google Scholar]
- [29].Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatric research. 2014;75:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Benavides A, Bell EF, Georgieff MK, Josephson CD, Stowell SR, Feldman HA, et al. Sex-specific cytokine responses and neurocognitive outcome after blood transfusions in preterm infants. Pediatric research. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lu H, Huang W, Chen X, Wang Q, Zhang Q, Chang M. Relationship between premature brain injury and multiple biomarkers in cord blood and amniotic fluid. J Matern Fetal Neonatal Med. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- [32].Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity. Developmental neuroscience. 2016;38:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Domellof M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, Hernell O. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–52. [DOI] [PubMed] [Google Scholar]
- [34].Tamura T, Hou J, Goldenberg RL, Johnston KE, Cliver SP. Gender difference in cord serum ferritin concentrations. Biol Neonate. 1999;75:343–9. [DOI] [PubMed] [Google Scholar]
- [35].Lan WC, Priestley M, Mayoral SR, Tian L, Shamloo M, Penn AA. Sex-specific cognitive deficits and regional brain volume loss in mice exposed to chronic, sublethal hypoxia. Pediatric research. 2011;70:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


