Abstract
Asthma, characterized by airway hyperresponsiveness, inflammation and remodeling, is a chronic airway disease with complex etiology. Severe asthma is characterized by frequent exacerbations and poor therapeutic response to conventional asthma therapy. A clear understanding of cellular and molecular mechanisms of asthma is critical for the discovery of novel targets for optimal therapeutic control of asthma. Metabolomics is emerging as a powerful tool to elucidate novel disease mechanisms in a variety of diseases. In this review, we summarize the current status of knowledge in asthma metabolomics at systemic and cellular levels. The findings demonstrate that various metabolic pathways, related to energy metabolism, macromolecular biosynthesis and redox signaling, are differentially modulated in asthma. Airway smooth muscle cell plays pivotal roles in asthma by contributing to airway hyperreactivity, inflammatory mediator release and remodeling. We posit that metabolomic profiling of airway structural cells, including airway smooth muscle cells, will shed light on molecular mechanisms of asthma and airway hyperresponsiveness and help identify novel therapeutic targets.
Keywords: Metabolism, Big data, Airway smooth muscle, Asthma
1. Introduction
Metabolome identifies the global collection of small molecules generated from cellular metabolic processes (Sun and Hu, 2016). Technically, metabolomics and metabonomics are used interchangeably. Metabolomics analyzes and defines the entire metabolome in organisms and multicellular systems, while metabonomics describes the metabolic changes in the system often in response to an evoked phenotype or interventions (Nicholson and Lindon, 2008). Compared to other “omics” approaches measuring global cellular changes, metabolomics characterizes the dynamic changes of small molecules generated from a multitude of complex intracellular processes (Fiehn et al.,2000). In fact, the metabolomic profile of a cell represents the downstream output of the transcriptomic and proteomic changes and the upstream input to the phenotype. Therefore, metabolomic profile encompasses information captured in transcriptomic and proteomic profiles of the cell.
2. Targeted vs untargeted metabolomics
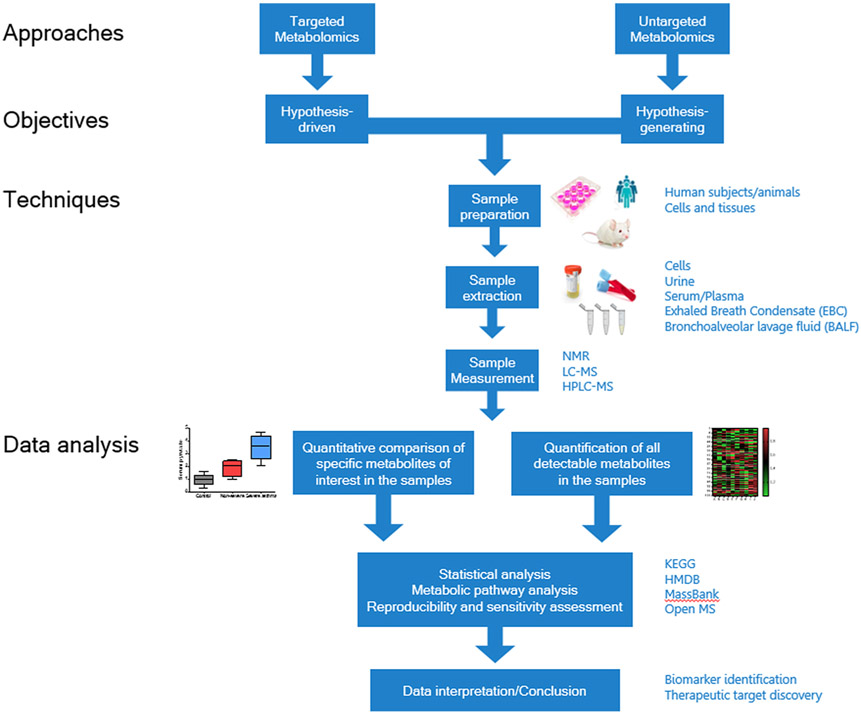
Based on analytical methods used, metabolomics can comprise untargeted and targeted metabolomics (Fig. 1). When it is driven by a specific hypothesis, metabolomic screening is conducted in a targeted manner for selected metabolites (Dudley et al., 2010). The targeted approach optimizes sample preparation and accurately identifies associations among pre-selected metabolites and a defined physiological context (Roberts et al., 2012). Toxicological studies in animal models and pharmacokinetic investigations in drug discovery benefit mostly from targeted metabolomic approaches (Kantae et al., 2017; Ramirez et al., 2013).
Fig. 1. Workflow of metabolomics-based asthma study.
A typical metabolomic workflow in asthma involves either targeted metabolomics to test a hypothesis or untargeted metabolomics approach to generate testable hypotheses. In targeted approach, quantitative comparison of pre-selected metabolites leads to identifying profiles related to disease states or treatment conditions. In the untargeted approach, disease or treatment-associated profiles can still be elucidated, however, bench validation is critical for testing hypotheses. The end result of both approaches is to identify disease-selective diagnostic biomarkers and understanding novel mechanisms of disease, which potentially lead to novel therapeutic targets. NMR – Nuclear Magnetic Resonance, LC-MS – Liquid Chromatography – Mass Spectrometry, HPLC-MS – High-Performance Liquid Chromatography – Mass Spectrometry, KEGG – Kyoto Encyclopedia of Genes and Genomes, HMDB – Human Metabolome Database, MassBank – MassBank Europe Spectral Database, OpenMS – Open-source software for mass spectrometry.
In untargeted metabolomics, the analysis is unbiased and screens all metabolites in the system in an exploratory manner to generate hypotheses. Untargeted metabolomic screening comprehensively reflects the metabolic profiles of the organism, tissue, or the selected cell types and facilitates identification of novel biomarkers and therapeutic targets (Patti et al., 2012).
3. Technical approaches in metabolomics
Currently, nuclear magnetic resonance (NMR) and mass spectrometry (MS) represent widely used analytical platforms in metabolomics. Generally, NMR is considered a non-destructive method with high reproducibility and capacity to identify chemical structures. In recent years, however, MS-based metabolomics has gained momentum over NMR due to improved sensitivity and wide availability (Dunn et al., 2005; Markley et al., 2017; Zhang et al., 2012). The MS platforms have advanced with recently-introduced cutting-edge technologies, such as BAYESIL system, matrix assisted laser desorption/ionization (MALDI), and desorption/ionization on silicon (DIOS) system (Greving et al., 2011; Ravanbakhsh et al., 2015; Warren G. Lewisa, 2003; Wittmann and Heinzle, 2001). The critical component of unbiased metabolomics is the data analysis with bioinformatic interfaces to derive meaningful biological inferences. The Kyoto Encyclopedia of Genes and Genomes (KEGG), Human Metabolome Database (HMDB), and Open MS are the prominent databases currently in use (Du et al., 2013; Fessenden, 2016).
In summary, metabolomics provides an integrative platform to understand the pathophysiologic basis of a variety of diseases (Cirulli et al., 2019; Guasch-Ferre et al., 2016). Studies suggest that asthma, especially the obesity-associated asthma, is driven by altered metabolic status (Cottrell et al., 2011; Singh et al., 2013). In this review, we will focus on the application of metabolomics in asthma studies, with an emphasis on airway structural cells.
4. Metabolomics studies in asthma
Asthma is a heterogeneous chronic airways disease, with a complex etiology (Diette et al., 2008; Ober and Hoffjan, 2006). Over the last three decades, “omics” approaches encompassing genomics, transcriptomics, epigenomics, and proteomics, have immensely helped understand the underlying disease mechanisms of asthma (Kan et al., 2017). However, the complex etiology and heterogeneity of asthma can also benefit from the use of novel tools such as metabolomics.
Recent research links metabolomic profiles to asthma by focusing on metabolites as systemic biomarkers (Kelly et al., 2017a; Licari et al., 2018; Pite et al., 2018; Snowden et al., 2012). We will review studies that have focused on asthma-derived biospecimens and metabolites associated with disease onset and severity.
4.1. Urine-based metabolomics
Because of its non-invasive and convenient sampling methods, urine is a widely used body fluid in metabolomic studies aimed to identify biomarkers of physiological status (Bouatra et al., 2013). A variety of metabolites in urine reportedly differentiate between asthma and healthy participants (Loureiro et al., 2016; Quan-Jun et al., 2017; Saude et al., 2011). According to urine-based metabolomic profiles, Citric Acid Cycle (Tricarboxylic Acid cycle, TCA cycle) appears to be a predominant pathway altered in asthma patients. TCA cycle is the terminal common pathway for oxidative catabolism of the three macronutrients, carbohydrates, lipids, and proteins. As the central metabolic hub in eukaryotic cells, the TCA cycle provides substrates for ATP generation and facilitates biosynthesis of non-essential fatty acids and amino acids (Jeremy, Berg, 2002; Victor W. Rodwell et al., 2018). In children with asthma, urinary metabolomic profiles, dominated by TCA metabolites, such as 2-oxoglutarate, succinate, threonine, and cis-aconitate, differentiated participants with stable and unstable asthma (Saude et al., 2011). These data suggest a perturbed TCA cycle is associated with childhood asthma and could serve as potential urinary biomarkers to predict poorly controlled pediatric asthma. However, potential confounding effects of asthma medications, such as glucocorticoids, should be carefully considered. Oxidative stress also represents a hallmark of airway inflammatory disorders, such as asthma and COPD (Kirkham and Rahman, 2006; MacNee, 2001). Mitochondrial electron transport chain (ETC) consists of a series of complexes transferring electrons at the inner membrane. Electrons leaked during ETC react with oxygen molecules to generate reactive oxygen species (ROS), causing oxidative stress in the cells (Pan et al., 2019). The lipids in membranes and biological fluids provide prime targets of oxidative damage induced by the ROS. Therefore, lipid peroxidation products are often measured to assess oxidative stress in vivo (Yoshida et al., 2013). In the asthma patients, lipid peroxidation products in urine, such as 2-methylpentane and octane, correlated with clinical parameters of asthma severity, including Forced Expiratory Volume in 1 s (FEV1) and eosinophilia (Loureiro et al., 2016). Eicosanoids-comprised of prostaglandins, cysteinyl leukotrienes and isoprostanes-are lipid mediators synthesized and rapidly metabolized in response to inflammatory stimuli and therefore studied in asthma as diagnostic and treatment response biomarkers (Sokolowska et al., 2021; von Moltke et al., 2012). For instance, elevated urinary leukotriene E4 (LTE4) and prominent PGD2 metabolites predicted severe and Th2-biased asthma in pediatric and adult patients. (Kolmert et al., 2021). On the other hand, urinary metabolomics can also be useful in measuring metabolic outcomes of asthma therapy. The conventional asthma treatment includes β2 adrenergic receptor (β2AR) agonists as bronchodilators and glucocorticoids as anti-inflammatory agents. Children with asthma treated with β2AR and glucocorticoids manifested urinary markers of altered TCA cycle and pyruvate metabolism, characterized by decreased citrate and increased lactate levels (Quan-Jun et al., 2017).
Although urine-based metabolomics plays an important role in characterizing both pulmonary and systemic metabolism, the quality and accuracy of urinary metabolomic screening can be compromised by multiple factors. Firstly, it is difficult to precisely control the dilution of urine samples, influenced by an array of factors including hydration status, diuretics use, collection time, and renal pathology. For instance, a study found almost a quarter of screened metabolites in urine showed a rhythmic variation during 24 h cycle (Giskeodegard et al., 2015). However, semi-quantitative strategies, such as normalization to total ion current, osmolality, and common components like creatinine, help optimize urinary metabolomic analysis (Miller et al., 2019; Warrack et al., 2009). Additionally, the pH values and ionic species variations in urine may chemically modify some urinary metabolites, and confound the original metabolomic profiles (Vincent M. Asiago et al., 2008). These variations can be minimized by introduction of buffering systems in sample collection and processing (Vincent M. Asiago et al., 2008; Xiao et al., 2009).
4.2. Serum/plasma metabolomics
Currently, serum and plasma are the predominant biological fluids examined in metabolomic studies. Blood-based metabolic profiling has identified metabolomic endotypes of asthma, including increased synthesis of bile acids and altered steroid and amino acid metabolism (Comhair et al., 2015). Amino acids and fatty acids are macromolecules critical for a variety of cellular functions, such as biosynthesis, inflammation, and homeostasis. Plasma or serum-based metabolomic studies in human and animal models have illustrated the association of amino acids, nucleic acid metabolites, and lipid derivatives with asthma and identified metabotypes of asthma. The serum concentrations of lipid mediators and hormones, such as oleoylethanolamide, dehydroepiandrosterone sulfate, and cortisone, corelate with asthma severity (Reinke et al., 2017). Plasma metabolomic profiles in ovalbumin (OVA)-induced allergic AHR mouse model showed dysregulated metabolisms of purine, glycerophospholipids, and tryptophan (Yu et al., 2016). In mammals, purine metabolism generates uric acid, which at its homeostatic levels scavenges oxidative free radicals and provides antioxidant defense (Peden et al., 1990). As an indicator of oxidative stress, serum uric acid can be used as a biomarker for asthma severity (Abdulnaby et al., 2016). Elevated serum uric acid levels are also detected during acute asthma exacerbation (Li et al., 2014). Arginine is another amino acid linked to antioxidant pathways through its metabolism to nitric oxide (NO). NO functions as an antioxidant to limit oxidative injuries in the cells that is associated with both broncho-dilatory and anti-inflammatory effects (Prado et al., 2011; Ricciardolo, 2003; Wink et al., 2001). Studies in human subjects have identified metabolomic signatures of asthma linked to NO. Disrupted arginine synthesis in asthma results in reduced arginine bioavailability and NO deficiency (Jung et al., 2013; Lara et al., 2008; Xu et al., 2017). The Fraction of exhaled NO (FeNO) is a biomarker for eosinophilic airway inflammation. Studies showed that serum levels of metabolites taurine and nicotinamide are elevated in severe asthma patients and correlate with high FeNO levels (Comhair et al., 2015). Interestingly, plasma metabolic profile is also useful to distinguish other allergic diseases, such as food allergy. Individuals with asthma showed decreased serum fatty acid levels, a finding similar to that in children with food allergy (Crestani et al., 2020). On the other hand, some biomarkers help differentiate between asthma and other allergic disorders. For instance, sphingomyelins are reported to be decreased in food allergy while increased in asthma (Crestani et al., 2020).
Nevertheless, various pre-analytical factors should be addressed in serum or plasma metabolomics. Blood coagulation is an important factor to consider in sample selection between serum and plasma. Collection tubes and collection time may modify the metabolic profiles, including the abundance of arachidonic acid, arginine, sarcosine, and kynurenine (Cruickshank-Quinn et al., 2018; Paglia et al., 2018). Additional confounding factors such as temperature and centrifugation may also contribute to unique metabolic patterns, due to metabolites released from platelets or enzymes activated in thrombosis (Liu et al., 2018b). Lastly, sample transportation and storage conditions should also be carefully controlled for optimal reproducibility (Gonzalez-Dominguez et al., 2020; Torell et al., 2017).
4.3. Exhaled breath condensate (EBC) metabolomics
Exhaled breath condensate (EBC) is a biospecimen relevant to airways diseases (Horvath et al., 2005). A variety of biomarkers, including hydrogen peroxide, leukotrienes, and nitrogen oxides, can be measured from EBC to assess airway inflammation and oxidative stress. Volatile organic compounds (VOCs) generated from tissue level metabolic processes are the primary foci in EBC-based metabolomic studies (Filipiak et al., 2012; Rufo et al., 2016). Inflammation, a hallmark of asthma, provides an important source of endogenous VOCs. Reactive oxygen species (ROS) induced by airway inflammation can degrade polyunsaturated fatty acids in membrane lipids that in turn generates VOCs (Dallinga et al., 2010). Specific EBC-based VOCs, such as butanoic acid and 3-(1-methylethyl)-benzene, can be used to diagnose pediatric asthma with high sensitivity and specificity (Dallinga et al., 2010). Furthermore, EBC-based VOC markers, such as 3,7-dimethylnonane, nonanal, and 1-propanol, can potentially distinguish between neutrophilic and eosinophilic types of asthma (Schleich et al., 2019). This distinction is critical to identify severe, corticosteroid-insensitive patients with asthma such that novel therapeutics can be developed (Carr et al., 2018; Fahy, 2009; Wang et al., 2016). Collectively, these studies suggest that EBC-derived VOCs may serve as promising biomarker candidates for diagnosing, phenotyping and monitoring therapeutic responses (Rufo et al., 2016). In addition to VOCs, EBC is also a source of other macromolecules and secretory markers relevant to lung pathology. For instance, the Vitamin A metabolite retinoic acid, prostaglandin derivatives (i.e.: 20-hydroxy-PGF2a, thromboxane B2), and nucleosides such as adenosine, are also present in EBC and their levels are altered in severe asthma patients (Carraro et al., 2013; Leung et al., 2013; Sinha et al., 2017).
Despite being a convenient and physiologically relevant sample for metabolomic screening, EBC has its limitations. Cysteinyl leukotrienes are elevated in EBC collected from moderate and severe asthma patients (Samitas et al., 2009). However, factors such as saliva contamination in EBC results in inaccurate measurements of metabolites, including amino acids and eicosanoids (Cruickshank-Quinn et al., 2017). Also, the metabolites in the bronchoalveolar region may not be accurately represented in the exhaled air captured from the upper airways. For instance, nonanal and decanal, two metabolites involved in lipid peroxidation and inflammation, significantly increased in the BALF from OVA-treated mice but remained low in EBC (Neuhaus et al., 2011). Thus, BALF from murine asthma models remains a preferred sample to investigate metabolomic basis of asthma pathogenesis. Mirroring the findings from other sample types, metabolites associated with energy, amino acid, lipid, and sterol metabolisms are altered in BALF from murine models of asthma (Ho et al., 2013; Jun Peng et al., 2014).
4.4. Metabolomics in translational medicine
The aforementioned studies clearly establish that asthma is associated with metabolomic changes, at the pulmonary and systemic levels (Table 1). The altered levels of metabolites detected in urine, blood, and EBC samples in asthma patients underscore the potential uses of these metabolites as diagnostic biomarkers and predictors of treatment outcome. The diagnostic biomarker application of metabolites in asthma was demonstrated in a recent pediatric asthma study, in which plasma sphingolipid levels at 6 months of age predicted early-onset asthma at the age of 3 years (Rago et al., 2021). Similarly, a survey-based study reported increased baseline bromotyrosine as a reliable indicator of poorly controlled asthma in children (Wedes et al., 2011).
Table 1.
Systemic metabolomics studies in asthma.
| Samples | Participants/Models | Metabolism Pathways | Distinct metabolites | Ref# |
|---|---|---|---|---|
| Urine | Children with non-stable asthma (n = 20), stable asthma (n = 73), or healthy controls (n = 42) | Citric Acid Cycle | 2-oxoglutarate, succinate, threonine, cis-aconitate | Saude et al. (2011) |
| Lipid metabolism | 2-hydroxyisobutyrate, 3-hydroxybutyrate, 3-methyladipate | |||
| Children with asthma acute exacerbation (n = 69) | Citric Acid Cycle and pyruvate metabolism | Cis-aconitate, lactate, formic acid, acetate, citrate | Quan-Jun et al. (2017) | |
| Arginine and proline metabolism | Citrulline, sarcosine, ornithine, creatine, creatinine | |||
| Adult subjects with or without asthma (n = 57 in total) | Lipid metabolism | 2,4-dimethylheptane, 4-methylheptane, octanal, heptano | Loureiro et al. (2016) | |
| Adult subjects with mild-to-moderate asthma (n = 86), severe asthma (n = 411), or healthy control (n = 100) | Eicosanoid metabolism | LTE4, TetranorPGDM, 2,3-dinor-11β-PGF2α. | Kolmert et al. (2021) | |
| Children with recurrent wheezing (n = 32) or healthy controls (n = 13) | Tryptophan metabolism | glutaric acid, 5-hydroxy-l-tryptophan, indole-3-acetamide, and 3-indoleacetic acid | Carraro et al. (2018) | |
| Lipid metabolism | N-acryloylglycine and tiglylglycine | |||
| Plasma/Serum | Adult subjects with non-severe asthma (n = 10), severe asthma (n = 10), and healthy controls (n = 10) | Taurine metabolism Bile acid metabolism |
Taurine, Arachidonate, β alanine, Taurocholate, glycodeoxycholate, lathosterol | Comhair et al. (2015) |
| Female, 4-6-week-old BALB/c mice, challenged with OVA or PBS (n = 16 in total) | Purine metabolism | Uric acid, inosine | Yu et al. (2016) | |
| Glycerophospholipid metabolism | Lysophosphatidylcholines, phosphatidylserine | |||
| Patients with acute asthma exacerbations (n = 120), and healthy controls (n = 120) | Uric acid metabolism | Uric acid | Abdulnaby et al. (2016)) | |
| Patients with acute asthma exacerbations (n = 217), and healthy controls (n = 142) | Uric acid metabolism | Uric acid | Li et al. 2014 | |
| Adult subjects with asthma (n = 232), or healthy controls (n = 26) | Arginine metabolism | Methylarginine, ornithine, citrulline | Lara et al. (2008) | |
| Adult subjects with asthma (n = 39), or healthy controls (n = 26) | Methyl transfer pathway | Formate, choline, methionine, O-phosphocholine, methanol | Jung et al. (2013) | |
| Arginine metabolism | Methylarginine, arginine | |||
| Children with asthma (n = 35), food allergy (n = 35), asthma and food allergy (n = 35), or healthy controls (n = 20) | Lipid metabolism Amino acid metabolism |
Glycerophosphoinositol, sphingomyelin, DHEA-S threonine, valine, glutamate | Crestani et al. (2020) | |
| Adult subjects with mild asthma (n = 12), moderate asthma (n = 20), severe asthma (n = 22), or healthy controls (n = 22) | Lipid metabolism | Cortisone, cortisol, DHEA-S Oleoylethanolamide, sphingosine-1-phosphate, 22-hydroxycholesterol, α-linolenic acid, N-palmitoyltaurine, sphinganine-1-phosphate, ceramides, sphingomyelins | Reinke et al. (2017) | |
| Pregnant women (n = 738) and their children (n = 700); Pregnant women (n = 881) and their children (n = 810) | Sphingolipid metabolism | Rago et al. (2021) | ||
| Adult subjects with asthma (n = 51) | Lipid metabolism | Lysophosphatidylglycerol, phosphatidylcholine | Gai et al. (2019) | |
| Adult subjects with asthma (n = 18) or healthy controls (n = 17) | Tryptophan metabolism | Tryptophan, kynurenine, quinolinic acid, anthranilic acid | van der Sluijs et al. (2013) | |
| EBC | Children with asthma (n = 63), or healthy controls (n = 57) | Lipid metabolism | Branched hydrocarbon (C13H28), 1-penten-2-on, butanoic acid, 3-(1-methylethyl)-benzene | Dallinga et al. (2010) |
| Adult subjects with eosinophil or neutrophil asthma (n = 521 in total) | Lipid metabolism | 3,7-dimethylnonane, 1-propanol, nonanal | Schleich et al. (2019) | |
| Children with non-severe asthma (n = 31), severe asthma (n = 11), or healthy controls (n = 15) | Vitamin metabolism | retinoic acid, vitamin D | Carraro et al. (2013) | |
| Adenosine metabolism | Deoxyadenosine | |||
| Children with asthma (n = 89), or healthy controls (n = 20) | Ketogenesis | Hydroxybutyrate, acetate, acetone | Sinha et al. (2017) | |
| Adult subjects with mild asthma (n = 16), moderate asthma (n = 12), severe asthma (n = 15), or healthy controls | Eicosanoid metabolism | 8-isoprostane, cysteinyl-leukotrienes | Samitas et al. (2009) | |
| Adult subjects with asthma (n = 18) or healthy controls (n = 17) | Tryptophan metabolism | Kynurenine, quinolinic acid | van der Sluijs et al. (2013) | |
| Arginine metabolism | Arginine, ornithine | |||
| BALF | Female, 5- to 6-week-old BALB/c mice challenged with | Lipid metabolism | Nonanal, decanal | Neuhaus et al. (2011) |
| Female, 6- to 8-week-old BALB/c mice from 4 groups (sensitized: n = 12, asthma model: n = 12, asthma with dexamethasone: n = 12, or naïve: n = 12) | Lipid metabolism | Choline, hexadecenoylcholine | Ho et al. (2013) | |
| Energy metabolism | Lactate, malate | |||
| Male, 10-week-old Brown Norway inflamed (n = 18), sensitized (n = 9) or naïve (n = 9) rats | Arginine-proline pathway | Arginine, proline | (Jun Peng et al., 2014) | |
| Male, 8-week-old BALB/c mice, sensitized with OVA or saline aerosol (n = 8 in total) | Arachidonic acid metabolism | Prostaglandin D2, 6-keto-prostaglandin F1alpha, 2S-hydroxy-5Z,8E,10E-heptadecatrienoic acid | Chiba et al. (2018) |
Another potential application of asthma metabolomics is identifying distinct endotypes and metabotypes that would aid in personalized treatments. Based on its etiology and diverse pathological features, asthma is classified into different phenotypes and endotypes (Kaur and Chupp, 2019). Recent studies in asthma metabolomics seek to subclassify these asthma phenotypes into metabotypes. Studies suggest that EBC-based VOC markers and serum glycerophospholipids can distinguish between neutrophilic and eosinophilic types of asthma (Gai et al., 2019; Schleich et al., 2019). Other important parameters used for classification of asthma, such as FEV1 and serum IgE levels, correlate well with the altered lipid and nucleotide metabolites (Kelly et al., 2017b; Loureiro et al., 2016). For instance, an untargeted metabolomic screening in urine clearly distinguished early-onset asthma from transient wheezing in preschool children, providing a valuable tool for personalized clinical management of these conditions (Carraro et al., 2018). Similarly, severity of rhinovirus-induced cold was positively correlated with systemic tryptophan catabolites in allergic asthma, suggesting blood screening for these catabolites has a prognostic value in rhinoviral infection and asthma exacerbations (van der Sluijs et al., 2013) Together, these studies support that metabolomics could be potentially used for the identification of asthma endotypes in practice.
Additionally, as previously mentioned, conventional asthma treatments can change urinary metabolomic profiles, suggesting that non-invasive metabolic assays can be developed to assess response to treatment (Kolmert et al., 2021; Quan-Jun et al., 2017). Supporting this potential, VOC profile measured by electronic nose distinguished steroid-responsive and steroid-unresponsive asthma, with relatively higher accuracy than traditional methods such as sputum eosinophils and FeNO (van der Schee et al., 2013). Therefore, further investigations integrating treatment-induced and disease-induced metabolomic profiles in asthma would benefit personalized and precision medicine in asthma.
While the aforementioned studies significantly contributed to biomarker discovery with potential diagnostic applications, elucidating the mechanistic link between asthma and metabolomic signature remains a challenge. In order to understand how metabolites modulate clinical and pathological features of asthma, metabolomic investigations at the cellular levels are essential.
5. Metabolomics in cells
As any cellular process, metabolism is a response to physiological and environmental cues. Metabolomic studies in homogenous cell populations represent a common investigative platform in a wide range of research areas, including drug discovery, diagnostic markers, toxicological assessment and signaling pathway identification (Hayton et al., 2017). Study of metabolomics in homogeneous cell cultures help identify signatures specific to a particular cell type, with minimal interference from other tissue components (Hayton et al., 2017). Single-cell metabolomics also facilitates the investigation of molecular mechanisms underlying the specific disease phenotypes manifested in the resident tissues. Therefore, cell-specific approaches to metabolomic screening offers opportunities to explore disease states driven by phenotypic modifications in single cell types (Table 2).
Table 2.
Cellular metabolomics studies related to asthma.
| Cell types | Metabolism Pathways | Distinct metabolites | Affected cellular functions | Ref# |
|---|---|---|---|---|
| Dendritic cells | Glucose metabolism | Glucose | Motility and migration | Guak et al. (2018) |
| Glucose, lactate | Activation and cytokine production | Krawczyk et al. (2010) | ||
| Macrophages | Glycolysis and citric acid cycle Purine and amino acid metabolism | D-Glucose, glyceraldehyde-3-P, citrate, Malate Carnitine, AMP, cysteine, allantoate, leucine | Proliferation and cytokine production | Mould et al. (2017) |
| Glucose metabolism | Itaconate, GABA, 2-hydroxyglutarate | Polarization | Puchalska et al. (2018) | |
| Amino sugar and nucleotide sugar metabolism | UDP-GlcNAc, UDP-glucose, and UDP-glucuronate | Polarization | Jha et al. (2015) | |
| Glutamine/glutamate metabolism | Glutamine, glutamate, AKG, ornithine | |||
| T-lymphocytes | Glycolysis | Lactate | Activation and proliferation | Ostroukhova et al. (2012) |
| Eosinophils | Lipid metabolism | Protectin D1, 4-hydroxy DHA, arachidonic acid, docosahexaenoic acid | Biosynthetic activities, migration, and adhesion | Miyata et al. (2013) |
| Platelets | Glycolysis and citric acid cycle | ATP | Energy production | Xu et al. (2015) |
| Airway epithelial cells | Glycolysis | Glucose, lactate, pantothenate, ADP | Cytokine production | Qian et al. (2018) |
| Citrate cycle | L-Malic acid, cis-aconitic acid | Energy production | (Qingyu Huang et al., 2015) | |
| Amino acid metabolism | Phenylalanine, tryptophan | Biosynthetic activities | ||
| Glutathione metabolism | Oxidized glutathione | Oxidative stress | ||
| Lipid metabolism | Phosphatidylcholine | Membrane remodeling | Nguyen et al. (2018) | |
| Type II pneumocytes | Lipid metabolism | Phosphatidylcholine | Biosynthetic activities | Liu et al. (1997) |
| Airway smooth muscle cells | Lipid metabolism | Sphingosine 1-phosphate | Proliferation, contraction, and cytokine secretion | (Ammit et al., 2001; Liu et al., 2018a) |
| Vitamin metabolism | Retinoic acid | Migration | Day et al. (2006) |
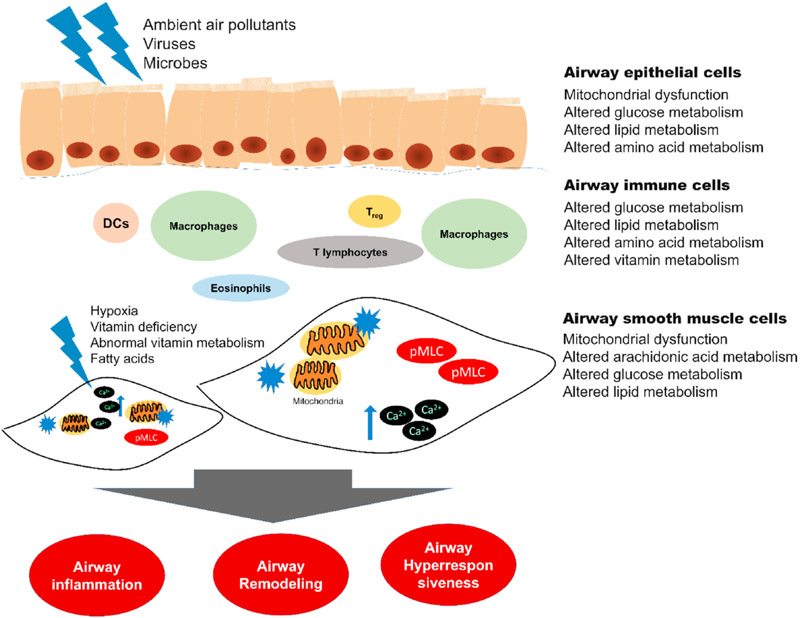
Airway hyperresponsiveness (AHR), inflammation, and airway remodeling are the three pivotal characteristics of asthma and a plethora of pulmonary cells contribute to these functions (Busse and Lemanske, 2001). The phenotypic heterogeneity of asthma presents a challenge for universally successful treatments. Mammalian lungs contain more than 40 different cell types, including airway smooth muscle (ASM) cells, immune cells, epithelial cells, and vascular smooth muscle cells (Franks et al., 2008). Therefore, we posit that single-cell metabolomics studies on select cell types may shed light on mechanisms of the three pivotal characteristics of asthma (Fig. 2).
Fig. 2. Cell types and their metabolic roles in asthma.
Each tissue in lungs plays its unique role in asthma pathology. Each tissue is composed of differentiated cell types, homogeneous or heterogeneous, with critical functions. In these cells, altered mitochondrial functions and metabolism of macro- and micro-nutrients -elaborated in the main text- modulate the specialized cellular functions. Airway smooth muscle (ASM) cells primarily dirve bronchial spasm in asthma, which is charaterized by increased cytosolic Ca2+ and myosin light chain (MLC) phosphorylation. Asthma is characterized by airway inflammtion, remodeling, and hyperresponsiveness. DC - dendritric cells, Treg - regulatory T-lymphocytes, Ca2+ - cytosolic Ca2+, pMLC - phosphorylated myosin light chain.
5.1. Inflammatory cells in asthma
Airway inflammation, a salient feature in asthma, contributes to airway hyperresponsiveness and remodeling (Chapman and Irvin, 2015; Murdoch and Lloyd, 2010). Stimulated by the allergens, antigen-presenting cells (APCs), such as dendritic cells (DCs), process and present the allergens to activate allergen-specific T cells (Gordon, 2002). Activated T helper cells, mostly T helper 2 (Th2) cells, release cytokines and chemokines to elicit cellular responses (Zimmermann et al., 2003). The key TH2 cytokines, IL-4, IL-13, and IL-5, regulate the pivotal events of allergic inflammation, such as B cell activation (IgE synthesis), eosinophil recruitment, and goblet cell metaplasia (Ingram and Kraft, 2012; Lorentz et al., 1999; Maes et al., 2012). Other cell types such as mast cells, regulatory T (Treg) lymphocytes, natural killer (NK) cells, and basophils are also actively involved (Murdoch and Lloyd, 2010). Compelling evidence shows that nutrient-derived metabolites regulate the functions of these inflammatory and immune cells (Ganeshan and Chawla, 2014; Veldhoen and Ferreira, 2015).
5.1.1. Antigen presenting cells (APCs)
As the first responders for injury, APCs, including DCs and macrophages, play fundamental roles in inflammatory responses. Studies showed that a bioenergetic switch from oxidative phosphorylation to glycolysis characterizes migration and differentiation of DCs (Guak et al., 2018; Mishra, 2017). The migration and differentiation of DCs are critical for the downstream interaction of DCs with other immune cells. Also, the inflammatory stimulation of DCs regulates subsequent NO production and reduces coupled respiration, which in turn promotes metabolic reprogramming towards glycolysis in DCs (Guak et al., 2018). This metabolic reprogramming of DCs is primarily supported by glycogen metabolism at the early stage, which can further increase inflammatory cytokine production, including TNF-α. (Krawczyk et al., 2010; Thwe et al., 2017). Another prominent APC in the lungs is the alveolar macrophage, which surveys and provides innate immune defense on alveolar surface. Key metabolic pathways, such as glycolysis, amino acid synthesis, and lipid metabolism have regulatory impact on proliferative and secretory response of the alveolar macrophages (Viola et al., 2019). Increased glycolysis and amino acid catabolism drive macrophages to adopt a pro-inflammatory M1 phenotype, while the pro-resolution M2 macrophages are dependent on oxidative phosphorylation in mitochondria (Puchalska et al., 2018; Viola et al., 2019). These differences may be due to the dependence of M2 macrophages on glutamine synthesis and N-glycosylation for their differentiation and polarization, which requires intermediates supplied with intact TCA cycles (Jha et al., 2015). Meanwhile, aerobic glycolysis is upregulated to meet elevated ATP demand of M1 macrophages during proliferation and differentiation (Koo and Garg, 2019; Van den Bossche et al., 2015; Yu et al., 2020). Resident alveolar macrophages showed increased proliferation that correlated with increase in TCA cycle and amino acid metabolism (Mould et al., 2017). A similar association between metabolism and function is demonstrated in macrophages in extra-pulmonary tissues as well (Liu et al., 2016). Clinical and epidemiological studies show that obesity, a clear outcome of metabolic perturbation, is linked to late-onset asthma and decreased response to mainstream asthma treatment (Kim et al., 2015; Orfanos et al., 2018; Peters et al., 2018). In obesity, elevated accumulation of pro-inflammatory M1 macrophages in adipose tissue is linked to low-grade chronic inflammation, which contributes to various obesity-related co-morbidities (Chawla et al., 2011; Weisberg et al., 2003).
5.1.2. T- lymphocytes
Various subsets of T-lymphocytes have pivotal roles in asthma. Studies have revealed unique metabolic signatures of T cells among asthma patients. Aerobic glycolysis was elevated in T-cells from asthma patients, characterized by increased lactate production, higher expression of hexokinase (HK) and phosphofructokinase (PFKFB) in T cells accompanied with elevated T cell proliferation (Ostroukhova et al., 2012). Pharmacological inhibition of aerobic glycolysis also attenuated airway inflammation and hyperreactivity in a murine model of asthma, suggesting that elevated glycolic pathways play a causal role in AHR (Ostroukhova et al., 2012). These observations resemble the Warburg effect, where neoplastic cells switch their metabolism from oxidative phosphorylation to aerobic glycolysis to enhance proliferation (Warburg et al., 1927). Functions of T regulatory (Treg) cells, which negatively regulate airway inflammation, are also be modulated by metabolites. 12, 13-diHOME, a metabolite of the unsaturated long chain fatty acid linoleic acid, reduced secretion of anti-inflammatory cytokines from DCs, via activation of peroxisome proliferator-activated receptor-γ (PPARγ). Subsequently, the PPARγ activation decreased the number of Treg cells and enhanced lung-resident T cells, neutrophils, and macrophages in an allergen-induced mouse model of asthma (Levan et al., 2019). In pediatric asthma, the serum levels of 12,13-diHOME were positively correlated with airway inflammation and atopy (Levan et al., 2019).
Downstream to APCs and T cells, granulocytic inflammatory cells drive airway inflammation. Eosinophils, a key granulocyte in Th2-driven inflammation, exhibited altered metabolic status in asthma. Protectin D1 (PD1) and 4-hydroxy DHA, metabolites of docosahexaenoic acid (DHA), was lower in asthma patients. Attenuated eosinophilic PD1 level appears to decrease its negative regulation on eosinophils and restrict the pro-resolving functions of eosinophils, which in turn exacerbate eosinophil inflammation in asthma (Miyata et al., 2013). Studies, although limited in number, show that even low-abundant cell types such as natural killer (NK) T cells adopt a unique metabolic profile in inflammatory diseases like asthma (O’Brien and Finlay, 2019). Evidence also shows that platelets, with their innate immune functions, show a metabolic shift characterized by increased TCA cycle in asthma (Li et al., 2012; Xu et al., 2015).
5.1.3. Airway epithelial cells
Airway epithelial cells serve as the first line of defense in lungs by providing physical barrier and their secretory and synthetic roles. Evidence shows metabolic dysfunctions in airway epithelial cells in human asthma and animal models of the disease. In asthma patients and HDM-induced murine models of asthma, airway epithelial cells manifested elevated glycolysis, characterized by activated IL-1/inhibitory kappa B kinase ε (IκBε) signaling (Qian et al., 2018). In a ragweed pollen extract-induced mouse model of allergic asthma, levels of carbonylated proteins were increased in mitochondrial respiratory complexes, leading to mitochondrial dysfunction and ROS generation, which further enhanced epithelial inflammation and airway hyperresponsiveness (Aguilera-Aguirre et al., 2009). This mitochondrial dysfunction has also been widely reported as a pivotal mechanism in lung diseases. Frequent exacerbation is a salient clinical feature of severe asthma and ambient air pollutant exposures are well known causes of this exacerbation. Human airway epithelial cells, following fine particulate matter (PM2.5) exposure, exhibited altered TCA cycle and amino acid biosynthesis, characterized by decreased levels of cis-aconitate and malate, and increased levels of phenylalanine and tryptophan (Qingyu Huang et al., 2015). Importantly, PM2.5 exposure also elevated oxidized GSH (GSSG), disturbed glutathione metabolism, and promoted oxidative damage of mitochondria in airway epithelial cells (Qingyu Huang et al., 2015). Taken together, these metabolic changes imply abnormal oxidative stress status in airway epithelial cells. Rhinovirus, another common cause of asthma exacerbation, modulates lipid metabolism in bronchial epithelium. In primary human bronchial epithelial cells, rhinovirus infection modified the activities of a multiple lipid-modifying enzymes, to alter fatty acid elongation and desaturation, suggesting a regulatory role for these long chain fatty acids in airway epithelial cell function in response to viral infection (Nguyen et al., 2018). Obesity, a risk factor for refractory asthma, is also associated with frequent exacerbations and is positively correlated with metabolic disorders (Baffi et al., 2015; Peters et al., 2018). The surfactant layer, composed of lipids and proteins, lines alveolar epithelia and is critical for normal lung function. Phospholipids are major components of alveolar surfactants, which are essential for surface-tension lowering and innate immunity in the lungs (Agassandian and Mallampalli, 2013). Compared to the lean subjects, those with higher BMI showed unique lipidomic compositions and increased sputum phospholipid concentrations, such as phosphocholines (PC) and phosphoglycerols (PG), in lung epithelial lining fluid (Brandsma et al., 2018). Increased concentrations of PG and phosphatidylethanolamine (PE) species are also found in the lungs from mice fed with obesogenic diet, suggesting a link between obesity and airway epithelial lining lipid composition in the context of obesity-associated asthma (Showalter et al., 2018). Due to their juxtaposition to a variety of other lung cells, airway epithelial cells are also regulated by external metabolites through paracrine signaling. For instance, 12/15-lipoxygenase, an enzyme in prostaglandin synthesis cascade, catalyzes arachidonic acid in alveolar macrophages and fibroblasts (Bryant et al., 1982; Mabalirajan et al., 2013). The metabolites generated from this catabolism, such as 12-S-HETE, act on surrounding airway structural cells and mediate epithelial injury response in airway diseases (Mabalirajan et al., 2013).
The type II pneumocytes lining alveoli secrete surfactants and play mechanical and defense roles in lungs (Andreeva et al., 2007). Phospholipid metabolism, including phosphatidylcholine synthesis, was attenuated in type II pneumocytes isolated from OVA-challenged guinea pigs (Liu et al., 1997). Investigations using gene knockout mouse models suggested a critical role for ATP-binding cassette transporter A1 (ABCA1) in maintaining the lipid homeostasis, including phospholipid and cholesterol content, in the type II pneumocytes (Bates et al., 2005). Repair and regeneration of airway epithelium are critical steps in lung injury response and a variety of airway progenitor cells drive these functions. A recent study showed that glycolysis was critical for proliferation of the airway progenitor cells, Club cells and variant Club cells. Blocking glycolysis attenuated proliferation of club cells but promoted their differentiation into goblet cells and ciliated epithelial cells, suggesting a critical role for metabolic signals in lung epithelial injury response (Li et al., 2019a). As far as we are aware, the metabolic profiles of goblet cells, a key cell type linked to innate defense mechanism, have not been investigated. Further investigations are needed to understand the role of macronutrient metabolism in these cell types in airway epithelium.
5.1.4. Airway smooth muscle cells
Airway smooth muscle (ASM) cells are the pivotal structural cells contributing to AHR. ASM cells isolated from asthma donors show amplified excitation-contraction coupling and agonist-induced contractile response, thus ASM remains as an important therapeutic target in asthma. Studies on ASM cell metabolism have focused on mitochondrial bioenergetics and described that ASM mitochondrial dysfunction is linked to airway diseases such as COPD and asthma (Delmotte and Sieck, 2019; Prakash et al., 2017). For instance, a cigarette smoke (CS) exposure model showed that mitochondrial fission/fusion proteins modulate the proliferation and apoptosis of ASM cells (Aravamudan et al., 2017). In another study to elucidate excitation-energy coupling, proinflammatory cytokine TNF-α attenuated mitochondrial mobility in human ASM cells to modulate cytosolic Ca2+ buffering functions (Delmotte et al., 2017). These observations posit a hypothesis that metabolic alterations in ASM cells contribute to AHR and asthma onset.
AHR, a salient clinical sign in asthma and chronic obstructive pulmonary disease (COPD), manifests as an amplified contractile response of ASM cells to contractile agonists (Chapman and Irvin, 2015). A variety of endogenous mediators, including cytokines and metabolites, are involved in amplifying the contractile response of ASM cells (Black et al., 2012). Products of arachidonic acid metabolism act as key mediators of AHR. Prostaglandin D2 (PGD2) is known as an inflammatory mediator functioning in several diseases, including asthma (Matsuoka et al., 2000; Mohri et al., 2006). In an OVA-induced allergic asthma mouse model, arachidonic acid (AA) metabolism is driven towards PGD2 production pathway by elevated prostaglandin D synthase expression in bronchial smooth muscle tissue, which elicited ASM hyperreactivity (Chiba et al., 2018). Cytosolic Ca2+ mobilization is a key component of excitation-contraction coupling in ASM cells. Agonist-induced cytosolic Ca2+ mobilization initiates a signaling cascade to activate myosin light chain kinase (MLCK) and MLC phosphorylation resulting in cell shortening in ASM cells (Kamm and Stull, 1985, 2001; Somlyo and Somlyo, 2003). In bronchial smooth muscle cells derived from asthma patients, calcium/calmodulin-dependent protein kinase IV (CaMK-IV) is activated to enhance calcium influx and increase mitochondrial biogenesis (Trian et al., 2007). The motility of mitochondria in ASM cells can module calcium uptake to modulate the contractile responses to agonists, such as histamine and acetylcholine (Delmotte et al., 2017). Cytosolic calcium levels are also modulated by various signaling cascades driven by a variety of metabolic by-products (Berridge, 2008; Somlyo and Himpens, 1989). Particularly, lipid metabolites regulate both calcium mobilization and sensitization pathways to modulate ASM cell shortening. Sphingosine 1-phosphate, the sphingolipid metabolite, is elevated in BALF following allergen challenge (Ammit et al., 2001), which further amplifies calcium mobilization and sensitization and evokes ASM hyperreactivity via inhibition of MLC phosphatase (Ammit et al., 2001; Kume et al., 2007; Rosenfeldt et al., 2003). In addition, there are multiple lines of evidence to suggest that dysfunctional metabolic status is associated with increased asthma incidence, severity, and resistance to treatment. Our studies showed that ASM cells obtained from obese human donors showed enhanced agonist-induced cell shortening accompanied with amplified MLC phosphorylation and calcium mobilization (Orfanos et al., 2018). Other investigators have reported distinct metabolic phenotypes among obese asthma patients, based on energy, amino acid, and lipid metabolisms (Maniscalco et al., 2017). Particularly, vitamin D deficiency has been identified as a risk factor of obesity-associated asthma (Mirzakhani et al., 2017; Vo et al., 2015). In ASM cells, vitamin D metabolism plays a role in decreasing AHR and airway remodeling through the inhibition of ASM cell growth and cytokine secretion (Damera et al., 2009; Hall et al., 2016; Himes et al., 2015).
Airway remodeling involves increased thickness of airway wall, characterized by myocyte hypertrophy, hyperplasia, angiogenesis, and increased extra-cellular matrix (ECM) deposition (Hirota and Martin, 2013; Lazaar and Panettieri, 2005). Mitochondrial energy metabolism is directly linked to cell proliferation in normal and cancer cell lines (Bhatti et al., 2017; Ganapathy-Kanniappan and Geschwind, 2013; Lunt and Vander Heiden, 2011; Vyas et al., 2016). In ASM cells, findings showed that increased mitochondrial mass and activity drive bronchial ASM cell proliferation in vitro (Trian et al., 2007). In fetal human ASM cells, hyperoxia decreased proliferation via modulating mitochondrial function, along with decreased fusion protein Mfn2 expression and increased fission protein Drp1 (Hartman et al., 2012). Furthermore, inhibition of glycolysis inhibits cell cycle progression and attenuates LPS-induced ASM cell proliferation (Zhang et al., 2019). Supporting a similar role for mitochondria in ASM cell proliferation, ASM cells from COPD patients showed reduced mitochondrial respiration with enhanced glycolysis. Inhibition of glycolysis attenuated proliferation of ASM cells obtained from COPD patients (Michaeloudes et al., 2017). Similar findings were reported in vascular smooth muscle cells obtained from subjects with pulmonary arterial hypertension (Hernandez-Saavedra et al., 2020). The upregulated glycolytic activity is likely an adaptive mechanism elicited by the mitochondrial dysfunction in highly proliferative ASM cells (Sutendra et al., 2010). These results underscore the metabolic basis for the phenotypic changes in smooth muscle cells in pulmonary disorders.
Lipid mediators also play a multitude of roles in airway remodeling. For instance, sphingosine 1 phosphate induces ASM cell proliferation via the activation or upregulation of the transcription factors, yes-associated protein (YAP) and forkhead box M1 (FOXM1) (Liu et al., 2018a). Sphingosine analog pre-treatment inhibited the proliferative capacity of ASM cells by interfering with oxidative phosphorylation and other metabolic processes (Blais-Lecours et al., 2020). Free fatty acids (FFAs) also elicit mitogenic effects in ASM cells. These free fatty acids act on nutrient-sensing G protein-coupled receptors (GPCRs) on target cells to elicit downstream effects (Thorburn et al., 2014). Both short-chain and long-chain FFAs, are associated with the activation of MAP kinases (MAPK), PI3 kinase (PI3K)/mTOR pathways downstream to the GPCRs (Thorburn et al., 2014; Yasuda et al., 2014). Free fatty acid receptor 1 (FFAR1) is reported to induce the proliferation of ASM cells via MAPK and PI3K pathways (Matoba et al., 2018).
ASM cell migration is another area where metabolic signaling is known to play a role. Cell migration is a multi-step process, coordinated by actin-driven elongation, adhesion, and contraction (Friedl and Wolf, 2009). Several endogenous mediators and exogenous substances, including lipid derivatives, vitamin metabolites and β2-adrenergic receptor agonists are associated with the migration of ASM cells (Day et al., 2006; Goncharova et al., 2012; Salter et al., 2017).
5.1.5. Other cells and lung tissue models
A small number of studies have characterized lung tissue to understand the metabolic regulation of asthma. L-ornithine-derived polyamines (PAs), derived from impaired L-arginine catabolism, are of particular interest in certain airway diseases. Ornithine-derived PAs are elevated in the sputum of patients with allergic asthma and in allergen-induced murine models of asthma (North et al., 2013). Altered metabolism of a multitude of PAs was reported in lung tissues isolated from murine models of asthma, characterized by increased levels of putrescine, N1-acetylputrescine, and N8-acetylspermidine (Lee et al., 2019). Since lung tissue provides an integrated, multi-cellular platform almost similar to the in vivo environment, metabolomic investigations in lung tissues will have significant translational value.
In addition to asthma, metabolomic profiles of human lung tissues and other cell types have also been studied in other lung diseases. In certain types of lung cancers, metabolic dysfunctions were characterized in human lung tissues and identified changes such as increased glycolysis and TCA cycle metabolites (Fan et al., 2009; Hensley et al., 2016). Similar hyper-glycolytic phenotypes were also identified in pleural effusions with a single-cell on-chip metabolic cytometry assay (Li et al., 2019b). Pulmonary hypertension (PH), although considered a vascular disease by etiology, is a disease with clinical consequences in lungs. Pulmonary artery endothelial cells (PAECs) obtained from PH patients showed elevated levels of fructose bisphosphate and succinate, suggesting increased glycolysis and glutamine-derived anaplerosis, respectively (Hernandez-Saavedra et al., 2020). Idiopathic pulmonary arterial hypertension impairs NO production, which in turn impairs mitochondrial biogenesis, resulting in a metabolic switch to glycolysis-dominant bioenergetics in PAECs (Xu et al., 2007). In summary, complex platforms such as lung tissue have been used in a small number of metabolomic studies. Ex vivo platforms such as precision-cut lung slices and organoids are yet to be utilized as metabolomic platforms to study pulmonary diseases such as asthma.
6. Conclusion
Over the recent years, metabolomics has emerged as a promising tool in multiple areas of biomedicine. A complex disease with multi-etiology, asthma is well-suited to be investigated by metabolomic approach to understand its pathophysiology. Currently, a vast majority of these investigations focus on distinct metabolite biomarkers for asthma diagnosis. We propose a highly focused metabolomic approach by targeting individual cell populations in lungs. ASM cells are primary drivers of bronchial spasm and show altered contractile phenotype in asthma. Therefore, investigating the distinct metabolic profile of ASM cells in asthma and obesity will be a fruitful endeavor. Finding from such studies will not only improve our understanding of obesity-associated asthma, but also potentially facilitate the optimization of asthma therapy with precision medicine (Wishart, 2016).
Arguably, there exists limitations in the metabolomics studies in asthma. First, there are high variabilities in findings among different studies due to confounding external environmental factors and methodological variations. To address this, internal and external validations should be used to confirm the findings (Kelly et al., 2017a; Kim H. Esbensen, 2010). Secondly, in order to minimize artificial inferences, one must ensure the “homogeneity” of the human samples used in the study. For instance, metabolites from xenobiotics may confound metabolomic profiles. Common asthma treatments include short-acting β-agonists (SABAs), long-acting β-agonists (LABAs), and glucocorticoids. Studies have suggested that albuterol administration has an impact on sphingolipid metabolism and associated changes in gene expressions (McGeachie et al., 2015). Thirdly, in disease states, compensatory mechanisms may drive certain metabolic resulting in findings that are not actually reflective of the pathological process. Most of these challenges can be rectified through an experimental design to address gender, ethnicity, and BMI variabilities of the donor samples. Despite limitations, cell- and tissue-based metabolomics may offer novel platforms to explore new therapeutic targets and or pathways to understand the pathogenesis of airways disease.
Acknowledgements
We wish to thank our funding sources from NIH: NCATS UL1TR003017 and NHLBI PO1HL114471.
References
- Abdulnaby NK, Sayed AO, Shalaby NM, 2016. Predictive value of serum uric acid in hospitalized adolescents and adults with acute asthma. Therapeut. Clin. Risk Manag 12, 1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agassandian M, Mallampalli RK, 2013. Surfactant phospholipid metabolism. Biochim. Biophys. Acta 1831 (3), 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I, 2009. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol 183 (8), 5379–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA Jr., 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb. J 15 (7), 1212–1214. [DOI] [PubMed] [Google Scholar]
- Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA, 2007. Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell Mol. Physiol 293 (2), L259–L271. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Thompson M, Sieck GC, Vassallo R, Pabelick CM, Prakash YS, 2017. Functional effects of cigarette smoke-induced changes in airway smooth muscle mitochondrial morphology. J. Cell. Physiol 232 (5), 1053–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiago Vincent M., Gowda GAN, Zhang Shucha, Shanaiah Narasimhamurthy, Clark Jason, Raftery Daniel, 2008. Use of EDTA to minimize ionic strength dependent frequency shifts in the 1H NMR spectra of urine. Metabolomics 328–336. [Google Scholar]
- Baffi CW, Winnica DE, Holguin F, 2015. Asthma and obesity: mechanisms and clinical implications. Asthma Res. Pract 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH, 2005. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am. J. Physiol. Lung Cell Mol. Physiol 289 (6), L980–L989. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, 2008. Smooth muscle cell calcium activation mechanisms. J. Physiol 586 (21), 5047–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti JS, Bhatti GK, Reddy PH, 2017. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 1863 (5), 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JL, Panettieri RA Jr., Banerjee A, Berger P, 2012. Airway smooth muscle in asthma: just a target for bronchodilation? Clin. Chest Med 33 (3), 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais-Lecours P, Laouafa S, Arias-Reyes C, Santos WL, Joseph V, Burgess JK, Halayko AJ, Soliz J, Marsolais D, 2020. Metabolic adaptation of airway smooth muscle cells to an SPHK2 substrate precedes cytostasis. Am. J. Respir. Cell Mol. Biol 62 (1), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, Baardman J, de Winther MP, 2015. Metabolic characterization of polarized M1 and M2 bone marrow-derived macrophages using real-time extracellular flux analysis. JoVE 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, Dame ZT, Poelzer J, Huynh J, Yallou FS, Psychogios N, Dong E, Bogumil R, Roehring C, Wishart DS, 2013. The human urine metabolome. PloS One 8 (9), e73076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J, Goss VM, Yang X, Bakke PS, Caruso M, Chanez P, Dahlen SE, Fowler SJ, Horvath I, Krug N, Montuschi P, Sanak M, Sandstrom T, Shaw DE, Chung KF, Singer F, Fleming LJ, Sousa AR, Pandis I, Bansal AT, Sterk PJ, Djukanovic R, Postle AD, Group, U.B.S, 2018. Lipid phenotyping of lung epithelial lining fluid in healthy human volunteers. Metabolomics 14 (10), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RW, Bailey JM, Schewe T, Rapoport SM, 1982. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxyeicosatetraenoic acid. J. Biol. Chem 257 (11), 6050–6055. [PubMed] [Google Scholar]
- Busse WW, Lemanske RF Jr., 2001. Asthma. N. Engl. J. Med 344 (5), 350–362. [DOI] [PubMed] [Google Scholar]
- Carr TF, Zeki AA, Kraft M, 2018. Eosinophilic and noneosinophilic asthma. Am. J. Respir. Crit. Care Med 197 (1), 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro S, Giordano G, Reniero F, Carpi D, Stocchero M, Sterk PJ, Baraldi E, 2013. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy 68 (1), 110–117. [DOI] [PubMed] [Google Scholar]
- Carraro S, Bozzetto S, Giordano G, El Mazloum D, Stocchero M, Pirillo P, Zanconato S, Baraldi E, 2018. Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr. Allergy Immunol 29 (4), 375–382. [DOI] [PubMed] [Google Scholar]
- Chapman DG, Irvin CG, 2015. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin. Exp. Allergy 45 (4), 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP, 2011. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol 11 (11), 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Suto W, Sakai H, 2018. Augmented Pla2g4c/Ptgs2/Hpgds axis in bronchial smooth muscle tissues of experimental asthma. PloS One 13 (8), e0202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, Kirkness EF, Spector TD, Caskey CT, Thorens B, Venter JC, Telenti A, 2019. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metabol. 29 (2), 488–500 e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA, McDunn J, Bennett C, Fettig J, Erzurum SC, Kalhan SC, 2015. Metabolomic endotype of asthma. J. Immunol 195 (2), 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G, 2011. Metabolic abnormalities in children with asthma. Am. J. Respir. Crit. Care Med 183 (4), 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani E, Harb H, Charbonnier LM, Leirer J, Motsinger-Reif A, Rachid R, Phipatanakul W, Kaddurah-Daouk R, Chatila TA, 2020. Untargeted metabolomic profiling identifies disease-specific signatures in food allergy and asthma. J. Allergy Clin. Immunol 145 (3), 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank-Quinn C, Armstrong M, Powell R, Gomez J, Elie M, Reisdorph N, 2017. Determining the presence of asthma-related molecules and salivary contamination in exhaled breath condensate. Respir. Res 18 (1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank-Quinn C, Zheng LK, Quinn K, Bowler R, Reisdorph R, Reisdorph N, 2018. Impact of blood collection tubes and sample handling time on serum and plasma metabolome and lipidome. Metabolites 8 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallinga JW, Robroeks CM, van Berkel JJ, Moonen EJ, Godschalk RW, Jobsis Q, Dompeling E, Wouters EF, van Schooten FJ, 2010. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 40 (1), 68–76. [DOI] [PubMed] [Google Scholar]
- Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA Jr., 2009. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br. J. Pharmacol 158 (6), 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RM, Lee YH, Park AM, Suzuki YJ, 2006. Retinoic acid inhibits airway smooth muscle cell migration. Am. J. Respir. Cell Mol. Biol 34 (6), 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P, Sieck GC, 2019. Endoplasmic reticulum stress and mitochondrial function in airway smooth muscle. Front. Cell Dev. Biol 7, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P, Zavaletta VA, Thompson MA, Prakash YS, Sieck GC, 2017. TNFalpha decreases mitochondrial movement in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol 313 (1), L166–L176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diette GB, McCormack MC, Hansel NN, Breysse PN, Matsui EC, 2008. Environmental issues in managing asthma. Respir. Care 53 (5), 602–615 discussion 616-607. [PMC free article] [PubMed] [Google Scholar]
- Du F, Virtue A, Wang H, Yang XF, 2013. Metabolomic analyses for atherosclerosis, diabetes, and obesity. Biomark. Res 1 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley E, Yousef M, Wang Y, Griffiths WJ, 2010. Targeted metabolomics and mass spectrometry. Adv. Protein Chem. Struct. Biol 80, 45–83. [DOI] [PubMed] [Google Scholar]
- Dunn WB, Bailey NJ, Johnson HE, 2005. Measuring the metabolome: current analytical technologies. Analyst 130 (5), 606–625. [DOI] [PubMed] [Google Scholar]
- Fahy JV, 2009. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc. Am. Thorac. Soc 6 (3), 256–259. [DOI] [PubMed] [Google Scholar]
- Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM, 2009. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol. Canc 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessenden M, 2016. Metabolomics: small molecules, single cells. Nature 540 (7631), 153–155. [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L, 2000. Metabolite profiling for plant functional genomics. Nat. Biotechnol 18 (11), 1157–1161. [DOI] [PubMed] [Google Scholar]
- Filipiak W, Ruzsanyi V, Mochalski P, Filipiak A, Bajtarevic A, Ager C, Denz H, Hilbe W, Jamnig H, Hackl M, Dzien A, Amann A, 2012. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res 6 (3), 036008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, Cardoso WV, Crystal RG, Drake CJ, Engelhardt J, Frid M, Herzog E, Mason R, Phan SH, Randell SH, Rose MC, Stevens T, Serge J, Sunday ME, Voynow JA, Weinstein BM, Whitsett J, Williams MC, 2008. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc. Am. Thorac. Soc 5 (7), 763–766. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K, 2009. Proteolytic interstitial cell migration: a five-step process. Canc. Metastasis Rev 28 (1–2), 129–135. [DOI] [PubMed] [Google Scholar]
- Gai XY, Zhang LJ, Chang C, Guo CL, Abulikemu M, Li WX, Wang J, Yao WZ, Zhang X, 2019. Metabolomic analysis of serum glycerophospholipid levels in eosinophilic and neutrophilic asthma. Biomed. Environ. Sci 32 (2), 96–106. [DOI] [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S, Geschwind JF, 2013. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol. Canc 12, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Chawla A, 2014. Metabolic regulation of immune responses. Annu. Rev. Immunol 32, 609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giskeodegard GF, Davies SK, Revell VL, Keun H, Skene DJ, 2015. Diurnal rhythms in the human urine metabolome during sleep and total sleep deprivation. Sci. Rep 5, 14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA Jr., 2012. beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am. J. Respir. Cell Mol. Biol 46 (1), 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dominguez R, Gonzalez-Dominguez A, Sayago A, Fernandez-Recamales A, 2020. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites 10 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111 (7), 927–930. [DOI] [PubMed] [Google Scholar]
- Greving MP, Patti GJ, Siuzdak G, 2011. Nanostructure-initiator mass spectrometry metabolite analysis and imaging. Anal. Chem 83 (1), 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM, Krawczyk CM, 2018. Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat. Commun 9 (1), 2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J, Hu FB, 2016. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39 (5), 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SC, Fischer KD, Agrawal DK, 2016. The impact of vitamin D on asthmatic human airway smooth muscle. Expet Rev. Respir. Med 10 (2), 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM, 2012. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol 303 (8), L711–L719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton S, Maker GL, Mullaney I, Trengove RD, 2017. Experimental design and reporting standards for metabolomics studies of mammalian cell lines. Cell. Mol. Life Sci 74 (24), 4421–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, McMillan E, Butt Y, Ni M, Oliver D, Torrealba J, Malloy CR, Kernstine K, Lenkinski RE, DeBerardinis RJ, 2016. Metabolic heterogeneity in human lung tumors. Cell 164 (4), 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Saavedra D, Sanders L, Freeman S, Reisz JA, Lee MH, Mickael C, Kumar R, Kassa B, Gu S, D’Alessandro A, Stenmark KR, Tuder RM, Graham BB, 2020. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Sci. Rep 10 (1), 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes BE, Koziol-White C, Johnson M, Nikolos C, Jester W, Klanderman B, Litonjua AA, Tantisira KG, Truskowski K, MacDonald K, Panettieri RA Jr., Weiss ST, 2015. Vitamin D modulates expression of the airway smooth muscle transcriptome in fatal asthma. PloS One 10 (7), e0134057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N, Martin JG, 2013. Mechanisms of airway remodeling. Chest 144 (3), 1026–1032. [DOI] [PubMed] [Google Scholar]
- Ho WE, Xu YJ, Xu F, Cheng C, Peh HY, Tannenbaum SR, Wong WS, Ong CN, 2013. Metabolomics reveals altered metabolic pathways in experimental asthma. Am. J. Respir. Cell Mol. Biol 48 (2), 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jobsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H, Condensate, A.E.T.F.o.E.B, 2005. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur. Respir. J 26 (3), 523–548. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Kraft M, 2012. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol 130 (4), 829–842 quiz 843-824. [DOI] [PubMed] [Google Scholar]
- Jeremy M Berg JLT, Stryer Lubert, 2002. Biochemistry. [Google Scholar]
- Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN, 2015. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42 (3), 419–430. [DOI] [PubMed] [Google Scholar]
- Jun Peng CDSL, Dean Befus A, Zhou Ruokun, Liang L, 2014. Metabolomic profiling of bronchoalveolar lavage fluids by isotope labeling liquid chromatography mass spectrometry: a promising approach to studying experimental asthma. Metabolomics 10 (6), 1305–1317. [Google Scholar]
- Jung J, Kim SH, Lee HS, Choi GS, Jung YS, Ryu DH, Park HS, Hwang GS, 2013. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy 43 (4), 425–433. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT, 1985. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol 25, 593–620. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT, 2001. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem 276 (7), 4527–4530. [DOI] [PubMed] [Google Scholar]
- Kan M, Shumyatcher M, Himes BE, 2017. Using omics approaches to understand pulmonary diseases. Respir. Res 18 (1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantae V, Krekels EHJ, Esdonk MJV, Lindenburg P, Harms AC, Knibbe CAJ, Van der Graaf PH, Hankemeier T, 2017. Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: towards personalized drug therapy. Metabolomics 13 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Chupp G, 2019. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J. Allergy Clin. Immunol 144 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- Kelly RS, Dahlin A, McGeachie MJ, Qiu W, Sordillo J, Wan ES, Wu AC, Lasky-Su J, 2017a. Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest 151 (2), 262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RS, Virkud Y, Giorgio R, Celedon JC, Weiss ST, Lasky-Su J, 2017b. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 1863 (6), 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Esbensen PG, 2010. Principles of Proper Validation: use and abuse of resampling for validation. J. Chemometr 24 (3–4), 168–187. [Google Scholar]
- Kim JY, Sohn JH, Lee JH, Park JW, 2015. Obesity increases airway hyperresponsiveness via the TNF-alpha pathway and treating obesity induces recovery. PloS One 10 (2), e0116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P, Rahman I, 2006. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol. Ther 111 (2), 476–494. [DOI] [PubMed] [Google Scholar]
- Kolmert J, Gomez C, Balgoma D, Sjodin M, Bood J, Konradsen JR, Ericsson M, Thorngren JO, James A, Mikus M, Sousa AR, Riley JH, Bates S, Bakke PS, Pandis I, Caruso M, Chanez P, Fowler SJ, Geiser T, Howarth P, Horvath I, Krug N, Montuschi P, Sanak M, Behndig A, Shaw DE, Knowles RG, Holweg CTJ, Wheelock AM, Dahlen B, Nordlund B, Alving K, Hedlin G, Chung KF, Adcock IM, Sterk PJ, Djukanovic R, Dahlen SE, Wheelock CE, U-Biopred Study Group, o.b.o.t.U.B.S.G, 2021. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type 2 inflammation. A clinical observational study. Am. J. Respir. Crit. Care Med 203 (1), 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SJ, Garg NJ, 2019. Metabolic programming of macrophage functions and pathogens control. Redox Biol. 24, 101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ, 2010. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 115 (23), 4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Takeda N, Oguma T, Ito S, Kondo M, Ito Y, Shimokata K, 2007. Sphingosine 1-phosphate causes airway hyper-reactivity by rho-mediated myosin phosphatase inactivation. J. Pharmacol. Exp. Therapeut 320 (2), 766–773. [DOI] [PubMed] [Google Scholar]
- Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, Bodine M, Levison BS, Hammel J, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Gaston B, Israel E, Jarjour N, Moore W, Peters SP, Teague WG, Wenzel S, Hazen SL, Erzurum SC, National Heart, L., Blood Institute’s Severe Asthma Research, P., 2008. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med 178 (7), 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaar AL, Panettieri RA Jr., 2005. Airway smooth muscle: a modulator of airway remodeling in asthma. J. Allergy Clin. Immunol 116 (3), 488–495 quiz 496. [DOI] [PubMed] [Google Scholar]
- Lee HS, Seo C, Hwang YH, Shin TH, Park HJ, Kim Y, Ji M, Min J, Choi S, Kim H, Park AK, Yee ST, Lee G, Paik MJ, 2019. Metabolomic approaches to polyamines including acetylated derivatives in lung tissue of mice with asthma. Metabolomics 15 (1), 8. [DOI] [PubMed] [Google Scholar]
- Leung TF, Ko FW, Wong GW, 2013. Recent advances in asthma biomarker research. Ther. Adv. Respir. Dis 7 (5), 297–308. [DOI] [PubMed] [Google Scholar]
- Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM, Boushey HA, Cabana MD, Johnson CC, Lynch SV, 2019. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol 4 (11), 1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, Chen P, Ni H, 2012. Crosstalk between platelets and the immune system: old systems with new discoveries. Adv. Hematol 2012, 384685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wan C, Wen F, 2014. An unexpected role for serum uric acid as a biomarker for severity of asthma exacerbation. Asian Pac. J. Allergy Immunol 32 (1), 93–99. [DOI] [PubMed] [Google Scholar]
- Li K, Li M, Li W, Yu H, Sun X, Zhang Q, Li Y, Li X, Li Y, Abel ED, Wu Q, Chen H, 2019a. Airway epithelial regeneration requires autophagy and glucose metabolism. Cell Death Dis. 10 (12), 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang Z, Tang Y, Lu X, Chen J, Dong Y, Wu B, Wang C, Yang L, Guo Z, Xue M, Lu S, Wei W, Shi Q, 2019b. Liquid biopsy-based single-cell metabolic phenotyping of lung cancer patients for informative diagnostics. Nat. Commun 10 (1), 3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL, Ciprandi G, 2018. Asthma endotyping and biomarkers in childhood asthma. Pediatr. Allergy Immunol. Pulmonol 31 (2), 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wang L, Holm BA, Enhorning G, 1997. Dysfunction of Guinea-pig pulmonary surfactant and type II pneumocytes after repetitive challenge with aerosolized ovalbumin. Clin. Exp. Allergy 27 (7), 802–807. [DOI] [PubMed] [Google Scholar]
- Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T, Milasta S, Wang J, Yang M, Liu G, Green DR, Wang R, 2016. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1alpha-dependent. Proc. Natl. Acad. Sci. U. S. A 113 (6), 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhai C, Pan Y, Zhu Y, Shi W, Wang J, Yan X, Su X, Song Y, Gao L, Li M, 2018a. Sphingosine-1-phosphate induces airway smooth muscle cell proliferation, migration, and contraction by modulating Hippo signaling effector YAP. Am. J. Physiol. Lung Cell Mol. Physiol 315 (4), L609–L621. [DOI] [PubMed] [Google Scholar]
- Liu X, Hoene M, Wang X, Yin P, Haring HU, Xu G, Lehmann R, 2018b. Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal. Chim. Acta 1037, 293–300. [DOI] [PubMed] [Google Scholar]
- Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC, 1999. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur. J. Immunol 29 (5), 1496–1503. [DOI] [PubMed] [Google Scholar]
- Loureiro CC, Oliveira AS, Santos M, Rudnitskaya A, Todo-Bom A, Bousquet J, Rocha SM, 2016. Urinary metabolomic profiling of asthmatics can be related to clinical characteristics. Allergy 71 (9), 1362–1365. [DOI] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG, 2011. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol 27, 441–464. [DOI] [PubMed] [Google Scholar]
- Mabalirajan U, Rehman R, Ahmad T, Kumar S, Leishangthem GD, Singh S, Dinda AK, Biswal S, Agrawal A, Ghosh B, 2013. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep 3, 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W, 2001. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol 429 (1–3), 195–207. [DOI] [PubMed] [Google Scholar]
- Maes T, Joos GF, Brusselle GG, 2012. Targeting interleukin-4 in asthma: lost in translation? Am. J. Respir. Cell Mol. Biol 47 (3), 261–270. [DOI] [PubMed] [Google Scholar]
- Maniscalco M, Paris D, Melck DJ, D’Amato M, Zedda A, Sofia M, Stellato C, Motta A, 2017. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol 139 (5), 1536–1547 e1535. [DOI] [PubMed] [Google Scholar]
- Markley JL, Bruschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS, 2017. The future of NMR-based metabolomics. Curr. Opin. Biotechnol 43, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba A, Matsuyama N, Shibata S, Masaki E, Emala CW Sr, Mizuta K, 2018. The free fatty acid receptor 1 promotes airway smooth muscle cell proliferation through MEK/ERK and PI3K/Akt signaling pathways. Am. J. Physiol. Lung Cell Mol. Physiol 314 (3), L333–L348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S, 2000. Prostaglandin D2 as a mediator of allergic asthma. Science 287 (5460), 2013–2017. [DOI] [PubMed] [Google Scholar]
- McGeachie MJ, Dahlin A, Qiu W, Croteau-Chonka DC, Savage J, Wu AC, Wan ES, Sordillo JE, Al-Garawi A, Martinez FD, Strunk RC, Lemanske RF Jr., Liu AH, Raby BA, Weiss S, Clish CB, Lasky-Su JA, 2015. The metabolomics of asthma control: a promising link between genetics and disease. Immun. Inflamm. Dis 3 (3), 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeloudes C, Kuo CH, Haji G, Finch DK, Halayko AJ, Kirkham P, Chung KF, Adcock IM, Copdmap, 2017. Metabolic re-patterning in COPD airway smooth muscle cells. Eur. Respir. J 50 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IJ, Peters SR, Overmyer KA, Paulson BR, Westphall MS, Coon JJ, 2019. Real-time health monitoring through urine metabolomics. NPJ Digit Med. 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzakhani H, O’Connor G, Bacharier LB, Zeiger RS, Schatz MX, Weiss ST, Litonjua AA, 2017. Asthma control status in pregnancy, body mass index, and maternal vitamin D levels. J. Allergy Clin. Immunol 140 (5), 1453–1456 e1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, 2017. Metabolic plasticity in dendritic cell responses: implications in allergic asthma. J. Immunol. Res 2017, 5134760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K, 2013. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol 131 (2), 353–360 e351-352. [DOI] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, Fukumoto N, Eguchi N, Kushi A, Sasai H, Kanaoka Y, Ozono K, Narumiya S, Suzuki K, Urade Y, 2006. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J. Neurosci 26 (16), 4383–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE, 2012. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490 (7418), 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould KJ, Barthel L, Mohning MP, Thomas SM, McCubbrey AL, Danhorn T, Leach SM, Fingerlin TE, O’Connor BP, Reisz JA, D’Alessandro A, Bratton DL, Jakubzick CV, Janssen WJ, 2017. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am. J. Respir. Cell Mol. Biol 57 (3), 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JR, Lloyd CM, 2010. Chronic inflammation and asthma. Mutat. Res 690 (1–2), 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus S, Seifert L, Vautz W, Nolte J, Bufe A, Peters M, 2011. Comparison of metabolites in exhaled breath and bronchoalveolar lavage fluid samples in a mouse model of asthma. J. Appl. Physiol 111 (4), 1088–1095, 1985. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Guedan A, Mousnier A, Swieboda D, Zhang Q, Horkai D, Le Novere N, Solari R, Wakelam MJO, 2018. Host lipidome analysis during rhinovirus replication in HBECs identifies potential therapeutic targets. J. Lipid Res 59 (9), 1671–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, 2008. Systems biology: metabonomics. Nature 455 (7216), 1054–1056. [DOI] [PubMed] [Google Scholar]
- North ML, Grasemann H, Khanna N, Inman MD, Gauvreau GM, Scott JA, 2013. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol 48 (6), 694–702. [DOI] [PubMed] [Google Scholar]
- O’Brien KL, Finlay DK, 2019. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol 19 (5), 282–290. [DOI] [PubMed] [Google Scholar]
- Ober C, Hoffjan S, 2006. Asthma genetics 2006: the long and winding road to gene discovery. Gene Immun. 7 (2), 95–100. [DOI] [PubMed] [Google Scholar]
- Orfanos S, Jude J, Deeney BT, Cao G, Rastogi D, van Zee M, Pushkarsky I, Munoz HE, Damoiseaux R, Di Carlo D, Panettieri RA Jr., 2018. Obesity increases airway smooth muscle responses to contractile agonists. Am. J. Physiol. Lung Cell Mol. Physiol 315 (5), L673–L681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, Alam R, 2012. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell Mol. Physiol 302 (3), L300–L307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia G, Del Greco FM, Sigurdsson BB, Rainer J, Volani C, Hicks AA, Pramstaller PP, Smarason SV, 2018. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin. Chim. Acta 486, 320–328. [DOI] [PubMed] [Google Scholar]
- Pan S, Conaway S Jr., Deshpande DA, 2019. Mitochondrial regulation of airway smooth muscle functions in health and pulmonary diseases. Arch. Biochem. Biophys 663, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, Siuzdak G, 2012. Innovation: metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol 13 (4), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden DB, Hohman R, Brown ME, Mason RT, Berkebile C, Fales HM, Kaliner MA, 1990. Uric acid is a major antioxidant in human nasal airway secretions. Proc. Natl. Acad. Sci. U. S. A 87 (19), 7638–7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters U, Dixon AE, Forno E, 2018. Obesity and asthma. J. Allergy Clin. Immunol 141 (4), 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pite H, Morais-Almeida M, Rocha SM, 2018. Metabolomics in asthma: where do we stand? Curr. Opin. Pulm. Med 24 (1), 94–103. [DOI] [PubMed] [Google Scholar]
- Prado CM, Martins MA, Tiberio IF, 2011. Nitric oxide in asthma physiopathology. ISRN Allergy 2011, 832560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Pabelick CM, Sieck GC, 2017. Mitochondrial dysfunction in airway disease. Chest 152 (3), 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalska P, Huang X, Martin SE, Han X, Patti GJ, Crawford PA, 2018. Isotope tracing untargeted metabolomics reveals macrophage polarization-state-specific metabolic coordination across intracellular compartments, iScience 9, 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Aboushousha R, van de Wetering C, Chia SB, Amiel E, Schneider RW, van der Velden JLJ, Lahue KG, Hoagland DA, Casey DT, Daphtary N, Ather JL, Randall MJ, Aliyeva M, Black KE, Chapman DG, Lundblad LKA, McMillan DH, Dixon AE, Anathy V, Irvin CG, Poynter ME, Wouters EFM, Vacek PM, Henket M, Schleich F, Louis R, van der Vliet A, Janssen-Heininger YMW, 2018. IL-1/inhibitory kappaB kinase epsilon-induced glycolysis augment epithelial effector function and promote allergic airways disease. J. Allergy Clin. Immunol 142 (2), 435–450 e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qingyu Huang JZ, Luo Lianzhong, Wang Xiaofei, Wang Xiaoxue, Alamdar Ambreen, Peng Siyuan, Liu Liangpo, Tian Meiping, Shen Heqing, 2015. Metabolomics reveals disturbed metabolic pathways in human lung epithelial cells exposed to airborne fine particulate matter. Toxicol. Res 4 (4), 939–947. [Google Scholar]
- Quan-Jun Y, Jian-Ping Z, Jian-Hua Z, Yong-Long H, Bo X, Jing-Xian Z, Bona D, Yuan Z, Cheng G, 2017. Distinct metabolic profile of inhaled budesonide and salbutamol in asthmatic children during acute exacerbation. Basic Clin. Pharmacol. Toxicol 120 (3), 303–311. [DOI] [PubMed] [Google Scholar]
- Rago D, Pedersen CT, Huang M, Kelly RS, Gurdeniz G, Brustad N, Knihtila H, Lee-Sarwar KA, Morin A, Rasmussen MA, Stokholm J, Bonnelykke K, Litonjua AA, Wheelock CE, Weiss ST, Lasky-Su J, Bisgaard H, Chawes BL, 2021. Characteristics and mechanisms of a sphingolipid-associated childhood asthma endotype. Am. J. Respir. Crit. Care Med 203 (7), 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez T, Daneshian M, Kamp H, Bois FY, Clench MR, Coen M, Donley B, Fischer SM, Ekman DR, Fabian E, Guillou C, Heuer J, Hogberg HT, Jungnickel H, Keun HC, Krennrich G, Krupp E, Luch A, Noor F, Peter E, Riefke B, Seymour M, Skinner N, Smirnova L, Verheij E, Wagner S, Hartung T, van Ravenzwaay B, Leist M, 2013. Metabolomics in toxicology and preclinical research. ALTEX 30 (2), 209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanbakhsh S, Liu P, Bjorndahl TC, Mandal R, Grant JR, Wilson M, Eisner R, Sinelnikov I, Hu X, Luchinat C, Greiner R, Wishart DS, 2015. Accurate, fully-automated NMR spectral profiling for metabolomics. PloS One 10 (5), e0124219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke SN, Gallart-Ayala H, Gomez C, Checa A, Fauland A, Naz S, Kamleh MA, Djukanovic R, Hinks TS, Wheelock CE, 2017. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J 49 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardolo FL, 2003. Multiple roles of nitric oxide in the airways. Thorax 58 (2), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Souza AL, Gerszten RE, Clish CB, 2012. Targeted metabolomics. Curr. Protocol Mol. Biol Chapter 30, Unit 30 32 31–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell Victor W., Bender DA, Kennelly Peter J., Anthony Weil P, 2018. Harper’s Illustrated Biochemistry, 31 ed. [Google Scholar]
- Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr., Spiegel S, 2003. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. Faseb. J 17 (13), 1789–1799. [DOI] [PubMed] [Google Scholar]
- Rufo JC, Madureira J, Fernandes EO, Moreira A, 2016. Volatile organic compounds in asthma diagnosis: a systematic review and meta-analysis. Allergy 71 (2), 175–188. [DOI] [PubMed] [Google Scholar]
- Salter B, Pray C, Radford K, Martin JG, Nair P, 2017. Regulation of human airway smooth muscle cell migration and relevance to asthma. Respir. Res 18 (1), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samitas K, Chorianopoulos D, Vittorakis S, Zervas E, Economidou E, Papatheodorou G, Loukides S, Gaga M, 2009. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir. Med 103 (5), 750–756. [DOI] [PubMed] [Google Scholar]
- Saude EJ, Skappak CD, Regush S, Cook K, Ben-Zvi A, Becker A, Moqbel R, Sykes BD, Rowe BH, Adarnko DJ, 2011. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J. Allergy Clin. Immunol 127 (3), 757–764 e751-756. [DOI] [PubMed] [Google Scholar]
- van der Schee MP, Palmay R, Cowan JO, Taylor DR, 2013. Predicting steroid responsiveness in patients with asthma using exhaled breath profiling. Clin. Exp. Allergy 43 (11), 1217–1225. [DOI] [PubMed] [Google Scholar]
- Schleich FN, Zanella D, Stefanuto PH, Bessonov K, Smolinska A, Dallinga JW, Henket M, Paulus V, Guissard F, Graff S, Moermans C, Wouters EFM, Van Steen K, van Schooten FJ, Focant JF, Louis R, 2019. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am. J. Respir. Crit. Care Med 200 (4), 444–453. [DOI] [PubMed] [Google Scholar]
- Showalter MR, Nonnecke EB, Linderholm AL, Cajka T, Sa MR, Lonnerdal B, Kenyon NJ, Fiehn O, 2018. Obesogenic diets alter metabolism in mice. PloS One 13 (1), e0190632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Prakash YS, Linneberg A, Agrawal A, 2013. Insulin and the lung: connecting asthma and metabolic syndrome. J. Allergy 627384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Desiraju K, Aggarwal K, Kutum R, Roy S, Lodha R, Kabra SK, Ghosh B, Sethi T, Agrawal A, 2017. Exhaled breath condensate metabolome clusters for endotype discovery in asthma. J. Transl. Med 15 (1), 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs KF, van de Pol MA, Kulik W, Dijkhuis A, Smids BS, van Eijk HW, Karlas JA, Molenkamp R, Wolthers KC, Johnston SL, van der Zee JS, Sterk PJ, Lutter R, team R.r., 2013. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus-induced asthma exacerbation: a prospective study with a parallel-group design. Thorax 68 (12), 1122–1130. [DOI] [PubMed] [Google Scholar]
- Snowden S, Dahlen SE, Wheelock CE, 2012. Application of metabolomics approaches to the study of respiratory diseases. Bioanalysis 4 (18), 2265–2290. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Rovati GE, Diamant Z, Untersmayr E, Schwarze J, Lukasik Z, Sava F, Angelina A, Palomares O, Akdis CA, O’Mahony L, Sanak M, Dahlen SE, Woszczek G, 2021. Current perspective on eicosanoids in asthma and allergic diseases: EAACI Task Force consensus report, part I. Allergy 76 (1), 114–130. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Himpens B, 1989. Cell calcium and its regulation in smooth muscle. Faseb. J 3 (11), 2266–2276. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV, 2003. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev 83 (4), 1325–1358. [DOI] [PubMed] [Google Scholar]
- Sun YV, Hu YJ, 2016. Integrative analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv. Genet 93, 147–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED, 2010. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci. Transl. Med 2 (44), 44ra58. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, Macia L, Mackay CR, 2014. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity 40 (6), 833–842. [DOI] [PubMed] [Google Scholar]
- Thwe PM, Pelgrom LR, Cooper R, Beauchamp S, Reisz JA, D’Alessandro A, Everts B, Amiel E, 2017. Cell-intrinsic glycogen metabolism supports early glycolytic reprogramming required for dendritic cell immune responses. Cell Metabol. 26 (3), 558–567 e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torell F, Bennett K, Rannar S, Lundstedt-Enkel K, Lundstedt T, Trygg J, 2017. The effects of thawing on the plasma metabolome: evaluating differences between thawed plasma and multi-organ samples. Metabolomics 13 (6), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, Berger P, 2007. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med 204 (13), 3173–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Ferreira C, 2015. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat. Med 21 (7), 709–718. [DOI] [PubMed] [Google Scholar]
- Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A, 2019. The metabolic signature of macrophage responses. Front. Immunol 10, 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo P, Bair-Merritt M, Camargo CA, 2015. The potential role of vitamin D in the link between obesity and asthma severity/control in children. Expet Rev. Respir. Med 9 (3), 309–325. [DOI] [PubMed] [Google Scholar]
- Vyas S, Zaganjor E, Haigis MC, 2016. Mitochondria and cancer. Cell 166 (3), 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gao P, Wu X, Chen Y, Feng Y, Yang Q, Xu Y, Zhao J, Xie J, 2016. Impaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthma. Respir. Res 17 (1), 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E, 1927. The metabolism of tumors in the body. J. Gen. Physiol 8 (6), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrack BM, Hnatyshyn S, Ott KH, Reily MD, Sanders M, Zhang H, Drexler DM, 2009. Normalization strategies for metabonomic analysis of urine samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 877 (5–6), 547–552. [DOI] [PubMed] [Google Scholar]
- Warren G, Lewisa ZS, Finn MG, Gary Siuzdak, 2003. Desorption/ionization on silicon (DIOS) mass spectrometry: background and applications. Int. J. Mass Spectrom 226 (1), 107–116. [Google Scholar]
- Wedes SH, Wu W, Comhair SA, McDowell KM, DiDonato JA, Erzurum SC, Hazen SL, 2011. Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J. Pediatr 159 (2), 248–255 e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr., 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest 112 (12), 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB, 2001. Mechanisms of the antioxidant effects of nitric oxide. Antioxidants Redox Signal. 3 (2), 203–213. [DOI] [PubMed] [Google Scholar]
- Wishart DS, 2016. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov 15 (7), 473–484. [DOI] [PubMed] [Google Scholar]
- Wittmann C, Heinzle E, 2001. Application of MALDI-TOF MS to lysine-producing Corynebacterium glutamicum: a novel approach for metabolic flux analysis. Eur. J. Biochem 268 (8), 2441–2455. [DOI] [PubMed] [Google Scholar]
- Xiao C, Hao F, Qin X, Wang Y, Tang H, 2009. An optimized buffer system for NMR-based urinary metabonomics with effective pH control, chemical shift consistency and dilution minimization. Analyst 134 (5), 916–925. [DOI] [PubMed] [Google Scholar]
- Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC, 2007. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. U. S. A 104 (4), 1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cardenes N, Corey C, Erzurum SC, Shiva S, 2015. Platelets from asthmatic individuals show less reliance on glycolysis. PloS One 10 (7), e0132007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Comhair SAA, Janocha AJ, Lara A, Mavrakis LA, Bennett CD, Kalhan SC, Erzurum SC, 2017. Arginine metabolic endotypes related to asthma severity. PloS One 12 (8), e0183066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Tanaka Y, Kume S, Morita Y, Chin-Kanasaki M, Araki H, Isshiki K, Araki S, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T, 2014. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim. Biophys. Acta 1842 (7), 1097–1108. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Umeno A, Shichiri M, 2013. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr 52 (1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Cui FX, Jia HM, Zhou C, Yang Y, Zhang HW, Ding G, Zou ZM, 2016. Aberrant purine metabolism in allergic asthma revealed by plasma metabolomics. J. Pharmaceut. Biomed. Anal 120, 181–189. [DOI] [PubMed] [Google Scholar]
- Yu Q, Wang Y, Dong L, He Y, Liu R, Yang Q, Cao Y, Wang Y, Jia A, Bi Y, Liu G, 2020. Regulations of glycolytic activities on macrophages functions in tumor and infectious inflammation. Front. Cell Infect. Microbiol 10, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Sun H, Wang P, Han Y, Wang X, 2012. Modern analytical techniques in metabolomics analysis. Analyst 137 (2), 293–300. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma C, Wang X, He S, Li Q, Zhou Y, Liu Y, Zhang M, Yu X, Zhao X, Li F, Zhu DL, 2019. Lipopolysaccharide-induced proliferation and glycolysis in airway smooth muscle cells via activation of Drp1. J. Cell. Physiol 234 (6), 9255–9263. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Hershey GK, Foster PS, Rothenberg ME, 2003. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol 111 (2), 227–242 quiz 243. [DOI] [PubMed] [Google Scholar]