Abstract
Background
Infective endocarditis is a severe infection arising in the lining of the chambers of the heart. It can be caused by fungi, but most often is caused by bacteria. Many dental procedures cause bacteraemia, which could lead to bacterial endocarditis in a small proportion of people. The incidence of bacterial endocarditis is low, but it has a high mortality rate.
Guidelines in many countries have recommended that antibiotics be administered to people at high risk of endocarditis prior to invasive dental procedures. However, guidance by the National Institute for Health and Care Excellence (NICE) in England and Wales states that antibiotic prophylaxis against infective endocarditis is not recommended routinely for people undergoing dental procedures. This is an update of a review that we first conducted in 2004 and last updated in 2013.
Objectives
Primary objective
To determine whether prophylactic antibiotic administration, compared to no antibiotic administration or placebo, before invasive dental procedures in people at risk or at high risk of bacterial endocarditis, influences mortality, serious illness or the incidence of endocarditis.
Secondary objectives
To determine whether the effect of dental antibiotic prophylaxis differs in people with different cardiac conditions predisposing them to increased risk of endocarditis, and in people undergoing different high risk dental procedures.
Harms
Had we foundno evidence from randomised controlled trials or cohort studies on whether prophylactic antibiotics affected mortality or serious illness, and we had found evidence from these or case‐control studies suggesting that prophylaxis with antibiotics reduced the incidence of endocarditis, then we would also have assessed whether the harms of prophylaxis with single antibiotic doses, such as with penicillin (amoxicillin 2 g or 3 g) before invasive dental procedures, compared with no antibiotic or placebo, equalled the benefits in prevention of endocarditis in people at high risk of this disease.
Search methods
An information specialist searched four bibliographic databases up to 10 May 2021 and used additional search methods to identify published, unpublished and ongoing studies
Selection criteria
Due to the low incidence of bacterial endocarditis, we anticipated that few if any trials would be located. For this reason, we included cohort and case‐control studies with suitably matched control or comparison groups. The intervention was antibiotic prophylaxis, compared to no antibiotic prophylaxis or placebo, before a dental procedure in people with an increased risk of bacterial endocarditis. Cohort studies would need to follow at‐risk individuals and assess outcomes following any invasive dental procedures, grouping participants according to whether or not they had received prophylaxis. Case‐control studies would need to match people who had developed endocarditis after undergoing an invasive dental procedure (and who were known to be at increased risk before undergoing the procedure) with those at similar risk who had not developed endocarditis.
Our outcomes of interest were mortality or serious adverse events requiring hospital admission; development of endocarditis following any dental procedure in a defined time period; development of endocarditis due to other non‐dental causes; any recorded adverse effects of the antibiotics; and the cost of antibiotic provision compared to that of caring for patients who developed endocarditis.
Data collection and analysis
Two review authors independently screened search records, selected studies for inclusion, assessed the risk of bias in the included study and extracted data from the included study. As an author team, we judged the certainty of the evidence identified for the main comparison and key outcomes using GRADE criteria. We presented the main results in a summary of findings table.
Main results
Our new search did not find any new studies for inclusion since the last version of the review in 2013.
No randomised controlled trials (RCTs), controlled clinical trials (CCTs) or cohort studies were included in the previous versions of the review, but one case‐control study met the inclusion criteria. The trial authors collected information on 48 people who had contracted bacterial endocarditis over a specific two‐year period and had undergone a medical or dental procedure with an indication for prophylaxis within the past 180 days. These people were matched to a similar group of people who had not contracted bacterial endocarditis. All study participants had undergone an invasive medical or dental procedure. The two groups were compared to establish whether those who had received preventive antibiotics (penicillin) were less likely to have developed endocarditis. The authors found no significant effect of penicillin prophylaxis on the incidence of endocarditis. No data on other outcomes were reported.
The level of certainty we have about the evidence is very low.
Authors' conclusions
There remains no clear evidence about whether antibiotic prophylaxis is effective or ineffective against bacterial endocarditis in at‐risk people who are about to undergo an invasive dental procedure. We cannot determine whether the potential harms and costs of antibiotic administration outweigh any beneficial effect. Ethically, practitioners should discuss the potential benefits and harms of antibiotic prophylaxis with their patients before a decision is made about administration.
Keywords: Humans; Anti-Bacterial Agents; Anti-Bacterial Agents/therapeutic use; Antibiotic Prophylaxis; Antibiotic Prophylaxis/adverse effects; Dentistry; Endocarditis, Bacterial; Endocarditis, Bacterial/drug therapy; Endocarditis, Bacterial/etiology; Endocarditis, Bacterial/prevention & control; Penicillins; Penicillins/therapeutic use
Plain language summary
Antibiotics for the prevention of bacterial endocarditis (severe infection or inflammation of the lining of the heart chambers) in dentistry
Review question
This Cochrane Review aimed to find out whether people with increased risk of bacterial endocarditis (a severe infection or inflammation of the lining of the heart chambers that can be fatal) should be given antibiotics routinely before invasive dental procedures to reduce the incidence of endocarditis, the number of deaths, and the amount of serious illness this group of people experiences.
Background
Bacterial endocarditis is an infection that tends to occur in previously damaged or malformed areas of the heart. It is usually treated with antibiotics. Though rare, bacterial endocarditis is potentially life‐threatening. Up to 30% of people who get it may die, even with antibiotic treatment.
Invasive dental procedures could cause bacterial endocarditis in people who are at risk of developing it. The number of cases of bacterial endocarditis (if any) directly caused in this way is unknown. Many dental procedures cause bacteraemia, which is the presence of bacteria in the blood. Although bacteraemia is usually dealt with quickly by the body’s immune system, some experts think that it may lead to bacterial endocarditis in some at‐risk people.
Guidelines in many countries have recommended that people at high risk of bacterial endocarditis be given antibiotics before undergoing invasive dental procedures. But other authorities have questioned the routine use of antibiotics, arguing that overprescription has resulted in the emergence of resistance to common antibiotics in many organisms, and also that the occasional adverse effects of antibiotics (severe allergic reactions) may outweigh the potential benefits.
In 2007, guidance from the American Heart Association changed to recommend that antibiotics be given only to people at high risk of developing bacterial endocarditis before dental interventions. Guidance from the National Institute for Health and Care Excellence (NICE) in England and Wales went further, advising against the routine prescription of preventive antibiotics for invasive dental or surgical procedures.
Study characteristics
There are no new studies to include in this updated review. Our original review included one study, based in the Netherlands, that compared the treatment of people at high risk of endocarditis who did or did not develop bacterial endocarditis. The authors collected information on 48 people who had contracted bacterial endocarditis over a specific two‐year period and had undergone a medical or dental procedure with an indication for prophylaxis within the past 180 days. These people were matched to a similar group of people who had not contracted bacterial endocarditis. All study participants had undergone an invasive medical or dental procedure. The two groups were compared to establish whether those who had received preventive antibiotics were less likely to have developed endocarditis.
Key results
It is unclear whether taking antibiotics as a preventive measure before undergoing invasive dental procedures is effective or ineffective against bacterial endocarditis in people at increased risk.
We found no studies that assessed numbers of deaths, serious adverse events requiring hospital admission, other adverse effects, or cost implications of treatment.
It is unclear whether the potential harms and costs of antibiotic administration outweigh any beneficial effects. Ethically, practitioners should discuss the potential benefits and harms of preventive antibiotic treatment with their patients before a decision is made about whether to prescribe it.
Limitations of the evidence
The evidence is based on one study that has some limitations in its design. For example, the participants who received antibiotics may have been in worse general health than those who did not. We are not confident about the evidence we found. We can only conclude that we do not know the effects of antibiotic prophylaxis for the prevention of bacterial endocarditis.
Date of the evidence
This review updates one carried out originally in 2004 and last revised in 2013. It is now up to date to 10 May 2021.
Summary of findings
Summary of findings 1. Summary of findings: antibiotic prophylaxis versus no antibiotic prophylaxis for preventing bacterial endocarditis in dentistry.
| Antibiotic prophylaxis compared with no antibiotic prophylaxis for the prevention of bacterial endocarditis in dentistry | ||||
|
Population: adults or children at risk of endocarditis Setting: dental setting Intervention: antibiotic prophylaxis Comparison: no antibiotic prophylaxis | ||||
| Outcome | Results | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Mortality or serious adverse events requiring hospitalisation | No data reported | 248 (1 study) |
‐ | ‐ |
| Development of endocarditis (in those with definite indication for prophylaxis) | There was no difference in the number of people (with a definitive indication for prophylaxis) who developed endocarditis between those receiving prophylaxis and those not receiving prophylaxis (OR 1.62; 95% CI 0.57 to 4.57). | 248 (1 study) |
⊕⊝⊝⊝ Very lowa | ‐ |
| Adverse effects of antibiotics | No data reported | 248 (1 study) |
‐ | ‐ |
| CI: confidence interval; OR: odds ratio | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded 3 levels for high risk of bias and serious imprecision.
Background
Description of the condition
Infective endocarditis is a rare disease caused by infected vegetations (growths) that often occur on previously damaged or congenitally malformed cardiac valves or endocardium (heart chamber lining). The infecting organisms are usually bacteria and less commonly fungi, particularly of the Candida species. Bacterial endocarditis is infective endocarditis caused by bacteria that enter the blood (bacteraemia). Bacteria may enter the blood through a variety of points of entry but especially mucosal surfaces. The gingiva (gums) and periodontal ligaments, which surround all teeth, experience an almost constant degree of inflammation and as such are a potential point of entry for bacteria to the blood. Indeed, everyday activities such as toothbrushing can cause bacteraemia (Lucas 2000; Roberts 1999). Bacterial endocarditis is a rare but potentially life‐threatening condition. A 2019 systematic review reported bacterial endocarditis incidence of 15 cases per 100,000 in the USA in 2011, with six‐month mortality of up to 30%, even with antibiotic treatment (Jamil 2019).
In the past, the majority of people who developed endocarditis had a known pre‐existing cardiac defect. More recently, however, this trend has shifted, with nearly half of endocarditis cases having no known previous cardiac disease (Duval 2012). The growth of the aging population with comorbidities and their subsequent increased interactions with healthcare may have contributed to the increased incidence of the disease (Jamil 2019).
Common cardiac conditions that put people at risk include previous endocarditis, prosthetic heart valves, valvular stenosis, ventricular septal defect and valvular damage following rheumatic fever (Danchin 2005; Farook 2012). In particular, people with previous endocarditis and prosthetic heart valves are considered to have a high risk of developing endocarditis (Durack 1994). These predisposing conditions either cause changes in the surface of the heart lining (endocardium) or changes in blood flow that damage the endocardium and enable organisms in the blood to adhere and multiply, forming bacterial vegetations. This leads to severe systemic illness and directly affects the functioning of the heart. Fragments of the vegetations may break away and become lodged elsewhere in the circulatory system, potentially resulting in an embolism.
Description of the intervention
Most dental procedures cause bacteraemia, which, it has been hypothesised, may lead to bacterial endocarditis. The proportion of bacterial endocarditis cases arising as a result of dental treatment is uncertain, though a recent study estimated it at 12% (Delahaye 2016). For many years, there was a well‐established practice of administering antibiotics, typically penicillins, to individuals at risk of developing bacterial endocarditis before any dental procedures that carried a risk of a bacteraemia developing. Early clinical guidelines supported this practice (Dajani 1997; EWP 1993), based on the rationale that a high circulating dose of antibiotic would prevent the development of an infected vegetation on damaged endocardium and thus prevent endocarditis.
Some population‐based case studies questioned the routine use of antibiotics for endocarditis prophylaxis (e.g. Strom 1998a), arguing that the adverse effects of antibiotics may outweigh their potential benefits. This point of view was given some support after the original 2004 publication of this review, which did not find sufficient evidence to draw any conclusions about the effectiveness or ineffectiveness of the intervention. Across Europe, the USA and Australia, clinical guidelines moved away from recommending antibiotic prophylaxis for all at‐risk patients, instead advising that they be given only to those at 'high risk' (Farook 2012), though 'high risk' was defined differently by different regulatory bodies (e.g. American Heart Association; European Society of Cardiology). In 2008, the National Institute for Health and Care Excellence (NICE) went even further when it published guidance for England and Wales stating that no antibiotic prophylaxis was required for any interventional procedure (NICE 2008). Initially, despite marked reductions in the use of prophylactic antibiotics, the incidence of bacterial endocarditis after dental treatment did not appear to increase (Duval 2012; Thornhill 2011). Recent evidence from a systematic review investigating the impact of guideline changes on the global incidence of infective endocarditis suggests that restricting prophylactic antibiotics to only high‐risk patients has not resulted in an increase in the incidence of streptococcal cases in North American populations; however, the authors indicate that further research is needed to clarify the impact of guideline changes in the UK and some European countries (Williams 2021). The NICE guidance was updated in 2016 (NICE 2008).
How the intervention might work
Antibiotic prophylaxis before invasive procedures has been a key strategy for preventing bacterial endocarditis for several decades, and remains so in many parts of the world (Thornhill 2011). Antibiotic therapy for the treatment of bacterial endocarditis is not in question: without antibiotic therapy, infective endocarditis is fatal (Durack 1994). However, the use of antibiotic prophylaxis for the prevention of bacterial endocarditis remains controversial.
Why it is important to do this review
In 2004, Cochrane Oral Health first undertook this systematic review, initially looking at penicillin for the prevention of bacterial endocarditis in people having dental treatment. The review was updated and expanded to include all antibiotics in 2008, and updated again in 2013. The review included only one case‐control study and was inconclusive about the place of antibiotic prophylaxis in the prevention of bacterial endocarditis. The initial review created much debate around the prescribing of antibiotic prophylaxis for the prevention of bacterial endocarditis. Changes in clinical guidelines in various countries led to restrictions in the use of prophylactic antibiotics, but many dentists were concerned that this could put patients at increased risk of bacterial endocarditis due to a dental intervention.
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of priority titles (Worthington 2015). This review was one of those identified at that time and its importance was confirmed in our second comprehensive prioritisation process, which was undertaken in 2020 (see Cochrane Oral Health priority review portfolio).
Objectives
Primary objective
To determine whether prophylactic antibiotic administration, compared to no antibiotic administration or placebo, before invasive dental procedures in people at risk or at high risk of bacterial endocarditis, influences mortality, serious illness or the incidence of endocarditis.
Secondary objectives
To determine whether the effect of dental antibiotic prophylaxis differs in people with different cardiac conditions predisposing them to increased risk of endocarditis, and in people undergoing different high risk dental procedures.
Harms
Had we found no evidence from randomised controlled trials or cohort studies on whether prophylactic antibiotics affected mortality or serious illness, and we had found evidence from these or case‐control studies suggesting that prophylaxis with antibiotics reduced the incidence of endocarditis, then we would also have assessed whether the harms of prophylaxis with single antibiotic doses, such as with penicillin (amoxicillin 2 g or 3 g) before invasive dental procedures, compared with no antibiotic or placebo, equalled the benefits in prevention of endocarditis in people at high risk of this disease.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) and controlled clinical trials (CCTs) where these were available, though we anticipated these may not have been possible due to the low incidence of bacterial endocarditis. We planned to include cohort and case‐control studies where suitably matched control or comparison groups had been studied.
Types of participants
RCTs and CCTs
Studies must have involved adults or children, or both, who had any of the following pre‐existing cardiac defects (i.e. patients known to be at risk): congenital heart defects, a history of rheumatic fever, prosthetic heart valves (tissue and mechanical) or previous endocarditis. We excluded studies of people with pacemakers (and no other risk factors).
The dental procedures that the participants may have undergone in the studies included: supragingival and subgingival scaling of teeth, extensive restorations of teeth, endodontics and oral surgery including dental extractions. We considered procedures performed under local and general anaesthetic.
Types of interventions
RCTs and CCTs
The intervention assessed was the administration of an antibiotic, compared with no such administration or placebo, before a dental procedure. We included studies in which an antibiotic was administered postoperatively if this was part of a protocol including preoperative administration. The antibiotics could be administered by oral, intravenous, or intramuscular routes, but not topically.
Co‐interventions could include preoperative use of mouthwash or mechanical cleaning of teeth.
Types of outcome measures
RCTs and CCTs
Mortality or serious adverse events (from any cause) requiring hospital admission
Development of endocarditis following any dental procedure in a defined time period
Development of endocarditis due to other non‐dental cause
Secondary outcomes
RCTs and CCTs
Any recorded adverse effects of the antibiotics
Cost implications of antibiotic provision for prophylaxis compared with the cost of care of patients who develop bacterial endocarditis
Assessment of harms would have included all studies where potentially serious adverse effects (such as would be expected to result in hospitalisation) or fatal adverse effects of a single antibiotic dose had been reported or assessed.
Cohort studies and case‐control studies
To be included, cohort studies would have had to fulfil the following criteria.
Participants would be people at increased risk of endocarditis (as above).
Their progress would be followed (no minimum time period) and invasive dental procedures carried out.
Use (or not) of prophylactic antibiotics at these visits and occurrence or not of bacterial endocarditis, death or serious illness would be recorded (as a minimum).
It would be possible to compare incidence of bacterial endocarditis, and death or serious illness in those who received invasive dental procedures with and without antibiotics.
The case‐control study included fulfilled the following criteria.
The groups that were compared included a group of people at increased risk of endocarditis who did develop bacterial endocarditis, and a group of people at increased risk of endocarditis who did not develop bacterial endocarditis.
Information was provided on the numbers of people in each group who had undergone an invasive dental procedure within a (stated) set period, and the numbers who had received antibiotic prophylaxis before the procedure.
We decided post hoc that studies that excluded cases when they died due to endocarditis would be excluded from our review as up to 30% of people who contract endocarditis will die of it and these participants may be different from those who survive.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 10 May 2021) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 4) in the Cochrane Library (searched 10 May 2021) (Appendix 2);
MEDLINE Ovid (1946 to 10 May 2021) (Appendix 3);
Embase Ovid (1980 to 10 May 2021) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. We opted not to use a filter to limit the search to randomised controlled trials as the yield from the subject search was low.
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 10 May 2021) (Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 10 May 2021) (Appendix 6).
For the 2013 update of this review, we had searched the metaRegister of Controlled Trials to 21 January 2013 (see Appendix 7), but this resource is no longer available.
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We checked that none of the included studies in this review had been retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts obtained from the searches. The review authors were not blinded to the authors, institution or journal. Full‐text papers that were retrieved were similarly screened for inclusion independently by two review authors. Any disagreements over inclusion would have been resolved by discussion between the review authors, with a third review author being consulted if necessary.
Data extraction and management
Two review authors independently extracted data and quality information onto a custom‐designed data collection form. In addition to bibliographic details of the paper, the key items of data we recorded were the study design, country of origin, details of the antibiotic intervention, type of dental procedure and study population details including risk factors. The outcome data collected from RCTs and CCTs would have included number of deaths; number of hospital admissions; number of serious illnesses that would be expected to result in hospital admission; number of cases of endocarditis; any other adverse events noted and number of people originally randomised to each group. The outcome data collected from cohort studies would have included the same information as for RCTs plus adjusted odds ratios or risk ratios and information about the factors for which adjustments were made. The outcome data collected from the case‐control study included the adjusted odds ratio of a person at increased risk of endocarditis having had antibiotic prophylaxis prior to invasive dentistry before either developing endocarditis (cases) or not (controls).
Where necessary, we contacted study authors for further details of their studies to assess inclusion.
Assessment of risk of bias in included studies
We planned to rank included studies according to study design: RCT, CCT, cohort study, case‐control study.
Two review authors independently assessed the risk of bias in the included study using the Cochrane risk of bias assessment tool for non‐randomised studies. The domains we assessed were: sequence generation, allocation concealment, confounding, blinding of outcome assessment, completeness of outcome data, risk of selective outcome reporting, and risk of other potential sources of bias.
We described the risk of bias in each domain for the included study, along with a judgement of low, high or unclear risk of bias. We considered the risk of bias overall according to whether there was a low risk of bias for all key domains (overall low), unclear risk of bias for one or more key domains (overall unclear) or high risk of bias for one or more key domains (overall high) (Higgins 2011).
Had data appeared ambiguous or incomplete, we would have contacted the study authors for clarification.
Measures of treatment effect
We planned to use the risk ratio as the effect estimate measure for dichotomous data, and the mean difference (or standardised mean difference) for continuous data.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We planned to contact study authors for missing data if required.
Assessment of heterogeneity
We planned to test for heterogeneity between trial results using a standard Chi2 test, considered significant where the P value was less than 0.1.
For case‐control studies, we planned that the odds of antibiotic prophylaxis before dental treatment in the previous three months for cases and controls would not be pooled with data from other types of studies. We planned to tabulate any harms data according to study design, but not to pool them.
Assessment of reporting biases
We did not assess publication bias.
Data synthesis
We planned to seek data on the number of participants with each outcome event, by allocated treatment group (RCTs) or quantile (cohort studies). We aimed to calculate a pooled estimate of the treatment effect for each outcome (separately) across RCTs, CCTs, cohort studies and case‐control studies in a random‐effects meta‐analysis as an odds ratio (the ratio of odds of developing bacterial endocarditis in the prophylaxis group to the odds in the no prophylaxis group), since the odds ratio is the only good measure of association that works across prospective studies and case‐control studies (Fleiss 1981).
Subgroup analysis and investigation of heterogeneity
If sufficient data had been identified, we would have conducted subgroup analysis according to:
different dosages, e.g. 2 g and 3 g amoxicillin;
different underlying causes of at‐risk and high‐risk status for endocarditis; and
different invasive dental techniques.
Sensitivity analysis
If sufficient data had been identified, we would have conducted a sensitivity analysis by removing any studies where risk factors for endocarditis were significantly different between the groups being compared and the trial authors had not adjusted adequately for this difference.
Summary of findings and assessment of the certainty of the evidence
We judged the certainty of the evidence we found according to GRADE criteria and created a summary of findings table to summarise our findings for the main comparison and our key outcomes of mortality, endocarditis and serious adverse events.
Results
Description of studies
Results of the search
For this update, we identified a total of 530 unique references between 21 January 2013 (which was the search date used in the 2013 version of this review) and 10 May 2021. None of the references were suitable for inclusion in the review. A flow diagram of this study selection combined with the study selection for the previous version is presented in Figure 1.
1.
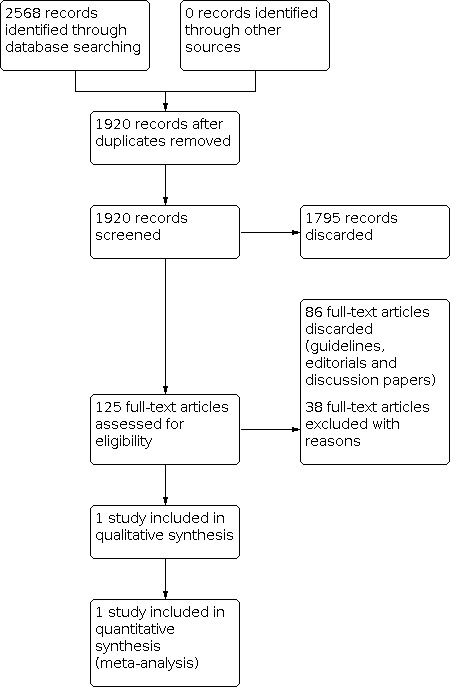
Study flow diagram
Included studies
We included one case‐control study in the original review (Van der Meer 1992a). Since the time of the last review, no further evidence has been produced to determine the effects of antibiotic prophylaxis for dental procedures.
Van der Meer 1992a involved 349 people with definite native‐valve endocarditis, 197 of whom had previous heart disease (proxy responders, i.e. spouses or general practitioners, were interviewed for 10 of these). Of these 197 at‐risk individuals, 54 had undergone a medical or dental procedure with an indication for prophylaxis within the past 180 days. Within this group, a causal relationship was ruled out in six people as the agent isolated from the blood was unlikely to have originated in the area of the procedure. Of the remaining 48 people with endocarditis, who formed the case group, 44 had undergone a dental procedure which the paper identified as having a definite (24) or possible (20) indication for prophylaxis (none of these cases had used a proxy responder). Indications for definite prophylaxis were dental extractions and dental root work, while indications for possible prophylaxis were defined as dental scaling.
Of 889 potential controls who were sent an introductory letter, 689 were ineligible (53 had died, 29 had a prosthetic heart valve, 62 could not be located, 102 could not be contacted by phone, and 418 had not undergone an invasive dental or medical procedure within the past 180 days). The remaining 200 were interviewed by phone two to five days later; 181 of these controls had undergone a dental procedure with definite (79) or possible (102) indications for prophylaxis.
Seven of 24 cases and 16 of 79 controls had had appropriate prophylaxis for a dental procedure requiring definite prophylaxis within the previous 180 days.
The characteristics of the cases and controls were not well described, as those who had received a dental procedure (rather than a medical one) were not separated out in the publication (the separated data were provided by Professor Van der Meer). The median time between a dental procedure requiring definite prophylaxis and onset of endocarditis was 10 days in the cases, and the median time between a dental procedure requiring definite prophylaxis and interview was 71 days in the controls (data missing for 12 controls). The following procedures were performed.
Apical surgery in one case (4%) and one control (1%)
Dental avulsion in one case (4%) and 12 controls (15%)
Dental extraction in nine cases (38%) and 15 controls (19%)
Dental abscess in one case (4%) and one control (1%)
Removal of subgingival calculus in three cases (13%) and eight controls (33%)
Removal of calculus plus polishing of teeth in six cases (25%) and 34 controls (43%)
Root canal therapy in three cases (13%) and eight controls (10%).
Including the cases and controls undergoing either definite or possible indication for prophylaxis, and including the four cases and 19 controls who underwent a non‐dental procedure, 69% of cases and 55% of controls were male. The median age of this larger group was 41 years for cases and 40 for controls (the controls were age‐matched).
Seven of 21 cases and 9 of 46 controls had had appropriate prophylaxis for a dental procedure requiring definite prophylaxis within the previous 90 days. Seven of 44 cases and 17 of 181 controls had had appropriate prophylaxis for a dental procedure requiring definite or possible prophylaxis within the previous 180 days. Seven of 32 cases and 9 of 100 controls had had appropriate prophylaxis for a dental procedure requiring definite or possible prophylaxis within the previous 90 days. No information was presented on the adjunctive use of mouthwash
Excluded studies
We did not identify any new 'excluded studies'. Details of previously excluded studies are presented in the Characteristics of excluded studies tables.
Risk of bias in included studies
Van der Meer 1992a, our one included study, used a case‐control design. Overall, the observational and retrospective nature of the design conferred a high risk of bias (Characteristics of included studies; Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Van der Meer 1992a used a case‐control design and therefore we judged it to be at high risk of selection bias.
Blinding
Outcome assessors in Van der Meer 1992a were not blinded and therefore we judged it to be at high risk of detection bias.
Incomplete outcome data
We judged Van der Meer 1992a to be at high risk of attrition bias. Potential cases who were very ill or who died were included in the selection process via the use of proxy responders (i.e. spouses or general practitioners); however, this did not occur for the 53/889 potential controls who died. In addition, it was unclear how similar the groups were with regard to proportions of males and females, different types of cardiac risk factor, and different dental interventions.
Selective reporting
Van der Meer 1992a reported expected outcomes in full and so we judged it to be at low risk of reporting bias.
Other potential sources of bias
We judged Van der Meer 1992a to be unclear in terms of other potential sources of bias. Participant sex and cardiac risk factor type were not described for the subgroup who had had a dental procedure, and the types of dental intervention appear to have been different for the cases and controls, although the two groups were matched for age. Both groups were required to have undergone invasive dental techniques within 180 days prior to onset of symptoms or interview and data were split by time period for both groups.
Effects of interventions
See: Table 1
We included one case‐control study (Van der Meer 1992a).
In each of the ways of assessing the data (as presented above under Included studies), the proportion of people receiving prophylaxis was greater in the cases than in the controls. When we calculated the odds of developing endocarditis in those receiving prophylaxis compared with those not receiving prophylaxis, we found an odds ratio (OR) that was not significantly different from the OR for any of the groupings (OR 1.62, 95% confidence interval (CI) 0.57 to 4.57 for those with a definite indication for prophylaxis within the previous 180 days).
Only four cases developed endocarditis following non‐dental medical interventions (within the past 180 days and with pre‐existing cardiac indications for the use of prophylaxis), so assessment of the effects of prophylaxis in these cases was not possible.
It was unclear whether antibiotic prophylaxis was effective or ineffective against bacterial endocarditis in people at risk who were about to undergo an invasive dental procedure.
The study did not provide any data on mortality, adverse events requiring hospitalisation, adverse effects of antibiotics or cost implications of treatment.
Because we observed no significant protective effect of antibiotic prophylaxis against endocarditis, we did not perform a wide‐ranging search to pool information on the potential harmful effects of antibiotic prophylaxis as prespecified in the protocol.
Discussion
Summary of main results
This review update has identified no additional studies that meet the review's inclusion criteria.
The one included case‐control study included all people in the Netherlands who developed endocarditis following an invasive dental procedure while at known cardiac risk over a two‐year period (24 individuals who underwent a procedure that definitely required prophylaxis, and a further 20 who may have required prophylaxis). The study provided no conclusive evidence about whether antibiotic prophylaxis is effective or ineffective against bacterial endocarditis in high risk individuals about to undergo an invasive dental procedure.
The evidence regarding the development of endocarditis over 180 days in those who received prophylaxis compared with those who did not receive prophylaxis is uncertain, with an odds ratio in favour of prophylaxis but with a confidence interval that includes both benefit and harm (OR 1.62, 95% CI 0.57 to 4.57).
There are currently insufficient primary data to determine whether antibiotic prophylaxis before invasive dental procedures in people at increased risk of endocarditis prevents endocarditis, deaths or other serious illness.
Overall completeness and applicability of evidence
As the usefulness of prophylaxis could not be established, we have not examined the harms of antibiotic administration in detail; this would be a systematic review in itself. Such a review would, however, be extremely valuable and could potentially be used by a wide spectrum of research workers and other systematic reviewers. In the absence of a systematic review on the harms of penicillins, the most authoritative source is Meyler's Side Effects of Drugs (Aronson 2006). The range of potential side effects from the administration of antibiotics is vast, and while the aetiology is largely hypersensitive, some direct toxic effects may also occur.
The effects of the NICE guidance on the incidence of endocarditis in the UK are going to be monitored using Hospital Episode Statistics (HES). Tracking endocarditis incidence rates over a number of years and comparing them with rates recorded in the pre‐NICE guideline era might be one method to answer this conundrum, albeit in a rather crude fashion.
Another underexplored area is the cost of prophylaxis, both in terms of finance and health. The financial cost to health services of providing large quantities of prophylactic antibiotics must be weighed against the cost of treating patients who develop endocarditis. Although endocarditis is a serious disease, it occurs in appreciably fewer patients than those potentially at risk. The health costs should also be considered, particularly the potential harms of administering antibiotics compared to endocarditis. The involvement of health economists would be beneficial. This was explored in depth in the NICE guidance (NICE 2008, updated 2016).
Despite the varying guidelines produced over the years and the change recommended by NICE, it is important for medical and dental practitioners to remember that patients remain at risk of developing endocarditis. Many patients will develop endocarditis via organisms that enter the blood through the oral cavity. Whilst there is no evidence that dental treatment is or is not directly related to the development of the disease, nor that prophylactic antibiotics can or cannot prevent the development of the disease, achieving and maintaining the highest level of oral health in at‐risk patients is a logical objective.
Following the update of the NICE guidance in 2016, the Scottish Dental Clinical Effectiveness Programme produced guidance for clinicians in Scotland to help overcome any concerns regarding implementation (SDCEP 2018).
Quality of the evidence
The overall certainty of the evidence is very low, as the evidence comes from a single study at high risk of bias. While it would be useful to have higher levels of certainty about the effectiveness of antibiotic prophylaxis of endocarditis in dentistry, it is not feasible because the incidence of endocarditis is so low. A randomised controlled trial run over two years would require approximately 60,000 participants with a cardiac risk factor for endocarditis (a cohort study over 10 years would require approximately 18,000 participants). Such a trial would require an intense international effort.
A larger, well conducted case‐control study might be more feasible, but would still require a large effort and multicentre participation. If including every endocarditis case in the Netherlands for two years produces only 24 appropriate cases, then the area or time span covered in a suitably sized study would be very large indeed. Selection of appropriate controls is probably the most challenging aspect; ideally, as in Van der Meer 1992a, they should have had dental treatment within a predefined time period and be matched very closely for sex, age and type of cardiac risk factor. Additionally, neither cases nor controls should be excluded for death or serious illness (use of proxy respondents would be ideal and this would require retrospective identification of controls as well as ongoing prospective identification of cases) and dental records should be available and be explicit about the use (or not) of prophylaxis. Full details should be collected on other factors that may compound the risk such as general well‐being, coexisting medical problems, socioeconomic status and oral health status.
The fact that neither of these study types has been attempted since this review began suggests it is highly unlikely that they ever will.
Potential biases in the review process
We identified no potential biases in the review process.
Agreements and disagreements with other studies or reviews
Most experts agree that there is little scientific evidence to support the effectiveness of antibiotic prophylaxis for the prevention of bacterial endocarditis (Cahill 2017; Duval 2012; Farook 2012; Thornhill 2011). This lack of evidence has led to variations in guideline recommendations with regard to who should or should not be prescribed antibiotic prophylaxis and who is or is not considered high‐risk for bacterial endocarditis. However, one area where most guidelines agree is with regard to the need for regular dental surveillance to promote good oral hygiene, thus reducing the need for invasive dental procedures and subsequently reducing the risk of bacterial endocarditis (SDCEP 2018).
Authors' conclusions
Implications for practice.
There is still no clear evidence to show whether antibiotic prophylaxis is effective or ineffective against bacterial endocarditis in people considered to be at increased risk of the disease who are about to undergo an invasive dental procedure. It is unknown whether any potential harms and the costs of antibiotic administration outweigh any beneficial effects.
Implications for research.
A randomised controlled trial (RCT) would only be feasible in extensive areas of very centralised and organised health care, due to the large numbers of participants with risk factors for endocarditis that would be required. A well‐designed multicentre cohort or case‐control study is possible, but would still require a very large and co‐ordinated effort, and a great deal of attention would need to be paid to recruiting suitable control participants. These studies would be most feasible in an area where registers exist so that investigators could easily identify all people with current risk factors for endocarditis before randomising them, or following their dental histories in detail to identify outcomes fully and accurately.
A systematic review of the harms and costs associated with antibiotic use is needed; such a review may be useful to assess the harms for a number of different interventions. It would be important to assess the effects of type of antibiotic, route of administration, dose, previous history of reaction, and duration of use on the side effects and adverse events experienced by people on antibiotic therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2022 | Amended | Broken hyperlink corrected |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 16 November 2021 | New citation required but conclusions have not changed | New author added. No new studies for inclusion |
| 10 May 2021 | New search has been performed | Updated search to 10 May 2021. Background updated |
| 31 July 2013 | New citation required but conclusions have not changed | The background and discussion have been updated to reflect recent literature in this area. 'Risk of bias' and 'Summary of findings' tables have been added. Change in authors. |
| 12 July 2013 | New search has been performed | Search updated to January 2013. |
| 24 July 2008 | New citation required but conclusions have not changed | Review updated and scope expanded to include all antibiotics and not just penicillins. Change in authors. |
| 24 July 2008 | New search has been performed | Search updated to June 2008. |
| 24 June 2008 | Amended | Converted to new review format. |
Acknowledgements
For this update, we thank Laura MacDonald, Anne Littlewood and Jan Clarkson of Cochrane Oral Health and Julia Turner for copy editing. For contributions to previous versions of this review, our gratitude goes to Dr Van der Meer, Dr Imperiale, Luisa Fernandez Mauleffinch, Anne Littlewood, Margaret Burke, Vittoria Lutje, Marco Esposito, Sylvia Bickley, Jan Clarkson, Paul Coulthard, Jayne Harrison, David Rickard, Robin Richardson, Jan van der Meer, Robin Seymour, Jeremy Bagg and the Cochrane Non‐Randomised Methods Group, and the Harms subgroup. In particular, we are grateful to Richard Oliver for initiating the review and for his involvement as an author in the protocol and previous versions of the review.
Appendices
Appendix 1. Cochrane Oral Health Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials
#1 (endocarditis) AND (INREGISTER) #2 ((endocardium and (inflamm* or infect*)):ti,ab) AND (INREGISTER) #3 ((ABE or SABE):ti,ab) AND (INREGISTER) #4 (#1 or #2 or #3) AND (INREGISTER) #5 ((antibiotic* or anti‐biotic* or antimicrobial* or anti‐microbial*):ti,ab) AND (INREGISTER) #6 ((antibacterial* or anti‐bacterial*):ti,ab) AND (INREGISTER) #7 ((penicillin* or amoxicillin* or amoxycillin* or amoxil or actimoxi or clamoxyl or hydroxyampicillin or penamox or trimox or wymox):ti,ab) AND (INREGISTER) #8 ((apocillin or beromycin or betapen or fenoxymethylpenicillin or "Pen VK" or phenoxymethylpenicillin or "V‐Cillin K" or vegacillin):ti,ab) AND (INREGISTER) #9 ((clindamycin or chlolincocin or cleocin or "Dalacin C"):ti,ab) AND (INREGISTER) #10 ((cephalexin or cefalexin* or ceporexine or Palitrex or cephahexin*):ti,ab) AND (INREGISTER) #11 ((azithromycin or azadose or azitrocin or azythromycin or gozal or sumamed or toraseptol or ultreon or vinzam or zentavion or zithromax or zitromax):ti,ab) AND (INREGISTER) #12 ((clarithromycin or biaxin):ti,ab) AND (INREGISTER) #13 ((cefazolin or ancef or cefamedin or cefamezine or cephamezine or cephazolin or gramaxin or kefzol or totacef):ti,ab) AND (INREGISTER) #14 ((ceftriaxone or benaxona or cefatriaxone or cefaxona or ceftrex or ceftriaxon* or lendacin or longacef or longaceph or rocefalin or rocefin or rocephin* or tacex or terbac):ti,ab) AND (INREGISTER) #15 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14) AND (INREGISTER) #16 (#4 and #15) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh Endocarditis] #2 endocarditis #3 (endocardium near/5 (inflamm* or infect*)) #4 (ABE or SABE):ti,ab #5 #1 or #2 or #3 or #4 #6 [mh "Dental prophylaxis"] #7 [mh ^"Dentistry, operative"] #8 [mh Endodontics] #9 [mh "Oral surgical procedures"] #10 ((oral or tooth or teeth) near/5 (surg* or extract* or restor* or invas* or scale or scaling or polish* or endodontic* or "root canal" or apicectom* or apicoectom*)) #11 (dental or dentist*) #12 #6 or #7 or #8 or #9 or #10 or #11 #13 [mh ^"Antibiotic prophylaxis"] #14 (antibiotic* or anti‐biotic* or antimicrobial* or anti‐microbial*) #15 [mh ^"Anti‐bacterial agents"] #16 [mh Penicillins] #17 (amoxicillin* or amoxycillin* or amoxil or actimoxi or clamoxyl or hydroxyampicillin or penamox or trimox or wymox) #18 ("penicillin v*" or apocillin or beromycin or betapen or fenoxymethylpenicillin or "Pen VK" or phenoxymethylpenicillin or "V‐Cillin K" or vegacillin) #19 (clindamycin or chlolincocin or cleocin or "Dalacin C") #20 (azithromycin or azadose or azitrocin or azythromycin or gozal or sumamed or toraseptol or ultreon or vinzam or zentavion or zithromax or zitromax) #21 (clarithromycin or biaxin) #22 (cefazolin or ancef or cefamedin or cefamezine or cephamezine or cephazolin or gramaxin or kefzol or totacef) #23 (ceftriaxone or benaxona or cefatriaxone or cefaxona or ceftrex or ceftriaxon* or lendacin or longacef or longaceph or rocefalin or rocefin or rocephin$ or tacex or terbac) #24 (cephalexin or cefalexin* or ceporexine or Palitrex or cephahexin*) #25 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #26 #5 and #12 and #25
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Endocarditis/ 2. endocarditis.tw. 3. (endocardium adj5 (inflamm$ or infect$)).tw. 4. (ABE or SABE).ti,ab. 5. or/1‐4 6. exp Dental prophylaxis/ 7. exp Dentistry, operative/ 8. exp Endodontics/ 9. exp Oral surgical procedures/ 10. ((oral or tooth or teeth) adj5 (surg$ or extract$ or restor$ or invas$ or scale or scaling or polish$ or endodontic$ or "root canal" or apicectom$ or apicoectom$)).tw. 11. (dental or dentist$).tw. 12. or/6‐11 13. Antibiotic prophylaxis/ 14. (antibiotic$ or anti‐biotic$ or antimicrobial$ or anti‐microbial$).tw. 15. Anti‐bacterial agents/ 16. exp Penicillins/ 17. (amoxicillin$ or amoxycillin$ or amoxil or actimoxi or clamoxyl or hydroxyampicillin or penamox or trimox or wymox).tw. 18. ("penicillin v$" or apocillin or beromycin or betapen or fenoxymethylpenicillin or "Pen VK" or phenoxymethylpenicillin or "V‐Cillin K" or vegacillin).tw. 19. Clindamycin/ 20. (clindamycin or chlolincocin or cleocin or "Dalacin C").tw. 21. Cephalexin/ 22. (cephalexin or cefalexin$ or ceporexine or Palitrex or cephahexin$).tw. 23. Azithromycin/ 24. (azithromycin or azadose or azitrocin or azythromycin or gozal or sumamed or toraseptol or ultreon or vinzam or zentavion or zithromax or zitromax).tw. 25. Clarithromycin/ 26. (clarithromycin or biaxin).tw. 27. Cefazolin/ 28. (cefazolin or ancef or cefamedin or cefamezine or cephamezine or cephazolin or gramaxin or kefzol or totacef).tw. 29. Ceftriaxone/ 30. (ceftriaxone or benaxona or cefatriaxone or cefaxona or ceftrex or ceftriaxon$ or lendacin or longacef or longaceph or rocefalin or rocefin or rocephin$ or tacex or terbac).tw. 31. or/13‐30 32. 5 and 12 and 31
Appendix 4. Embase (Ovid) search strategy
1. exp Endocarditis/ 2. endocarditis.tw. 3. (endocardium adj5 (inflamm$ or infect$)).tw. 4. (ABE or SABE).ti,ab. 5. or/1‐4 6. exp Dental surgery/ 7. exp Endodontics/ 8. exp Oral surgery/ 9. ((oral or tooth or teeth) adj5 (surg$ or extract$ or restor$ or invas$ or scale or scaling or polish$ or endodontic$ or "root canal" or apicectom$ or apicoectom$)).tw. 10. (dental or dentist$).tw. 11. or/6‐10 12. Antibiotic prophylaxis/ 13. (antibiotic$ or anti‐biotic$ or antimicrobial$ or anti‐microbial$).tw. 14. Antiinfective agent/ 15. exp Penicillin derivative/ 16. (amoxicillin$ or amoxycillin$ or amoxil or actimoxi or clamoxyl or hydroxyampicillin or penamox or trimox or wymox).tw. 17. ("penicillin v$" or apocillin or beromycin or betapen or fenoxymethylpenicillin or "Pen VK" or phenoxymethylpenicillin or "V‐Cillin K" or vegacillin).tw. 18. Clindamycin/ 19. (clindamycin or chlolincocin or cleocin or "Dalacin C").tw. 20. Cefalexin/ 21. (cephalexin or cefalexin$ or ceporexine or Palitrex or cephahexin$).tw. 22. Azithromycin/ 23. (azithromycin or azadose or azitrocin or azythromycin or gozal or sumamed or toraseptol or ultreon or vinzam or zentavion or zithromax or zitromax).tw. 24. Clarithromycin/ 25. (clarithromycin or biaxin).tw. 26. Cefazolin/ 27. (cefazolin or ancef or cefamedin or cefamezine or cephamezine or cephazolin or gramaxin or kefzol or totacef).tw. 28. Ceftriaxone/ 29. (ceftriaxone or benaxona or cefatriaxone or cefaxona or ceftrex or ceftriaxon$ or lendacin or longacef or longaceph or rocefalin or rocefin or rocephin$ or tacex or terbac).tw. 30. or/12‐29 31. 5 and 11 and 30
Appendix 5. US National Institutes of Health Trials Register search strategy
endocarditis AND dental AND prophylaxis
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
endocarditis AND dental AND prophylaxis
Appendix 7. metaRegister of Controlled Trials search strategy
endocarditis AND dental AND prophylaxis
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Van der Meer 1992a.
| Study characteristics | ||
| Methods | Case‐control study | |
| Participants | All 349 people who developed definite native‐valve endocarditis in the Netherlands over a 2‐year period (1 November 1986 to 1 November 1988) were collected. Cases (n = 48) were eligible if they had previously had congenital heart disease, coarctation of the aorta, rheumatic or other valvular dysfunction, or mitral valve prolapse with mitral regurgitation. Proxy responders (spouses or general practitioners) were used where cases were too ill to be interviewed or had died. Controls (n = 200) had not been diagnosed with endocarditis but had 1 of the cardiac conditions and were outpatients at a cardiology department of 1 of 5 hospitals. Controls were matched for age (within the same 5‐year age category). A random sample of potential controls was drawn, and, where there were at least 4 controls per case, all were contacted. Where there were fewer than 4 controls, a further random sample was drawn. | |
| Interventions | Cases and controls had to have undergone a medical or dental procedure that required antibiotic prophylaxis within 180 days prior to the onset of symptoms of endocarditis (cases) or their interview (controls). Of the participants who underwent a dental procedure with definite indication for prophylaxis:
Median time from dental procedure to onset of endocarditis in cases was 10 days, range 0 to 175, and for the 7 who received antibiotics median time to onset was 18 days, range 7 to 60. Median time from dental procedure to interview in controls was 71 days, range 0 to 179 (12 missing values ignored), and for the controls who received antibiotics the median was 83 days, range 5 to 151 (1 missing value ignored). For both groups, all information about invasive procedures and use of prophylaxis was checked with medical or dental specialists and pharmacists. |
|
| Outcomes | Of the 349 people with definite native‐valve endocarditis, 197 had previous heart disease (10 proxy responders). Of these, 54 had undergone a medical or dental procedure with an indication for prophylaxis within the past 180 days. A causal relationship was ruled out in 6 of these 54 potential cases as the agent isolated from the blood was unlikely to have originated in the area of the procedure. Of the remaining 48 people with endocarditis, 44 had undergone a dental procedure with a definite (24) or possible (20) indication for prophylaxis (none of these cases had used a proxy responder). Of 889 potential controls who were sent an introductory letter, 689 were ineligible (53 had died, 29 had a prosthetic heart valve, 62 could not be located, 102 could not be contacted by phone and 418 had not undergone an invasive dental or medical procedure within the past 180 days) and the remaining 200 were interviewed by phone 2 to 5 days later. 181 of these controls had undergone a dental procedure with definite (79) or possible (102) indication for prophylaxis. The authors ensured that controls had not developed endocarditis, as defined by the diagnostic criteria of Von Reyn 1981. They also checked the appropriateness of antibiotic prophylaxis with medical, dental and pharmacy staff and against the Netherlands Heart Foundation recommendations, finding that 7 of 24 cases and 16 of 79 controls had had appropriate prophylaxis for a dental procedure requiring definite prophylaxis within the previous 180 days. |
|
| Notes | The published paper provided data on participants who had both medical and dental invasive procedures. The author kindly separated out those who had had invasive dental interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not undertaken (case‐control study). It is possible that, as dentists decide whether to give prophylaxis or not on the basis of the information about the patient in front of them, those patients appearing more frail may have been more likely to receive prophylaxis. |
| Allocation concealment (selection bias) | High risk | Not undertaken (case‐control study) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Having died was an exclusion criterion for controls but not for cases, who could be included through a proxy. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes and exposure reported |
| Confounding | Unclear risk | Participant sex and cardiac risk factor type was not described for the subgroup who had had a dental procedure, and the type of dental intervention appeared to be different in the cases and controls, although cases and controls were matched for age. Both groups were required to have undergone invasive dental techniques within 180 days prior to onset of symptoms/interview and data were split by time period for both groups. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Karaawi 2001 | Retrospective analysis of cumulative exposure to bacteraemia following various dental procedures in children with severe congenital heart disease but no cases of endocarditis |
| Anonymous 1992 | Economic analysis of the cost‐effectiveness of using prophylactic antibiotics using same data as Bonhomme 1992 |
| Archard 1966 | 2 case studies of high risk patients developing endocarditis after dental treatment with antibiotic prophylaxis |
| Bayliss 1983 | Not all cases at risk and no controls |
| Bennis 1995 | No control group |
| Bhat 1996 | Retrospective analysis of 28 cases of endocarditis, no controls |
| Biron 1997 | Case report |
| Bonhomme 1992 | Economic analysis of the cost‐effectiveness of using prophylactic antibiotics based on published data |
| Caretta 1988 | No control group |
| Clemens 1982 | Assessment of the effect of mitral valve prolapse on risk of endocarditis (rather than assessment of the effect of prophylaxis), case‐control design |
| Conner 1967 | Participants not at high risk of endocarditis |
| Gersony 1977 | Cohort study, but it was not stated how many patients had preceding dental treatment, only two cases with preceding dental treatment and no prophylaxis |
| Herr 1976 | Case report (German) |
| Hess 1983 | All children with cardiac disease received antibiotic prophylaxis before dental extraction, no controls |
| Horstkotte 1986 | Retrospective study of a group of people at high risk of endocarditis who had had appropriate prophylaxis for medical and dental interventions, and a group of people at similar risk who did not have appropriate prophylaxis for such interventions. It was not possible to ascertain how many of the cases or controls had had dental interventions, and the source of the 2 groups is unclear. |
| Imperiale 1990 | Case‐control study: people with endocarditis (cases) who died were excluded, although the mortality rate in the cases was much higher (20%) than was likely in the control group, thus making the 2 groups incomparable. |
| Khairat 1966 | CCT, but participants not at high risk of endocarditis and no relevant outcomes measured |
| Lacassin 1995 | Case‐control study: people with endocarditis (cases) who died were excluded, although the mortality rate in the cases was much higher (20%) than was likely in the control group, thus making the 2 groups incomparable. |
| Lauridson 1984 | Case reports |
| Lecointre 1981 | Cohort study of patients having dental extractions but all patients received antibiotics |
| McGowan 1978 | Letter on failures of prophylaxis on a case by case basis, not RCT, CCT, cohort or case‐control design |
| McGowan 1982 | Case reports |
| Pogrel 1975 | Retrospective study of cases of endocarditis but no controls |
| Rahn 1988 | Serological study of bacteraemia following penicillin versus administration and tooth extraction |
| Rahn 1993 | Not an assessment of antibiotic prophylaxis (concerned with adjunctive use of antiseptic solution) |
| Schirger 1964 | Case series |
| Shanson 1980 | No at‐risk patients; examined serum levels of amoxicillin in healthy volunteers |
| Strom 1998b | Case‐control study based in the USA of 273 hospital patients with endocarditis. Not all the cases (38%) or controls (6%) had a previously known risk of endocarditis. |
| Tozer 1966 | No dental interventions, and participants not at high risk of endocarditis |
| Tzukert 1984 | Same group of patients as Tzukert 1986 |
| Van der Meer 1992b | Epidemiological study of endocarditis in the Netherlands, no controls |
| Woodman 1985 | Basic science research paper |
| Yoshimura 1985 | Cohort study of 17 patients undergoing dental extractions; all received antibiotics |
CCT: controlled clinical trial; RCT: randomised controlled trial
Differences between protocol and review
We modified the title from Antibiotics for the prophylaxis of bacterial endocarditis in dentistry to Antibiotic prophylaxis for preventing bacterial endocarditis following dental procedures. We updated the Background and added more detail of planned analyses under updated data analysis subheadings.
Contributions of authors
Samantha J Rutherford: revised the Background text for the 2021 update and screened updated search results. Anne‐Marie Glenny: led the updates to the review, screened updated search results, amended background and discussion to reflect recent literature, added risk of bias tables and summary of findings tables in the 2013 update. Lee Hooper: was involved in the design of the first published version of the review, writing of the protocol, searching, duplication of assessment of titles and abstracts, inclusion and exclusion of full text papers, data extraction and quality assessment of included studies, data analysis and interpretation. Graham Roberts: provided background information for the protocol and review and provided feedback on update. Helen V Worthington: provided methodological and statistical support and screened updated search results.
Sources of support
Internal sources
Central Manchester and Manchester Children's University Hospitals NHS Trust, UK
-
School of Dentistry, The University of Manchester, UK
Support to Cochrane Oral Health and Anne‐Marie Glenny
Eastman Dental Institute, UK
-
Manchester Academic Health Sciences Centre (MAHSC), UK
Cochrane Oral Health is supported by MAHSC and the NIHR Manchester Biomedical Research Centre
External sources
Department of Health Cochrane Review Incentive Scheme 2007, UK
-
Cochrane Oral Health Global Alliance, UK
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships-alliances). Contributors in recent years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society of Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the NHS, or the Department of Health and Social Care.
Declarations of interest
Samantha J Rutherford: no interests to declare. I work for the Scottish Dental Clinical Effectiveness Programme and was involved in producing implementation guidance for the NICE guideline. Anne‐Marie Glenny: no interests to declare. I am one of two Co‐ordinating Editors of Cochrane Oral Health. Graham Roberts: no interests to declare. Lee Hooper: no interests to declare. Helen V Worthington: no interests to declare. I am an Editor with Cochrane Oral Health and was Co‐ordinating Editor until 2020.
Neither Anne‐Marie Glenny nor Helen V Worthington were involved in the editorial process for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Van der Meer 1992a {published and unpublished data}
- Van der Meer JT, Wijk W, Thompson J, Vandenbroucke JP, Valkenburg HA, Michel MF.Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992;339(8786):135-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Karaawi 2001 {published data only}
- Al Karaawi ZM, Lucas VS, Gelbier M, Roberts GJ.Dental procedures in children with severe congenital heart disease: A theoretical analysis of prophylaxis and non-prophylaxis procedures. Heart 2001;85(1):66-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1992 {published data only}
- Anonymous.Prophylaxis of infectious endocarditis. Medecine et Maladies Infectieuses 1992;22(Suppl Apr):1-12. [Google Scholar]
Archard 1966 {published data only}
- Archard HO, Roberts WC.Bacterial endocarditis after dental procedures in patients with aortic valve prostheses. Journal of the American Dental Association 1966;72:648-52. [DOI] [PubMed] [Google Scholar]
Bayliss 1983 {published data only}
- Bayliss R, Clarke C, Oakley C.The teeth and infective endocarditis. British Heart Journal 1983;50(6):506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennis 1995 {published data only}
- Bennis A, Zahraoui M, Izzouzi L, Soulami S, Mehadji BA, Tahiri A, et al.Bacterial endocarditis in Morocco [ ]. Annales de Cardiologie et D'Angeiologie 1995;44(7):339-44. [PubMed] [Google Scholar]
Bhat 1996 {published data only}
- Bhat AW, Jalal S, John V, Bhat AM.Infective endocarditis in infants and children. Indian Journal of Pediatrics 1996;63(2):204-9. [DOI] [PubMed] [Google Scholar]
Biron 1997 {published data only}
- Biron CR.Despite diligent staff, infective endocarditis surfaces during periodontal treatment. (Erratum in:RDH 1997 Jun;17(6):67) [ ]. RDH 1997;17(3):42-8. [PubMed] [Google Scholar]
Bonhomme 1992 {published data only}
- Bonhomme I, Briancon S, Fagnani F.Exploratory economic appraisal of prophylaxis of infective endocarditis. Medecine et Maladies Infectieuses 1992;22(Special Issue Dec):1084-91. [Google Scholar]
Caretta 1988 {published data only}
- Caretta Q, Chiarini F, Di Rocco V, Brandimarte C, Biggio S, Alessandri N, et al.Anti-infectious prophylaxis in patients at risk for endocarditis in the treatment of the oral cavity. Importance of the antibiotic combination [ ]. Cardiologia 1988;33(6):577-81. [PubMed] [Google Scholar]
Clemens 1982 {published data only}
- Clemens JD, Horwitz RI, Jaffe CC.A controlled evaluation of the risk of bacterial endocarditis in persons with mitral-valve prolapse. New England Journal of Medicine 1982;307(13):776-81. [DOI] [PubMed] [Google Scholar]
Conner 1967 {published data only}
- Conner HD, Haberman S, Collings CK, Winford TE.Bacteremias following periodontal scaling in patients with healthy appearing gingiva. Journal of Periodontology 1967;38(6):466-72. [DOI] [PubMed] [Google Scholar]
Gersony 1977 {published data only}
- Gersony WM, Hayes CJ.Bacterial endocarditis in patients with pulmonary stenosis, aortic stenosis, or ventricular septal defect. Circulation 1977;56 Suppl 1:I84-7. [PubMed] [Google Scholar]
Herr 1976 {published data only}
- Herr HB.Fever after dental treatment. Schweizerische Rundschau fur Medizin Praxis 1976;65(38):1152-4. [PubMed] [Google Scholar]
Hess 1983 {published data only}
- Hess J, Holloway Y, Dankert J.Incidence of postextraction bacteremia under penicillin cover in children with cardiac disease. Pediatrics 1983;71(4):554-8. [PubMed] [Google Scholar]
Horstkotte 1986 {published data only}
- Horstkotte D, Friedrichs W, Pippert H, Bircks W, Loogen F.Benefits of endocarditis prevention in patients with prosthetic heart valves [Nutzen der endokarditisprophlaxe bei pateinten mit prothetischen herzklappen]. Zeitschrift fur Kardiologie 1986;75(1):8-11. [PubMed] [Google Scholar]
- Horstkotte D, Rosin H, Friedrichs W, Loogen F.Contribution for choosing the optimal prophylaxis of bacterial endocarditis. European Heart Journal 1987;8 Suppl J:379-81. [Google Scholar]
Imperiale 1990 {published data only}
- Imperiale TF, Horwitz RI.Does prophylaxis prevent postdental infective endocarditis? A controlled evaluation of protective efficacy. American Journal of Medicine 1990;88(2):131-6. [DOI] [PubMed] [Google Scholar]
- Imperiale TF.Effectiveness of antibiotic prophylaxis for postdental infective endocarditis. Cardiology Board Review 1991;8(3):18-27. [Google Scholar]
Khairat 1966 {published data only}
- Khairat O.An effective antibiotic cover for the prevention of endocarditis following dental and other post-operative bacteraemias. Journal of Clinical Pathology 1966;19(6):561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacassin 1995 {published data only}
- Hoen B, Lacassin F, Briancon S, Selton-Suty C, Goulet V, Delahaye F, et al.Procedures at risk for infective endocarditis [Gestes a risque d'endocardite infectieuse. Une enquete cas-temoins]. Medecine et Malades Infectieuses 1992;22( ):1010-22. [Google Scholar]
- Lacassin F, Hoen B, Leport C, Selton-Suty C, Delahaye F, Goulet V, et al.Procedures associated with infective endocarditis in adults - a case control study [ ]. European Heart Journal 1995;16(12):1968-74. [DOI] [PubMed] [Google Scholar]
Lauridson 1984 {published data only}
- Lauridson JR, Rainer WG, Merrick TA.The dental patient with artificial heart valves. The importance of antibiotic prophylaxis prior to dental surgery [ ]. Journal of the Colorado Dental Association 1984;62(5):5-6. [PubMed] [Google Scholar]
Lecointre 1981 {published data only}
- Lecointre C, Aupois R.Prevention using antibiotics of infectious endocarditis after tooth extraction. Chirurgien-Dentiste de France 1981;51(126):41-2. [PubMed] [Google Scholar]
McGowan 1978 {published data only}
- McGowan DA.Failure of prophylaxis of infective endocarditis following dental treatment. Journal of Antimicrobial Chemotherapy 1978;4(6):486-8. [DOI] [PubMed] [Google Scholar]
McGowan 1982 {published data only}
- McGowan DA.Endodontics and infective endocarditis. International Endodontic Journal 1982;15(3):127-31. [DOI] [PubMed] [Google Scholar]
Pogrel 1975 {published data only}
- Pogrel MA, Welsby PD.The dentist and prevention of infective endocarditis. British Dental Journal 1975;139(1):12-6. [DOI] [PubMed] [Google Scholar]
Rahn 1988 {published data only}
- Rahn R, Shah PM, Schafer V, Muggenthaler F, Frenkel G, Knothe H.Oral endocarditis prophylaxis in dental-surgery operations. Schweizer Monatsschrift fur Zahnmedizin 1988;98(5):478-81. [PubMed] [Google Scholar]
Rahn 1993 {published data only}
- Rahn R.Review presentation on povidone-iodine antisepsis in the oral cavity. Postgraduate Medical Journal 1993;69 Suppl 3:S4-S9. [PubMed] [Google Scholar]
Schirger 1964 {published data only}
- Schirger A, Martin WJ, Royr RQ, Needham GM.Phenoxymethylpenicillin (penicillin V) for prophylaxis prior to oral surgery in patients with heart mumurs. Mayo Clinic Proceedings 1964;39(5):359-62. [PubMed] [Google Scholar]
Shanson 1980 {published data only}
- Shanson DC, Ashford RF, Singh J.High-dose oral amoxycillin for preventing endocarditis [ ]. British Medical Journal 1980;280(6212):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strom 1998b {published data only}
- Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al.Dental and cardiac risk factors for infective endocarditis: a population-based, case-control study. Annals of Internal Medicine 1998;129(10):761-9. [DOI] [PubMed] [Google Scholar]
- Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al.Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation 2000;102(23):2842-8. [DOI] [PubMed] [Google Scholar]
Tozer 1966 {published data only}
- Tozer RA, Boutflower S, Gillespie WA.Antibiotics for prevention of bacterial endocarditis during dental treatment. The Lancet 1966;1(7439):686-8. [DOI] [PubMed] [Google Scholar]
Tzukert 1984 {published data only}
- Tzukert A, Leviner E.Prevention of infective endocarditis in dental care: not by antibiotics alone. Lancet 1984;1(8387):1190-1. [DOI] [PubMed] [Google Scholar]
Van der Meer 1992b {published data only}
- Van der Meer JT, Thompson J, Valkenburg HA, Michel MF.Epidemiology of bacterial endocarditis in the Netherlands. II. Antecedent procedures and use of prophylaxis [ ]. Archives of Internal Medicine 1992;152(9):1869-73. [DOI] [PubMed] [Google Scholar]
Woodman 1985 {published data only}
- Woodman AJ, Vidic J, Newman HN, Marsh PD.Effect of repeated high dose prophylaxis with amoxycillin on the resident oral flora of adult volunteers. Journal of Medical Microbiology 1985;19(1):15-23. [DOI] [PubMed] [Google Scholar]
Yoshimura 1985 {published data only}
- Yoshimura Y, Kishimoto H, Matsuura R, Oka M, Matsumoto K.Dental extraction in patients with prosthetic heart valves: antibiotic prophylaxis of prosthetic valve endocarditis. Journal of the Osaka University Dental School 1985;25:153-9. [PubMed] [Google Scholar]
Additional references
American Heart Association
- Infective Endocarditis. www.heart.org/en/health-topics/infective-endocarditis.
Aronson 2006
- Aronson JK.Meyler's Side Effects of Drugs. 15th edition. Amsterdam: Elsevier Science, 2006. [Google Scholar]
Cahill 2017
- Cahill TJ, Harrison JL, Jewell P, Onakpoya I, Chambers JB, Dayer M, et al.Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart 2017;103:937-44. [DOI: 10.1136/heartjnl-2015-309102] [DOI] [PubMed] [Google Scholar]
Dajani 1997
- Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al.Prevention of bacterial endocarditis - recommendations by the American Heart Association. JAMA 1997;277(22):1794-801. [PubMed] [Google Scholar]
Danchin 2005
- Danchin N, Duval X, Leport C.Prophylaxis of infective endocarditis: French recommendations 2002. Heart 2005;91(6):715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delahaye 2016
- Delahaye F, M'Hammedi A, Guerpillon B, Gevigney G, Boibieux A, Dauwalder O, et al.Systematic search for present and potential portals of entry for infective endocarditis. Journal of American College of Cardiology 2016;67(2):151-8. [DOI: 10.1016/j.jacc.2015.10.065] [PMID: ] [DOI] [PubMed] [Google Scholar]
Durack 1994
- Durack DT.Infective and noninfective endocarditis. In: Schlant R, Alexander RW, editors(s). The Heart: Arteries and Veins. 8th edition. New York: McGraw-Hill, 1994:1681-709. [Google Scholar]
Duval 2012
- Duval X, Delahaye F, Alla F, Tattevin P, Obadia JF, Le Moing V, et al.Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. Journal of the American College of Cardiology 2012;59(22):1968-76. [DOI] [PubMed] [Google Scholar]
European Society of Cardiology
- Summary Card for General Practice - Infective Endocarditis. www.escardio.org/static-file/Escardio/Guidelines/Publications/Summary%20card/IE_2016-_Summary_Card.pdf.
EWP 1993
- Endocarditis Working Party of the British Society of Antimicrobial Chemotherapy.Recommendations for endocarditis prophylaxis. Journal of Antimicrobial Chemotherapy 1993;31:437-8. [DOI] [PubMed] [Google Scholar]
Farook 2012
- Farook SA, Davis AK, Khawaja N, Sheikh AM.NICE guideline and current practice of antibiotic prophylaxis for high risk cardiac patients (HRCP) among dental trainers and trainees in the United Kingdom (UK). British Dental Journal 2012;213(4):E6. [DOI] [PubMed] [Google Scholar]
Fleiss 1981
- Fleiss JL.Sampling method II: prospective and retrospective studies. In: Statistical Methods for Rates and Proportions. New York: John Wiley and Sons, 1981:83-99. [Google Scholar]
Jamil 2019
- Jamil M, Sultan I, Gleason TG, Navid F, Fallert MA, Suffoletto MS, et al.Infective endocarditis: trends, surgical outcomes, and controversies. Journal of Thoracic Disease 2019;11(11):4875-85. [DOI: 10.21037/jtd.2019.10.45] [PMCID: 6940216] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 2000
- Lucas V, Roberts GJ.Odontogenic bacteremia following tooth cleaning procedures in children. Pediatric Dentistry 2000;22(2):96-100. [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence.Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. NICE Clinical Guideline No 64 2008 (updated 2016). [PubMed]
Roberts 1999
- Roberts GJ.Dentists are innocent! 'Everyday' bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatric Cardiology 1999;20(5):317-25. [DOI] [PubMed] [Google Scholar]
SDCEP 2018
- Antibiotic prophylaxis against infective endocarditis: implementation advice for National Institute for Health and Care Excellence (NICE) Clinical Guideline 64 Prophylaxis Against Infective Endocarditis. August 2018. www.sdcep.org.uk/published-guidance/antibiotic-prophylaxis/ (accessed 20 November 2021).
Strom 1998a
- Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al.Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Annals of Internal Medicine 1998;129:761-9. [DOI] [PubMed] [Google Scholar]
Thornhill 2011
- Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ, et al.Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ 2011;342:d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Reyn 1981
- Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS.Infective endocarditis: an analysis based on strict case definitions. Annals of Internal Medicine 1981;94:505-18. [DOI] [PubMed] [Google Scholar]
Williams 2021
- Williams ML, Doyle MP, McNamara N, Tardo D, Mathew M, Robinson B.Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review. Therapeutic Advances in Cardiovascular Disease 2021;15:17539447211002687. [DOI: 10.1177/17539447211002687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J.Priority oral health research identification for clinical decision-making. Evidence-based Dentistry 2015;16(3):69-71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Glenny 2013
- Glenny A-M, Oliver R, Roberts GJ, Hooper L, Worthington HV.Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2013, Issue 10. Art. No: CD003813. [DOI: 10.1002/14651858.CD003813.pub4] [DOI] [PubMed] [Google Scholar]
Oliver 2002
- Oliver RJ, Hooper L, Roberts G.Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD003813. [DOI: 10.1002/14651858.CD003813] [DOI] [PubMed] [Google Scholar]
Oliver 2004
- Oliver RJ, Roberts GJ, Hooper L.Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD003813. [DOI: 10.1002/14651858.CD003813.pub2] [DOI] [PubMed] [Google Scholar]
Oliver 2008
- Oliver R, Roberts GJ, Hooper L, Worthington HV.Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD003813. [DOI: 10.1002/14651858.CD003813.pub3] [DOI] [PubMed] [Google Scholar]


