Abstract
Endometriosis (EM) is a hormone-dependent gynecological disease associated with chronic pelvic pain and altered immuno-inflammatory processes. It shares some cancer-like characteristics such as increased proline biosynthesis and activated glutaminolysis. Both proline and glutamine are interconvertible metabolically, and studies have shown their roles in cancer cell metabolic reprogramming, redox homeostasis, occurrence/development of endometrial carcinoma, and its further progression toward the malignant state. So based on this, we hypothesized that the circulatory proline to glutamine ratio (PQR) would be altered in EM and may serve as an indicative biomarker to improve the clinical diagnosis of EM. In present study, the circulatory-PQR levels were estimated for 39 EM patients and 48 age matched healthy female subjects using 800 MHz NMR spectroscopy. Among 39 EM patients, 15 were in the clinical stages I to II and referred to here as moderate EM (MEM) patients and 24 were in the clinical stages III to IV and referred here as severe EM (SEM) patients. The circulatory-PQR levels were significantly increased in EM patients (0.99 ± 0.13 μM in MEM; 1.39 ± 0.22 μM in SEM) compared to normal control (NC) subjects (0.52 ± 0.05 μM in NC). Further, the circulatory PQR levels exhibit the highest diagnostic potential with area under receiver operating characteristic (AUROC) curve values equal to 0.87 ± 0.04 [95%CI = 0.79–0.96] for MEM and 0.89 ± 0.04 [95% CI = 0.82–0.96] for SEM. These results suggested that circulatory-PQR has significant potential to serve as a noninvasive biomarker for diagnostic/prognostic screening of EM and further underscored the importance of these two nonessential amino acids (proline and glutamine) in cancer metabolism.
Introduction
Endometriosis (EM) is a gynecological disease associated with chronic pain and affecting women in their reproductive age.1 It shares many similarities with metastatic cancer2 and involves the growth of uterine lining (glandular and stromal endometrium endometrial tissues) in regions other than the uterus. The frequency of occurrence ranges from 5 to 10% worldwide in reproductively active and infertile women.3 Although it is considered as a benign gynecological condition, it is often associated with an increased risk of malignant transformation. Several factors are involved in the chronic inflammatory process of endometriosis, such as hormones, growth or adhesion factors, antigenic glycoproteins (e.g., Zn-alpha2-glycoprotein, CA125, and CA19.9), cytokines, chemokines, and oxidative stress.4−6 The two most accepted theories for endometriosis, the retrograde mensuration theory7 and the metaplasia theory,8 describe the origin of this enigmatic gynecological disease, due to an abnormal defect in the clearance of menstrual efflux in reproductively active women.9 Women suffering with endometriosis disease most often have symptoms of chronic pelvic pain, fatigue, dysmenorrhea (pain during periods), dyspareunia (pain during intercourse), heavy menstrual bleeding, and bleeding between periods.10 Moreover, approximately 47% of women with chronic pelvic pain and subfertility have chances of chronic endometriosis condition.11 Clinical diagnosis of this condition is being done via familial and physical examination, transvaginal or ultrasonography, noninvasive biomarker analysis, or laparoscopy.12 So far, laparoscopy has been defined as the gold standard for diagnosis of endometriosis and also used for the surgical treatment of it for years. The revised American Society of Reproductive Medicine (rASRM) standard has been used for classification of endometriosis, which requires description of the lesions and their extent to identify the severity of the disease and to decrease false positive results by clinicians.13 As such, EM lesions and symptoms overlap with those of many other uterine/ovarian medical conditions, causing a delay in diagnosis; therefore, new noninvasive biomarkers for differentiating EM from other uterine medical conditions are urgently needed.
Metabolomics represents a useful analytical tool for quantitative and comparative analysis of metabolites in biological samples (blood plasma, urine, serum, follicular and endometrial fluid etc.) and identifying new promising biomarkers. The metabolic profiling capabilities of NMR in combination with statistical analysis tools have successfully been applied for understanding the pathogenesis of many diseases as well as for identifying metabolic signatures for early diagnosis, therapeutic monitoring, and predicting disease severity, progression, and recurrence.14 The increasing popularity of NMR in clinical metabolomics studies is because of its high-throughput nature (>100 samples can be analyzed per day), minimal sample preparation requirement, inherently noninvasiveness, nondestructive and nonselective nature, and, on top of all this, high level of experimental reproducibility.14 Despite several advantages, the discoveries of NMR based metabolomics studies focusing on disease diagnostics still lack the clinical translations. A major bottleneck for translational applications focusing on disease diagnostics is the lack of a suitable internal standard for reliable quantitation of metabolites, especially in blood plasma/serum samples. Scientific efforts have already been started in this direction, and recently, circulatory metabolites (fumaric acid and maleic acid) have been reported as reliable internal standards for NMR analysis of protein precipitated plasma and serum samples.15 Alternatively in our lab, we are using formate as an internal calibration standard for NMR analysis of normal plasma and serum samples, and the resulting concentration profiles (estimated w.r.t. formate) are then used to estimate the metabolic ratios (ratiometric parameters).14,16−20 As compared to conventionally used normalized spectral features, the circulatory metabolic ratios were found to be more reliable in reflecting the pathophysiological state of the patients and correlate well with the clinical parameters or disease severity scores.16−20 The approach has been extended here further for investigating the circulatory levels of proline and glutamine and the proline to glutamine ratio (PQR) in female endometriosis (EM) patients with respect to normal control (NC) female subjects. The purpose of selecting these two specific amino acids (i.e., proline and glutamine) is that EM is often associated with altered inflammatory and immune processes and shares some cancer-like characteristics such as activated glutaminolysis21,22 and increased proline biosynthesis.23−25 Both proline and glutamine are interconvertible metabolically, and studies have shown their roles in cancer cell metabolic reprogramming, redox homeostasis, occurrence/development of cancer (including endometrial carcinoma), and its further progression toward the malignant state.22−31 So based on these pathophysiological hallmarks, we hypothesized that the circulatory proline to glutamine ratio (PQR) would be altered in EM and may serve as an indicative biomarker to improve the clinical diagnosis of EM. The metabolic alterations will further underscore the importance of these two nonessential amino acids in cancer metabolism and rational design of a new therapeutic strategy for EM.
Results
Patient Characteristics
The present study involved 39 endometriosis (EM) patients and 48 normal control (NC) subjects, and all were in their reproductive age. Table 1 represents the physical and clinical features of all the subjects (total N = 87) involved in this study. Out of 39 EM patients, 15 were diagnosed with stages I and II; however, 24 were diagnosed with stages III and IV, referred to here as moderate EM (MEM) and severe EM (SEM), respectively. Consistent with various previous reports, the circulatory glycoprotein antigen CA-125 levels were significantly higher in EM and so were various other parameters including BMI and hormonal profiles32 (Table 1).
Table 1. Demographic and Clinical Features of Endometriosis Patients and Healthy Controlsa.
| Clinical Parameter | Endometriosis (N = 39) | Control (N = 48) | p-Value |
|---|---|---|---|
| BMI (kg/m2) | 25.00 ± 1.80 | 19.98 ± 0.99 | <0.001*** |
| Age (years <32) | 26.37 ± 2.68 | 27.02 ± 2.92 | 0.528ns |
| Age (years >32) | 42.53 ± 4.53 | 39.93 ± 6.19 | 0.200ns |
| Age at Menarche (AAM) years | 14.64 ± 1.25 | 14.03 ± 1.18 | 0.363ns |
| CA-125 (U/mL) | 46.44 ± 6.85 | 27.42 ± 2.14 | <0.001*** |
| Sugar (fasting; mg/dL) | 103.6 ± 54.00 | 89.40 ± 5.77 | 0.319ns |
| Sugar (Post Prandial; mg/dL) | 140.8 ± 43.8 | 97.77 ± 9.97 | 0.001* |
| Prolactin (PRL; ng/dL) | 12.35 ± 1.76 | 8.016 ± 0.94 | <0.001*** |
| Triiodothyronine (T3; ng/dL) | 135.67 ± 23.53 | 106.29 ± 10.00 | 0.002* |
| Thyroxine (T4, μg/dL) | 9.73 ± 2.02 | 8.56 ± 0.92 | 0.095ns |
| Thyroid-stimulating hormone (TSH; μIU/mL) | 4.69 ± 0.89 | 2.48 ± 0.49 | <0.001*** |
| Luteinizing hormone (LH; mIU/mL) | 24.40 ± 7.76 | 4.41 ± 1.40 | <0.001*** |
| Follicle-stimulating hormone (FSH; mIU/mL) | 13.57 ± 3.01 | 5.90 ± 1.01 | <0.001*** |
| LH/FSH | 1.77 ± 0.42 | 0.73 ± 0.10 | <0.001*** |
| Estradiol (E2; pg/dL) | 419.27 ± 18.45 | 46.88 ± 2.84 | <0.001*** |
| Testosterone (T; ng/dL) | 0.45 ± 0.08 | 0.43 ± 0.058 | 0.722ns |
| E2/T ratio | 0.97 ± 0.19 | 0.12 ± 0.01 | <0.001*** |
| Interleukin 6 (IL-6) | 442.92 ± 9.50 | 16.15 ± 1.26 | <0.001*** |
| Cortisol | 110.22 ± 5.69 | 91.94 ± 3.16 | <0.001*** |
N = total number of subjects, ns = not significant, p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Student’s t test was used to compare the mean for the endometriosis and control subjects. Two-tailed p-values less than 0.05 were considered statistically significant. Abbreviations used: BMI, body mass index; kg, kilogram; mg, milligram; μg, microgram; ng, nanogram; pg, pictogram; mL, milliliter; dL, deciliter; mIU, milli-international units; μIU, micro-international units.
Targeted NMR Based Profiling of Circulatory Proline and Glutamine
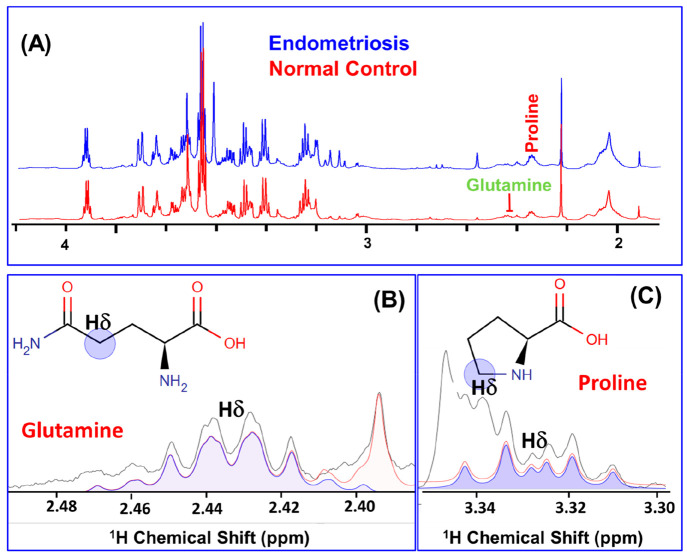
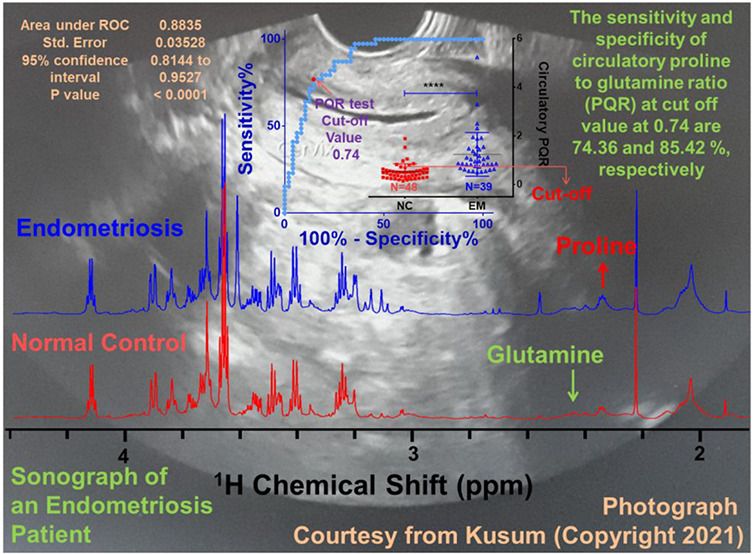
Figure 1A shows the stack plot of the 1D 1H CPMG NMR spectra recorded on serum samples obtained from EM and NC subjects. The present study involves concentration profiling of proline and glutamine in the serum samples of EM patients and NC subjects following a targeted 1H NMR based metabolomics approach as described previously.16−18,20 For this, first we have identified the NMR signals corresponding to proline and glutamine and assigned by following a chemical shift and peak pattern matching procedure employing the 800 MHz spectral database of metabolites in the NMR suite of CHENOMX software; the selected spectral regions are shown in Figure 1B (for glutamine) and Figure 1C (for proline). To minimize the experimental/methodological deviations, the proline and glutamine concentrations were explicitly measured using formate as an internal reference (explicit details are described in the Materials and Method).
Figure 1.
(A) Stack plot of cumulative 1D 1H CPMG NMR spectra recorded on serum samples obtained from 39 endometriosis (EM, blue) patients and 48 normal control (NC, red) subjects. The NMR signals corresponding to proline and glutamine were identified and assigned by matching their specific chemical shifts and peak patterns employing the NMR suite of CHENOMX software. (B and C) Selected regions from the 1D 1H CPMG NMR spectra of serum samples displaying the Hδ peaks of (B) glutamine and (C) proline selected in this study for their targeted concentration profiling. Blue lines in parts B and C represent the fitting lines showing pattern matching with the experimental peak pattern (black).
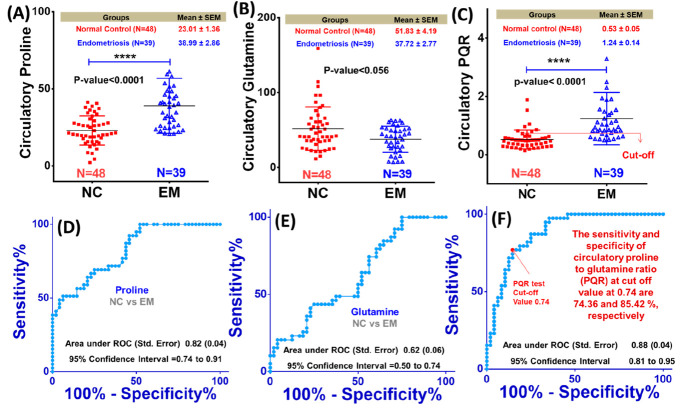
The estimated circulatory concentrations of proline and glutamine were then used to estimate the proline to glutamine ratio (PQR; P and Q represent proline and glutamine, respectively). Figure 2A–C shows the box plots comparing the circulatory concentrations of proline and glutamine and the PQR between the EM and NC groups. As evident, the circulatory proline level and the PQR were significantly increased in EM patients (with their mean values Pro = 38.99 ± 2.86 μM and PQR = 1.24 ± 0.14 μM) compared to NC subjects (Pro = 23.01 ± 1.36 μM and PQR = 0.53 ± 0.05) with p-value < 0.0001, respectively (Figure 2A and C). On the other hand, the circulating glutamine levels were decreased in EM patients (Gln = 37.72 ± 2.77 μM) compared to NC subjects (Gln = 51.83 ± 4.19 μM) with nearly significant p-value equal to 0.056 (Figure 2B). The decreased circulatory glutamine levels might be related to active glutaminolysis in EM patients. It is important to mention here that both the proline and glutamine levels have been estimated with respect to formate (as an internal reference), and possibly, this may have impacted the quantitative profiles of these metabolites, rendering a nearly significant change in the glutamine levels between the EM and NC groups (p-value < 0.056, Figure 2B). In order to check this possibility, we additionally performed a small exercise if formate levels change in EM with respect to NC subjects. For this, the reference concentration of TSP was calibrated to 100 μM (in the software program CHENOMX) and the concentration levels of formate, glutamine, and proline were estimated and compared between NC subjects and EM patients (the results are presented in Figure S2). Compared to NC subjects, the serum proline levels were significantly increased in EM patients (66.88 ± 4.07 μM in EM vs 37.88 ± 1.86 μM in NC with p-value < 0.0001), whereas the changes in the serum levels of glutamine (decreased in EM) and formate (increased in NC) were found to be insignificant between EM and NC patients (see the values in Figure S2). The insignificant changes in formate levels are also evident from Figure S3 comparing the cumulative NMR signal of formate between EM and NC subjects with respect to TSP.
Figure 2.
(A–C) Box-cum-whisker plot comparing the circulatory proline and glutamine levels and the proline to glutamine ratio (PQR) between EM and NC subjects. (D–F) Plots representing the receiver operating characteristic (ROC) curve analysis performed for evaluating the diagnostic potential of these metabolic features in differentiating EM patients from NC subjects. The AUROC values (area under ROC curve) with standard error and 95% confidence interval (CI) are shown in blue for each ROC plot.
To be mentioned here is that the formate levels have been reported to be decreased in endometrial tissue,33 and if we consider this decrease in EM patients here as well, the estimated glutamine level (technically the glutamine to formate ratio) may not change between the study groups. Indeed metabolomics studies have shown that the circulatory formate level decreases in lung cancer and breast cancer patients relative to healthy controls.34 Contrary to glutamine, the circulatory proline level (technically proline to formate ratio) and PQR were found to be elevated in EM patients, which might be related to disturbed proline metabolism in EM patients.
These metabolic features were then tested for their diagnostic potential using ROC curve analysis, and the results are shown in Figure 2D–F. Of the three, the circulatory PQR demonstrated the highest diagnostic potential with area under ROC (AUROC) curve values equal to 0.88 ± 0.04 [95% CI = 0.81–0.95] (Figure 2F). The AUROC values for proline and glutamine were 0.82 ± 0.04 [95% CI = 0.74–0.91] and 0.62 ± 0.06 [95% CI = 0.50–0.74], respectively (Figure 2D and E). These observations were found in well concordance with various metabolic studies on endometriosis patients, suggesting the potential of these metabolites, particularly of their PQRs, to serve as noninvasive diagnostic and prognostic markers.35−38
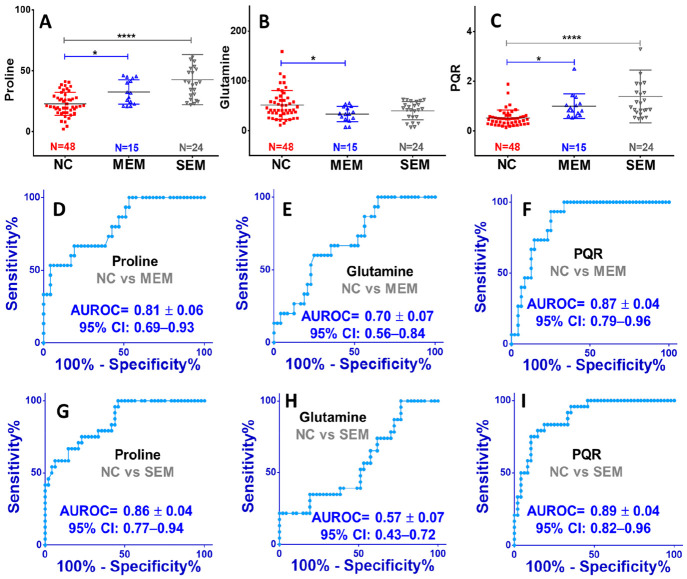
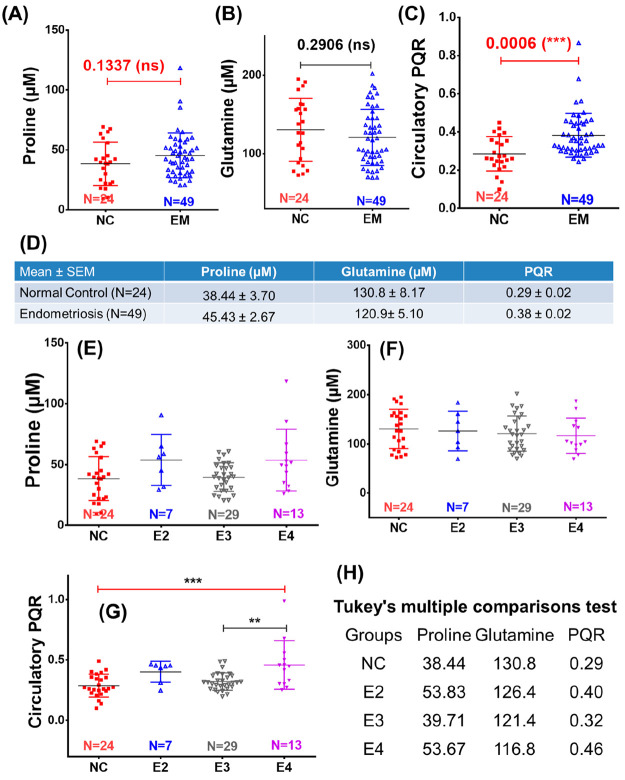
Further, we compared the circulatory proline and glutamine levels and PQR between moderate EM (MEM) and severe EM (SEM) patients w.r.t. the NC subjects (Figure 3). The circulating levels of proline were evidently and significantly increased in MEM and SEM with their mean values equal to 32.75 ± 2.58 μM and 42.89 ± 4.20 μM, respectively, compared to NC subjects (23.01 ± 1.36 μM) (Figure 3A). The p-values for NC vs MEM and for NC vs SEM comparisons were found to be <0.05 and <0.0001, respectively (Figure 3A). Inversely, the circulatory levels of glutamine were decreased in MEM and SEM patients with their mean values equal to 33.57 ± 3.93 μM and 40.74 ± 3.74 μM, respectively, compared to NC subjects (51.83 ± 4.19 μM) (Figure 3B). The p-value analysis, however, revealed the statistically significant difference between NC and MEM, whereas significant differences were found neither between NC and SEM nor between MEM and SEM (Figure 3B). Finally, the circulatory PQR levels were compared and showed a progressive and significant increase from NC to MEM (with p-value < 0.05) and from MEM to SEM (with p-value < 0.0001) with their mean values equal to 0.52 ± 0.05 μM for NC, 0.99 ± 0.13 μM for MEM, and 1.39 ± 0.22 μM for SEM (Figure 3C). The ROC curve analysis was further used to evaluate the diagnostic potential of these metabolic features, and the results are summarized in Figure 2D–I. It is clearly evident that the circulatory PQR levels exhibited the highest AUROC values equal to 0.89 ± 0.04 [95% CI = 0.82–0.96] for comparison between NC and SEM (Figure 3F) and 0.87 ± 0.04 [95% CI = 0.79–0.96] for comparison between NC and MEM (Figure 3I). Following the PQR, the circulatory proline levels also showed strong diagnostic potential AUROC values equal to 0.86 ± 0.04 [95% CI = 0.77–0.94] for comparison between NC and SEM (Figure 3G) and 0.81 ± 0.06 [95% CI = 0.69–0.93] for comparison between NC and MEM (Figure 3I). These findings were further cross-validated using the NMR spectral data set of a previously reported plasma metabolomics study.39 The results summarized in Figure 4 reveal that the circulatory PQRs are significantly elevated in EM (0.38 ± 0.02) compared to NC (0.29 ± 0.02) with a statistical p-value equal to 0.0006 (Figure 4C). The circulatory PQRs were also found significantly elevated (with p-value < 0.05) in stage IV endometriosis patients (E4, 0.46 ± 0.21) compared to stage III endometriosis patients (E3, 0.32 ± 0.07) and NC (0.29 ± 0.10) (Figure 4C). However, the circulatory proline and glutamine levels failed to show a statistically significant difference between different study groups (Figure 4A and B).
Figure 3.
Box plots showing comparison of the circulatory levels of (A) proline and (B) glutamine and (C) the proline to glutamine ratio in moderate endometriosis (MEM) and severe endometriosis (SEM) patients compared to normal control (NC) subjects. The symbols * and **** represent the p-values <0.05 and <0.0001, respectively, derived from the unpaired statistical t test for each metabolic comparison. The plots in parts D–F and G–I represent the receiver operating characteristic (ROC) curve analysis performed for evaluating the diagnostic potential in moderate endometriosis and severe endometriosis patients compared to NC subjects, respectively. The AUROC values (area under ROC curve) with standard error and 95% confidence interval (CI) are shown in blue for each ROC plot.
Figure 4.
(A–C) Box plots showing a comparison of the circulatory (A) proline and (B) glutamine levels and (C) proline to glutamine ratio (PQR) between endometriosis (EM) patients and normal control (NC) subjects. (D) Mean values (with standard error of mean (SEM)) of these circulatory parameters (i.e., proline, glutamine, and PQR) in EM patients and NC subjects shown in tabulated form. (E–G) Box plots showing a comparison of the circulatory (E) proline and (F) glutamine levels and (G) proline to glutamine (PQR) ratio between stage II, III, and IV endometriosis patients (E2, E3, and E4, respectively) with respect to normal control (NC) subjects. (H) Mean values of these circulatory parameters (i.e., proline, glutamine, and PQR) in NC subjects and E2, E3, and E4 patients shown in tabulated form. For each box plot, the boxes denote interquartile ranges, the horizontal line inside the box denotes the median, and the bottom and top boundaries of the boxes are the 25th and 75th percentiles, respectively. Lower and upper whiskers are 5th and 95th percentiles, respectively. Note: the NMR spectral data used for cross-validation purpose corresponds to that used in the previous plasma based clinical metabolomics study by Vicente-Muñoz et al.39
Discussion
A noninvasive test for endometriosis (EM) would be useful for nearly all women with minimal to mild stage EM and/or subfertility with normal ultrasound.40 This would also benefit women with moderate to severe stage EM without clearly visible ovarian endometrioma.40 Metabolomics analysis of blood plasma/serum can provide noninvasive biomarkers for diagnostic and therapeutic monitoring and also an emerging approach for gaining better understanding of the pathophysiology of this disease.35,41 Considering the relevance of this omics approach, several metabolomics studies have been carried out in the recent past showing alterations in the blood plasma/serum levels of various amino acids in EM patients (with different disease stages) compared to healthy controls.33,35,37,42
Recent studies suggested that epigenetic modifications (such as DNA methylation, histone modifications, and noncoding microRNAs (miRNAs)) are involved in the malignant transformation of EM in ovarian tumorigenesis.43 The microRNA miR-23b* was reported to be overexpressed during tumor progression and markedly suppress the expression of POX enzyme.31 Besides its role as tumor suppressor, POX enzyme has an important role in the proline cycle where it converts proline into delta1-pyrroline-5-carboxylate (P5C), an intermediate which spontaneously changes into glutamic-gamma-semialdehyde (GSA).31 This GSA produces glutamate with the help of P5C dehydrogenase enzyme and enters in the TCA cycle for energy production. However, due to decreased expression of POX in EM, this pathway remains suppressed, causing increased availability of circulatory proline in the endometrial tissue of EM patients. In this targeted 1H NMR based metabolomics study, we performed concentration profiling of proline and glutamine in serum samples of normal healthy women and women with different stages of endometriosis (moderate, stages I and II; severe, stages III and IV). The comparison (based on univariate statistical analysis) revealed that the circulatory proline levels were significantly elevated in EM patients whereas the circulatory glutamine levels were found to be decreased. The reduced glutamine concentration in the serum of patients with endometriosis reflected activated glutaminolysis in EM to fulfill the higher energy requirements of endometriotic cells which share similar proliferative capacity to cancer cells.21,22 The increased proline38 and reduced glutamine35 levels were well supported with previous serum/plasma metabolomics characteristics of EM patients, suggesting an amplified utilization of glutamine via glutaminolysis and suppressed proline cycle probably due to increased expression of an mRNA, i.e. MiR-23b.31
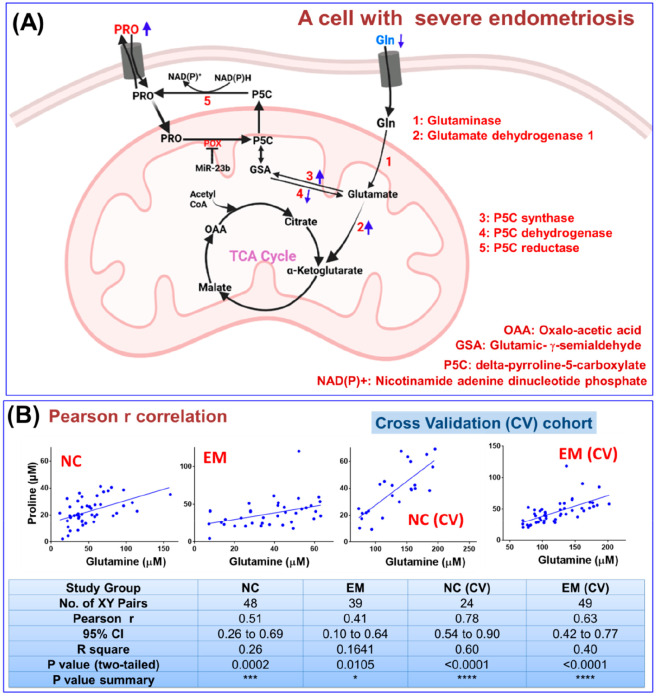
Figure 5A represents interconnected glutaminolysis and the proline cycle within the cells of endometrial tissue, and it is shown here that glutamine is converted into glutamate, which is finally utilized in energy production via the TCA cycle in cells with endometriosis.22 Briefly, glutamine (Gln) is transported into the cell and converted into glutamate, which fulfills the high energy demands of proliferating tumor (malignant) cells via the TCA cycle. Additionally, the glutamate participates into the proline cycle, where it is reduced into glutamic-gamma-semialdehyde (GSA) by P5C synthase and spontaneously converted into delta-pyrroline-5-carboxylate (P5C), which is further reduced by P5C reductase (also known as PYCR) into proline (Figure 5A).
Figure 5.
(A) Schematic showing interconnected proline (Pro) and glutamine (Gln) metabolism in a cell with severe endometriosis; both are linked through an intermediate glutamic-γ-semialdehyde (GSA) as depicted. Messenger RNA 23b (MiR-23b) is reported to be overexpressed during tumor progression, which markedly suppresses the expression of POX enzyme. (B) Pearson r correlation analysis performed between circulatory proline and glutamine levels measured in endometriosis (EM) patients and normal control (NC) subjects (including the cross-validation cohort).
Considering that glutamine and proline are biosynthetically linked, their circulatory levels are expected to correlate positively if the metabolic pathway is operating within the cells of endometrial tissue. The statistical correlation plot between the circulatory glutamine and proline levels in EM patients was generated using the Pearson r method and is shown in Figure 5B. As evident from the figure, there is a statistically significant and positive correlation of proline with glutamine; therefore, the conjecture that proline and glutamine are biosynthetically linked is well supported.24 The Pearson r correlation value was found to be decreased in EM patients (r = 0.41 (95% CI: 0.10 to 0.64), p-value = 0.0105) compared to NC subjects (r = 0.52 (95% CI: 0.26 to 0.69), p-value = 0.0002), suggesting disturbed utilization of proline or glutamine in EM. For the validation cohort as well, the Pearson r correlation value was found to be decreased in EM patients (r = 0.63 (95% CI: 0.42 to 0.77), p-value <0.0001) compared to NC subjects (r = 0.78 (95% CI: 0.54 to 0.90), p-value < 0.0001) (Figure 5A).
The nutritional and hormonal regulation of amino acid homeostasis is a well established physiological phenomenon and is achieved through exchange of essential amino acids with nonessential amino acids and the transfer of amino groups from oxidized amino acids to amino acid biosynthesis.28,44,45 Both proline and glutamine are nonessential amino acids and interconvertible metabolically.24 Various studies in the recent past have shown that increased proline biosynthesis plays an important role in cancer cell metabolic reprogramming, the occurrence/development of cancer, and its further progression toward the malignant state.23,25 Of the two, glutamine (the most abundant amino acid in the circulatory system) is known to serve as an anaplerotic substrate to replenish tricarboxylic acid (TCA) cycle intermediates during growth of cancer cells and tumor progression.27,46 The glutamate produced from glutamine is further utilized in part for proline biosynthesis to support anaplerosis, ATP production, protein and nucleotide synthesis, and redox homeostasis in cancer cells.24 As proline is a major and essential constituent in collagen, the elevated PQR levels in EM might be related to increased proline biosynthesis and its further utilization in collagen synthesis as support.30 There are some previous studies suggesting that the collagen concentration is significantly higher in chronic endometriosis associated fibrosis and that it may have a major role in endometriosis.47 On the other hand, the cellular processes mediating tumor progression in EM are utilizing glutamine at a higher rate, rendering its decreased circulatory levels in the sera of EM patients (Figures 2B and 3B). The result of this metabolic derangement is the elevated proline to glutamine ratio (PQR) which has been demonstrated in this paper. The results are well in line with previous clinical/preclinical studies suggesting mitochondrial dysfunction and decreased energy production in uterine endometriosis tissue,48 as proline catabolism in mitochondria serves as an important source of energy production and previous transcriptomics studies have demonstrated that there is reduced expression of proline oxidase (POX, a mitochondrial inner-membrane flavoenzyme involved in the catabolic degradation of the proline) due to overexpression of microRNA (known as MiR-23b).31,49 Epigenetics studies on clinical samples have demonstrated that miRNA-23b exhibits regulatory roles, especially in the development of cancer,50,51 and its overexpression is negatively correlated with the expression of tumor suppressor gene TUSC7 in endometrial carcinoma.52 Studies have shown that miRNA-23b directly binds the POX mRNA 3′-untranslated region; thus, the overexpression of miRNA-23b is directly correlated with downregulation of mitochondrial proline oxidase (POX), as schematically depicted in (Figure 5A).53 Alternatively, the elevated circulatory proline levels can be because of increased expression of PYCR (i.e., P5C reductase), as proposed in some of the previous studies.25
In order to find clinical correlates of circulatory PQR, we performed statistical correlation analysis (employing the Spearman method) for PQR with various clinical parameters estimated for EM patients. The results are summarized in Table 2 and show that circulatory PQR levels do not correlate significantly with most of the clinical parameters; rather, the PQR shows significant correlations with proline (r = 0.43, 95% CI: 0.12 to 0.66, P = 0.0069) and glutamine (r = −0.53, 95% CI: −0.73 to −0.25, P = 0.0005). In an example clinical study, the diagnostic potential of Zn-alpha2-glycoprotein has been compared with the other antigenic glycoproteins CA125 and CA19.9. The sensitivities have been reported to be 69.4%, 33%, and 13%, respectively.54 Compared to all these, the cumulative sensitivity and specificity of circulatory PQR (at the cutoff value 0.74) have been found to be 74.36% and 85.42% (Figure 2F), suggesting this metabolic ratio has apposite potential to serve as a surrogate marker to improve the clinical diagnosis of EM.
Table 2. Spearman Correlation Estimated for the Proline to Glutamine Ratio (PQR) with Different Metabolic and Clinical Parametersa.
| PQR vs Other Parameters | Number of XY Pairs | Spearman r (95% Confidence Interval) | p-Value (Two-Tailed) | p-Value Summary |
|---|---|---|---|---|
| Proline | 39 | 0.43 (0.12 to 0.66) | 0.0069 | Significant (**) |
| Glutamine | 39 | –0.53 (−0.73 to −0.25) | 0.0005 | Significant (***) |
| CA-125 | 30 | 0.15 (−0.23 to 0.50) | 0.4227 | Not Significant |
| Fasting Sugar (mg/dL) | 35 | 0.09 (−0.26 to 0.42) | 0.5949 | Not Significant |
| Post-Prandial Sugar (mg/dL) | 34 | 0.05 (−0.31 to 0.39) | 0.7899 | Not Significant |
| Triiodothyronine (T3, ng/dL) | 35 | –0.1 (−0.42 to 0.25) | 0.5839 | Not Significant |
| Thyroxine (T4, μg/dL) | 35 | –0.14 (−0.46 to 0.21) | 0.42 | Not Significant |
| Thyroid-stimulating hormone (TSH; μLu/mL) | 35 | 0.11 (−0.24 to 0.44) | 0.5336 | Not Significant |
Abbreviations used: mg, milligram; μg, microgram; ng, nanogram; mL, milliliter; dL, deciliter; μIU, micro-international units.
Conclusion
EM is a chronic, hormone-dependent gynecologic disease which, though considered benign, is associated with an increased risk of malignant transformation and involves various mechanisms of disease progression and development.33,42,43,55 Putative biomarkers such as antigenic glycoproteins (e.g., Zn-alpha2-glycoprotein, CA125, and CA19.9), growth or adhesion factors, hormones, or proteins related to immunology or angiogenesis have failed to successfully diagnose the disease.40 So far, there is no single biomarker or panel of biomarkers in the blood that has been validated as a clinical test for the diagnosis of endometriosis or definition of its stages.40 In this targeted metabolomics study, an attempt has been made to validate the previously reported hallmark of endometriosis; that is, the microRNA named miR-23b is overexpressed during EM tumor progression,49 which markedly suppresses the expression of mitochondrial POX enzyme (a novel tumor suppressor), rendering decreased utilization of proline in the generation of reactive oxygen species (ROS, critical for regulation of cell growth and apoptosis).53 The availability of proline, on the other hand, induces collagen synthesis—a process essential for tumor growth and progression.26 The present study will serve as a proof of the principal demonstrating that epigenetic/transcriptomic changes altering the metabolic profiles can be evaluated or assessed using metabolomics approaches. The elevated PQRs in EM have also been cross-validated on NMR spectral data recorded on another cohort of EM patients.39 Overall, the present study will serve as a basis for future studies aiming to develop diagnostic/prognostic tests based on circulatory PQRs. Further, the reprogramming of circulatory PQRs will serve as an indicator for the therapeutic efficacy against EM.
Material and Methods
Study Design and Sample Collection
All patients (N = 39) were recruited from the outpatient department of the Sir Sunderlal Hospital, Department of Obstetrics and Gynecology, Institute of Medical Sciences, Banaras Hindu University (BHU), Varanasi, India, during March 2017 to March 2020, and belonging to the province of Eastern Uttar Pradesh, India. Participants were included after filling out the informed consent, proforma, and questionnaire. Demographic parameters, clinical symptoms, and physical examination findings of patients suffering with endometriosis were recorded. The ethical approval was granted by the Institutional Ethical Committee (ref No: I. Sc./ECM-IX/2016-17/04). Female participants (N = 48) included in the control group were having healthy medical examinations, normal reproductive cycles, and ≥2 pregnancies without history of pregnancy-related complications. And in the case group, only those who have been diagnosed for endometriosis by ultrasonography, had no endocrinal radiation or chemical therapy or had not taken oral contraceptives 3 months prior to their admission, or not having any other disease history were included.
Further, diagnosis of endometriosis patients was done via laparoscopic inspections of the pelvis, preferably with histological biopsy confirmation, and stages were defined based on rASRM. From each subject (EM patient or normal control), approximately 2.0 mL of blood sample was drawn from the medial cubital vein, and the collected blood was kept at room temperature for 30–40 min for coagulation and then centrifuged at 3000 rpm, 15 min, at 4 °C. Supernatant serum was isolated and stored at −80 °C in an ultradeep refrigerator freezer.
NMR Sample Preparation
Before starting NMR experiments, the stored serum samples were withdrawn from −80 °C and thawed at room temperature. Each NMR sample was prepared by mixing 300 μL of sodium phosphate buffer (0.9% saline, buffer strength 50 mM prepared in 100% D2O, pH 7.4) with serum samples (300 μL in each case) and centrifuged at 16,278g for 5 min. After that, 450 μL of each prepared sample was transferred to a 5 mm NMR tube (Wilmad Glass, USA). A coaxial NMR tube containing 1.0 mM TSP (sodium salt of 3-trimethylsilyl-(2,2,3,3)-propionic acid-d4) dissolved in D2O was inserted separately that served as an external reference (offering a final apparent concentration approximately equal to 0.1 mM). Deuterium oxide (D2O) and the sodium salt of trimethylsilylpropionic acid-d4 (TSP) used for NMR spectroscopy were purchased from Sigma–Aldrich (St. Louis, MO, USA).
NMR Measurements
NMR spectra of the prepared serum samples, collected from healthy normal control and EM patients, were acquired using an 800 MHz Bruker Avance III NMR spectrometer equipped with a TCI cryogenic probe. A one dimensional Bruker standard CPMG (Carr–Purcell–Maiboom–Gill) spin–echo pulse sequence with water presaturation and a T2 filter for suppressing broad signals of protein and other macromolecules was used to record the spectra at 300k.56,57 The total time of the T2 filter (i.e., [τ–180°−τ]n) used was ∼80 ms with the spin echo time (2τ) used equal to 600 μs, 180° RF pulse equal to 25 μs, and loop counter (n) equal to 128. The other acquisition parameters used were as follows: 128 transients with 64k data points, relaxation delay of 5 s, and spectral width of 20 ppm with an acquisition time per scan of 15 min. Each spectrum was then manually phased and baseline corrected using TopSpin 3.6.1. Afterward, the spectrum was opened in the PROCESSOR module of CHENOMX NMR Suite 8.6 software and further better corrected for baseline and calibrated with respect to the formate peak at 8.43 ppm. For concentration profiling, formate was also used as an internal reference and the concentration was set to 10 μM (i.e., nearly close to the detection limit of metabolites in the CPMG NMR spectra of serum samples recorded at 800 MHz NMR spectrometer).58,59 The advantage of selecting formate as an internal reference has already been demonstrated in previous methodological studies60,61 including recent metabolomics studies from our lab.16−18,20 Studies have shown that, unlike TSP, formate does not interact with serum proteins/macromolecules60−62 and, therefore, has legitimate potential to serve as an internal reference for quantitative profiling of metabolites from blood serum (and eventually other biological fluids) in normal and diseased conditions not involving disorders of endogenous formate metabolism. After data processing, the spectrum was imported to the PROFILER-Module of CHENOMX and the concentrations of selected metabolites (i.e., proline and glutamine) were estimated in all the serum samples of EM patients and NC subjects. It is to be mentioned here that the present study is the very first part of the ongoing clinical metabolomics study on EM patients; the NMR spectra recorded using TSP as an external reference signal will be used for calibration in future studies.
Statistical Analysis
The statistical analysis was performed using the software program GraphPad Prism v6.01. The circulatory metabolic concentrations between the study groups were compared using the unpaired student t test analysis method, and the change was considered statistically significant if the test p-value was <0.05. The differences in the levels of significantly altered metabolites were visualized using box plots. The diagnostic potential of circulatory metabolites was evaluated using receiver operating characteristic (ROC) curve analysis and the area under the ROC curve (AUROC), with value ≥0.85 considered as the criterion for diagnostic significance. The correlations of circulatory metabolites with various clinical parameters were evaluated based on the Spearman correlation coefficient (r). The categorical variables were expressed as percentage and continuous variables as mean ± SD.
Acknowledgments
We all would like to acknowledge the Department of Medical Education, Government of Uttar Pradesh, for supporting the high-field NMR facility at the Centre of Biomedical Research, Lucknow, India. D.K. acknowledges the Department of Science and Technology for financial assistance under the SERB EMR Scheme (ref. No.: EMR/2016/001756). R.R. acknowledges the receipt of a SRF fellowship from CSIR, India. K.K. is a recipient of a CSIR-SRF fellowship, Department of Zoology, Banaras Hindu University. This work was partially supported by a UGC grant to R.C.
Glossary
Abbreviations
- NMR
nuclear magnetic resonance
- EM
endometriosis
- CI
confidence interval
- CPMG
Carr–Purcell–Meiboom–Gill
- T2DM
type II diabetic mellitus
- AUROC
area under ROC curve
- Glu
glutamate
- Pro
proline
- POX
proline oxidase
- P5C
delta1-pyrroline-5-carboxylate
- PYCR
pyrroline-5-carboxylate reductase
- MEM
moderate endometriosis
- TSP
trimethylsilylpropionic acid
- TSH
thyroid-stimulating hormone
- AAM
age at menarche
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- NC
normal control
- PQR
proline to glutamine ratio
- 1D
one dimensional
- VLDL
very low-density lipoproteins
- ROC
receiver operating characteristic
- Gln
glutamine
- GSA
glutamic-gamma-semialdehyde
- TCA
tricarboxylic acid
- MiR-23b
microRNA-23b
- SEM
severe endometriosis
- D2O
deuterium oxide
- PRL
prolactin
- BMI
body mass index
- T3
triiodothyronine
- T4
thyroxine
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00332.
Figure S1: Cumulative 1D 1H CPMG NMR spectra of serum samples of endometriosis (blue) and NC (red) study groups showing the resonances of formate and other serum metabolites. Figure S2: Box plots showing comparison of the circulatory levels of formate, glutamine, and proline between NC and EM subjects. Figure S3: Cumulative NMR signal of formate compared between endometriosis (EM, red) and normal control (NC, blue) groups with respect to the trimethyl peak of TSP at 0.00 ppm (PDF)
Author Contributions
R.C. and D.K.: Study design and conceptualization. K.K., A.A., and S.R.: Study approval, clinical evaluation, selection of subjects, compilation of clinical and anatomical details, and sample collection. R.R. and P.P.: Preparation of serum samples for NMR studies, NMR data collection, and NMR spectral analysis. S.V.: NMR spectral data for cross-validation and revising the manuscript draft critically for important intellectual content. D.K., K.K., R.C., and R.R.: Interpretation of data and drafting the manuscript.
The authors declare no competing financial interest.
Notes
The data that support the findings of this study has been uploaded on ZENODO (https://zenodo.org/record/5179906) and is available without undue reservation for further studies on request to the corresponding author. The DOI for the data set is 10.5281/zenodo.5179906.
Supplementary Material
References
- Vercellini P.; Vigano P.; Somigliana E.; Fedele L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol 2014, 10, 261–275. 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- Stejskalova A.; Fincke V.; Nowak M.; Schmidt Y.; Borrmann K.; von Wahlde M. K.; Schafer S. D.; Kiesel L.; Greve B.; Gotte M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci. Rep 2021, 11, 1–15. 10.1038/s41598-021-83645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusum K.; Rai S.; Singh K.; Chaube R. Endometriosis: pronouncing in reproductive women with role of estrogen and aromatase. Proc. Zool. Soc. India 2019, 18, 1–7. [Google Scholar]
- May K. E.; Conduit-Hulbert S. A.; Villar J.; Kirtley S.; Kennedy S. H.; Becker C. M. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update 2010, 16, 651–674. 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho L. M.; Ferreira M. C.; Rocha A. L.; Carneiro M. M.; Reis F. M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. 10.1016/bs.acc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Fassbender A.; Vodolazkaia A.; Saunders P.; Lebovic D.; Waelkens E.; De Moor B.; D’Hooghe T. Biomarkers of endometriosis. Fertil. Steril 2013, 99, 1135–1145. 10.1016/j.fertnstert.2013.01.097. [DOI] [PubMed] [Google Scholar]
- Sampson J. A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 1927, 3, 93. [PMC free article] [PubMed] [Google Scholar]
- Vinatier D.; Orazi G.; Cosson M.; Dufour P. Theories of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 21–34. 10.1016/S0301-2115(00)00405-X. [DOI] [PubMed] [Google Scholar]
- Maybin J. A.; Critchley H. O. Menstrual physiology: implications for endometrial pathology and beyond. Hum. Reprod. Update 2015, 21, 748–761. 10.1093/humupd/dmv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi M.; Lazzeri L.; Perelli F.; Reis F. M.; Petraglia F. Dysmenorrhea and related disorders. F1000Research 2017, 6, 1645. 10.12688/f1000research.11682.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman C.; Vandenabeele B.; Fieuws S.; Spiessens C.; Timmerman D.; D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril 2009, 92, 68–74. 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Riazi H.; Tehranian N.; Ziaei S.; Mohammadi E.; Hajizadeh E.; Montazeri A. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health 2015, 15, 1–12. 10.1186/s12905-015-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canis M.; Donnez J. G.; Guzick D. S.; Halme J. K.; Rock J. A.; Schenken R. S.; Vernon M. W. Revised American society for reproductive medicine classification of endometriosis: 1996. Fertility and Sterility 1997, 67, 817–821. 10.1016/S0015-0282(97)81391-X. [DOI] [PubMed] [Google Scholar]
- Guleria A.; Kumar A.; Kumar U.; Raj R.; Kumar D. NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr. Trends Med. Chem. 2018, 18, 1827–1849. 10.2174/1568026619666181120141603. [DOI] [PubMed] [Google Scholar]
- Nagana Gowda G. A.; Hong N. N.; Raftery D. Evaluation of Fumaric Acid and Maleic Acid as Internal Standards for NMR Analysis of Protein Precipitated Plasma, Serum, and Whole Blood. Anal. Chem. 2021, 93, 3233–3240. 10.1021/acs.analchem.0c04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U.; Kumar A.; Singh S.; Arya P.; Singh S. K.; Chaurasia R. N.; Singh A.; Kumar D. An elaborative NMR based plasma metabolomics study revealed metabolic derangements in patients with mild cognitive impairment: a study on north Indian population. Metab. Brain Dis 2021, 36, 957–968. 10.1007/s11011-021-00700-z. [DOI] [PubMed] [Google Scholar]
- Arya P.; Kumar U.; Sharma S.; Durgappa M.; Guleria A.; Raj R.; Pande G.; Kumar D. Targeted NMR-based serum metabolic profiling of serine, glycine and methionine in acute-on-chronic liver failure patients: Possible insights into mitochondrial dysfunction. Anal. Sci. Adv. 2021, 2, 536. 10.1002/ansa.202000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U.; Mehta P.; Kumar S.; Jain A.; Guleria A.; Kumar V. R.; Misra R.; Kumar D. Circulatory histidine levels as predictive indicators of disease activity in takayasu arteritis. Anal. Sci. Adv. 2021, 2, 527. 10.1002/ansa.202000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L.; Guleria A.; Rawat A.; Kumar D.; Aggarwal A. NMR based clinical metabolomics revealed distinctive serum metabolic profiles in patients with spondyloarthritis. Magn. Reson. Chem. 2021, 59, 85–98. 10.1002/mrc.5083. [DOI] [PubMed] [Google Scholar]
- Kumar U.; Sharma S.; Durgappa M.; Gupta N.; Raj R.; Kumar A.; Sharma P. N.; Krishna V. P.; Kumar R. V.; Guleria A. Serum metabolic disturbances associated with acute-on-chronic liver failure in patients with underlying alcoholic liver diseases: An elaborative NMR-based metabolomics study. J. Pharm. BioAllied Sci. 2021, 13, 276. 10.4103/jpbs.JPBS_333_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiersz L. M. Role of endometriosis in cancer and tumor development. Ann. N.Y. Acad. Sci. 2002, 955, 281–292. 10.1111/j.1749-6632.2002.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Jin L.; Alesi G. N.; Kang S. Glutaminolysis as a target for cancer therapy. Oncogene 2016, 35, 3619–3625. 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Le A.; Hancock C.; Lane A. N.; Dang C. V.; Fan T. W. M.; Phang J. M. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl. Acad. Sci. 2012, 109, 8983–8988. 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang J. M.; Liu W.; Hancock C. N.; Fischer J. W. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71. 10.1097/MCO.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. J.; Fendt S. M.; Becker D. F. The proline cycle as a potential cancer therapy target. Biochemistry 2018, 57, 3433–3444. 10.1021/acs.biochem.8b00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aniello C.; Patriarca E. J.; Phang J. M.; Minchiotti G. Proline metabolism in tumor growth and metastatic progression. Front. Oncol 2020, 10, 776. 10.3389/fonc.2020.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise D. R.; Thompson C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J. Nutr. 2008, 138, 2003S–2007S. 10.1093/jn/138.10.2003S. [DOI] [PubMed] [Google Scholar]
- Yang L.; Venneti S.; Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- Palka J.; Oscilowska I.; Szoka L. Collagen metabolism as a regulator of proline dehydrogenase/proline oxidase-dependent apoptosis/autophagy. Amino Acids 2021, 53, 1917. 10.1007/s00726-021-02968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang J. M.; Liu W. Proline metabolism and cancer. Front. Biosci. -Landmark 2012, 17, 1835. 10.2741/4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol B. W.; Bayram N.; Lijmer J. G.; Wiegerinck M. A.; Bongers M. Y.; Van Der Veen F.; Bossuyt P. M. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil. Steril 1998, 70, 1101–1108. 10.1016/S0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- Dutta M.; Singh B.; Joshi M.; Das D.; Subramani E.; Maan M.; Jana S. K.; Sharma U.; Das S.; Dasgupta S. Metabolomics reveals perturbations in endometrium and serum of minimal and mild endometriosis. Sci. Rep 2018, 8, 1–9. 10.1038/s41598-018-23954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzke M.; Arroyo S. F.; Sumpton D.; Mackay G. M.; Martin-Castillo B.; Camps J.; Joven J.; Menendez J. A.; Vazquez A. Stratification of cancer and diabetes based on circulating levels of formate and glucose. Cancer Metab. 2019, 7, 1–11. 10.1186/s40170-019-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maignien C.; Santulli P.; Kateb F.; Caradeuc C.; Marcellin L.; Pocate-Cheriet K.; Bourdon M.; Chouzenoux S.; Batteux F.; Bertho G. Endometriosis phenotypes are associated with specific serum metabolic profiles determined by proton-nuclear magnetic resonance. Reprod. BioMed. Online 2020, 41, 640–652. 10.1016/j.rbmo.2020.06.019. [DOI] [PubMed] [Google Scholar]
- Marianna S.; Alessia P.; Susan C.; Francesca C.; Angela S.; Francesca C.; Antonella N.; Patrizia I.; Nicola C.; Emilio C. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. BioSyst 2017, 13, 1213–1222. 10.1039/C7MB00181A. [DOI] [PubMed] [Google Scholar]
- Troisi J.; Sarno L.; Landolfi A.; Scala G.; Martinelli P.; Venturella R.; Di Cello A.; Zullo F.; Guida M. Metabolomic signature of endometrial cancer. J. Proteome Res. 2018, 17, 804–812. 10.1021/acs.jproteome.7b00503. [DOI] [PubMed] [Google Scholar]
- Ihata Y.; Miyagi E.; Numazaki R.; Muramatsu T.; Imaizumi A.; Yamamoto H.; Yamakado M.; Okamoto N.; Hirahara F. Amino acid profile index for early detection of endometrial cancer: verification as a novel diagnostic marker. Int. J. Clin. Oncol 2014, 19, 364–372. 10.1007/s10147-013-0565-2. [DOI] [PubMed] [Google Scholar]
- Vicente-Muñoz S.; Morcillo I.; Puchades-Carrasco L.; Payá V.; Pellicer A.; Pineda-Lucena A. Pathophysiologic processes have an impact on the plasma metabolomic signature of endometriosis patients. Fertil. Steril 2016, 106, 1733–1741. 10.1016/j.fertnstert.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Fassbender A.; Burney R. O.; O D. F.; D’Hooghe T.; Giudice L. Update on biomarkers for the detection of endometriosis. BioMed. Res. Int. 2015, 2015, 130854. 10.1155/2015/130854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z.; Wang H.; Yin X.; Deng P.; Jiang W. Application of NMR metabolomics to search for human disease biomarkers in blood. Clin. Chem. Lab. Med. 2019, 57, 417–441. 10.1515/cclm-2018-0380. [DOI] [PubMed] [Google Scholar]
- Li J.; Guan L.; Zhang H.; Gao Y.; Sun J.; Gong X.; Li D.; Chen P.; Liang X.; Huang M. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod. Biol. Endocrinol 2018, 16, 1–10. 10.1186/s12958-018-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.; Chang W.; Feng C.; Cui M.; Xu T. Endometriosis malignant transformation: Epigenetics as a probable mechanism in ovarian tumorigenesis. Int. J. Genomics 2018, 2018, 1465348. 10.1155/2018/1465348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B.; Balasubramaniam M.; Lebowitz J. J.; Taylor A.; Villalta F.; Khoshbouei H.; Grueter C.; Grueter B.; Dash C.; Pandhare J. Activation of proline biosynthesis is critical to maintain glutamate homeostasis during acute methamphetamine exposure. Sci. Rep 2021, 11, 1–16. 10.1038/s41598-020-80917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S.; Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott A. J.; Maimouni S.; Zong W. X. The pleiotropic effects of glutamine metabolism in cancer. Cancers 2019, 11, 770. 10.3390/cancers11060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila T.; Kauppila S.; Bode M.; Tapanainen J.; Risteli J.; Risteli L.; Kauppila A.; Stenbäck F. Synthesis and maturation of type I and type III collagens in endometrial adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 66–74. 10.1016/S0301-2115(02)00406-2. [DOI] [PubMed] [Google Scholar]
- Hsu A. L.; Townsend P. M.; Oehninger S.; Castora F. J. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil. Steril 2015, 103, 347–352. 10.1016/j.fertnstert.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Lee K. E. Comparison of the miR-23b and miR-203 Expressions in Endometrial Cancer. Korean Journal of Clinical Laboratory Science 2017, 49, 455–459. 10.15324/kjcls.2017.49.4.455. [DOI] [Google Scholar]
- Guo Y.-X.; Wang N.; Wu W.-C.; Li C.-Q.; Chen R.-H.; Zhang Y.; Li X. The Role of miR-23b in Cancer and Autoimmune Disease. J. Oncol 2021, 2021, 6473038. 10.1155/2021/6473038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi I.; Salvi A.; Baiocchi G.; Portolani N.; De Petro G. Functional role of microRNA-23b-3p in cancer biology. Microrna 2018, 7, 156–166. 10.2174/2211536607666180629155025. [DOI] [PubMed] [Google Scholar]
- Shang C.; Lang B.; Ao C. N.; Meng L. Long non-coding RNA tumor suppressor candidate 7 advances chemotherapy sensitivity of endometrial carcinoma through targeted silencing of miR-23b. Tumor Biol. 2017, 39, 1010428317707883. 10.1177/1010428317707883. [DOI] [PubMed] [Google Scholar]
- Liu W.; Zabirnyk O.; Wang H.; Shiao Y. H.; Nickerson M. L.; Khalil S.; Anderson L. M.; Perantoni A. O.; Phang J. M. miR-23b* targets proline oxidase, a novel tumor suppressor protein in renal cancer. Oncogene 2010, 29, 4914–4924. 10.1038/onc.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorile P. G.; Baldi A. Serum biomarker for diagnosis of endometriosis. J. Cell. Physiol 2014, 229, 1731–1735. 10.1002/jcp.24620. [DOI] [PubMed] [Google Scholar]
- Anastasiu C. V.; Moga M. A.; Elena Neculau A.; Bâlan A.; Scârneciu I.; Dragomir R. M.; Dull A. M.; Chicea L. M. Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int. J. Mol. Sci. 2020, 21, 1750. 10.3390/ijms21051750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S. Quantitative metabolomics using NMR. TrAC trends in analytical chemistry 2008, 27, 228–237. 10.1016/j.trac.2007.12.001. [DOI] [Google Scholar]
- Beckonert O.; Keun H. C.; Ebbels T. M.; Bundy J.; Holmes E.; Lindon J. C.; Nicholson J. K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc 2007, 2, 2692. 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- Crook A. A.; Powers R. Quantitative NMR-based biomedical metabolomics: Current status and applications. Mol. 2020, 25, 5128. 10.3390/molecules25215128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontiers in Drug Design & Discovery; Atta-ur-Rahman, Chaudhary M. I., Eds.; Bentham Books, 2016; Vol. 7. [Google Scholar]
- Ando I.; Hirose T.; Nemoto T.; Totsune K.; Imai Y.; Takeuchi K.; Fujiwara M. Quantification of molecules in 1H-NMR metabolomics with formate as a concentration standard. Journal of Toxicological Sciences 2010, 35, 253–256. 10.2131/jts.35.253. [DOI] [PubMed] [Google Scholar]
- Kriat M.; Confort-Gouny S.; Vion-Dury J.; Sciaky M.; Viout P.; Cozzone P. J. Quantitation of metabolites in human blood serum by proton magnetic resonance spectroscopy. A comparative study of the use of formate and TSP as concentration standards. NMR in Biomedicine 1992, 5, 179–184. 10.1002/nbm.1940050404. [DOI] [PubMed] [Google Scholar]
- Kumar U.; Jain A.; Guleria A.; Misra D. P.; Goel R.; Danda D.; Misra R.; Kumar D. Circulatory glutamine/glucose ratio for evaluating disease activity in Takayasu arteritis: a NMR based serum metabolomics study. J. Pharm. Biomed. Anal. 2020, 180, 113080. 10.1016/j.jpba.2019.113080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.