Abstract
The effectiveness of newer macrolides in acute Q fever for 113 patients was recorded. The mean times to defervescence were 2.9 days for doxycycline and 3.3, 3.9, 3.9, and 6.4 days for clarithromycin, roxithromycin, erythromycin, and β-lactams, respectively (P < 0.01 for macrolides versus β-lactams). We conclude that macrolides may be an adequate empirical antibiotic therapy for acute Q fever.
Acute Q fever disease is a zoonosis with a worldwide distribution and is caused by Coxiella burnetii, an obligate intracellular parasite. It is characterized by a wide variety of clinical manifestations, such as prolonged fever, pneumonia, granulomatous hepatitis, and meningoencephalitis (1, 3, 8, 12, 13, 16). The treatment of choice for acute Q fever is considered doxycycline (8, 15), but the diagnosis often is missed, and macrolides and other antibiotics considered ineffective in vitro are usually used. (17) Respiratory involvement with an atypical pneumonia syndrome is the predominant clinical presentation of Q fever in most published studies (3, 10, 14, 16).
Our knowledge about various features of acute and chronic disease, such as who will develop chronic disease, remains incomplete. Furthermore, information about the course of untreated infection, or infection treated with ineffective antibiotics, is also lacking.
In order to assess the clinical responsiveness of C. burnetii to the new macrolides, we reviewed the clinical features, antibiotic treatments, and outcomes of 113 patients hospitalized between 1989 and 1996 with acute Q fever infection. To our knowledge, this is the largest study assessing the efficacy of macrolides, especially the newer members of this family, against acute Q fever infection.
Patients were identified from a retrospective study of adults hospitalized for a febrile illness associated with serological evidence of acute C. burnetii infection.
Over a period of 7 years (1989 to 1996), serum samples from 3,300 patients suspected of being infected by C. burnetii were assayed for the presence of antibodies against antigen phase II of the microorganism, using the indirect immunofluorescence antibody technique. In order to increase the specificity of this technique, we considered titers of immunoglobulins G and M of 1/960 and 1/400 and/or a fourfold increase of the titer between two assays (titers should be at least 1/200) a strong indication of acute infection.
We defined cases of acute Q fever as serologic findings consistent with acute C. burnetii infection and one or more of the following manifestations: (i) fever (≥38°C); (ii) respiratory disease (dyspnea, expectoration, cough, and chest pain, with positive X-ray findings); (iii) hepatitis (a more-than-twofold increase in serum glutamic oxalacetic transaminase and/or serum glutamic pyruvic transaminase levels); and (iv) central nervous system involvement (neurological symptoms associated with normal or abnormal cerebrospinal fluid findings).
Medical records for 113 patients were reviewed. These patients were divided into three groups corresponding to doxycycline, macrolide, and β-lactam treatment. Doxycycline was administered at 100 mg twice a day (BID). Patients in the macrolide group were further subdivided into three subgroups: those who received erythromycin at 1 g three times a day (TID) intravenously or 500 mg TID per os, those who received clarithromycin at 500 mg BID per os, and those who received roxithromycin at 150 mg BID. Medical records of all 113 patients were reviewed with regard to clinical features, antibiotic treatment, and outcome.
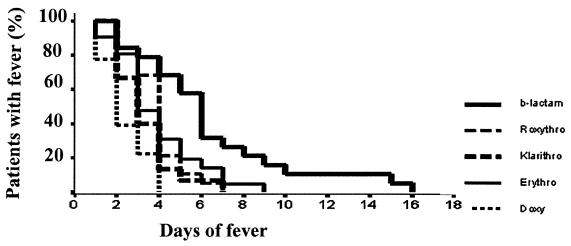
Statistical analysis was performed with the SPSS 8.0 statistical program. Antibiotics were compared pairwise in terms of time to defervescence using the log rank test, and the curves for fever responses to various antibiotics were determined by a Kaplan-Meier plot (Fig. 1).
FIG. 1.
Kaplan-Meier plot showing the relationship between antibiotics used and duration of fever in 113 patients with acute Q fever infection.
Eighty-four patients (74.3%) were men, and 29 (25.7%) were women. The mean age of all the patients was 36.4 ± 14.6 (15 to 77) years. The seasonal distribution of Q fever cases in our area from 1989 to 1996 showed a higher incidence of infection during the period from January to June than in the period from July to December, resulting in a significant difference between the two semesters of the year (x2 = 17.2; P < 0.001) (16). A difference was also observed among various age groups (x2 = 40.3; P < 0.01). Subjects belonging to the 20- to 39-year and 80- to 89-year age groups were shown to be at a higher risk (16). Forty-one of 113 patients (36.3%) had a history of contact with animals or ingestion of unpasteurized milk or fresh cheese. Since only 18.7% of the Cretan population is stockbreeders, contact with animals represents a significant risk factor (x2 = 16.4; P < 0.001) (16).
Table 1 summarizes demographic and common clinical manifestations of acute Q fever. Of 113 patients receiving antibiotics, 18 (15.9%) received doxycycline while, the majority (n = 95; 84.1%) were treated with macrolides and β-lactam antibiotics. Forty-two patients (37.2%) were treated with erythromycin, 19 (16.8%) were treated with roxithromycin, and 15 (13.3%) were treated with clarithromycin (Table 2).
TABLE 1.
Clinical and demographic data of 113 cases of acute Q fever
| Characteristic | No. (%) of patientsa |
|---|---|
| Male | 84 (74.3) |
| Female | 29 (25.7) |
| Pneumonia | 107 (94.7) |
| Febrile illness | 6 (5.3) |
| Splenomegaly | 6 (5.3) |
| Rash | 5 (4.4) |
| Lymphadenopathy | 7 (6.2) |
The mean age for all patients was 37.6 (15 to 91) years.
TABLE 2.
Antibiotics used in 113 patients with acute Q fever
| Antibiotic | No. (%) of patients | Mean days to defervescence |
|---|---|---|
| Doxycycline | 18 (15.9) | 2.39 (1.88–2.89) |
| Erythromycin | 42 (37.2) | 3.93 (3.32–4.53) |
| Clarithromycin | 15 (13.3) | 3.33 (2.63–4.04) |
| Roxithromycin | 19 (16.8) | 3.89 (3.32–4.47) |
| β-Lactams | 19 (16.8) | 6.42 (4.66–8.18) |
Fever subsided after a mean of 2.39 (1.88 to 2.89) days after administration of doxycycline. Mean times to defervescence were 3.33 (2.63 to 4.04) days with clarithromycin, 3.89 (3.32 to 4.47) days with roxithromycin, and 3.93 (3.32 to 4.53) days with erythromycin. In patients receiving β-lactams, fever subsided after a mean of 6.42 (4.66 to 8.18) days (Table 2).
The clinical response of C. burnetii to erythromycin and roxithromycin, in terms of days to apyrexia, differed significantly from the response to doxycycline (P < 0.001). Clarithromycin was also significantly different from doxycycline (P < 0.05), but macrolides did not differ from each other. β-Lactams differed significantly from both doxycycline and macrolides (P < 0.001). All patients within the doxycycline group became afebrile on day 4, while patients in the macrolide group became afebrile on day 9. Fever subsided in all patients treated with β-lactams on day 16 of treatment (Fig. 1).
There were no relapses or complications registered independently of the antibiotic administration or deaths related to infection, although one patient did die from an unrelated tumor and another with a serious underlying disease.
This study provides evidence that although tetracycline is the treatment of choice in acute Q fever infection, macrolides (mainly the newer ones) could be a valuable alternative. β-Lactams seem to have no role in the treatment of C. burnetii, as previously described (11, 17). Coxiella multiplies in the phagolysosomes of infected cells at a pH lower than 5, making it unlikely for the antibiotic to be active (13). The in vitro activities of various antibiotics against C. burnetii were well studied by Yeaman et al. (17, 18) and others (5, 6, 9). However, data about the clinical activity of drugs with high intracellular concentrations, such as quinolones and macrolides, are lacking.
The treatment of choice for C. burnetii is doxycycline, which does not have a bactericidal effect against the microorganism (8, 15). The treatment must be initiated within the first 3 days of illness in order to be effective. Its use is limited because of the late diagnosis, depending mainly on serology, and the atypical presentation of the acute illness. Other antibiotics, such as chloramphenicol, co-trimoxazole, quinolones, and rifampin, are effective in vitro against C. burnetii and have been used, but few clinical data are available. (17, 18)
The role of macrolides in the treatment of Q fever is not clear. In a previous work, the MIC of clarithromycin for C. burnetii was found to range from 2 to 4 μg/ml. (5). There are also few reports of erythromycin inactivity in vitro against C. burnetii (9). A limited number of publications about its clinical response, especially in severe cases, have come up with contradictory results (8). Since atypical pneumonia is the most frequent clinical presentation of Q fever in areas of endemicity (3, 8, 16), C. burnetii infection is empirically treated with erythromycin and other new macrolides.
Our data suggest that erythromycin and other new macrolides could be considered a reasonable treatment for acute C. burnetii infection. Pharmacokinetic factors may account for this clinical efficacy of macrolides. Factors related to liposolubility and high intracellular concentrations might influence the potential of this class of drugs to treat intracellular microorganisms. Data on erythromycin have been presented in other published reports but are lacking for the newer macrolides (1, 2, 4, 6, 13). It is well known that the newer macrolides, such clarithromycin (MIC, 1.0 to 4.0 mg/liter), attain higher intracellular concentrations than erythromycin and could potentially be more effective against C. burnetii infection (5, 6, 9).
To our knowledge the use of clarithromycin in acute Q fever has been described once for only four patients (7), and there are no clinical data in the international literature about the clinical activity of roxithromycin against C. burnetii.
In this study, clinical improvement, apyrexia, and shortening of acute disease were achieved in 3.33 and 3.89 days, respectively, when clarithromycin and roxithromycin were used. We believe that the 1-day delay to apyrexia with clarithromycin compared to doxycycline is a clinically meaningful end point. On the other hand, all newer macrolides were more efficacious than β-lactams in terms of fever duration (P < 0.001).
In summary, in acute Q fever infection, a relatively frequent cause of community-acquired pneumonia in areas of endemicity, macrolides—especially the newer ones, such as clarithromycin and roxithromycin, which have the advantages of fewer side effects and higher intracellular concentrations—seem to be adequate alternative treatments.
Further prospective studies are needed to better evaluate the efficacy and safety of the macrolides in the treatment of acute Q fever infection.
REFERENCES
- 1.Caron F, Meurice J C, Ingrand P, Bourgoin A, Masson P, Roblot P, Patte F. Acute Q fever pneumonia: a review of 80 hospitalized patients. Chest. 1998;114:808–813. doi: 10.1378/chest.114.3.808. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelo L J, Hetherington R. Q fever treated with erythromycin. Br Med J. 1979;ii:305–306. doi: 10.1136/bmj.2.6185.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuis G, Peter O, Pedroni D, Petite J. Aspects clinics observes lors d'une epidemie de 415 cas de fievre Q. Schweiz Med Wochenschr. 1985;115:814–818. [PubMed] [Google Scholar]
- 4.Ellis M E, Dumbar E M. In vivo response of acute Q fever to erythromycin. Thorax. 1982;37:867–868. doi: 10.1136/thx.37.11.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gikas A, Spyridaki I, Psaroulaki A, Kofteridis D, Tselentis Y. In vitro susceptibility of Coxiella burnetii to trovafloxacin in comparison with susceptibilities to pefloxacin, ciprofloxacin, ofloxacin, doxycycline, and clarithromycin. Antimicrob Agents Chemother. 1998;42:2747–2748. doi: 10.1128/aac.42.10.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keysary A, Itzhaki A, Rubinstein E, Oron C, Keren G. The in vitro anti-rickettsial activity of macrolides. J Antimicrob Chemother. 1996;38:727–731. doi: 10.1093/jac/38.4.727. [DOI] [PubMed] [Google Scholar]
- 7.Ko W C, Liu J W, Chuang Y C. Acute Q fever as a cause of acute febrile illness of unknown origin in Taiwan: report of seven cases. J Formos Med Assoc. 1997;96:295–297. [PubMed] [Google Scholar]
- 8.Marrie T J. Coxiella burnetii. In: Mandell G L, Douglas R G J, Bennett J E, editors. Principles and practice of infectious disease. 3rd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 1472–1476. [Google Scholar]
- 9.Maurin M, Raoult D. In vitro susceptibilities of spotted fever group rickettsiae and Coxiella burnetii to clarithromycin. Antimicrob Agents Chemother. 1993;37:2633–2637. doi: 10.1128/aac.37.12.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montejo Baranda M, Corral Carranceja J, Aguirre Errasti C. Q fever in the Basque Country: 1981–1984. Rev Infect Dis. 1985;7:700–701. doi: 10.1093/clinids/7.5.700. [DOI] [PubMed] [Google Scholar]
- 11.Perez-del-Molino A, Aguado J M, Riancho J A, Sampedro I, Matorras P, Gonzalez-Macias J. Erythromycin and the treatment of Coxiella burnetii pneumonia. J Antimicrob Chemother. 1991;28:455–459. doi: 10.1093/jac/28.3.455. [DOI] [PubMed] [Google Scholar]
- 12.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier P E, Bernit E, Stein A, Nesri M, Harle J R, Weiller P J. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysomotropic agents on Coxiella burnetii in P338D1 cells. Antimicrob Agents Chemother. 1990;34:1512–1514. doi: 10.1128/aac.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly S, Northwood J L, Caul E O. Q fever in Plymouth, 1972–88. A review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408. doi: 10.1017/s095026880004797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spelman D W. Q fever: a study of 111 consecutive cases. Med J Aust. 1982;1:547–553. doi: 10.5694/j.1326-5377.1982.tb124169.x. [DOI] [PubMed] [Google Scholar]
- 16.Tselentis Y, Gikas A, Kofteridis D, Kyriakakis E, Lydataki N, Bouros D, Tsaparas N. Q fever in the Greek island of Crete. Epidemiology, clinical and therapeutic data from 98 cases. Clin Infect Dis. 1995;20:1311–1316. doi: 10.1093/clinids/20.5.1311. [DOI] [PubMed] [Google Scholar]
- 17.Yeaman M R, Baca O G. Antibiotic susceptibility of Coxiella burnetii. In: Marrie T J, editor. Q fever. The disease. Vol. 1. Boca Raton, Fla: CRC Press; 1990. pp. 213–223. [Google Scholar]
- 18.Yeaman M R, Mitscher L A, Baca O G. In vitro susceptibility of Coxiella burnetii to antibiotics, including several quinolones. Antimicrob Agents Chemother. 1987;31:1079–1084. doi: 10.1128/aac.31.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]