Abstract
At present, plastic waste accumulation has been observed as one of the most alarming environmental challenges, affecting all forms of life, economy, and natural ecosystems, worldwide. The overproduction of plastic materials is mainly due to human population explosion as well as extraordinary proliferation in the global economy accompanied by global productivity. Under this threat, the development of benign and green alternative solutions instead of traditional disposal methods such as conversion of plastic waste materials into cherished carbonaceous nanomaterials such as carbon nanotubes (CNTs), carbon quantum dots (CQDs), graphene, activated carbon, and porous carbon is of utmost importance. This critical review thoroughly summarizes the different types of daily used plastics, their types, properties, ways of accumulation and their effect on the environment and human health, treatment of waste materials, conversion of waste materials into carbon-based compounds through different synthetic schemes, and their utilization in energy storage devices particularly in supercapacitors, as well as future perspectives. The main purpose of this review is to help the targeted audience to design their futuristic study in this desired field by providing information about the work done in the past few years.
1. Introduction
In the last couple of decades, a growing concern for researchers is electronic waste, as it is a profligate waste on the planet. According to analysis ∼44.7 million tons of e-waste was engendered in 2016, and it is predicted that it will reach ∼52.2 per million tons in 2021 by following a growth rate of 3–4% per annum. E-waste comprises various constituents containing glass, ceramics, metals, and polymers.1
Polymers further comprise plastics, cellulose, rubbers, and wax macromolecules composed of smaller units called monomers and are broadly categorized into different classes based on their origin as natural, artificial (synthetic), and semisynthetic molecules. Plastics are artificial/synthetic polymers containing a wide range of synthetic/semisynthetic organic malleable molecules which can be turned into solid objects. Since 1907, after the production of the first synthetic plastic in New York, plastics have occupied an important place in the routine life of humans. Almost all facets of routine life involve plastics, such as clothing, transport, telecommunications, footwear, and packaging materials that enable the transport of a broad range of drinks, eatables, and other goods. Moreover, their desirable properties, such as economical nature, low maintenance requirements, lightweight, stability, weathering resistance, transparency, decreased toxicity, good electrical insulation, excellent thermal properties, and design flexibility compared to other solid components, expedite the practical applications of plastics in various commercial, industrial, and agricultural activities. There is further room for novel applications of plastics that will be quite beneficial in the future, such as the development of renewable energy sources, novel medical applications, and reduced energy utilization in transport.2,3 In the last 60 years, due to the large-scale utilization of plastic in different applications, it is ranked as the most widely employed material in the world, with global use surpassing 260 million tons/annum, and it now accounts for about 10% of total generated waste.4,5
According to the latest survey, only in 2019, plastic global production was ≈370 million metric tons, and among these 36.4% are anticipated to be incinerated; a similar fraction (36.4%) is expected to be dumped into landfills or excreted in the environment, and only 27.2% will be consumed in recycling. These forecasts undoubtedly project the plastic wastes’ mismanagement due to improper recycling/reusing of these materials, which consequently leads to soil, freshwater, and ocean contamination worldwide via plastic wastes. Due to their lightweight, durable nature, plastics have become a widespread element of marine litter with succeeding influences on a natural ecosystem and all living organisms.6,7
Currently, preventive strategies to lessen the plastic wastes discharged to the environment (upstream responses) and strategies to diminish their impact after their release into the environment (downstream responses) are considered important tools to tackle the crisis of global plastic waste, and proper waste management comes into the category of upstream responses. Conversely, the plastic wastes’ recycling process has several limitations, one of which results in the product’s economic attractiveness.8−15
2. Types of Plastic
Plastics are macromolecules prepared by the polymerization process and can be molded by applying the appropriate amount of pressure, heat, or some other external force. Polymerization is the process of linking individual units of identical or different molecules (“mers”) through chemical reactions to develop the long-chain structure of macromolecules accompanied by properties quite different from those of starting molecules. Different types of polymers based on their properties and nature are categorized as rubbers or elastomers, plastics, and fibers.16−20
Two main types of plastics are thermoplastics and thermosetting plastics (Figure 1).
-
(i)
Thermoplastics
Thermoplastics are composed of linear chains of molecules held by weak Van der walls forces and, once prepared, can be molded repeatedly into different shapes by applying temperature until they lose their identity, as they are flexible.
Examples: polyethylene, polystyrene, polypropylene, polycarbonate, nylon, etc., where polystyrene, polyethylene, and polypropylene fall into the category of addition polymers while nylon and polycarbonate are types of condensation polymers.
Daily life applications: polystyrene cups, polyethylene buckets, nylon ropes, etc.
-
(ii)
Thermosetting Plastics
Thermosetting plastics are fabricated by the route of irreversible polymerization; once designed or shaped they are unable to be softened again by thermal reactions due to strong attractive forces and a highly cross-linked structure, and material charring occurs on heating.
Examples: urea formaldehyde, phenol formaldehyde, melamine formaldehyde, thermosetting polyester, etc. All of these are types of condensation polymers.
Applications: Formica tabletops, Bakelite electrical switches, and melamine cutlery.20−22
Figure 1.

Commonly used plastics in our daily life. Reproduced with permission from ref (22). Open access article 2017, RSC.
2.1. Types of Thermoplastic Polymers
The ideal properties of thermoplastic polymers, such as corrosion resistance, low density, user-friendly design, and high strength, are responsible for the greater demand for plastics than for aluminum and other metals. The three subtypes of thermoplastics are mentioned below:
-
(i)
Crystalline Thermoplastics
A material with highly ordered molecular chains and translucent properties is called a crystalline thermoplastic. High mechanical impact resistance as compared to other types is the salient feature of these materials.
Examples: high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP).
-
(ii)
Amorphous Thermoplastics
Materials with randomly arranged molecular chains and transparent nature are amorphous thermoplastics.
Examples: poly(methyl methacrylate) (PMMA), polyvinyl chloride (PVC), polycarbonate (PC), acrylonitrile butadiene styrene (ABS), and polystyrene (PS).
-
(iii)
Semicrystalline Polymers
The properties of semicrystalline materials are a combination of the properties of both the above-mentioned types.
Examples: polyamide imide (PAI) and polybutylene terephthalate (PBT).
The most widely utilized plastics in our routine life are thermoplastics, and among them the most common are PE (polyethylene) 29.6%, PP (polypropylene) 18.9%, PVC (polyvinyl chloride) 10.4%, PUR (polyurethane) 7.4%, PS (polystyrene) 7.1%, and PET (polyethylene terephthalate) 6.9%, respectively.23
3. Physical Properties of Different Types of Plastics
Plastics have characteristic physical characteristics, essential to be considered during the processing of any product. The physical data for numerous commercially available plastics are provided in Table 1.24
Table 1. Commonly Used Plastics and Their Physical Propertiesa.
| Plastic | Thermal properties |
Strength |
Density | ||||
|---|---|---|---|---|---|---|---|
| Chemical name | Tm (°C) | Tg (°C) | Td (°C) | Cte (°C) | Tensile psi | Compressive psi | (g/cm3) |
| LDPE (Low-density polyethylene) | 122 | 1900 | 0 | 0.914–0.940 | |||
| 124 | 4000 | ||||||
| HDPE (High-density polyethylene) | 130 | 79 | 59 | 3200 | 2700 | 0.952–0.965 | |
| 137 | 91 | 110 | 4500 | 3600 | |||
| PP (Polypropylene) | 168 | –20 | 107 | 81 | 4500 | 5500 | 0.900–0.910 |
| 175 | 121 | 100 | 6000 | 8000 | |||
| PS (Polystyrene) | 74 | 68 | 50 | 5200 | 12000 | 1.04–1.05 | |
| 105 | 96 | 83 | 7500 | 13000 | |||
| PVC (Polyvinyl chloride) | 75 | 57 | 50 | 5900 | 8000 | 1.30–1.58 | |
| 105 | 82 | 100 | 7500 | 13000 | |||
| PI (Polyimide) | 310 | 277 | 45 | 10500 | 30000 | 1.36–1.43 | |
| 365 | 360 | 56 | 17100 | 40000 | |||
| PMMA (Poly(methyl methacrylate)( | 85 | 79 | 50 | 7000 | 10500 | 1.17–1.20 | |
| 105 | 107 | 90 | 11000 | 18000 | |||
| PC (Polycarbonate) | 150 | 138 | 68 | 9500 | 12500 | 1.2 | |
Tm, crystalline melting temperature; Td, heat distortion temperature (66 psi load); Cte coefficient of linear thermal expansion; Tg, glass transition temperature (the plastic becomes brittle below this temperature); compressive psi, compressive strength (load necessary to crush a sample of the plastic); tensile psi, tensile strength (load necessary to pull a sample of the plastic apart).
4. Impact of Plastic Accumulation
Plastics are non-biodegradable, mostly composed of hydrogen, carbon, and other elements, such as nitrogen, chlorine, etc., and are the main reason for the municipal waste management problem (Figure 2). According to the latest survey of the United Nations, the annual production of plastic in 2020 is over 400 Mt, and it is expected to be double (800 Mt) in 2035 and to increase to the value to 1600 Mt by 2050. The manufacturing process of plastic leads to the release of an excessive amount of detrimental gaseous substances in the air such as Dioxin, carbon monoxide, hydrogen cyanide, and nitrogen oxides, which have serious/detrimental effects on human health and the environment. Furthermore, 4.5 billion tons of plastic wastes dumped in landfills or excreted in the environment harm the soil’s microbial content. In 2018, it was reported that the intensity of trace gases generated from low-density polyethylene increases with the increase of incubation time.25,26
Figure 2.
Plastic waste and its harmful effects on the environment and human health. Reproduced with permission from ref (80). Copyright 2021 Elsevier.
4.1. Impact of Plastic Accumulation on the Environment and Human Health
According to the 2020 survey, 195 countries were found to be responsible for the production of 400 Mt of plastic waste, and ≈8.8 Mt was observed to be released in the ocean. Plastic wastes are the main source of organic pollutants, heavy metals, chemicals, and pathogens. Furthermore, a toxic compound released after abiotic degradation of waste plastic badly affects the quality of water and soil, and the hydrophobic nature of plastics enhances their accumulation with other contaminants, such as organic pollutants, polyaromatic hydrocarbons (PAHs), and polychlorinated biphenyls (Table 2). There are numerous chemicals added to plastics during the manufacturing process to modify their properties, such as phthalates, bisphenol A, and flame retardants, which harm animal and human health and predominantly affect the endocrine systems. Additionally, the toxic monomers also tend to be cross-linked to create cancer and reproductive issues.27
-
(i)
The major concern of society in the present era is water contamination via plastic wastes as they deteriorate the fishing industry, are detrimental for ocean animals, and devastate the aquatic environment. The studies have revealed that 60–80% of marine debris comprises plastic wastes that are widespread on sea beds and shorelines, in the marine environment, and at the sea surface. Additionally, ingestion of microplastics and their long-lasting accretion affect the food chain, and abiotic degradation through rain, wind, and the sun is responsible for the leaching of chemicals from plastics and leads to consequent ancillary pollution problems within groundwater, surface water, and marine water bodies.28−30
-
(ii)
Regarding soil contamination, settlement of various plastic wastes on the soil surface or their penetration into soil layers from sludge, irrigation via wastewater, fertilizers, biosolids, or landfilling is the main source of soil pollution and could result in declined crop production as well as soil infertility due to release of toxins, and over 500 years is required for complete plastic decomposition. The plastic wastes dumped in landfills not only occupy a huge area of land but also inevitably discharge large quantities of chemicals comprising catalyst remnants, oligomers, and polymerization solvents along with a broad range of plastic additives. These hazardous chemicals influence the quality of the soil and surrounding groundwater.31,32
-
(iii)
The third incineration of plastic wastes releases numerous pollutants into the atmosphere and leads to air pollution, another severe environmental issue. In the process of solid waste management and disposal, plastic solid waste incineration with municipal solid waste (MSW) releases harmful chemicals including metals, aldehydes (-CHO), particulate matters (PMs), methane (CH4), carbon monoxide (CO), nitrogen oxides (NOx), carbon dioxide (CO2), polyaromatic hydrocarbons (PAHs), furan (C4H4O), volatile organic compounds (VOCs), and other solid materials (i.e., residue and ash) which intensify the levels of inorganic salts, heavy metals, and several organic compounds in the environment. The most alarming issue is the discharge of some carcinogenic substances such as PAHs, dioxins, and nitro-PAHs, through incineration of PET, PVC, PE, and PS. Moreover, an excessive amount of tenacious free radicals (highly reactive and unstable) in both the solid residual ash and soot creates adverse health effects particularly to human lungs.33−38
-
(iv)
Plastic disposal significantly affects human health, directly or indirectly via digestion and inhalation, respectively. Specifically, the tenacity of microplastics is responsible for multiple biological responses such as inflammation, apoptosis, genotoxicity, oxidative stress, necrosis, tissue damage, carcinogenesis, and fibrosis in the case of uninterrupted exposure. The leaching of residual monomers and unbound chemicals and the desorption of the associated hydrophobic organic contaminants also result in worsening of human health conditions. Moreover, all plastics generate free radicals by C–H bond dissociation during interaction of reactive oxygen species (ROS) with light and transition metal carcinogenic resin monomers by decomposition of polyvinyl chloride, polystyrene polymer, and epoxy resin. Thus, keeping in mind the plastic mismanagement wastes and the worldwide impact of plastics on human health, wildlife, and the environment, there is an exigent requirement for novel and effective technologies for plastic wastes’ disposal and treatment.39−41
Table 2. Effect of Plastic Accumulation on Human Health and Environmental Health.
| Sr. No | Effect on aquatic life | Effect on soil | Effect on environment | Effect on humans |
|---|---|---|---|---|
| 1 | Deteriorated fishing industry | Decreased crop production | Polluted air | Inflammation |
| 2 | Polluted water bodies | Soil infertility | Release of toxic gases | Tissue damage |
| 3 | Polluted aquatic environment | Contaminated soil and groundwater | High concentration of heavy metals, salts, and organic compounds | Oxidative stress |
| 4 | Change in soil pH | Release of carcinogenic substances and highly reactive free radicals in the air | Carcinogenesis |
5. Types of Waste Plastics and Their Recyclability
Among the different types of commonly used plastics, some are more smoothly recyclable than others. However, the quality of recycling is greatly affected by the contaminants present in the form of dirt, paper, printing inks, additives, metals, foil, pesticides, foreign bodies, and partially oxidized polymers easily noticeable in HDPE and PET bottles collected from pavements. For some commonly consumable plastics, their tendency to be recycled and their accumulated contaminants are stated in Table 3.24
Table 3. Commonly Used Plastics, Their Recyclable Nature, and Their Contaminants.
| Type | Abbreviation | Recyclabilty | Description | Contamination |
|---|---|---|---|---|
| Type 1 | PET | Yes | Polyethylene terephthalate | PVC, green PET, Al, water, glue, oligomers |
| Beverage bottles | ||||
| Type 2 | HDPE | Yes | High-density polyethylene | PP, milk residue, pigments, paper, EPS, cork |
| Milk, detergent, and oil bottles, toys, containers, plastic bags | ||||
| Type 3 | PVC | Yes, but not common | Polyvinyl chloride | PET, PE, paper, Al foil, PP |
| Food wraps, vegetable oil bottles, blister packages or automotive parts, beverage bottles | ||||
| Type 4 | LDPE | Yes | Low-density polyethylene | Paper receipts, printing ink, food scraps |
| Plastic bags, wraps, garment bags | ||||
| Type 5 | PP | Yes | Polypropylene | Pb, Cu, acid, grease, dirt |
| Refrigerated containers, bottle tops, some carpets, food wraps, battery cases | ||||
| Type 6 | PS | Yes, but not common | Through away utensils, meat packing, protective packing | |
| Type 7 | Some | Others | ||
| Layered or mixed plastic |
5.1. Management of Waste Plastic
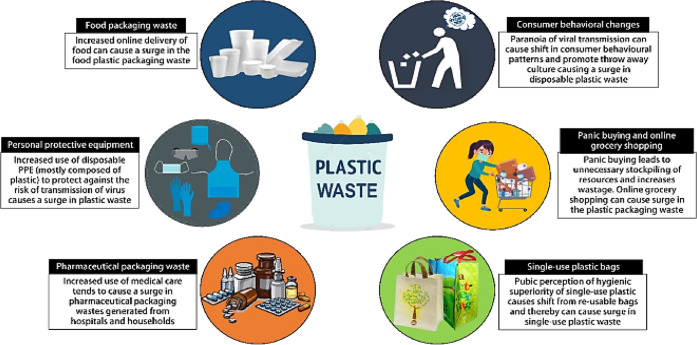
According to a survey, more than 40% of produced plastic is utilized as single use packaging materials, including food containers, baby bottles, and materials for medical applications. Due to excessive utilization of plastic products, the plastic particles can be easily detected in air, rain, soil, snow, tap/bottled water, salt, tea, beer, and food. In addition, an ongoing pandemic condition due to COVID-19 has made the plastic waste management process more complicated (Figure 3). The transmission fear and global administrative regulations have led to the elevated consumption of personal protective equipment, personal hygiene products, single use bags, and containers and have resulted in the overall increase in plastic waste. Furthermore, national lockdowns, as well as home quarantine orders, have motivated the augmented reliance on online distribution of food and some other essential grocery items, which has persuaded a plausible rise in the generation of plastic packaging waste. Although this paradigm underscores the worth of plastics at the public level, it also underlines our vulnerabilities toward pollution.42,43
Figure 3.
Accumulation of plastic waste through different sources during COVID 19. Reproduced with permission from ref (40). Copyright 2021 Elsevier.
Management of waste plastics is an important process to effectively dispose of plastics or to manufacture new products. Some of the important techniques for the management of waste plastics are given in Figure 4 and Table 4.28
Figure 4.
Conventional and new technologies for waste plastic management.
Table 4. Pros and Cons of Different Solid Waste Management Techniques.
| Sr. No | Solid waste management technique | Advantages | Disadvantages |
|---|---|---|---|
| 1 | Landfilling | (i) Cheap | (i) Production of toxic chemicals |
| (ii) Deal with a large volume of waste | (ii) Occupy large volume | ||
| (iii) Reduced transportation distance | (iii) Leeching of heavy metal to ground, soil pollution | ||
| (iv) Applicable to nonrecyclable waste | (iv) Deforestation | ||
| 2 | Incineration | (i) Reduction of waste volume | (i) Costly |
| (ii) Devastation of combustible toxins | (ii) High energy input | ||
| (iii) Energy recovery | (iii) Cause air pollution | ||
| (iv) Minimum area of land occupied | (iv) Not applicable to all types of materials | ||
| 3 | Pyrolysis | (i) Ideal for waste plastic management | (i) Technology in the evolution process |
| (ii) Reduced greenhouse gas emission | (ii) Relatively cheap | ||
| (iii) Procession of the large variety of feedstock | (iii) Relatively expensive compared to landfilling | ||
| (iv) High energy recovery | (iv) Thermoplastic effectively treated |
5.1.1. Landfilling
The most conventional strategy employed for waste management in most countries is landfilling. The addition of additives such as antioxidants and stabilizers supports the waste plastic polymers discarded in landfills to slow down the plastic biodegradation process for a long time. Thus, landfilling seems not to be an appropriate methodology for waste plastic management, and its utilization should be limited due to (i) the long-term firmness of plastics, (ii) their slow kinetics for biodegradation, (iii) limited available sites for landfilling, (iv) complete utilization of starting materials, and (v) health issues arising due to groundwater contamination by leeching and inclusion of toxic chemicals as well as their tendency to leach from landfill sites to groundwater.29−31
5.1.2. Incineration
Waste plastics’ incineration is responsible for the excessive release of distinct matter along with harmful gases. The partial combustion of polypropylene (PP), polyethylene (PE), and polystyrene (PS) during thermal treatment may cause high concentrations of CO (carbon monoxide) and noxious substances to be discharged as combustion of polyethylene (both LDPE and HDPE) at different temperatures releases volatile organic compounds (VOCs) and semi-VOCs, particularly paraffin, olefins, aldehydes, and short-chain hydrocarbons. Benzene, among VOCs, is a known carcinogen and is released during plastic combustion, while PVC engenders carbon black, dioxins, and aromatic compounds such as chrysene and pyrene. Moreover, hazardous materials’ emissions may comprise color pigments and halides that encompass heavy metals such as copper, chromium, cobalt, lead, selenium, and cadmium. Hence, incineration is also not considered to be a good approach for plastic waste management. Plastics also contain certain additives such as plasticizers, antioxidants, fire retardants, light and thermal stabilizers, pigments, lubricants, and antistatic agents depending on the plastic type, its application, and its service circumstance. Research has revealed that most of these additives tend to mimic, interfere with, or block the hormonal system of the endocrine system and are termed endocrine disordering chemicals (EDCs). Persistent organic pollutants (POPs), also known as “forever chemicals”, is the term used for many of them. Mostly flame retardants, such as polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDs), hexachlorobenzene, hexabromobiphenyls (HBBs), short-chain chlorinated paraffin (SCCP), hexabromocyclododecane (HBCD), and fluorinated tensides such as perfluorooctanoic acid (PFOA), all are included in this class. These organic compounds show resistance toward environmental degradation via biological, chemical, and photolytic processes.44
5.1.3. Mechanical Recycling
As compared to the incineration and landfilling processes, the better technique for waste management is waste plastics’ recycling.34 Generally, recycled polymers are more economical than new plastic, but actually, the price variation depends on the category of polymer and the polymer quantity in the plastic in the range of 20–100%. The recycling process is divided into four main classes: primary, secondary, tertiary, and quaternary recycling.14,45−47
Mechanical recycling is subdivided into two types: upcycling and downcycling of waste plastic.
5.1.3.1. Downcycling of Plastic Waste
The downcycling technique is employed for plastics inferior in quality and functionality as compared to the original plastic. However, contrary to other materials such as metal and glass, recycled plastics are unable to persistently perform the same function after recycling, as usually plastics lose certain qualities, such as optical clarity, mechanical integrity, and other inherent features, which tends to make them unfit for their preliminary applications. The physical approaches which are part of this process include collection, isolation, washing, cleaning, desiccation, chipping/sizing, filling, dyeing, accumulation, and pelletization, respectively. The deterioration of recycled products and change in the properties owing to the decrease in molecular weight after successive cycles due to chain scissions are the foremost constraints of this process. So, this process is most appropriate for the recycling of thermoplastics such as PET, LDPE, and HDPE because they can be remelted and then turned into novel products. On the contrary, materials such as thermosetting plastics, hybrids, and high-melting-point viscous polymers are not fit for downcycling.48,49
5.1.3.2. Upcycling of Plastic Waste
Upcycling, also termed creative reuse, is the progression of waste plastic materials’ transformation into fresh materials/products of better quality for artistic value/environmental value, for instance, upcycling of plastic bottles into flower pots, garden sprinklers, bird feeders, green parking canopies, Christmas trees, chandeliers, kid’s toys, and much more. Chemical processing is the method of upcycling waste single as well as mixed plastics. The facile and economical nature of the process is the main advantage of this process. However, the limitation of this process is that multiple solid waste plastics cannot be recycled due to the presence of impurities. Moreover, an increased content of waste plastics in the feed mixture leads to the declining quality of the output product. To enhance the efficacy of the process, mandatory steps need to be performed, such as (a) the segregation of wastes based on particular resins and colors, followed by thorough washing and (b) the re-extrusion of plastic in the form of pellets due to good melting properties and mixing with the original resin to get better quality products. Plastic bottles manufactured from a blend of recycled PET and new PET are an example of this process.50−52
5.1.4. Pyrolysis
Pyrolysis is the process of thermal degradation (tertiary recycling) of solid waste plastic at the temperature range 300–900 °C in a nonoxygenated environment to produce gaseous fuel and liquid. Plastics are polymeric substances made up of monomers, and pyrolysis is a process of transformation of used and discarded plastics into valuable products in the form of fuel, monomers, and other beneficial resources. Pyrolysis is a comparatively advantageous technique of waste management as compared to other conventional approaches in the following ways: (a) Most of the waste plastics downcycled during recycling produce articles of the lower process and produce poor quality products as plastic losing its salient features of clarity, flexibility, and strength during multiple cycling is avoided in pyrolysis. (b) The high cost is associated with sorting, blending, and washing before waste plastics’ mechanical recycling is completely excluded in the pyrolysis technique. Additionally, (c) mechanical recycling comprises the melting and remolding of discarded or used plastics into fresh articles. So, only thermoplastic waste articles can be effectively utilized in this technique. On the contrary, in the pyrolysis process, both thermoplastic and thermosetting plastics are utilized as feedstock. Moreover, (d) the pyrolysis technique is highly proficient toward the treatment of novel material composites, particularly in the evolving technology to replace traditional materials with polymeric composites in the of majority applications. In conclusion, depending on the composition of the feedstock, the reaction conditions such as temperature, reacting gas, heating rate, and catalytic process (in the presence of a catalyst) or thermal pyrolysis (in the absence of a catalyst), final product, yield, and composition can be varied.53−55
5.2. Classification of Pyrolysis Based on Heating Rates
The pyrolysis process can be classified into multiple categories depending on the operating conditions, such as pyrolytic temperature, volatile matter residence time, and heating rate. The three primary types of pyrolysis based on heating rate are fast pyrolysis, slow pyrolysis, and flash pyrolysis (Figure 5).56
Figure 5.
Different types of pyrolysis along with controlling factors and obtained products. Reproduced with permission from ref (56). Copyright 2021 Elsevier.
5.2.1. Slow Pyrolysis (Nonisothermal)
Slow pyrolysis is the process of slow heating (heating rate of 10 °C/s) of the precursors in a nonoxygenated environment. Instead of complete combustion, the volatile parts from the organic material are evaporated partly, and a char (product) remains behind and consists of 80% carbon. Carbonization is another term used for slow pyrolysis accompanied by char as the main product as compared to the liquid product in fast pyrolysis.
5.2.2. Fast Pyrolysis (Isothermal)
Fast pyrolysis comprises the rapid heating (heating rate is 100 °C/s) of the feedstock at moderate temperatures (400–600 °C) for a short residence time to get a huge amount of liquid fuel during pyrolysis in a reactor operated isothermally. It is the most conventional technique utilized for plastic waste pyrolysis in research and practical work.
5.2.3. Ultrafast/Flash Pyrolysis
Ultrafast or flash pyrolysis is a tremendously prompt thermal decomposition technique for pyrolysis accompanied by a high heating rate, and the final products are bio-oil and gases. Heating rates range from 100 to 10,000 °C/s along with short-term residence time.
5.3. Other Classifications of Pyrolysis
5.3.1. Thermal Pyrolysis Technique
Thermal pyrolysis or cracking, also called thermochemical treatment (TCT), is the process of depolymerization/cracking of the resources of the plastic through thermal treatment in the range 350–900 °C in the presence of a low oxygen concentration or the absence of oxygen. Because of cleavage of chains and bonds as well as breakage of intramolecular and intermolecular forces, mostly the required elevated temperature is up to 900 °C. The products are obtained in all three states of matter: solid (char), liquid fuel, and gases. The liquid fuel is generally extracted from the condensable segment of the volatile product and is usually a blend of olefins, paraffin, isoparaffins, and aromatic fractions such as naphthalene, while the residual noncondensable fraction is a gas with a high calorific value.33
5.3.2. Microwave-Assisted Pyrolysis Technique
Microwave pyrolysis, termed microwave-assisted pyrolysis, is the pyrolysis technique that comprises microwave dielectric heating. During pyrolysis, the interaction of microwaves with diverse materials occurs in three different ways, (i) transmitted through perfect insulators, (ii) reflected by conductors, or (iii) absorbed and decomposed on the way inside the targeted materials based on their dielectric feature, and the heat is engendered inside dielectric materials as a result of molecules’ agitation by applying alternating electromagnetic fields. In the case of plastic materials, the mechanism of microwave pyrolysis depends on the magnitude of the microwave energy absorbed by the absorbent and the subsequent thermal heat transferred to the plastic by conduction. Moreover, the uniform distribution of heat depends on the physical properties as well as the absorbent volume ratio, and the different powers of the microwaves completely control the different product distributions.
It has been observed that as compared to catalytic and thermal pyrolysis, the main advantage of microwave-induced pyrolysis is to get desired chemicals and fuels with great ease from plastic waste.42 The literature proves the microwave pyrolysis of the majority of single plastic types such as polyethylene (PE), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC), and poly(ethylene terephthalate) (PET) in the presence of microwave absorbent.57
Undri et al. in 2014 pyrolyzed PP and HDPE by using carbon and tire as the microwave absorbers. The HDPE complete decomposition was achieved by the interaction of plastic with the microwaves of power 6 kW, and 37 wt % was the yield of liquid product, while in the case of PP, a low-density liquid product was obtained at any reaction condition because inferior and complete degradation of material was achieved after an exposure time of 50 min. In 2012, Hussain et al. pyrolyzed the polystyrene foam waste material by interacting it with radiation of frequency 2450 MHz by using a domestic oven. The high temperature (≈ melting point of aluminum) was attained by interacting the microwaves with the closely positioned aluminum coil. The product obtained consists of liquid fuel (88 wt %), gases (9–10 wt %), along with residual char. The enactment of microwave-assisted pyrolysis of HDPE and toothpaste tubing (aluminum/polymer laminates) was studied in a semibatch apparatus by providing a mixed feedstock during the experiment. The pyrolysis temperature was in the range 500–700 °C, and the uncontaminated aluminum was obtained from the toothpaste tubing along with hydrocarbons. The same products with equal molecular weight distribution were obtained from the pyrolysis of HDPE and toothpaste tubes. The main products (81–93%) were linear hydrocarbons, alkanes, alkenes, and dialkenes; the remaining was a complex blend of cyclic, branched aliphatic, and aromatic compounds.58,59
5.3.3. Catalytic Pyrolysis Technique
Catalytic pyrolysis involves the degradation of the polymeric material through thermal treatment in the presence of a catalyst and the absence of oxygen. The primary function of a catalyst is to obtain the desired product with a specific composition by less consumption of energy and in a lower processing time. The most commonly utilized catalysts for plastic waste product pyrolysis described in the literature consist of alumina, silica, zeolites, clay, fluid catalyst converter, and mobile classification of materials (MCM-41). Higher conversion efficiency is usually attained with zeolitic catalysts as compared to nonzeolitic catalysts owing to their greater acid strength. It was recommended that moderate acidity and large pore size of the silica–alumina catalyst loaded in the upper layer favorably catalyze the conversion of polyethylene into liquid hydrocarbons. Recently, many researchers implemented clay as a catalyst for the pyrolysis of plastic. Catalytic cracking of PE over clay catalysts and its comparison with an ultrastable zeolite were examined by Manos et al., and they stated that at the temperature 600 K, the zeolite was found to be more stable than clay and to completely decompose the polyethylene. The liquid products obtained over clay catalysts were found to be heavier due to the milder acidity of clay.57,60−62
5.3.4. Factors Affecting the Pyrolysis Process and Product of Pyrolysis
The operational factors which typically affect the process to be carried out include pressure, temperature, residence time, type of reactor, nature of the feedstock, and experimental conditions. It is indispensable to ponder the limits of the experiment process and to recognize the controlling factors of the process, such as feedstock and other essential factors that can influence the process as optimized parameters and result in the maximum yields of byproducts in pyrolysis. Thus, by regulating and amending the process parameters (Figure 6), the anticipated end products can effectively be produced.63
Figure 6.
Different parameters affecting the pyrolysis product.
5.3.4.1. Influence of Plastic Types
In pyrolysis, the nature of the feedstock material and its purity influence the product distribution and can limit the process for a requisite time for conversion and to get the desired products.
LPDE and HDPE both are examples of polyethylene plastics but possess different amounts of branching as well as cross-linking, as LDPE, with a high magnitude of branching and cross-linking, restricts the polymer chains’ close packing and leads to a plastic product with low density. Pyrolysis of both these thermoplastics produces an oil/wax or gas product, and the hydrocarbon composition depends on the polymer’s original structure. For example, PE is thermally decomposed by polymeric chain random scission to produce a broad range of hydrocarbon chains that become stable and generate a series of n-alkanes (C1–C60) as well alkenes and alkadienes at lower concentrations. Polypropylene, a type of polyalkene plastic, identically produces a mixture of alkanes, alkadienes, and alkenes. At a low temperature of ∼500 °C, an oil/wax is obtained with a high concentration of an aliphatic product rather than aromatic fragments, while at high temperature, the aromatic content tends to increase rather than the aliphatic part.
Polystyrene (PS), an aromatic polymer, on thermal cracking produces an aromatic nature product with a maximum amount of styrene (∼50–79 wt %) along with a dimer and trimer of styrene accompanied by other aromatic compounds, such as xylene, toluene, and alkylated benzenes.
Polyvinyl chloride (PVC) polymer has a chainlike structure, and the expected product is aliphatic. However, dechlorination and removal of HCl from the polymer chain at ∼300 °C lead to C=C bonds which through cyclization give aromatic compounds. Desorbed HCl is absorbed by additives of the process such as FeOOH or Fe3O4.
Polyethylene terephthalate (PET) comprises aromatic, linear, and oxygenated hydrocarbon groups, which on thermal scission of the polymer during thermal degradation produce benzoic acid and terephthalic acid along with gaseous products such as CO and CO2.64,65
The substantial amounts of hydrochloric acid and benzoic acid produced during the pyrolysis of PVC and PET are corrosive and toxic to the reactors. So, both of these polymers are typically omitted from the pyrolysis process.53
5.3.4.2. Influence of Catalyst
Among the distinctive catalysts for the plastic pyrolysis process, e.g., HZSM-5, FCC, MCM-41, mordenite, zeolites, and amorphous alumina-silica, the acidic zeolites are the most studied catalysts. Zeolite catalysts prove to be efficient catalysts for cracking, aromatization, and isomerization due to their definite physicochemical properties, such as microporous crystalline structure and strong acidity. The catalyzed pyrolysis of plastic products, particularly in the presence of HZSM5, produces more aromatic hydrocarbons than the uncatalyzed pyrolysis process. Furthermore, enhanced cracking of polymers in the presence of a zeolite catalyst typically produces gases while amorphous silica–alumina pyrolysis catalysts are responsible for the production of light olefins, without altering the formation of aromatic compounds. ZSM-5 promotes the development of both branched and aromatics hydrocarbons, along with a substantial amount of gaseous hydrocarbons. Catalytic reforming of plastic over Al-MCM-41 actively plays its role in gasoline production due to the feebler acid properties along with the larger pore size of the catalyst. In the presence of both Y-zeolite and, ZSM-5 catalyst decreased the oil yield dramatically decreases in favor of gas production. As compared to the ZSM-5 catalysts, Y-zeolite favors the production of more and more aromatic compounds due to distinctive physical and chemical catalytic properties, such as surface area, pore size, and surface acidity.66−69
5.3.4.3. Influence of Temperature
Temperature is the major controlling factor of the pyrolysis process irrespective of the nature of the feedstock. The extent of bond scission and, hence, the proportion of oil and gas produced depends mainly on the pyrolysis temperature, where higher temperatures lead to higher levels of bond scission and, hence, more C1–C4 gases. In the process of plastic waste pyrolysis, high temperature results in a greater concentration of gaseous fragments due to enhanced cracking reactions accompanied by decreased oil/wax yield. The yield of propylene and ethylene tends to increase with the increase in temperature. Temperature not only affects the yields but also affects the quality of the product, by altering the kinetic mechanism of the pyrolysis process. The general observation is that high temperature leads to the formation of more oily compounds due to the transformation of large size paraffin/olefins into shorter molecules along with less yield of solid residue.70
The importance of catalysts for product distribution and liquid yield becomes less substantial when the temperature is increased, as the reaction becomes identical to the thermal degradation process. Principally, the catalytic pyrolysis proceeds at a much lower temperature with a higher cracking rate than thermal pyrolysis under identical reaction conditions.71,72 For different plastics, it has been observed that HDPE degradation started above 325 °C followed by complete degradation at temperatures ≥467 °C. Rapid heating also accelerates the degradation process and consequently leads to faster kinetics. On the other hand, in the case of LDPE, the degradation starts at 360 °C. In 2010, Jung et al. reported that the PP degradation at a temperature underneath 400 °C is less than that of PE. However, PS degradation starts at the lowermost temperature among commonly studied plastics, as it starts to degrade around 300 °C, even lower than the preliminary decomposition of PET. So, the conclusion is that induced breakage of carbon chains is mainly controlled by the temperature condition of pyrolysis to a large extent. Furthermore, in the case of low-density polyethylene, the pyrolytic reaction in a fluidized bed at 700 °C with long-term vapor residence time mainly produces an oil/wax (28.6 wt %) with a high concentration of benzene, toluene, xylene, and polycyclic aromatic compounds. In addition, a high temperature of 740 °C and pyrolysis product recycling lead to a higher concentration of aromatic compounds.70,73
5.3.4.4. Influence of Vapor Residence Time
Another factor along with temperature which significantly affects the composition and yield of the process of pyrolysis is the vapor’s residence time in a reactor. A literature survey revealed that the different waste plastics’ flash pyrolyses in micro- and macroscale reactors designate that a short-term vapor residence time produces a high-olefin-content gas. The controlled residence time is a big challenge for reactor designers. Milne, and colleagues invented and utilized a fenestrated centrifugal riser terminator in a conventional fluid bed reactor. This terminator tends to separate solid products from gases in less than 20 min with ≈99.5% separation efficiency. Such quick isolation is critically very important when the main objective is to rapidly quench the reaction and to carry it out at high temperatures with reduced vapor residence time.74 Al-Salem et al. in 2009 thoroughly studied the impact of residence time and temperature on the degradation of PS and LDPE. It was witnessed that secondary reactions proceed at long residence time accompanied by the conversion of oil products into gas and char. In the case of LDPE, 91.1 wt % oil product along with 8.70 wt % gas was obtained at zero residence time at 450 °C while oil production was reduced to 61 wt % along with 28.5 wt % gaseous fractions when the residence time was increased to 120 min. On the other hand, in the case of PS, it was completely degraded to liquid products and a minimum amount of char and gas even at zero vapor residence time.27
5.3.4.5. Effect of a Flow Rate of Nitrogen Gas
Nitrogen, being an inert gas, does not show so much interference in the pyrolysis process carried out inside the reactor, but it augments the transfer of the volatiles’ fraction into the condenser and leads to the gases provision for condensation into liquid fuel. According to the literature, the nitrogen flow rate of 10 mL/s is mostly applied for the majority of the plastic pyrolysis process.75
5.3.4.6. Effect of Polymer to Catalyst Ratio
According to the literature, another important factor significantly affecting the composition and yield of the pyrolysis process is the polymer to catalyst ratio. The survey concludes that the conversion efficiency is not directly proportional to the catalyst amount. The conversion tendency is enhanced up to a certain limit with the increase of the catalyst amount, and after that, no appreciable change is observed with an increase of the catalyst amount. Akpanudoh et al. analyzed the relationship of polymer to catalyst with the polymer degradation process in the presence of highly stable Y-zeolite catalysts. After an initial increase in the conversion process, a decline was observed with the further upsurge in catalyst amount, and the maximum conversion efficiency was obtained with 7% of pure catalyst.76
5.3.4.7. Effect of Pressure
Mostly the pyrolysis processes are carried out at atmospheric pressure, so the impact of pressure needs to be fully understood to fulfill a major research gap of pyrolysis studies. Murata et al. (2004) studied the HDPE pyrolysis process in the pressure range 0.1–0.8 MPa in a continuous stirred tank reactor and observed that with the increase of pressure, the gas production increases from 6 to 13 wt % at 410 °C while at 440 °C the gas production was enhanced from 4 to 6 wt %. The study concludes that at low temperature, the influence of pressure is significant and it tends to diminish with an increase of temperature. It was also described that the gaseous product’s average molecular weight decreases with the upsurge of operating pressure. Moreover, it was further observed that the kinetics of double bond formation reduces at high pressure because of the relationship of C–C bond breaking with the double bond establishment. Thermal degradation of plastic is due to two types of C–C bond scission occurring simultaneously: (a) random chain scission, where facile product formation occurs due to reduction in the molecular weight of the polymer (the rate of this pressure-independent process is proportional to the number of C–C links) and (b) chain-end scission, where reactor content dissipation gives volatile products (pressure-dependent chain-end scission takes place at a liquid/gas interface and produces volatile products).77
5.3.4.8. Type of Reactor
Until now, multiple reactors have been designed for the conversion of a variety of plastic wastes into three types of products, solids, liquids, and gases, as the yields of particular products depend on the reactor design as well as the operating parameters. Several methods are proposed for the categorization of the process and apparatus designs. Currently conical spouted bed, batch, semibatch, fixed bed, rotary kiln, and fluidized bed reactors are available reactors for the pyrolytic reaction.78
6. Conversion of Waste Plastic to Carbon-Based Compounds
Waste plastic can be converted into a variety of products, among which the most valued product is carbon. For carbon-containing value-added products, a rich source of carbon is waste plastic because of its high carbon content.79,80 The transformation of solid waste plastic into carbon-based materials was first reported in 2004, by Parra colleagues. During this study, a series of activated carbons were obtained by PET pyrolysis under an inert atmosphere and then were successively activated with CO2 to prepare materials with high-H2-adsorption tendencies. Since 2004, numerous reported data reveal the production of many other carbon-based materials from plastics, such as carbon microspheres, carbon nanotubes (CNTs), carbon nanofibers (CNFs), 2D graphene-based materials, fullerene graphite, and composite materials.81
The reason for the growing interest in carbon materials is their exclusive properties, such as high surface area, electronic conductivity, porosity, rich and flexible surface chemistry, and stable structure at high temperatures. Consequently, carbon-based materials are increasingly employed in different fields, such as catalysis, biomedical, environmental, energy, analytical, and electronic applications. Moreover, the latest invention of consumer products revealed that at present carbon-based materials are the second most commonly used class of nanomaterials, being exceeded only by metal nanoparticles.82−86
6.1. Synthesis Schemes to Obtain Carbon-Based Materials from Waste Plastic
This category of carbon-based materials consists of activated carbon (AC), graphite, graphene, fullerene, and carbon nanotubes (CNTs). Among them, carbon nanotubes (CNTs) are the material of interest for researchers due to their distinctive characteristics and their potential use in a wide range of applications. CNTs are physically as well as chemically stable materials with extraordinary electrical conductivity along with tensile strength greater than 100 times that of stainless steel. Carbon nanotubes are extensively utilized in composite materials as conductive fillers or to enhance the composite strength, as conductive coatings and paints, in transistors for semiconductors and microelectronics, in energy storage, in biosensors for environmental applications, and in medical devices.87−90
6.1.1. Catalytic Pyrolysis
The main techniques employed for CNTs’ synthesis include arc discharge, laser ablation, and the CVD method (Table 5). However, to fabricate CNTs from solid waste plastic, different approaches are utilized. For example, polyethylene is used as a carbon source to synthesize carbon nanotubes through a two-step pyrolysis process in the presence of nickel plate catalysts retained inside a quartz tube reactor maintained at moderate temperature. Defect-rich CNTs of 10–20 nm in diameter accompanied by carbon nanofibers were obtained through this process. The advantages of this technique are the low temperature and energy requirements with the controlled composition of the product, while the high cost of the catalyst and low yield of the product are the main disadvantages of this approach.91
Table 5. Comparison of Carbon-Based Materials’ Synthesis Approachesa.
| Synthetic approach | Advantages | Disadvantages |
|---|---|---|
| Catalytic pyrolysis | (i) Low temperature and energy requirement | (i) High cost of catalyst |
| (ii) Controlled composition of the product | (ii) Low yield of the product | |
| CVD | (i) High growth rate | (i) Complicated process |
| (ii) Good yield of product | (ii) high-temperature requirement | |
| (iii) Better option for the development of the epitaxial thin film | ||
| Pyrolysis deposition CVD | (i) Simple and facile | (i) Structure destruction during the template etching technique |
| (ii) Reuse of plastic with a lower amount of solvent | ||
| (iii) Ordered porous structure | ||
| Thermal decomposition | (i) Material with controlled pore size, high surface area, and conductivity | (i) High temperature results in large pore volume, low energy density, and conductivity |
| Activation method | (i) One-pot process with low-temperature requirement | (i) Low product purity |
| (ii) Ordered structure with good porosity and high surface area | (ii) Large volume of water required for washing purpose | |
| Template-based method | (i) Highly ordered structure | (i) Structure collapse or disorder |
| (ii) Pore blockage during etching | ||
| Hydrothermal carbonization | (i) Simple approach to get valuable materials with oxygen functionalities | (i) High cost and undeveloped porosity |
| One-pot synthesis | (i) Simple, affordable, and reproducible nature of the process | (i) Polymer amount and the nature of the reactor |
| Thermochemical conversion | (i) Fast conversion rate | (i) High temperature |
| (ii) High surface area | (ii) Uncontrolled morphology | |
| (iii) Environmentally benign product by complete conversion of organic parts into carbon-based materials | ||
| Stepwise cross-linking | (i) Favorable technique for the fabrication of a thermally stable cross-linked structure | (i) Partial deterioration of structure upon heating |
| (ii) Moderate porosity |
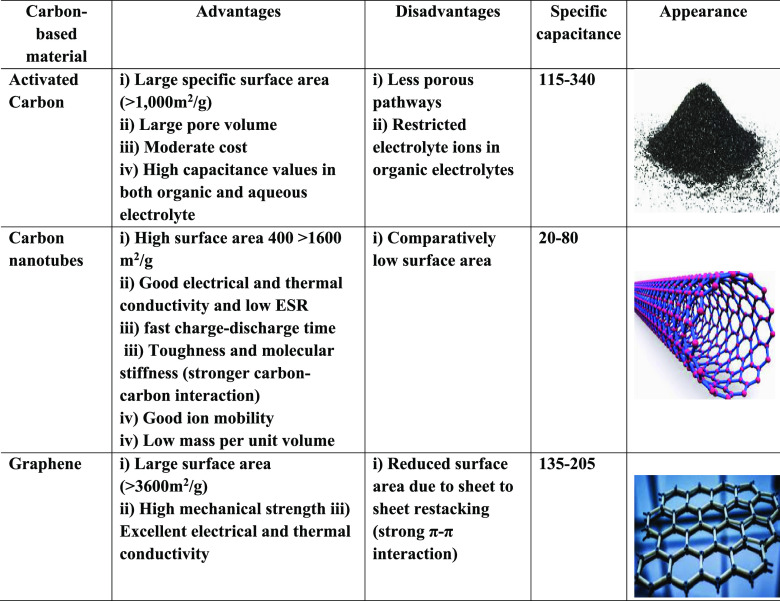
Here is a comparison of different techniques utilized for the synthesis of carbonaceous materials such as activated carbon, graphene, and carbon nanotubes.
6.1.2. Batch Pyrolysis–Catalysis
A joint, fixed bed, batch pyrolysis–catalysis reactor system is another option to get CNTs by catalytic pyrolysis of waste polypropylene (PP) in the presence of nickel catalyst at a temperature above 600 °C. Multiwalled carbon nanotubes (MWCNTs) of diameters 10–25 nm along with lengths around 100 μm are easy to prepare by this approach.92
6.1.3. CVD Method
CNTs can also be synthesized by the combination of the pyrolysis process with the chemical vapor deposition method. The overall process of plastic waste conversion into CNTs consists of two consecutive stages. The first stage consists of the conversion of waste plastic into volatile vapors in a nonoxygenated environment at a moderate temperature. In the second stage, these vapors at high pressure (1 MPa) and temperature in the presence of Ni-based catalyst are converted into CNTs through the chemical vapor deposition technique. The positive points of CVD are the high growth rate, good yield of product, and better option for the development of the epitaxial thin film. However, a complicated process with a high-temperature requirement is the negative point of this technique.93
Furthermore, single layer graphene was prepared via mixed plastic waste packaging material enclosing PS and PE. The hydrocarbon-rich gas produced in the furnace at 500 °C is directed toward a CVD system working under atmospheric pressure on a copper foil at 1020 °C in an Ar/H2 atmosphere. The PMMA film-coated monolayer graphene conveyed to a SiO2/Si substrate develops hexagonal-shaped crystals.94
6.1.4. Pyrolysis–Deposition Followed by CVD
Another way to obtain mesoporous carbon is to combine the pyrolysis–deposition approach with the CVD technique. In situ pyrolysis of polystyrene provides waste polystyrene as a rich carbon source in the presence of Fe(NO3)3 catalyst and a hard template of SBA-15. A mixture of Fe-modified SBA-15 and PS was then pyrolyzed in a tube furnace. The small organic gases generated during pyrolysis were absorbed and deposited into Fe-SBA-15 mesochannels by capillary action and then changed to carbon–silica composites. Later on, the etching of Fe and silica produced ordered mesoporic carbons (OMCs) with morphology identical to SBA-15. This method is highly recommended, as it is simple, is facile, uses less solvent, and reuses plastic waste to obtain an ordered structure with high porosity. However, the destruction of a structure during template etching restrains this technique.95
6.1.5. Thermal Decomposition
A facile, reproducible, and economical thermal decomposition approach was used to prepare graphene from PET obtained from waste bottles. In this process, raw PET was torn and screened to get the appropriate size particles in the range 1–3 mm with the help of a traditional sieve shaker. After that, waste PET was transferred to a stainless steel autoclave and then pyrolyzed in a tube furnace at 800 °C for 1 h. The product was then cooled down overnight to obtain graphene. This technique is recommended when the requirement is to obtain material with controlled pore size, high surface area, and high conductivity. However, at high temperature, large pore volume leads to low energy density and conductivity, which restrict its application at high-temperature conditions.96
6.1.6. Activation Methods
Activation is deliberated as a method for turning industrial raw materials into active materials. The process of pyrolysis is commonly utilized to turn industrial waste into carbon-based materials at high temperatures in the absence/less amount of oxygen. The activation method is also employed for the synthesis of carbonaceous materials, and it consists of pyrolysis followed by activation of material. Furthermore, a one-pot process with a low-temperature requirement and an ordered structure with good porosity and high surface area are the salient features of this process, but the drawback of this process is that product purity is low and a large volume of water is required for washing purposes.97
6.1.6.1. Physical Activation Method
Physical activation usually takes place right away from the step of pyrolysis carried out at elevated temperatures of about 1200 °C in the presence of air/steam/carbon dioxide or a mixture of them. The air, CO2, and steam act as activating agents during the above-mentioned process, and the most commonly available activating agent utilized for the conversion process is the steam due to its low cost and no further treatment being required for the elimination of useless products. During the activation process, the steam is generally amalgamated with pyrolysis in one pot, and the oxygen-functionalities-rich surface produced during the reaction enhances the wettability and specific capacitance of the manufactured carbonaceous materials.
The process of physical activation consists of two steps: (a) the first step is the heating of the carbon source in an inert environment by heating between 400 and 900 °C to eliminate bulky volatile materials; (b) the second step is the partial gasification step employing an oxidizing agent in the temperature range 350–1000 °C. The active oxygen groups in the oxidizing agents burn away the side products of the pyrolysis process entrenched in the pores and consequently lead to the opening of clogged pores. Moreover, the microporous structure is obtained when the more reactive areas of the carbon framework are burned away and produce CO and CO2. The magnitude of annealing depends on the activation temperature and used gas.98−100
6.1.6.2. Chemical Activation Method
In the one-pot chemical activation process, an activating agent, such as a strong base (KOH, NaOH), an acid (H3PO4, H2SO4), or a salt (K2CO3, ZnCl2, FeCl3), is implanted into the pores of carbon precursor before pyrolysis and then heated in the temperature range 450–900 °C. It is a laboratory-level process and provides good control of the pore size distribution. On the contrary, the chemical activation processes’ downsides are that plenty of water is needed to flush away the impurities produced during the activation step and polluted water management. The most commonly utilized alkali for the industrial waste materials’ activation is KOH due to its short activation time, low-temperature requirement, high yield, and distinct micropore size distribution, as well as the huge specific surface area of the resultant porous carbon structure with excellent charge storage capacity. The most important controlling factors for the KOH activation process include (i) the mass ratio of carbon precursor/KOH, (ii) the rate of heating (3–10 °C/min), (iii) the activation temperature, and (iv) the activation time (1–4 h). Time is the main factor, as excessive activation leads to large pore volume, which is responsible for decreased conductivity, low energy density, and loss of power capability of the activated carbon. So a magnificently tailored microporous structure and good surface chemistry of prepared porous carbons via the KOH activation process are decisive to fabricate high-performance supercapacitors. Extensive efforts have been made by researchers for the synthesis of tailored microstructured carbon materials for energy storage applications. For example, the conclusions of deep analysis of the study of KOH activation of carbonaceous materials are that (i) the high temperature and greater carbon/KOH ratio lead to activated carbon with large micropores, (ii) the micropore integration and presence of small mesopores in the carbon nanostructured framework intensify the porosity and interconnected porous networks with original textural properties, and (iii) the pore size, carbon structure, and surface functionalities are among the crucial factors which control the performance of a supercapacitor, as normalized capacitance drops with the rise of micropore size. Furthermore, heteroatom incorporation through oxygen-/nitrogen-rich precursors can proliferate the specific capacitance via the pseudocapacitance effect.101−103
Activated carbon can be produced from different types of plastics, and its properties depend on the precursors and synthetic scheme. A good precursor is that which contains high carbon content and low concentration of inorganic matter content, as in the case of PET, which is available in the pure state. The AC is produced from PET by the eminent process of physical and chemical activation. The process consists of two steps: (i) carbonization in the N2 environment and (ii) activation by air, CO2, or steam and then impregnation with H3PO4, ZnCl2, or KOH.104,105
6.1.7. Template-Based Method
Different templates, such as soft templates, hard templates, or the combination of both templates, are utilized for the preparation of an activated carbon-based material. Moreover, zeolites, porous sacrificial structure controlling agents, are also functional materials for the conversion of industrial waste into carbonaceous materials for supercapacitor applications. Desired and highly ordered structures can be obtained by this technique. However, the limitation of this process is the structure collapse or disorder and pore blockage during etching of a template or in the case of a soft template.
6.1.7.1. Hard Template-Based Method
The hard template method, as the name indicates, uses hard particles such as polymer colloids and SiO2 as a sacrificial stencil to develop a macroporous structure. Usually, the voids of colloidal particles in the templates are filled by fluid-like carbon raw materials that pierce into the templates and then turn to a solid. Afterward, in pyrolysis treatment, the solid fillers are converted into a solid carbon framework under an inert environment, and then the sacrificial templates surrounding the air holes left in the initial positions of the solid particles are excluded by the pyrolysis treatment or by chemical etching. A subsequent KOH activation process produces a hierarchically porous carbon structure, and a combination of the heteroatom-rich compound with a precursor develops a heteroatom-doped ordered porous carbon structure.106
6.1.7.2. Soft Template-Based Method
The soft template method is an attractive approach due to its appealing benefits, such as the ability to produce carbon-based materials of different morphologies under less harsh experimental settings. Moreover, the heteroatom-rich compound can also be utilized for the synthesis of the doped ordered porous carbonaceous material for application as a supercapacitor electrode. To prepare the hierarchical porous carbon-based materials accompanied by mesopores and macropores to get good electrochemical performances in supercapacitors, a better option is to mix both the soft and hard template methods. Generally, the triblock copolymer micelles and hard particles are used as mesoporous and macroporous templates, and during the preparation, the cavities of the hard templates are occupied by the soft templates. The macropore-based ordered porous carbon supercapacitor electrode shows excellent performance due to facilitated ion transport and electrolyte access and enhances the loading and dispersal of electroactive materials.
6.1.7.3. Template-Free Method
Till now, extensive work has been done by researchers to get porous activated carbon by coupling carbon precursors with innumerable templates for applications in supercapacitors. However, it is also possible to prepare porous hierarchical activated carbon materials without introducing any templates. The main advantages of template-free methods are their ease, short times, and affordable prices as compared to template-based methods.107
6.1.8. Hydrothermal Carbonization Method
The hydrothermal carbonization approach produces hydro char, a moderately carbonized product, which possesses a high degree of oxygen functionalities and a low degree of condensation. The hydro char accompanied by porosity and tunable surface functionalities is a tempting source of carbon yield. The hydrothermally synthesized hydro char possesses low porosity as well as insufficient specific surface area. Consequently, to improve the physical and chemical properties of as-synthesized materials, activation/carbonization is required. Moreover, the porous carbon materials with highly ordered structures obtained from hydro char possess a large number of heteroatoms. The different carbon-based electrode materials with surface functionalities may foster the capacitive behavior in both aqueous and organic electrolytes while the electrochemically active functional groups augment the carbon electrode wettability and enhance the specific capacitance by fully utilizing accessible ions and surface groups. It is a highly efficient simple approach to obtain valuable materials with oxygen functionalities. However, high costs and undeveloped porosity limit the application of this technique.107−110
6.1.9. One-Pot Synthesis
Among the different processes utilized for the conversion of waste plastics to CNTs, one-pot synthesis of carbon nanotubes starts from solid polymers mixed with catalysts followed by heat treatment to catalytically decompose the plastics through pyrolysis. The liquid or gaseous product obtained by decomposition serves as a carbon source for the development of CNTs on the surface of the catalyst. Polymers such as polypropylene (PP), polyethylene (PE), polystyrene (PS), polyvinyl chloride (PVC), poly(vinyl alcohol) (PVA), polyethylene terephthalate (PET), polytetrafluoroethylene (PTFE), polycarbosilane (PCS), and phenol-formaldehyde (PF) are extensively studied through this method, and various catalysts are utilized, such as transition metals either in elemental form (iron, nickel, etc.) or in the form of chemical compounds (nickel oxides, ferrous chloride, ferrocene, cobalt acetate, etc.). The main source of heat is either electric furnaces (autoclaves, fixed beds, fluidized beds) or fuel combustion. The simple, affordable, and reproducible nature of the process is its salient feature, but different factors, such as the amount of polymer and the nature of the reactor, also matter.92,111−114
6.1.10. Thermochemical Conversion
The thermochemical transformation of polymer solid wastes into activated carbon with good adsorption tendency as well as energy is an auspicious way of conversion. Polymeric wastes are very significant raw materials to produce economically activated carbon materials. Polymeric waste in the form of polyolefin wax (POW) with average molecular mass 1100 and melting point around 115 °C when heated in the air at 360 °C decomposes to low-molecular-weight products, which slowly evaporate. During the synthesis of activated carbon, 200 g of POW is heated up to 115 °C (melting point) and then 98% H2SO4 is added dropwise with continuous stirring followed by heating up to 160 °C. The obtained solid product after washing and drying at 150 °C is carbonized at 600 °C. The carbonized product of POW was then subjected to the process of water steam activation for 60 min at 800 °C. The obtained solid product with a high surface area due to porosity is termed POW-AC. Faster conversion rate, high surface area, environmentally benign nature, and complete conversion of organic parts into carbon-based materials are the pros of this process, while high temperature and uncontrolled morphology are the cons of this technique.115
6.1.11. Stepwise Cross-linking
A novel strategy of stepwise cross-linking is used to prepare porous nitrogen-doped porous carbon (NPC) through the controlled pyrolysis of polyethylene terephthalate by using a mixture of melamine with the molten salt NaCl/ZnCl2. During the conversion process, a robust cross carbon framework is attained by elimination of weak bonds of the cross-linked structure via NaCl/ZnCl2. The resultant material possesses a large surface area of 1173 m2/g along with rich nitrogen content, without any activation process, and shows a good tendency toward CO2 adsorption and generation of solar-driven steam. This green technique is highly favorable for the fabrication of a thermally stable cross-linked structure. However, partial deterioration of structure upon heating and consequent moderate porosity restricts its application.116
7. Different Applications of Carbon-Based Materials
Figure 7 not only represents the current trends and research track in effective utilization of a wide variety of carbon-based nanomaterials (CNMs) but also specifies the important role of solid waste management in futuristic progress and innovations. The high carbon content of solid waste materials is an attraction seeking factor that encourages the scientific community to transform these waste materials into diverse CNMs such as activated carbon, graphene, CQDs, and CNTs. Furthermore, in the past few years, scientists have paid attention to doping (metal oxides, conducting polymers, organic reagents) and functionalization of these materials to make them more effective. The solid reasons for extensive utilization of CNMs in different areas of science and technology are their excellent properties and surface modifications to get the specific properties, which make them effective in different fields, such as supercapacitor electrode materials, batteries, solar cells, catalyst supports for proton-exchange membrane fuel cells (PEMFC), drug delivery, bioimaging, aerospace, medicinal biology, and the latest weapon technology.117−137
Figure 7.

Multiple applications of solid waste-derived carbon-based materials. Reproduced with permission from ref (137). Copyright the authors 2021 open access.
7.1. Applications of Carbon-Based Materials in Energy Storage Devices
The most promising way of obtaining carbon precursors is plastic wastes, due to their high carbon content, abundance, and low cost. The percentages of carbon content in the different available plastics are as follows: polypropylene (86%), polyethylene (86%), polyvinyl chloride (PVC) and polystyrene (92%), low-density polyethylene (LDPE), and polyacrylonitrile (PANI) (68%) as well as polyethylene terephthalate (≈63%). Among these sources, the cheapest source of high carbon content is polyethylene in plastic bags. Carbon-based materials synthesized from waste plastic by different techniques are efficiently utilized for sensors, biomedical applications, energy conversion processes, and energy storage devices such as supercapacitors and batteries.
It is a well-known fact that carbon-based nanomaterials are the most commonly used electrode materials in electrochemical capacitors, due to their high surface area, diversity of structures, good electrical conductivity, and highly porous textures. Other salient features of nickel cobalt manganese (NCMs) are high thermal and chemical stability, controlled surface chemistry and morphology, and wide operating voltage window. Moreover, on the commercial level, their nontoxic economical nature and easy availability make them a potential candidate for the electrode material in supercapacitors.138
The utilization of waste plastic in Li-ion batteries results in drastically reduced priced Li-ion batteries and enlarges their utilization in a wide variety of buses and electric cars. The reduced battery price makes it feasible to effectively utilize them in large-scale wind and solar power plants. Plastic products such as polyethylene plastic (shopping bags), with a useful life of a few minutes and a non-biodegradable nature, can be effectively employed as the anode material in Li-ion batteries by the pyrolysis of sulfonated plastic. During carbonization, high-temperature treatment results in cross-linked sulfonated PE at a temperature around 500 °C, and then the plastic is decomposed to carbon chips. The finely ground carbon chips are effectively utilized as the anode material in the lithium-ion battery as it is composed of 80% carbon derived from polyethylene.139
7.1.1. Supercapacitors, Their Types, and Different Parameters to Express the Efficiency of the Supercapacitor
Among the innumerable unconventional electric-power-derived devices, such as batteries, supercapacitors (SCs), and fuel cells, which are being utilized in various fields, e.g., hybrid cars, portable electronic devices, and electric mass transit-based vehicles, etc., SCs have caught the attention of researchers due to their exceptional electrochemical performances in terms of high specific power, excellent cyclic stability, and a prompt charge–discharge process.140,141 SCs are a kind of capacitor that are also recognized as ultracapacitors, electrical double layer capacitors (EDLCs), electrochemical capacitors (ECs), or pseudocapacitors. The main components of a SC configuration include the current collector, electrolyte, and electrodes with a huge specific surface area (SSA), responsible for the 1000 times greater capacitance than conventional capacitors.142−145 The energy storage process based on the charge–discharge mechanism is identical in both capacitors and supercapacitors. However, the capacity of charge storage in supercapacitors is in the range 100–1000 F in a device possessing low equivalent series resistance accompanied by good energy and power density. SCs also show more safety, device stability, a low level of heating, and a flexible and lightweight nature The specific energy (energy density) determines how long the energy storage device can be utilized, and the specific power (power density) signifies how rapidly a device can deliver the energy.146−148
Based on the charge storage principle, supercapacitors are divided into three main types, such as (i) EDLCs, that store the charge produced in a non-Faradaic reaction through a double layer, (ii) pseudocapacitors (PCs), that store the charge produced through a Faradaic reaction (redox reaction) at various potentials, and (iii) asymmetric supercapacitors (ASSCs), where the charge storage mechanism depends on both Faradaic as well as non-Faradaic reactions.149,150 The different parameters used to assess the practical applications of supercapacitor include energy density, power density, voltage window, time, impedance, and cell capacitance. The energy density represents the tendency of a material to store energy for a long time, and the power density represents the joint effect of the energy density and device efficacy to deliver the energy from the device, while capacitance is the material’s tendency to collect energy and then store it as an electrical charge on the surface of the conductor at a different potential. The controlling factors for both energy and power density are the electrolyte stability along with the utilization of electroactive material. The major limitation observed in the commercial application of supercapacitors is their inferior energy density value, which can be improved by using (a) the electrode possessing high specific capacitance, (b) electrolytes working in a wide potential range, and (c) an integrated systems optimized structure. Charge–discharge cycle, electrochemical impedance, and cyclic voltammetry are the three different means of electrochemical testing. The different factors affecting the capacitive behavior of supercapacitors comprise the following: (a) the surface area of the electrode directly affects the capacitance of the material; (b) pore structure features, such as the pore size, pore shape, and pore distribution, strongly influence the capacitance of the electrode material by varying the ion and the charge transport process; (c) the electrode conductive nature and the insertion of conducting material with binding properties into the electroactive material effectively elevate its electrochemical response by preventing its volume expansion; (d) an impure electrode or electrolyte results in current leakage followed by self-discharge of the supercapacitor; (e) contrary to organic and aqueous electrolytes, ionic liquids show reluctance toward the redox process; (f) aqueous electrolytes are comparatively more stable than organic and ionic liquids; (g) the capacitor resistance mainly relies on the resistivity of the electrolyte as well as ions of different sizes diffusing toward and out of the electrode; (h) structural defects, such as vacancies, defected surfaces, and basal edges of the electrodes, serve as electroactive sites for ions’ adsorption and consequent enhanced capacitance; (i) as the energy is stored on the electrode surface, a porous nature of the electrode gives outstanding performance; (j) well-defined and preferably crystalline facets intensely enhance the storage tendency of materials.151−157
7.2. Carbon-Based Materials Utilized in Supercapacitors and Different Ways to Improve Their Efficiencies
Various materials, such as carbon-based materials, metal oxides, and conducting polymers, are utilized in different types of supercapacitors. However, among these options, carbon-based materials are the best option for energy storage devices such as supercapacitors, as mentioned in Table 6.158
Table 6. Comparison of the Physicochemical Properties of Materials Used in Supercapacitors (Open Access).
| Physiochemical properties | Carbon-based materials | Metal oxides | Conducting polymers |
|---|---|---|---|
| Non-Faradaic capacitance | Very high | medium | Medium |
| Faradaic capacitance | Low | Very high | Very high |
| Conductivity | Very high | Low | Very high |
| Energy density | Low | High | Medium |
| Power density | High | Low | Medium |
| Cost | Low | High | Low |
| Chemical stability | Very high | Low | High |
| Cycle life | Very high | Medium | Medium |
| Easy fabrication process | Medium | Very low | High |
Among the different carbon-based materials effectively utilized in supercapacitors as electrode material, some commonly employed materials are activated carbon, graphene, quantum dots, and CNTs (Table 7).
Table 7. Comparison of Carbon-Based Compounds Used as the Electrode Material in Supercapacitors.
7.2.1. Activated Carbon
The most conventional carbon-based material used as an electrode in capacitors is activated carbon (AC) because of its large specific surface area (>1,000m2/g), large pore volume, moderate cost, and appropriate electrical properties with high capacitance values in both organic and aqueous electrolytes of 120 F/g and >200 F/g, respectively (range is 115–340 F/g). Activated carbon shows plenty of physicochemical properties based on the carbon precursors used and activation methods that have a strong influence on its surface area, porous structure, and pore size distribution. A promising strategy to improve the material’s capacitance is to increase the specific surface area up to certain limits. Wang et al. reported an increase in capacitance from 17.68 F/g to 171.2 F/g with an increase of specific surface area from 621 m2/g to 2685 m2/g.159,160 ACs synthesized by physical/chemical activation of solid waste (carbon precursors) possess narrow micropores (<0.5 nm) and less porous pathways, which leads to restricted electrolyte ion transport, particularly in organic electrolytes, and results in reduced capacitance. To diminish this problem, carbon-based materials with mesopores (2–50 nm), named well-ordered mesoporous carbons, with improved accessibility of ions are prepared by the soft or hard template approach, and they show remarkable electrochemical activities at high current densities, but their low surface areas harm capacitance. Alternatively, zeolite-templated carbon (ZTC) equipped with intertwined pores and a huge specific surface area (SSA) ∼ 3000 m2/g can be used to increase the capacitance by exhibiting high SSA. Keeping in mind that meso-macropores facilitate the diffusion of ions at high current density and micropores augment the capacitance, the NCMs with well-interconnected micromesoporous structures would be a promising alternate for ECs.
Hierarchically porous graphitic carbons (HPGCs) accompanied with micropores, mesoporous walls, and macropores exhibit a high capacitance value of 220 F/g in 6 M KOH solution along with 2-fold power and energy density values of 10 kW/kg and 5.7 Wh/kg, respectively, as compared to a viable AC studied under the same conditions, as reported by many researchers.161−170 In 2022 Singh et al. reported an electrolyte containing a methyl cellulose-based biopolymer and an ionic liquid (1-ethyl-3-methylimidazolium tricyanomethanide). The resultant electrolyte with a maximum conductivity of 1.93–10–2 S/cm shows a high specific capacitance of 38 F/g at 5 mV/s along with an efficiency of 0.46%.171
The different strategies employed to improve the electrochemical responses of the ACs include the following: (i) modification of the Fermi level position of the ACs via ultrasonic radiation treatment, (ii) AC surface modification by an oxidation process, (iii) incorporation of heteroatoms (S, O, N) in the spongy structure of activated carbon, (iv) development of a composite of AC with other carbon-based nanomaterials (e.g., CNTs, CNF, etc.), and (v) formation of a composite electrode by insertion of polymers in the carbon substrate.172−175
7.2.2. Carbon Nanotubes
Another important carbon-based material extensively studied by researchers is carbon nanotubes which possess high electrical conductivity and diminished resistance in the device. The purity and morphology of the CNTs are the two critical factors that can change the specific capacitance of the material. Based on structural alignment, CNTs are of two types: (a) multiwalled carbon nanotubes (MWCNTs) and (b) single walled carbon nanotubes (SWCNTs). The latter one consists of in-phase cylindrical graphene sheets and out-of-phase sp2 hybridized carbon atoms. Further types of SWCNTs based on conductivity, structural patterns, and chirality are metallic SWCNTs, semimetallic SWCNTs, and semiconducting SWCNTs. The SWCNTs’ codimensional arrangement leads to MWCNTs with a huge difference of diameter compared to SWCNTs. The theoretical SSA reported for SWCNTs is about 1315 m2/g, while for MWCNTs the SSA is smaller than ACs, which leads to a lower energy density for CNT than AC. The electrochemical behavior of the SWCNTs was strongly affected by the electrolyte ionic size and ease of access of the electrode materials while in the MWCNTs the mesoporous structure is responsible for faster movement of ions across the electrode–electrolyte interface.176 The CNT’s stronger carbon–carbon bond is responsible for the toughness and molecular stiffness of the CNTs. However, their comparatively low surface area, which caught up their further progress in this application, can be overcome by chemical/electrochemical treatments or through the synthesis of arrays/spongelike foams with open porous structure, greater than achieved from CNT films, which is responsible for high capacitance.177−179 The specific capacitance of CNTs is in the range 20–80 F/g in both aqueous and organic media. They show enhanced charge storage tendency due to extensive electron delocalization as a result of the conjugated carbon chain. Moreover, a high aspect ratio of CNTs enables them to get entangled to develop a porous structure equipped with a nanotube network. It also develops a mesoporous central canal in the case of MWCNTs, which is responsible for the facile movement of electrolyte ions between the electrode–electrolyte interface during charge–discharge cycles. The CNT electrodes display lower ESR and subsequently higher power density than ACs due to the facile movement of ionic electrolytes as a result of a network-like structure of CNTs. The CNTs and CNT composites with other materials, such as polymer, graphene, MXene, and metal oxides, can be directly employed as electrode material. Moreover, the CNT can also be directly deposited onto the surface of conductive substrates, where CNTs function as a mechanical support and, in turn, facilitate the electron transport and reduce the contact resistance between the electroactive material and the current collectors.176,180−182
7.2.3. Graphene
Graphene is a single layer form of graphite and the thinnest allotrope of carbon with a 2D skeleton with the maximum specific surface area, tremendous thermal and electrical conductivity, exceeding the mechanical strength owing to a C–C σ bond linkage, and high chemical stability and carrier mobility. All these features make graphene a potential candidate for its use in various applications. Graphene is the better option for a supercapacitor electrode material than CNTs and ACs, as in graphene-based electrodes the pore distribution in the solid state is not a crucial factor.128,129 A further advantage is the exposure of both exterior surfaces of graphene toward electrolytes. However, the application of graphene for electronic applications entails the defined functionalization of graphene sheets at the molecular level into various devices. Researchers have formulated various covalent and noncovalent interactions to modify the surface and bulk properties of graphene for energy storage and conversion applications. Particularly, graphene (2-D NCM) is studied as electrode material in electrochemical capacitors.183 This material, with theoretically high SSA (2600 m2/g), huge electrical conductivity, high mechanical strength, and the tendency to develop, is revealed to be a good candidate to fabricate binder-free electrodes, in which the presence of a collector or even an electrical conductivity promoter can be avoided by different architectures. Graphene nanosheets (GNSs) can be effectively utilized as an electrode material for supercapacitors in the form of 2D and 3D structures. However, in the case of the 2D structure of graphene, the layers restack due to the interplanar π–π interactions along with van der Waals forces present between the graphene nanosheets, which results in decreased SSA, restricted ion diffusion, and consequent depressed electrochemical performance. The different reported strategies to resolve this issue comprise (a) template-assisted growth and (b) addition of spacers and crumpling of the graphene layers.184−186 Moreover, this problem can be minimized by using graphene-related materials, primarily reduced graphene oxide (rGO) with a notable energy density of 9.7Wh/kg, although the same value is usually obtained for very thin electrodes and its comparison with other carbon-based materials is not accurate. The specific capacitance shown by graphene and rGO is in the range 135–205 F/g in all types of electrolytes.187−193
7.3. Different Strategies to Improve the Efficiency of Carbon-Based Materials
Besides the inherent nature and properties of carbon-based materials, some other strategies are adopted to improve the capacitance of carbon-based materials, which are described below:
7.3.1. Extent of Carbonization
An important factor affecting the electrochemical properties of carbon-based materials is the degree of carbonization, as carbonization alters the comprehensive resistance, rate capacity, power density, power delivery, cyclic stability, and energy efficiency. Carbonization improves the aqueous electrolytes’ hydrophilicity, which principally improves the diffusion of the ions accompanied by the transport number and’ in turn, augments the capacitive activity of the manufactured devices.123 The low resistivity proposes the sample’s high conductivity is related to a greater extent of carbon activation/carbonization. However, it is observed that at elevated carbonization and graphitization, the structure and pore volume get distorted owing to a lesser specific area. Thus, to optimize the electrochemical performance, there is a need to invent the compromising link between the extent of carbonization and the developed pore structure. High-temperature pyrolysis under an inert environment usually lessens the material’s functional moieties, which limits the exposure of the exact specific surface area of the corresponding material. Carbonization at a high temperature of 1000 °C or above results in outstanding conductivity. So, the prepared carbonaceous materials work as excellent candidates for supercapacitor electrodes, semiconductors, and temperature-resistant electrocatalyst carriers. Typically, the conductivity rises to 1000 °C due to the relative decline in the diameter of denser carbonaceous materials, availability of moving electrons, corresponding increase in the specific surface area, and progressive graphitization with the increase in carbonic weight percentage. Conversely, as the carbonic layers’ favorite alignment with a flat surface escalates significantly, the cavity volume drops efficiently along with a corresponding increase in the crystallite dimension.194−197
7.3.2. Porosity and Surface Framework
Solid waste-derived carbon-based materials with unique structural features, such as spherical tubes, honeycomb lattice, hierarchical spheres, carbon onion, and vertical flower, are prepared through different synthetic approaches while the electrical activity of the supercapacitors is usually enhanced with an increase of the specific surface area of the electroactive material along with some alterations. Besides the surface area, the other factors affecting the electrochemical response of the device are pore volume, pore size, and pore distribution (Table 8). In the fabricated material, the macropores (i.e., size 450 nm) work as a storage site for diverse metallic/nonmetallic ions and mesopores (2–50 nm) provide channels for the charge transfer process while micropores ensure a huge specific surface area and promote the electrical double layer capacitance. So, to achieve optimized performance, the researcher’s prime interest is to synthesize hierarchically porous carbonaceous materials comprising both the micropores and mesopores by modifying the experimental parameters such as the (a) amount of actuating reagent, (b) reaction conditions, and (c) activating reagents.198−202 Thangavel et al. studied the relationship of the electrochemical activity with the designed structure of the carbonic materials, synthesized by using different activating agents such as ZnCl2, KOH, and H3PO4, and described a strong relationship between pore structure modification and activating agents: KOH activated electroactive materials possess large, open, and hierarchical pores, ZnCl2 actived materials’ pores were invisible, and H3PO4 activation results in pores inserted in different cavities.203 Porous carbon materials with different structural frameworks are better described as three-dimensional (3D) networks, two-dimensional (2D) nanosheets, one-dimensional (1D) nanofibers and nanotubes, and zero-dimensional (0D) hollow structure materials, and a direct relationship between the ionic diffusion path, the pore shape, the pore size, and their distribution is observed as a crucial factor for the enrichment of the capacitors’ performance. The porous and hierarchical carbonic network in the form of 0D, 1D, 2D, and 3D structures usually tends to reduce the ionic diffusion distance from electrolyte toward electrodes and eventually lessens the diffusion time.
Table 8. Comparison of the Electrochemical Responses of Different Carbon-Based Materials Derived from Waste Plastics.
| Electrode materials | Plastic source | Electrolyte | Specific capacitance (F/g) | Cycle life (cycles) | Current density | Capacitance retained after cyclic stability test (%) | Ref |
|---|---|---|---|---|---|---|---|
| GNs | Mixed plastic | 1 M H3PO4 | 273.7 | 1.0 A/g | (1) | ||
| PE-HPC-900NH3 | PE | 6 M KOH | 244.0 | 10,000 | 0.2 A/g | 97.1 | (2) |
| PE-HPC-900NH3 | PE | 1 M TEABF4 | 68 | 0.5 A/g | (205) | ||
| PE-HPC-900NH3 | PE | 1 M TEABF4 + EMIMBF4 | 100 | 0.5 A/g | (205) | ||
| G@PE40MC700 | PE | 6 M KOH | 239 | 10,000 | 1.0 A/g | 93.8 | (3) |
| G@PE40MC700 | PE | 1 M EMIMBF4 | 114.0 | 5,000 | 2.0 A/g | 89.3 | (206) |
| HPC-6 | LDPE | 6 M KOH | 355 | 5,000 | 0.2 A/g | 82.4 | (4) |
| PCNS | PET | 6 M KOH | 169 | 5,000 | 0.2 A/g | ≈97.0 | (5) |
| PCNS | PET | 1 M TEATFB/PC | 121 | 5,000 | 0.2 A/g | 78.5 | (209) |
| 4NG | PET | 6 M KOH | 405.0 | 5,000 | 1.0A/g | 87.7 | (6) |
| PCS-MnO2-2 | PET | 6 M KOH | 210.5 | 5,000 | 10 A/g | 90.1 | (7) |
| HPC-4 | PET | 6 M KOH | 413.0 | 0.5 A/g | 77.3 | (8) | |
| PTFE-1:3-700 | PTFE | 6 M KOH | 313.7 | 5,000 | 0.5 A/g | 93.1 | (9) |
| PW-C | PVC | 6 M KOH | 399.0 | 1,000 | 1.0 A/g | 92.0 | (10) |
| PW-C | PVC | 1 M H2SO4 | 363 | 1,000 | 1.0A/g | 92.0 | (215) |
| C-Co | PVC | IL: PVDF-HFP | 294 | 0.004 A | (11) | ||
| Carbon-Mn2 | PVC | 6 M KOH | 751.5 | 5,000 | 1 A/g | 83.1 | (12) |
| 600-NS-DCM | PVDC | 1 M Na2SO4 | 427.0 | 1,000 | 1.0 A/g | 90.0 | (13) |
| C-800-4 | PVDF | 2 M KOH | 252.8 | 5,000 | 5.0 A/g | 96.9 | (14) |
| ACNS-800 | PS | 6 M KOH | 323.0 | 10,000 | 0.5 A/g | 92.6 | (15) |
| ACNS-800 | PS | 1 M EMIMBF4/PC | 10,000 | 81.5 | (218) | ||
| PC | PS | 6 M KOH | 208.0 | 5,000 | 1.0 A/g | 94.3 | (16) |
| PCF-MnO2 | PS | 6 M KOH | 247.0 | 10,000 | 1.0 A/g | 93.4 | (17) |
| PSC-3-700 | PS | 1 M H2SO4 | 327.0 | 10,000 | 1.0 A/g | ≈100.0 | (18) |
| PS-C | PS | 1 M KOH | 482.0 | 5,000 | 1.0 A/g | 95.0 | (19) |
| PCS-3 | PS | 1 M H2SO4 | 135.0 | 10,000 | 1.0 A/g | 92.4 | (20) |
| OMCs | PS | 6 M KOH | 118.0 | 5,000 | 0.2 A/g | 87.2 | (21) |
| ACNS-4 | PP | 6 M KOH | 349.0 | 10,000 | 0.5 A/g | 99.0 | (22) |
| MWCNTs | PP | 1 N KOH | 58.9 | 5 mV/s | (23) | ||
| CMS-3 | PP | 6 M KOH | 328.9 | 10,000 | 1 A/g | 90.4 | (24) |
| PCNSs | Mixed plastic | 6 M KOH | 207.0 | 0.2 A/g | 79.2 | (25) | |
| FMWCNTs-3 | Mixed plastic | 6 M KOH | 33.5 | 0.5 A/g | (26) | ||
| P-E-S3CB5Ni | Mixed plastic | 6 M KOH | 140 | 1,000 | 20 mV/s | 99.2 | (27) |
| GNs | Mixed plastic | PVA + H3PO4 | 38.78 | 0.1 A/g | (28) |
0D Carbon Nanomaterials
Usually, the connected hollow zero-dimensional carbon spheres provide continuous open ionic diffusion and ionic transfer at the triggered surface area and ensure charging/discharging with good chemical activation during the electrochemical testing of the developed devices.
1D Carbon Nanomaterials
One-dimensional carbonaceous materials generally differ from zero-dimensional materials due to a higher aspect ratio, which leads to the relatively increased surface area. The 1D materials most commonly utilized for electrochemical studies include carbon nanofibers and carbon nanotubes, and the most simple and facile approach for the synthesis of 1D materials is electrochemical spinning with a size in the range 1–100 nm. Hollow interconnected nanotubes furnished with good porosity normally consist of 20–50 mm structural length. Inclusively, both the 1D nanotubular structure and nanofibers recommend a new track for faster charge transfer accompanied by minimized ionic diffusion during the process of electrochemical activation.137,204
2D Carbon Nanomaterials
An easy, green, and effective pathway to promote the electrochemical properties of 2D materials is solid waste materials cracking at high temperatures. Different structures provide new options for promising ionic transport and empower the 2D materials to work as excellent materials for energy storage applications. Usually, the layer-like arrangement and their amalgamation with the metal ions are mainly responsible for faster ionic diffusion, which leads to enhanced electrochemical capacitance.205
3D Carbon Nanomaterials
3D porous and ordered materials in supercapacitor devices with excellent capacitance performance, good energy density, and high power density are achieved through good charge accumulation via surface contact between the electrode and electrolyte. Due to the extraordinary conductivity, good thermal, and electrochemical stability, hierarchical carbonaceous nanomaterials are recognized as potential candidates to resolve the issue of low energy density. The hierarchical porous carbon network is essential for faster ionic diffusion and transportation accompanied by the charge-storage process. The mesoporous nanomaterials possess a cylindrical porous surface along with a wormhole-like structure. So, it is assumed that ion transportation takes place via pores in electric double layer capacitors (EDLCs). However, in micropores, due to the small pore size, the electric wire can be developed by the electrolyte ions in the cylindrical capacitor network.137,204
7.3.3. Surface Functionalization by Doping of Heteroatoms
Modification of the carbon framework through surface functionalization by the introduction of different heteroatoms such as O, N, H S, P, B, Si, etc. is an effective strategy to improve the capacitance of the material. Among these heteroatoms, the most commonly used heteroatoms to synthesize the heteroatom-doped NCMs as an electrode in ECs are nitrogen and oxygen. Incorporation of an oxygen-containing functional group is easily carried out by chemical oxidation or reaction with an activating agent, and these groups enhance the conductivity reactivity and wettability of the surface, which ultimately leads to high capacitance in both acidic and basic electrolytes.206−209
Regarding the nitrogen-containing functional groups, they have captured the attention of researchers, as they modify the carbon framework and improve the pseudocapacitance and double layer capacitance, elevate the surface wettability, and increase the stability and electron-donating tendency.210−214 Different nitrogen functional groups, such as pyrrole, quaternary N, and pyridine, can be easily introduced through different methods, such as (i) reaction with nitrogen sources (urea, NH3, NO, etc.), (ii) thermal treatment, (iii) and carbonization of nitrogen-containing compounds such as pyridine, melamine, etc. It is extensively recognized that pseudocapacitance is linked with pyrrolic or pyridinic groups positioned at the graphene layer edges while a quaternary nitrogen positive charge density leads to enhanced capacitance and promotes electron transfer.212,215−217
In 2014, Sun et al. reported an N-doped porous graphitic carbon (NPGCs) with high nitrogen content (7.7 wt %) and a high surface area of 1,027 m2/g. These NPGCs exhibit high capacitance (293 F/g) and greater energy density (8.1 Wh/kg) in 6 M KOH solution. This outstanding performance is specifically attributed to the nitrogen functionalities, which enriched the wettability, electron transfer, and electrical conductivity.218
To study the effect of the nitrogen functionalities, Zhao et al. in 2015 prepared highly ordered N-doped carbon nanocages (hNCNCs), and it was observed that these hNCNCs displayed an extraordinary specific capacitance of 313 F/g along with an energy density value of 10.9 Wh/kg, better than undoped hCNCs in 6 M KOH solution. The better hNCNCs’ activity was linked with N-doping that upgrades the hydrophilicity with consequently increased ion accessibility and capacitance. In conclusion, nitrogen doping augments the capacitance via Faradaic reactions as well as improves the electron transfer and hydrophilicity, which in turn improves the capacitive performance.219
The doping effect of other heteroatoms needs further clarification and research. However, the codoping of some other heteroatoms with oxygen or nitrogen functional groups has revealed the synergistic effect. In 2017, Yan et al. presented that despite their low surface area (<60 m2/g), N, P codoped carbon nanofibers (nonporous) with C–O–P groups and pyrolytic nitrogen exhibit a huge specific capacitance of 224 F/g, due to the synergistic effect where the N-group is responsible for the high pseudocapacitance and phosphorus contributes to the enhanced capacitance by enhancing the wettability. The electrochemical performance of N, P codoped graphene monoliths was tested by Wen et al. in 2016. The synthesized N/P graphene sheets with different concentrations of N and P lead to a stable capacitive response at 1.6 V in acidic electrolyte and show a high energy density response of 11.33 Wh/kg, higher than N-doped graphene, proving the synergistic effect of phosphorus with nitrogen. In conclusion, the inclusion of heteroatoms into the carbon framework is a promising approach to increase the energy density value in electrochemical capacitors. Similarly, the effect of N–S, N–B codoping is also studied by many researchers to improve the material’s capacitance.209,220−222
7.3.4. Composites of Carbon-Based Materials
Another way to improve the capacitance of carbon-based materials is to make their composites with transition metal oxides (MnO2, RuO2, V2O5, etc.), metal hydroxides (Ni/Co(OH)2), and spinal oxides (MnCo2O4 or NiCo2O4), which increases the capacitance through different Faradaic reactions.223,224
In the case of transition metal oxides, RuO2-based materials are considered the best electrode material in capacitors because of their exceptional gravimetric capacitance. The novel work about RuO2-based materials in electrochemical capacitors represents the maximum capacitance value of 720 F/g, and since then RuO2 has been an extensively studied material for electrochemical capacitors. In 2016, Shen et al. reported RuO2 nanodots/graphene electrode tested in an ionic liquid electrolyte and showed a high energy density value of 103Wh/kg by working in a voltage window of 3.8 V.1000 In an alkaline medium, metal oxide NPs on graphene (RuO2/G) were studied by Rakhi et al. in 2011 in a two-electrode system that exhibits an energy density of 50.6 Wh/kg. According to these energy density values, RuO2-based composites are very good potential candidates as electrode materials, but their high price, as well as scarcity, hampers their commercialization. So, at this point, the interesting alternative to RuO2 is MnO2, as MnO2/G composite-based electrochemical capacitors exhibit a high magnitude of energy density, 33.1Wh/kg. Furthermore, RuO2 composites with low-cost metal oxides (such as TiO2, MoO3, VOx, and SnO2) with high surface area and great electrochemical activity have also been projected. Recently, Ni and Co oxides were also evaluated as good electrode materials for electrochemical capacitors because of their pseudocapacitance and extraordinary electrical conductivity that facilitate the capacitive processes. In short, metal oxide-modified carbon-based materials (CMs) can be promising materials for supercapacitor electrodes due to their double layer contributions and Faradaic reactions from the metal oxide united with the currently explained properties of CMs which lead to enhanced electrochemical performance.225−231
Coordination polymers (CPs), polyaniline (PANI), polythiophene (PTh), polypyrrole (PPy), and poly(3,4-ethylene dioxythiophene) (PEDOT) are some commonly used polymers with distinctive properties, favorable for application in electrochemical capacitors. These properties comprise pseudocapacitance originating from redox activity, large electrochemical stability, and high electrical conductivity. However, the foremost drawbacks that hinder their implementation as capacitors are (a) their difficult processability and (b) the electrodes’ poor mechanical stability due to the volume changes throughout the doping/dedoping processes. Keeping in mind all these things, there is a need to amalgamate the CPs with some other materials to produce suitable electrodes for capacitors. So, carbonaceous materials are utilized as interesting supports to lessen the volume changes and to increase stability.232 Among these CPs, PANI and PPy are extensively studied because of their facile synthesis, low cost, and environmental stability. In 2006, Gupta and Miura tested SWCNT/PANI composites as electrode material in the capacitor by procuring the excellent capacitance of 485 F/g while Mini et al. in 2011 fabricated a thin film of PPy on graphene via an electropolymerization process and reported an extraordinary specific capacitance value of 1510 F/g. Moreover, Zhou et al. in 2014 presented capacitors based on aligned CNTs as well as PEDOT/CNTs composites in an organic electrolyte which can deliver an outstanding volumetric energy density value of 82.8Wh/L up to a voltage of 4.0 V.233−235
In conclusion, the amalgamation of NCMs and CPs is a motivating method to escalate the energy density values with the help of the pseudocapacitance contribution from coordination polymers as well as improved mechanical properties which increases the cyclic stability of a material.236,237
An economical practical strategy is implemented for the fabrication of a silicon/carbon nanofibers/carbon (Si/CNF/C) hybrid for Li-ion batteries by utilizing self-prepared micron-sized silicon and high-density polyethylene (HDPE) plastic waste as precursors. The Si/CNF/C hybrid obtained through a simple pyrolysis approach works as a conductive carbon network and confines the silicon volumetric expansion during Li-ion inclusion/exclusion processes and demonstrates extraordinary Coulombic efficiency.238
In 2021, Pandey et al. reported the conversion of waste plastic into graphene nanosheets (GNs) through the upcycling process and their succeeding applications in supercapacitors and dye-sensitized solar cells (DSSCs). Furthermore, bentonite nano clay is used as an activating agent for waste plastics’ degradation via two-step pyrolysis processes at temperatures of 450 and 945 °C in an inert environment of N2 gas to get GNs, and their successful synthesis and the presence of functional groups were ensured through Raman spectroscopy, HRTEM, XRD, EDX, and FTIR analyses. The application of GNs as an active layer-like material for supercapacitor electrodes presents a high specific capacitance value of 398 F/g at a scan rate of 0.005 V/s. The supercapacitor also shows significant energy density and power density values of 38Wh/kg and 1009.7 W/kg, respectively.239
7.4. Polyethylene (PE) Plastic Bag
Over time, the production of ethylene has increased, but simultaneously it has also become harder to recycle. Several years ago, PE was considered a cheap (1 $/kg) carbon source with high carbon content (≈86%), and conversion of waste polyethylene plastic bags into active electrode materials for supercapacitors was a great accomplishment and economic strategy for the green environment and, particularly, the development of renewable energy sources. The most effective electrode material utilized in supercapacitors is porous carbon due to its high surface area, low price, excellent conductivity, and good physical and chemical stability along with its tailorable size combined with the incorporation of heteroatoms into the lattice.
Recently, Lian and colleagues proposed an economical strategy for conversion of waste polyethylene bags into hierarchical and porous activated carbon materials by mixing the polyethylene bag waste with magnesium carbonate pentahydrate (fire retardant) followed by subsequent activation in ammonia to get PE-HPC-900NH3 and to use it in a supercapacitor. The PE-HPC-900NH3 possesses a huge specific surface area (1219 m2/g), large mesopores (1.97 cm3/g), high purity, and a trivial amount of O/N. Furthermore, the PE-HPC-900NH3 presented the specific capacitance value of 244 F/g at 0.2 A/g, cyclic stability ≈ 97.1%, and retention of its original capacitance even after 10,000 cycles at 2 A/g in 6 M KOH solution. Besides, a high energy density value of 14.6 Wh/kg along with a power density of 389 W/kg was attained by the PE-HPC-900NH3-based symmetrical supercapacitor in 1 M TEABF4 electrolyte while the capacitance shown was 68 F/g at 0.5 A/g. To further increase the energy density, the EMIMBF4 (1-ethyl-3-methylimidazolium tetrafluoroborate, ionic liquid) was added to the above-mentioned organic electrolyte and then the capacitance exhibited by the material was 100 F/g at 0.5 A/g along with an energy density of 43 Wh/kg at a high voltage value of 4.0 V.240
Later on, Lian and coauthors developed a green and cost-effective technique and more powerful strategy than a traditional method to prepare a graphene/mesoporous carbon composite (designated as G@PE40-MC700), an electrode material (symmetric supercapacitor) obtained from polyethylene plastic waste amalgamated with a graphene oxide additive and flame retardant through a low-temperature carbonization process at 700 °C without incorporation of any activating agent. The G@PE40-MC700 possesses a high surface area (1175 m2/g), a large number of mesopores (2.3 m3/g), and a specific capacitance of 239 F/g at 2 A/g, along with an energy density of 18.7 Wh/kg at a power density value of 0.75 KW/kg. The total capacitance retained by the material was 93.8% after 10,000 cycles. On the other hand, in the case of 1 M EMIMBF4, the as-reported material exhibits a higher energy density value of 63.3 Wh/kg at a high voltage of 4.0 V and shows an enhanced cyclic stability of 89.3% even after 5000 cycles. Their detailed study exposes that the maximum capacitance of 114 F/g at 1A/g and the high rate capability of G@PE40-MC700 were mainly due to the synergies between mesoporous carbon and graphene which result in high specific surface area, facile ionic diffusion, and greater conductivity.241
7.4.1. Low-Density Polyethylene (LDPE)
Low-density polyethylene (LDPE), one of the extensively used plastics in routine life, is mainly utilized in shopping bags, housewares, food packaging, and agriculture film and is usually thrown out as a solid waste after a short lifespan/after a single use. LDPE, due to the high carbon content of 85.7%, is a promising carbon source. However, traditional thermal and catalytic pyrolysis of LDPE is quite unsuitable because of the complete depolymerization of LDPE at approximately 450 °C, so some suitable methods are required for carbon recovery.
Zhang et al. in 2019 reported a hierarchical porous carbon (HPC) obtained from LDPE via autogenic pressure carbonization assisted by the KOH activation process and employed it as a supercapacitor electrode. During the carbonization step, a high-pressure atmosphere was developed by small molecules generated by the thermal decomposition of LDPE, and these short-chain nonaromatic hydrocarbons in the closed vessel endured further reaction. Through polycondensation and aromatization, the carbon layers in the form of hexagons were developed, which work as a unit cell for carbon materials and change into HPC after KOH activation. The as-synthesized HPCs with hierarchical porous structure and plentiful surface functionalities display a notable capacitive performance by exhibiting a high specific capacitance of 355 F/g at 0.2 A/g, low resistance, and good cyclic stability along with high energy and power density of 9.81 Wh/kg and 450 W/kg, respectively.242
7.4.2. Polyethylene Terephthalate (PET) Waste
Polyethylene terephthalate (PET), consisting of polymerized ethylene terephthalate units, is a synthetic thermoplastic polymer that is extensively utilized for a wide range of applications. In the last few decades, there is a fast-growing increase in PET consumption due to the latest enlargement of the PET bottle industry. Consequently, PET has become an abundant industrial and municipal waste, and its disposal with low photo- and biodegradability represents a thoughtful challenge for industrial countries globally. Different disposal options contain recovery and recycling, coincineration, landfilling, and thermal processes.243
The treatment of PET bottles’ waste accumulated in the environment and ocean is a big challenge. However, the conversion of waste PET into valuable carbon materials and utilization in energy storage devices is attracting the attention of researchers owing to its huge specific surface area, stable physicochemical properties, and high electrical conductivity. A facile approach was established by Wen and colleagues in 2020 to efficiently convert the beverage bottles’ waste PET to PCNS (porous carbon nanosheets) through the processes of catalytic carbonization assisted by KOH activation. The as-prepared PCNS possess an ultrahigh surface area of 2236 m2/g and hierarchically porous structure, along with a large pore volume of 3.0 cm3/g. Such tremendous physicochemical properties conjointly are responsible for the outstanding supercapacitance of 169 F/g in 6 M KOH solution and 135 F/g in 1 M Na2SO4 solution along with a minimum resistance of 11.4 ohm and maximum stability of 90.6% even after 5000 cycles in an aqueous electrolyte. Furthermore, PCNS display a high specific capacitance of 121 F/g along with an energy density of 30.6 Wh/kg at 0.2 A/g in an organic electrolyte (1 M TEATFB/PC) and a high current density value of 10 A/g; the capacitance shown by the material was 95 F/g, reflecting the remarkable rate capability. The high specific surface area, hierarchically porous structure, and large pore volume synergistically contribute to the excellent performance of a material in supercapacitors. The main advantages of this strategy include (i) the carbon precursor from cheaper and abundantly available waste PET beverage bottles, (ii) the facile and easily operated synthetic scheme contributing to mitigated environmental problems, and (iii) the high performance of as-synthesized PCNS in electrical double layer supercapacitors.244
Another strategy was adopted by Elessawy et al. in 2019 to tackle the PET waste by preparing 3D N-doped graphene nanosheets (NGs) through a scalable green simple one-step process. The morphology, crystal structure, and specific surface area as well as the surface functional groups of the synthesized NG were tailored by governing the synthetic conditions such as thermal degradation temperature and urea dose. The nitrogen-doped unique 3D porous architecture provides fluent ion transport via an open porous structure along with a huge surface area and excellent electrical conductivity, maintaining the high specific capacitance, stability, and rate performance. Cyclic voltammetry (CV) and impedance spectroscopy (EIS) measurements exposed that nitrogen fixation affects the morphology and structure of prepared materials and improves ion propagation and ion diffusion. The prepared materials display outstanding performance by showing the specific capacitance of 405 F/g at 1 A/g along with maximum power and energy density values of 558.5 W/kg and 68.1 W h/kg, respectively, in 6 M KOH solution for the optimum sample. Moreover, the prepared NG samples show cyclic stability by retaining 87.7% capacitance even after 5000 cycles at a current density of 4 A/g with long-term charge/discharge cycles.245
Mu and colleagues in 2020 carbonized PET plastic in a single step by using MgO/Co(acac)3 as a catalyst to get a high yield (36.4 wt %) of 3D porous carbon (PC). After uniform incorporation of MnO2 nanoflakes on the carbon nanosheets via redox reaction, the as-synthesized PCS-MnO2-2 composite showed excellent capacitance in a supercapacitor. Due to the appropriate pore size, high surface area, and uniform distribution of MnO2 electroactive sites, the PCS-MnO2-2 composite exhibits a specific capacitance of 210.5 F/g, the areal capacitance of 0.33 Fm–2, and excellent cyclic stability after 5000 cycles at 10 A/g.246
In 2020, the coetching effect of sp2/sp3 hybridized carbon was used by Liu et al. to produce hierarchical porous carbon (HPC) through the KOH activation method after carbonizing the PET waste at 500 °C in a quartz tube. In electrochemical testing in 6 M KOH solution, the as-synthesized HPC with high surface area (2238 m2/g) and small macropores/mesopores (<0.2 cm3/g) possesses a high specific capacitance of 413 F/g, and the symmetric supercapacitor displays a good energy density of 25 Wh/kg.247
7.4.3. Plastic Waste-Based Fluorine and Chloride
Plastic wastes containing halogens, such as fluorine- and chlorine-like polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), and polytetrafluoroethylene (PTFE), are also promising carbon precursors for synthesizing carbon materials for supercapacitors. PVC plastics play a great role in numerous areas, such as healthcare, packaging, and construction materials. After their use, a large amount of PVC wastes is greatly scattered in the environment and causes environmental problems. The landfilling and incineration of PVC wastes are not satisfactory technologies because they release other byproducts such as chlorine, hydrogen chloride, and organochlorine, which are harmful to human life and the environment and hard to tackle.
Chen et al. in 2014 convert halogen-containing plastic materials into nanoporous carbon through the template carbonization method, by using zinc powder as a proficient hard template. The mass ratio between zinc powder and plastics as well as carbonization conditions effectively controls the carbon structures and subsequent electrochemical performances. Among the halogen-containing compounds, the PTFE-1:3-700 sample obtained by the carbonizing of zinc powder and polytetrafluoroethylene powder (mass ratio of 3:1) at 700 °C possesses a large surface area of 800.5 m2/g and a high pore volume of 1.59 cm3/g and delivers a tremendous specific capacitance value of 313.7 F/g at 0.5 A/g. Moreover, it shows superior cyclic stability with a tendency to retain 93.1% capacitance after 5000 cycles.248
In 2018, Chang et al. reported an effective scheme for the green disposal of PVC plastic wastes into valuable carbon materials. The developed method was smoothly carried out at room temperature by simple grinding followed by washing without any hazardous byproducts such as Cl2. Furthermore, the resultant carbonaceous material, when applied as an electrode in a supercapacitor, displayed excellent capacitive performance by exhibiting a specific capacitance of 399 F/g at 1.0 A/g in an alkaline electrolyte and maintained over 92% of the initial capacitance even after 1000 cycles at a current density of 5.0 A/g, while in an acidic electrolyte, the PW-C specific capacitance was dropped to 363 F/g. The merits of high specific capacitance, greater rate capability, long-term cycling stability, and mainly the low costs may enable the profligate implantation of doped carbonaceous materials obtained from PVC plastic wastes.249
Singh and colleagues in 2020 prepared porous carbon from PVC and then cobalt enriched porous carbon material “C–Co” by using a similar precursor. The porous nature of the prepared material was confirmed by BET results, and C–Co material was implemented as an electrode material to fabricate the supercapacitor in the presence of PVDF-HFP (polyvinylidene fluoride cohexafluoropropylene) doped with ionic liquid (1-ethyl-3-methylimidazolium thiocyanate) working as an electrolyte. The value of the specific capacitance calculated for the cobalt enriched carbon cell in the EDLC was 294 F/g at 5 mV/s (42 times greater than the calculated specific capacitance value of pure carbon), and it was cyclically stable until 800 s. The main reason for this outstanding capacitance was (a) the porosity of the cobalt enriched carbon and (b) the wet surface of the sandwiched electrolyte present between two symmetrical electrodes.250
Cheng et al. in 2015 demonstrated a template carbonization technique to convert the waste polyvinyl chloride (PVC) into nanoporous and amorphous carbon by using inexpensive Mg(OH)2 as a hard template and providing a carbonization temperature of 700 °C. The resultant carbon black exhibits a large BET surface area (958.6 m2/g), high pore volume (3.56 cm3/g), and highly ordered pore size distribution. To further increase the electrochemical activity, different amounts of MnOx nanoparticles were integrated into the carbon via direct redox reaction of carbon black with KMnO4 at 70 °C. The optimized carbon-Mn2 sample shows a specific capacitance of 751.5 F/g at 1.0 A/g, minimum resistance, high energy density, and tendency to retain 83.1% of the original capacitance after 5000 cycles.251
Chang et al. in 2018 developed an effective methodology for the ecofriendly conversion of PVC plastic wastes into value-added carbon-based materials. The developed synthetic scheme was simple grinding and washing followed by KOH activation at room temperature without any hazardous side product such as Cl2. Regarding application as an electrode material in a supercapacitor, the PVC wrap transformed carbon (PW-C) exhibits an excellent specific capacitance value of 399 F/g at 1.0 A/g in an alkaline medium and retains more than 92% of the original capacitance after 1000 cycles at a current density of 5.0 A/g, while in acidic media, the displayed capacitance was 363 F/g. The large specific capacitance, stable cycling stability, high rate capability, and low cost may empower the fast implantation of doped carbon-based materials obtained from PVC plastic into energy storage devices.249
In 2017, Chang and colleagues developed a facile synthetic approach to develop multiply doped carbonaceous materials at ambient conditions through in situ doping of polyvinylidene chloride (PVDC) dehalogenation attained carbon with heteroatom sources. N, S doped carbon materials (NS-DCM) were fabricated by dehalogenation of PVDC in the presence of two common solvents, DMSO and DMF, followed by KOH activation. The synthesized NS-DCM was further subjected to annealing to gain a large specific surface area and good conductivity. During electrochemical testing, the resultant 600-NS-DCM exhibited a high specific capacitance of 427 F/g at 1.0 A/g and good cyclic stability with retention of 90% of the capacitance at a current density value of 100.0 A/g in 1.0 M H2SO4 solution after 1000 cycles. Furthermore, in capacitive deionization (CDI) measurements, long-term stability and high efficiency were shown by attaining a desalination capability of 32.3 mg/g in 40.0 mg/L NaCl solution (Figure 8).252
Figure 8.
(a) Conversion process from PET to mesoporous carbon. Reproduced with permission from ref (240). Copyright 2019 Elsevier. (b) Developmental strategy for the graphene/mesoporous carbon composite and its electrochemical response in 6 M KOH solution. Reproduced with permission from ref (242). Copyright 2019 Elsevier. (C) Complete synthetic scheme for the preparation of PCS from PET and its capacitive performance in 6 M KOH solution. Reproduced with permission from ref (246). Copyright the authors 2020, MDPI. (d) Illustration of the synthetic process of HPC-4 from PET and its capacitive response in 6 M KOH solution. Reproduced with permission from ref (247). Copyright 2020 Elsevier. (e) Description of the synthetic scheme of PCF-MnO2 from PS and its electrochemical activity in 6 M KOH solution. Reproduced with permission from ref (256). Copyright 2019 Elsevier. (f) Synthetic process detail and GCD curves along with the cyclic stability test of 600-NS-DCM in different electrolytes, Reproduced with permission from ref (252). Copyright 2017 American Chemical Society.
The nanoporous graphitic carbonaceous material (C-800) was prepared by Cheng et al. in 2015 by using polyvinylidene fluoride (PVDF) waste as a carbon source and Ni(NO3)2·6H2O as a graphitic catalyst. Detailed analysis reveals that the carbonization temperature mainly controls the pore structures and, in turn, affects the electrochemical performances. The research work concluded that with the increase of carbonization temperature from 800 to 1200 °C, the porosity tends to decrease with a corresponding increase in the degree of graphitization. Moreover, the electrochemical performance was improved by the addition of different amounts of unique redox additives of 4-(4-nitrophenylazo)-1-naphthol, termed as NPN, in 2 M KOH solution. Therein, by the addition of 4 M NPN, the specific capacitance was 2.98 times greater than the pristine material.253
7.4.4. Polystyrene (PS)
Polystyrene, currently used as a prevalent packaging/insulating material, is the polymer of styrene molecules (monomer) and has produced severe environmental hitches owing to its disproportionate use and ineffective recycling process. Conversion of PS into functional carbonaceous materials and application in supercapacitors is one of the economic strategies to recycle polystyrene and other types of waste plastics into electroactive materials.
In 2019, polystyrene waste has been converted into mesoporous carbon nanosheets (CNS) through the template method by Ma and colleagues. The further modification of the porous structure of the prepared CNS was done by KOH activation in the presence of a MgO template to develop ordered porous carbon sheets possessing a surface area of 2650 m2/g along with a pore volume of 2.43 cm3/g. The ordered porous carbon sheets also posses multiple unique properties, such as the following: (i) The porous MgO template controls the morphology of the carbonaceous materials and their carbonization yield. (ii) MgO is easily removed from a noncorrosive acid. (iii) The carbon materials have a high yield and purity due to the autogenic high pressure developed in the closed reactor owing to gas release during the decomposition of the polymer. (iv) The tailored pore size and improved surface area due to KOH both contribute to the electrochemical performance of the material. Benefiting from the above unique properties, in a three-electrode setup, the ordered porous carbon sheets exhibit a specific capacitance of 323 F/g at 0.5 A/g in a 6 M KOH solution, a good rate capability of 222 F/g at 20 A/g, and cyclic stability by maintaining 92.6% of capacitance after 10,000 cycles. To work on a broader voltage window and to get high energy and power density, the same material was tested in the organic electrolyte EMIMBF4/PC at 3.0 V. The high energy and power density of 44.1 Wh/kg and 757.1 W/kg, respectively, were shown for the sample along with a tendency to retain 88% and 81.5% capacitance after 5000 and 10,000 cycles, respectively. In summary, the present strategy validates a facile method for the recycling of plastic waste into highly valuable products.254
Zhang et al. in 2018 successfully synthesized a 3D porous carbon architecture via the Friedel–Crafts reaction by consuming waste polystyrene foam (carbon source) and silica particles to generate a rich porous structure. The as-synthesized carbon material possesses a huge specific surface area of 620 m2/g along with an even distribution of mesopores in the bulk phase. Furthermore, the 3D porous structure and high specific surface area result in the outstanding electrochemical capacitance of ≈208 F/g at 1 A/g. Additionally, a superior energy density (22.5 Wh/kg), an excellent power density (1024.4 W/kg), and a superb capacitance retention of 94.3% after 5000 cycles at 5 A/g are other salient features of the tested electrode materials.255
In 2019, Min and colleagues prepared a hybrid electrode material of PCF-MnO2 by the carbonization of polystyrene foam waste via a MgO template. First of all, porous carbon flakes (PCFs) were fabricated by the direct pyrolysis of PS foam in an autoclave by using a flake-like MgO template followed by subsequent deposition of MnO2 nanosheets on the resultant PCFs’ surface to form a hybrid electrode PCF-MnO2. Due to the (i) high specific surface area of 1087 m2/g, (ii) excellent conductivity of the PCFs, (iii) inherent huge specific capacity of MnO2, and (iv) positive synergistic effect between MnO2 and PCF, the resultant hybrid material displayed an ultrahigh capacitance value of 247 F/g at 1 A/g and 308 F/g at 1 mV/s in LiCl electrolyte. In an asymmetric supercapacitor device, a remarkable long-term stability was shown by retaining 93.4% capacitance after 10,000 cycles at a current density value of 10 A/g. In conclusion, the high conductivity (less resistivity) and hierarchical porous structure provide a fast ion diffusion channel along with shorter diffusion distance owing to the connected micro-macro-mesopores and results in remarkable capacitance.256
Liang and colleagues in 2019 reported a facile and effective approach for fabrication of ordered mesoporous carbons (OMCs) through a pyrolytic deposition strategy by using waste PS (carbon precursor) and a Fe-modified SBA-15 template. During the synthetic process, the deposition and pyrolysis proceeded synchronously in the carbonization process and the morphology of fabricated OMCs was completely replicated with the SBA-15 template. The effects of the mass ratio of PS/Fe-SBA-15, amount of Fe(NO3)3, metal catalyst, and pyrolysis temperature on the structural parameters of OMCs were thoroughly investigated. By using the optimized conditions of (a) 5% Fe(NO3)3, (b) Fe-SBA-15/PS mass ratio of 1, and (c) pyrolysis temperature of 800 °C, the as-synthesized OMCs displayed a morphology identical to the SBA-15 template, the huge specific surface area of 1156 m2/g, the maximum pore volume of 1.23 cm3/g, a specific capacitance of 118 Fg–1 at 0.2 A/g, and a tendency to retain 87.2% of the initial capacitance even after 5000 cycles, demonstrating that OMCs have good cyclic stability and rate capability. This relatively simple method avoids the usage of organic solvents and achieves good control of the morphology and structure of synthesized OMCs.95
Deka and colleagues in 2020 synthesized a series of hierarchical N-doped porous carbons derived from expanded polystyrene foam (EPS). The polymeric material was obtained by a simple Friedel-Craft reaction of EPS with cyanuric chloride. The polymeric material carbonization in the presence of KOH (activating agent) was performed at different temperatures followed by characterization by different analytical techniques. The optimized sample, PSC-3-700, with high specific surface area (810 m2/g), appropriate pore sizes, and high content of heteroatoms, proves to be a potential candidate for capacitive deionization and supercapacitive applications. The specific capacitance shown by the sample was 327 F/g in 1 M H2SO4 solution. In a symmetric supercapacitor device the gravimetric specific capacitance shown by PSC-3-700 was 294 F/g along with an energy density of 10.21 Wh/kg at a current density of 0.5 A/g. The material also exhibits approximately 100% retention of capacitance after 10,000 cycles accompanied by an electrosorption tendency of 34.8 mg/g at an applied potential of 1.6 V.257
Sahoo et al. in 2021 described the large-scale production of N-doped porous carbon (PS-C) from polystyrene foam through a thermal plasma approach with a short processing time of 5 min. The as-fabricated PS-C displayed a capacitance of 482 F/g at 1 A/g and a tendency to retain 95% of the specific capacitance at 6 A/g even after 5000 charge–discharge cycles, indicating the stable nature of the material. This reported cyclic performance and specific capacitance were attributed to the nitrogen doping, morphology, and structural integrity originating from the synergies of the material. In the light of the detailed analysis, it was suggested that (a) a hierarchal mesoporous structure made available a broadly exposed surface area, facilitating the electrolytic ion’s infiltration with minimized diffusion pathways and (b) N-dopant in the porous carbon network enhanced the electrical conductivity which boosted the charge carriers’ transport at the electrode and electrolyte interface and consequently led to notable charge storage performance.258
In 2019, porous carbon sheets (CSs) were fabricated from polystyrene through a MgO template tied with the KOH activation process. The effect of the KOH ratio on the surface area, morphology, and pore structure of the synthesized carbon sheets was examined for symmetric supercapacitors. In 1 M H2SO4 solution, KOH activated optimized PCSs (PCS-3) showed excellent electrochemical performance by displaying specific capacitances of 97 and 135 F/g at the current density of 1 A/g and a scan rate of 1 mV/s. At a high current density of 20 A/g, the specific capacitance retained by the sample was 82.9%. Moreover, the energy density value of 3.4 Wh/kg at the corresponding power density value of 250 W/K was attained by the sample in the aqueous electrolyte along with excellent stability by retaining 92.41% of the capacitance after 10,000 charge/discharge cycles.259
7.4.5. Polypropylene (PP)
Polypropylenes (PPEs) are mainly composed of 72% PP polymer, which is a thermoplastic material and is produced by polymerizing propylene molecules (monomer). It is a saturated polymer that consists of a linear chain of hydrocarbon, that is thermally and chemically resistant with a melting point of 160 °C. The tremendous and desirable physical, thermal, and mechanical properties at room temperature explain its wide applications in the plastic industry, storage boxes, furniture, stationery, and car bumpers.56
At present, the transformation of waste plastic into 2D carbon nanosheets (CNS) is regarded as an auspicious way to resolve environmental issues, and it is a rich carbon source. Liu et al. in 2020 reported the fabrication of CNS by the proficient carbonization of waste polypropylene (PP) by using a combined catalyst of sulfur and ferrocene. The carbonization process performed in a sealed container confirmed an ultrahigh yield of carbon (62.8%) in the form of thin CNS (4–4.5 nm), even though a minute amount of catalyst was used. After the activation step, the prepared activated carbon nanosheets (ACNS), by possessing a distinct hierarchical porous structure accompanied by a huge specific surface area (3200 m2/g) and big pore volume (3.71 cm3/g), delivered a huge specific capacitance of 349 F/g at a current density of 0.5 A/g. Moreover, the designed symmetric supercapacitor demonstrated a high energy density value of 23 Wh/kg at a power density value of 225 W/kg. In this work, the ACNS-4 electrode (optimized sample) manifested a good rate capability by showing a capacitance of 262 F/g at 10 A/g, and even at an elevated current density of 20 A/g, the capacitance shown by the ACNS-4-based electrode was 251 F/g with 72% retention of capacitance, which can be ascribed to the fast ion migration rate throughout the electrode. Furthermore, the cyclic stability test performed at 5 A/g showed a 99% capacitance retention even after 10,000 cycles, and EIS results proved the important role of macropores/mesopores in carbon nanosheets that ensures the electrolyte ion flows vertically between the contiguous carbon nanosheets and leads to good rate capability. The good electrochemical activity of the ACNS-4-based device probably stems from the synergistic effects: (a) the large SSA ensure excellent ion storage capacity is responsible for the high capacitance; (b) the 2D nanosheets permit the horizontal movement of electrolyte ions; (iii) the meso-/macropores present in the carbon nanosheets minimize the ion transfer resistance by facilitating the vertical movement of the electrolyte ions across the contiguous carbon sheets; and (iv) better wettability due to oxygen functionalities on the carbon surface leads to exceptional electrochemical performance.260
The multiwall carbon nanotubes (MWCNTs) were prepared at 800 °C from waste polypropylene plastic (WPP) by the chemical vapor deposition (CVD) technique in the presence of a nickel catalyst by Mishra and colleagues in 2013. The WPP derived CNTs’ 1 N KOH solution was observed to be a suitable electrode material in EDLC, as the specific capacitance shown by the sample was 59 F/g at of 5 mV/s for an optimized material, while the specific capacitance shown by CNTs prepared at 700 and 600 °C was 37 and 44 F/g at the same scan rate, respectively. The conclusions of the whole research work were the following:
A direct relationship of the surface area of carbonaceous materials and capacitance was observed. However, significant deviations from the observed trend confirm that some other factors, such as temperature and pore size, are also controlling factors.
It was witnessed that the current response of CNTs fabricated at 700 °C at a higher scan rate was greater than CNTs prepared at 800 and 600 °C, which might be due to the pseudocapacitive behavior of some metal particles in these CNTs.
Capacitor cells and electrodes can be easily prepared by this carbonaceous material without any binder.
With the increase of scan rate, the currents tend to increase but specific capacitance tends to decrease linearly.261
In 2021, Hu and colleagues reported a novel dense hollow fiber and porous carbon electrode material successfully synthesized by sulfonation followed by the carbonization method. The as-prepared carbon electrode exhibited high specific capacitance applied to the supercapacitor. The solid-state capacitor amassed with CMS-3 (electrode) and PVA/KOH (electrolyte) shows a high capacitance value of 52.1 F/g at 1 A/g, along with a good energy density value of 10.4 W h/kg at a value of power density of 600 W/kg, while in the case of 6 M KOH solution, the specific capacitance shown by the same material was 328.9 F/g at 1A/g. After 10,000 cycles the capacitance retained by the sample was 90.4%, which indicates its stable nature, and EIS testing indicates the capacitive nature of the material at low frequency. Due to the synergistic effect of the sulfonic acid groups as well as KOH activation, a dense porous structure was developed on the carbon surface which promotes the ions’ rapid diffusion. Moreover, the unique sulfur doping plus carbonization technology results in an extraordinary carbon yield and consequent good capacitive enactment of the as-prepared carbon electrode.262
7.4.6. Mixed Plastic Waste
At present, sustainable development accompanied by a serious energy crisis needs appropriate management of extensively available industrial and municipal waste plastics and the development of advanced and economical materials for energy storage purposes. However, the waste plastics’ complex nature significantly obstructs the application of conventional methods, and certain attention has been paid to the effective utilization of plastics-derived carbon for energy storage applications. Porous carbon nanosheets (PCNS) were produced and reported by catalytic carbonization of the ‘‘real-world” combined waste plastics on organically modified montmorillonite (OMMT) with subsequent KOH activation. PCNS possess (a) hierarchically micro-/mesoporous structures accompanied by pore sizes of 3.63, 1.42, and 0.57 nm, (b) a large pore volume of 3.026 cm3/g, (c) incompletely exfoliated graphitic layers, and (d) a huge surface area of 2198 m2/g. As a result of these remarkable properties, PCNS displayed a grander performance for supercapacitors by exhibiting high specific capacitances of 120 and 207 F/g at 0.2 A/g in organic and aqueous electrolytes, respectively. Significantly, at a high current density of 10 A/g, the specific capacitances retained by the sample were 95 F/g (79.2%) and 150 F/g (72.5%) in organic and aqueous electrolytes, respectively.263
In 2021, functionalized multiwalled carbon nanotubes (FMWCNTs) were prepared from plastic packaging material by a three-step synthetic process followed by surface functionalization of MWCNTs with concentrated HNO3 for 3–6 h by Abbas and colleagues. Among the synthesized samples, the optimized sample of FMWCNTs-3 possesses the largest integration area due to its wide surface functionalization and facile approach of electrolyte ions toward electrochemically active sites. So, it was determined that the FMWCNT-3-based electrode displays good wettability and conductivity. The specific capacitance shown by FMWCNT-3 was significantly upgraded, and the displayed range was 25–33.5 F/g with maximum retention of capacitance at high current density. On the other hand, the Nyquist plot in the high-frequency domain shows the lowest ohmic resistance while the smallest semicircle in the low-frequency region indicates the lowermost charge transfer resistance at the interface and, in turn, the maximum capacitance.264
Pandey et al. in 2011 reported the conversion of waste plastics into value-added graphene nanosheets (GNs) and through the up-cycling process their subsequent applications in supercapacitors and dye-sensitized solar cells (DSSCs). The waste plastic degradation was carried out in the presence of Bentonite nanoclay by two-step pyrolytic processes at 450 and 945 °C, respectively, in the presence of N2 gas to fabricate the GNs. The formation of few-layer-thick GNs was confirmed by XRD, Raman spectroscopy, and HRTEM analyses. Further, EDX and FT-IR analyses identify the functional groups in GNs. The GNs thus synthesized from plastic waste have been used for the fabrication of DSSCs and supercapacitors. In the case of a supercapacitor, the implementation of GNs as an active electrode layer material offered a maximum specific capacitance of 398 F/g at a scan rate of 0.005 V/s along with a significant power and energy density of 1009.74 W/kg and 38 Wh/kg, respectively. Thus, the process explained the utilization of waste plastics upcycling for maintenance of EEE, i.e., ecology, energy, and economy, for a better tomorrow.239
In 2014, Wen et al. studied the influence of Ni2O3 and nanosized carbon black (CB) as an innovative combined catalyst system for catalytic carbonization of mixed plastic. This combined catalyst was highly efficient to promote the conversion of polymers into a good yield of high-quality CNTs. Catalytic pyrolysis along with model carbonization experiments represented the dependence of the carbonization mechanism on the synergies of the combined catalysts. The mixed plastic was degraded to aromatic compounds, which were then dehydrogenated and converted to CNTs through the catalytic reaction of CB with Ni particles. As an electrode material, the synthesized CNTs (P-E-S3CB5Ni) displayed a high specific capacitance (140 F/g) and long-term stability compared to commercial CB and CNTs due to the relatively greater specific surface areas of synthesized CNTs and their oxygen functionalities.265
In 2020, Karakoti and colleagues reported a facile and benign upcycling route for the conversion of plastic waste to graphene nanosheets (GNs) through a two-step pyrolysis process. To analyze the use of synthesized GNs, their supercapacitive performance was studied by using different current collectors, such as copper tape (CuT), graphite sheet (GS), indium tin oxide glass (ITO), and aluminum sheet (AlS), in the presence of PVA-H3PO4 gel electrolyte. The testing process confirms the high specific capacitance of 38.78 F/g at 0.1 A/g though an AlS current collector, minimum resistance, and maximum charge–discharge response.266
8. Conclusion and Future Perspectives
Plastics are one of the most indispensable materials utilized in our daily life due to their distinctive properties. However, continuous increases in plastic consumption and accumulation pose a serious health and environmental threat. This review article describes the types of commonly used plastics, ways of accumulation, detrimental effects of mishandled plastic wastes on human health and the environment, the need for a green processing practice, conversion of polymeric waste in valuable materials, and their effective utilization in energy storage devices. The development of cost-effective methodologies for the fabrication of carbon-based materials of different morphologies such as zero to three dimensions with high surface area, good conductivity, facile electrolyte diffusion, the inclusion of different functional groups through the incorporation of heteroatoms, controlled carbonization conditions, and composites of carbonaceous materials for better electrochemical response is thoroughly discussed in this review. The electrochemical responses of 3D porous materials and their hybrids with transition metals and metal oxides make potential candidates for energy storage devices because of their facile and sufficient electronic transition. Our prevailing plastic waste management setup and infrastructure are constrained by inefficiencies and inadequacies to deal with excessive waste generation.
-
(1)
There is a need to pay more attention to innovations in current products and technologies to inspire environmental and economic efficiency. Moreover, the incorporation of novel sustainable technologies into the prevailing waste management process would envisage a future where all plastic wastes would be either reprocessed or recycled.
-
(2)
Furthermore, there is a need to further explore the synthesis of different carbon-based materials and their composites by novel and environment-friendly conversion techniques, doping of heteroatoms, metal ions, metal oxides/hydroxides, and polymers to modify the architecture and properties of synthesized materials, in-depth study of the relationship between the physical modification and mechanical bonding of carbon-based materials, and visualization of the doping pattern through computational studies.
-
(3)
The new emergent technology of “microfactories” can transform waste plastics into valued products by utilizing several attributes of waste plastics, such as carbon content, chain structure, binding properties, and mechanical and thermal properties
-
(4)
Pyrolysis, one of the commonly used techniques for the conversion of waste plastics into carbon-based materials (CNTs, graphene, activated carbon, and quantum dots), needs to be carried out under controlled conditions (temperature, pressure, inert gas flow rate, reactor type, etc) to get materials with desired features for an enhanced electrochemical response.
-
(5)
The consumption of local products with tax cuts will help to reduce the accumulation of plastic packaging waste.
-
(6)
For long-term improvement in the efficiency of the plastic waste recycling process, there is a need to formulate policies to reduce multilayer packaging and to promote homogeneous plastic packaging, which is easier to recycle.
Acknowledgments
We want to acknowledge the School of Natural Sciences, NUST, Islamabad, School of Chemical and Materials Engineering, NUST, Islamabad, and U.S. Pakistan Center for Advanced Studies in Energy (USPCAS-E), NUST, Islamabad.
Author Contributions
Conceptualization and data collection, L.Y.; supervision, T.N. and N.I.; original draft preparation, L.Y.; writing reviews and editing, all authors.
The authors declare no competing financial interest.
References
- Balde C. P.; Forti V.; Gray V.; Kuehr R.; Stegmann P.. The global e-waste monitor 2017: Quantities, flows and resources:; United Nations University, International Telecommunication Union, 2017. [Google Scholar]
- Panda A. K.; Singh R. K.; Mishra D. Thermolysis of waste plastics to liquid fuel: A suitable method for plastic waste management and manufacture of value added products-A world prospective. Renew.Sust.EnergyRev. 2010, 14, 233–248. 10.1016/j.rser.2009.07.005. [DOI] [Google Scholar]
- Sharuddin S. D. A.; Abnisa F.; Daud W. M. A. W.; Atoua M. K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manage. 2017, 148, 925–934. 10.1016/j.enconman.2017.06.046. [DOI] [Google Scholar]
- Derraik J. G. The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 2002, 44, 842–852. 10.1016/S0025-326X(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Thompson R. C.; Moore C. J.; Vom Saal F. S.; Swan S. H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2153–2166. 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R.Production, use, and fate of synthetic polymers. Plastic waste and recycling; Elsevier, 2020; pp 13–32. [Google Scholar]
- Miranda M. N.; Silva A. M.; Pereira M. F. R. Microplastics in the environment: A DPSIR analysis with focus on the responses. Sci. Total Environ. 2020, 718, 134968. 10.1016/j.scitotenv.2019.134968. [DOI] [PubMed] [Google Scholar]
- Vieira O.; Ribeiro R. S.; Diaz de Tuesta J. L.; Gomes H. T.; Silva A. M. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chem. Eng. J. 2022, 428, 131399. 10.1016/j.cej.2021.131399. [DOI] [Google Scholar]
- Shahbaz M.; Al-Ansari T.; Inayat M.; Sulaiman S. A.; Parthasarathy P.; McKay G. A critical review on the influence of process parameters in catalytic co-gasification: current performance and challenges for a future prospectus. Renew. Sust. Energy Rev. 2020, 134, 110382. 10.1016/j.rser.2020.110382. [DOI] [Google Scholar]
- Zhao D.; Wang X.; Miller J. B.; Huber G. W. The chemistry and kinetics of polyethylene pyrolysis: a process to produce fuels and chemicals. ChemSusChem. 2020, 13, 1764–1774. 10.1002/cssc.201903434. [DOI] [PubMed] [Google Scholar]
- Tian B.; Li J.; Li Z.; Xu N.; Yao G.; Zhang N. Synergistic lignin construction of a long-chain branched polypropylene and its properties. RSC Adv. 2020, 10, 38120–38127. 10.1039/D0RA06889F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V.; Baurai K.; Sanjay M.; Siengchin S. Mechanical, microstructural, and thermal characterization insights of pyrolyzed carbon black from waste tires reinforced epoxy nanocomposites for coating application. Polym.Compos. 2020, 41, 338–349. 10.1002/pc.25373. [DOI] [Google Scholar]
- Zhuo C.; Levendis Y. A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131, 2346–2389. 10.1002/app.39931. [DOI] [Google Scholar]
- Okan M.; Aydin H. M.; Barsbay M. Current approaches to waste polymer utilization and minimization: a review. J. Chem. Technol. Biotechnol. 2019, 94, 8–21. 10.1002/jctb.5778. [DOI] [Google Scholar]
- Nyakuma B. B.; Ivase T. J. P. Emerging trends in sustainable treatment and valorisation technologies for plastic wastes in Nigeria: A concise review. Environ. Prog. Sustain.Energy 2021, 40, e13660. 10.1002/ep.13660. [DOI] [Google Scholar]
- Amin S.; Amin A. Thermoplastic elastomeric (TPE) materials and their use in outdoor electrical insulation. Rev. Adv. Mater. Sci. 2011, 29, 15–30. [Google Scholar]
- Ribeiro M.; Fiuza A.; Ferreira A.; Dinis M.; Meira Castro A.; Meixedo J.; Alvim M. Recycling approach towards sustainability advance of composite materials’ industry. Recycling 2016, 1, 178–193. 10.3390/recycling1010178. [DOI] [Google Scholar]
- Brazel C. S.; Rosen S. L.. Fundamental principles of polymeric materials, 3rd ed.; John Wiley & Sons, 2012; pp456–460. [Google Scholar]
- Yang Y.; Boom R.; Irion B.; van Heerden D.-J.; Kuiper P.; de Wit H. Recycling of composite materials. Chem. Eng. Process. 2012, 51, 53–68. 10.1016/j.cep.2011.09.007. [DOI] [Google Scholar]
- Grigore M. E. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2017, 2, 24. 10.3390/recycling2040024. [DOI] [Google Scholar]
- Schlosser S.; Nass B.; Wanzke W.. Flame retardant combination for thermoplastic polymers L. US 6547992 B1, 2003.
- Nicholson J.The chemistry of polymers; Royal Society of Chemistry, 2017; pp 345–349. [Google Scholar]
- Yang S.; Brandon A. M.; Xing D.; Yang J.; Pang J.; Criddle C.. Progresses in polystyrene biodegradation and prospects for solutions to plastic waste pollution. IOP Conference Series: Earth and Environmental Science; 2018; p 012005.
- Das S.; Pandey S.. Pyrolysis and catalytic cracking of municipal plastic waste for recovery of gasoline range hydrocarbons. Thesis, 2007, pp 567–580.
- Leonard S.; Barra R.. Plastics and the circular economy. Scientific and Technical Advisory Panel to the Global Environment. 2018.
- Royer S.-J.; Ferron S.; Wilson S. T.; Karl D. M. Production of methane and ethylene from plastic in the environment. PLoS One 2018, 13, 1–13. 10.1371/journal.pone.0200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Salem S.; Lettieri P.; Baeyens J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste management 2009, 29, 2625–2643. 10.1016/j.wasman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Jambeck J. R.; Geyer R.; Wilcox C.; Siegler T. R.; Perryman M.; Andrady A. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Cózar A.; Echevarría F.; González-Gordillo J. I.; Irigoien X.; Úbeda B.; Hernández-León S. Plastic debris in the open ocean. Proc. Acad. Sci. 2014, 111, 10239–10244. 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusher A. L.; Mchugh M.; Thompson R. C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. 10.1016/j.marpolbul.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Lithner D.; Larsson A.; Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Crompton T. R.Additive migration from plastics into foods: a guide for analytical chemists; iSmithers Rapra Publishing, 2007. [Google Scholar]
- Koller M.; Braunegg G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. Euro.Biotechnol. J. 2018, 2, 89–103. 10.2478/ebtj-2018-0013. [DOI] [Google Scholar]
- Galloway T. S.; Lewis C. N. Marine microplastics spell big problems for future generations. Proc. Nat. Acad. Sci. 2016, 113, 2331–2333. 10.1073/pnas.1600715113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C.; Dai L.; Ma L.; Guo R. T. Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 2020, 4, 114–126. 10.1038/s41570-020-0163-6. [DOI] [PubMed] [Google Scholar]
- Sabiha-Javied; Tufail M.; Khalid S. Heavy metal pollution from medical waste incineration at Islamabad and Rawalpindi, Pakistan. Microchem. J. 2008, 90, 77–81. 10.1016/j.microc.2008.03.010. [DOI] [Google Scholar]
- Verma R.; Vinoda K.; Papireddy M.; Gowda A. Toxic pollutants from plastic waste-a review. Procedia Enviro. Sci. 2016, 35, 701–708. 10.1016/j.proenv.2016.07.069. [DOI] [Google Scholar]
- Schug T. T.; Janesick A.; Blumberg B.; Heindel J. J. Endocrine disrupting chemicals and disease susceptibility. J.Steroid. Biochem. Mol.Biol. 2011, 127, 204–215. 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J. C.; da Costa J. P.; Lopes I.; Duarte A. C.; Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Wright S. L.; Kelly F. J. Plastic and human health: a micro issue?. Environ. Sci. Technol. 2017, 51, 6634–6647. 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Gewert B.; Plassmann M. M.; MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci: Processes Impacts 2015, 17, 1513–1521. 10.1039/C5EM00207A. [DOI] [PubMed] [Google Scholar]
- Andreani G.; Cannavacciuolo A.; Menotta S.; Spallucci V.; Fedrizzi G.; Carpene E. Environmental exposure to non-essential trace elements in two bat species from urbanised (Tadarida teniotis) and open land (Miniopterus schreibersii) areas in Italy. Environ. Pollut. 2019, 254, 113034. 10.1016/j.envpol.2019.113034. [DOI] [PubMed] [Google Scholar]
- Vanapalli K. R.; Sharma H. B.; Ranjan V. P.; Samal B.; Bhattacharya J.; Dubey B. K. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ. 2021, 750, 141514. 10.1016/j.scitotenv.2020.141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A.; Iliopoulos N.; Gotsis G.; Fiotakis K. Persistent free radicals, heavy metals and PAHs generated in particulate soot emissions and residue ash from controlled combustion of common types of plastic. J. hazard. Mater. 2008, 156, 277–284. 10.1016/j.jhazmat.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Hopewell J.; Dvorak R.; Kosior E. Plastics recycling: challenges and opportunities. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2115–2126. 10.1098/rstb.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brems A.; Baeyens J.; Dewil R. Recycling and recovery of post-consumer plastic solid waste in a European context. Therm. Sci. 2012, 16, 669–685. 10.2298/TSCI120111121B. [DOI] [Google Scholar]
- Munir D.; Irfan M. F.; Usman M. R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. 10.1016/j.rser.2018.03.034. [DOI] [Google Scholar]
- Patni N.; Shah P.; Agarwal S.; Singhal P. Alternate strategies for conversion of waste plastic to fuels. Int. Sch. Res. Notices 2013, 2013, 1245–1278. 10.1155/2013/902053. [DOI] [Google Scholar]
- Karayıldırım T.; Yanik J.; Ucar S.; Sağlam M.; Yüksel M. Conversion of plastics/HVGO mixtures to fuels by two-step processing. Fuel Process. Technol. 2001, 73, 23–35. 10.1016/S0378-3820(01)00192-8. [DOI] [Google Scholar]
- Miknius L.; Butkute R. Properties of residual marine fuel produced by thermolysis from polypropylene waste. Mater. Sci. 2015, 21, 249–254. 10.5755/j01.ms.21.2.6105. [DOI] [Google Scholar]
- Yu J.; Sun L.; Ma C.; Qiao Y.; Yao H. Thermal degradation of PVC: A review. Waste. Manag. 2016, 48, 300–314. 10.1016/j.wasman.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Rahimi A.; García J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 1–11. 10.1038/s41570-017-0046. [DOI] [Google Scholar]
- Miandad R.; Barakat M.; Aburiazaiza A. S.; Rehan M.; Nizami A. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. 10.1016/j.psep.2016.06.022. [DOI] [Google Scholar]
- Qureshi M. S.; Oasmaa A.; Pihkola H.; Deviatkin I.; Tenhunen A.; Mannila J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis. 2020, 152, 104804. 10.1016/j.jaap.2020.104804. [DOI] [Google Scholar]
- Teuten E. L.; Saquing J. M.; Knappe D. R.; Barlaz M. A.; Jonsson S.; Björn A. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2027–2045. 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harussani M.; Sapuan S.; Rashid U.; Khalina A.; Ilyas R. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. 10.1016/j.scitotenv.2021.149911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze W. U.; Umunakwe R.; Obasi H. C.; Ugbaja M. I.; Uche C. C.; Madufor I. C. Plastics waste management: A review of pyrolysis technology. Clean Technologies and Recycling 2021, 1, 50–69. 10.3934/ctr.2021003. [DOI] [Google Scholar]
- Hussain Z.; Khan K. M.; Perveen S.; Hussain K.; Voelter W. The conversion of waste polystyrene into useful hydrocarbons by microwave-metal interaction pyrolysis. Fuel process. Technol. 2012, 94, 145–150. 10.1016/j.fuproc.2011.10.009. [DOI] [Google Scholar]
- Undri A.; Rosi L.; Frediani M.; Frediani P. Efficient disposal of waste polyolefins through microwave assisted pyrolysis. Fuel. 2014, 116, 662–671. 10.1016/j.fuel.2013.08.037. [DOI] [Google Scholar]
- Panda A. K.; Singh R. Catalytic performances of kaoline and silica alumina in the thermal degradation of polypropylene. J. Fuel Chem. Technol. 2011, 39, 198–202. 10.1016/S1872-5813(11)60017-0. [DOI] [Google Scholar]
- Praveen Kumar K.; Srinivas S. Catalytic co-pyrolysis of biomass and plastics (polypropylene and polystyrene) using spent FCC catalyst. Energy Fuels 2020, 34, 460–473. 10.1021/acs.energyfuels.9b03135. [DOI] [Google Scholar]
- Manos G.; Yusof I. Y.; Papayannakos N.; Gangas N. H. Catalytic cracking of polyethylene over clay catalysts. Comparison with an ultrastable Y zeolite. Ind. Eng. Chem. Res. 2001, 40, 2220–2225. 10.1021/ie001048o. [DOI] [Google Scholar]
- Hidalgo J. P.; Gerasimov N.; Hadden R. M.; Torero J. L.; Welch S. Methodology for estimating pyrolysis rates of charring insulation materials using experimental temperature measurements. J. Build. Eng. 2016, 8, 249–259. 10.1016/j.jobe.2016.09.007. [DOI] [Google Scholar]
- Faussone G. C. Transportation fuel from plastic: Two cases of study. Waste Manag. 2018, 73, 416–423. 10.1016/j.wasman.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Kunwar B.; Cheng H.; Chandrashekaran S. R.; Sharma B. K. Plastics to fuel: a review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. 10.1016/j.rser.2015.10.015. [DOI] [Google Scholar]
- Mastral J.; Berrueco C.; Gea M.; Ceamanos J. Catalytic degradation of high density polyethylene over nanocrystalline HZSM-5 zeolite. Polym. Degrad. Stab. 2006, 91, 3330–3338. 10.1016/j.polymdegradstab.2006.06.009. [DOI] [Google Scholar]
- Marcilla A.; Beltrán M.; Navarro R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. 10.1016/j.apcatb.2008.07.026. [DOI] [Google Scholar]
- Lopez G.; Artetxe M.; Amutio M.; Bilbao J.; Olazar M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. 10.1016/j.rser.2017.01.142. [DOI] [Google Scholar]
- Zhang X.; Lei H.; Yadavalli G.; Zhu L.; Wei Y.; Liu Y. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel. 2015, 144, 33–42. 10.1016/j.fuel.2014.12.013. [DOI] [Google Scholar]
- Jung S. H.; Cho M. H.; Kang B. S.; Kim J. S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. 10.1016/j.fuproc.2009.10.009. [DOI] [Google Scholar]
- Chen D.; Yin L.; Wang H.; He P. Pyrolysis technologies for municipal solid waste: a review. Waste Manage. 2014, 34, 2466–2486. 10.1016/j.wasman.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Achilias D.; Roupakias C.; Megalokonomos P.; Lappas A.; Antonakou E. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazaed. Mater. 2007, 149, 536–542. 10.1016/j.jhazmat.2007.06.076. [DOI] [PubMed] [Google Scholar]
- Chin B. L. F.; Yusup S.; Al Shoaibi A.; Kannan P.; Srinivasakannan C.; Sulaiman S. A. Kinetic studies of co-pyrolysis of rubber seed shell with high density polyethylene. Energy Convers. Manag. 2014, 87, 746–753. 10.1016/j.enconman.2014.07.043. [DOI] [Google Scholar]
- Milne B. J.; Behie L. A.; Berruti F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166. 10.1016/S0165-2370(99)00014-5. [DOI] [Google Scholar]
- Cleetus C.; Thomas S.; Varghese S. Synthesis of petroleum-based fuel from waste plastics and performance analysis in a CI engine. J. Energy 2013, 2013, 125–129. 10.1155/2013/608797. [DOI] [Google Scholar]
- Akpanudoh N. S.; Gobin K.; Manos G. Catalytic degradation of plastic waste to liquid fuel over commercial cracking catalysts: effect of polymer to catalyst ratio/acidity content. J. Mol. Catal. A: Chem. 2005, 235, 67–73. 10.1016/j.molcata.2005.03.009. [DOI] [Google Scholar]
- Lopez G.; Amutio M.; Elordi G.; Artetxe M.; Altzibar H.; Olazar M.. A conical spouted bed reactor for the valorisation of waste tires. The 13th International Conference on Fluidization - New Paradigm in Fluidization Engineering, 2010; Vol. 34, pp 456–460.
- Kassargy C.; Awad S.; Burnens G.; Kahine K.; Tazerout M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. 10.1016/j.jaap.2017.09.005. [DOI] [Google Scholar]
- Ma J.; Liu J. J.; Song; Tang T. Pressurized carbonization of mixed plastics into porous carbon sheets on magnesium oxide. RSC adv. 2018, 8, 2469–2476. 10.1039/C7RA12733B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. S.; Elsamahy T.; Koutra E.; Kornaros M.; El-Sheekh M.; Abdelkarim E. Degradation of conventional plastic wastes in the environment. A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. 10.1016/j.scitotenv.2020.144719. [DOI] [PubMed] [Google Scholar]
- Parra J.; Ania C.; Arenillas A.; Rubiera F.; Palacios J.; Pis J. Textural development and hydrogen adsorption of carbon materials from PET waste. J. Alloys Compod. 2004, 379, 280–289. 10.1016/j.jallcom.2004.02.044. [DOI] [Google Scholar]
- Figueiredo J. L. Functionalization of porous carbons for catalytic applications. J. of Mater.Chem. A 2013, 1, 9351–9364. 10.1039/c3ta10876g. [DOI] [Google Scholar]
- Lalović B.; Đurkić T.; Vukčević M.; Janković-Častvan I.; Kalijadis A.; Laušević Z. Solid-phase extraction of multi-class pharmaceuticals from environmental water samples onto modified multi-walled carbon nanotubes followed by LC-MS/MS. Environ. Sci. Pollut. Res. 2017, 24, 20784–20793. 10.1007/s11356-017-9748-0. [DOI] [PubMed] [Google Scholar]
- Yun Y. S.; Yoon G.; Kang K.; Jin H. J. High-performance supercapacitors based on defect-engineered carbon nanotubes. Carbon 2014, 80, 246–254. 10.1016/j.carbon.2014.08.063. [DOI] [Google Scholar]
- Virji S.; Fowler J. D.; Baker C. O.; Huang J.; Kaner R. B.; Weiller B. H. Polyaniline nanofiber composites with metal salts: chemical sensors for hydrogen sulfide. Small 2005, 1, 624–627. 10.1002/smll.200400155. [DOI] [PubMed] [Google Scholar]
- Agüí L.; Yáñez-Sedeño P.; Pingarrón J. M. Role of carbon nanotubes in electroanalytical chemistry: a review. Anal. Chim. Acta 2008, 622, 11–47. 10.1016/j.aca.2008.05.070. [DOI] [PubMed] [Google Scholar]
- De Volder M. F.; Tawfick S. H.; Baughman R. H.; Hart A. J. Carbon nanotubes: present and future commercial applications. Science 2013, 339, 535–539. 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Dai L.; Chang D. W.; Baek J. B.; Lu W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. 10.1002/smll.201101594. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Sharma K.; Dixit A. R. Carbon nanotube-and graphene-reinforced multiphase polymeric composites: review on their properties and applications. J. Mater. Sci. 2020, 55, 2682–2724. 10.1007/s10853-019-04196-y. [DOI] [Google Scholar]
- Bassyouni M.; Mansi A.; Elgabry A.; Ibrahim B. A.; Kassem O. A.; Alhebeshy R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A: Mater. Sci. Process. 2020, 126, 1–33. 10.1007/s00339-019-3211-7. [DOI] [Google Scholar]
- Jorio A.; Dresselhaus G.; Dresselhaus M. S.. Carbon nanotubes: advanced topics in the synthesis, structure, properties and applications; Springer Science and Business Media, 2007; Vol. 111, pp 223–234. [Google Scholar]
- Mishra N.; Das G.; Ansaldo A.; Genovese A.; Malerba M.; Povia M. Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J. Anal. Appl. Pyrolysis 2012, 94, 91–98. 10.1016/j.jaap.2011.11.012. [DOI] [Google Scholar]
- Wang J.; Shen B.; Lan M.; Kang D.; Wu C. Carbon nanotubes (CNTs) production from catalytic pyrolysis of waste plastics: The influence of catalyst and reaction pressure. Catal. Today 2020, 351, 50–57. 10.1016/j.cattod.2019.01.058. [DOI] [Google Scholar]
- Jung I.; Pelton M.; Piner R.; Dikin D. A.; Stankovich S.; Watcharotone S. Simple approach for high-contrast optical imaging and characterization of graphene-based sheets. Nano Lett. 2007, 7, 3569–3575. 10.1021/nl0714177. [DOI] [Google Scholar]
- Liang K.; Liu L.; Wang W.; Yu Y.; Wang Y.; Zhang L. Conversion of waste plastic into ordered mesoporous carbon for electrochemical applications. J. Mater. Res. 2019, 34, 941–949. 10.1557/jmr.2018.493. [DOI] [Google Scholar]
- El Essawy N. A.; Ali S. M.; Farag H. A.; Konsowa A. H.; Elnouby M.; Hamad H. A. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 2017, 145, 57–68. 10.1016/j.ecoenv.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wei F.; Sui Y.; Qi J.; He Y.; Meng Q. A novel core-shell polyhedron Co3O4/MnCo2O4.5 as electrode materials for supercapacitors. Ceram. Int. 2019, 45, 12558–12562. 10.1016/j.ceramint.2019.03.010. [DOI] [Google Scholar]
- Jiang H.; Lee P. S.; Li C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. 10.1039/C2EE23284G. [DOI] [Google Scholar]
- Sevilla M.; Mokaya R. Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. 10.1039/C3EE43525C. [DOI] [Google Scholar]
- Rajagopal R. R.; Rajarao R.; Cholake S. T.; Sahajwalla V. Sustainable composite panels from non-metallic waste printed circuit boards and automotive plastics. J. Clean. Prod. 2017, 144, 470–481. 10.1016/j.jclepro.2016.12.139. [DOI] [Google Scholar]
- Sun L.; Wang C.; Zhou Y.; Zhang X.; Qiu J. KOH-activated depleted fullerene soot for electrochemical double-layer capacitors. J. Appl. Electrochem. 2014, 44, 309–316. 10.1007/s10800-013-0636-0. [DOI] [Google Scholar]
- Wang J.; Kaskel S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. 10.1039/c2jm34066f. [DOI] [Google Scholar]
- Yan J.; Miao L.; Duan H.; Zhu D.; Lv Y.; Xiong W. Core-shell hierarchical porous carbon spheres with N/O doping for efficient energy storage. Electrochim. Acta 2020, 358, 136899. 10.1016/j.electacta.2020.136899. [DOI] [Google Scholar]
- Adibfar M.; Kaghazchi T.; Asasian N.; Soleimani M. Conversion of poly (ethylene terephthalate) waste into activated carbon: chemical activation and characterization. Chem. Eng. Technol. 2014, 37, 979–986. 10.1002/ceat.201200719. [DOI] [Google Scholar]
- Djahed B.; Shahsavani E.; Khalili Naji F.; Mahvi A. H. A novel and inexpensive method for producing activated carbon from waste polyethylene terephthalate bottles and using it to remove methylene blue dye from aqueous solution. Desalination. Water Treat. 2016, 57, 9871–9880. 10.1080/19443994.2015.1033647. [DOI] [Google Scholar]
- Abudu P.; Wang L.; Xu M.; Jia D.; Wang X.; Jia L. Hierarchical porous carbon materials derived from petroleum pitch for high-performance supercapacitors. Chem. Phys. Lett. 2018, 702, 1–7. 10.1016/j.cplett.2018.04.055. [DOI] [Google Scholar]
- Utetiwabo W.; Yang L.; Tufail M. K.; Zhou L.; Chen R.; Lian Y. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin. Chem. Lett. 2020, 31, 1474–1489. 10.1016/j.cclet.2020.01.003. [DOI] [Google Scholar]
- Jain A.; Balasubramanian R.; Srinivasan M. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. 10.1016/j.cej.2015.08.014. [DOI] [Google Scholar]
- Sevilla M.; Fuertes A. B.; Mokaya R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. 10.1039/c0ee00347f. [DOI] [Google Scholar]
- Gu W.; Sevilla M.; Magasinski A.; Fuertes A. B.; Yushin G. Sulfur-containing activated carbons with greatly reduced content of bottle neck pores for double-layer capacitors: a case study for pseudocapacitance detection. Energy Environ. Sci. 2013, 6, 2465–2476. 10.1039/c3ee41182f. [DOI] [Google Scholar]
- Pol V. G.; Thiyagarajan P. Remediating plastic waste into carbon nanotubes. J. Environ. Monit. 2010, 12, 455–459. 10.1039/B914648B. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Du j.; Qian Y.; Xiong S. Synthesis, characterization and properties of carbon nanotubes microspheres from pyrolysis of polypropylene and maleated polypropylene. Mater. Res. Bull. 2010, 45, 15–20. 10.1016/j.materresbull.2009.09.007. [DOI] [Google Scholar]
- Gong J.; Liu J.; Jiang Z.; Wen X.; Chen X.; Mijowska E. Effect of the added amount of organically-modified montmorillonite on the catalytic carbonization of polypropylene into cup-stacked carbon nanotubes. Chem. Eng. J. 2013, 225, 798–808. 10.1016/j.cej.2013.03.112. [DOI] [Google Scholar]
- Maafa I. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. 10.3390/polym13020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoycheva I. G.; Tsyntsarki B. G.; Petrova B. N.; Budinova T. K.; Petrov N. V. New carbon adsorbent from polymer waste for effective removal of mercury from water. Desalination Water Treat. 2016, 57, 15435–15444. 10.1080/19443994.2015.1073178. [DOI] [Google Scholar]
- Song C.; Zhang B.; Hao L.; Min J.; Liu N.; Niu R. Converting poly (ethylene terephthalate) waste into N-doped porous carbon as CO2 adsorbent and solar steam generator. Green Energy Environ. 2022, 7, 411–422. 10.1016/j.gee.2020.10.002. [DOI] [Google Scholar]
- Luo X.; Yang Q.; Dong Y.; Huang X.; Kong D.; Wang B. Maximizing pore and heteroatom utilization within N, P-co-doped polypyrrole-derived carbon nanotubes for high-performance supercapacitors. J. Mater. Chem. A 2020, 8, 17558–17567. 10.1039/D0TA06238C. [DOI] [Google Scholar]
- Yang Y.; Liu Y. X.; Li Y.; Deng B. W.; Yin B.; Yang M. B. Design of compressible and elastic N-doped porous carbon nanofiber aerogels as binder-free supercapacitor electrodes. J. Mater. Chem. A 2020, 8, 17257–17265. 10.1039/D0TA05423B. [DOI] [Google Scholar]
- You S.; Xi X.; Zhang X.; Wang H.; Gao P.; Ma X. Long-term stable and highly efficient perovskite solar cells with a formamidinium chloride (FACl) additive. J. Mater. Chem. A 2020, 8, 17756–17764. 10.1039/D0TA05676F. [DOI] [Google Scholar]
- Liu K.; Wu M.; Jiang H.; Lin Y.; Zhao T. An ultrathin, strong, flexible composite solid electrolyte for high-voltage lithium metal batteries. J. Mater. Chem. A 2020, 8, 18802–18809. 10.1039/D0TA05644H. [DOI] [Google Scholar]
- Li G.; Wang Y.; Guo H.; Liu Z.; Chen P.; Zheng X. Direct plasma phosphorization of Cu foam for Li ion batteries. J. Mater. Chem. A 2020, 8, 16920–16925. 10.1039/D0TA02512G. [DOI] [Google Scholar]
- Paquin F.; Rivnay J.; Salleo A.; Stingelin N.; Silva C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. 2015, 3, 10715. 10.1039/C5TC02043C. [DOI] [Google Scholar]
- Li X.; Gittleson F.; Carmo M.; Sekol R. C.; Taylor A. D. Scalable fabrication of multifunctional freestanding carbon nanotube/polymer composite thin films for energy conversion. ACS Nano 2012, 6, 1347–1356. 10.1021/nn2041544. [DOI] [PubMed] [Google Scholar]
- Wu H.; Wang K.; Meng Y.; Lu K.; Wei Z. An organic cathode material based on a polyimide/CNT nanocomposite for lithium ion batteries. J. Mater. Chem. A 2013, 1, 6366–6372. 10.1039/c3ta10473g. [DOI] [Google Scholar]
- Liu G.; Shen H.; Mao J.; Zhang L.; Jiang Z.; Sun T. Transferrin modified graphene oxide for glioma-targeted drug delivery: in vitro and in vivo evaluations. ACS Appl. Mater. interfaces 2013, 5, 6909–6914. 10.1021/am402128s. [DOI] [PubMed] [Google Scholar]
- Wang C.; Li J.; Amatore C.; Chen Y.; Jiang H.; Wang X. M. Gold nanoclusters and graphene nanocomposites for drug delivery and imaging of cancer cells. Angew. Chem. 2011, 123, 11848–11852. 10.1002/ange.201105573. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Li X.; Tabakman S. M.; Jiang K.; Fan S.; Dai H. Multiplexed multicolor Raman imaging of live cells with isotopically modified single walled carbon nanotubes. J. Am. Chem. Soc. 2008, 130, 13540–13541. 10.1021/ja806242t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohardani O.; Elola M. C.; Elizetxea C. Potential and prospective implementation of carbon nanotubes on next generation aircraft and space vehicles: A review of current and expected applications in aerospace sciences. Prog. Aerosp. Sci. 2014, 70, 42–68. 10.1016/j.paerosci.2014.05.002. [DOI] [Google Scholar]
- Natsuki T.; Tsuchiya T.; Ni Q. Q.; Endo M. Torsional elastic instability of double-walled carbon nanotubes. Carbon 2010, 48, 4362–4368. 10.1016/j.carbon.2010.07.050. [DOI] [Google Scholar]
- Zhao Y.; Nakamura R.; Kamiya K.; Nakanishi S.; Hashimoto K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 1–7. 10.1038/ncomms3390. [DOI] [PubMed] [Google Scholar]
- Yu D.; Nagelli E.; Du F.; Dai L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. 10.1021/jz100533t. [DOI] [Google Scholar]
- Yang Z.; Nie H.; Chen X.; Huang S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. 10.1016/j.jpowsour.2013.02.057. [DOI] [Google Scholar]
- Clancy A. J.; Bayazit M. K.; Hodge S. A.; Skipper N. T.; Howard C. A.; Shaffer M. S. Charged carbon nanomaterials: redox chemistries of fullerenes, carbon nanotubes, and graphenes. Chem. Rev. 2018, 118, 7363–7408. 10.1021/acs.chemrev.8b00128. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Li M.; Zhang L.; Dai L.; Xia Z. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal–air batteries. Adv. Mater. 2015, 27, 6834–6840. 10.1002/adma.201503211. [DOI] [PubMed] [Google Scholar]
- Pang R.; Li M.; Zhang C. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: diffusional limitation investigation. Talanta. 2015, 131, 38–45. 10.1016/j.talanta.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Ryan J. D.; Lund A. I.; Hofmann R.; Kroon R.; Sarabia-Riquelme M. C.; Weisenberger A. All-organic textile thermoelectrics with carbon-nanotube-coated n-type yarns. ACS Appl. Energy Mater. 2018, 1, 2934–2941. 10.1021/acsaem.8b00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatrari G.; Karakoti M.; Tewari C.; Pandey S.; Bohra B. S.; Dandapat A. Solid waste-derived carbon nanomaterials for supercapacitor applications: a recent overview. Mater. Adv. 2021, 2, 1454–1484. 10.1039/D0MA00871K. [DOI] [Google Scholar]
- Salinas-Torres D.; Ruiz-Rosas R.; Morallon E.; Cazorla-Amoros D. Strategies to enhance the performance of electrochemical capacitors based on carbon materials. Front. Mater. 2019, 6, 115. 10.3389/fmats.2019.00115. [DOI] [Google Scholar]
- Villagómez-Salas S. I.; Manikandan P.; Acuna Guzman S. F.; Pol V. G. Amorphous carbon chips li-ion battery anodes produced through polyethylene waste upcycling. ACS Omega 2018, 3, 17520–17527. 10.1021/acsomega.8b02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P.; Gogotsi Y.; Dunn B. Where do batteries end and supercapacitors begin?. Science 2014, 343, 1210–1211. 10.1126/science.1249625. [DOI] [PubMed] [Google Scholar]
- Li C.; Islam M.; Moore J.; Sleppy J.; Morrison C.; Konstantinov K. Wearable energy-smart ribbons for synchronous energy harvest and storage. Nat. Commun. 2016, 7, 1–10. 10.1038/ncomms13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski A.; Galinski M. Practical and theoretical limits for electrochemical double-layer capacitors. J. Power Sources 2007, 173, 822–828. 10.1016/j.jpowsour.2007.05.062. [DOI] [Google Scholar]
- Bohlen O.; Kowal J.; Sauer D. U. Ageing behaviour of electrochemical double layer capacitors: Part II. Lifetime simulation model for dynamic applications. J. Power Sources 2007, 173, 626–632. 10.1016/j.jpowsour.2007.07.059. [DOI] [Google Scholar]
- Lu Z.; Raad R.; Safaei F.; Xi J.; Liu Z.; Foroughi J. Carbon nanotube based fiber supercapacitor as wearable energy storage. Front. Mater. 2019, 6, 138. 10.3389/fmats.2019.00138. [DOI] [Google Scholar]
- Lokhande P. E.; Chavan U. S.; Pandey A. Materials and fabrication methods for electrochemical supercapacitors: overview. Electrochem. Energy Rev. 2020, 3, 155–186. 10.1007/s41918-019-00057-z. [DOI] [Google Scholar]
- Li J. Review of electrochemical capacitors based on carbon nanotubes and graphene. Graphene 2012, 1, 1. 10.4236/graphene.2012.11001. [DOI] [Google Scholar]
- Pan H.; Li J.; Feng Y. Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. 10.1007/s11671-009-9508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forse A. C.; Merlet C.; Griffin J.; Grey C. P. New perspectives on the charging mechanisms of supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744. 10.1021/jacs.6b02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W.; Ahn T.; Kim J. H.; Ko M.; Kim J. D. Nanosheets based mesoporous NiO microspherical structures via facile and template-free method for high performance supercapacitors. Electrochim. Acta 2011, 56, 4849–4857. 10.1016/j.electacta.2011.02.116. [DOI] [Google Scholar]
- Xing Z.; Chu Q.; Ren X.; Ge C.; Qusti A. H.; Asiri A. M. Ni3S2 coated ZnO array for high-performance supercapacitors. J. Power Sources 2014, 245, 463–467. 10.1016/j.jpowsour.2013.07.012. [DOI] [Google Scholar]
- Lobato B.; Suárez L.; Guardia L.; Centeno T. A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434–445. 10.1016/j.carbon.2017.06.083. [DOI] [Google Scholar]
- Bai S.; Tang Y.; Chen G.; Wu Y.; Liang Y.; Yang S. Phosphor copper-based flexible high voltage supercapacitors fabricated via laser irradiation and three-dimensional packaging. J. Power Sources 2021, 507, 230257. 10.1016/j.jpowsour.2021.230257. [DOI] [Google Scholar]
- Choi J. H.; Kim Y.; Kim B. S. Multifunctional role of reduced graphene oxide binder for high performance supercapacitor with commercial-level mass loading. J. Power Sources 2020, 454, 227917. 10.1016/j.jpowsour.2020.227917. [DOI] [Google Scholar]
- Xia L.; Yu L.; Hu D.; Chen G. Z. Electrolytes for electrochemical energy storage. Mater. Chem. Front. 2017, 1, 584–618. 10.1039/C6QM00169F. [DOI] [Google Scholar]
- Deb Nath N. C.; Shah S. S.; Qasem M. A.; Zahir M. H.; Aziz M. A. Defective carbon nanosheets derived from syzygium cumini leaves for electrochemical energy-storage. Chem. Select. 2019, 4, 9079–9083. 10.1002/slct.201900891. [DOI] [Google Scholar]
- Baumann A. E.; Burns D. A.; Liu B.; Thoi V. S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 1–14. 10.1038/s42004-019-0184-6. [DOI] [Google Scholar]
- Deng T.; Zhang W.; Arcelus O.; Wang D.; Shi X.; Zhang X. Vertically co-oriented two dimensional metal-organic frameworks for packaging enhanced supercapacitive performance. Commun. Chem. 2018, 1, 1–9. 10.1038/s42004-017-0005-8. [DOI] [Google Scholar]
- Shown I.; Ganguly A.; Chen L. C.; Chen K. H. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. 10.1002/ese3.50. [DOI] [Google Scholar]
- Wang C.; Wen C.; Che Y.; Chang J.; Ho Y. C.; Kao K. S.. The influence of specific surface area on the capacitance of the carbon electrodes supercapacitor. The Proceedings of the Second International Conference on Industrial Application Engineering, 2015; pp 439–442.
- Simon P.; Burke A. Nanostructured carbons: double-layer capacitance and more. Electrochem. Soc. Interface 2008, 17, 38. 10.1149/2.F05081IF. [DOI] [Google Scholar]
- Cazorla-Amorós D.; Lozano-Castelló D.; Morallón E.; Bleda-Martínez M.; Linares-Solano A.; Shiraishi S. Measuring cycle efficiency and capacitance of chemically activated carbons in propylene carbonate. Carbon. 2010, 48, 1451–1456. 10.1016/j.carbon.2009.12.039. [DOI] [Google Scholar]
- Falco C.; Marco-Lozar J.; Salinas-Torres D.; Morallón E.; Cazorla-Amorós D.; Titirici M. Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature. Carbon. 2013, 62, 346–355. 10.1016/j.carbon.2013.06.017. [DOI] [Google Scholar]
- Liu H. J.; Wang J.; Wang C. X.; Xia Y. Y. Ordered hierarchical mesoporous/microporous carbon derived from mesoporous titanium-carbide/carbon composites and its electrochemical performance in supercapacitor. Adv. Energy Mater. 2011, 1, 1101–1108. 10.1002/aenm.201100255. [DOI] [Google Scholar]
- Jiang L.; Yan J.; Xue R.; Sun G.; Yi B. Partially graphitized ordered mesoporous carbons for high-rate supercapacitors. J. Solid State Electrochem. 2014, 18, 2175–2182. 10.1007/s10008-014-2458-3. [DOI] [Google Scholar]
- Yoshida N.; Hirota Y.; Uchida Y.; Asada T.; Kobayashi K.; Nishiyama N. Solvent-free synthesis and KOH activation of mesoporous carbons using resorcinol/Pluronic F127/hexamethylenetetramine mixture and their application to EDLC. Micropor. Mesopo. Mater. 2018, 272, 217–221. 10.1016/j.micromeso.2018.06.028. [DOI] [Google Scholar]
- Itoi H.; Nishihara H.; Ishii T.; Nueangnoraj K.; Berenguer-Betrian R.; Kyotani T. Large pseudocapacitance in quinone-functionalized zeolite-templated carbon. Bull. Chem. Soc. Jpn. 2014, 87, 250–257. 10.1246/bcsj.20130292. [DOI] [Google Scholar]
- Qie L.; Chen W.; Xu H.; Xiong X.; Jiang Y.; Zou F. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497–2504. 10.1039/c3ee41638k. [DOI] [Google Scholar]
- Valero-Romero M.; Márquez-Franco E.; Bedia J.; Rodríguez-Mirasol J.; Cordero T. Hierarchical porous carbons by liquid phase impregnation of zeolite templates with lignin solution. Microporous Mesoporous Mater. 2014, 196, 68–78. 10.1016/j.micromeso.2014.04.055. [DOI] [Google Scholar]
- Salinas-Torres D.; Ruiz-Rosas R.; Valero-Romero M. J.; Rodríguez-Mirasol J.; Cordero T.; Morallón E. Asymmetric capacitors using lignin-based hierarchical porous carbons. J. Power Sources 2016, 326, 641–651. 10.1016/j.jpowsour.2016.03.096. [DOI] [Google Scholar]
- Ruiz-Rosas R.; Valero-Romero M. J.; Salinas-Torres D.; Rodríguez-Mirasol J.; Cordero T.; Morallon E. Electrochemical performance of hierarchical porous carbon materials obtained from the infiltration of lignin into zeolite templates. ChemSusChem 2014, 7, 1458–1467. 10.1002/cssc.201301408. [DOI] [PubMed] [Google Scholar]
- Singh D.; Kumar S.; Singh A.; Sharma T.; Dhapola P. S.; Konwar S. Ionic liquid–biopolymer electrolyte for electrochemical devices. Ionics. 2022, 28, 759–766. 10.1007/s11581-021-04372-8. [DOI] [Google Scholar]
- Grishchenko L. M.; Tsapyuk G. G.; Ricco M.; Diyuk V. E.; Boldyrieva O. Y.; Mariychuk R.. Enhancing the Performance of Supercapacitor Activated Carbon Electrodes by Oxidation. 2020 IEEE 40th International Conference on Electronics and Nanotechnology (ELNANO), 2020; pp 173–177.
- Maria Sundar Raj F. R.; Jaya N. V.; Boopathi G.; Kalpana D.; Pandurangan A. S-doped activated mesoporous carbon derived from the Borassus flabellifer flower as active electrodes for supercapacitors. Mater. Chem. Phys. 2020, 240, 122151. 10.1016/j.matchemphys.2019.122151. [DOI] [Google Scholar]
- Cheng F.; Yang X.; Zhang S.; Lu W. Boosting the supercapacitor performances of activated carbon with carbon nanomaterials. J. Power Sources 2020, 450, 227678. 10.1016/j.jpowsour.2019.227678. [DOI] [Google Scholar]
- Inal I. I. G.; Gokce Y.; Aktas Z.. Waste tea derived activated carbon/polyaniline composites as supercapacitor electrodes. 2016 IEEE International Conference on Renewable Energy Research and Applications (ICRERA), 2016; pp 458–462.
- Forouzandeh P.; Kumaravel V.; Pillai S. C. Electrode materials for supercapacitors: a review of recent advances. Catalysts 2020, 10, 969. 10.3390/catal10090969. [DOI] [Google Scholar]
- Futaba D. N.; Hata K.; Yamada T.; Hiraoka T.; Hayamizu Y.; Kakudate Y. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. 10.1038/nmat1782. [DOI] [PubMed] [Google Scholar]
- Hsia B.; Marschewski J.; Wang S.; Carraro C.; Poulikakos D.; Grigoropoulos C.; Maboudian R. Highly flexible, all solid-state micro-supercapacitors from vertically aligned carbon nanotubes. Nanotechnology 2014, 25, 9–12. 10.1088/0957-4484/25/5/055401. [DOI] [PubMed] [Google Scholar]
- Ye J. S.; Cui H. F.; Liu X.; Lim T. M.; Zhang W. D.; Sheu F. S. Preparation and characterization of aligned carbon nanotube–ruthenium oxide nanocomposites for supercapacitors. Small 2005, 1, 560–565. 10.1002/smll.200400137. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M.; Bharathi Sankar A.; Sri Rohita D.; Rao T. N.; Karthik M. Conversion of biomass waste into high performance supercapacitor electrodes for real-time supercapacitor applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. 10.1021/acssuschemeng.9b03568. [DOI] [Google Scholar]
- Khalid M.; Bhardwaj P.; Varela H. Carbon-based composites for supercapacitor. Sci. Technol. Adv. Appl. Supercapacitors 2018, 28, 1–18. 10.5772/intechopen.80393. [DOI] [Google Scholar]
- Liu Q.; Yang J.; Luo X.; Miao Y.; Zhang Y.; Xu W. Fabrication of a fibrous MnO2@ MXene/CNT electrode for high-performance flexible supercapacitor. Ceram. Int. 2020, 46, 11874–11881. 10.1016/j.ceramint.2020.01.222. [DOI] [Google Scholar]
- Ramakrishna Matte H.; Subrahmanyam K.; Rao C. Synthetic aspects and selected properties of graphene. Nanomater. Nanotechnol. 2011, 1, 3–13. 10.5772/50945. [DOI] [Google Scholar]
- Shabangoli Y.; Rahmanifar M. S.; Noori A.; El-Kady M. F.; Kaner R. B.; Mousavi M. F. Nile blue functionalized graphene aerogel as a pseudocapacitive negative electrode material across the full pH range. ACS Nano 2019, 13, 12567–12576. 10.1021/acsnano.9b03351. [DOI] [PubMed] [Google Scholar]
- Karnan M.; Subramani K.; Sathish M. Template assisted synthesis of nitrogen doped 3d-graphene for supercapacitor applications. Mater.Today: Proceedings 2017, 4, 12144–12151. 10.1016/j.matpr.2017.09.143. [DOI] [Google Scholar]
- Xu L.; Cheng C.; Yao C.; Jin X. Flexible supercapacitor electrode based on lignosulfonate-derived graphene quantum dots/graphene hydrogel. Org. Electron. 2020, 78, 105407. 10.1016/j.orgel.2019.105407. [DOI] [Google Scholar]
- Lee Y. H.; Chang K. H.; Hu C. C. Differentiate the pseudocapacitance and double-layer capacitance contributions for nitrogen-doped reduced graphene oxide in acidic and alkaline electrolytes. J. Power Sources 2013, 227, 300–308. 10.1016/j.jpowsour.2012.11.026. [DOI] [Google Scholar]
- Yoon Y.; Lee K.; Kwon S.; Seo S.; Yoo H.; Kim S. Vertical alignments of graphene sheets spatially and densely piled for fast ion diffusion in compact supercapacitors. ACS Nano 2014, 8, 4580–4590. 10.1021/nn500150j. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Tian J.; Yin Z.; Cui C.; Qian W.; Wei F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: a review. Carbon 2019, 141, 467–480. 10.1016/j.carbon.2018.10.010. [DOI] [Google Scholar]
- Miller J. R.; Outlaw R. A. Vertically-oriented graphene electric double layer capacitor designs. J. Electrochem. Soc. 2015, 162, 5077. 10.1149/2.0121505jes. [DOI] [Google Scholar]
- Akbar F.; Kolahdouz M.; Larimian S.; Radfar B.; Radamson H. Graphene synthesis, characterization and its applications in nanophotonics, nanoelectronics, and nanosensing. J. Mater. Sci. 2015, 26, 4347–4379. 10.1007/s10854-015-2725-9. [DOI] [Google Scholar]
- Pop E.; Varshney V.; Roy A. K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. 10.1557/mrs.2012.203. [DOI] [Google Scholar]
- Pham K. C.; McPhail D. S.; Wee A. T.; Chua D. H. Amorphous molybdenum sulfide on graphene-carbon nanotube hybrids as supercapacitor electrode materials. RSC adv. 2017, 7, 6856–6864. 10.1039/C6RA27901E. [DOI] [Google Scholar]
- Sun L.; Tian C.; Li M.; Meng X.; Wang L.; Wang R. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. 10.1039/c3ta10897j. [DOI] [Google Scholar]
- Liu C. K.; Feng Y.; He H. J.; Zhang J.; Sun R. J.; Chen M. Y. Effect of carbonization temperature on properties of aligned electro spun polyacrylonitrile carbon nanofibers. Mater. Des. 2015, 85, 483–486. 10.1016/j.matdes.2015.07.021. [DOI] [Google Scholar]
- Hao P.; Zhao Z.; Tian J.; Li H.; Sang Y.; Yu G. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. 10.1039/C4NR03574G. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Liu P.; Zhu B.; Chen S.; Yao K.; Han R. Microporous carbon derived from acacia gum with tuned porosity for high-performance electrochemical capacitors. Int. j. Hydrog. Energy 2015, 40, 6188–6196. 10.1016/j.ijhydene.2015.03.090. [DOI] [Google Scholar]
- Lin X.-Q.; Lü Q.-F.; Li Q.; Wu M.; Liu R. Fabrication of low-cost and ecofriendly porous biocarbon using konjaku flour as the raw material for high-performance supercapacitor application. ACS omega 2018, 3, 13283–13289. 10.1021/acsomega.8b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Yu F.; Wang G.; Chen L.; Dai B.; Peng S. Nitrogen and sulfur self-doped activated carbon directly derived from elm flower for high-performance supercapacitors. ACS Omega 2018, 3, 4724–4732. 10.1021/acsomega.8b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Liu L.; Zhang P.; Wang J.; Xu M.; Deng Q. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem.Eng. J. 2019, 355, 309–319. 10.1016/j.cej.2018.08.169. [DOI] [Google Scholar]
- Wang J.; Zhang X.; Li Z.; Ma Y.; Ma L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794. 10.1016/j.jpowsour.2020.227794. [DOI] [Google Scholar]
- Mondal A. K.; Kretschmer K.; Zhao K.; Liu H.; Wang C.; Sun B. Nitrogen-Doped Porous Carbon Nanosheets from Eco-Friendly Eucalyptus Leaves as High Performance Electrode Materials for Supercapacitors and Lithium Ion Batteries. Chem.—Eur. J. 2017, 23, 3683–3690. 10.1002/chem.201605019. [DOI] [PubMed] [Google Scholar]
- Thangavel R.; Kaliyappan K.; Ramasamy H. V.; Sun X.; Lee Y. S. Engineering the Pores of Biomass-Derived Carbon: Insights for Achieving Ultrahigh Stability at High Power in High-Energy Supercapacitors. ChemSusChem. 2017, 10, 2805–2815. 10.1002/cssc.201700492. [DOI] [PubMed] [Google Scholar]
- Wang D. W.; Liu F.; Li M.; Lu G. Q.; Cheng H. M. Mesopore-aspect-ratio dependence of ion transport in rodtype ordered mesoporous carbon. J. Phys. Chem. C 2008, 112, 9950–9955. 10.1021/jp800173z. [DOI] [Google Scholar]
- Long C.; Chen X.; Jiang L.; Zhi L.; Fan Z. Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 2015, 12, 141–151. 10.1016/j.nanoen.2014.12.014. [DOI] [Google Scholar]
- Liu Z.; Fu X.; Li M.; Wang F.; Wang Q.; Kang G. Novel silicon-doped, silicon and nitrogen-codoped carbon nanomaterials with high activity for the oxygen reduction reaction in alkaline medium. J. Mater. Chem. A 2015, 3, 3289–3293. 10.1039/C4TA05937A. [DOI] [Google Scholar]
- Mostazo-López M. J.; Ruiz-Rosas R.; Castro-Muñiz A.; Nishihara H.; Kyotani T.; Morallón E. Ultraporous nitrogen-doped zeolite-templated carbon for high power density aqueous-based supercapacitors. Carbon 2018, 129, 510–519. 10.1016/j.carbon.2017.12.050. [DOI] [Google Scholar]
- Inagaki M.; Konno H.; Tanaike O. Carbon materials for electrochemical capacitors. J. Power Sources. 2010, 195, 7880–7903. 10.1016/j.jpowsour.2010.06.036. [DOI] [Google Scholar]
- Xiang S.; Yang X.; Lin X.; Chang C.; Que H.; Li M. Nitrogen and sulfur co-doped polyurethane-based porous carbon materials as supercapacitors exhibit excellent electrochemical performance. J. Solid State. Electrochem. 2017, 21, 1457–1465. 10.1007/s10008-017-3505-7. [DOI] [Google Scholar]
- Lei Z.; Christov N.; Zhang L. L.; Zhao S. S. Mesoporous carbon nanospheres with an excellent electrocapacitive performance. J. Mater. Chem. 2011, 21, 2274–2281. 10.1039/C0JM03322G. [DOI] [Google Scholar]
- Ornelas O.; Sieben J. M.; Ruiz-Rosas R.; Morallon E.; Cazorla-Amorós D.; Geng J. On the origin of the high capacitance of nitrogen-containing carbon nanotubes in acidic and alkaline electrolytes. Chem. Commun. 2014, 50, 11343–11346. 10.1039/C4CC04876H. [DOI] [PubMed] [Google Scholar]
- Salinas-Torres D.; Shiraishi S.; Morallón E.; Cazorla-Amorós D. Improvement of carbon materials performance by nitrogen functional groups in electrochemical capacitors in organic electrolyte at severe conditions. Carbon 2015, 82, 205–213. 10.1016/j.carbon.2014.10.064. [DOI] [Google Scholar]
- Wei X.; Jiang X.; Wei J.; Gao S. Functional groups and pore size distribution do matter to hierarchically porous carbons as high-rate-performance supercapacitors. Chem. Mater. 2016, 28, 445–458. 10.1021/acs.chemmater.5b02336. [DOI] [Google Scholar]
- Wang X.; Liu C. G.; Neff D.; Fulvio P. F.; Mayes R. T.; Zhamu A. Nitrogen-enriched ordered mesoporous carbons through direct pyrolysis in ammonia with enhanced capacitive performance. J. Mater. Chem. A 2013, 1, 7920–7926. 10.1039/c3ta11342f. [DOI] [Google Scholar]
- Nishihara H.; Kyotani T. Templated nanocarbons for energy storage. Adv. Mater. 2012, 24, 4473–4498. 10.1002/adma.201201715. [DOI] [PubMed] [Google Scholar]
- Mostazo-López M. J.; Ruiz-Rosas R.; Morallón E.; Cazorla-Amorós D. Generation of nitrogen functionalities on activated carbons by amidation reactions and Hofmann rearrangement: Chemical and electrochemical characterization. Carbon 2015, 91, 252–265. 10.1016/j.carbon.2015.04.089. [DOI] [Google Scholar]
- Quílez-Bermejo J.; González-Gaitán C.; Morallón E.; Cazorla-Amorós D. Effect of carbonization conditions of polyaniline on its catalytic activity towards ORR. Some insights about the nature of the active sites. Carbon 2017, 119, 62–71. 10.1016/j.carbon.2017.04.015. [DOI] [Google Scholar]
- Sun L.; Tian C.; Fu Y.; Yang Y.; Yin J.; Wang L. Nitrogen-doped porous graphitic carbon as an excellent electrode material for advanced supercapacitors. Chem.—Eur. J. 2014, 20, 564–574. 10.1002/chem.201303345. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Lai H.; Lyu Z.; Jiang Y.; Xie K.; Wang X. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. 10.1002/adma.201500945. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Rufford T. E.; Hulicova-Jurcakova D.; Wang L. Nitrogen and phosphorous co-doped graphene monolith for supercapacitors. ChemSusChem. 2016, 9, 513–520. 10.1002/cssc.201501303. [DOI] [PubMed] [Google Scholar]
- Chen Y. M.; Li Z. W.; Lou W. General formation of MxCo3- xS4 (M= Ni, Mn, Zn) hollow tubular structures for hybrid supercapacitors. Angew. Chem. 2015, 127, 10667–10670. 10.1002/ange.201504349. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Zheng L.; Ma Y.; Lei L.; Li Q.; Li Y. Synthesis of nitrogen-and sulfur-codoped 3D cubic-ordered mesoporous carbon with superior performance in supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 2657–2665. 10.1021/am405128j. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Li Y.; Liu J.; Huang X.; Yuan C.; Lou W. X. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. 10.1002/adma.201202146. [DOI] [PubMed] [Google Scholar]
- Augustyn V.; Simon P.; Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. 10.1039/c3ee44164d. [DOI] [Google Scholar]
- Shen B.; Zhang X.; Guo R.; Lang J.; Chen J.; Yan X. Carbon encapsulated RuO2 nano-dots anchoring on graphene as an electrode for asymmetric supercapacitors with ultralong cycle life in an ionic liquid electrolyte. J. Mater. Chem. A 2016, 4 (21), 8180–8189. 10.1039/C6TA02473D. [DOI] [Google Scholar]
- Chen Y.; Zhang J.; Li M.; Yang C.; Zhang L.; Wang C. Strong interface coupling and few-crystalline MnO2/Reduced graphene oxide composites for supercapacitors with high cycle stability. Electrochim. Acta 2018, 292, 115–124. 10.1016/j.electacta.2018.09.131. [DOI] [Google Scholar]
- Lai F.; Feng J.; Yan R.; Wang G. C.; Antonietti M.; Oschatz M. Breaking the Limits of Ionic Liquid-Based Supercapacitors: Mesoporous Carbon Electrodes Functionalized with Manganese Oxide Nanosplotches for Dense, Stable, and Wide-Temperature Energy Storage. Adv. Funct. Mater. 2018, 28, 1801298. 10.1002/adfm.201801298. [DOI] [Google Scholar]
- Faraji S.; Ani F. N. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors-a review. J. Power Sources 2014, 263, 338–360. 10.1016/j.jpowsour.2014.03.144. [DOI] [Google Scholar]
- Rakhi R.; Chen W.; Cha D.; Alshareef H. N. High performance supercapacitors using metal oxide anchored graphene nanosheet electrodes. J. Mater. Chem. 2011, 21, 16197–16204. 10.1039/c1jm12963e. [DOI] [Google Scholar]
- Zhang J.; Jiang J.; Li H.; Zhao X. A high-performance asymmetric supercapacitor fabricated with graphene-based electrodes. Energy Environ. Sci. 2011, 4, 4009–4015. 10.1039/c1ee01354h. [DOI] [Google Scholar]
- Jo K.; Gu M.; Kim B. S. Ultrathin supercapacitor electrode based on reduced graphene oxide nanosheets assembled with photo-cross-linkable polymer: conversion of electrochemical kinetics in ultrathin films. Chem. Mater. 2015, 27, 7982–7989. 10.1021/acs.chemmater.5b03296. [DOI] [Google Scholar]
- Fang Q.; Evans D.; Roberson S.; Zheng J. Ruthenium oxide film electrodes prepared at low temperatures for electrochemical capacitors. J. Electrochem. Soc. 2001, 148, A833. 10.1149/1.1379739. [DOI] [Google Scholar]
- Bose S.; Kuila T.; Mishra A. K.; Rajasekar R.; Kim N. H.; Lee J. H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. 10.1039/C1JM14468E. [DOI] [Google Scholar]
- Mini P.; Balakrishnan A.; Nair S. V.; Subramanian K. Highly super capacitive electrodes made of graphene/poly (pyrrole). Chem. Commun. 2011, 47, 5753–5755. 10.1039/c1cc00119a. [DOI] [PubMed] [Google Scholar]
- Gupta V.; Miura N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. 10.1016/j.electacta.2006.01.074. [DOI] [Google Scholar]
- Zhou Y.; Xu H.; Lachman N.; Ghaffari M.; Wu S.; Liu Y. Advanced asymmetric supercapacitor based on conducting polymer and aligned carbon nanotubes with controlled nanomorphology. Nano Energy 2014, 9, 176–185. 10.1016/j.nanoen.2014.07.007. [DOI] [Google Scholar]
- Ghosh A.; Lee Y. H. Carbon-based electrochemical capacitors. ChemSusChem. 2012, 5, 480–499. 10.1002/cssc.201100645. [DOI] [PubMed] [Google Scholar]
- Vlad A.; Singh N.; Melinte S.; Gohy J. F.; Ajayan P. Carbon redox-polymer-gel hybrid supercapacitors. Sci. Rep. 2016, 6, 1–6. 10.1038/srep22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T.; Zhang Z.; Zhu Z.; Zhou X.; Wang Y.; Wang Y. Recycling of waste plastics and scalable preparation of Si/CNF/C composite as anode material for lithium-ion batteries. Ionics 2019, 25, 1523–1529. 10.1007/s11581-019-02892-y. [DOI] [Google Scholar]
- Pandey S.; Karakoti M.; Surana K.; Dhapola P. S.; SanthiBhushan B.; Ganguly S. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 2021, 11, 1–17. 10.1038/s41598-021-83483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Y.; Ni M.; Huang Z.; Chen R.; Zhou L.; Utetiwabo W. Polyethylene waste carbons with a mesoporous network towards highly efficient supercapacitors. Chem. Eng. J. 2019, 366, 313–320. 10.1016/j.cej.2019.02.063. [DOI] [Google Scholar]
- Lian Y. M.; Utetiwabo W.; Zhou Y.; Huang Z.; Huang L.; Zhou L.; Faheem M. From upcycled waste polyethylene plastic to graphene/mesoporous carbon for high-voltage supercapacitors. J. Colloid Interface Sci. 2019, 557, 55–64. 10.1016/j.jcis.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhou X. L.; Shao L. M.; Lü F.; He P. J. Hierarchical porous carbon spheres from low-density polyethylene for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3801–3810. 10.1021/acssuschemeng.8b04539. [DOI] [Google Scholar]
- Adame-Pereira M.; Alexandre-Franco M.; Fernández-González C.; Gómez-Serrano V.. Adsorption of bisphenol A by activated carbon developed from PET by KOH activation; Springer, 2021; Vol. 28, pp 24342–24354. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Kierzek K.; Min J.; Chen X.; Gong J.; Niu R. Porous carbon nanosheet with high surface area derived from waste poly (ethylene terephthalate) for supercapacitor applications. J. Appl. Polym. Sci. 2020, 137, 48338. 10.1002/app.48338. [DOI] [Google Scholar]
- Elessawy N.; El Nady A. J.; Wazeer W.; Kashyout A. Development of high-performance supercapacitor based on a novel controllable green synthesis for 3D nitrogen doped graphene. Sci. Rep. 2019, 9, 1–10. 10.1038/s41598-018-37369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X.; Li Y.; Liu X.; Ma C.; Jiang H.; Zhu J. Controllable carbonization of plastic waste into three-dimensional porous carbon nanosheets by combined catalyst for high performance capacitor. Nanomaterials 2020, 10, 1097. 10.3390/nano10061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wen Y.; Chen X.; Tang T.; Mijowska E. Co-etching effect to convert waste polyethylene terephthalate into hierarchical porous carbon toward excellent capacitive energy storage. Sci. Total Environ. 2020, 723, 138055. 10.1016/j.scitotenv.2020.138055. [DOI] [PubMed] [Google Scholar]
- Chen X. Y.; Cheng L. X.; Deng X.; Zhang L.; Zhang Z. J. Generalized conversion of halogen-containing plastic waste into nanoporous carbon by a template carbonization method. Ind. Eng. Chem. Res. 2014, 53, 6990–6997. 10.1021/ie500685s. [DOI] [Google Scholar]
- Chang Y.; Pang Y.; Dang D.; Kumar A.; Zhang G.; Chang Z. Converting polyvinyl chloride plastic wastes to carbonaceous materials via room-temperature dehalogenation for high-performance supercapacitor. ACS Appl. Energy Mater. 2018, 1, 5685–5693. 10.1021/acsaem.8b01252. [DOI] [Google Scholar]
- Singh A.; Dhapola P. S.; Singh S. S.; Singh P. K.; Samsudin A.; Sahoo N. G. Highly conducting polymer electrolyte-ionic liquid and porous carbon material for sandwich electric double layer capacitor. High Perform. Polym. 2021, 33, 469–475. 10.1177/0954008320964535. [DOI] [Google Scholar]
- Cheng L. X.; Zhang L.; Chen X. Y.; Zhang Z. J. Efficient conversion of waste polyvinyl chloride into nanoporous carbon incorporated with MnOx exhibiting superior electrochemical performance for supercapacitor application. Electrochim. Acta 2015, 176, 197–206. 10.1016/j.electacta.2015.07.007. [DOI] [Google Scholar]
- Chang Y.; Zhang G.; Han B.; Li H.; Hu C.; Pang Y. Polymer dehalogenation-enabled fast fabrication of N, S-codoped carbon materials for superior supercapacitor and deionization applications. ACS Appl. Mater. Interfaces 2017, 9, 29753–29759. 10.1021/acsami.7b08181. [DOI] [PubMed] [Google Scholar]
- Cheng L. X.; Zhu Y. Q.; Chen X. Y.; Zhang Z. J. Polyvinylidene Fluoride-Based Carbon Supercapacitors: Notable Capacitive Improvement of Nanoporous Carbon by the Redox Additive Electrolyte of 4-(4-Nitrophenylazo)-1-naphthol. Ind. Eng. Chem. Res. 2015, 54, 9948–9955. 10.1021/acs.iecr.5b02490. [DOI] [Google Scholar]
- Ma C.; Liu X.; Min J.; Li J.; Gong J.; Wen X. Sustainable recycling of waste polystyrene into hierarchical porous carbon nanosheets with potential applications in supercapacitors. Nanotechnology 2020, 31, 035402. 10.1088/1361-6528/ab475f. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Shen Z.; Yu Y.; Liu L.; Wang G.; Chen A. Porous carbon derived from waste polystyrene foam for supercapacitor. J. Mater. Sci. 2018, 53, 12115–12122. 10.1007/s10853-018-2513-z. [DOI] [Google Scholar]
- Min J.; Zhang S.; Li J.; Klingeler R.; Wen X.; Chen X. From polystyrene waste to porous carbon flake and potential application in supercapacitor. Waste Manag. 2019, 85, 333–340. 10.1016/j.wasman.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Deka N.; Barman J.; Kasthuri S.; Nutalapati V.; Dutta G. K. Transforming waste polystyrene foam into N-doped porous carbon for capacitive energy storage and deionization applications. Appl. Surf. Sci. 2020, 511, 145576. 10.1016/j.apsusc.2020.145576. [DOI] [Google Scholar]
- Sahoo R. K.; Chitumalla R. K.; Das A.; Mohapatra M.; Yun J. M.; Jang J. Scalable Processing Method using Waste Polystyrene to Produce Nitrogen-enriched Porous Carbon for Boosting Supercapacitor Performance. Mater. Lett. 2021, 300, 130135. 10.1016/j.matlet.2021.130135. [DOI] [Google Scholar]
- Wen Y.; Wen X.; Wenelska K.; Chen X.; Mijowska E. Novel strategy for preparation of highly porous carbon sheets derived from polystyrene for supercapacitors. Diam. Relat,Mater. 2019, 95, 5–13. 10.1016/j.diamond.2019.03.015. [DOI] [Google Scholar]
- Liu X.; Ma C.; Wen Y.; Chen X.; Zhao X.; Tang T. Highly efficient conversion of waste plastic into thin carbon nanosheets for superior capacitive energy storage. Carbon 2021, 171, 819–828. 10.1016/j.carbon.2020.09.057. [DOI] [Google Scholar]
- Mishra N.; Shinde S.; Vishwakarma R.; Kadam S.; Sharon M.; Sharon M.. MWCNTs synthesized from waste polypropylene plastics and its application in super-capacitors. AIP Conference Proceedings, 2013; pp 228–236.
- Hu X.; Lin Z. Transforming waste polypropylene face masks into S-doped porous carbon as the cathode electrode for supercapacitors. Ionics 2021, 27, 2169–2179. 10.1007/s11581-021-03949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y.; Kierzek K.; Chen X.; Gong J.; Liu J.; Niu R. Mass production of hierarchically porous carbon nanosheets by carbonizing “real-world” mixed waste plastics toward excellent-performance supercapacitors. Waste Manag. 2019, 87, 691–700. 10.1016/j.wasman.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Abbas A.; Yi A. M.; Saleem F.; Jin Z.; Veksha A.; Yan Q. Multiwall carbon nanotubes derived from plastic packaging waste as a high-performance electrode material for supercapacitors. Int. J. Energy Res. 2021, 45, 19611–19622. 10.1002/er.6967. [DOI] [Google Scholar]
- Wen X.; Chen X.; Tian N.; Gong J.; Liu J.; Rümmeli M. H. Nanosized carbon black combined with Ni2O3 as “universal” catalysts for synergistically catalyzing carbonization of polyolefin wastes to synthesize carbon nanotubes and application for supercapacitors. Environ. Sci. Technol. 2014, 48, 4048–4055. 10.1021/es404646e. [DOI] [PubMed] [Google Scholar]
- Karakoti M.; Pandey S.; Jangra R.; Dhapola P. S.; Singh P. K.; Mahendia S. Waste plastics derived graphene nanosheets for supercapacitor application. Mater. Manuf. Processes 2021, 36, 171–177. 10.1080/10426914.2020.1832680. [DOI] [Google Scholar]