Abstract

Steel hydrochloric acid pickling sludge (SHPS), containing the heavy metals Fe, Zn, and Ni and a high chloride salt content, is considered a type of hazardous solid waste because of its potential harm to human health and the environment. In addition, the SHPS yield is large, but the main treatment currently used is only safe for landfills. Although studying the composition and leaching toxicity of SHPS is of great importance, only a small amount of related literature is available. This paper can help compensate for this deficiency. SHPS is analyzed from the aspects of its formation mechanism, pH, moisture content, elemental concentration, phase composition, microstructure, and leaching toxicity. The results show that its pH ranges from 2.25 to 11.11, and the moisture content ranges from 45.47% to 83.34%. Additionally, the concentration of Fe is the highest, with values from 29.80% to 50.65%, while other alkali metal elements, namely, Ca, K, and Na, have values of 0.36% to 23.07%, 0.02% to 19.82%, and 0.38% to 3.31%, respectively. Heavy metal elements, namely, Zn, Ni, Mn, Cr, and Pb, have values of 0.02% to 14.88%, 0.001% to 0.05%, 0.03% to 0.38%, 0.01% to 0.09%, and 0.02% to 0.19%, respectively. Anions, namely, SO42–, Cl–, F–, and NO3–, have contents of 0.09% to 0.34%, 0.54% to 5.73%, 0.001% to 0.04%, and 0.01% to 0.15%, respectively. X-ray diffraction (XRD) analysis shows that Fe and Zn are mainly present in oxides, Ca is present as CaO and CaCO3, and chlorine is present in NaCl. Moreover, scanning electron microscopy (SEM) analysis shows that the microscopic structure consists mainly of bright and fluffy irregular spheres; stripes; flakes; and dark, very small irregular particles. The leaching toxicity test based on HJ/T 299-2007 (China) was performed, where SHPS samples were treated with a mixed solution of sulfuric acid, nitric acid, and pure water (pH = 3.20 ± 0.05) at a liquid-to-solid ratio of 10:1 for a period of 18 h. The leachate was filtered and analyzed for Cr, Ni, Mn, Zn, etc. The leaching results indicate that Zn and Ni are the main elements that cause SHPS to be hazardous to the environment. These research results can provide a reference for later researchers studying the effective treatment of SHPS, such as more effective treatments for reducing toxicity and resource utilization.
1. Introduction
Pickling is one of the most commonly applied methods in sheet mills or metal fabrication plants to remove rust or scale, impurities, and inorganic contaminants from the surface of parts of hot-rolled or annealed stainless steel products (e.g., strips) by passing them through an acid bath prior to shaping, cold-rolling, or metal-finishing operations, such as galvanization.1−5 The pickling process not only improves the metal surface quality but also prepares the metal for further processing. In the past, sulfuric acid pickling was used for ordinary steel and ordinary low-alloy steel because the boiling point of hydrochloric acid is low and a corrosive acid mist will be produced during pickling and heating. However, with the improvement in material anticorrosion technology, effective use of hydrochloric acid pickling (HAP) mist inhibitors, and research on waste acid regeneration equipment, the shortcomings of HAP have been overcome.6−8 Moreover, compared with sulfuric acid pickling, HAP has the following advantages: the use of inexpensive hydrochloric acid, a faster pickling speed, easier cleaning, lower acid and heat consumption, less acid pickling waste liquid, greater versatility, and more uniform product quality. Therefore, since 1964, a large number of steel pickling plants have adopted the use of hydrochloric acid instead of sulfuric acid for pickling.9
The pickling liquid used in the iron and steel HAP process normally dilutes industrial hydrochloric acid from a concentration of 32% to 18% to 20%, and then the liquid acid is heated to 50–80 °C, at which time the liquid acid can reach the optimal state for pickling clean steel surfaces. When the concentration of acid falls below 10% (or when the concentration of Fe2+ is higher than 120 g/L), the pickling speed and pickling effect cannot meet the requirements. At this point, the liquid is discharged as hydrochloric pickling waste liquid (HPWL), which includes acidic rinsewater, iron chlorides, and metallic salts together with low concentrations of organic compounds, such as HAP mist inhibitors. According to the China National List of Hazardous Waste (2021 version),100 HPWL is considered a hazardous waste. The direct discharge of HPWL will cause not only waste of a large amount of resources but also serious, irreversible pollution of the ecological environment and water environment. Thereby, some researchers and technologists have exerted a large amount of effort to find novel and efficient methods for HPWL treatment and disposal and resource treatment and utilization, e.g., distillation,10−12 high temperature roasting13/pyrohydrolysis,14,15 crystallization,16,17 solvent extraction,18−23 electrolysis,24−30 ion-exchange/acid-retardation methods,31 electrodialysis/membrane separation/diffusion dialysis,5,32−35 a microwave-hydrothermal method,36 selective carbochlorination,37 adsorption,38 neutralization/precipitation,39 flocculant production for water treatment,40−42 iron pigment production, ferrite production,43,44 nanosized iron oxide production,45−48 Fe3O4 magnetic powder production,49−52 and schwertmannite@akaganeite core/shell nanostructure production.53
Although there are various methods for treating and recycling HPWL, some of which are also applied in engineering, some deficiencies remain. However, the traditional neutralization precipitation (NP) treatment process can not only neutralize the acidity of the waste liquid but also remove Fe, Ni, Zn, and other pollutant metal ions in the waste liquid by precipitation. Furthermore, this method has the advantages of high efficiency and low cost. Thus, NP has been extensively adopted in many small and medium-sized steel pickling plants to treat HPWL, particularly in current small hot-dip galvanization plants. However, the NP method will generate a certain amount of toxic and harmful steel hydrochloric acid pickling sludge (SHPS) that contains Fe and Zn compounds and residual acid, which may lead to serious water and air pollution if not properly disposed, whether it is landfilled or simply buried.15,54 In China, as per the National List of Hazardous Waste (2021 version), SHPS is a hazardous waste.100
Commonly, the yield of SHPS is approximately 30–50 kg/ton of steel.55−57 Regarding SHPS, to treat its toxicity, recover its resources, or assess its environmental risks, Fekete et al.58 studied the separation of zinc and iron from SHPS by sulfation. Arsenovic et al.59 studied the removal of toxic metals from SHPS by fixing it in a brick structure. Ferreira et al.60 evaluated the drying of SHPS using an active integrated solar dryer. Sikalidis et al.61 investigated the phase changes of SHPS after thermal treatments, aiming to determine the appropriate conditions for its transformation into useful iron oxide pigment powders. Zhou et al.62 analyzed SHPS from the aspects of its metal speciation, concentration, leaching toxicity, and risk assessment. However, as mentioned above regarding the existing literature, most of the present work on SHPS lacks systematic and relatively comprehensive research on its composition, character, and leaching toxicity. In addition, the SHPS yield is large, but its main treatment makes it safe only for landfills. Therefore, the systematic study of the properties of SHPS presented in this article is necessary to provide support for the efficient resource utilization of SHPS and realize cleaner production in metal surface treatment industries.
The objective of the present study is to systematically investigate the composition and leaching toxicity of SHPS, focusing on its color and appearance, pH, moisture content, chemical (including the contents of nonmetal elements, metal elements, and anions) and phase compositions, microstructure, and leaching toxicity. The mechanism for formation of SHPS has also been reported. In addition, the research results tested in each part are also analyzed carefully and discussed.
2. Experiment
2.1. Preparation of SHPS Samples
Six typical SHPS samples were collected from the Jinghai District of Tianjin in China, as shown in Figure 1. Sludges 1 and 2 were from two hot-dip galvanized steel wire manufacturers, while sludges 3 and 4 were from two hot-dip galvanized steel pipe manufacturers, and sludges 5 and 6 were from two hot-dip galvanized steel sheet manufacturers. The SHPS generation of these six manufacturers was basically the same except for each processing condition. There are many hot-dip galvanized steel plants in the Jinghai District; thus, the environmental problems caused by SHPS are very prominent in this district.
Figure 1.

Photographs of the sludge samples after drying and crushing.
Two sampling methods, systematic sampling and multipoint sampling, were adopted when collecting and preparing samples after referring to the technical specifications on sampling and sample preparation from industrial solid waste (HJ/T 20-1998, China).101
The drying process of the samples referred to the iron ores—determination of moisture content—gravimetric method (GB/T6730.2-2018, China).102 Briefly, 1 kg of each sample material was weighed, placed on a tray, flattened to a thickness of 30 mm or less, and placed in a 105 °C oven to measure the moisture content. Weight loss was recorded every 30 min until a constant weight was reached. To perform XRD and other analyses, the dried SHPS was ground into a powder and sieved by passing it through a 200 mesh (size approximately <74 μm).
2.2. Analytical Methods and Instruments
The elemental content of the samples was determined by microwave digestion. A dry sample weighing 0.100–0.200 g was placed in a 50 mL Teflon digestion tube, and 5 mL of nitric acid, 2 mL of perchloric acid, and 2 mL of hydrofluoric acid were added. The mixture was covered until the resulting white smoke was exhausted, 0.5 mL of boric acid was added, the mixture was placed in a graphite digestion instrument at 160 °C overnight, and then it was transferred to a volume of 50 mL. Then, the elemental content was tested three times by inductively coupled plasma mass spectrometry (an Agilent ICP-MS 7700 instrument made in America). The chlorine content in the powder samples was analyzed using an ARLAdvantX Intellipower TM3600 X-ray fluorescence (XRF) spectrometer (Thermo Fisher, USA). Before the XRF test, an ultrahigh-pressure sample (UHPS) preparation system was used to directly press the sample powder into discs, and then the XRF test was carried out. The anions (Cl–, SO42–, F–, NO3–) were determined by ion chromatography (IC) using Shimadzu LC20ADsp with a Shodex IC SI-52 4E anion column. Before the IC test, the samples were centrifuged and filtered with an On-Guard H column and a 0.45 μm filter membrane and then injected for analysis. X-ray diffraction (XRD) patterns of the samples between 10 and 80° (2θ) at a step size of 0.02° were obtained with a Rigaku Ultimate-IV X-ray diffractometer utilizing a Cu Kα radiation source operated at 40 kV and 100 mA to determine the crystalline phases. Before the XRD test, the sample was prepared by the tablet pressing method: The sample powder was sprinkled into the window of the sample preparation frame as evenly as possible, chopped gently with the knife edge of a small spatula to spread the powder evenly, and stacked inside the window hole. Then, the powder was pressed gently with a small spatula, and finally the excess protruding powder was scraped off with a safety blade. The sample preparation frame was then carefully picked up from the glass plane to obtain a very flat plane of sample powder. Scanning electron microscopy (SEM) images were recorded by a Zeiss Sigma 300 scanning electron microscope operated at 2.0 kV to observe the morphology. Before the SEM test, a conductive adhesive was prepared, the sample powder was poured on the conductive adhesive, the base was held in the hand and vibrated on a table to disperse and spread the powder, and then the sample was purged to ensure that all particles were in full contact with the conductive adhesive and were distributed in a single layer without stacking. Finally, a thin layer of sample was obtained. In addition, the SHPS samples have good conductivity, so coating with a thin conductive layer was not necessary. The pH values were analyzed by a pH analyzer (Lei Magnetic Precision Benchtop pH Meter PHS-25, China). All experiments were conducted in duplicate, and the averaged results were used for analysis. The variation between the replicates for each analysis, including the blank control, was below 5%.
2.3. Leaching Toxicity Test Experiments
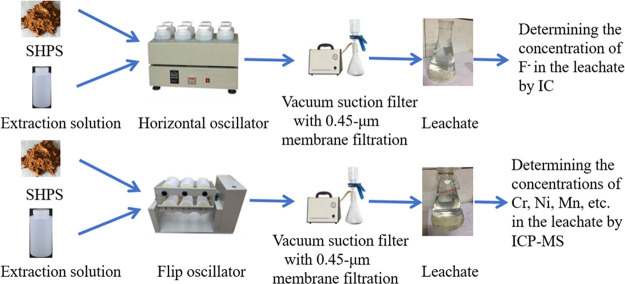
The leaching toxicity of fluorine ions in the samples was studied by using the solid-waste leaching toxicity method—the horizontal oscillation method (HJ 557-2010, China).103 Each sample was treated with an extraction solution (pure water) and at a liquid-to-solid ratio of 10:1 in a bottle. After the bottle cap was closed, the bottle was fixed vertically on a horizontal oscillation device, the oscillation frequency was adjusted to 110 ± 10 times/min, and the amplitude was adjusted to 40 mm. After shaking at room temperature for 8 h, the extraction bottle was removed and allowed to stand for 16h. A 0.45 μm microporous filter membrane was installed on a vacuum filtration device, and the leaching solution was filtered and collected. The leachate was analyzed for F– by IC.
The leaching toxicity test for metals was conducted as per the solid-waste extraction procedure for leaching toxicity—sulfuric acid/nitric acid method (HJ/T 299-2007, China).104 Each sample was treated with an extraction solution (a mixture of sulfuric acid, nitric acid, and pure water) at a pH of 3.20 ± 0.05 and a liquid-to-solid ratio of 10:1 in a bottle. The bottle was flipped in an end-over-end extractor at 30 rpm and 23 °C for 18 h. At the end of the extraction, the leachate was filtered through a 0.45 μm membrane filter to remove suspended solids. The leachate was analyzed for Cr, Ni, Mn, etc. by an Agilent ICP-MS 7700 instrument made in America. Figure 2 shows a schematic image of the leaching apparatus for this experiment.
Figure 2.
Schematic diagram of the leaching toxicity test unit.
3. Results and Discussion
3.1. Color and Appearance of Samples
Figure 1 shows a picture of the sludge samples after drying and crushing, and it shows that the color of the SHPS samples varies from red to dark brown, depending on the composition and free water content of SHPS after drying.63 Sludge 1, sludge 2, and sludge 6 are dark brown to black, which may be related to the certain amount of black Fe3O4 contained in these samples. Sludge 3 and sludge 4 are slightly yellow in color, possibly because they contain yellow FeO(OH). Sludge 5 contains a large amount of red Fe2O3, which may be the reason why sample 5 shows a bright red color. SHPS is formed by the agglomeration of very fine particles, which are soft and easy to break.
3.2. pH and Moisture Content of the Samples
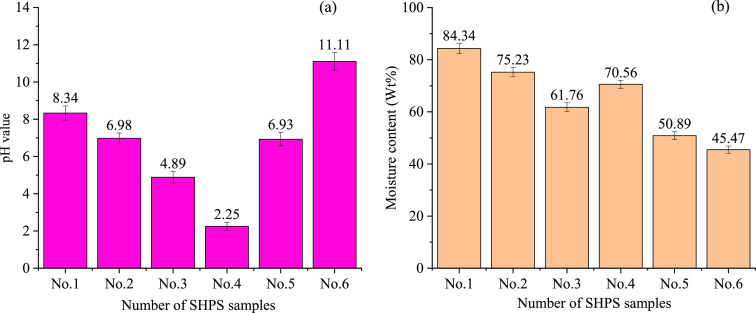
The pH and moisture content of the SHPS samples are shown in Figure 3. The pH is related to the degree of effective reaction between the acid and the neutralizing base agent added during the neutralization process, and eqs 1–3 give the reaction of the alkaline reagents to neutralize HPWL. Equations 4–9 show that heavy metal cations in HPWL combine with the hydroxide ions in the alkaline substances to form precipitates that can be removed, and the anions in HPWL combine with the cations in the alkaline substances to form salts; thus, the pH of the neutralized solution reaches 7–8. Figure 4 shows a schematic diagram of the production process of SHPS. The test results (Figure 3a) indicate that the pH of samples 1, 2, and 5 is approximately 7, ranging from 6.93 to 8.34. The pH of sample 3 is 4.89, which is slightly acidic, and the source of acidity is presumably the free acid generated by the hydrolysis of FeCl2. The reaction equation is shown in eq 10. Sludge 4 has a pH below 2.5, which may be due to the added alkali neutralizing reagent not being enough, or the addition is a sufficient amount, but the degree of the acid and base neutralization reaction is not high enough, resulting in the acid neutralization not being complete. Obviously, both sludge 3 and sludge 4 contain a certain amount of HCl residual acid. Although the reasons why they contain residual acid may be different, residual acid is a harmful waste that will cause potential environmental problems (serious water and air pollution) and threaten human health.64 The pH of sample 6 is 11.11 because the enterprise added an excess amount of neutralizing agents during the HPWL neutralization stage, resulting in the pH of the sludge being strongly alkaline. Excessive addition of a neutralizer will cause a large amount of neutralizer to be wrapped by other flocs and then sink in the sludge without participating in the reaction, resulting in a large sludge yield. Therefore, the dose of neutralizer should be controlled during neutralization.
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
Figure 3b shows that the moisture content of the samples ranges from 45.47% to 83.34%, which mainly includes free water and a small amount of bound water.65 The different water contents of the SHPS samples may be related to the steel pickling process, HPWL treatment process, and types and use of filter presses in the various enterprises. At present, SHPS dewatering is generally conducted with plate-and-frame filters, while a few enterprises use belt-filter presses for dewatering. These different treatment processes led to the different water contents of the SHPS samples. When SHPS is roasted, the free water rapidly volatilizes from ambient temperature to 100 °C, and when SHPS is heated to 300 °C, the bound water in the SHPS volatilizes.
Figure 3.
pH and moisture content of the SHPS samples: (a) pH and (b) moisture content (%).
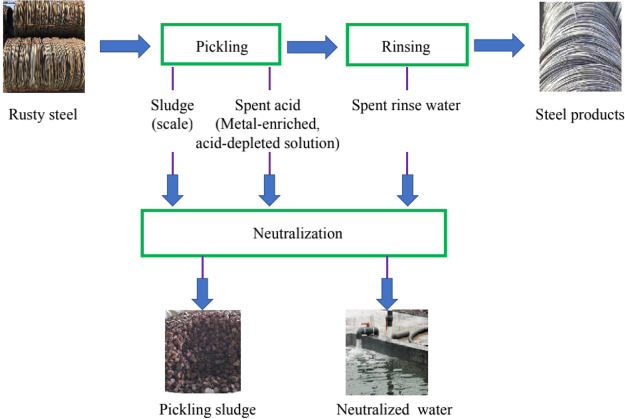
Figure 4.
Generation of SHPS in pickling lines.
3.3. Chemical and Phase Composition of Samples
Figure 5 and Figure 6 show the major elements in SHPS. Different steel plants may have different pickling processes, thereby producing different types of sludge; however, the main chemical components of sludge can be predicted on the basis of the steel composition, pickling agents, and HPWL treatment reagents used. As the test results (Figure 5 and Figure 6) show, the SHPS samples from different manufacturers were detected to contain Si, Al, K, Na, Ca, Cl, P, S, Fe, Zn, Mn, Pb, Cr, Hg, Ni, and As to different degrees. Table 1 shows the categories and main chemical components (except iron) of the steel to be pickled by these six hot-dip galvanizing manufacturers.
Figure 5.
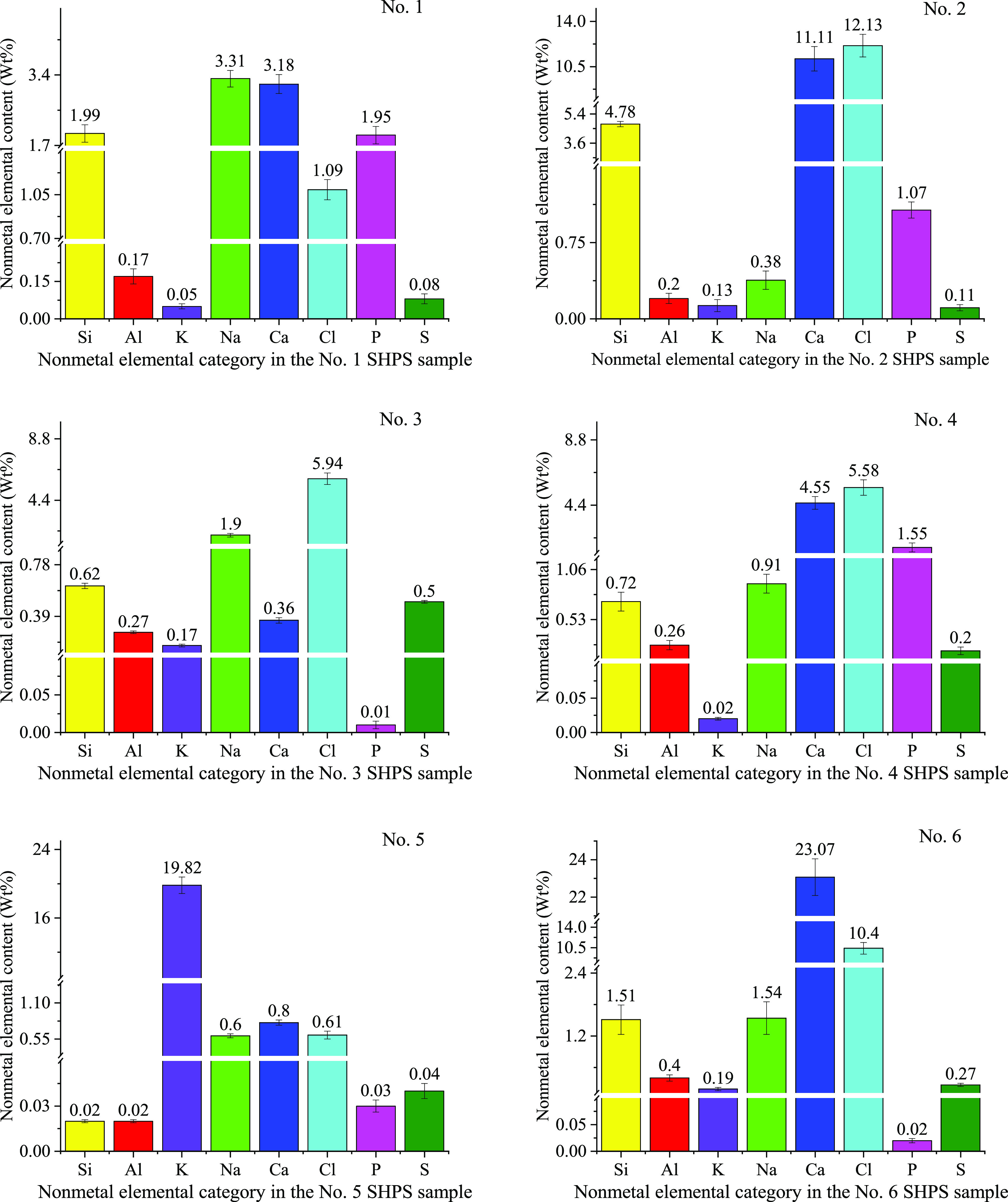
Analysis results of the nonmetal elements in the SHPS samples (wt %).
Figure 6.
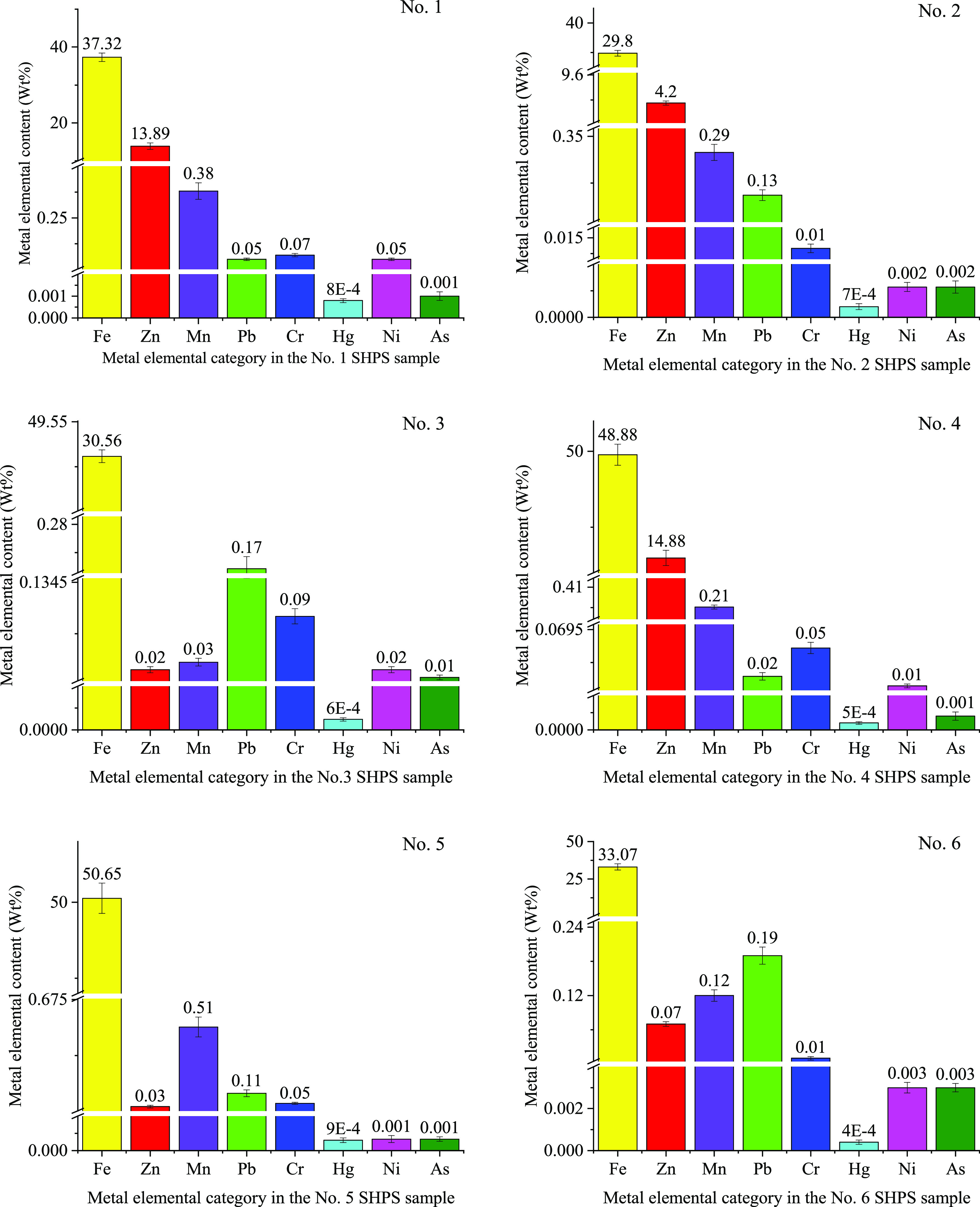
Metal contents of the SHPS samples.
Table 1. Types and Components of Steel Used by Six Hot-Dip Galvanizing Manufacturers (wt %).
| steel
composition |
||||||
|---|---|---|---|---|---|---|
| manufacturer no. | steel category | C | Si | Mn | P | S |
| manufacturer 1 (steel wire) | SWRH82B | 0.80–0.85 | 0.12–0.32 | 0.30–0.60 | <0.025 | <0.025 |
| manufacturer 2 (steel wire) | SWRH77B | 0.74–0.81 | 0.15–0.35 | 0.60–0.90 | <0.030 | <0.030 |
| manufacturer 3 (steel pipe) | Q215B | 0.24–0.32 | 0.17–0.37 | 0.80–1.10 | <0.035 | <0.035 |
| manufacturer 4 (steel pipe) | Q235A | ≤0.22 | ≤0.35 | 0.30–0.65 | ≤0.045 | ≤0.045 |
| manufacturer 5 (steel sheet) | DX51D | ≤0.07 | ≤0.07 | ≤0.50 | <0.025 | <0.025 |
| manufacturer 6 (steel sheet) | SGCC | ≤0.10 | ≤0.05 | ≤0.50 | <0.035 | <0.025 |
Table 1 shows that the steel used by these hot-dip galvanizing manufacturers belongs to carbon steel (with an alloy content of less than or equal to 6%), and the difference in composition is not very large. Therefore, the difference in the chemical and phase composition of SHPS samples produced by these manufacturers is mainly related to the pickling process and the treatment process of pickling waste liquid.
Since hydrochloric acid is used in pickling, and sodium hydroxide, lime, or potassium hydroxide is used in neutralization, the contents of chlorine, calcium, sodium, and potassium in the SHPS samples are extremely high, while the content of sulfur is relatively low, as shown in Figure 5. According to an investigation of the conducted neutralization steps, samples 1, 4, and 6 were treated with lime and sodium hydroxide, so the Ca and Na contents in these three samples are high. The Ca content of sample 2 is 11.11 wt % because the enterprise only uses lime in the neutralization process. The Na content of sample 3 is 1.9 wt % because the enterprise only adds sodium hydroxide for precipitation. The K content of sample 5 is 19.82 wt % because the enterprise only uses potassium hydroxide in the HPWL neutralization stage. In addition to neutralizers, the manufacturers of sludge 1, sludge 2, sludge 5, and sludge 6 also add small amounts of oxidants, flocculants, and other reagents. Si and Al in SHPS samples may generally come from a silica-particle flocculant and a polyaluminum chloride (PAC) flocculant that are separately used in HPWL treatment. Sometimes, in addition to a flocculant, some manufacturers also add polyacrylamide (PAM) coagulant aids to play a synergistic role, making it easier to settle and separate the precipitates.66−69
The major metal element contents of SHPS are shown in Figure 6. The content of iron is high in SHPS, whereas the contents of manganese, lead, chromium, mercury, nickel, and arsenic are relatively low. The metal content distribution in the SHPS samples mainly arises because the steel pickled with hydrochloric acid is usually ordinary carbon steel, which has the highest iron content and contains less Mn, Ni, Cr, and other metals; carbon steel is usually composed of 0.07–0.85 wt % C, 0.30–1.10 wt % Mn, 0.05–0.37 wt % Si, 0.025 wt % S, 0.0045 wt % P, and a balance of Fe.9
The zinc content is high in samples 1, 2, and 4 but relatively low in samples 3, 5, and 6. The reason for this result is that usually when steel is subsequently galvanized and a coating too poor for applications is produced, stripping is required to rework and reproduce the product. Stripping is usually carried out in a 1:1 hydrochloric acid solution. The waste liquid produced by stripping usually contains high concentrations of zinc, iron, and free acid. The main chemical composition of the waste liquid from a galvanization plant in Jieyang, Guangdong Province, China, is shown in Table 2. Some small manufacturers mix the washing water and stripping solution from the galvanization process with the HPWL from the pickling process and then treat the mixed waste liquid with the NP method; when this method is utilized, the SHPS produced by NP will have a high zinc content.68−71 SHPS samples with a high zinc content are generally taken from metal surface processing plants that perform both pickling and galvanization processes, such as wire rope hot-dip galvanization plants and automotive component galvanization plants.68,69 Of course, some large-scale manufacturers also separate pickling waste liquid from stripping waste liquid to facilitate treatment, so the zinc content of the produced pickling sludge is low.71,72 In addition, in a hot-dip galvanization factory, when hanging steel during pickling, the zinc covering the hanging piece (such as hooks, jigs, cages, or baskets) may be partially dissolved into the pickling solution, which will clearly cause the sludge sample to contain trace amounts of zinc.5,73
Table 2. Main Chemical Composition of the Stripping Waste with Hydrochloric Acid.
| item | W(Zn) | W(Fe 2+) | W(Fe 3+) | acidity (via HCl) |
|---|---|---|---|---|
| content (%) | 4.25 | 9.31 | 0.10 | 4.11 |
Anion chromatography was used to test the anions in the samples, and the results are provided in Figure 7. The species and content of the anions in SHPS directly reflect the acid used by the plant in the pickling process. The anionic content in the SHPS varies greatly, and the content of chloride ions is the highest, followed by that of sulfate ions, fluoride ions, and nitrate ions.
Figure 7.

Analysis results of the anions in the SHPS samples.
To determine the phases in the SHPS samples, the XRD results of the samples are shown in Figure 8. The XRD results show that the diffraction peaks are complicated, and NaCl, FeO(OH), Fe3O4, Fe2O3, ZnFe2O4, ZnO, CaO, and CaCO3 are the predominant phases in SHPS. Table 3 summarizes the common occurrence states of the major elements in accordance with Figure 8. No nickel-, manganese-, or chromium-containing phases are observed in the XRD patterns because of the poor crystallization of their hydroxides; additionally, their contents in SHPS are too low to be detected.65
Figure 8.

XRD patterns of the SHPS samples after drying at 105 °C.
Table 3. Occurrence States of the Major Elements in the SHPS Samples.
| element | occurrence states of phases |
|---|---|
| Fe | FeO(OH), Fe3O4, Fe2O3, ZnFe2O4 |
| Zn | ZnFe2O4, ZnO, Fe2ZnO4 |
| Cl | NaCl |
| Ca | CaO, CaCO3 |
| Si | SiO2 |
As listed in Table 3, Fe and Zn are mainly present in complex oxides. This state of existence is mainly due to the dehydration of the hydroxides of Fe and Zn during the drying process of wet sludge, which generates their corresponding metal oxides. Regarding wet sludge samples, the relevant chemical reactions that mainly occur during the drying process are shown in eqs 11–14. Cl is found in NaCl, Ca is mainly found in CaO and CaCO3, and Si is mainly found in SiO2. CaCO3 forms to some extent as a result of the reaction between Ca(OH)2 and CO2. The corresponding chemical reaction is shown in eq 14. It is also possible that the addition of CaCO3 is wrapped by other flocculants and deposited in the sludge before it can participate in the reaction.
| 11 |
| 12 |
| 13 |
| 14 |
3.4. Microstructure of the Pickling Samples
The morphology of the pickling sludge samples is presented in Figure 9.
Figure 9.
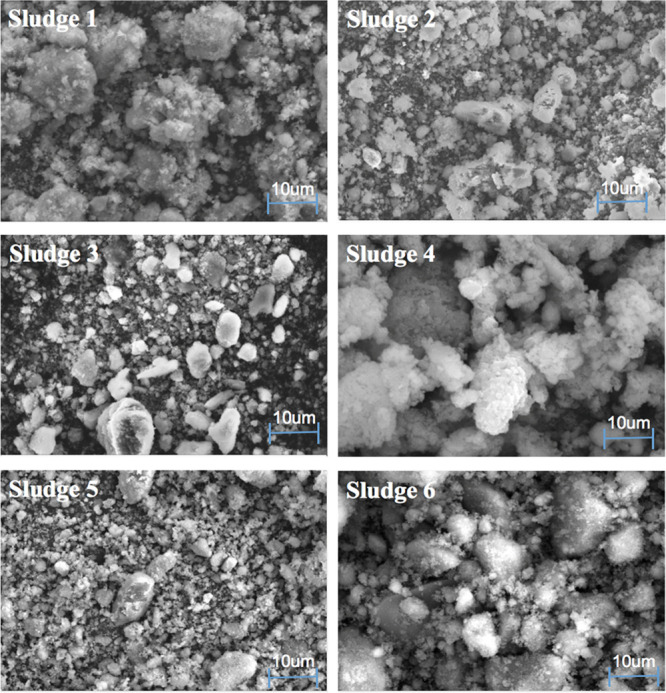
Scanning electron micrographs of SHPS samples.
As shown in Figure 9, the SHPS has a microscopic structure of bright and fluffy irregular spheres, stripes, flakes; dark and very small irregular particles; and the structure of the sludge is complicated. The SEM results in Figure 9 reveal that the particle sizes of the NaOH- and KOH-derived iron oxides are mostly in the range of 10 to 50 μm. Obviously, the agglomerates in the sample must be dispersed if the valuable elements in SHPS are to be recovered.
3.5. Leaching Toxicity of the Samples
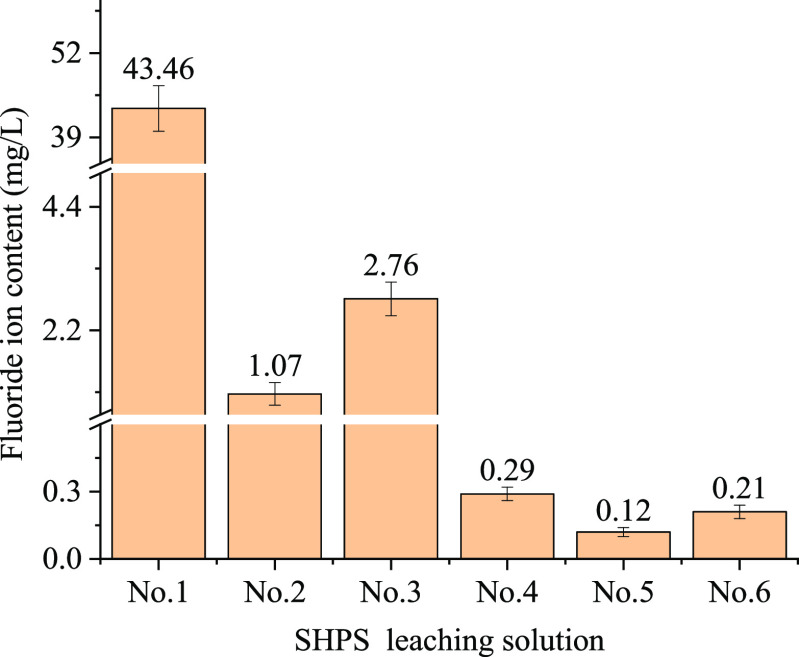
According to the national standard, the technical code for the identification of hazardous waste (HJ/T 298-2019, China) was adopted,105 and the leaching toxicity of fluorine in the samples was studied by using the solid-waste leaching toxicity method—horizontal oscillation method (HJ 557-2010, China).103 Furthermore, an IC test was carried out on the leaching solution using a Shimadzu LC20ADsp. The content of fluoride ions in the leaching solution is presented in Figure 10.
Figure 10.
Analysis results of the fluoride ions in the leaching solutions.
To estimate the toxicity effects of metals, namely, chromium, zinc, manganese, nickel, lead, and arsenic, in SHPS, the solid-waste extraction procedure for leaching toxicity—sulfuric acid/nitric acid method (HJ/T 299-2007, China) was adopted.104Figure 11 shows the concentrations of heavy metals, namely, zinc, manganese, lead, chromium, nickel, and arsenic, in the leachates. The detected concentrations of barium and mercury are <0.001 and <0.001 mg/L, and although the alkyl mercury content was also tested, no alkyl mercury was detected; thus, the leaching concentrations of these three toxic heavy metals are not shown in Figure 11.
Figure 11.

Heavy metal contents in the different SHPS samples (quantities in mg/L).
For comparison of these results with standard regulatory limits, the values from the identification standards for hazardous wastes—identification for extraction toxicity (GB5085.3-2007, China)106 are provided in Table 4.
Table 4. Regulatory Limits Provided by GB5085.3-2007 for Hazardous Wastes (Quantities in mg/L).
| F | Zn | Pb | Cr | Hg | alkyl Hg | Be | Ni | As |
|---|---|---|---|---|---|---|---|---|
| 100 | 100 | 5 | 15 | 0.1 | not detectable | 0.02 | 5 | 5 |
From Figures 10 and 11 and Table 4, the contents of fluoride ions and most metal elements are clearly lower than the emission requirements of GB5085.3-2007;106 however, Zn in sample 1 and sample 4 and Ni in sample 1 are beyond the permissible levels of less than 100 and 5 mg/L, respectively, indicating that Zn and Ni are the most significant heavy metals in SHPS. The XRD results of the samples after leaching by the sulfuric acid/nitric acid method are shown in Figure 12.
Figure 12.

XRD patterns of the SHPS samples after leaching.
Compared with the XRD diffraction patterns of the samples in Figure 8, the NaCl peaks disappear, and the diffraction peaks of CaCO3, Fe3O4, Fe2O3, ZnO, and ZnFe2O4 significantly decrease, as shown in Figure 12. This may occur because NaCl is easily dissolved in the leaching solution, and CaCO3, Fe3O4, Fe2O3, ZnO, and ZnFe2O4 can react with the acid. This is consistent with the findings of Brück et al.66 Zhang and Liu’s research shows that when a sulfuric acid concentration of 20% is applied to SHPS with a w/v of 1:1.5, a reaction time of 30 min, and a reaction temperature of 20 °C, 96.20% of SHPS is extracted and most metal elements in SHPS can be leached except for Pb.74 These results clearly reveal that SHPS can be partially dissolved in weak acids and largely dissolved in concentrated sulfuric acids; a large amount of Zn in SHPS can be leached by sulfuric acid under appropriate reaction conditions.
The HJ/T 299-2007 method uses a nitric acid/sulfuric acid mixed solution as the extraction solution to simulate the process of hazardous components leaching from waste and entering the environment under the influence of acid precipitation when waste is landfilled or stacked or when land is used after harmless treatment.104 The SHPS leaching toxicity test results above reveal that SHPS is a hazardous waste with leaching toxicity. Pinto et al.75 evaluated the bioavailability and genotoxicity to cells of SHPS. They found a decrease in the germination speed of Lactuca sativa seeds and the appearance of chromosomal aberrations in cytotoxicity tests. They noted that an increase in the SHPS concentration in soil decreases the speed of germination and increases the number of chromosomal aberrations related to aneugenic phenomena that promote tumor development. Thus, SHPS is a serious environmental hazard and cannot be discarded without the use of prevention and control measures. Once SHPS has been improperly disposed of, it can cause soil and water contamination. Mixed residual hydrochloric acid and dissolved metal from SHPS can be absorbed by plants and aquatic animals and even be ingested in soluble form, after which these materials can be bioaccumulated and biomagnified.64,76
4. Conclusions
Based on the test results, the following conclusions can be drawn:
-
(1)
The colors of the SHPS samples vary from red to dark brown. SHPS is formed by agglomeration of very fine particles, which are soft and easy to break. The pH of the SHPS ranges from 2.25 to 11.11, and the moisture content is approximately 46% to 84%. Due to the high moisture content of SHPS, energy-saving dehydration should be considered. The contents of Cl, Ca, K, and Na range from 0.61% to 12.13%, 0.36% to 23.07%, 0.02% to 19.82%, and 0.38% to 3.31%, respectively. Additionally, Fe has the highest concentration with values from 29.8% to 50.6%, while other heavy metal elements, namely, Zn, Ni, Mn, Cr, and Pb, have contents of 0.02% to 14.9%, 0.001% to 0.05%, 0.03% to 0.38%, 0.01% to 0.09%, and 0.02% to 0.19%, respectively. Anions, namely, SO42–, Cl–, F–, and NO3–, have contents of 0.09% to 0.34%, 0.54% to 5.73%, 0.001% to 0.04%, and 0.01% to 0.15%, respectively. XRD analysis shows that Fe and Zn are mainly present as oxides, Ca is present as CaO and CaCO3, and chlorine is present as NaCl. Moreover, SEM analysis shows that the microscopic structure consists mainly of bright and fluffy irregular spheres; stripes; flakes; and dark, very small irregular particles. The SHPS characterization results demonstrate that the material has an inorganic composition comprising metallic oxides (principally Fe, Zn, and Mn) and water-soluble chlorides. The chemical composition of SHPS generated from different pickling processes varies widely, and such SHPS samples should be collected and utilized separately.
-
(2)
The leaching toxicity test performed on the SHPS sample shows that the concentrations of Zn and Ni in the leaching solution exceed the limits defined in GB5085.3-2007 and that Zn and Ni will pollute the environment as a result of water leaching.106 Proper sludge management is needed, including waste minimization, metal recovery, and safe disposal of sludge. Care should be taken in handling SHPSs in the metal pickling industry to avoid health hazards. Effective extraction of the harmful metals Zn and Ni from SHPS and recovery of a large proportion of Fe are good measures for realizing harmless utilization of SHPS resources.
Acknowledgments
This work was supported by the National Key R&D Plan (2019YFC1904600), the National Natural Science Foundation of China (Grant No. 51704016, 51074170), the State Key Laboratory of Environmental Protection in the Iron and Steel Industry (Grant No. Yzc2017Ky03), the Key Laboratory for Exploration and Comprehensive Utilization of Coal Resources of the Ministry of Land and Resources Open Research Topic (Grant No. KF2016-3), Xinjiang Uygur Autonomous Region University Scientific Research Plan Project (Grant No. XJEDU2019Y073), and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Grant No. 2021D01F37). The authors express their thanks to all the scientists associated with this article for their encouragement and assistance.
Glossary
Acronyms
- SHPS
steel hydrochloric acid pickling sludge
- HAP
hydrochloric acid pickling
- HPWL
hydrochloric pickling waste liquid
- NP
neutralization precipitation
The authors declare no competing financial interest.
References
- Chandra-Ambhorn S.; Tungtrongpairoj J.; Jutilarptavorn A.; Nilsonthi T.; Somphakdee T. On the hot-rolled recycled carbon steels: their oxide formation, pickling ability and scale adhesion. Anti-Corros. Methods Mater. 2019, 66, 294–299. 10.1108/ACMM-07-2018-1974. [DOI] [Google Scholar]
- Cunha T. N. D.; Trindade D. G.; Canesin M. M.; Effting L.; De -Moura A. A.; MoiséS M. P.; Costa Junior I. L.; Bail A. Reuse of Waste Pickling Acid for the Production of Hydrochloric Acid Solution, Iron(II) Chloride and Magnetic Iron Oxide: An Eco-Friendly Process. Waste Biomass Valor. 2021, 12, 1517–1528. 10.1007/s12649-020-01079-1. [DOI] [Google Scholar]
- Ding J.; Tang B.; Li M.; Feng X. F.; Fu F. L.; Bin L. Y.; Huang S. S.; Su W.; Li D. N.; Zheng L. C. Difference in the characteristics of the rust layers on carbon steel and their corrosion behavior in an acidic medium: Limiting factors for cleaner pickling. J. Clean Prod. 2017, 142, 2166–2176. 10.1016/j.jclepro.2016.11.066. [DOI] [Google Scholar]
- Regel-Rosocka M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. 10.1016/j.jhazmat.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Culcasi A.; Gueccia R.; Randazzo S.; Cipollina A.; Micale G. Design of a novel membrane-integrated waste acid recovery process from pickling solution. J. Clean Prod. 2019, 236, 117623. 10.1016/j.jclepro.2019.117623. [DOI] [Google Scholar]
- Krishnan M.; Subramanian H.; Dahms H. U.; Sivanandham V.; Seeni P.; Gopalan S.; Mahalingam A.; Rathinam A. J. Biogenic corrosion inhibitor on mild steel protection in concentrated HCl medium. Sci. Rep. 2018, 8, 2609. 10.1038/s41598-018-20718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odewunmi N. A.; Mazumder M. A. J.; Ali S. A.; Aljeaban N. A.; Alharbi B. G.; Al-Saadi A. A.; Obot I. B. Impact of Degree of Hydrophilicity of Pyridinium Bromide Derivatives on HCl Pickling of X-60 Mild Steel: Experimental and Theoretical Evaluations. Coatings 2020, 10, 185. 10.3390/coatings10020185. [DOI] [Google Scholar]
- Tang B.; Su W.; Wang J.; Fu F. L.; Yu G. J.; Zhang J. Y. Minimizing the creation of spent pickling liquors in a pickling process with high-concentration hydrochloric acid solutions: Mechanism and evaluation method. J. Environ. Manage. 2012, 98, 147–154. 10.1016/j.jenvman.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Agrawal A.; Sahu K. K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. 10.1016/j.jhazmat.2009.06.099. [DOI] [PubMed] [Google Scholar]
- Ministry of Ecology and Environment of the People's Republic of China. China National List of Hazardous Waste (2021 version). Order No. 15 of the Ministry of Ecology and Environment; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 2020.
- Cai J. C.; Guo F. Mass transfer during membrane distillation treatment of wastewater from hot-dip galvanization. Sep. Purif. Technol. 2020, 235, 116164. 10.1016/j.seppur.2019.116164. [DOI] [Google Scholar]
- Leonzio G. Recovery of metal sulphates and hydrochloric acid from spent pickling liquors. J. Clean Prod. 2016, 129, 417–426. 10.1016/j.jclepro.2016.04.037. [DOI] [Google Scholar]
- Tomaszewska M.; Gryta M.; Morawski A. W. Recovery of hydrochloric acid from metal pickling solutions by membrane distillation. Sep. Purif. Technol. 2001, 22–23, 591–600. 10.1016/S1383-5866(00)00164-7. [DOI] [Google Scholar]
- Ferreira A. S.; Mansur M. B. Statistical analysis of the spray roasting operation for the production of high quality Fe2O3 from steel pickling liquors. Process Saf. Environ. Protect. 2011, 89, 172–178. 10.1016/j.psep.2010.11.005. [DOI] [Google Scholar]
- Lv C. Numerical Simulation on the Recovery Process of Acid Pickling Waste Liquor by Jet-Flow Pyrolysis. JOM. 2019, 71, 4944–4949. 10.1007/s11837-019-03827-8. [DOI] [Google Scholar]
- Stocks C.; Wood J.; Guy S. Minimisation and recycling of spent acid wastes from galvanizing plants. Resour. Conserv. Recycl. 2005, 44, 153–166. 10.1016/j.resconrec.2004.11.005. [DOI] [Google Scholar]
- Leonzio G. Mathematical modeling and environmental analysis of heat pumps integrated in aprocess to treat spent pickling liquors. J. Clean Prod. 2016, 133, 835–849. 10.1016/j.jclepro.2016.05.185. [DOI] [Google Scholar]
- Özdemir T.; Öztin C.; Kincal N. S. Treatment of waste pickling liquors: process synthesis and economic analysis. Chem. Eng. Commun. 2006, 193, 548–563. 10.1080/00986440500192238. [DOI] [Google Scholar]
- Wang Y. M.; He Y.; Yin S. H.; Long H. L.; Li S. W. Research on extraction of zinc from spent pickling solution using Aliquat 336. Hydrometallurgy 2020, 193, 105322. 10.1016/j.hydromet.2020.105322. [DOI] [Google Scholar]
- Carrillo-Abad J.; Garcia-Gabaldon M.; Ortiz-Gandara I.; Bringas E.; Urtiaga A. M.; Ortiz I.; Perez-Herranz V. Selective recovery of zinc from spent pickling baths by the combination of membrane-based solvent extraction and electrowinning technologies. Sep. Purif. Technol. 2015, 151, 232–242. 10.1016/j.seppur.2015.07.051. [DOI] [Google Scholar]
- Lum K. H.; Stevens G. W.; Kentish S. E. Development of a process for the recovery of zinc sulphate from hot-dip galvanizing spent pickling liquor via two solvent extraction steps. Hydrometallurgy 2014, 142, 108–115. 10.1016/j.hydromet.2013.11.016. [DOI] [Google Scholar]
- Sinha M. K.; Sahu S. K.; Meshram P.; Pandey B. D. Solvent extraction and separation of zinc and iron from spent pickle liquor. Hydrometallurgy 2014, 147–148, 103–111. 10.1016/j.hydromet.2014.05.006. [DOI] [Google Scholar]
- Regel-Rosocka M.; Wisniewski M. Selective removal of zinc(II) from spent pickling solutions in the presence of iron ions with phosphonium ionic liquid Cyphos IL 101. Hydrometallurgy 2011, 110, 85–90. 10.1016/j.hydromet.2011.08.012. [DOI] [Google Scholar]
- Mansur M. B.; Rocha S. D. F.; Magalhães F. S.; Benedetto J. S. B. Selective extraction of zinc(II) over iron(II) from spent hydrochloric acid pickling effluents by liquid-liquid extraction. J. Hazard. Mater. 2008, 150, 669–678. 10.1016/j.jhazmat.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Chehade G.; Alrawahi N.; Yuzer B.; Dincer I. A photoelectrochemical system for hydrogen and chlorine production from industrial waste acids. Sci. Total Environ. 2020, 712, 136358. 10.1016/j.scitotenv.2019.136358. [DOI] [PubMed] [Google Scholar]
- Carrillo-Abad J.; García-Gabaldón M.; Pérez-Herranz V. pH effect on zinc recovery from the spent pickling baths of hot dip galvanizing industries. Sep. Purif. Technol. 2017, 177, 21–28. 10.1016/j.seppur.2016.12.034. [DOI] [Google Scholar]
- Carrillo-Abad J.; García-Gabaldón M.; Pérez-Herranz V. Study of the zinc recovery from spent pickling baths by means of an electrochemical membrane reactor using a cation-exchange membrane under galvanostatic control. Sep. Purif. Technol. 2014, 132, 479–486. 10.1016/j.seppur.2014.05.052. [DOI] [Google Scholar]
- Carrillo-Abad J.; García-Gabaldón M.; Pérez-Herranz V. Treatment of spent pickling baths coming from hot dip galvanizing by means of an electrochemical membrane reactor. Desalination 2014, 343, 38–47. 10.1016/j.desal.2013.11.040. [DOI] [Google Scholar]
- Carrillo-Abad J.; García-Gabaldón M.; Ortega E.; Pérez-Herranz V. Recovery of zinc from spent pickling solutions using an electrochemical reactor in presence and absence of an anion-exchange membrane: Galvanostatic operation. Sep. Purif. Technol. 2012, 98, 366–374. 10.1016/j.seppur.2012.08.006. [DOI] [Google Scholar]
- Carrillo-Abad J.; García-Gabaldón M.; Ortega E.; Pérez-Herranz V. Electrochemical recovery of zinc from the spent pickling baths coming from the hot dip galvanizing industry. Potentiostatic operation. Sep. Purif. Technol. 2011, 81, 200–207. 10.1016/j.seppur.2011.07.029. [DOI] [Google Scholar]
- Collivignarelli M. C.; Abbà A.; Bestetti M.; Crotti B. M.; Miino M. C. Electrolytic Recovery of Nickel and Copper from Acid Pickling Solutions Used to Treat Metal Surfaces. Water Air Soil Pollut. 2019, 230, 101. 10.1007/s11270-019-4158-1. [DOI] [Google Scholar]
- Csicsovszki G.; Kékesi T.; Török T. I. Selective recovery of Zn and Fe from spent pickling solutions by the combination of anion exchange and membrane electrowinning techniques. Hydrometallurgy 2005, 77, 19–28. 10.1016/j.hydromet.2004.10.020. [DOI] [Google Scholar]
- Gueccia R.; Randazzo S.; Chillura Martino D.; Cipollina A.; Micale G. Experimental investigation and modeling of diffusion dialysis for HCl recovery from waste pickling solution. J. Environ. Manage. 2019, 235, 202–212. 10.1016/j.jenvman.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Paquay E.; Clarinval A.-M.; Delvaux A.; Degrez M.; Hurwitz H. D. Applications of electrodialysis for acid pickling wastewater treatment. Chem. Eng. J. 2000, 79, 197–201. 10.1016/S1385-8947(00)00208-4. [DOI] [Google Scholar]
- San Román M. F.; Ortiz Gándara I.; Ibañez R.; Ortiz I. Hybrid membrane process for the recovery of major components (zinc, iron and HCl) from spent pickling effluents. J. Membr. Sci. 2012, 415–416, 616–623. 10.1016/j.memsci.2012.05.063. [DOI] [Google Scholar]
- Carrera J. A.; Muñoz E.; Bringas E.; San Román M. F.; Ortiz I. Influence of operation variables on the recovery of zinc from spent pickling effluents using the emulsion pertraction technology. Desalination 2009, 245, 675–679. 10.1016/j.desal.2009.02.036. [DOI] [Google Scholar]
- Ciminelli V. S. T.; Dias A.; Braga H. C. Simultaneous production of impurity-free water and magnetite from steel pickling liquors by microwave-hydrothermal processing. Hydrometallurgy 2006, 84, 37–42. 10.1016/j.hydromet.2006.03.058. [DOI] [Google Scholar]
- Hamann C.; Spanka M.; Stolle D.; Auer G.; Weingart E.; Al-Sabbagh D.; Ostermann M.; Adam C. Recycling of blast-furnace sludge by thermochemical treatment with spent iron(II) chloride solution from steel pickling. J. Hazard. Mater. 2021, 402, 123511. 10.1016/j.jhazmat.2020.123511. [DOI] [PubMed] [Google Scholar]
- Bao S. Y.; Tang L. H.; Li K.; Ning P.; Peng J. H.; Guo H. B.; Zhu T. T.; Liu Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4 @SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. 10.1016/j.jcis.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Pathak A.; Roy A.; Manna M. Recovery of zinc from industrial waste pickling liquor. Hydrometallurgy 2016, 163, 161–166. 10.1016/j.hydromet.2016.04.006. [DOI] [Google Scholar]
- Yang S.; Li W.; Zhang H. J.; Wen Y. B.; Ni Y. H. Treatment of paper mill wastewater using a composite inorganic coagulant prepared from steel mill waste pickling liquor. Sep. Purif. Technol. 2019, 209, 238–245. 10.1016/j.seppur.2018.07.049. [DOI] [Google Scholar]
- Lan W.; Qiu H. Q.; Zhang J.; Yu Y. J.; Yang K. L.; Liu Z. Z.; Ding G. J. Characteristic of a novel composite inorganic polymer coagulant-PFAC prepared by hydrochloric pickle liquor. J. Hazard. Mater. 2009, 162, 174–179. 10.1016/j.jhazmat.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Huang X. S.; Zhang Y. J.; Li X.; Duan J. G.; Xu B.; Xia S. B.; Dong P. Direct preparation of polysilicic acid flocculant by using pickling waste liquor of metal plate. J. Water. Process. Eng. 2020, 36, 101267. 10.1016/j.jwpe.2020.101267. [DOI] [Google Scholar]
- Chen D.; Hou J.; Yao L. H.; Jin H. M.; Qian G. R.; Xu Z. P. Ferrite materials prepared from two industrial wastes: Electroplating sludge and spent pickle liquor. Sep. Purif. Technol. 2010, 75, 210–217. 10.1016/j.seppur.2010.07.009. [DOI] [Google Scholar]
- Liu C. W.; Lin C. H.; Fu Y. P. Characterization of Mn-Zn Ferrite Prepared by a Hydrothermal Process From Used Dry Batteries and Waste Steel Pickling Liquor. J. Am. Ceram. Soc. 2007, 90, 3349–3352. 10.1111/j.1551-2916.2007.01902.x. [DOI] [Google Scholar]
- Lian J. T.; Ouyang Q.; Tsang P. E.; Fang Z. Q. Fenton-like catalytic degradation of tetracycline by magnetic palygorskite nanoparticles prepared from steel pickling waste liquor. Appl. Clay Sci. 2019, 182, 105273. 10.1016/j.clay.2019.105273. [DOI] [Google Scholar]
- Tang J. Z.; Pei Y. W.; Hu Q. H.; Pei D. D.; Xu J. P. The Recycling of Ferric Salt in Steel Pickling Liquors: Preparation of Nano-sized Iron Oxide. Procedia Environ. Sci. 2016, 31, 778–784. 10.1016/j.proenv.2016.02.071. [DOI] [Google Scholar]
- Sinha M. K.; Sahu S. K.; Meshram P.; Prasad L. B.; Pandey B. D. Low temperature hydrothermal synthesis and characterization of iron oxide powders of diverse morphologies from spent pickle liquor. Powder Technol. 2015, 276, 214–221. 10.1016/j.powtec.2015.02.006. [DOI] [Google Scholar]
- Huang R. X.; Fang Z. Q.; Fang X. B.; Tsang E. P. Ultrasonic Fenton-like catalytic degradation of bisphenol A by ferroferric oxide (Fe3O4) nanoparticles prepared from steel pickling waste liquor. J. Colloid Interface Sci. 2014, 436, 258–266. 10.1016/j.jcis.2014.08.035. [DOI] [PubMed] [Google Scholar]
- Wang X. Y.; Lv F. F. Synthesis of Fe3O4 Magnetic Powder from Spent Pickling Liquors. Trans. Tianjin Univ. 2018, 24, 45–50. 10.1007/s12209-017-0095-5. [DOI] [Google Scholar]
- Zhang W. X.; Lu B.; Tang H. H.; Zhao J. X.; Cai Q. H. Reclamation of acid pickling waste: A facile route for preparation of single-phase Fe3O4 nanoparticle. J. Magn. Magn. Mater. 2015, 381, 401–404. 10.1016/j.jmmm.2015.01.037. [DOI] [Google Scholar]
- Fang X. B.; Fang Z. Q.; Tsang P. K. E.; Cheng W.; Yan X. W.; Zheng L. C. Selective adsorption of Cr(VI) from aqueous solution by EDA-Fe3O4 nanoparticles prepared from steel pickling waste liquor. Appl. Surf. Sci. 2014, 314, 655–662. 10.1016/j.apsusc.2014.06.191. [DOI] [Google Scholar]
- Tang B.; Yuan L. J.; Shi T. H.; Yu L. F.; Zhu Y. C. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical coprecipitation. J. Hazard. Mater. 2009, 163, 1173–1178. 10.1016/j.jhazmat.2008.07.095. [DOI] [PubMed] [Google Scholar]
- Kang F.; Wu M.; Xiao B.; Chen R.; Wei Y.; Liu H.; Wu N. Facile synthesisof schwertmannite@akaganeitecore/shell nanostructure from pickling waste liquor: Formation mechanism and potential application. J. Clean Prod. 2020, 260, 120961. 10.1016/j.jclepro.2020.120961. [DOI] [Google Scholar]
- Vijay R.; Sihorwala T. A. Identification and leaching characteristics of sludge generated from metal pickling and electroplating industries by Toxicity Characteristics Leaching Procedure (TCLP). Environ. Monit. Assess. 2003, 84, 193–202. 10.1023/A:1023363423345. [DOI] [PubMed] [Google Scholar]
- Li X. M.; Wang S. J.; Zhao J. X.; Cui Y. R.; Hou S. B. A Review on the treatments and minimization techniques of stainless steel pickling sludge. Advanced Materials Research. Trans. Technol. Publ. 2011, 194–196, 2072–2076. 10.4028/www.scientific.net/AMR.194-196.2072. [DOI] [Google Scholar]
- Zhang S. R.; Jiang X. G.; Liu B. X.; Lv G. J.; Jin Y. Q.; Yan J. H. Co-combustion of bituminous coal and pickling sludge in a drop-tube furnace: thermodynamic study and experimental data on the distribution of Cr, Ni, Mn, As, Cu, Sb, Pb, Cd, Zn, and Sn. Energy Fuel 2017, 31, 3019–3028. 10.1021/acs.energyfuels.6b02872. [DOI] [Google Scholar]
- Junxue Z.; Zhongyu Z.; Ruimeng R.; Xiaoming L.; Yaro C. Issues Relevant to Recycling of Stainless-Steel Pickling Sludge. JOM 2018, 70, 2825–2836. 10.1007/s11837-018-3168-6. [DOI] [Google Scholar]
- Fekete F.; Lázár K.; Keszler A. M.; Jánosity A.; Zhibin L.; Szilágyi I. M.; Kótai L. Recycling the industrial waste ZnFe2O4 from hot-dip galvanization sludge. J. Therm. Anal. Calorim. 2018, 134, 1863–1872. 10.1007/s10973-018-7849-8. [DOI] [Google Scholar]
- Arsenovic M.; Radojevic Z.; Stankovic S. Removal of toxic metals from industrial sludge by fixing in brick structure. Constr. Build. Mater. 2012, 37, 7–14. 10.1016/j.conbuildmat.2012.07.002. [DOI] [Google Scholar]
- Ferreira A. G.; Gonçalves L. M.; Maia C. B. Solar drying of a solid waste from steel wire industry. Appl. Therm. Eng. 2014, 73, 104–110. 10.1016/j.applthermaleng.2014.07.047. [DOI] [Google Scholar]
- Sikalidis C.; Zorba T.; Chrissafis K.; Paraskevopoulos K. M. Iron oxide pigmenting powders produced by thermal treatment of iron solid wastes from steel mill pickling lines. J. Therm. Anal. Calorim. 2006, 86, 411–415. 10.1007/s10973-005-7168-8. [DOI] [Google Scholar]
- Zhou C. L.; Ge S. F.; Yu H.; Zhang T. Q.; Cheng H. L.; Sun Q.; Xiao R. Environmental risk assessment of pyrometallurgical residues derived from electroplating and pickling sludges. J. Clean Prod. 2018, 177, 699–707. 10.1016/j.jclepro.2017.12.285. [DOI] [Google Scholar]
- Ministry of Ecology and Environment of the People's Republic of China. Technical specifications on sampling and sample preparation from industry solid waste. Chinese Standard HJ/T 20-1998; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 1998.
- Standardization Administration Committee of the People’s Republic of China. Iron Ores—Determination of Moisture Content—Gravimetric Method. Chinese Standard GB/T6730.2-2018; Standardization Administration Committee of the People’s Republic of China: Beijing, China, 2018.
- Ministry of Ecology and Environment of the People's Republic of China. Solid-waste Leaching Toxicity Method- Horizontal Vibration Method. Chinese Standard HJ 557-2010; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 2010.
- Ministry of Ecology and Environment of the People's Republic of China. Solid-waste Extraction Procedure for Leaching Toxicity-Sulfuric Acid/Nitric Acid Method. Chinese Standard HJ/T 299-2007; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 2007.
- Li X. M.; Lv M.; Yin W. D.; Zhao J. X.; Cui Y. R. Desulfurization thermodynamics experiment of stainless steel pickling sludge. J. Iron Steel Res. Int. 2019, 26, 519–528. 10.1007/s42243-018-0113-4. [DOI] [Google Scholar]
- Fang B. B.; Chu Z.; Yang Y.; Sun X. Y.; Huang W. P.; Li X. F.; Wang L. J. Characterization of Stainless Steel and Wire Rope Pickling Sludge. Adv. Mater. Res. 2013, 726–731, 2130–2134. 10.4028/www.scientific.net/AMR.726-731.2130. [DOI] [Google Scholar]
- Li G. H.; Wang J.; Rao M. J.; Luo J.; Zhang X.; You J. X.; Peng Z. W.; Jiang T. Coprocessing of Stainless-Steel Pickling Sludge with Laterite Ore via Rotary Kiln-Electric Furnace Route: Enhanced Desulfurization and Metal Recovery. Process Saf. Environ. Protect. 2020, 142, 92–98. 10.1016/j.psep.2020.06.014. [DOI] [Google Scholar]
- Brück F.; Fritzsche A.; Totsche K. U.; Weigand H. Steel pickling rinse water sludge: Concealed formation of Cr(VI) driven by the enhanced oxidation of nitrite. J. Environ. Chem. Eng. 2017, 5, 2163–2170. 10.1016/j.jece.2017.04.002. [DOI] [Google Scholar]
- Tsarev N.; Aksenov V.; Tatyannikova E. Aggressive wastewater sludges conditioning with polyelectrolytes. AIP Conference Proceedings 2019, 2174, 020259. 10.1063/1.5134410. [DOI] [Google Scholar]
- Zueva S. B.; Ferella F.; Ippolito N. M.; Ruduka E.; De Michelis I. Wastewater Treatment from Galvanization Industry with Zinc recovery. E3S Web of Conferences 2021, 247, 01064. 10.1051/e3sconf/202124701064. [DOI] [Google Scholar]
- Zueva S. B.; Ferella F.; Innocenzi V.; De Michelis I.; Corradini V.; Ippolito N. M.; Vegliò F. Recovery of Zinc from Treatment of Spent Acid Solutions from the Pickling Stage of Galvanizing Plants. Sustainability 2021, 13, 407. 10.3390/su13010407. [DOI] [Google Scholar]
- Pozdniakova T. A.; Mazur L. P.; Boaventura R. A. R.; Vilar V. J. P. Brown Macro-algae as Natural Cation Exchangers for the Treatment of Zinc Containing Wastewaters Generated in the Galvanizing Process. J. Clean Prod. 2016, 119, 38–49. 10.1016/j.jclepro.2016.02.003. [DOI] [Google Scholar]
- Liang Y. W.; Liu W. R. Extraction-electrolysis combined process for treating galvanized deplating waste hydrochloric acid. China Res. Comp. Util. 2018, 36, 58–61. (in Chinese). [Google Scholar]
- Zhang Y. Z.; Ding H.; He H. L.; Xia Q. H.; Wang L. Q. Process of N-503 solvent extraction of zinc from degalvanizing waste liquid. Tianjin Chem. Ind. 2001, 3, 15–17. (in Chinese). [Google Scholar]
- Pietrelli L.; Ferro S.; Vocciante M. Raw Materials Recovery From Spent Hydrochloric Acid-Based Galvanizing Wastewater. Chem. Eng. J. 2018, 341, 539–546. 10.1016/j.cej.2018.02.041. [DOI] [Google Scholar]
- Ministry of Ecology and Environment of the People's Republic of China. Technical Specifications on Identification for Hazardous Waste. Chinese Standard HJ/T 298-2019; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 2019.
- Ministry of Ecology and Environment of the People's Republic of China. Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. Chinese Standard GB5085.3-2007; Ministry of Ecology and Environment of the People's Republic of China: Beijing, China, 2007.
- Zhang L.; Liu Y. S. A novel method for harmless disposal and resource reutilization of steel wire rope sludges. Environ. Sci. Pollut. Res. Int. 2016, 23, 19797–19805. 10.1007/s11356-016-7197-9. [DOI] [PubMed] [Google Scholar]
- Pinto F. M.; Pereira R. A.; Souza T. M.; Saczk A. A.; Magriotis Z. M. Treatment, reuse, leaching characteristics and genotoxicity evaluation of electroplating sludge. J. Environ. Manage. 2021, 280, 111706. 10.1016/j.jenvman.2020.111706. [DOI] [PubMed] [Google Scholar]
- Yang J.; Zhang S. G.; Pan D. A.; Liu B.; Wu C. L.; Volinsky A. A. Treatment method of hazardous pickling sludge by reusing as glass-ceramics nucleation agent. Rare Met. 2016, 35, 269–274. 10.1007/s12598-015-0673-4. [DOI] [Google Scholar]