Abstract
Discharge of nanoparticles (NPs) into aquatic and terrestrial ecosystems during manufacturing processes and from various commercial goods has become a significant ecotoxicological concern. After reaching soil systems, NPs cause deleterious effects on soil fertility, microbial activity, and crop productivity. Taking into consideration the medicinal importance of Withania somnifera (L.) (ashwagandha), the present study assessed the potential hazards of silver nanoparticles (Ag-NPs) and the toxicity amelioration by a metal-tolerant plant growth-promoting rhizobacterium (PGPR). Bacillus mojavensis BZ-13 (NCBI accession number MZ950923) recovered from metal-polluted rhizosphere soil, tolerated an exceptionally high level of Ag-NPs. The growth-regulating substances synthesized by B. mojavensis were increased with increasing concentrations (0–1000 μg mL–1) of Ag-NPs. Also, strain BZ-13 had the ability to form biofilm, produce alginate and exopolysaccharides (EPSs), as well maintain swimming and swarming motilities in the presence of Ag-NPs. Soil application of varying concentrations of Ag-NPs resulted in a dose-related reduction in growth and biochemical features of ashwagandha. In contrast, following soil inoculation, B. mojavensis relieved the Ag-NPs-induced phytotoxicity and improved plant productivity. Root, shoot length, dry biomass, and leaf area increased by 13, 17, 37, 25%, respectively, when B. mojavensis was applied with 25 mg/kg Ag-NPs when compared to noninoculated controls. Furthermore, the soil plant analysis development (SPAD) index, photosystem efficiency (Fv/Fm), PS II quantum yield (FPS II), photochemical quenching (qP), non-photochemical quenching (NpQ), and total chlorophyll and carotenoid content of BZ-13-inoculated plants in the presence of 25 mg Ag-NPs/kg increased by 33, 29, 41, 47, 35, 26, and 25%, respectively, when compared to noninoculated controls that were exposed to the same amounts of NPs. In addition, a significant (p ≤ 0.05) increase in 48, 18, 21, and 19% in withaferin-A (alkaloids), flavonoids, phenols, and tannin content, respectively, was recorded when plants were detached from bacterized and Ag-NP-treated plants. Leaf gas exchange parameters were also modulated in the case of inoculated plants. Furthermore, bacterial inoculation significantly decreased proline, lipid peroxidation, antioxidant enzymes, and Ag-NP’s absorption and build-up in phyto-organs. In conclusion, soil inoculation with B. mojavensis may possibly be used as an alternative to protect W. somnifera plants in soil contaminated with nanoparticles. Therefore, phytohormone and other biomolecule-synthesizing and NP-tolerant PGPR strains like B. mojavensis might serve as an agronomically significant and cost-effective remediation agent for augmenting the yield and productivity of medicinally important plants like ashwagandha raised in soil contaminated with nanoparticles in general and Ag-NPs in particular.
Introduction
The fast growth of nanotechnology has resulted in the development, use, and commercialization of a wide range of goods using engineered nanoparticles.1 Contamination with nanoparticles (NPs) arises from different anthropogenic sources including residential wastewater effluents, treatment plants, and industries such as agriculture, biomedicine, electronics, pharmaceuticals, and aerospace.2 Nanoparticles are discharged into the environment (both deliberately and unintentionally) and then cause harmful consequences to agricultural crops. Silver nanoparticles (Ag-NPs) are a kind of NP that are widely employed in a variety of commercial (textiles/clothing, household appliances like refrigerators, furniture, cosmetics, and even children toys)3 and medicinal (surgical and nonsurgical equipment like wound dressings, bandages, catheters, etc.) applications.4−6
The discharge of Ag-NPs into aquatic and terrestrial ecosystems during manufacturing processes and from various commercial goods has become a significant ecotoxicological concern. Specifically, the increased production, use, and release of Ag-NPs from diverse goods have caused concerns about their harmful effects on agricultural crops. The NPs’ prolonged durability7 and low disintegration rate, in addition to their buildup in terrestrial ecosystems, exacerbate the phytotoxicity issues. The NPs physically interact with plants and are absorbed by their roots, resulting in plant organ absorption and accumulation. Plant accumulation of NPs is affected by exposure period, exposure medium, and size of nanoparticles, dose rates, and chemistry of surface. Higher NPs accumulation in plant organs, as well as translocation to edible plant parts can limit growth, biomass, and crop production while also transferring NPs to the next trophic level.8 Reports on phytotoxic effects of Ag-NPs on various agronomically important crops are available. For instance, Rani et al.9 reported that following accumulation in root systems, Ag-NPs caused production of reactive oxygen species (ROS) in Pisum sativum (L.) that ultimately caused cell death. Likewise, exposure to excess concentrations of Ag-NPs exhibited delayed germination, reduced flowering and floral development, decreased the viability of petal and pollen formation, and decreased pod production in Arabidopsis thaliana.10 In an study, a Ag-NP concentration-dependent decrease in root biomass and plant length and changes in structural responses of Hordeum vulgare (L.) were observed by González Linares et al.11 Furthermore, Ag-NPs caused severe phytotoxic effects on Triticum aestivum and decreased the length, biomass, micronutrients, protein, and amino acids and increased the bioconcentration of Ag in different plant organs.12 In addition, studies by Nair and Chung13 on various levels of Ag-NPs showed the toxic effects on Vigna radiata (L.) seedlings by altering their physiological and molecular processes.
As a result, new, ecologically friendly, and cost-effective techniques for avoiding the consequences of nanoparticles must be devised. One of these might be the utilization of indigenous rhizosphere bacteria capable of stimulating plant development through the synthesis of growth-promoting bioactive compounds that could reduce nanophytotoxicity. The current investigation’s goal was to assess a metal-tolerant plant growth-promoting rhizobacterial (PGPR) strain under nanoparticle stress and its bioinoculation effect on ashwagandha plants. The strain was chosen because soil microorganisms (i) have a variety of methods for surviving extended periods of metal exposure in soils and (ii) carry out the biological transformation or conversion of metals to a less harmful form. Numerous root colonizing PGPR such as Azotobacter salinestris,14Bacillus cereus, Pseudomonas fluorescens and Planomicrobium chinense,15Bacillus cereus,16Pseudomonas sp.,17 and Rhizobium meliloti(18) have been used for cleanup of polluted soil and reduction of metal stress in crops. The strategies used by rhizosphere bacteria that can lead to plant protection include (i) chelating metals in the rhizosphere via overproduction of exopolysaccharides and other metal-chelating compounds and (ii) intracellular metal fraction pumping.19 Bacterial exopolysaccharides (EPSs) produce compounds that interact with metals and inhibit the entry of metal into the root system, decreasing metal toxicity to rhizosphere bacteria and also protecting plants from abiotic stressors.20
Withania somnifera L. Dunal (Ashwagandha) is a popular medicinal plant cultivated throughout the world. The primary active components identified and described from Withania somnifera are alkaloids and withanolides. The therapeutic benefits of leaves including antibacterial, antitumor, and anti-inflammatory characteristics are linked to withanolide-D, which has a strong antitumor activity. The main withanolides extracted from the plant include withanolide-E, which has immune-suppressive properties. Also, several illnesses such as leprosy, neurological problems, intestinal infections, and rheumatism are treated with leaf extracts of ashwagandha.21 The berries and seeds are diuretics that are also used to alleviate chest problems. Additionally, ashwagandha has been found in studies to be helpful in the treatment of tardive dyskinesia, inflammation, stroke, and osteoarthritis. Ashwagandha is utilized to relax the mind, reduce weakness and nervous weariness, increase sexual vitality, and promote restful sleep.22 Many research investigations have shown that the fruits and leaves of ashwagandha contain a variety of phytochemicals with antioxidant activities, which are responsible for their good health effects.23
In light of these considerations, a series of experiments was devised to achieve the following objectives: (i) assess the toxicity of Ag-NPs in W. somnifera (L.) grown in vitro, (ii) isolate and identify soil bacteria and determine their tolerance toward Ag-NPs, (iii) assess the different rates of Ag-NPs on biofilm-associated traits and plant growth-regulating substances in B. mojavensis BZ 13 strain, (iv) assess the impact of B. mojavensis BZ-13 strain on dry biomass, leaf chlorophyll fluorescence, and carotenoid contents in the leaves of W. somnifera (L.) treated with different amounts of Ag-NPs, (v) determine the alkaloid and polyphenol contents in roots of bioinoculated and NP-treated W. somnifera (L.), (vi) determine the leaf exchange parameters and Ag-NPs uptake in different organs of B. mojavensis-inoculated and Ag-NP-treated W. somnifera (L.), and (vii) determine the proline, MDA, and antioxidant activity in root tissues of bacteria-inoculated and NP-treated plants.
Results and Discussion
Toxicity of Ag-NPs to W. somnifera (L.) in Vitro
Germination, Length, and Vigor Indices
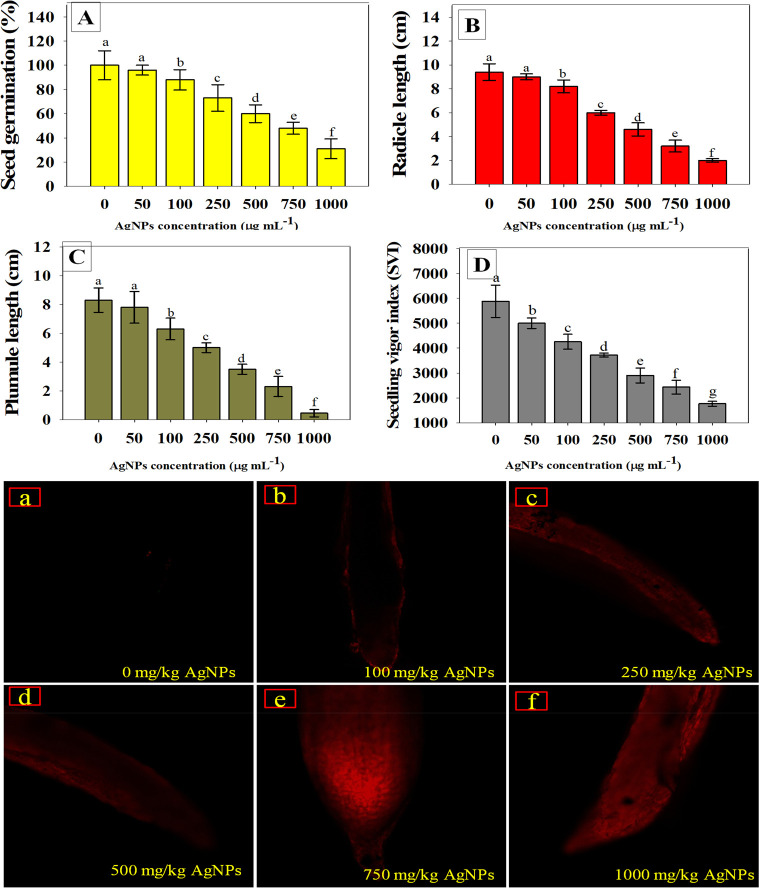
Seed germination is, undoubtedly, the initial step toward crop establishment success. As a result, the most important physiognomies of the seeds to be employed for cultivation are seed germination and seedling vigor index. Seeds that germinate quickly and aggressively in controlled circumstances are likely to generate robust seedlings in the field as well. Hence, seed germination has been frequently utilized as an indicator to assess Ag-NP-induced phytotoxicity. Higher dosages exhibited the most deleterious impact on seedling biological characteristics among the concentrations used in the present work. As an example, germination rate (Figure 1A), radicle length (Figure 1B), plumule length (Figure 1C), and vigor index (Figure 1D) of W. somnifera (L.) were maximally reduced by 69, 78, 90, and 70%, respectively, when exposed to higher (1000 μg mL–1) concentrations of Ag-NPs. Changes in seed germination of several plant species caused by various NPs have been reported to confirm the present findings. For instance, when exposed to 2000 mg L–1 cerium dioxide (CeO2) NPs, a considerable decrease in germination efficiency of edible crops (tomato, maize, and cucumber) was recorded as reported by López-Moreno et al.24 Similarly, metal oxide nanoparticles (MONPs) viz., TiO2, Al2O3, and CuO, reduced growth features of Allium cepa under in vitro conditions.25
Figure 1.
Toxicity evaluation of Ag-NPs using Withania somnifera (L.) plants in vitro; seed germination (A), radicle length (B), plumule length (C), and seedling vigor index (D) of W. somnifera (L.) seeds germinated on soft agar plates treated with different rates of Ag-NPs. Panels a–f represent CLSM images of NP-induced cellular permeability in the roots of W. somnifera (L.) treated with 0, 100, 250, 500, 750, and 1000 mg kg–1 Ag-NPs, respectively. In panels A–D, the bar diagrams represent the mean values of five replicates (n = 5). Corresponding error bars represent standard deviation (SD) of three replicates (SD, n = 5).
Changes in Root Mitochondrial Membrane Potential (ΔΨm)
Using in vivo histochemical staining with Rhodamine 123 (Rh123), the influence of various doses of Ag-NPs on the mitochondrial membrane potential of roots of W. somnifera (L.) plants was qualitatively evaluated. Here, as a result of exposure to increasing concentrations of Ag-NPs, changes in mitochondrial membrane potential (ΔΨm) were seen in the roots of Ag-NP-exposed plants, as shown by enhanced Rh123 fluorescence (Figure 1a–f). Since mitochondria are the origins of cell ROS production, excess ROS generation may have resulted from Ag-NPs interactions with the mitochondrial transport system26 that resulted in decrease in root ΔΨm as evident by increasing the level of dye Rh123. In line with the current finding, Nair and Chung13 found that exposure to Ag-NPs caused substantial alterations in ΔΨm in Vigna radiata (L). Similarly, Faisal et al.27 has shown that exposure to nickel oxide nanoparticles caused changes in ΔΨm in Solanum lycopersicum (L.) seedling roots, resulting in increased Rh123 fluorescence due to alterations in mitochondrial membrane permeability.
Biochemical Characterization, Ag-NPs Tolerance, and Molecular Identification of BZ-13
Because nanoparticles are often discharged into the soil environment, it is critical to understand some of the negative consequences of nanoparticles in agriculturally important crops including medicinal plants. When entered into soil systems, NPs adversely affect the physiological and biochemical features of crops. To address this issue, we attempted to identify metal-tolerant soil bacteria, which may be a microbiological resource, and it has the potential to be utilized to increase agricultural performance by reducing the metal toxicity. In this work, 20 rhizopheric isolates were collected, and their morphological and biochemical features were investigated. Bacterial isolates showed a varied response toward the biochemical tests. When grown on Ag-NP-supplemented agar plates, strain BZ-13 withstood the highest concentration (1600 μgmL–1) of Ag-NPs among PGPR isolates. Strain BZ-13 was chosen for inoculation in crop experiments based on its Ag-NPs tolerance characteristics. Bacterial strain BZ-13 showed a positive response toward a number of biochemical reactions. This strain (BZ-13) belonged to the genus Bacillus on the basis of physiological and cultural factors. In addition, 16S rRNA partial gene sequencing was used to identify Bacillus BZ-13 at the species level. The strain BZ-13’s 16S rRNA nucleotide sequences (about 1400 bp in size) were deposited to GenBank (NCBI Accession Number MZ950923.1). For a similarity search, BLASTn software was employed that revealed BZ-13 strain was nearly and strongly related to Bacillus mojavensis NRLL-B 14698 (EU138460). Because of the maximum similarity, strain BZ-13 was identified and confirmed as Bacillus mojavensis. Furthermore, using the 16S rRNA partial sequences already present in the NCBI database, a phylogenic tree was constructed for Bacillus mojavensis BZ-13 (Figure S1). In this regard, a number of metal/nanoparticle-tolerant agriculturally important soil bacteria have been isolated from different metal-polluted areas and characterized using partial gene sequencing.28−31
PGP Substances of B. mojavensis under Ag-NPs Stress
Indole-3-acetic Acid, Siderophore, ACC Deaminase, P-Solubilization, NH3, and HCN
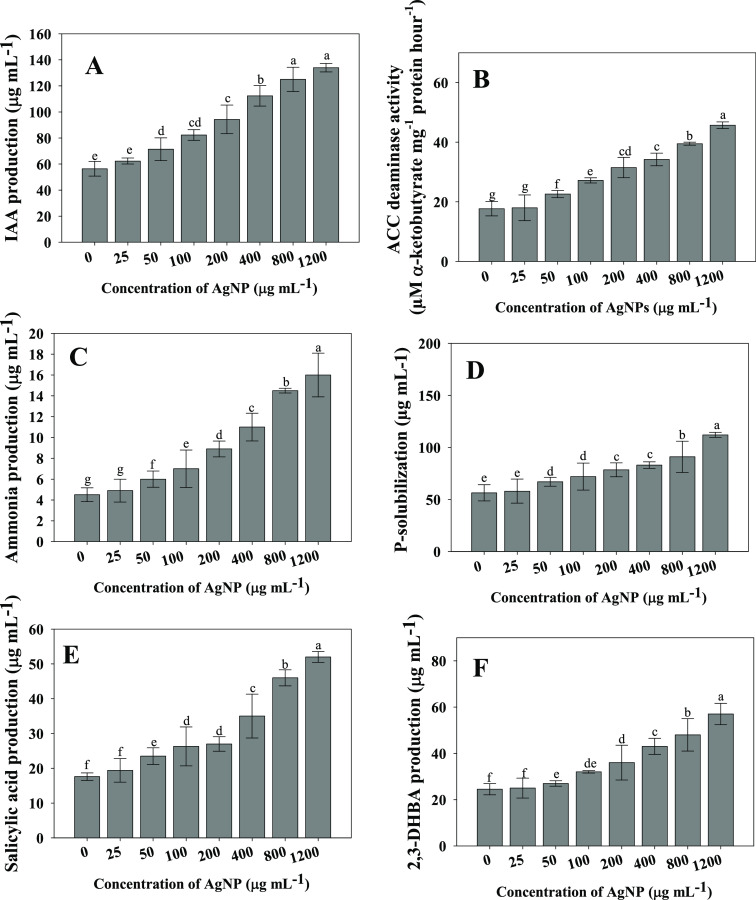
The plant growth-regulating bioactive molecule produced by Bacillus mojavensis strain BZ-13 was evaluated by growing cells in Ag-NP-supplemented growth medium. It was observed that even at a higher Ag-NPs dosage, IAA synthesized by Bacillus mojavensis was not significantly reduced. However, production of IAA was increased with increasing concentrations of Ag-NPs (Figure 2A). Bacillus mojavensis BZ-13 synthesized 56.4 ± 5.7 μgIAAmL–1 in the absence of NP stress, which, however, increased with the Ag-NP concentration. For example, at 1200 μg mL–1 Ag-NP concentration, IAA produced by BZ-13 strain increased by 58% over controls. Plant beneficial bacteria reduce the negative impacts of environmental contaminants and, as a result, improved the crop yield in stressed soils.32 In a similar investigation, Sphingomonas sp. produced the phytohormone gibberellins, which significantly enhanced the growth indices of plants.33 Because Ag-NPs, even after prolonged exposure, may have no effect on the functional profile of the soil microbial population, IAA generation remained unaffected.34
Figure 2.
Plant growth-regulating (PGR) substances synthesized by Bacillus mojavensis BZ-13 strains under different concentrations (0–1200 μg mL–1) of Ag-NPs stress; indole-3-acetic acid (A), 1-aminocyclopropane carboxylate (ACC) deaminase (B), ammonia (C), phosphate solubilization (D), siderophore: salicylic acid (E) and 2,3-dihdroxy benzoic acid, DHBA (F). Here, bar diagrams represent the mean values of three replicates (n = 3). Mean values followed by different letters are significantly different at p ≤ 0.05 according to Duncan’s multiple range (DMRT) test. Vertical and scattered bars represent the means ± SD (n = 3).
Even when grown on medium added with increasing rates of Ag-NPs solution, strain BZ-13 exhibited a good sensitivity to ACC deaminase. B. mojavensis generated 17.7 μM α-ketobutyrate mg–1 protein h–1 at a higher concentration (1200 μg mL–1 Ag-NPs), which is 61% (45.7 μM α-ketobutyrate mg–1 protein h–1) higher than the amount of ACC deaminase produced at a lower concentration (0 μg mL–1 Ag-NPs) (Figure 2B). Similarly, two nitrogen-fixing PGPR isolates (A. nigricans and Azorhizophilus paspali) have been shown to produced ACC deaminase activity under metal-stressed conditions that could help to manage the growth and health of Helianthus anuus.35 Furthermore, chitosan NP-resistant and ACC deaminase producing soil bacterium Bacillus licheniformis protected plants from damaging effects of phytopathogens and improved the overall crop growth.36
Similarly, the increasing concentrations of Ag-NPs did not show any adverse impacts on NH3 (Figure 2 panel C) and HCN production. At all concentrations, B. mojavensis exhibited HCN and ammonia production. After Nessler’s reagent was added, a yellow-brown tint color developed, suggesting that the ammonia production test was positive. Plant growth is said to be influenced by ammonia production in an indirect way. Many soil-dwelling bacteria produce HCN (a well-known secondary metabolite), which is thought to have an imperative role in the biological control of plant diseases. Certain species of Bacillus have been implicated in the control of soil-borne diseases by producing HCN.37,38 Similarly, several studies have shown that metal-resistant soil bacteria cultivated in stressed medium produce ammonia and cyanogenic compounds.39−41
Phosphatic fertilizers (PF) are used often in agricultural regions to compensate for soil P shortage.42 Phosphate fertilizers are absorbed in smaller amounts by plants, and the rest is quickly transformed into insoluble complexes in the soil. However, using phosphate fertilizers on a regular basis is not only expensive but also harmful to the environment.43 As a result, researchers are looking for an environmentally friendly and cost-effective way to boost crop output in low-phosphorus soils. So, organisms with P-solubilizing activity, also known as phosphate-solubilizing microorganisms (PSM), may feed plants with the accessible forms of P and hence serve as a viable alternative to chemical fertilizers.44,45 Phosphate-solubilizing bacteria (PSB) are regarded promising biofertilizers because they may feed plants with P from sources that are ordinarily inaccessible to plants through diverse mechanisms.46 Naturally, inorganic phosphorus is solubilized as a result of the action of low molecular weight organic acids generated by diverse soil microorganisms. Considering the importance of P, the amount of solubilized P by strain BZ-13 was assessed under NPs stress, and the quantum of solubilized P was increased with increasing Ag-NPs concentrations (Figure 2D).
In iron (Fe)-deficient situations, siderophore, a low molecular weight (LMW) Fe-chelating molecule produced by soil microbial species, supplies Fe to plants. Siderophore synthesis by bacterial strains, like IAA, has risen with increasing rates of Ag-NPs. As an example, BZ-13 synthesized 17.6 ± 1.1 μgmL–1 salicylic acid (SA), which, however, increased by 66% (52 ± 1.56 μg mL–1) at 1200 μg mL–1 Ag-NPs (Figure 2E). Also, the amount of 2,3-dihydroxy benzoic acid consistently increased with the enhancement in cumulative Ag-NP concentration (Figure 2F). Likewise, different concentrations of nanoparticles (CuO and ZnO) differentially affect the fluorescent siderophore synthesized by a NP-resistant and root colonizing soil bacterium Pseudomonas chlororaphis strain O6.47
Biofilm Formation and Related Traits under Ag-NPs Stress
Under in vitro conditions, the effect of varying levels of Ag-NPs on biofilm formation, cell adhesion ability to hydrocarbons, and motility of B. mojavensis BZ-13 was investigated. In the context of Ag-NPs stress, the formation of bacterial biofilm was enhanced in a dose-dependent way (Table 1). The influence of Ag-NPs stress on EPS synthesis by B. mojavensis was investigated in order to better understand the biological consequences of extra polymeric substances (EPS) produced by a range of soil bacterial communities. Strain BZ-13 synthesized a substantial quantity of EPS (112.0 ± 7.7 g mL–1) in controlled conditions (NP free media), which, however, increased dramatically with increasing Ag-NP concentrations. It has been demonstrated that polymeric compounds generated and released under extracellular conditions by soil bacteria play a significant role in crop protection against abiotic stresses.48 In certain investigations, bacterial EPS has also been shown to protect agricultural plants against the harmful effects of phagocytosis and infections.49,50
Table 1. Effect of Different Concentrations (0–1000 μg mL–1) of Ag-NPs on Biofilm Development and Its Related Activities of Bacillus mojavensis BZ-13 Strains under in vitro Conditionsa.
| Ag-NPs (μg mL–1) | biofilm development | EPS production (μg mL–1) | production of alginate (μg mL–1) | swarming motility (mm) | swimming motility (mm) | cell surface hydrophobicity (%) |
|---|---|---|---|---|---|---|
| 0 | 0.53f ± 0.04 | 112g ± 7.7 | 87f ± 5.2 | 34d ± 3.6 | 30d ± 00 | 54e ± 3.8 |
| 25 | 0.57f ± 0.05 | 122f ± 3.4 | 91f ± 2.8 | 34d ± 0.7 | 32c ± 2.1 | 58e ± 2.3 |
| 50 | 0.63e ± 0.01 | 145e ± 6.8 | 98e ± 1.7 | 36c ± 2.7 | 33c ± 4.5 | 62d ± 4.7 |
| 100 | 0.72d ± 0.08 | 167d ± 11.2 | 106d ± 4.5 | 37c ± 2.2 | 37b ± 2.2 | 67d ± 7.2 |
| 250 | 0.82c ± 0.03 | 189c ± 8.2 | 118c ± 5.0 | 42b ± 5.3 | 38b ± 1.6 | 71c ± 5.2 |
| 750 | 0.93b ± 0.05 | 202b ± 7.1 | 134b ± 3.9 | 42b ± 4.4 | 40a ± 3.5 | 74b ± 3.1 |
| 1000 | 1.12a ± 0.02 | 219a ± 5.4 | 168a ± 2.6 | 45a ± 3.2 | 40a ± 5.2 | 84a ± 7.2 |
Each value is the mean of five replicates (n = 5). Mean values are significant at p ≤ 0.05. Means followed by different letters are significantly different from each other according to the DMRT test.
The synthesis of alginate, ability to form swimming and swarming motility, and the CSH of strain BZ-13, like other biofilm characteristics, increased with increasing concentrations of Ag-NPs. The swarming and swimming motility of the BZ-13 strain were measured as 34 mm and 30 mm, respectively, at 0 μg Ag-NP mL–1, but increased by 24% and 25%, respectively, when the Ag-NP concentration was increased 0 to 1200 μg mL–1 (Table 1). The hydrophobicity of bacterial cell surfaces is connected to bacterial cell adhesion and aggregation, as well as biofilm development. The capacity of the metal/NP-tolerant PGPR strain to form biofilms and produce related characteristics even at greater concentrations of Ag-NPs is a strong indicator that it may tolerate even harsher nanoparticle stress conditions and enhance the growth of plants.
Likewise, the biofilm-forming and EPS-producing soil bacterium Azotobacter salinestris protected Lycopersicum esculentum (L.) crops from adverse impacts of different metal oxide NPs and augmented the physiological and biochemical characteristics of crops.14
Bioinoculation Impact of B. mojavensis on Physiological and Biochemical Features of Ag-NP-Treated W. somnifera (L.) Plants
Plant Length, Leaf Number, Leaf Area, and Berry Number
The effect of Ag-NP-tolerant B. mojavensis on the germination rate and biological characteristics (Figure 3A,B) of W. somnifera (L.) seedlings exposed to different concentrations of Ag-NPs in sandy clay loam soil was dissimilar. In general, B. mojavensis BZ-13 improved seed germination even in NP-affected soil. For instance, at 100 mg Ag-NPs kg–1 soil, strain BZ-13 increased the germination efficiency by 19%, over noninoculated controls (Figure 3C). Plant growth characteristics were reduced by increasing the dosage of Ag-NPs, but in the presence of B. mojavensis BZ-13 inoculum, growth was improved over noninoculated plants. In the presence of 1000 mg Ag-NPs kg–1, roots, shoots length, leaf number, leaf area and number of berries/plants were greatly decreased by 51, 47, 73, 70 and 79%, respectively compared with control. Following B. mojavensis in the presence of 100 mg Ag-NPs kg–1, the lengths of roots and shoots were increased by 12 and 15%, respectively, over noninoculated treatments (Figure 3D). It is possible that NP-tolerant B. mojavensis might promote root development by modifying some of the intrinsic processes of root growth control, resulting in increased nutrient absorption from soil. Similarly, leaf number (Figure 3F), leaf area (Figure 3G), and number of berries (Figure 3H) were significantly (p ≤ 0.05) increased by 26, 22, and 19%, respectively, when B. mojavensis was inoculated to W. somnifera (L.) plants cultivated in soils treated with 100 mg Ag-NPs kg–1, compared with plants noninoculated but treated with identical Ag-NP concentrations. Likewise, NPs-tolerant Bacillus cereus LPR2 exhibited a positive effect on growth, plant length, and physiology in Zea mays (L.) cultivated in soil supplemented with different amounts of Ag-NPs.16
Figure 3.
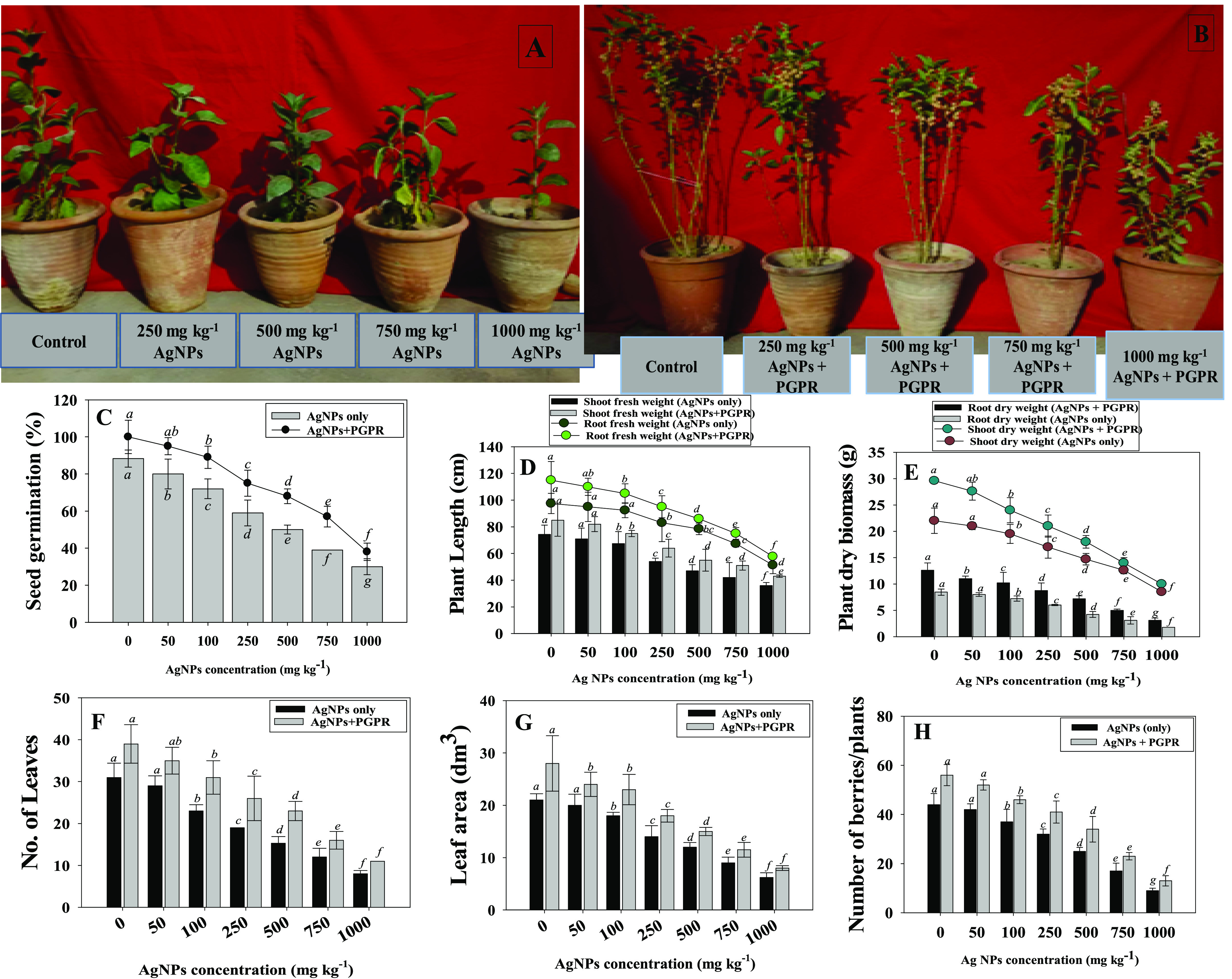
Panel A depicts W. somnifera (L.) plants grown in soils treated with increasing concentrations of Ag-NPs. Panel B depicts the inoculation effect of Bacillus mojavensis BZ-13 on W. somnifera raised in soil added with different concentrations of Ag-NPs. The bioinoculation impact of NP-tolerant B. mojavensis on seed germination efficiency (C), plant length (D), dry biomass (E), number of leaves (F), leaf area (G), and number of berries (H) of W. somnifera (L.) plants raised in pot-house conditions supplemented with different concentrations of Ag-NPs. Here, bar and scatter diagrams represent the mean values of five replicates (n = 5). Mean values followed by different letters are significantly different at p ≤ 0.05 according to DMRT tests. Vertical and scattered bars represent means ± SD (n = 5).
Dry Biomass Accumulation
Here, even in the presence of three dosages of Ag-NPs, biomass accumulation in W. somnifera (L.) plants was generally enhanced after B. mojavensis inoculation. However, increasing the dosage of NPs resulted in a progressive reduction in dry biomass in the absence of bacterial inoculants. For example, Ag-NPs at 1000 mg kg–1 maximally and significantly reduced the dry biomass accumulated in roots (74% reduction) and shoots (65% reduction), compared with controls. In contrast, B. mojavensis inoculation increased the dry biomass of roots and shoots by 30 and 21%, respectively, when applied to soils indigenously contaminated with 100 mg Ag-NPs kg–1 (Figure 3E). The NP-tolerant B. mojavensis BZ-13, which was utilized as a bacterial inoculant, also led to a substantial increase in the function of plants, which might be related to the production of plant growth regulators. These growth regulators, such as IAA, increase cell elongation and division while also promoting root growth. The plant absorbs more water and nutrients as a result of its enlarged roots, resulting in increased growth.
Chlorophyll Fluorescence and Leaf Pigments
In this study, the increasing concentration of Ag-NPs reduced the SPAD index of W. somnifera (L.) over untreated controls, whereas bacterial-inoculated plants treated with 100 mg Ag-NPs kg–1 growing under NPs stress showed a significant increase in leaf chlorophyll fluorescence parameters. For instance, the SPAD index, photo system efficiency (Fv/Fm), PS II quantum yield (FPSII), photochemical quenching (qP), and non-photochemical quenching (NpQ) declined with increasing concentrations of Ag-NPs. However, inoculation of NP-tolerant B. mojavensis BZ-13 to W. somnifera (L.) plants treated with different Ag-NPs amounts modulated all the parameters (Figure 4A–E). Likewise, leaf pigments accumulating in ashwagandha foliage declined with increasing Ag-NP concentrations. However, the application of NP-resistant PGPR to soil showed an improvement in leaf pigments. For example, chl a (Figure 4F), chl b (Figure 4G), total chlorophyll (Figure 4 panel H), and carotenoid content (Figure 4I) of ashwagandha plants were significantly (p ≤ 0.05) increased by 37, 33, 15, and 10%, respectively when B. mojavensis was inoculated to plants raised in soils treated with 25 mg Ag-NPs kg–1 over noninoculated control. The amount of chlorophyll in plants is an important indication of their resistance to Ag-NP stress. The chlorophyll fluorescence process was verified to be a responsive approach for recognizing and evaluating alternative ways supported in the photosynthetic machinery. To conclude the NP-resistant B. mojavensis-triggered improvement in the photosynthetic apparatus, Fv/Fm, Fv/F0, qP, and NPQ values were also evaluated in the present study. Ag-NPs present in the soils lowered the values of Fv/Fm, Fv/F0, and NPQ; however, inoculation of NP-resistant B. mojavensis on Ag-NP-treated W. somnifera (L.) plants compensated for these negative effects. These findings suggest that NP-tolerant strains can boost fluorescence pigments and safeguard the photosynthetic mechanism of plants cultivated in soils contaminated with Ag-NPs. Similar to this observation, the inoculation of NP-resistant PGPR strains Chryseobacterium(51) and Azotobacter chroococcum(52) increased the chlorophyll content of iron-deficient Lycopersicum esculentum (L.) plants by generating siderophores and other biomolecules.
Figure 4.
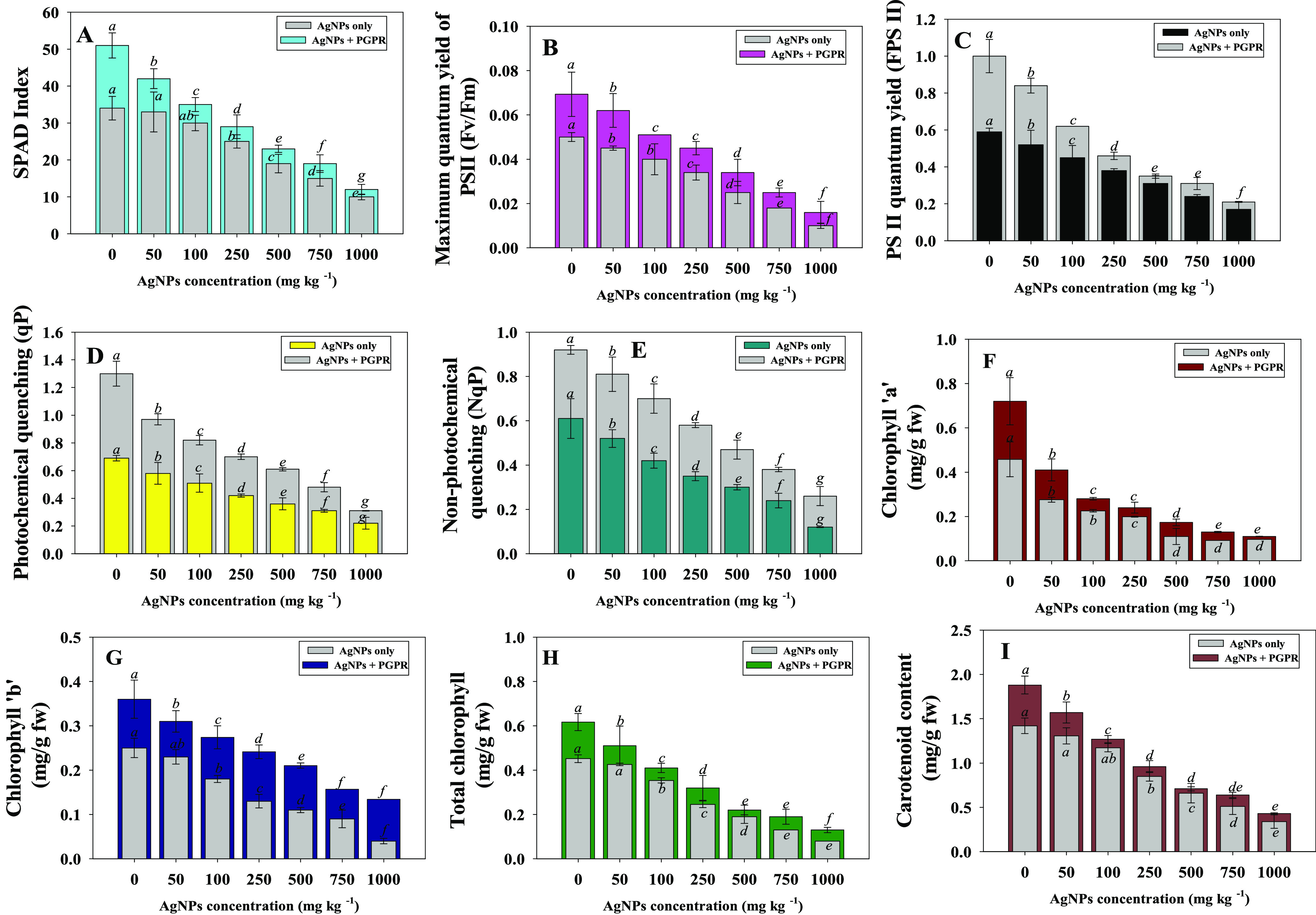
Bioinoculation impact of B. mojavensis BZ-13 on chlorophyll fluorescence; SPAD index (A), maximum quantum yield (B), PS II quantum yield (C), photochemical quenching (D), non-photochemical quenching (E) and photosynthetic pigments; chlorophyll a (F), chlorophyll b (G), total chlorophyll (H), and carotenoid content (I) in W. somnifera (L.) plants raised in loamy soils added with different concentrations of Ag-NPs under pot-house conditions. Values are the mean of five replicates (n = 5) with standard errors (SE). Different bar letters show significant differences among treatments separately.
B. mojavensis Modulates Gas Exchange Parameters of W. somnifera (L.) under Ag-NPs
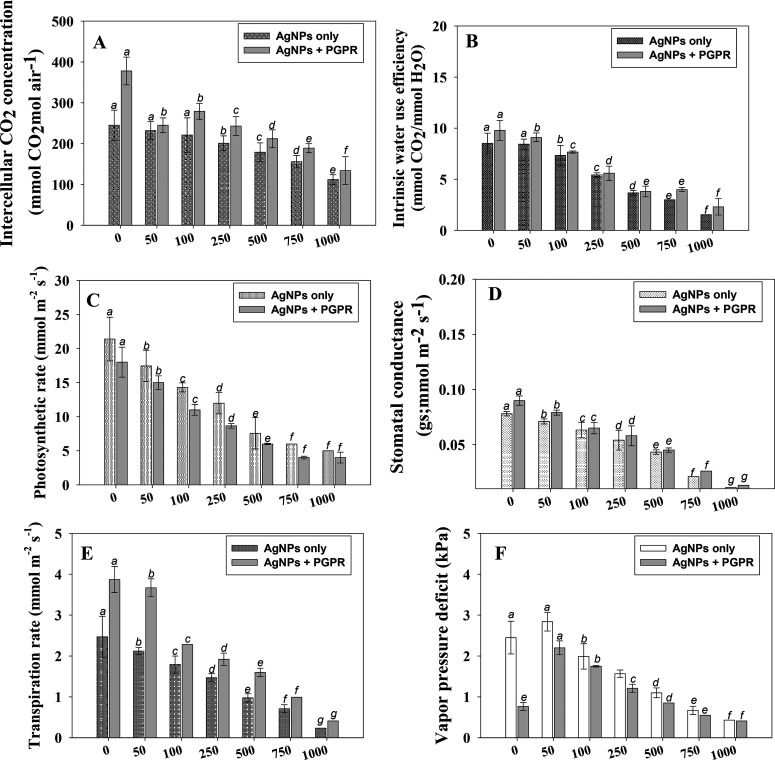
The physiological response of the plant under Ag-NPs stress was revealed by gas exchange characteristics. Like other parameters, Ag-NPs modulate leaf gas exchange attributes, viz., stomatal conductance (gs), intercellular CO2 concentration (Ci), rate of transpiration (E), vapor pressure deficit (VpDL), intrinsic water use efficiency (iWUE), and photosynthetic rate (PN) of W. somnifera (L.). Among the concentrations used, 1000 mg Ag-NPs kg–1 had the maximum adverse impact on leaf gas exchange characteristics of plants. However, greater improvements in the above parameters were recorded when seedlings were inoculated with NP-resistant PGPR strain B. mojavensis. For example, following the inoculation of PGPR strain into ashwagandha plants grown in soil even supplemented with 100 mg Ag-NPs kg–1, the Ci (Figure 5A), iWUE (Figure 5B), PN (Figure 5C), gs (Figure 5D), E (Figure 5E), and VpDL (Figure 5F) of foliage were substantially (p ≤ 0.05) improved by 21, 10, 23, 9, 24, and 12%, respectively, over noninoculated controls. Because of its high Ag-NPs tolerance ability, resilience and efficient rhizosphere colonization, and production of various growth-regulating substances, the B. mojavensis strain enhanced the gas exchange properties of plants by decreasing the Ag-NP-induced phytotoxicity.
Figure 5.
Gas exchange attributes; intracellular CO2 concentration (A), intrinsic water use efficiency (B), photosynthetic rate (C), stomatal conductance (D), transpiration rate (E), and vapor pressure deficit (F) in B. mojavensis BZ-13-inoculated and Ag-NP-treated W. somnifera (L.) plants raised in pot soil. Values are the mean of five replicates (n = 5) with standard errors (SE). Different bar letters show significant differences among treatments separately.
Alkaloids and Polyphones in B. mojavensis and Ag-NP-Treated W. somnifera (L.)
Plant polyphenols have been shown to be effective at scavenging free radicals. Many pharmacological effects exhibited by plant-based formulations are attributable to the presence of polyphenols. Flavonoids and other phenolic compounds of plant origin have been documented as free radical scavengers. Because of their possible therapeutic effects, dietary polyphenols and alkaloids derived from medicinal plants have recently attracted the interest of scientists and consumers. They acts as powerful antioxidants that help the body fight oxidative stress generated by reactive oxygen species (ROS), which has been linked to a variety of illnesses. Considering these properties, alkaloids and polyphenols content were determined in roots tissues of ashwagandha to assess the effects of bioinoculants as well as Ag-NPs. Alkaloids and polyphones extracted from root samples of W. somnifera (L.) were significantly (p ≤ 0.05) decreased as the concentrations of Ag-NPs increased from 25 to 1000 mg Ag-NPs kg–1 soil. For example, higher NP rates (1000 mg Ag-NPs kg–1 soil) had the maximum adverse effect where it decreased the total phenols, tannins, flavonoids, and alkaloids content maximally by 57, 77, 61, and 56%, respectively over untreated controls. Contrarily, inoculation of the NP-tolerant B. mojavensis BZ-13 strain resulted in an increase in these contents by detoxifying the Ag-NPs-induced toxicity. For instance, total phenols (Figure 6A) and tannins (Figure 6B), flavonoids (Figure 6C), and alkaloids (Figure 6D) in bioinoculated plants increased to 25 mg GA equivalents g–1 extracts (14%), 6.5 mg tannic acid equivalents g–1 extracts (12%), 18 mg quercetin equivalents g–1 extracts (11%), and 1.46 mg g–1 dry root wt (44%), respectively, when compared with noninoculated and 100 mg Ag-NPs kg–1 soil-treated plants. Similarly, NP-tolerant Pseudomonas spp. increased the contents of secondary metabolites in silicon nanoparticle-treated Melissa officinalis (L.) plants following their soil inoculation.53
Figure 6.
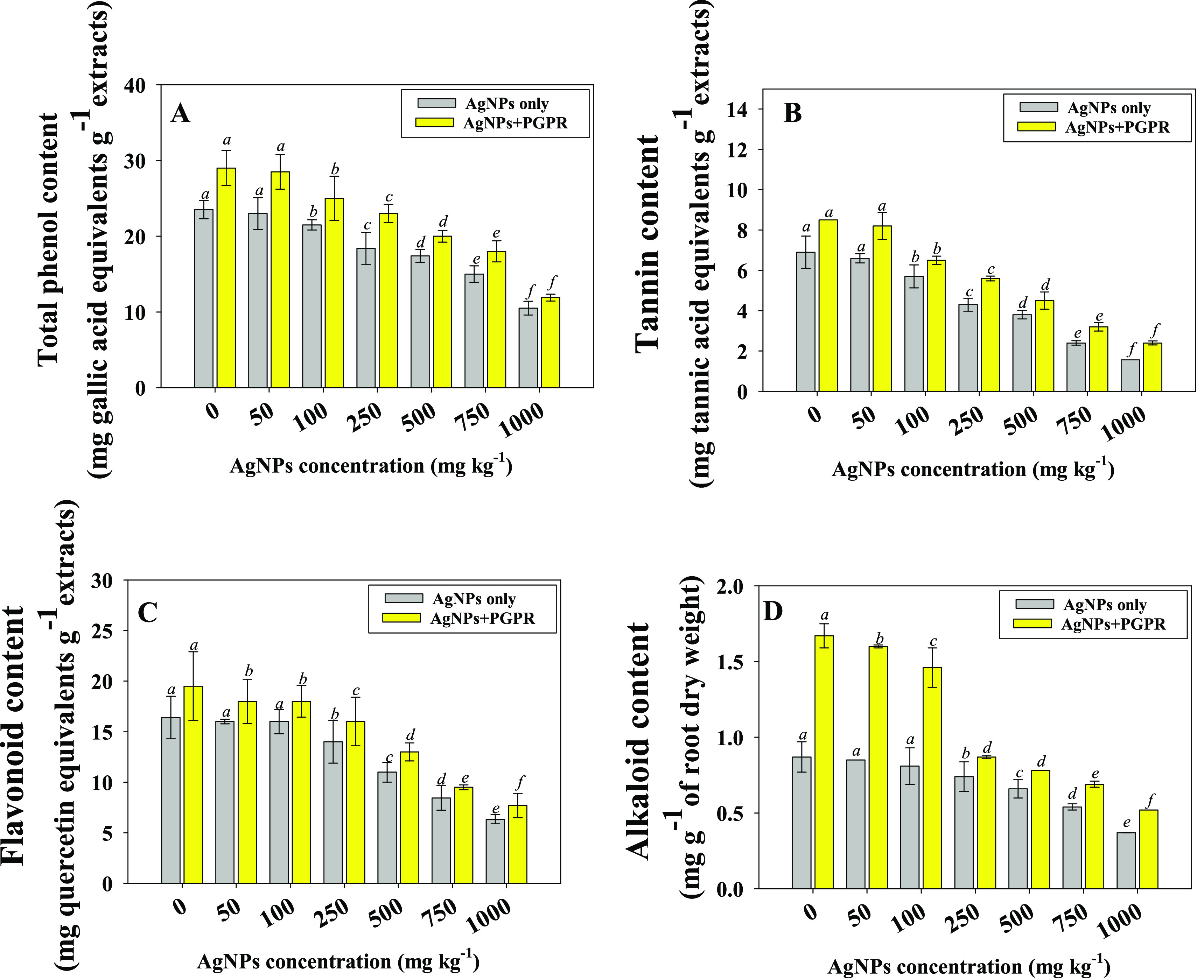
Impact of the B. mojavensis BZ-13 strain on total phenol content (A), tannin content (B), flavonoid content (C), and alkaloid content (D) extracted from root tissues of W. somnifera (L.) plants grown in loamy soils added with different concentrations of Ag-NPs under pot-house conditions. Values are the mean of five replicates (n = 5) with standard errors (SE). Different bar letters show significant differences among treatments separately.
Proline, MDA, and Antioxidant Activity
Proline (a water-soluble amino acid) generated by plants in harsh environments is regarded as an essential biomolecule that acts as a scavenger to protect membranes from the damaging effects of stressors.54 Furthermore, it functions as a compatible osmolyte, assisting in the storage of carbon and nitrogen. Aside from that, proline may act as an ROS scavenger, a molecular chaperone that stabilizes protein structure, and it can assist in maintaining cytosolic pH and the cell redox state. As a result, in the majority of plant species, the increase in free cellular proteins released in response to various environmental stressors has been shown to perform a variety of defensive activities. In this study, we discovered a significant buildup of proline in ashwagandha plants cultivated in soil polluted with different amounts of Ag-NPs. The maximum proline accumulation (56 μg g–1 fw), was recorded at 1000 mg Ag-NPs kg–1 soil. On the other hand, following soil inoculation of NP-tolerant PGPR, the proline content dropped significantly. For instance, when B. mojavensis was inoculated with 100 mg Ag-NPs kg–1, the proline concentration decreased by 41% (from 19 to 11.2 μg g–1 fw), when compared to noninoculated but NP-treated plants (Table 2). The proline concentration in plants exposed to Ag-NPs was typically reduced when B. mojavensis inoculation was done.
Table 2. Inoculation Effect of Nanoparticle-Tolerant Bacillus mojavensis BZ-13 Strains on Antioxidant Enzyme Activities, Proline, and MDA Content of W. somnifera (L.) Plants Raised in a Soil System Supplemented with Different Concentrations of Ag-NPsa.
| antioxidant
enzymes (μ mol min–1 mg–1 protein) |
||||||||
|---|---|---|---|---|---|---|---|---|
| treatment | Ag-NPs concentration (mg kg–1) | CAT | SOD | POX | APX | GR | proline content (μg g–1 fw) | MDA content (μ mol g–1 fw) |
| noninoculated | 0 mg kg–1 | 122 ± 13.5 (h) | 76 ± 4.6 (g) | 12 ± 0.3 (g) | 22.3 ± 1.1 (h) | 16 ± 0.7 (h) | 11 ± 0.8 (h) | 3.6 ± 0.08 (f) |
| 50 mg kg–1 | 134 ± 22.4 (g) | 79 ± 5.3 (g) | 17 ± 0.5 (f) | 25.0 ± 2.1 (g) | 20 ± 1.0 (g) | 14 ± 0.8 (f) | 3.9 ± 0.4 (f) | |
| 100 mg kg–1 | 156 ± 11.2 (f) | 86 ± 6.2 (f) | 25 ± 0.8 (e) | 29.3 ± 2.4 (f) | 26 ± 1.4 (f) | 19 ± 1.6 (e) | 4.2 ± 0.6 (e) | |
| 250 mg kg–1 | 176 ± 20.0 (e) | 97 ± 7.1 (e) | 36 ± 1.3 (d) | 34.6 ± 00 (e) | 34 ± 1.8 (e) | 26 ± 1.9 (d) | 6.1 ± 0.6 (c) | |
| 500 mg kg–1 | 214 ± 12.6 (d) | 112 ± 8.2 (d) | 43 ± 2.5 (c) | 42.1 ± 3.0 (c) | 43 ± 2.3 (d) | 34 ± 2.5 (c) | 7.8 ± 0.7 (b) | |
| 750 mg kg–1 | 267 ± 24.0 (c) | 136 ± 9.1 (c) | 51 ± 4.7 (b) | 50.2 ± 3.2 (b) | 56 ± 2.8 (b) | 42 ± 2.7 (b) | 9.2 ± 0.8 (b) | |
| 1000 mg kg–1 | 334 ± 27.8 (a) | 174 ± 12 (a) | 73 ± 6.0 (a) | 57.2 ± 4.6 (a) | 71 ± 4.2 (a) | 56 ± 5.0 (a) | 11.7 ± 1.0 (a) | |
| inoculated with PGPR | 0 mg kg–1 + strain BZ-13 | 107 ± 9.7 (i) | 52 ± 6.7 (i) | 7.3 ± 0.7 (hi) | 12.4 ± 1.6 (j) | 8.8 ± 0.7 (j) | 4.6 ± 0.8 (i) | 1.23 ± 0.2 (i) |
| 50 mg kg–1 + strain BZ-13 | 111 ± 14.7 (i) | 62 ± 5.8 (h) | 10.5 ± 1.5 (h) | 19.2 ± 2.7 (i) | 13.5 ± 1.0 (i) | 9.3 ± 0.8 (h) | 2.1 ± 0.6 (h) | |
| 100 mg kg–1 + strain BZ-13 | 124 ± 10.2 (g) | 65 ± 8.3 (h) | 19.2 ± 2.8 (f) | 23.5 ± 2.9 (g) | 20.2 ± 1.4 (g) | 11.2 ± 1.6 (g) | 2.43 ± 0.09 (g) | |
| 250 mg kg–1 + strain BZ-13 | 146 ± 16.8 (f) | 84 ± 5.8 (f) | 27.3 ± 3.2 (e) | 29.3 ± 3.0 (f) | 27.4 ± 1.8 (f) | 21.2 ± 1.9 (e) | 4.2 ± 0.12 (e) | |
| 500 mg kg–1 + strain BZ-13 | 179 ± 13.7 (e) | 93 ± 9.0 (e) | 38.2 ± 4.0 (d) | 39.2 ± 3.1 (d) | 34.6 ± 2.3 (e) | 26.4 ± 2.5 (d) | 5.3 ± 0.23 (d) | |
| 750 mg kg–1 + strain BZ-13 | 213 ± 37.0 (d) | 114 ± 8.8 (d) | 47.2 ± 4.2 (b) | 45.5 ± 4.3 (c) | 47.9 ± 2.8 (c) | 36.2 ± 2.7 (c) | 8.2 ± 0.45 (b) | |
| 1000 mg kg–1 + strain BZ-13 | 312 ± 28.5 (b) | 145 ± 9.3 (b) | 70.1 ± 5.3 (a) | 54.3 ± 7.2 (a) | 68.2 ± 4.2 (a) | 53.2 ± 5.0 (a) | 10.3 ± 0.76 (a) | |
Each value is a mean of five replicates where each replicate constitutes five plants/pot (n = 5). Mean values are significant at p ≤ 0.05. Means followed by different letters are significantly different from each other according to DMRT test.
Malondialdehyde (MDA) is a byproduct of lipid peroxidation in cell membranes that may be used to assess the severity of cell membrane damage.55 Ag-NPs stress considerably raised MDA levels in W. somnifera (L.) in this study. The soil inoculation of NP-tolerant B. mojavensis, on the other hand, decreased the MDA buildup in Ag-NPs-stressed plants, suggesting that the bacterial strain can prevent lipid peroxidation.
Antioxidant mechanisms have evolved in plants to protect them from the detrimental effects of oxidative stress. Some of the most frequent ROS scavenging enzymes released by various cell organelles, such as chloroplast and mitochondria, are SOD, POD, CAT, APX, GPX, and GR. For example, superoxide dismutase (SOD) and catalases (CAT) work collectively to change [O–O]2– and hydrogen peroxide (H2O2) to molecular oxygen (O2) and water (H2O), as well as to decrease •OH radicals, while POD serves as an ROS scavenger.56 Here, the amount of antioxidant enzymes extracted from root tissues of ashwagandha increased significantly with increasing Ag-NPs concentrations. However, the application of the NP-tolerant PGPR strain lowered the level of antioxidant enzymes. For instance, CAT, SOD, POX, APX, and GR activities of ashwagandha plants maximally and significantly declined by 17, 21, 41, 24, and 32%, respectively when B. mojavensis BZ-13 was used for inoculation in the presence of 50 mg Ag-NPs kg–1 soil compared to plants that were noninoculated but treated with a similar amount of NPs (Table 2).
Accumulation of Ag-NPs in Bioinoculated W. somnifera (L.)
Ag-NPs internalization in different organs (roots, shoots, and leaves) of ashwagandha plants increased with increasing concentrations in sandy clay loam soil. In general, roots accumulated the largest concentration of Ag-NPs. As an example, Ag from 1000 mg Ag-NPs kg–1 soil was deposited in roots up to 423 μg g–1 followed by shoots (111 μg g–1) and leaves (45 μg g–1) (Figure 7A–C). W. somnifera (L.) plants grown concurrently with NP-tolerant B. mojavensis BZ-13, on the other hand, showed a substantial reduction in Ag bioaccumulation, with a maximum drop in leaves. Overall, the organ uptake of Ag followed the order: roots > shoots > leaves. B. mojavensis reduced the bioaccumulation of Ag-NPs in roots and shoots by 20% and 25%, respectively, over noninoculated and treated 50 mg Ag-NPs kg–1 soil with control in terms of percent improvement. B. mojavensis may have used metal-chelating processes other than synthesis of growth regulating substances to do this, such as siderophore excretion in order to make the nanoparticles biounavailable to plants. Madhaiyan et al.57 showed that metal-tolerant PGPR strains (Methylobacterium oryzae and Burkholderia sp.) decreased the phytotoxicity of nanoparticles in L. esculentum (L.) and improved tomato growth in gnotobiotic and pot culture tests, which is similar to the current work.
Figure 7.
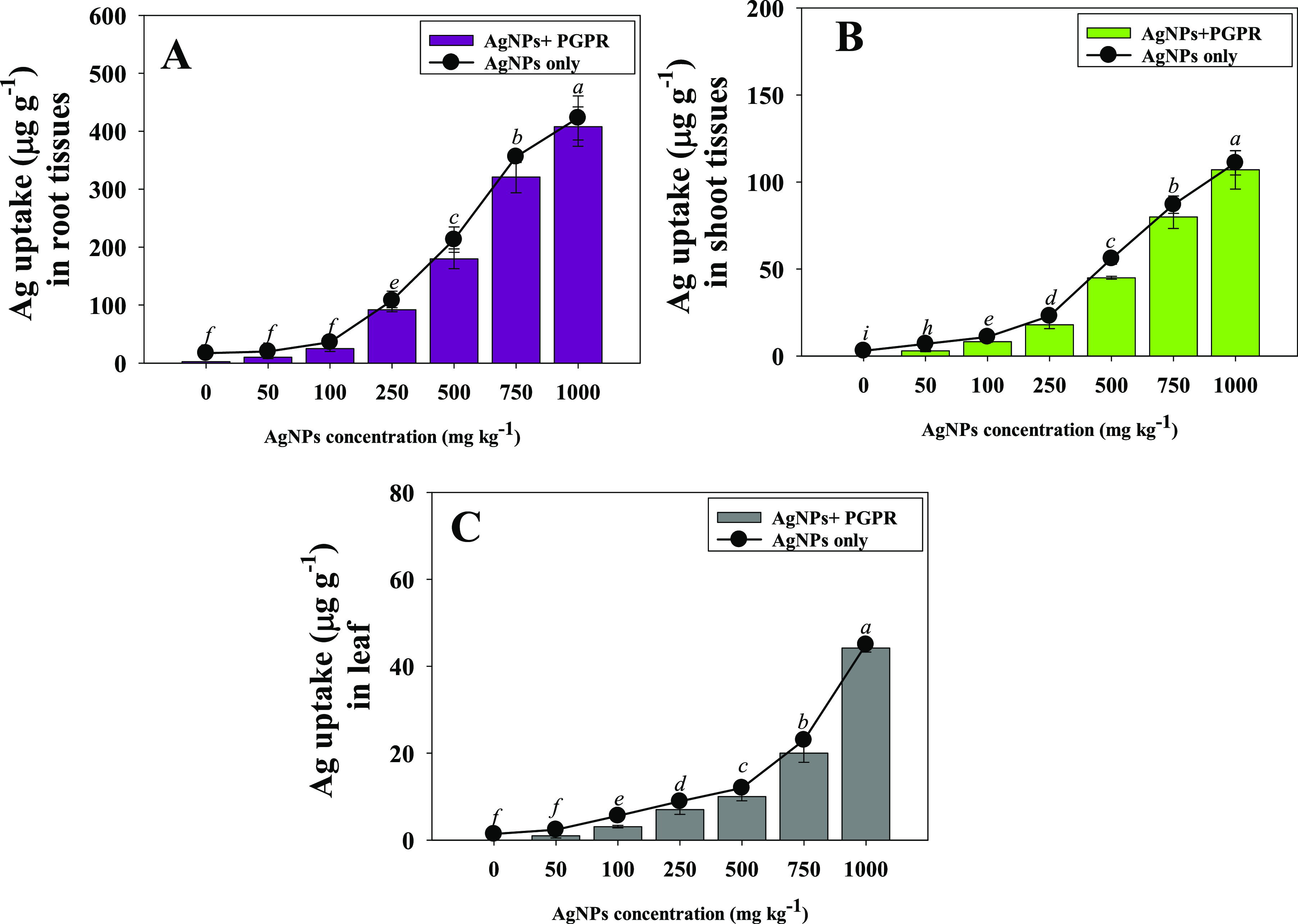
Bioaccumulation of Ag in different organs: root (A), shoots (B), and leaf (C) tissues of W. somnifera (L.) plants treated with different concentrations of Ag-NPs and inoculated with Bacillus mojavensis BZ-13 under pot-house conditions. Values are the mean of five replicates (n = 5) with standard errors (SE). Different bar letters show significant differences among treatments separately.
Conclusion
The current study emphasized the harmful influence of varied levels of Ag-NPs on several biological and physiological characteristics of Withania somnifera (L.) plants cultivated in a pot-house environment. Because of considerable Ag-NPs accumulation and translocation, NP-treated W. somnifera (L.) plants demonstrated significantly lower crop performance. The bacterial inoculant Bacillus mojavensis strain BZ-13, which is NP-resistant, synthesizes indole-3-acetic acid, EPSs, and other plant growth-promoting chemicals, and was utilized to circumvent the harmful effects of Ag-NPs. Inoculation with B. mojavensis enhanced the growth of W. somnifera (L.) crops cocultivated with Ag-NPs in a soil environment by increasing the germination rates, length, dry biomass, chlorophyll fluorescence, alkaloids, and leaf exchange parameters and lowering the level of proline, MDA, and antioxidant activity, and decreasing Ag-NPs absorption by roots. The improved W. somnifera (L.) performance indicates that B. mojavensis may possibly colonize and established itself in the plant rhizosphere while sustaining physiological activity in Ag-NP-contaminated soil. B. mojavensis might serve as an agronomically significant and cost-effective remediation agent for Ag-NPs polluted soils in terms of increasing W. somnifera (L.) output due to its NPs stress reduction and plant growth enhancing characteristics. In the presence of lower Ag-NPs doses, B. mojavensis BZ-13 had a maximum positive effect; therefore, a lower level of NPs would be desirable in future investigations, and applications of higher concentrations of NPs in agricultural practices should be avoided. Furthermore, more research is needed to determine the effect of B. mojavensis seed inoculation on soil biological activities under different experimental settings.
Experimental Section
Chemicals (Ag-NPs) Used
Silver nanoparticles (Ag-NPs) were procured from Sisco Research Laboratories Pvt. Ltd., (Mumbai, India) with an approximate size of 50–60 nm (TEM analysis revealed they are round and polydispersed) with 99% purity.
Phytotoxicity Assessment of Ag-NPs in W. somnifera (L.) Grown in Vitro
Germination Percentage, Length, and Vigor Index
W. somnifera (L.) seeds were soaked for 24 h in double distilled (DD) deionized water. Seeds were cleaned with a solution of 3% sodium hypochlorite (NaOCl) and washed with sterile water. Soft agar (Hi-Media Pvt. Ltd. Mumbai, India) (0.7%) plates amended with different concentrations of Ag-NPs were prepared. Seeds were planted on agar plates and kept at room temperature (28 ± 2 °C) for 3–4 days. Percentage of germination, root and shoot lengths, and plantlet vigor index were measured after 4 days.
In Vivo Detection of Changes in Mitochondrial Membrane Potential (ΔΨm) in Ag-NP-Treated Roots Using CLSM
In order to evaluate the cytotoxic behavior of Ag-NPs, ashwagandha seeds were grown on NP-supplemented soft agar plates. After emergence, seedlings were carefully detached, and Ag-NPs-exposed roots were stained with 1.0 g mL–1 of rhodamine 123 (Rh-123; a fluorescent dye purchased from Sigma-Aldrich) for qualitative investigation of alterations in the mitochondrial membrane potential (ΔΨm) and cellular death in roots and examined under a confocal laser scanning microscope (LSM-780, Leica Confocal microscope, Zeiss, Oberkochen, Germany).27
Bacterial Isolation and Biochemical Characterization
Soil samples for recovery of bacterial isolates were obtained from vegetable grown areas near Aligarh’s lock factories (GPS location; 27.937343, 78.141488), where effluent from industries is utilized to irrigate agricultural fields. The top layer was scraped off, and dirt was collected from at least 12 cm beneath the surface and delivered to the lab in sterile plastic bags (20–30 cm in diameter). For microbiological testing, soil samples were equally mixed and serially diluted in normal saline solution (NSS). A 100 μL aliquot of soil suspension was spread on nutrient agar (NA) (Hi-Media Pvt. Ltd., Mumbai, India) plates and incubated at 28 ± 2 °C for 2–3 days. After the population density was determined, microbial cultures were selected and restreaked three times on the same medium to get pure cultures. The recovered soil isolates were stored on the same nutrient medium until it was required. Standard morphological and biochemical techniques were used to identify the bacterial strain.58
Bacterial Tolerance to Ag-NPs
To investigate bacterial resistance or sensitivity, cells were treated with different concentrations of Ag-NPs. In 50 mL of nutrient broth (NB), a single colony from the plate culture was inoculated in liquid broth added with 0–1600 μg Ag-NPs mL–1 to determine the CFU count. Under shaking (120 r/min) conditions, NP-untreated/treated cells were incubated (for 72 h at 28 °C). A 0.1 mL culture filtrate was uniformly dispersed on NB plates and allowed to grow at 28 °C. Following incubation, CFUs were calculated after counting the number of viable cells.14
Molecular Identification and Construction of Phylogenetic Tree
Following cultural and biochemical analysis, strain BZ-13 was further molecularly sequenced based on 16S rRNA partial gene sequencing utilizing the universal primers 785F and 907R for species-level molecular identification (refer to section in Supporting Information).
Effect of Ag-NPs on Plant Growth-Promoting Activities (IAA, Siderophore, ACC Deaminase, Ammonia and HCN) of B. mojavensis Strain BZ-13
The plant hormone indole-3-acetic acid (IAA) produced by the Ag-NP-tolerant strain B. mojavensis BZ-13 was quantified using the globally adapted Brick technique.59 Briefly, the strain BZ-13 was grown in Luria–Bertani (LB) broth (Hi-Media Pvt. Ltd. India) supplemented with a fixed concentration of tryptophan (Hi-Media Pvt. Ltd. Mumbai, India) and treated with various levels of Ag-NPs (0–1200 μg mL–1),, and the produced IAA was determined. Furthermore, siderophore production activity was assessed both qualitatively and quantitatively. Spot inoculation of BZ-13 isolates on Ag-NP-supplemented universal chrome azurol S (CAS) agar (Hi-Media Pvt. Ltd. India) plates were tested for siderophore synthesis.60,61 Then, the amount of phenolate siderophore (salicylic acid and 2,3-dihydroxy benzoic acid) was quantified by culturing the cells of B. mojavensis in Ag-NP-treated liquid medium. To check the bacterial synthesis of 1-amino cyclopropane 1-carboxylate (ACC) deaminase, bacterial cultures were raised in liquid medium treated with increasing concentrations of Ag-NPs, and the amount of ACC (μM α-ketobutyrate mg–1 protein h–1) was quantified according to the methods of Honma and Shimomura,62 and Penrose and Glick.63 Synthesis of cyanogenic compound (HCN)64 and ammonia65 by B. mojavensis BZ-13 was also assayed by growing the cells in HCN-induction medium and peptone water, respectively.
Assessment of Biofilm Development and Their Associated Traits under Ag-NP Stress
The development of biofilm by strain BZ-13 in the absence/presence of Ag-NPs was assessed on 96-well plates using 1% crystal violet (CV; Hi-Media Pvt. Ltd. Mumbai, India) following the standard procedure of O’Toole66 (refer to section in Supporting Information). Furthermore, swimming and swarming motilities were assayed.67 For this, spot inoculation of freshly produced cells was done on Ag-NPs supplemented at 0.3% and 0.5% (w/v) on NA plates and incubated at 28 ± 2 °C for 2 days. Following incubation, by zone measurement scale, bacterial motilities were measured as their swarming diameter and represented in millimeters (mm). In the presence of Ag-NP stress, the extracellular polymeric substances (EPSs) generated by the BZ-13 strain were quantified68 Furthermore, growing cells in liquid medium supplemented with varying amounts of Ag-NPs were used to measure alginate synthesis69 by strain BZ-13 (refer to section in Supporting Information). Furthermore, the bacterial strain’s cell surface hydrophobicity (CSH) was determined by culturing the cells with various Ag-NP concentrations and using the microbial adherence to hydrocarbons (MATH) method.70
Impact of Ag-NPs on B. mojavensis BZ-13 Inoculated W. somnifera (L.): Pot-House Study
Preparation of Bacterial Inoculum, Ag-NPs Treatment, and Soil Application
For inoculum build-up, B. mojavensis BZ-13 was cultured in nutrient broth (NB) at 28 ± 2 °C for 3–4 days. The healthy and uniform in sized seeds of W. somnifera were surface sterilized for 3 min with 2% sodium hypochlorite (NaOCl; Hi-Media Pvt. Ltd. Mumbai, India) and then washed with sterile water and dried in the shade. Seeds were then immersed in liquid broth of B. mojavensis (∼1 × 107–8 CFU mL–1 inoculum suspensions) for 2–3 h using 1% gum acacia powder (Sisco Research Laboratory Pvt. Ltd., Mumbai, India) as an adhesive. The bacteria-treated and control ashwagandha seeds were sown in pot soils treated with 0–1000 mg/kg Ag-NPs solution (the NP solution was prepared by mixing the powdered nanoparticles in water). Two days before planting the seeds, particles were added into the experimental soils (refer to Supplementary Table S1). The pot soils were homogenized after the amendment of Ag-NPs. The seeds that had not been coated with bacterial culture were dipped into sterile water and used as a control. Pots were maintained in an open field condition until the crops were matured and harvested. The higher values (500 and 1000 mg kg–1) were used under the assumption that NPs would remain in agricultural soils in excess of normal levels due to unfettered release through home and industrial wastewater over time.
Seed Germination, Growth Measurement, Biomass Accumulation, Leaf Number and Area, Berry Number
After 8 days of sowing (DAS), the germination percentage was recorded. Biological parameters like root and shoot length, number and area of leaves, and number of berries were recorded at harvest. After the plants were carefully removed, they were gently separated into roots and shoots, and a measuring scale was used to measure the lengths. After adherent soil particles were carefully removed, multiple washings were done with tap water, and following drying on tissue sheets, the dry matter buildup in roots was evaluated. Root samples were dried in a ventilated oven (York Scientific Industries, Pvt. Ltd. India) at 80 °C, and the accumulated biomass was recorded. Further, the leaf number, leaf area, and berry number per plant were determined.
Determination of Photosynthetic Pigments (chl a, b, Total Chlorophyll, and Carotenoid)
The formation of chlorophylls (chl a, chl b, total chlorophyll) and carotenoid content in leaf tissues of B. mojavensis BZ-13-inoculated and Ag-NPs-treated W. somnifera (L.) plants were extracted and measured using the previously described methods of Arnon71 and Kirk and Allen72 (refer to section in Supporting Information).
Measurement of SPAD Index and Chlorophyll Fluorescence
The soil plant analysis development (SPAD) index was measured using a SPAD chlorophyll meter (SPAD, Minolta Camera Co., Osaka, Japan). The SPAD value was taken from randomly selected plant samples.73 Chlorophyll fluorescence parameters including photosystem II efficiency (Fv/Fm), PS II quantum yield (FPSII), photochemical quenching (qP), and non-photochemical quenching (NpQ) in bacteria-inoculated and Ag-NP-treated ashwagandha plants were calculated by using a chlorophyll fluorometer.74
Gas Exchange Characteristics of Ag-NP-Treated and Bioinoculated W. somnifera (L.)
Gas exchange parameters stomatal conductance (gs) including the transpiration rate (E), internal CO2 concentration (Ci), net photosynthetic rate (PN), and vapor pressure deficit (kPa) were measured with a Li-COR 6400 portable photosynthesis system (Li-COR, Lincoln, NE, USA). After 45 days postsowing (DAS), fully developed leaves from each treatment were carefully detached (between 9:00–10:00 a.m.) and cleaned, and gas exchange parameters were determined.75
Alkaloid and Polyphenol Contents in Ag-NP-Treated and Bioinoculated W. somnifera
For extraction and measurement of withaferin-A (alkaloid content) in root tissues, Ag-NP-treated and bioinoculated ashwagandha plants were detached. The method of extraction and determination was adopted as previously described by Lin et al.76 The total phenolics content in roots was measured by the Folin-Ciocalteu technique77 using 100 μL of the plant extract. A total of 900 μL of methanol was used to dilute the extract 10 times. Folin-Ciocalteu reagent (0.5 mL) was added, and the mixture was thoroughly mixed. Then, 1.0 mL of saturated disodium carbonate (Na2CO3; Hi-Media Pvt. Ltd., Mumbai, India) was added after 6 min. The reaction mixture was allowed to settle for at least 2–3 h after the total volume was adjusted to 7.5 mL using DDW. The absorbance was measured at 680 nm against a blank solution after 2–3 h. Using a gallic acid (200–1000 g) calibration curve, the total phenol content was reported as mg of gallic acid equivalents (GAE)/g of extract. The total flavonoid concentration was determined using the aluminum chloride colorimetric test, as described by Kim et al.78 For quantification, in a test tube, 100 μL of the plant extract was diluted with 0.9 mL. Using DDW, the volume was reduced to 4 mL. Then, 300 μL of 5% Na2NO3 was added to this and left for 5 min (for detailed methodology, refer to the section in Supporting Information). In order to determine total tannins accumulated in B. mojavensis inoculated and Ag-NP-treated roots of ashwagandha plants, the method of Folin-Denis was applied.79 For extraction, 900 μL of methanol was added to 100 μL of extract, and the final volume of solution was made to 7.5 mL by adding DDW. After this, 0.5 mL of Folin-Denis reagent was added along with 1.0 mL of saturated Na2CO3. Using DDW, total volume was adjusted to 10 mL. After the reaction mixture was allowed to sit for a couple of hours, the absorbance was measured at 680 nm against a blank solution. Using a tannic acid (20–100 g) calibration curve, the quantity of tannins was quantified as mg of tannic acid equivalents/gram of extract.
Proline, MDA, and Antioxidant Enzymes Determination in Ag-NP-Treated and Bioinoculated Plants
The method of Bates et al.80 was applied to determine proline content accumulated in root tissues of B. mojavensis and Ag-NPs-treated ashwagandha plants. For quantification, 50 mg of root samples was extracted in sulfosalicylic acid (Sisco Research Laboratory Pvt. Ltd., Mumbai, India) and combined with the same quantity of glacial acetic acid (Sisco Research Laboratory Pvt. Ltd., Mumbai, India) and ninhydrin solutions (Hi-Media Pvt. Ltd., Mumbai, India). A total of 5.0 mL of toluene was added to the sample after it was heated to 100 °C. Using a spectrophotometer (UV–visible spectrophotometer UV-2450, Shimadzu, Tokyo, Japan), the aspired layer’s absorbance was measured at 528 nm.
The previously used methods of Shahid et al.81−85 and Khan et al.86 was employed to determine lipid peroxidation as measured by the MDA content. For the assay, roots were homogenized in 0.1% trichloroacetic acid (TCA; Sisco Research Laboratory Pvt. Ltd., Mumbai, India) and centrifuged for 15 min at 10000g. The supernatant was combined with a 20% TCA solution containing 0.5% thiobarbituric acid (TBA; Sisco Research Laboratory Pvt. Ltd., Mumbai, India). Then, the solution was warmed for 30 min at 95 °C. The supernatant was centrifuged at 1000g for 15 min at 4 °C after chilling. The mixture’s absorbance was measured at 532 nm.87
For determination of antioxidant enzymes like catalase (CAT), peroxidase (POX), and superoxide dismutase (SOD), roots (0.5 g) were homogenized in a 50 mM phosphate buffer (pH = 7.0) of 1% polyvinylpyrrolidone (PVP; Hi-Media Pvt. Ltd., Mumbai, India). The mixture solutions were homogenized (at 15000g for 10 min and at 4 °C), and the obtained filtrate was used for determination of antioxidant enzymes. The enzyme extract (0.1 mL) was combined in a reaction mixture of pyrogallol, phosphate buffer (pH = 6.8), and 1% H2O2 to determine the peroxidase (POX) activity. A UV–visible spectrophotometer was used to calculate absorbance at 420 nm.88 For the catalase (CAT) estimation, phosphate buffer (pH = 6.8), 0.1 M hydrogen peroxide (H2O2; Hi-Media Pvt. Ltd., Mumbai, India), and enzyme extract (100 μL) were mixed together.89 Beauchamp and Fridovich’s technique90 was used to determine the superoxide dismutase (SOD) activity. The reaction combination needed 50 mM phosphate buffer (pH = 7.8), 20 μM riboflavin (Hi-Media Pvt. Ltd., Mumbai, India), 75 mM nitroblue tetrazolium (NBT; Hi-Media Pvt. Ltd., Mumbai, India), 13 mM methionine (Hi-Media Pvt. Ltd., Mumbai, India), and 0.1 mM ethylene diamine tetra acetic acid (EDTA; Hi-Media Pvt. Ltd., Mumbai, India). A UV–visible spectrophotometer was used to measure absorbance at 560 nm after the combination was illuminated for 10 min in two fluorescent light tubes.
Uptake of Ag-NPs in B. mojavensis-Inoculated W. somnifera (L.) Plants
The accumulation of Ag-NPs was measured at the harvest of ashwagandha crops. W. somnifera (L.) plants were divided into three parts: roots, shoots, and leaves, when detached from pots cultivated with varying rates of Ag-NPs. Plant tissues were dried in a vacuum oven at 60 °C until they reached a consistent weight and then were crushed into a fine powder. Acid digestion was done in 10 mL of nitric acid and perchloric acid (Sisco Research Laboratory Pvt. Ltd., Mumbai, India) (at a ratio of 4:1) using 1.0 g of dried powder. The remaining solution was filtered using Whatman No. 2 filter paper after complete digestion, and the volume was increased to 100 mL using DDW. Inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer’s NexION ICP) was used to determine the amount of Ag in the sample. For calibration, we employed blank and varied concentrations of standard solutions made from 1000 mg L–1 stock.
Statistical Analysis
Each ashwagandha crop experimental treatment was reproduced at least five times. Because the data were homogeneous, the data from each crop were combined and statistically evaluated using one-way and two-way analysis of variance (ANOVA) with the statistical program Minitab 17.0. For two variables (PGPR inoculation and Ag-NP dosage), a two-way ANOVA was used. For each measured parameter, the data in the figures are displayed as mean ± SD (n = 5).
Acknowledgments
The authors extend their appreciation to the researchers supporting Project No. RSP-2021/229, King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00262.
Details on how to use 16S rRNA gene sequencing to identify rhizobacterial strains, detailed methodologies for biofilm assessment, alginate production, and total flavonoid content. Table S1 presenting the physicochemical properties of experimental soil. Figure S1 showing the neighbor-joined phylogenetic tree of Bacillus mojavensis BZ-13 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hobson D. W. Commercialization of Nanotechnology. WIREs Nanomedicine and Nanobiotechnology 2009, 1 (2), 189–202. 10.1002/wnan.28. [DOI] [PubMed] [Google Scholar]
- Bundschuh M.; Filser J.; Lüderwald S.; McKee M. S.; Metreveli G.; Schaumann G. E.; Schulz R.; Wagner S. Nanoparticles in the Environment: Where Do We Come from, Where Do We Go To?. Environ. Sci. Eur. 2018, 30 (1), 6. 10.1186/s12302-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltenrich N. Nanosilver: weighing the risks and benefits. Environ. Health Perspect. 2013, 121, a220 10.1289/ehp.121-a220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strużyńska L.; Skalska J. Mechanisms Underlying Neurotoxicity of Silver Nanoparticles 2018, 1048, 227–250. 10.1007/978-3-319-72041-8_14. [DOI] [PubMed] [Google Scholar]
- Singh R.; Singh D. Chitin membranes containing silver nanoparticles for wound dressing application. Int. Wound J. 2014, 11 (3), 264–268. 10.1111/j.1742-481X.2012.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Diao J.; Zhang J.; Liu J. Fabrication of new chitosan-based composite sponge containing silver nanoparticles and its antibacterial properties for wound dressing. J. Nanosci. Nanotech 2011, 11 (6), 4733–4738. 10.1166/jnn.2011.4179. [DOI] [PubMed] [Google Scholar]
- Paci B.; Spyropoulos G. D.; Generosi A.; Bailo D.; Albertini V. R.; Stratakis E.; Kymakis E. Enhanced structural stability and performance durability of bulk heterojunction photovoltaic devices incorporating metallic nanoparticles. Adv. Funct. Mater. 2011, 21 (18), 3573–3582. 10.1002/adfm.201101047. [DOI] [Google Scholar]
- Hong J.; Wang C.; Wagner D. C.; Gardea-Torresdey J. L.; He F.; Rico C. M. Foliar Application of Nanoparticles: Mechanisms of Absorption, Transfer, and Multiple Impacts. Environ. Sci. Nano 2021, 8 (5), 1196–1210. 10.1039/D0EN01129K. [DOI] [Google Scholar]
- Rani P.; Gaurav S. S.; Trivedi L.; Singh A.; Shukla G. Assessment of nanotoxicity of silver nanoparticles on Pea (Pisum sativum) grown under ex situ conditions. J. Stress Physiol. Biochem. 2021, 17, 20–34. [Google Scholar]
- Ke M.; Li Y.; Qu Q.; Ye Y.; Peijnenburg W. J. G. M.; Zhang Z.; Xu N.; Lu T.; Sun L.; Qian H. Offspring Toxicity of Silver Nanoparticles to Arabidopsis Thaliana Flowering and Floral Development. J. Hazard. Mater. 2020, 386, 121975. 10.1016/j.jhazmat.2019.121975. [DOI] [PubMed] [Google Scholar]
- González Linares M.; Jia Y.; Sunahara G. I.; Whalen J. K. Barley (Hordeum Vulgare) Seedling Growth Declines with Increasing Exposure to Silver Nanoparticles in Biosolid-Amended Soils. Can. J. Soil Sci. 2020, 100 (3), 189–197. 10.1139/cjss-2019-0135. [DOI] [Google Scholar]
- Yang J.; Jiang F.; Ma C.; Rui Y.; Rui M.; Adeel M.; Cao W.; Xing B. Alteration of Crop Yield and Quality of Wheat upon Exposure to Silver Nanoparticles in a Life Cycle Study. J. Agric. Food Chem. 2018, 66 (11), 2589–2597. 10.1021/acs.jafc.7b04904. [DOI] [PubMed] [Google Scholar]
- Nair P. M. G.; Chung I. M. Physiological and Molecular Level Studies on the Toxicity of Silver Nanoparticles in Germinating Seedlings of Mung Bean (Vigna Radiata L.). Acta Physiol. Plant. 2015, 37 (1), 1719. 10.1007/s11738-014-1719-1. [DOI] [Google Scholar]
- Ahmed B.; Syed A.; Rizvi A.; Shahid M.; Bahkali A. H.; Khan M. S.; Musarrat J. Impact of Metal-Oxide Nanoparticles on Growth, Physiology and Yield of Tomato (Solanum Lycopersicum L.) Modulated by Azotobacter Salinestris Strain ASM. Environ. Pollut. 2021, 269, 116218. 10.1016/j.envpol.2020.116218. [DOI] [PubMed] [Google Scholar]
- Khan N.; Bano A. Modulation of Phytoremediation and Plant Growth by the Treatment with PGPR, Ag Nanoparticle and Untreated Municipal Wastewater. Int. J. Phytoremediation 2016, 18 (12), 1258–1269. 10.1080/15226514.2016.1203287. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Pahal V.; Gupta A.; Vadhan R.; Chandra H.; Dubey R. C. Effect of Silver Nanoparticles and Bacillus Cereus LPR2 on the Growth of Zea Mays. Sci. Rep. 2020, 10 (1), 20409. 10.1038/s41598-020-77460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S.; Bano A. Effects of PGPR (Pseudomonas Sp.) and Ag-Nanoparticles on Enzymatic Activity and Physiology of Cucumber. Recent Pat. Food. Nutr. Agric. 2020, 11 (2), 124–136. 10.2174/2212798410666190716162340. [DOI] [PubMed] [Google Scholar]
- Askary M.; Talebi S.; Shafieigavari M. Effect of Iron Oxid Nanoparticle on the Growth and Physiology of Inoculated Alfalfa (Medicago Sativa L.) with Rhizobium Meliloti. J. Cell Tissue 2020, 11 (1), 25–43. 10.52547/JCT.11.1.25. [DOI] [Google Scholar]
- Saif S.; Khan M. S.; Zaidi A.; Rizvi A.; Shahid M.. Metal Toxicity to Certain Vegetables and Bioremediation of Metal-Polluted Soils. In Microbial Strategies for Vegetable Production; Springer International Publishing: Cham, 2017; pp 167–196. 10.1007/978-3-319-54401-4_8. [DOI] [Google Scholar]
- Morcillo R.; Manzanera M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11 (6), 337. 10.3390/metabo11060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahiruddin S.; Basist P.; Parveen A.; Parveen R.; Khan W.; Gaurav; Ahmad S. Ashwagandha in Brain Disorders: A Review of Recent Developments. J. Ethnopharmacol. 2020, 257, 112876. 10.1016/j.jep.2020.112876. [DOI] [PubMed] [Google Scholar]
- Singh G.; Sharma P. K.; Dudhe R.; Singh S. Biological activities of Withania somnifera. Ann. Biol. Res. 2010, 1 (3), 56–63. [Google Scholar]
- Singh O. S.; Pant N. C.; Laishram L.; Tewari M.; Dhoundiyal R.; Joshi K.; Pandey C. Effect of CuO nanoparticles on polyphenols content and antioxidant activity in Ashwagandha (Withania somnifera L. Dunal). J. Pharmacogn. Phytochem. 2018, 7 (2), 3433–3439. [Google Scholar]
- López-Moreno M. L.; de la Rosa G.; Hernández-Viezcas J. Á.; Castillo-Michel H.; Botez C. E.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Evidence of the Differential Biotransformation and Genotoxicity of ZnO and CeO 2 Nanoparticles on Soybean (Glycine Max) Plants. Environ. Sci. Technol. 2010, 44 (19), 7315–7320. 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed B.; Shahid M.; Khan M. S.; Musarrat J. Chromosomal Aberrations, Cell Suppression and Oxidative Stress Generation Induced by Metal Oxide Nanoparticles in Onion (Allium Cepa) Bulb. Metallomics 2018, 10 (9), 1315–1327. 10.1039/C8MT00093J. [DOI] [PubMed] [Google Scholar]
- Nair P. M. G.; Chung I. M. Assessment of Silver Nanoparticle-Induced Physiological and Molecular Changes in Arabidopsis Thaliana. Environ. Sci. Pollut. Res. 2014, 21 (14), 8858–8869. 10.1007/s11356-014-2822-y. [DOI] [PubMed] [Google Scholar]
- Faisal M.; Saquib Q.; Alatar A. A.; Al-Khedhairy A. A.; Hegazy A. K.; Musarrat J. Phytotoxic Hazards of NiO-Nanoparticles in Tomato: A Study on Mechanism of Cell Death. J. Hazard. Mater. 2013, 250–251, 318–332. 10.1016/j.jhazmat.2013.01.063. [DOI] [PubMed] [Google Scholar]
- Fatemi H.; Esmaiel Pour B.; Rizwan M. Isolation and Characterization of Lead (Pb) Resistant Microbes and Their Combined Use with Silicon Nanoparticles Improved the Growth, Photosynthesis and Antioxidant Capacity of Coriander (Coriandrum Sativum L.) under Pb Stress. Environ. Pollut. 2020, 266, 114982. 10.1016/j.envpol.2020.114982. [DOI] [PubMed] [Google Scholar]
- Ayano H.; Miyake M.; Terasawa K.; Kuroda M.; Soda S.; Sakaguchi T.; Ike M. Isolation of a Selenite-Reducing and Cadmium-Resistant Bacterium Pseudomonas Sp. Strain RB for Microbial Synthesis of CdSe Nanoparticles. J. Biosci. Bioeng. 2014, 117 (5), 576–581. 10.1016/j.jbiosc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Soltani Nezhad S.; Rabbani Khorasgani M.; Emtiazi G.; Yaghoobi M. M.; Shakeri S. Isolation of Copper Oxide (CuO) Nanoparticles Resistant Pseudomonas Strains from Soil and Investigation on Possible Mechanism for Resistance. World J. Microbiol. Biotechnol. 2014, 30 (3), 809–817. 10.1007/s11274-013-1481-3. [DOI] [PubMed] [Google Scholar]
- Khan S.; Mukherjee A.; Chandrasekaran N. Silver Nanoparticles Tolerant Bacteria from Sewage Environment. J. Environ. Sci. 2011, 23 (2), 346–352. 10.1016/S1001-0742(10)60412-3. [DOI] [PubMed] [Google Scholar]
- Rostami S.; Azhdarpoor A. The Application of Plant Growth Regulators to Improve Phytoremediation of Contaminated Soils: A Review. Chemosphere 2019, 220, 818–827. 10.1016/j.chemosphere.2018.12.203. [DOI] [PubMed] [Google Scholar]
- Khan A. L.; Waqas M.; Kang S.-M.; Al-Harrasi A.; Hussain J.; Al-Rawahi A.; Al-Khiziri S.; Ullah I.; Ali L.; Jung H.-Y.; Lee I.-J. Bacterial Endophyte Sphingomonas Sp. LK11 Produces Gibberellins and IAA and Promotes Tomato Plant Growth. J. Microbiol. 2014, 52 (8), 689–695. 10.1007/s12275-014-4002-7. [DOI] [PubMed] [Google Scholar]
- Karimi E.; Mohseni Fard E. Nanomaterial Effects on Soil Microorganisms 2017, 48, 137–200. 10.1007/978-3-319-46835-8_5. [DOI] [Google Scholar]
- Rezayatmand Z.; Doudi M. The Effect of Silver Nanoparticles on the Growth of Nitrogen Fixing and ACC Deaminase Producing Bacteria Isolated from Sunflower Rhizosphere. Int. J. Mol. Clin. Microbiol. 2018, 8 (1), 912–921. [Google Scholar]
- Panichikkal J.; Puthiyattil N.; Raveendran A.; Nair R. A.; Krishnankutty R. E. Application of Encapsulated Bacillus Licheniformis Supplemented with Chitosan Nanoparticles and Rice Starch for the Control of Sclerotium Rolfsii in Capsicum Annuum (L.) Seedlings. Curr. Microbiol. 2021, 78 (3), 911–919. 10.1007/s00284-021-02361-8. [DOI] [PubMed] [Google Scholar]
- Kumar P.; Pahal V.; Gupta A.; Vadhan R.; Chandra H.; Dubey R. C. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep. 2020, 10 (1), 1–10. 10.1038/s41598-020-77460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M.; Khan M. S. Cellular destruction, phytohormones and growth modulating enzymes production by Bacillus subtilis strain BC8 impacted by fungicides. Pestic. Biochem. Physiol. 2018, 149, 8–19. 10.1016/j.pestbp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Al-Shwaaiman H. A.; Shahid M.; Elgorban A. M.; Siddique K. H.; Syed A. Beijerinckia fluminensis BFC-33, a novel multi-stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum L. (wheat). Chemosphere 2022, 295, 133843. 10.1016/j.chemosphere.2022.133843. [DOI] [PubMed] [Google Scholar]
- Syed A.; Zeyad M. T.; Shahid M.; Elgorban A. M.; Alkhulaifi M. M.; Ansari I. A. Heavy metals induced modulations in growth, physiology, cellular viability, and biofilm formation of an identified bacterial isolate. ACS omega 2021, 6 (38), 25076–25088. 10.1021/acsomega.1c04396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj S. R.; Karthik C.; Kadirvelu K.; Arulselvi P. I.; Shanmugasundaram T.; Bruno B.; Rajkumar M. Understanding the Molecular Mechanisms for the Enhanced Phytoremediation of Heavy Metals through Plant Growth Promoting Rhizobacteria: A Review. J. Environ. Manage. 2020, 254, 109779. 10.1016/j.jenvman.2019.109779. [DOI] [PubMed] [Google Scholar]
- Cordell D.; Drangert J. O.; White S. The story of phosphorus: global food security and food for thought. Global Environ. Change 2009, 19 (2), 292–305. 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- Jiao W.; Chen W.; Chang A. C.; Page A. L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: a review. Environ. Polym. 2012, 168, 44–53. 10.1016/j.envpol.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Zaidi A.; Khan M. S.; Rizvi A.; Saif S.; Ahmad B.; Shahid M.. Role of phosphate-solubilizing bacteria in legume improvement. In Microbes for Legume Improvement; Springer: Cham, 2017; pp 175–197. [Google Scholar]
- Sharma S. B.; Sayyed R. Z.; Trivedi M. H.; Gobi T. A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2 (1), 1–14. 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abawari R. A.; Tuji F. A.; Yadete D. M. Phosphate solubilizing bio-fertilizers and their role in bio-available P nutrient: an overview. Int. J. Appl. Agric. Sci. 2020, 6, 162–171. 10.11648/j.ijaas.20200606.11. [DOI] [Google Scholar]
- Dimkpa C. O.; Mclean J. E.; Britt D. W.; Anderson A. J. CuO and ZnO Nanoparticles Differently Affect the Secretion of Fluorescent Siderophores in the Beneficial Root Colonizer, Pseudomonas Chlororaphis O6. Nanotoxicology 2012, 6 (6), 635–642. 10.3109/17435390.2011.598246. [DOI] [PubMed] [Google Scholar]
- Costa O. Y. A.; Raaijmakers J. M.; Kuramae E. E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 9. 10.3389/fmicb.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.; Cai Z.; Chen Y.; Zhu H.; Chang X.; Wang X.; Liu Z.; Zhang J.; Nie G. Effects of an Exopolysaccharide from Lactococcus Lactis Z-2 on Innate Immune Response, Antioxidant Activity, and Disease Resistance against Aeromonas Hydrophila in Cyprinus Carpio L. Fish Shellfish Immunol. 2020, 98, 324–333. 10.1016/j.fsi.2020.01.037. [DOI] [PubMed] [Google Scholar]
- Király Z.; El-Zahaby H. M.; Klement Z. Role of Extracellular Polysaccharide (EPS) Slime of Plant Pathogenic Bacteria in Protecting Cells to Reactive Oxygen Species. J. Phytopathol. 1997, 145 (2–3), 59–68. 10.1111/j.1439-0434.1997.tb00365.x. [DOI] [Google Scholar]
- Radzki W.; Gutierrez Mañero F. J.; Algar E.; Lucas García J. A.; García-Villaraco A.; Ramos Solano B. Bacterial Siderophores Efficiently Provide Iron to Iron-Starved Tomato Plants in Hydroponics Culture. Antonie Van Leeuwenhoek 2013, 104 (3), 321–330. 10.1007/s10482-013-9954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan W.; David J.; Bashir F. ACC-Deaminase and/or Nitrogen-Fixing Rhizobacteria and Growth Response of Tomato (Lycopersicon Pimpinellfolium Mill.). J. Plant Interact. 2014, 9 (1), 869–882. 10.1080/17429145.2014.964785. [DOI] [Google Scholar]
- Hatami M.; Khanizadeh P.; Bovand F.; Aghaee A. Silicon Nanoparticle-Mediated Seed Priming and Pseudomonas Spp. Inoculation Augment Growth, Physiology and Antioxidant Metabolic Status in Melissa Officinalis L. Plants. Ind. Crops Prod. 2021, 162, 113238. 10.1016/j.indcrop.2021.113238. [DOI] [Google Scholar]
- Shahid M.; Khan M. Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech 2018, 8 (2), 1–17. 10.1007/s13205-018-1145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschwege P.; Paradis V.; Conti M.; Holstege A.; Richet F.; Deteve J.; Menager P.; Legrand A.; Jardin A.; Bedossa P.; Benoit G. In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J. Urol. 1999, 162 (2), 553–557. 10.1016/S0022-5347(05)68626-0. [DOI] [PubMed] [Google Scholar]
- Tian Z.; Wang F.; Zhang W.; Liu C.; Zhao X. Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hortic. Environ. Biotechnol. 2012, 53 (3), 183–192. 10.1007/s13580-012-0069-4. [DOI] [Google Scholar]
- Madhaiyan M.; Poonguzhali S.; Sa T. Metal Tolerating Methylotrophic Bacteria Reduces Nickel and Cadmium Toxicity and Promotes Plant Growth of Tomato (Lycopersicon Esculentum L.). Chemosphere 2007, 69 (2), 220–228. 10.1016/j.chemosphere.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Holt J.; Krieg N. R.; Sneath P. H. A.; Stanley J. T.; William S. T.. Bergey’s Manual of Determinative Microbiology; Williams and Wilkins: Baltimore, 1994; pp 786–788. [Google Scholar]
- Bric J. M.; Bostock R. M.; Silverstone S. E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57 (2), 535–538. 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya H. B.; Rao K. K. Production of Pyoverdine, the Fluorescent Pigment of Pseudomonas Aeruginosa PAO1. FEMS Microbiol. Lett. 1985, 27 (2), 233–235. 10.1111/j.1574-6968.1985.tb00673.x. [DOI] [Google Scholar]
- Schwyn B.; Neilands J. B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160 (1), 47–56. 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Honma M.; Shimomura T. Metabolism of 1-Aminocyclopropane-1-Carboxylic Acid. Agric. Biol. Chem. 1978, 42 (10), 1825–1831. 10.1080/00021369.1978.10863261. [DOI] [Google Scholar]
- Penrose D. M.; Glick B. R. Methods for Isolating and Characterizing ACC Deaminase-Containing Plant Growth-Promoting Rhizobacteria. Physiol. Plant. 2003, 118 (1), 10–15. 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Bakker A. W.; Schippers B. Microbial Cyanide Production in the Rhizosphere in Relation to Potato Yield Reduction and Pseudomonas SPP-Mediated Plant Growth-Stimulation. Soil Biol. Biochem. 1987, 19 (4), 451–457. 10.1016/0038-0717(87)90037-X. [DOI] [Google Scholar]
- Dye D. W.The Inadequacy of the Usual Determinative Tests for the Identification of Xanthomonas Spp. New Zeal. J. Sci. 1962, 5 ( (4), ). [Google Scholar]
- O’Toole G. A.Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, (47), . 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in Bacteria. Science (80-.). 1966, 153 (3737), 708–716. 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Mody B.; Bindra M.; Modi V. Extracellular Polysaccharides of Cowpea Rhizobia: Compositional and Functional Studies. Arch. Microbiol. 1989, 153 (1), 38–42. 10.1007/BF00277538. [DOI] [Google Scholar]
- Wozniak D. J.; Wyckoff T. J. O.; Starkey M.; Keyser R.; Azadi P.; O’Toole G. A.; Parsek M. R. Alginate Is Not a Significant Component of the Extracellular Polysaccharide Matrix of PA14 and PAO1 Pseudomonas Aeruginosa Biofilms. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (13), 7907–7912. 10.1073/pnas.1231792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.; Judes H.; Weiss E. Cell Surface Hydrophobicity of Dental Plaque Microorganisms in Situ. Infect. Immun. 1983, 42 (2), 831–834. 10.1128/iai.42.2.831-834.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24 (1), 1–15. 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. O.; Allen R. L. Dependence of Chloroplast Pigment Synthesis on Protein Synthesis: Effect of Actidione. Biochem. Biophys. Res. Commun. 1965, 21 (6), 523–530. 10.1016/0006-291X(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Faizan M.; Rajput V. D.; Al-Khuraif A. A.; Arshad M.; Minkina T.; Sushkova S.; Yu F. Effect of Foliar Fertigation of Chitosan Nanoparticles on Cadmium Accumulation and Toxicity in Solanum Lycopersicum. Biology (Basel). 2021, 10 (7), 666. 10.3390/biology10070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco W. F.; Queiroz A. M.; Fernandes J.; Botero E. R.; Falcão E. A.; Guimarães F. E. G.; M’Peko J.-C.; Oliveira S. L.; Colbeck I.; Caires A. R. L. Interaction between Chlorophyll and Silver Nanoparticles: A Close Analysis of Chlorophyll Fluorescence Quenching. J. Photochem. Photobiol. A Chem. 2015, 299, 203–209. 10.1016/j.jphotochem.2014.12.001. [DOI] [Google Scholar]
- Falco W. F.; Scherer M. D.; Oliveira S. L.; Wender H.; Colbeck I.; Lawson T.; Caires A. R. L. Phytotoxicity of Silver Nanoparticles on Vicia Faba: Evaluation of Particle Size Effects on Photosynthetic Performance and Leaf Gas Exchange. Sci. Total Environ. 2020, 701, 134816. 10.1016/j.scitotenv.2019.134816. [DOI] [PubMed] [Google Scholar]
- Lin G.; Rose P.; Chatson K. B.; Hawes E. M.; Zhao X. G.; Wang Z. T. Characterization of Two Structural Forms of Otonecine-Type Pyrrolizidine Alkaloids from Ligularia Hodgsonii by NMR Spectroscopy. J. Nat. Prod. 2000, 63 (6), 857–860. 10.1021/np9906119. [DOI] [PubMed] [Google Scholar]
- Swain T.; Hillis W. E. The Phenolic Constituents Of Prunus Domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10 (1), 63–68. 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Kim D.-O.; Jeong S. W.; Lee C. Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81 (3), 321–326. 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Ganjiwale R.; Wadher S.; Yeole P.; Polshettiwar S. Spectrophotometric Estimation of Total Tannins in Some Ayurvedic Eye Drops. Indian J. Pharm. Sci. 2007, 69 (4), 574. 10.4103/0250-474X.36949. [DOI] [Google Scholar]
- Bates L. S.; Waldren R. P.; Teare I. D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39 (1), 205–207. 10.1007/BF00018060. [DOI] [Google Scholar]
- Shahid M.; Khan M. S.; Syed A.; Marraiki N.; Elgorban A. M. Mesorhizobium Ciceri as Biological Tool for Improving Physiological, Biochemical and Antioxidant State of Cicer Aritienum (L.) under Fungicide Stress. Sci. Rep. 2021, 11 (1), 9655. 10.1038/s41598-021-89103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M.; Manoharadas S.; Chakdar H.; Alrefaei A. F.; Albeshr M. F.; Almutairi M. H. Biological Toxicity Assessment of Carbamate Pesticides Using Bacterial and Plant Bioassays: An in-Vitro Approach. Chemosphere 2021, 278, 130372. 10.1016/j.chemosphere.2021.130372. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Khan M. S.; Kumar M. Kitazin-Pea Interaction: Understanding the Fungicide Induced Nodule Alteration, Cytotoxicity, Oxidative Damage and Toxicity Alleviation by Rhizobium Leguminosarum. RSC Adv. 2019, 9 (30), 16929–16947. 10.1039/C9RA01253B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M.; Ahmed B.; Zaidi A.; Khan M. S. Toxicity of Fungicides to Pisum Sativum: A Study of Oxidative Damage, Growth Suppression, Cellular Death and Morpho-Anatomical Changes. RSC Adv. 2018, 8 (67), 38483–38498. 10.1039/C8RA03923B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M.; Khan M. S. Fungicide tolerant Bradyrhizobium japonicum mitigate toxicity and enhance greengram production under hexaconazole stress. J. Environ. Sci. 2019, 78, 92–108. 10.1016/j.jes.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Khan S.; Shahid M.; Khan M. S.; Syed A.; Bahkali A. H.; Elgorban A. M.; Pichtel J. Fungicide-tolerant plant growth-promoting rhizobacteria mitigate physiological disruption of white radish caused by fungicides used in the field cultivation. Int. J. Environ. Res. Public Health 2020, 17 (19), 7251. 10.3390/ijerph17197251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. L.; Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophy 1968, 125 (1), 189–198. 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jurkow R.; Pokluda R.; Sekara A.; Kalisz A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biology 2020, 20 (1), 1–12. 10.1186/s12870-020-02490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico C. M.; Morales M. I.; McCreary R.; Castillo-Michel H.; Barrios A. C.; Hong J.; Tafoya A.; Lee W.-Y.; Varela-Ramirez A.; Peralta-Videa J. R.; Gardea-Torresdey J. L. Cerium Oxide Nanoparticles Modify the Antioxidative Stress Enzyme Activities and Macromolecule Composition in Rice Seedlings. Environ. Sci. Technol. 2013, 47 (24), 14110–14118. 10.1021/es4033887. [DOI] [PubMed] [Google Scholar]
- Beauchamp C.; Fridovich I. A Mechanism for the Production of Ethylene from Methional. J. Biol. Chem. 1970, 245 (18), 4641–4646. 10.1016/S0021-9258(18)62842-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.