Abstract

Gravity override and viscous fingering are inevitable in gas flooding for improving hydrocarbon production from petroleum reservoirs. Foam is used to regulate gas mobility and consequently improve sweep efficiency. In the enhanced oil recovery process, when the foam is introduced into the reservoir and exposed to the initial saline water saturation and pH condition, selection of the stable foam is crucial. Salinity and pH tolerance of generated foams are a unique concern in high salinity and pH variable reservoirs. NaOH and HCl are used for adjusting the pH, and NaCl and CaCl2 are utilized to change salinity. Through analyzing these two factors along with surfactant concentration, we have instituted a screening scenario to optimize the effects of salinity, pH, surfactant type, and concentration to generate the most stable state of the generated foams. An anionic (sodium dodecyl sulfate) and a nonionic (lauric alcohol ethoxylate-7) surfactants were utilized to investigate the effects of the surfactant type. The results were applied in a 40 cm synthetic porous media fully saturated with distilled water to illustrate their effects on water recovery at ambient conditions. This most stable foam along with eight different stabilities and foamabilities and air alone was injected into the sand pack. The results show that in optimum surfactant concentration, the stability of LA-7 was not highly changed with salinity alteration. Also, we probed that serious effects on foam stability are due to divalent salt and CaCl2. Finally, we found the most water recovery that was obtained by the three most stable foams by the formula of 1 cmc SDS + 0.5 M NaCl, 1 cmc SDS + 0.01 M CaCl2, and LA-7@ pH ∼ 6 from porous media flooding. Total water recovery for the most stable foam increased by an amount of 65% compared to the state of air alone. A good correlation between foam stability and foamability at higher foam stabilities was observed.
1. Introduction
Foam is a nonequilibrium dispersion of the gaseous phase within a continuous aqueous phase (generally containing surface-active agents) composed mainly of thin liquid films known as lamellaes. The long-term stability of foam is a result of the stability of these lamellae. This stability is implemented by adsorption of surfactants or nanoparticles at the gas/liquid interfaces.1−3 Foam stability turns out to be important in the petroleum industry such as enhanced oil recovery (EOR) and drilling engineering, and its importance in food, pharmaceutical, and detergent industries is also well known.4
An issue related to many secondary and tertiary gas injection projects is the unfavorable mobility condition, which results in poor sweep efficiency and reduction of oil recovery due to viscous fingering or unfavorable mobility condition.5,6 Foam flooding can significantly improve both macrosweeping volume and microdisplacement efficiency. This improvement is implemented by increasing the effective viscosity and blocking the high permeable swept zones, redirecting the fluid front into various pore sizes and also reducing the capillary forces owing to the presence of surfactants.3,6−8 In higher-permeability layers, the foam has lower mobility and will thus effectively block/hinder flow in these layers in favor of low-permeability layers.9
The greater recovery of oil by foam displacement is primarily due to foam stability.10 Knowing that the stability of foams for various applications is significant. To be an efficient recovery or blocking agent, the foam must remain stable in the porous formation, or to have an appropriate sweep of oil in the reservoir, it is required to stabilize the foam at the reservoir condition.1,11
Surfactant flooding is a chemical EOR technique for lowering the oil–water interfacial tension (IFT) and directing residual oil into producing wells.12 Surfactant molecules that adsorb at the liquid/gas contact stabilize the lamellae of foam.13 The IFT is greatly lowered during emulsification and entrainment. The water-to-oil mobility ratio is reduced as a result of the crude oil droplets being emulsified into the water phase, and surfactant solution is diverted to upswept areas, improving the areal and vertical sweep efficiencies.14
Foam stability is characterized as the height of foam after a certain period.5,15 Due to the complexity of the foam system, its behavior in porous media depends on various factors, in particular, on the composition of the continuous liquid phase (surfactant type, surfactant concentration, extra additive, salinity, pH, oil presence, etc.), micellar stability, foam quality, and internal gas-phase properties.16−19
The presence of oil is a remarkable concern regarding the stability of the foam. In order to be successful in achieving good mobility control, the foam must remain stable when it comes into contact with oil. Some studies have reported that the addition of small traces of oil or hydrophobic particles destabilizes the generated foam. It has been proposed that the stability of foam depends on the composition of the oil process, so that the presence of light components is detrimental to the stability of the foam.8,20
The stability of foam may decrease as the temperature increases. Under different pressures, foam has varying stabilities. The more pressure applied, the more stable the foam becomes.21
Adding surfactants as stabilizers can increase foam stability; however, surfactant-generated foams have a shorter life span due to an unstable interface. Surfactants are also more likely to be retained and chemically degraded in porous media, especially at harsh reservoir conditions. Nanoparticles (NPs) can be an excellent foam stabilizing agent due to their surface chemistry and high adsorption energy. When compared to surfactant-stabilized foams, NP-generated foams are more stable and provide superior mobility control. The colloidal stability of NPs as foam stabilizers is one restriction, limiting the usage of low-cost NPs. Surfactants have the advantage of improving foam stability, increasing sweep efficiency, recovering more residual oil due to saponification, and decreasing IFT. Surfactant addition not only improves foam stability, but it also alters wettability, which speeds up oil flow by reducing capillary forces.22 During foam flooding in porous media, the production of thin liquid coatings known as lamellas blocks the gas phase flow in some areas. Foam stability is provided by the ability of hydrophilic surfactant molecules to adsorb on the gas–water interface, while foam movement is regulated by surfactant-generated lamellae in reservoir micropores. Surfactants can effectively enhance foam viscosity, resulting in more consistent and piston-like oil front displacement.23
Anionic and nonionic surfactants are the commonly used foaming agents in foam generation. Some common anionic surfactants are sodium dodecyl benzene sulfonate (ABS), sodium lauryl sulfate (SDS), sodium dodecyl sulfate (AS), and so forth. Abundance, good foaming performances, and low cost are some of their advantages, but they exhibit poor tolerance against salinity. On the other hand, nonionic surfactants such as lauryl alcohol-7 (LA-7) have poor foaming performances, but they properly tolerate saline conditions.24
Due to the adverse effect of oil existence on foam stability of water-based foams, it is proposed to inject a slug of water before injection of foam and use foam to displace oil indirectly. Thus, we manipulated the foam injection process in a way that direct contact of foam–oil is avoided, that is, foam pushes water in a piston-liked manner and consequently water displaces oil by the same favorable displacement condition.25
The influences of salinity, pH, surfactant type, and concentration on generating the most stable condition of created foams that maximizes total water recovery were studied. The primary purpose of this analysis was to determine the efficacy of the stability of foam on the foam flooding process in displacing fresh water from a fully saturated sand pack column and evaluating the recovery performance of the foam. An attempt was made to find the most stable foam and the foam with the highest foamability by adjusting surfactant concentration, salinity, and pH for two different types of surfactants. Both anionic (SDS) and nonionic (LA-7) surfactants were utilized. The foaming properties and foamability of aqueous foam solutions owing to the addition of NaCl and CaCl2 were persuaded. Optimum foam conditions were selected by screening criteria; first, the optimum surfactant concentration to generate both the most stable foam and the foam with the highest foamability was obtained, and at this surfactant concentration, NaCl and CaCl2 were added to screen the effect of salinity, and then, the most stable foam and foam with the highest stability were selected by adjusting salinity, and this foam was the candidate for pH adjustments. At the end, the effect of pH adjustments on the foam generation was investigated to find the optimum conditions of foam stability. The major goals of this study were to see how salinity, pH, surfactant type, and concentration can affect foam properties. The impacts of foam stability and foamability on the foam flooding process in displacing freshwater from a fully saturated sand pack column were investigated in this study. Then, 10 different scenarios consisting of the most stable foam along with the lower stable foams, the air alone, and also the foams with higher formability were injected into the sand pack to investigate the effects of foam stability and foamability on the recovery factor of fresh water in the foam injection process. The unique characteristics of this work were the simultaneous investigation of foam stability and formability, both of which are intrinsic features of any foam in foam flooding, which makes foam selection competitive, and their importance in the recovery factor is well known.
2. Theoretical Background
Disjoining pressure, Marangoni effect, and bulk and surface viscosities therewith cmc and surface tension influence the stability of aqueous foams.26
Based on the classical form of DLVO theory, the long-term stability of the colloidal system is due to the interplay between the repulsive electrostatic Πel and the attractive van der Waals ΠVW components of the disjoining pressure27
| 1 |
The electrostatic disjoining pressure, Πel, arises to be28
| 2 |
The inverse Debye length 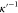 is given by28
is given by28
| 3 |
where R is the universal
gas constant, Cel represents the molar
concentration of electrolytes in the solution, Ψs stands for interfacial potential, ε0 and εr are the permittivities of vacuum and the dielectric constant
of water, respectively, NA represents
Avogadro’s number, e is the electrical charge, k represents the Boltzmann constant, I is
the ionic strength, and T stands for the absolute
temperature. D represents surface separation. The
Debye length is the characteristic length of the diffuse electric
double layer. For 1:1 electrolytes (e.g., NaCl), 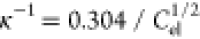 nm, and for 2:1 and
1:2 electrolytes (CaCl2 and Na2SO4),
nm, and for 2:1 and
1:2 electrolytes (CaCl2 and Na2SO4), 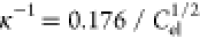 nm, where the unit of Cel is mol/L.
nm, where the unit of Cel is mol/L.
According to the DLVO theory, in the foams with the ionic surfactant, adding the electrolyte suppresses the electrostatic repulsion between the surfaces in the lamella.29,30 By screening the electrostatic forces, only attraction van der Waals remains left. This attraction causes to rupture the film but the addition of monovalent ions such as NaCl improves surface tension of air solution of SDS, which improves the stability of foam films.
Equation 2 indicates that increasing Cel, that is, bivalent ions, increases Debye length, which means that the range of electrostatic force becomes shorter by adding the electrolyte. Thus, the bivalent electrolytes further suppress the electrostatic compared to monovalent electrolytes.
Due to the existence of negative surface charge as a result of adsorption of negative OH– ions onto the surface, electrostatic force presents in some cases in aqueous films, which are stabilized by nonionic surfactants. Also, in nonionic surfactants, the increasing pH increases the surface charge density (q0).31,32 At constant ionic strength, any decline in the pH of the solution causes surface charge density to be disappeared (q0 = 0) at a certain pH value, which is called the isoelectric point.33 This point is the no foam point.29,32
The effect of micelle stability on foam stability and foam height (foamability) is well known. The concentrations of surfactants, type of counterions, and electrolyte influence the micelle stability.34 The presence of either CaCl2 or NaCl eventuates the more stability of micelles, but in the case of CaCl2, the micelles aggregate more compactly and form more stable micelle structures. It has been indicated that very stable micelles cannot create more monomer flux, which results in less foamability. By investigating the SDS surfactant, it has been shown that the most stable micelles (for SDS) occur when the concentration of the solution is 200 mM. At this concentration, foam height becomes the lowest amount.35
The Gibbs–Marangoni theory explains the foam stability and rupture due to the thin film elasticity. Due to the film’s restricted extension compared to its size, Gibbs elasticity refers to the increase in film surface tension caused by a reduction in surfactant concentration inside the interlamellar fluid. The deterioration of the interstitial surfactant solution causes the film elasticity in the Gibbs process, with the assumption that the lamellae thickness is relatively small. The Marangoni elasticity, also known as the Marangoni effect, comes from the movement of surfactant molecules from the surrounding bulk phase to the interface under the nonequilibrium state of thick foam sheets.36 It means that owing to film elasticity, it could deform without rupturing, and this phenomenon stabilizes the foam film. Based on this theory, any enhancement of surfactant concentration enhances the foam stability but this enhancement is accomplished up to an extent that is often close to critical micelle concentration (cmc). In other words, only at an intermediate surfactant concentration, maximum foam is generated, and at any surfactant concentration below and above this intermediate, concentration foam stability is deteriorated.37
3. Experimental Section
3.1. Materials
The two investigated surfactants in this work were acquired commercially from Merck-Germany and used without previous purification. Table 1 illustrates the specifications of these surfactants.
Table 1. Characteristics of Selected Surfactants.
| surfactant | type | abbreviation | molecular weight (g/mol) | cmc (M) |
|---|---|---|---|---|
| lauryl alcohol ethoxylate-7 (CH3(CH2)10CH2OH)O(CH2CH2O)7H | nonionic | LA-7 | 494 | 0.001417 |
| SDS C12H25So4Na | anionic | SDS | 288.38 | 0.00832 |
At 25 °C, the cmc of SDS, which has been reported in different studies,15,17,38−40 is in the range of 7.6–8.3 mM in the absence of the added electrolyte.
The utilized electrolytes were NaCl and CaCl2, which were bought from Merck-Germany (99% purity). The addition of CaCl2 leads to precipitation of the SDS solutions and nonionic surfactants. The desired pH value was adjusted by the addition of small aliquots of %37 M NaOH (sodium hydroxide, Merck) or %10 M HCl (hydrochloric acid, Merck) into the surfactant solution. To distinguish surfactant solution from water, methylene blue was used in order to set the color of the surfactant solution to blue. All solutions were prepared with deionized water having a specific resistivity of 18.2 MΩ·cm (Milli-Q purification system). The working temperature was 25 °C, and all experiments were performed at atmospheric pressure.39,40
3.2. Surface Tension Measurement
The surface tension isotherms of the SDS and LA-7 solutions were determined using the drop weight method both in the presence and absence of NaCl and CaCl2. Each surfactant solution was prepared at least 1 day before the experiment and used within 1 week to reduce hydrolysis. In order to eliminate the effect of measurement error, both tests were carried out at least three times, and the mean values were recorded.25
The cmc was determined from the surface tension versus log concentration curves, corresponding to the inflection point on the curves. Note that the SDS was used without any purification. The observed variance could therefore be due to the existence of impurities and/or the disparity in measurement methods.41
3.3. Foam Stability and Foamability Test
Foam stability is measured as the required time that given foam is demolished by half. In this work, the modified Ross–Miles method was used to generate foam. In the modified Ross–Miles method, a 30 mL aqueous surfactant solution is placed in a titration pipette (Figure 1) and it is allowed to fall from a distance of about 1 cm above the cylindrical vessel onto 25 mL of the same solution, which is included in a cylindrical vessel at ambient temperature, 25 °C. As the titration pipette valve is opened, the foam would generate due to a clash of two fluids. The height of the foam formed in the cylindrical vessel is calculated immediately after all the solution has run out of the titration pipette. The stability of the foam is therefore estimated by calculating the foam’s half-life; the time is taken for the foam height to become half of its original height. All tests were conducted at least four times, and in this analysis, the quoted results are the average of four measurements.
Figure 1.
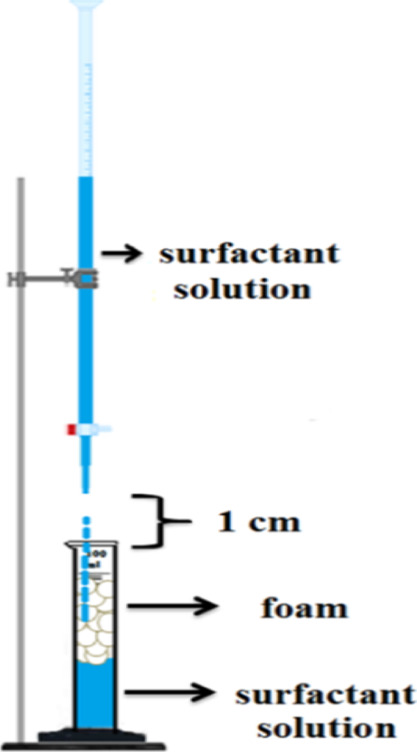
Schematic of the foam generation setup (modified Ross–Miles method).
3.4. Foam Flooding Setup
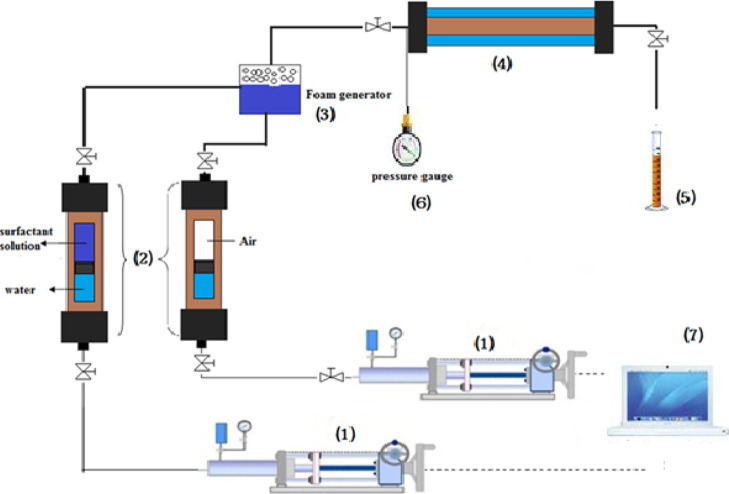
In Figure 2, the experimental setup used to conduct the experiments is shown schematically. It consists of four sections: foam generation section, foam flooding section, pressure controlling section, and data acquisition section.
Figure 2.
Schematic representation of the experimental setup used for foam generation, flooding, and recording. (1) high-precision double-effect piston displacement pump, (2) high-pressure sample cylinder-floating piston cylinder, (3) foam generation section, (4) core holder, (5) graduated cylinder, (6) high-precision pressure gauge, and (7) data acquisition section.
In the foam generation section, located before the inlet of the core holder, gas is mixed with the surfactant solution to generate foam. In order to ensure that gas is supplied at a stable rate, the gas flow rate is controlled by using a high-precision needle valve, and by using a gas flow meter, it is tracked. Two high-precision double-effect piston displacement pumps are used to inject the surfactant solution and air at a constant rate. The foam generation section includes numbers 1, 2, and 3 in Figure 2.
In the flooding section, the sand pack is placed inside a cylindrical core holder. The foam is introduced from the injection tube, and the liquid output is stored in a graduated cylinder. One high-precision pressure gauge is located at the inlet to evaluate the drop in pressure along the pipe and the outlet section is introduced to the atmosphere. In Figure 2, numbers 4, 5, and 6 represent this section.
The data acquisition unit was used to record the flow rate of injection, pressure, and total volume of fluid. Liquid production data were collected manually using a graduated cylinder. All experiments were conducted under isothermal conditions and atmospheric pressure. The experiment temperature was 25 °C and remained constant. In comparison to the gas injection, two pumps are used for the injection of surfactant solution; one for the injection of surfactant solution (which is shut off by gas injection) and the other for the injection of air. The surfactant solution was colored blue with methylene blue, so that it could be identified by the outlet water as the foam falls out of the core outlet.
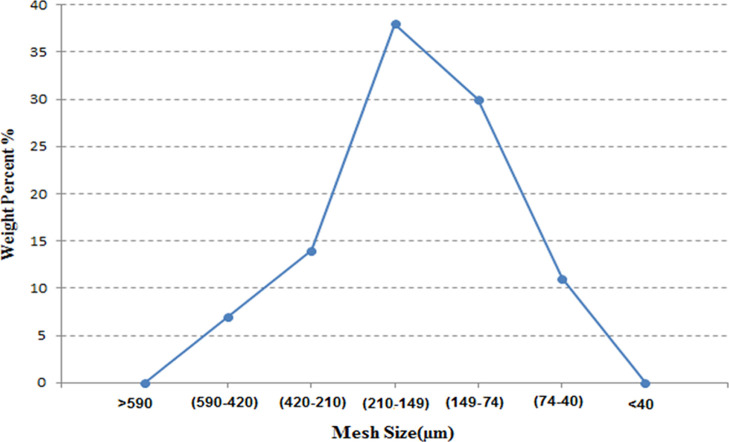
An experimental sand pack model was prepared using screened glass beads and the desired range of sizes. In order to make the model heterogeneous, the entire range of glass beads was used in the sand pack. Figure 3 shows the weight percent of glass bead meshes used in the sand pack. A majority of bead diameters were in the range of 149–210 μm.
Figure 3.
Mesh size characterization of sand pack and distribution.
After filling the sleeve with the specified bead meshes, it was vibrated for 30 min using the pneumatic vibrator to get good packing. Better compaction was achieved using hammer shocks; stainless screens were used to avoid bead migration. The sand pack was first evacuated and then saturated with water using a core holder and a vacuum pump (a confining pressure of 700 psi was applied to approach the reservoir condition). Porosity was calculated using the pumped water, and absolute permeability was measured using some rates of water. Then, this system was connected to an air tank and the air was injected by a very small pressure and water produced from another side of the sand pack. This process was followed till no water was produced and there was only air to be produced. Then, injection was ended up and the initial water saturation of the system was calculated using produced water. As shown in Table 2, the porosity (ϕ) and absolute permeability of the system were measured as 31% and 0.99 darcy, respectively. Also, pore volume (PV) was estimated to be 145 cm3.
Table 2. Values of the Initial Parameters of the Sand Pack.
| (%) ϕ | absolute permeability (D) | PV (pore volume) (cm3) |
|---|---|---|
| 31 | 0.99 | 145 |
The experiments were carried out to compare the generated foams from preceding experiments on ultimate recovery; to do so, the amount of injected foam or changing injection parameters such as pressure, rate, and type of porous media has no significant effect on results, and thus it is accomplished using one constant injection state. The air injection rate was 150 cm3/h and the surfactant rate was 15 cm3/h. Two pumps were used in surfactant injection; one to inject the surfactant (which was off in air injection) and the other one to inject air. The color of the surfactant solution was set as blue, to be recognized when it leaves the core holder. Human error, instability of laboratory ambient temperature, impurity of surfactant, salts and additives, not totally vacuum in porosity measurements, mechanical error in flooding, and foam creation are the primary sources of errors in this study, which have been tried to minimize.
4. Results and Discussion
4.1. Surface Tension
4.1.1. Effect of Surfactant Concentration on Surface Tension
Figure 4 represents the experimental results for the effect of surfactant concentration on surface tension for both nonionic LA-7 surfactant and ionic SDS surfactant solutions. Increasing the surfactant concentration reduces surface tension to an extent and then it moderately stabilizes. The surface tension has no minimum in the vicinity of the cmc. Here, the amounts of cmc for LA-7 and SDS are 0.07 g/100 cm3 and 0.024 g/100 cm3, respectively, which are equal to those reported by the vendor. In Figure 4, it is observed that the nonionic surfactant (LA-7) encounters a more dramatic decline in surface tension than the ionic surfactant (SDS) (Table 3).
Figure 4.
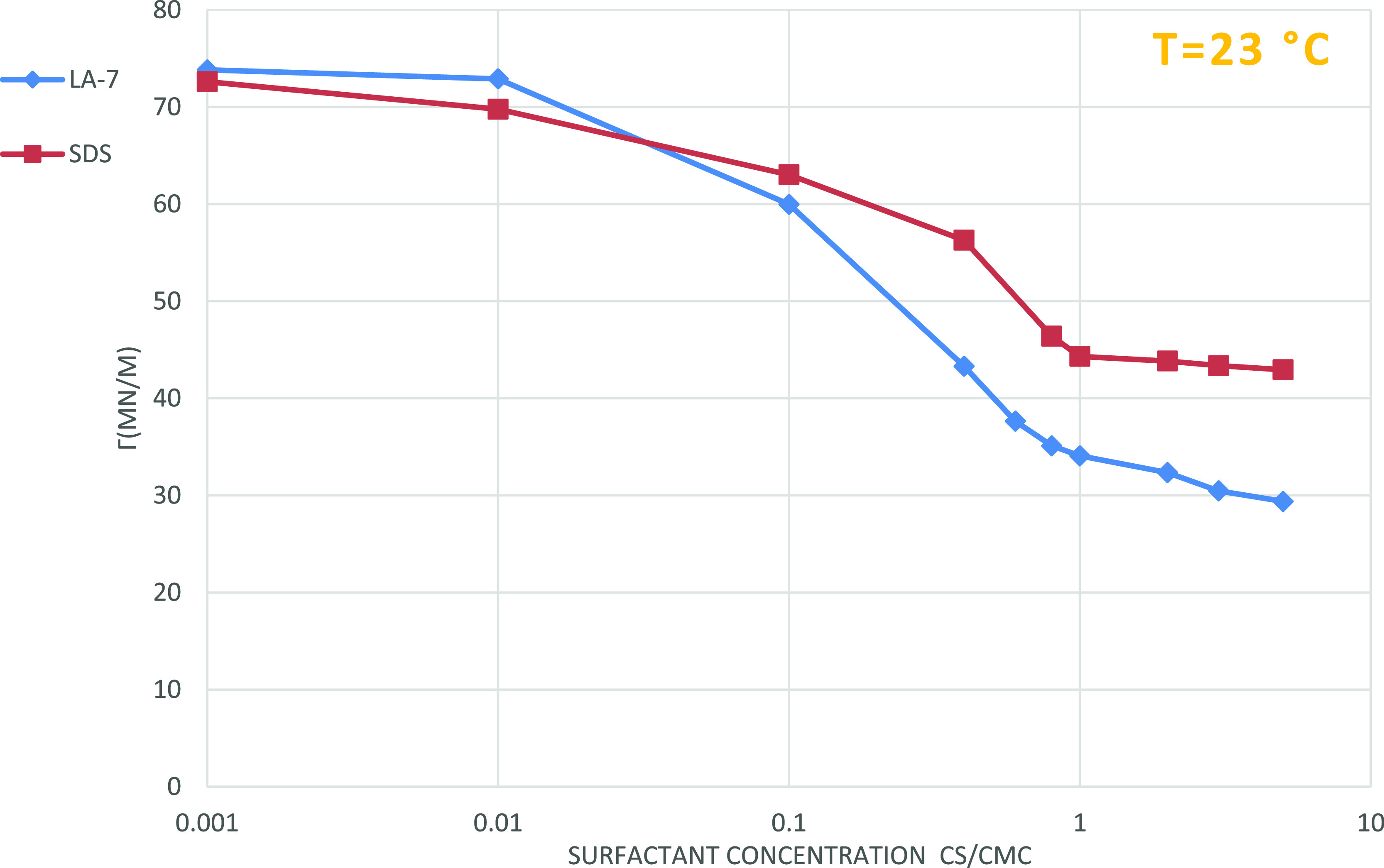
Effect of surfactant concentration on surface tension for LA-7 and SDS solutions; impact of LA-7 is more than SDS to mitigate the surface tension.
Table 3. Surfactant Concentration and Surface Tension Values for LA-7 and SDS Solutions at 23 °C.
| cmc LA-7g/100 cm3 | surface tension (mN/m) | cmc SDSg/100 cm3 | surface tension (mN/m) |
|---|---|---|---|
| 0.001 | 73.83 | 0.001 | 72.60 |
| 0.01 | 72.89 | 0.01 | 69.77 |
| 0.1 | 59.98 | 0.1 | 63.03 |
| 0.4 | 43.30 | 0.4 | 56.28 |
| 0.6 | 37.64 | 0.8 | 46.39 |
| 0.8 | 35.11 | 1 | 44.32 |
| 1 | 34.07 | 2 | 43.83 |
| 2 | 32.35 | 3 | 43.36 |
| 3 | 30.47 | 5 | 42.94 |
| 5 | 29.38 |
4.1.2. Effect of Salinity on Surface Tension
Figure 5 represents the experimental results for the effect of salinity on surface tension for both nonionic LA-7 surfactant and ionic SDS surfactant solutions. Increasing the NaCl concentration decreases the SDS (ionic surfactant) surface tension, while the addition of CaCl2 increases the SDS surface tension. Increasing the NaCl electrolyte to SDS surfactant solution causes reduction of co-ion repulsion of the SDS surfactant heads, and according to the Gouy–Chapman equation, more surfactants absorb on the surface, which leads to a fall in cmc and surface tension but the addition of CaCl2 to the solution of the SDS surfactant leads to SDS deposition, which, in turn, causes a rise in surface tension.
Figure 5.
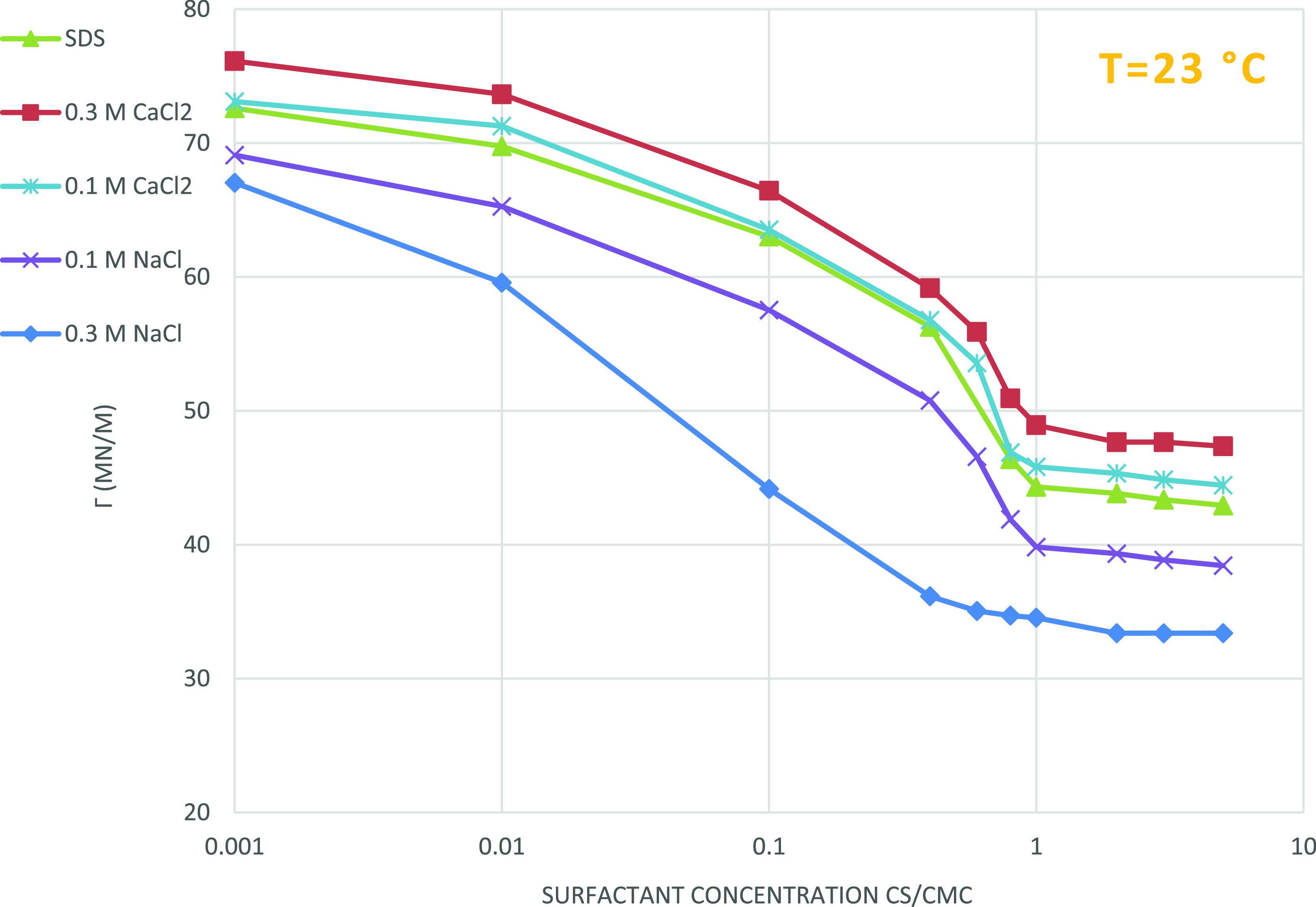
Effect of salinity on surface tension for the SDS surfactant and salinity. Surfactant concentration has a uniform effect on surface tension.
Figure 6 represents the effect of salinity on the surface tension of a nonionic surfactant (LA-7) solution. The addition of two salts (NaCl and CaCl2) at high concentrations (0.3 M) causes growth in surface tension, but at low concentrations (0.1 M), it reduces the surface tension. Surprisingly, at lower surfactant concentrations, addition of salinity is limited to an extent. The increasing ionic strength is a result of the addition of salts, which can effectively reduce hydrogen bonding dominance between intermolecular head groups of the LA-7 monomer on the surface, and enhances the adsorption of LA-7, which reduces the surface tension. However, the increasing salinity reduces the solubility of the surfactant in the solution more than enough, which results in a rise in surface tension.
Figure 6.
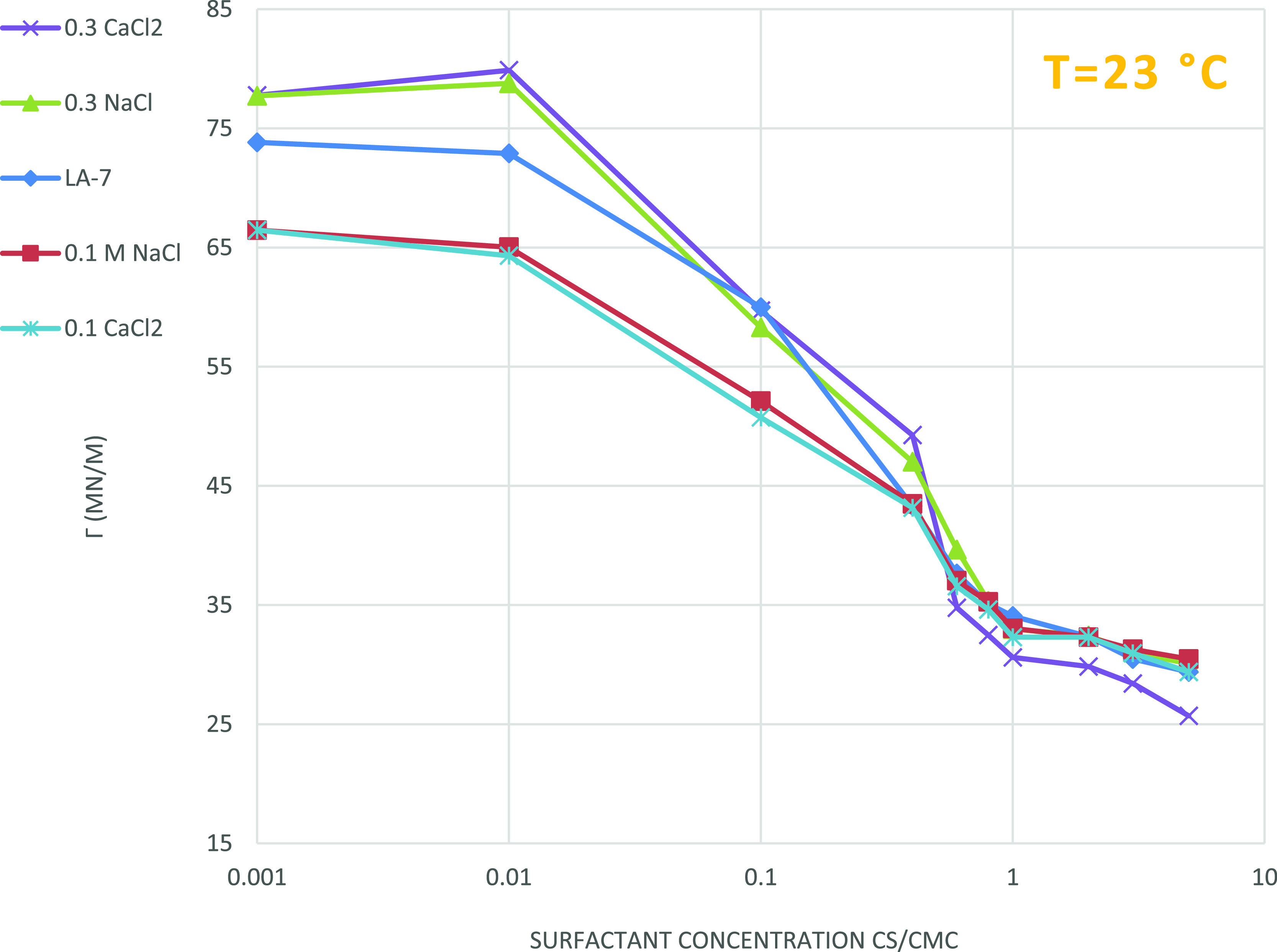
Effect of salinity on surface tension for the LA-7 surfactant; the addition of salts can only be effective up to a limit, and after that limit, salinity has an adverse influence on surface tension.
4.1.3. Effect of pH on Surface Tension
Figures 7 and 8 represent the effect of pH on the surface tension of two surfactants solutions. No tangible changes in surface tension are observed if a very small amount of acid or base is added.
Figure 7.
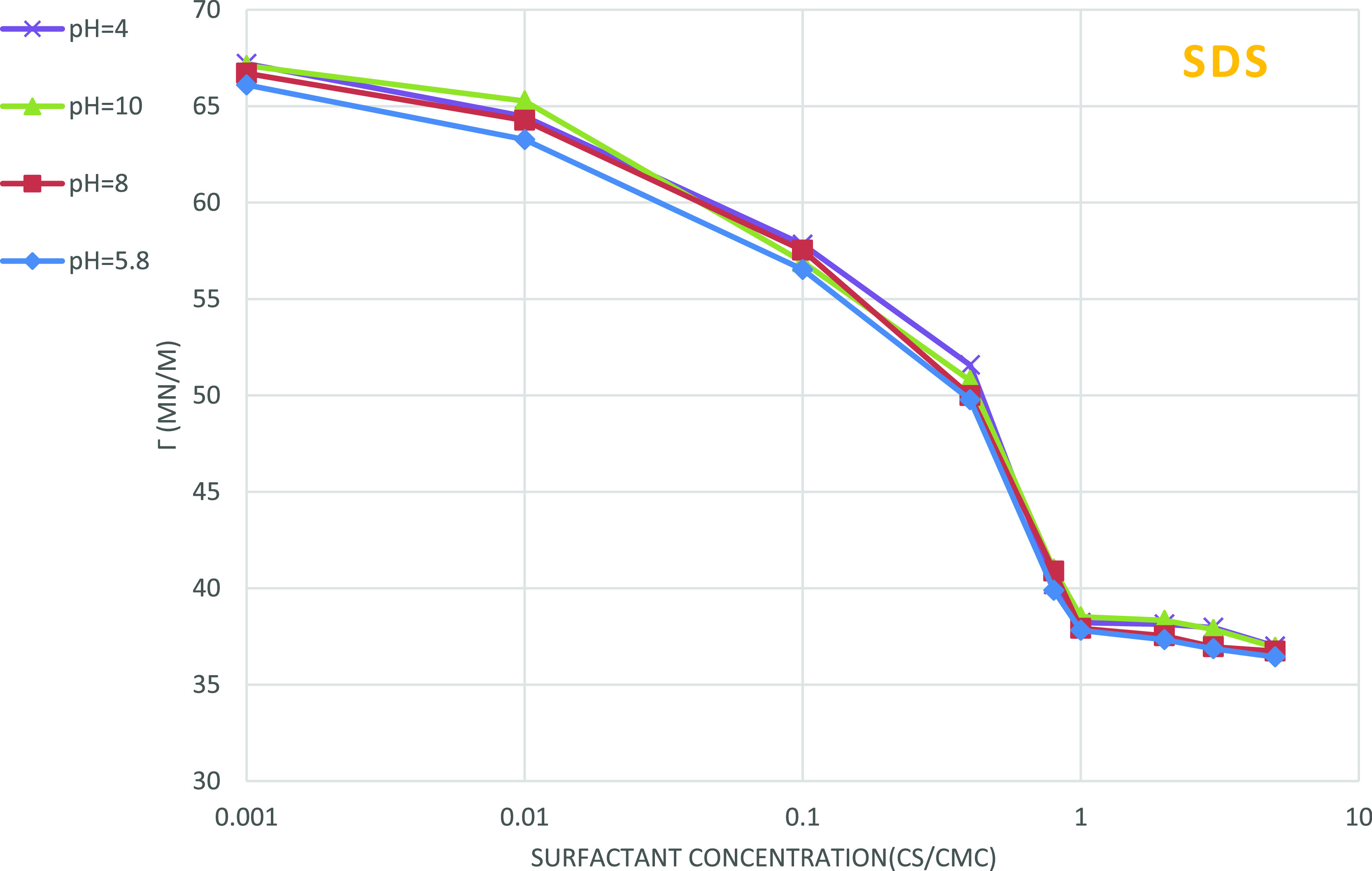
Effect of pH on the surface tension of SDS solution; the impact of pH changes on the surface tension of SDS surfactant is ignorable.
Figure 8.
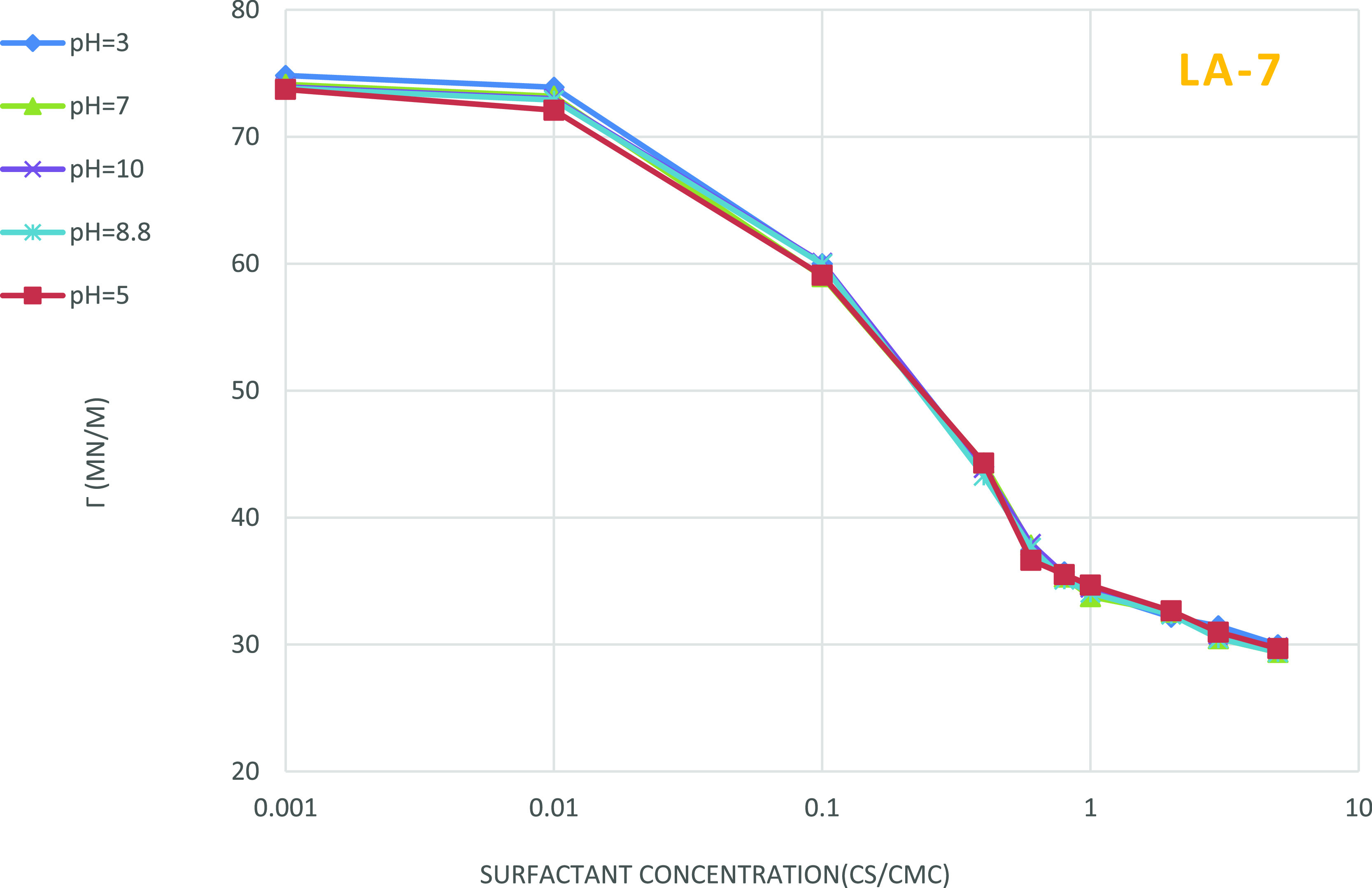
Effect of pH on the surface tension of nonionic LA-7 solution, the impact of pH changes on the surface tension of LA-7 surfactant is ignorable.
4.2. Foam Height and Stability Measurements
4.2.1. Effect of Surfactant Concentration on Foam Height and Foam Stability
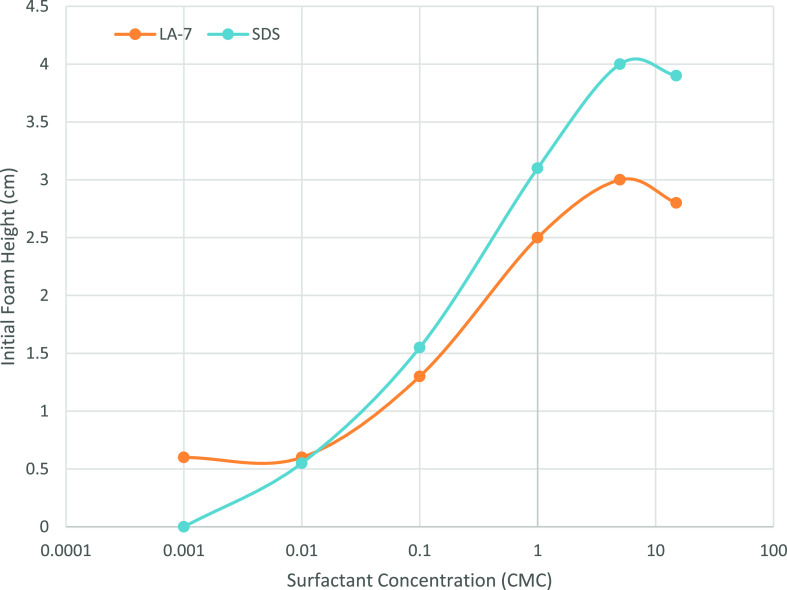
The cmc for a surfactant is an essential parameter because the surfactant will begin to aggregate and form micelles at this concentration. Thus, the cmc is defined as the maximum solubility of monomers in a particular solution. Because the physicochemical properties of the surfactant vary considerably above and below the cmc, the effect of the surfactant on the stability of the foam can differ above and below the cmc. Figure 9 shows the initial foam height prepared by SDS and LA-7 concentrations below and above the cmc.
Figure 9.
Effect of surfactant concentration on the initial foam height for two surfactant types (LA-7 and SDS).
In all experiments, surfactant concentration was reported as a factor of the cmc. At points below the cmc, reduction of surfactant concentration for both LA-7 and SDS surfactants leads to the destruction of foam; it is due to low production of monomers. At points above the cmc, there is an increase in the initial foam height as SDS and LA-7 concentrations change. This indicates a surfactant concentration dependent on foam volume produced. However, the amount of foam volume growth (due to growth of surfactant monomers) is only to an extent and then its trend inverses; it is owing to micelle stability, which was mentioned in preceding paragraphs.17 Many investigations in the course of the effect of surfactant concentration on micelles stability have been carried out, which reveal the fact that increasing surfactant concentration causes the stability of micelles.19 If the micelles in the solution are very stable, they cannot easily supply the surfactant monomers to the newly formed surfaces; hence foaming ability would be poor. However, if the micelles are relatively unstable, their breakdown produces surfactant monomers that can easily adsorb on newly formed surfaces. This could increase micellar solutions’ foamability, but the impact of a micellar lifetime on micellar solutions’ foamability has never been considered or established. At concentrations higher than 5 cmc, due to the stability of micelles, foam generation reduces. Figure 9 shows the initial foam height as a function of surfactant concentration. Experiments show that ionic SDS surfactant results in higher foam height. It is also obvious that at 0.001 cmc for SDS surfactant solution, no foam is generated.
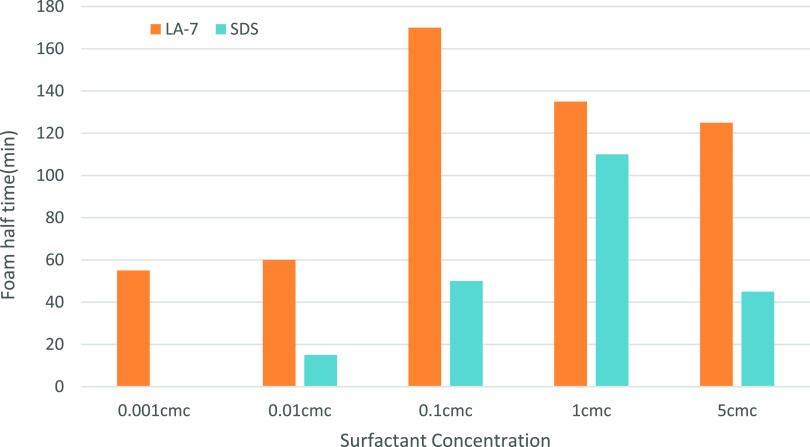
Figure 10 shows the effect of the concentration of the surfactant on the stability of the foam. According to this figure, foam stability for nonionic LA-7 surfactant is much more than SDS. As known from literature32 and as Figure 10 also shows, SDS surfactant solution has the most stable foam at 1 cmc; reducing the SDS concentration below and above the cmc results in the rapid reduction of foam stability; for LA-7 at 0.1 cmc, maximum foam stability is established; and for concentrations other than this point, stability reduces. Marangoni theory explains the higher foam stability at surfactant cmc regions.38
Figure 10.
Effect of surfactant concentration on foam stability (foam half time) for both LA-7 and SDS. For LA-7, the most stable foam is attained at 0.1 cmc, and for SDS, the most stable foam is attained at 1 cmc.
4.2.2. Effect of Salinity on Foam Height and Stability
To investigate the effect of salinity on foam stability, surfactant solutions of 1 cmc for SDS and 0.1 cmc for LA-7, were selected, which have the most foam stability at these concentrations. Table 4 indicates the amount of salt used.
Table 4. Amount of Salt Used in Surfactant Solution Preparation.
| salt |
||
|---|---|---|
| concentration | NaCl (g in 100cm3) | CaCl2 (g in 100cm3) |
| 0.001 M | 0.005844 | 0.0111 |
| 0.01 M | 0.05844 | 0.111 |
| 0.1 M | 0.5844 | 1.11 |
| 0.5 M | 2.922 | 5.55 |
| 1 M | 5.844 | 11.1 |
Figure 11 illustrates the effect of salinity on foam height for SDS at 1 cmc and LA-7 at 0.1 cmc. Increasing salinity reduces foaming ability (foamability). According to Figure 11, ionic SDS surfactant generates more foam compared to a nonionic LA-7 surfactant; also the addition of NaCl to both surfactants solutions has a little effect on foam generation, and in contrast, addition of CaCl2 to SDS solution reduces the foam generation to its least possible level. In Figure 5, it is observed that the addition of NaCl to SDS solution results in a decline in surface tension where more foam is generated. However, increasing salinity more than enough results in stability of micelles and less generation of foam. As CaCl2 is added to surfactant solution, SDS is precipitated and foam generation shrinks. The addition of both NaCl and CaCl2 salts up to 0.1 M into LA-7 solution results in a reduction of surface tension and more foam generation, but after this value, foam generation stops to rise. The trend of all graphs in Figure 11 is in this way: first, the foam generation is upward to a maximum level due to lower surface tension, and then, it shifts downward owing to the rise in surface tension.
Figure 11.

Effect of salinity on foam height for both SDS and LA-7 solutions, increasing salinity has a good effect on foam height improvement, which has an optimum point at about 0.001 M for both SDS and LA-7 surfactants.
The optimum salt concentration for two salts is about 0.001 M. Addition of salt to SDS surfactant solution causes micelle to be more stable compared to the primary state, which increases the foam stability and reduces the foaming ability, because the more stable the micelles, the less generation of the foam.17
Results of salinity in foam stability tests seem interesting. We can say that salinity represents different characteristics in different media, that is, different surfactants. It is observed that the addition of NaCl to SDS solution up to 0.001 M reduces the foam stability and increases the salinity from 0.001 to 0.5 M and also increases the foam stability, and after that, a sudden stability reduction happens. The addition of CaCl2 to SDS solution increases the foam stability, but after this limit, a rapid decline in foam stability is observed. The addition of both NaCl and CaCl2 to LA-7 solution has a similar influence on foam stability, that is, foam system encounters a gradual decline in foam stability, but for the case of CaCl2, this reduction is a little more than NaCl. Figure 12 shows the effect of salt concentration on foam half time (foam stability). The addition of NaCl and CaCl2 to nonionic LA-7 solution significantly reduces the foam stability. The reason is the devastation of electrostatic forces between foam lamellae.15 Salt (salinity) diminishes disjoining pressure, which increases bubble rupture and, as a result, decreases foam stability. CaCl2 has a higher disjoining pressure and less foam stability than NaCl because it is divalent rather than monovalent. To some extent, adding salt to the ionic SDS solution diminishes foam stability, but after that, foam stability begins to improve. CaCl2 infuses the SDS deposition, resulting in increased surface tension and a decrease in foam stability. The foam film will stabilize as a result of the addition of salt to the SDS solution, which reduces hydrophobic forces and increases disjoining pressure.
Figure 12.

Effect of salt concentration on foam half time (foam stability) of both SDS and LA-7 solutions.
0.5 M NaCl and 0.01 M CaCl2 are the optimal salt concentrations for the SDS solution. The presence of NaCl in SDS solution also enhances surfactant adsorption in the air–liquid interface, resulting in increased foam stability. Thus, adding salt to SDS solution reduces electrostatic forces between surfactant–surfactant and enhances monomer aggregation at the air–liquid interface, but adding salt in excess (in this case more than 0.5 M NaCl and 0.01 M CaCl2) screens the repulsion forces between two finite substances.39,42 For LA-7 solution, optimum salinity occurs at the state of no salt where maximum foam stability is observed.
4.2.3. Effect of pH Changes on Foam Stability and Foam Height
The pH of the solution changes when surfactant is added to deionized water. At 1 cmc, the primary pH of SDS solution is 5.8, whereas at 0.1 cmc, the primary pH of LA-7 solution is 8.8. The pH modifications are commonly 1 cmc SDS + 0.5 M NaCl, 1 cmc SDS + 0.01 M CaCl2, and 0.1 cmc LA-7 + no salt at salt concentrations where foam has the best stability. Figure 13 illustrates the effect of pH changes on foam stability (at optimum surfactant concentration and salinity). Acidifying the LA-7 solution reduces the foam stability down to isoelectric pH (about 2) where there is no foam generated. The dashed zone in Figure 13 represents the pH* (isoelectric pH).
Figure 13.
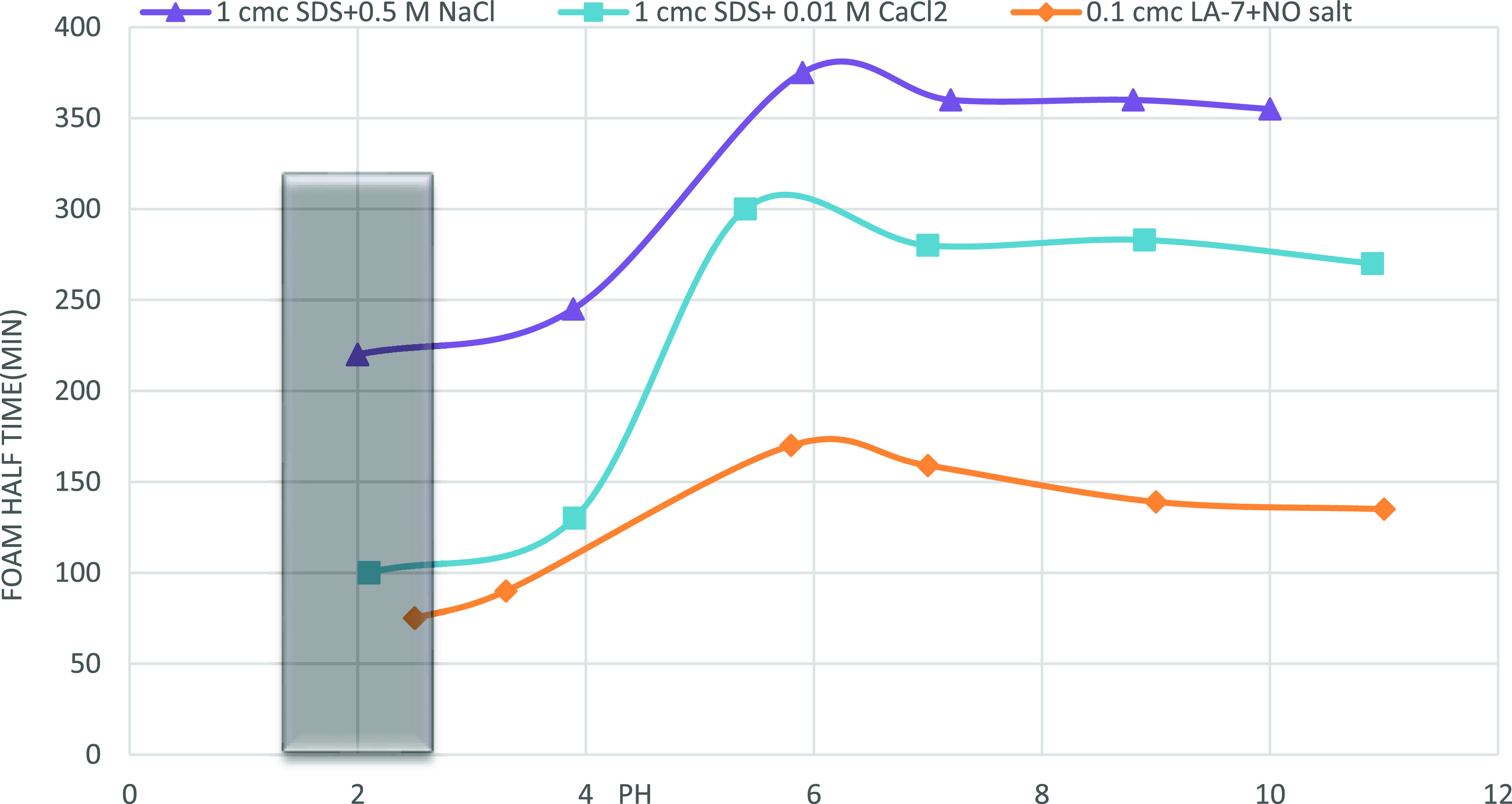
Effect of pH on foam half-time; optimum pH for both surfactants solutions is near 6 where the highest stability in the foam is observed.
Acidifying the solution (pH less than 6) increases the amount of H+ in the solution, which causes more H+ to be adsorbed onto an interface, lowering the monomer concentration and reducing foam stability. When the pH is reduced, the bulk concentration of H+ ions increases, which increases H+ adsorption at the solution/air contact. More H+ ions in the bulk and their adsorption at the interface result in recombination with OH– ions, and the negative charge is eventually destroyed. Electrostatic forces are created by increasing OH– ions at the air–water interface in nonionic surfactant solutions. For nonionic surfactants, the only source of charge is adsorption of OH– ions, whereas for the ionic surfactants, it is the surfactant itself that holds the charge.11,18,33 The belief that electrostatic repulsion is related to the particular adsorption of hydroxide ions at the water–air interface in nonionic foam films is supported by pH-dependent measurements.
Basifying the solution (pH’s more than 8) has no significant effect on the foam stability of both surfactants. According to Figure 14, pH ≈ 6 is the best pH for surfactant solutions where foams have the highest stability. Figure 14 depicts the impact of pH on foam height (foamability). It has been found out that pH has no effect on foam height. However, it is thought that decreasing the pH (acidifying the foam) of SDS + 0.01 M CaCl2 solution lowers the foam height and increasing the pH raises it. The initial foam height of LA-7 solution is unaffected by pH changes.
Figure 14.
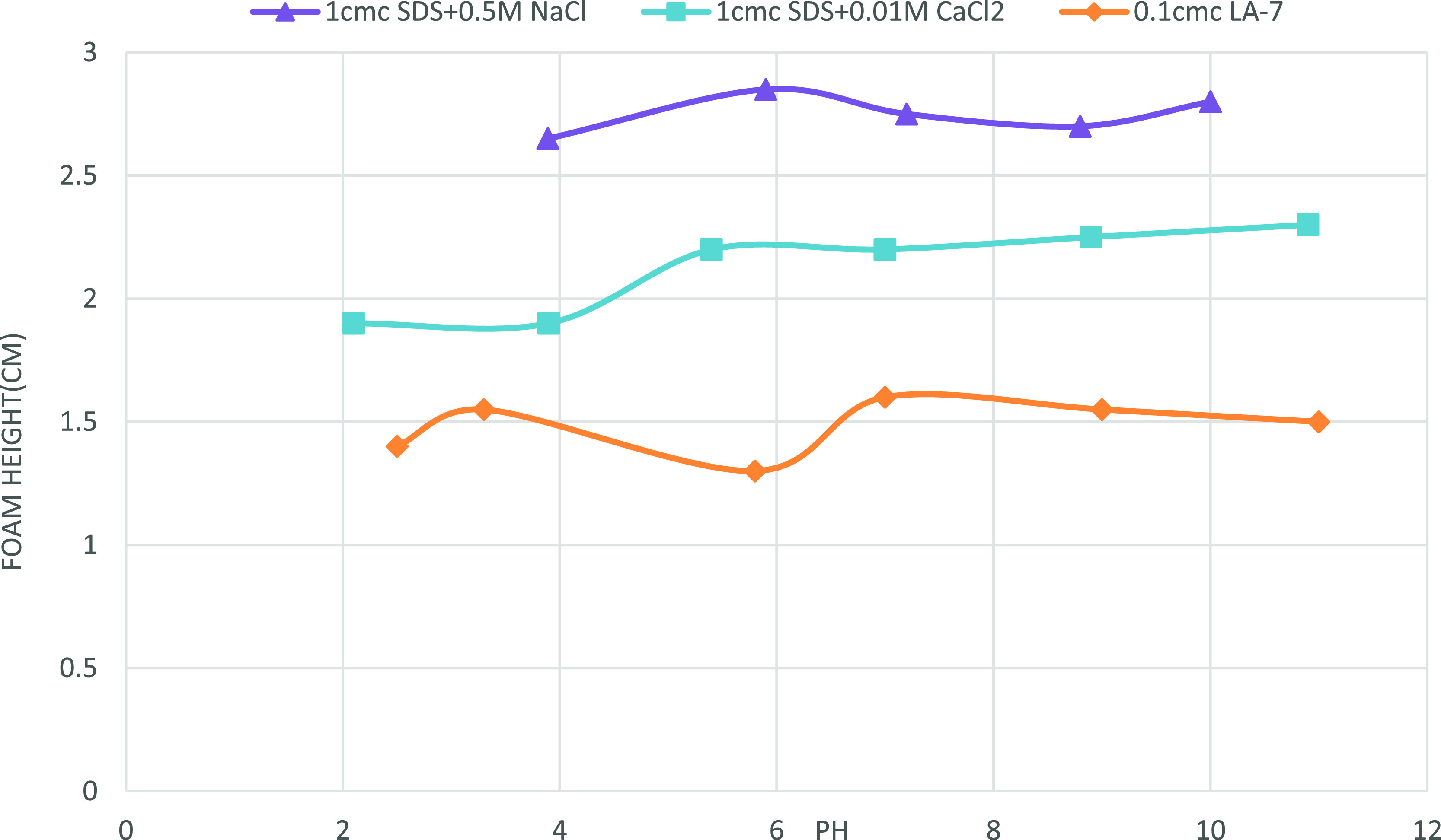
Effect of pH on foam height; no significant impact on foam height is observed owing to pH alteration.
4.2.4. Recovery Performance of Water by Foam Injection
In the final step, the ability of the foam to remove water from a fully saturated sand pack was investigated. The selected foams which are going to be injected into the sand pack are tabulated in Table 5 to evaluate the performance of optimum foam in water recovery; some other foams and the state of no foam (air alone) were also selected. Selected foams are in the following categories:
Three different foams with high foam stability and good foam height (foam number 1, 2, and 3)
Three different foams with medium stability (foam number 4, 5, and 6)
Three different foams with low foam stability and low foam height (foam number 7, 8, and 9)
Table 5. Characteristics of Nine Different Selected Foams along with the State of No Foam (Air Alone).
| injected fluid | material used | surfactant concentration | salt concentration | pH | foam half-time stability (min) | foam initial height (cm) |
|---|---|---|---|---|---|---|
| air | air | |||||
| foam 1 | air + SDS | 1 cmc | 0.5 MNaCl | 6 | 375 | 2.8 |
| foam 2 | air + SDS | 1 cmc | 0.01 M CaCl2 | 6 | 310 | 2.25 |
| foam 3 | air + LA-7 | 0.1 cmc | 6 | 170 | 1.5 | |
| foam 4 | air + SDS | 5 cmc | 0.001 M CaCl2 | 8.5 | 150 | 4.5 |
| foam 5 | air + LA-7 | 5 cmc | 0.001 M NaCl | 7.5 | 125 | 4 |
| foam 6 | air + SDS | 5 cmc | 0.001 M NaCl | 6 | 100 | 3.5 |
| foam 7 | air + LA-7 | 0.01 cmc | 1 M CaCl2 | 4 | 25 | 0.5 |
| foam 8 | air + SDS | 0.01 cmc | 0.001 M CaCl2 | 2 | 25 | 0.4 |
| foam 9 | air + SDS | 0.01 cmc | 0.001 M CaCl2 | 2 | 20 | 0.4 |
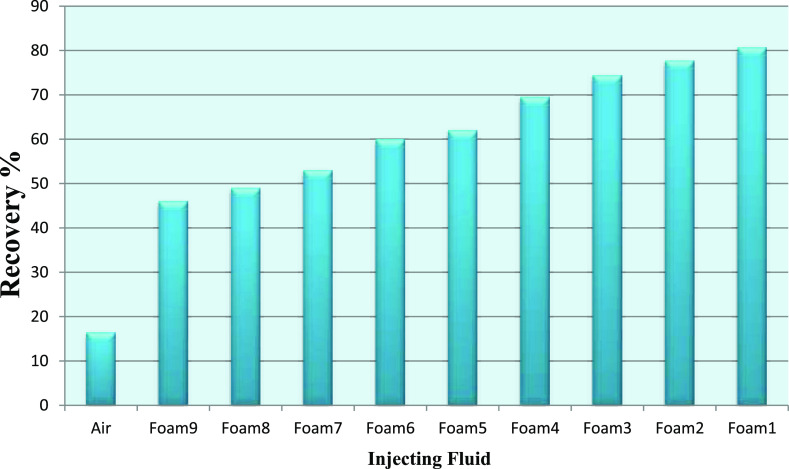
Figure 15 illustrates the water recovery versus pore volume injected. In these experiments, the gas flow rate is equal to 150 cm3/h and the surfactant injection flow rate is equal to 15 cm3/h. For the case of air injection, the breakthrough time is about 35 min. Due to gravity override and fingering effect water recovery is the lowest. In the case of foam injection (optimum foam) due to reduction of gravity override and fingering effects, the breakthrough time is about 235 min. Good results are observed in optimum foam (foam with the highest stability) compared to other foams. Foam 1 which has the highest half time is the most stable foam. Reducing foam stability reduces the water recovery severely. Foam 4 has the highest foam height but lower foam stability compared to foam numbers 1, 2, and 3; this foam has a lower water recovery compared to these foams (1, 2, and 3), but its recovery is more than foam numbers 5 and 6, which is a result of its higher foam height than foam numbers 5 and 6. These results show that foam stability has more impact on water recovery than foam height.
Figure 15.

Recovery performance of water as a result of foam injection; the foam with the highest stability (foam 1) has the highest ability to remove water from the sand pack.
Of the 10 injection scenarios studied, foam 1 was the most successful solution and was considered for the removal of water from the sand pack. The foam front finally arrived at the outlet after 4–5 pore volume of foam was injected. After about 4 pore volume foam injection, there was no significant increase in water recovery. In the case of air alone, it was observed that after less than one pore volume, air breaks through the sand pack outlet. The effective permeability of the porous medium at each point is significantly reduced when the foam is present, relative to the permeability measured in the absence of foam. The foam may therefore be expected to decrease the channeling flow effect in a reservoir by decreasing the permeability of the aqueous displacement phase. This increases the mobility ratio and hence the flood’s homogeneity.
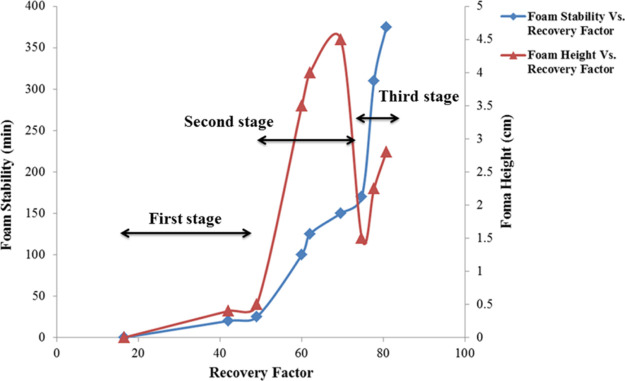
4.2.5. Foam Stability Versus Foam Height
In this part, we decided to assess the effects of both foam height and stability on the foam flooding process. As mentioned in the former section, foam stability is more effective than foam height in removing water from the porous sand pack. However, the effect of foam height on foam flooding is also inevitable. Figure 16 shows the effect of both foam height and foam stability on water recovery performance. The whole range of recovery factor was divided into three different stages; in the “first stage,” a good collaboration between foam stability and foam height is observed, that is, they are both increasing (foam height 0–0.5 cm and foam stability 0–25 min); hence the slope of recovery performance is sharp and ongoing. In the second stage, a similar trend is observed, that is, foam height 0.5–4.5 cm and foam stability 25–170 min and a wide range of recovery rises. At the start of the third stage, foam height encounters a rapid decline (4.5–1.5), but it continues to rise in the lower level and lower foam height (1.5–2.8). Though the foam stability increases drastically (170–375), this lower level of foam height causes recovery performance to have a modest increase. In Figure 17, it is concluded that in order to have good recovery performance, the collaboration between foam stability and foam height is required, though foam stability plays a more significant role than foam height.
Figure 16.
Graph that shows the collaboration of foam stability and foam height in recovery performance of water from porous media.
Figure 17.
Impact of injection of various foams on the recovery of water.
5. Conclusions and Recommendation
During CO2 flooding and steam flooding, conformance improvement oilfield foams are recommended as a mobility control agent. They are also used as blocking/plugging agents around producing wells in conjunction with gas flooding. Due to the great mobility of gas, recovery factors attained during gas injection are lower than expected, as gas tends to overwhelm the water and oil in place. Additionally, viscous fingering and gas channeling through high-permeability streaks enhance the porous medium’s poor volumetric sweep efficiency. Foam is utilized to control gas mobility, which improves sweep efficiency. In this work, the effects of both foam stability and foamability on the foam flooding process in displacing freshwater from a fully saturated sand pack column were investigated. Experiments comparing foam efficiency in water recovery have shown that foam stability plays a more important role than foam volume. Foam stability has a direct relationship with the recovery of water; in other words, increasing the stability of foam increases the amount of water recovery. Most stable foam and the foam with the highest foamability were selected by adjusting surfactant concentration, salinity, and pH for two different types of anionic (SDS) and nonionic (LA-7) surfactants. The type of surfactant plays an important role in foam stability. In this study, the foam formed by the nonionic surfactant LA-7 was expected to have good salt stability, but it was observed that the stability of the foam decreases by increasing a small amount of salt. From this foam behavior obtained by the nonionic surfactant LA-7, it is concluded that the air–water interface of the nonionic surfactant solution is not free of charge and, due to its ion charge, has electrostatic force on the surface and that electrostatic force with increasing salt disappears or decreases. The foam formed by the SDS ionic surfactant has good stability against the NaCl and CaCl2 salts. Optimal salinity and pH were achieved for SDS surfactant foam. The highest stability of foam at this optimum salinity and pH was obtained that results in the most water recovery. Despite the highest impact of foam stability on foam flooding, it was observed that foam flooding performance is more effective in scenarios where good collaboration between foam stability and foamability is observed.
Combining nanomaterials with surfactants in a laboratory experiment for flooding could be helpful for future studies. Another topic of investigation might be the recovery factor in the presence of various types of oils with different APIs. The roles of high temperatures and pressures in foam stability have not been adequately described in the literature. Another aspect that can be investigated is the impact of rock materials on the foam’s stability.
Glossary
Nomenclature
- Å
angstrom (metres: 10–10 m)
- C
interaction constant
- Cel
electrolyte molar concentration (mol/L)
- D
surface separation
- E
surface elasticity
- I
ionic strength
- e
electron charge
- H
surface-to-surface separation distance (ft)
- He
equilibrium thickness (ft)
- k′
permeability (darcy)
- k
Boltzmann constant
- NA
Avogadro’s number, 6.02 × 1023 mol–1
- ε0
dialectic permittivity in vacuum, 8.854 × 10–12 C2 J–1 m–1 (SI)
- P
pressure (psi)
- PV
volume of pore space (cm3)
- R
gas constant 10.73 (psi ft3/lb-mol R)
- εr
relative dialectic permittivity of water, 78.5 at 25 °C
- T
temperature absolute
- σ,γ
surface tension (dynes/cm)
- ϕ
porosity
- ψ
electric potential of surface
- Π
disjoining pressure (psi)
- Πel
electrostatic disjoining pressure (psi)
- Πvw
van der Waals disjoining pressure (psi)
The authors declare no competing financial interest.
References
- Farajzadeh R.; Andrianov A.; Krastev R.; Hirasaki G. J.; Rossen W. R. Foam–oil interaction in porous media: implications for foam assisted enhanced oil recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13. 10.1016/j.cis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- a Miquelim J. N.; Lannes S. C. S.; Mezzenga R. pH Influence on the stability of foams with protein-polysaccharide complexes at their interfaces. Food Hydrocolloids 2010, 24, 398–405. 10.1016/j.foodhyd.2009.11.006. [DOI] [Google Scholar]; b Jha B. K.; Patist A.; Shah D. O. Effect of antifoaming agents on the micellar stability and foamability of sodium dodecyl sulfate solutions. Langmuir 1999, 15, 3042–3044. 10.1021/la981523b. [DOI] [Google Scholar]; c Müller H. J.; Rheinländer T. Anomalous thickness variation of nonionic surfactant foam films with salt concentration. Langmuir 1996, 12, 2334–2339. 10.1021/la950314d. [DOI] [Google Scholar]
- Angarska J. K.; Dimitrova B. S.; Danov K. D.; Kralchevsky P. A.; Ananthapadmanabhan K. P.; Lips A. Detection of the hydrophobic surface force in foam films by measurements of the critical thickness of the film rupture. Langmuir 2004, 20, 1799–1806. 10.1021/la0357514. [DOI] [Google Scholar]
- Sammalkorpi M.; Karttunen M.; Haataja M. Ionic surfactant aggregates in saline solutions: sodium dodecyl sulfate (SDS) in the presence of excess sodium chloride (NaCl) or calcium chloride (CaCl2). J. Phys. Chem. B 2009, 113, 5863–5870. 10.1021/jp901228v. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhang T.; Ge J.; Wang Y.; Ding L.; Zhang G. The CO2-in-water foam stabilized with the mixture of CO2-soluble surfactant and nonionic surfactant. J. Pet. Sci. Eng. 2020, 198, 108117. 10.1016/j.petrol.2020.108117. [DOI] [Google Scholar]
- a Du D.-X.; Beni A. N.; Farajzadeh R.; Zitha P. L. J. Effect of water solubility on carbon dioxide foam flow in porous media: an X-ray computed tomography study. Ind. Eng. Chem. Res. 2008, 47, 6298–6306. 10.1021/ie701688j. [DOI] [Google Scholar]; b Stubenrauch C.; Von Klitzing R. Disjoining pressure in thin liquid foam and emulsion films—new concepts and perspectives. J. Phys.: Condens. Matter 2003, 15, R1197. 10.1088/0953-8984/15/27/201. [DOI] [Google Scholar]
- a Krejca M. M.; Wüstner C.; Goedel W. A. Pickering Membranes Stabilized by Saturn Particles. Langmuir 2017, 33, 10772–10781. 10.1021/acs.langmuir.7b01852. [DOI] [PubMed] [Google Scholar]; b Aarra M. G.; Skauge A.; Solbakken J.; Ormehaug P. A. Properties of N2-and CO2-foams as a function of pressure. J. Pet. Sci. Eng. 2014, 116, 72–80. 10.1016/j.petrol.2014.02.017. [DOI] [Google Scholar]; c Guerrini M. M.; Lochhead R. Y.; Daly W. H. Interactions of aminoalkylcarbamoyl cellulose derivatives and sodium dodecyl sulfate. 2. Foam stabilization. Colloids Surf., A 1999, 147, 67–78. 10.1016/s0927-7757(98)00751-1. [DOI] [Google Scholar]; d Tambe D. E.; Sharma M. M. Hydrodynamics of thin liquid films bounded by viscoelastic interfaces. J. Colloid Interface Sci. 1991, 147, 137–151. 10.1016/0021-9797(91)90142-u. [DOI] [Google Scholar]
- Exerowa D.; Zacharieva M.; Cohen R.; Platikanov D. Dependence of the equilibrium thickness and double layer potential of foam films on the surfactant concentration. Colloid Polym. Sci. 1979, 257, 1089–1098. 10.1007/bf01761121. [DOI] [Google Scholar]
- a Qiao X.; Miller R.; Schneck E.; Sun K. Influence of salt addition on the surface and foaming properties of silk fibroin. Colloids Surf., A 2021, 609, 125621. 10.1016/j.colsurfa.2020.125621. [DOI] [Google Scholar]; b Li Y.; Zhang P.; Zhao G.-Q.; Cao X.-L.; Wang Q.-W.; Wang H.-Y. Effect of equilibrium and dynamic surface activity of surfactant on foam transport in porous medium. Colloids Surf., A 2006, 272124–272129. 10.1016/j.colsurfa.2005.07.037. [DOI] [Google Scholar]; c Velev O. D.; Gurkov T. D.; Chakarova S. K.; Dimitrova B. I.; Ivanov I. B.; Borwankar R. P. Experimental investigations on model emulsion systems stabilized with non-ionic surfactant blends. Colloids Surf., A 1994, 83, 43–55. 10.1016/0927-7757(93)02639-v. [DOI] [Google Scholar]
- a Pu W.; Jiang R.; Pang S.; Qiu T.; Sun Z.; Shen C.; Wei P. Experimental investigation of surfactant-stabilized foam stability in the presence of light oil. J. Dispersion Sci. Technol. 2020, 41, 1596–1606. 10.1080/01932691.2019.1632208. [DOI] [Google Scholar]; b Al-Attar H. H. Evaluation of oil foam as a displacing phase to improve oil recovery: a laboratory study. J. Pet. Sci. Eng. 2011, 79, 101–112. 10.1016/j.petrol.2011.08.013. [DOI] [Google Scholar]; c Karakashev S. I.; Manev E. D. Frothing behavior of nonionic surfactant solutions in the presence of organic and inorganic electrolytes. J. Colloid Interface Sci. 2001, 235, 194–196. 10.1006/jcis.2000.7288. [DOI] [PubMed] [Google Scholar]
- Rattanaudom P.; Shiau B.-J.; Suriyapraphadilok U.; Charoensaeng A. Effect of pH on silica nanoparticle-stabilized foam for enhanced oil recovery using carboxylate-based extended surfactants. J. Pet. Sci. Eng. 2021, 196, 107729. 10.1016/j.petrol.2020.107729. [DOI] [Google Scholar]
- Belhaj A. F.; Elraies K. A.; Mahmood S. M.; Zulkifli N. N.; Akbari S.; Hussien O. S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. 10.1007/s13202-019-0685-y. [DOI] [Google Scholar]
- a Micheau C.; Rosenberg E.; Barré L.; Pannacci N. Microfluidic comparative study of foam flow between a classical and a pH sensitive surfactant. Colloids Surf., A 2016, 501, 122–131. 10.1016/j.colsurfa.2016.04.061. [DOI] [Google Scholar]; b Micheau C.; Bauduin P.; Diat O.; Faure S. Specific salt and pH effects on foam film of a pH sensitive surfactant. Langmuir 2013, 29, 8472–8481. 10.1021/la400879t. [DOI] [PubMed] [Google Scholar]
- a Massarweh O.; Abushaikha A. S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. 10.1016/j.egyr.2020.11.009. [DOI] [Google Scholar]; b Goodarzi F.; Zendehboudi S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309. 10.1002/cjce.23336. [DOI] [Google Scholar]; c Nie C.; Han G.; Ni J.; Guan S.; Du H.; Zhang Y.; Wang H. Stability Dynamic Characteristic of Oil-in-Water Emulsion from Alkali–Surfactant–Polymer Flooding. ACS Omega 2021, 6, 19058–19066. 10.1021/acsomega.1c02367. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zendehboudi S.; Ahmadi M. A.; Rajabzadeh A. R.; Mahinpey N.; Chatzis I. Experimental study on adsorption of a new surfactant onto carbonate reservoir samples—application to EOR. Can. J. Chem. Eng. 2013, 91, 1439–1449. 10.1002/cjce.21806. [DOI] [Google Scholar]
- Schelero N.; Hedicke G.; Linse P.; Klitzing R. v. Effects of counterions and co-ions on foam films stabilized by anionic dodecyl sulfate. J. Phys. Chem. B 2010, 114, 15523–15529. 10.1021/jp1070488. [DOI] [PubMed] [Google Scholar]
- a Singh R.; Panthi K.; Weerasooriya U.; Mohanty K. K. Multistimuli-responsive foams using an anionic surfactant. Langmuir 2018, 34, 11010–11020. 10.1021/acs.langmuir.8b01796. [DOI] [PubMed] [Google Scholar]; b Xu Q.; Nakajima M.; Ichikawa S.; Nakamura N.; Roy P.; Okadome H.; Shiina T. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J. Colloid Interface Sci. 2009, 332, 208–214. 10.1016/j.jcis.2008.12.044. [DOI] [PubMed] [Google Scholar]; c Pugh R. J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. 10.1016/0001-8686(95)00280-4. [DOI] [Google Scholar]; d Aveyard R.; Clint J. H. Foam and thin film breakdown processes. Curr. Opin. Colloid Interface Sci. 1996, 1, 764–770. 10.1016/s1359-0294(96)80079-3. [DOI] [Google Scholar]
- Wang L.; Yoon R.-H. Hydrophobic forces in the foam films stabilized by sodium dodecyl sulfate: effect of electrolyte. Langmuir 2004, 20, 11457–11464. 10.1021/la048672g. [DOI] [PubMed] [Google Scholar]
- Pandey S.; Bagwe R. P.; Shah D. O. Effect of counterions on surface and foaming properties of dodecyl sulfate. J. Colloid Interface Sci. 2003, 267, 160–166. 10.1016/j.jcis.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Derjaguin B. V.Theory of Stability of Colloids and Thin Films; Consultants Bureau New York, 1989; Vol. 1, pp 258–267. [Google Scholar]
- a AlYousef Z. A.; Almobarky M. A.; Schechter D. S. The effect of nanoparticle aggregation on surfactant foam stability. J. Colloid Interface Sci. 2018, 511, 365–373. 10.1016/j.jcis.2017.09.051. [DOI] [PubMed] [Google Scholar]; b Simjoo M.; Rezaei T.; Andrianov A.; Zitha P. L. J. Foam stability in the presence of oil: effect of surfactant concentration and oil type. Colloids Surf., A 2013, 438, 148–158. 10.1016/j.colsurfa.2013.05.062. [DOI] [Google Scholar]
- Da C.; Chen X.; Zhu J.; Alzobaidi S.; Garg G.; Johnston K. P. Elastic gas/water interface for highly stable foams with modified anionic silica nanoparticles and a like-charged surfactant. J. Colloid Interface Sci. 2022, 608, 1401–1413. 10.1016/j.jcis.2021.10.058. [DOI] [PubMed] [Google Scholar]
- Rezaei F.; Rezaei A.; Jafari S.; Hemmati-Sarapardeh A.; Mohammadi A. H.; Zendehboudi S. On the evaluation of interfacial tension (IFT) of CO2–paraffin system for enhanced oil recovery process: comparison of empirical correlations, soft computing approaches, and parachor model. Energies 2021, 14, 3045–3059. 10.3390/en14113045. [DOI] [Google Scholar]
- Issakhov M.; Shakeel M.; Pourafshary P.; Aidarova S.; Sharipova A. Hybrid surfactant-nanoparticles assisted CO2 foam flooding for improved foam stability: A review of principles and applications. Pet. Res. 2021, In press 10.1016/j.ptlrs.2021.10.004. [DOI] [Google Scholar]
- Zhao L.; Li A.; Chen K.; Tang J.; Fu S. Development and evaluation of foaming agents for high salinity tolerance. J. Pet. Sci. Eng. 2012, 81, 18–23. 10.1016/j.petrol.2011.11.006. [DOI] [Google Scholar]
- Abd Rahim N. S.; Saaid I. M.; Umar A. A. Evaluation of foam performance at different temperature for enhanced oil recovery process. World J. Eng. 2019, 16, 412–418. 10.1108/wje-06-2018-0210. [DOI] [Google Scholar]
- a Kanokkarn P.; Shiina T.; Santikunaporn M.; Chavadej S. Equilibrium and dynamic surface tension in relation to diffusivity and foaming properties: Effects of surfactant type and structure. Colloids Surf., A 2017, 524, 135–142. 10.1016/j.colsurfa.2017.04.043. [DOI] [Google Scholar]; b Israelachvili J.Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, 1992; Vol. 1, pp 15–22. [Google Scholar]
- a Yekeen N.; Manan M. A.; Idris A. K.; Samin A. M. Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. J. Pet. Sci. Eng. 2017, 149, 612–622. 10.1016/j.petrol.2016.11.018. [DOI] [Google Scholar]; b Farajzadeh R.; Muruganathan R.; Rossen W.; Krastev R. Effect of gas type on foam film permeability and its implications for foam flow in porous media. Adv. Colloid Interface Sci. 2011, 168, 71–78. 10.1016/j.cis.2011.03.005. [DOI] [PubMed] [Google Scholar]; c Karraker K.; Radke C. Disjoining pressures, zeta potentials and surface tensions of aqueous non-ionic surfactant/electrolyte solutions: theory and comparison to experiment. Adv. Colloid Interface Sci. 2002, 96, 231–264. 10.1016/s0001-8686(01)00083-5. [DOI] [PubMed] [Google Scholar]; d Bergeron V.; Waltermo Å.; Claesson P. M. Disjoining pressure measurements for foam films stabilized by a nonionic sugar-based surfactant. Langmuir 1996, 12, 1336–1342. 10.1021/la950594x. [DOI] [Google Scholar]; e Kruglyakov P.Equilibrium properties of free films and stability of foams and emulsions. Thin Liquid Films; Marcell Dekker Inc., 1988; Vol. 1, pp 35–44. [Google Scholar]; f De Feijter J. A.; Vrij A. Contact angles in thin liquid films. II. Contact angle measurements in Newton black soap films. J. Colloid Interface Sci. 1978, 64, 269–277. 10.1016/0021-9797(78)90362-4. [DOI] [Google Scholar]
- Manev E. D.; Pugh R. J. Diffuse layer electrostatic potential and stability of thin aqueous films containing a nonionic surfactant. Langmuir 1991, 7, 2253–2260. 10.1021/la00058a046. [DOI] [Google Scholar]
- Yekeen N.; Manan M. A.; Idris A. K.; Samin A. M.; Risal A. R. Experimental investigation of minimization in surfactant adsorption and improvement in surfactant-foam stability in presence of silicon dioxide and aluminum oxide nanoparticles. J. Pet. Sci. Eng. 2017, 159, 115–134. 10.1016/j.petrol.2017.09.021. [DOI] [Google Scholar]
- a Patist A.; Jha B. K.; Oh S.-G.; Shah D. O. Importance of micellar relaxation time on detergent properties. J. Surfactants Deterg. 1999, 2, 317–324. 10.1007/s11743-999-0083-6. [DOI] [Google Scholar]; b Bibette J.; Calderon F. L.; Poulin P. Emulsions: basic principles. Rep. Prog. Phys. 1999, 62, 969–1033. 10.1088/0034-4885/62/6/203. [DOI] [Google Scholar]; c Khristov K.; Exerowa D.; Yankov R. The isoelectric state at the solution/air interface—effect on the stability of foams and foam films from nonionic surfactants. Colloids Surf., A 1997, 129–130, 257–266. 10.1016/s0927-7757(97)00042-3. [DOI] [Google Scholar]
- a AlYousef Z.; Ayirala S.; Gizzatov A.; Kokal S. Evaluating foam stability using tailored water chemistry for gas mobility control applications. J. Pet. Sci. Eng. 2020, 195, 107532. 10.1016/j.petrol.2020.107532. [DOI] [Google Scholar]; b Rossen W. R.Foams in enhanced oil recovery. Foams: Theory, Measurements and Applications; Taylor & Francis, 1996; Vol. 57, pp 413–464. [Google Scholar]; c Oh S. G.; Shah D. O. Relationship between micellar lifetime and foamability of sodium dodecyl sulfate and sodium dodecyl sulfate/1-hexanol mixtures. Langmuir 1991, 7, 1316–1318. 10.1021/la00055a004. [DOI] [Google Scholar]
- Rusanov A. I.; Krotov V. V.; Nekrasov A. G. Extremes of some foam properties and elasticity of thin foam films near the critical micelle concentration. Langmuir 2004, 20, 1511–1516. 10.1021/la0358623. [DOI] [PubMed] [Google Scholar]
- Wang L. G.; Yoon R. H. Stability of foams and froths in the presence of ionic and non-ionic surfactants. Miner. Eng. 2006, 19, 539–547. 10.1016/j.mineng.2005.09.006. [DOI] [Google Scholar]
- Dehdari B.; Parsaei R.; Riazi M.; Rezaei N.; Zendehboudi S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755. 10.1016/j.molliq.2020.112755. [DOI] [Google Scholar]
- a Aziz U. A.; Adnan N.; Sohri M.; Mohshim D.; Idris A.; Azman M. Characterization of Anionic–Nonionic Surfactant Mixtures for Enhanced Oil Recovery. J. Solution Chem. 2019, 48, 1617–1637. 10.1007/s10953-019-00902-1. [DOI] [Google Scholar]; b Stellner K. L.; Scamehorn J. F. Hardness tolerance of anionic surfactant solutions. 1. Anionic surfactant with added monovalent electrolyte. Langmuir 1989, 5, 70–77. 10.1021/la00085a014. [DOI] [Google Scholar]
- Wang J.Effects of reagents and solid particles on drainage and stability of liquid film, foam and froth. Ph.D. Thesis, The University of Queensland, 2015. [Google Scholar]
- Almubarak M.; AlYousef Z.; Almajid M.; Almubarak T.; Ng J. H.. Enhancing Foam Stability Through a Combination of Surfactant and Nanoparticles. Abu Dhabi International Petroleum Exhibition & Conference, 2020.
- a Hadian Nasr N.; Mahmood S. M.; Akbari S.; Hematpur H. A comparison of foam stability at varying salinities and surfactant concentrations using bulk foam tests and sandpack flooding. J. Pet. Explor. Prod. Technol. 2020, 10, 271–282. 10.1007/s13202-019-0707-9. [DOI] [Google Scholar]; b Huang C.-W.; Chang C.-H. A laboratory study on foam-enhanced surfactant solution flooding in removing n-pentadecane from contaminated columns. Colloids Surf., A 2000, 173, 171–179. 10.1016/s0927-7757(00)00604-x. [DOI] [Google Scholar]
- Persson C. M.; Jonsson A. P.; Bergström M.; Eriksson J. C. Testing the Gouy-Chapman theory by means of surface tension measurements for SDS-NaCl-H2O mixtures. J. Colloid Interface Sci. 2003, 267, 151–154. 10.1016/s0021-9797(03)00761-6. [DOI] [PubMed] [Google Scholar]
- Sedev R.; Exerowa D. DLVO and non-DLVO surface forces in foam films from amphiphilic block copolymers. Adv. Colloid Interface Sci. 1999, 83, 111–136. 10.1016/s0001-8686(99)00007-x. [DOI] [Google Scholar]
- Ali N.; Bilal M.; Khan A.; Ali F.; Iqbal H. M. N. Effective exploitation of anionic, nonionic, and nanoparticle-stabilized surfactant foams for petroleum hydrocarbon contaminated soil remediation. Sci. Total Environ. 2020, 704, 135391. 10.1016/j.scitotenv.2019.135391. [DOI] [PubMed] [Google Scholar]
- Ivanov I. B.; Kralchevsky P. A. Stability of emulsions under equilibrium and dynamic conditions. Colloids Surf., A 1997, 128, 155–175. 10.1016/s0927-7757(96)03903-9. [DOI] [Google Scholar]