Abstract

In this study, three types of electrospun scaffolds, including furfuryl-gelatin (f-gelatin) alone, f-gelatin with polycaprolactone (PCL) in a 1:1 ratio, and coaxial scaffolds with PCL (core) and f-gelatin (sheath), were developed for tissue engineering applications. Scaffolds were developed through single nozzle electrospinning and coaxial electrospinning, respectively, to serve as scaffolds for cardiac tissue engineering. Uniform fibrous structures were revealed in the scaffolds with significantly varying average fiber diameters of 760 ± 80 nm (f-gelatin), 420 ± 110 nm [f-gelatin and PCL (1:1)], and 810 ± 60 nm (coaxial f-gelatin > PCL) via scanning electron microscopy. The distinction between the core and the sheath of the fibers of the coaxial f-gelatin > PCL electrospun fibrous scaffolds was revealed by transmission electron microscopy. Thermal analysis and Fourier transformed infrared (FTIR) spectroscopy revealed no interactions between the polymers in the blended electrospun scaffolds. The varied blending methods led to significant differences in the elastic moduli of the electrospun scaffolds with the coaxial f-gelatin > PCL revealing the highest elastic modulus of all scaffolds (164 ± 3.85 kPa). All scaffolds exhibited excellent biocompatibility by supporting the adhesion and proliferation of human AC16 cardiomyocytes cells. The biocompatibility of the coaxial f-gelatin > PCL scaffolds with superior elastic modulus was assessed further through adhesion and functionality of human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes, thereby demonstrating the potential of the coaxially spun scaffolds as an ideal platform for developing cardiac tissue-on-a-chip models. Our results demonstrate a facile approach to produce visible light cross-linkable, hybrid, biodegradable nanofibrous scaffold biomaterials, which can serve as platforms for cardiac tissue engineered models.
1. Introduction
Myocardial infarction (MI) prevails as one of the leading causes of morbidity and mortality worldwide, caused by the irreversible death of cardiomyocytes in the heart wall.1 The loss of these terminally differentiated cardiomyocytes during MI leads to a significant reduction in contractile efficiency after damage to the myocardium in the heart, subsequently leading to heart failure in the long term.2 The deficiency of reliable human tissue-based model systems for studying cardiac diseases has posed a severe challenge in the understanding of the molecular mechanisms and cellular processes during the progression of heart disease.3 Studies have attempted to model cardiac tissues through traditional tissue engineering approaches with cells seeded on scaffolds to understand heart diseases at their initial stage.4−6 Tissue engineering includes the development of functional and supportive scaffolds for the targeted regeneration of damaged tissues and organs.7 Nanofiber-based scaffolds serve as a suitable environment for cell attachment and proliferation because of their resemblance to a natural extracellular matrix (ECM).8−10 However, traditional hydrogel-based scaffolds often have inherently low mechanical and handling properties, which poses a disadvantage.11 Electrospinning is a simple and versatile method for generating nanofibers from a variety of materials that include polymers, composites, and ceramics.12 Electrospun fibrous scaffolds with high surface area to volume ratio and superior mechanical properties have been applied in wound healing, tissue engineering, and drug delivery.13−15 Moreover, ECM-mimicking electrospun scaffolds laden with stem cells bear promise toward in vivo transplantation as they reduce the probability of an immune response since autologous cells are obtained from patients.16−18
Our goal in this study was to develop a set of furfuryl-gelatin (f-gelatin)-based electrospun scaffolds for the modeling of cardiac tissues. Gelatin is most favored for the preparation of cell-based scaffolds in tissue engineering because of its biodegradability, cell binding ability due to the presence of an Arg-Gly-Asp (RGD)-sequence, and its widespread availability at a low cost.19 Moreover, its lower immunogenicity and its ability to be mixed with other materials such as alginate in order to enhance its mechanical properties make it an ideal polymer biomaterial for applications in tissue engineering and regenerative medicine.20−24 Cross-linking of gelatin, enzymatically or with chemical agents like glutaraldehyde, usually render the resultant compound toxic.25,26 In order to overcome this drawback, Son et al. developed visible light cross-linkable gelatin through the introduction of furfuryl groups.27 We had previously adopted this f-gelatin for the biofabrication of scaffolds to study the interactions between STO fibroblasts and C2C12 cells over a sustained period.28 However, the rheological analysis performed on the aforementioned scaffolds revealed an elastic modulus of 1.7 kPa, which is significantly lower than that of the native ECM present in the myocardium. Hence, a platform with an elastic modulus closely mimicking the mechanical properties of the native cardiac tissue needs to be developed to serve as an ideal scaffold for modeling of cardiac tissue. Thus, we hypothesized that blending of the hydrophilic f-gelatin with the hydrophobic polycaprolactone (PCL) would enhance the elastic modulus of the resultant scaffolds, as well as improve its structural stability in order to be used as platforms for modeling of cardiac tissues.29 PCL is an optimal hydrophobic biodegradable polymer, which has been used as a component for making cardiac tissue scaffolds.30,31 Although PCL is mechanically stable and rigid, it does not confer bioreactivity as it lacks the innate sites for cell adhesion. Being hydrophobic in nature, PCL also tends to attract platelet and plasma protein adhesion, leading to a prolonged inflammatory response from the host.32 Hence, we postulated that combining PCL with f-gelatin would ensure that the hydrophobic PCL component would confer mechanical stability to the scaffold, while the f-gelatin would enhance biocompatibility to mimic the ECM properties of native biological tissue. Although others before have done this in different ratios (70% gelatin and 30% PCL33), our study reports the creation of hybrid scaffolds with f-gelatin and PCL in a 1:1 ratio with the goal of enhancing the structural stability of both blended and coaxially electrospun fibers for evaluation of their microscopic structure, surface and bulk properties, and biocompatibility of the resultant scaffolds.
Thus, we developed three types of electrospun scaffolds based on f-gelatin, including (1) f-gelatin electrospun scaffolds, (2) f-gelatin with PCL in a ratio of 1:1, and (3) coaxial PCL (core, inside) with f-gelatin (sheath, outside) (coaxial f-gelatin > PCL), were developed through single nozzle electrospinning and coaxial electrospinning, respectively. To our knowledge, this is the first study reporting the development and establishment of f-gelatin-based scaffolds via electrospinning. Material characterization and biocompatibility evaluation were then performed on these hybrid electrospun scaffolds as platforms for growing cardiac cells. The electrospinning parameters were optimized for all three scaffolds and their structural and mechanical integrity were characterized through electron microscopy and rheological studies. The interaction between the blended electrospun scaffolds (f-gelatin with PCL 1:1, coaxial f-gelatin > PCL) were assessed through thermal analysis and attenuated total reflection–Fourier transform infrared (ATR-FTIR) spectroscopy. The biodegradation behavior of all scaffolds were analyzed through swelling studies accompanied by scanning electron microcopy (SEM). The biocompatibility of all the electrospun scaffolds was analyzed initially with human AC16 cardiomyocytes. The biocompatibility of the coaxial f-gelatin > PCL scaffolds with an expected higher elastic modulus was assessed further through adhesion and functionality of human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes, thereby helping establish these scaffolds as an ideal platform for developing cardiac tissue-on-a-chip models. The hiPSC cardiomyocytes were adopted in this study due to their prior use in the modeling and study of cardiac diseases such as cardiomyopathies and ischemic heart disease.34
The results obtained from this study can support the use of these electrospun scaffolds as in vitro preclinical platforms for modeling of healthy and diseased cardiac tissue states beneficial for drug screening or regenerative engineering applications. This study collectively demonstrates a facile approach to produce visible light cross-linkable, hybrid, biodegradable nanofibrous scaffold biomaterials, which can be adopted as versatile cardiac tissue engineering platforms.
2. Materials and Methods
2.1. Chemicals
Furfuryl-gelatin (f-gelatin) was prepared by homogeneous addition of furfuryl amine to a porcine gelatin solution as described in previously published works.28 Phosphate buffered saline 10× solution and 1,1,1,3,3,3 hexafluoroisopropanol (HFP) was purchased from Fisher Bioreagents, USA. Polycaprolactone (PCL) of average Mn 80 000 was purchased from Sigma-Aldrich, St Louis, MO, USA.
2.2. Cell Studies
Two different cardiac cell types were utilized for this study. Human AC16 cardiomyocyte cell lines and Cellartis human iPSC derivative cardiomyocytes were utilized to determine the biocompatibility of the electrospun scaffolds.
The AC16 human cardiomyocyte cell lines (SCC109, EMD Millipore, Burlington, MA, USA) were cultured and expanded in Dulbecco’s Modified Eagle Medium (DMEM/F12, Sigma-Aldrich) containing 2 mM l-glutamine (EMD Millipore), 10% fetal bovine serum (FBS) (EMD Millipore), and 1× penicillin-streptomycin solution (EMD Millipore). PKH26 Red Fluorescent Cell Linker Mini Kit (Sigma-Aldrich, St. Louis, MO, USA), and 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, USA) were used as cell labeling dyes. In addition, 48- and 24-well flat-bottom plates (Thermo Fisher Scientific, USA) were used for in vitro cultures. Trypsin-ethylene diamine tetra acetic acid (EDTA, 0.25%, phenol red, ThermoFisher) was used for cell detachment.
Cellartis cardiomyocytes (from ChiPSC22) are human cardiomyocytes derived from induced pluripotent stem cells (iPSCs) and were obtained from Takara Bio, USA. These cells were cultured in Cellartis culture base with 10% FBS and stabilized prior to experiment. Both AC16 human cardiomyocytes and Cellartis cardiomyocytes were stained with PKH26 dye (Sigma-Aldrich, St. Louis, MO) prior to seeding on electrospun scaffolds, as per manufacturer recommendations.
2.3. Synthesis of F-Gelatin
F-gelatin was synthesized and characterized as previously reported.28 Porcine gelatin (4 g, Sigma-Aldrich) was dissolved in 300 mL of milli-Q water. N-Hydroxysuccinimide (NHS) (3.9 g, Tokyo Chemical Industry, Tokyo, Japan) and 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) (4.8 g, Tokyo Chemical Industry), dissolved in 100 mL of milli-Q water, and furfurylamine (1.5 g, Sigma-Aldrich) were added slowly to the gelatin solution, and then the solution was stirred at 40 °C for 24 h. After the reaction, the solution was filtrated and then dialyzed using a dialysis membrane with a molecular weight cutoff of 3500 Da (Spectrum Laboratories, Rancho Dominguez, CA) against water for 3 days in dark conditions. The f-gelatin was recovered by lyophilization and was used for further studies.
2.4. Electrospinning of F-Gelatin-Based Scaffolds
The apparatus used for obtaining coaxial fibers was developed in-house.12 F-gelatin polymer solutions of 10% w/v concentration were prepared by dissolving 1 g of dried f-gelatin in 10 mL of HFP. The visible light cross-linking photoinitiator, riboflavin (RF), was procured from Thermo Fisher Scientific (Waltham, MA). To the f-gelatin solution was added 100 μL of RF (5% w/v in HFP). Blended polymer solutions of 10% w/v were obtained by individually dissolving 5% w/v of dried f-gelatin and 5% w/v of PCL in 10 mL of HFP. The dissolved solution was further mixed and stirred for 6–8 h to obtain a blended solution of PCL and f-gelatin in a ratio of 1:1. The polymer solution was then loaded in a 5 mL syringe with 24 G needle connected to the positive terminal of a high voltage DC supply (ES30P 10 W power supply, Gamma High Voltage Research, Ormond Beach, FL). In case of coaxial electrospinning, the polymer solutions were loaded in different syringes. PCL solution was passed through an inner needle of 22 G (0.71 mm internal diameter) and the sheath f-gelatin solution was passed through the outer needle of 16 G (1.65 mm internal diameter).35 The RF solution was added to the f-gelatin solution before electrospinning. The fibers were deposited onto a grounded aluminum substrate placed at a distance of 10 cm perpendicular to the needle. Fibers were electrospun at room temperature (26 °C) and a relative humidity of 78%. The resulting electrospun fibers were then cross-linked by immediately exposing them to visible light for 2 min (400 nm wavelengths at 100% intensity, Intelli-Ray 600, Uvitron International, West Springfield, MA). After the cross-linking process, the electrospun scaffolds were rinsed with phosphate buffered saline (pH 7.4) and used for further studies. The obtained samples were stored at room temperature until further use.
2.5. Characterization of the Electrospun Fibers
The physiochemical characterization of the scaffolds was performed to analyze and choose an optimal scaffold as a potential platform for cardiac tissue modeling.
2.5.1. Scanning Electron Microscopy (SEM)
SEM was performed to analyze the surface morphology of the electrospun scaffolds. The samples were mounted on brass stubs, were sputter-coated with gold/palladium (2–3 min) in a sputter coater (Gatan Model 682 Precision etching coating system, Pleasantown, CA, USA), and were visualized using SEM (S-4800, Hitachi, Japan) at 7 kV voltage and current of 5 μA at varying magnifications. The diameters of about 50 different fiber samples were measured in each sample group using ImageJ to obtain their average diameter.
2.5.2. Transmission Electron Spectroscopy (TEM)
TEM was performed on coaxial f-gelatin > PCL electrospun scaffolds using an H9500 TEM (Hitachi Ltd., Tokyo, Japan), operating with an accelerating potential of 100 kV, to analyze their internal structure because it cannot be revealed using SEM. The electrospun fiber samples for TEM observation were prepared by directly depositing the as-spun fibers on Formvar-coated Cu TEM grids (Ted Pella Inc., Redding, CA, USA).
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR was performed on the electrospun scaffolds to analyze the interaction between the blended polymers during the electrospinning process. Measurements were carried out on the fibrous scaffolds using a Thermo Mattison spectrometer (Thermo Mattison, Waltham, MA) equipped with a ZnSe ATR crystal. Typically, 32 scans were signal-averaged to reduce spectral noise. The spectrum of the samples was recorded from 400 to 4000 cm–1 to assess the interaction between the polymers.
2.5.4. Thermal Analysis by Thermogravimetric (TGA) Analysis and Differential Scanning Calorimetry (DSC)
Thermal analysis was performed to elucidate the thermal stability of the electrospun scaffolds and further discern the interaction or lack of interaction between the blended polymer systems. TGA of the fibers was performed using a universal Mettler TGA Analyzer, Model TGA/DSC 1 (Mettler-Toledo, Columbus, OH). About 5 mg of the samples was heated at 10 °C min–1 in a temperature range of 0–600 °C using platinum crucibles. DSC analysis of the fibers was performed from 0 to 300 °C at 10 °C min–1 using the Mettler TGA Analyzer, Model TGA/DSC 1. The instrument was calibrated using an indium standard, and the calorimeter cell was flushed with liquid nitrogen at 20 mL min–1.
2.5.5. Rheological Analysis
The elastic modulus of the electrospun scaffolds was elucidated by studying their rheological properties. To examine the rheological properties of the electrospun scaffold, the samples were soaked in 1× PBS for 24 h before testing. Circular samples of electrospun scaffold mats were made with dimensions of 25 mm diameter and 1 mm thickness for these experiments in accordance with previously published works.36 An oscillatory shear stress rheometric study was performed using an Anton-Paar MCR 92 rheometer (Anton-Paar, Austria) with a PP25/S measuring system at 1% strain with a frequency range between 0.5 and 50 Hz.28,37 Analysis for frequency and strain was conducted within the viscoelastic range of the samples.28,37 The storage/loss moduli, complex viscosity, and elastic modulus were measured at 1.99 Hz, as previously done and reported.28,37
2.5.6. Swelling and Morphological Analysis of Electrospun Scaffolds
Swelling studies were performed on electrospun scaffolds to assess their structural stability during long-term in vitro studies. Prior to performing these studies, the hydrophilicity of all samples were determined using contact angle measurement with an in-house video recorder. The cross-linked electrospun scaffolds were cut into dimensions of 2 × 2 cm2 for in vitro swelling studies. The isolated specimens were placed in Petri plates containing DMEM/F12 with 2 mM l-glutamine, 10% FBS, and 1× penicillin–streptomycin solution at 37 °C humidified with 5% CO2 for 14 and 21 days, respectively. The specimens were recovered at the end of each incubation period and were analyzed using SEM.
2.6. Biological Assessment of the Electrospun Scaffolds
2.6.1. Human AC16 Cardiomyocytes Cell Culture and Adhesion on Electrospun Scaffolds
AC16 cardiomyocytes were prestained using the PKH26 cytoplasmic staining dye; seeded on the fibrous scaffolds placed in a 24 well culture plate (Corning, NY, USA); and maintained in DMEM/F12 containing 2 mM l-glutamine, 10% FBS, and 1X Penicillin-Streptomycin Solution at 37 °C humidified with 5% CO2. The cell seeding density was 25 000 cells/cm2 on scaffolds each approximately 1.9 cm2 in area that were designed to fit within the 24 wells. After 24 h of culture, the cell-laden constructs were fixed using 4% PFA, mounted on glass slides, and imaged using a confocal fluorescence microscope (Olympus IX81 inverted fluorescence motorized microscope, Japan) to confirm the adhesion and retention of viable cells on the electrospun scaffolds.
2.6.2. Flow Cytometry Analysis (FACS)
To estimate cell proliferation and overall biocompatibility of the electrospun scaffolds, the AC16 human cardiomyocytes were prestained using Cell Trace Violet proliferation kit (Invitrogen, Carlsbad, CA) using manufacturer’s protocols for this experiment. These prestained cells were seeded on the electrospun scaffolds (25 000 cells/cm2 on a total area of 1.9 cm2) and cultured for 24 h and 7 days, respectively (37 °C, 5% CO2). After 24 h and 7 days, cells on the electrospun scaffolds were treated using Trypsin-EDTA (0.25%, phenol red) to detach and extract the cells for FACS analysis. Extracted cells were fixed with 4% PFA for 15 min at room temperature, added to their designated FACS analysis falcon tubes, and analyzed using a Beckman Coulter Gallios Flow Cytometer (Brea, CA, USA) with excitation and emission wavelengths of 405 and 450 nm, respectively. Positive controls included prestained cells grown on plastic Petri dishes for 48 h. Negative controls included nonstained cells grown on plastic Petri dishes for 48 h.
2.6.3. Culture of Human hiPSC-Derived Cardiomyocytes
Because of their expected superior mechanical properties, the coaxial f-gelatin > PCL electrospun scaffolds were chosen for further studies with hiPSC-derived cardiomyocytes for developing a cardiac tissue model. Cellartis cardiomyocytes (10 000 cells/0.95 cm2) were seeded on coaxial f-gelatin > PCL scaffolds placed in 48-well plates and cultured in Cellartis culture base with 10% FBS at 37 °C humidified with 5% CO2. After 48 h of culture, the cell-laden constructs were fixed using 4% PFA, mounted on glass slides, and imaged using a confocal fluorescence microscope (Olympus IX81 inverted fluorescence motorized microscope, Japan) to confirm the adhesion and retention of cells on the electrospun scaffolds.
Moreover, to assess the long-term biocompatibility of the coaxial electrospun scaffolds, a Live/Dead assay kit (Thermo Fisher Scientific, USA) was used according to the protocol provided by the manufacturer after 7 days of culture of the hiPSC-derived cardiomyocytes atop such scaffolds. Calcein AM (green) represented live cells while ethidium homodimer (red) represented dead cells. The viability of the cells was quantified using the eq 1 below.
| 1 |
2.7. Statistical Analysis
The IBM SPSS software package was used for all statistical analysis reported in this study. All experiments were performed in triplicate, and numerical data are reported as mean ± standard deviation. All data were compared using ANOVA with p < 0.05 considered to be statistically significant.
3. Results
3.1. Morphological and Structural Analysis
SEM images of the cross-linked electrospun samples are shown in Figure 1A–I. Well-formed, robust fibers were observed in all electrospun scaffolds, and their average fiber diameters are tabulated in Table 1.
Figure 1.

Electron microscopy images of f-gelatin, f-gelatin blended with PCL (1:1), and coaxial f-gelatin > PCL electrospun scaffolds. In images A–I, SEM images are presented, and in J, a TEM image is presented. In A–C the cross section is presented at a lower magnification (scale bar = 1 mm). In D–F, higher magnification cross-sectional images are shown (scale bar = 100 μm). In G–I, high-magnification images from the surface are presented (scale bar = 20 μm). Panel J shows a representative TEM image of coaxial f-gelatin > PCL electrospun scaffolds (scale bar = 100 nm).
Table 1. Optimized Parameter for Electrospinning F-Gelatin-Based Fibers.
| scaffold type | f-gel | f-gelatin and PCL (1:1) | coaxial f-gelatin > PCL |
|---|---|---|---|
| concentration of polymer(s) | 10% w/v (f-gel) | 5% w/v (f-gel) | 5% w/v (f-gel) |
| 5% w/v (PCL) | 5% w/v (PCL) | ||
| solvent | HFP | HFP | HFP |
| flow rate | 0.5 mL/h | 1 mL/h | core-0.5 mL/h |
| sheath-0.5 mL/h | |||
| accelerating voltage | 1.5 kV/cm | 1.5 kV/cm | 1.5 kV/cm |
| distance between tip and collector | 10 cm | 10 cm | 10 cm |
| average fiber diameter (nm) | 760 ± 80 nm | 420 ± 110 nm | 810 ± 60 nm |
Figures 1A–F show the cross-sectional images of the electrospun scaffolds in low (Figure 1A–C) and high magnification (Figure 1D–F), respectively. The thickness of the electrospun scaffolds varied between 50 and 75 μm, and these scaffolds were used as such for further characterization. The yellow color present in the non-cross-linked scaffolds was due to the presence of RF, which was used as an initiator for visible light cross-linking (Supplementary Figure S1). The fading of the yellow color confirmed the completion of the photo-cross-linking process (optimized in our laboratory; results not included) in all the electrospun scaffolds. The average diameter of the blended f-gelatin and PCL (1:1) scaffolds was found to be the lowest (420 ± 110 nm), and it was noted to be the highest (810 ± 60 nm) for the blended coaxial f-gelatin > PCL electrospun scaffolds. The optimized parameters for electrospinning, as tabulated in Table 1, outline that the distance between the tip and collector, the accelerating voltage, and the total concentration of the polymer solutions were all maintained constant. Hence, the significant change in average fiber diameters was due to the flow rate and addition of PCL in samples containing the polymer solution. The viscosity of the polymer solutions influences the average fiber diameters of the electrospun scaffolds in a proportional manner.7,12 Hence, the significant difference in the scaffold diameters was due to the lowering of the viscosity (results not included). In case of the coaxially spun f-gelatin > PCL scaffolds, the polymer solutions were not blended conventionally, thereby leading to a corresponding increase in average fiber diameter.
Coalescence of the fiber junctions was found to be predominant in coaxial f-gelatin > PCL electrospun scaffolds (Figure 1I). During coaxial electrospinning, the evaporation of the core polymer solvent occurs through the sheath. The latent evaporation of the core solvent through the sheath led to formation of coalescent junctions in coaxial f-gelatin > PCL electrospun scaffolds.12 Coalescence at the fiber junctions in coaxial f-gelatin > PCL electrospun scaffolds is advantageous because the fiber junctions enhance the elastic modulus of the scaffolds.7,12,38Figure 1J represents a TEM image of the coaxial f-gelatin > PCL electrospun scaffolds that clearly indicates the core–shell structure of the coaxial f-gelatin > PCL fibers. The core diameter of the coaxial f-gelatin > PCL fibers was found to be significantly lower in comparison with the sheath diameter (p = 0.03). Since the PCL polymer solution (core) had significantly lower viscosity than the f-gelatin (sheath) polymer solution, this resulted in a higher diameter of the sheath.38
3.2. Thermal Stability and Interaction
In order to determine the chemical modifications due to addition of PCL to f-gelatin during electrospinning, ATR-FTIR analysis was performed initially to confirm their blending. The pure f-gelatin fibers with and without RF were also analyzed in this experiment. Figure 2 shows the ATR-FTIR spectra of all electrospun scaffolds. No apparent peaks of the solvent were observed, which demonstrates a complete evaporation of solvent during the electrospinning process. All the commonly associated peaks corresponding to both PCL and f-gelatin, namely, the carbonyl, amide I, and amide II peaks, were observed.7,12,38 Few peaks of PCL and f-gelatin within 1160–1200 cm–1 were found to be overlapping.7,12,38 The presence of f-gelatin in the sample was confirmed from four different regions in the spectra and the two regions that included the 1656–1644 cm–1 (amide I)39 and 1560–1335 cm–1 (amide II).40 The electrospun spectra showed bands of increasing intensity for amides I and II in the case of the f-gelatin alone system, while the peaks were significantly lower in intensity for the blended systems. The absence of peaks in the amide III region (1240–670 cm–1) is related to the loss of the triple-helix state during denaturation of collagen to gelatin.7,12,38 The carbonyl C=O double bond stretching mode, with contributions from in-phase bending of the N—H bond and stretching of the C—N bond, occurs in the frequency region of 1660–1620 cm–1, which is often referred to as the amide I band. The frequency range 1660–1650 cm–1 was known as α-helical, and 1640–1620 cm–1 was known as β-sheet structures. The frequency range of 1550–1520 cm–1 is due to the amide II with an α-helical structure between 1550 and 1540 cm–1 and β-sheets at 1525–1520 cm–1.7,12,38,40 The amide II vibration is caused by deformation of the N—H bonds.41 The characteristic absorption band at 1730 cm–1 is mainly due to an ester carbonyl group, and the band at 1283 cm–1 corresponds to the −CH group of PCL.7,12,32,38 The most eminent peaks are listed in Table 2.
Figure 2.
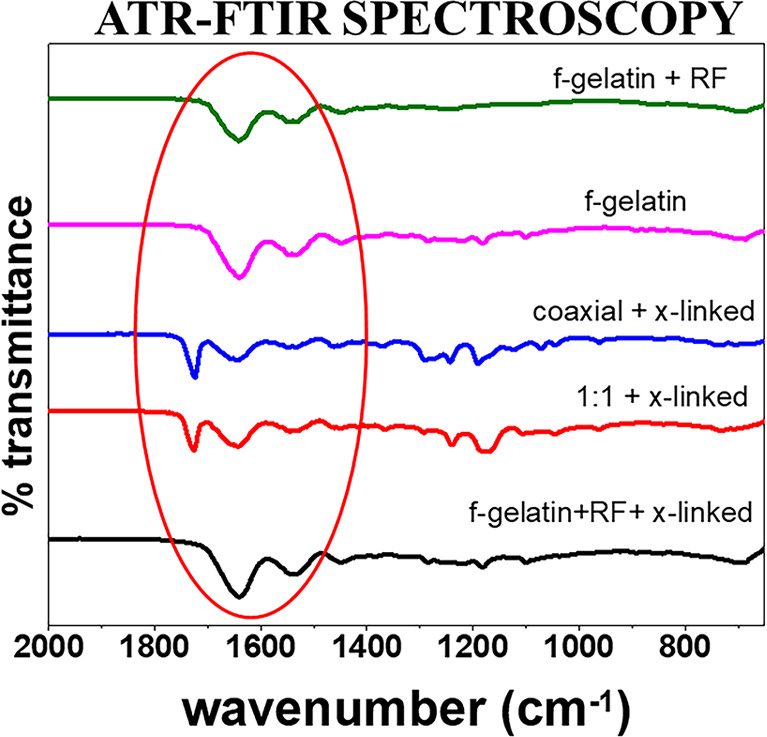
ATR-FTIR spectroscopy of all electrospun scaffolds.
Table 2. Prominent Peaks of F-Gelatin-Based Electrospun Fibers.
| wavelength (cm–1) | designation |
|---|---|
| 1700–1750 | C=O stretching |
| 1226–1280 | O—C—O stretching |
| 1636–1640 | C—O stretching of amide I in gelatin |
| 1542–1548 | N—H and C—H stretching in amide II |
The extent of interaction between the polymers of the blended electrospun systems could not be determined as all peaks corresponding to both PCL and f-gelatin were observed. Since no interactive peaks were observed, and because the interactive peaks may overlap with individual band vibrations of PCL and f-gelatin, thermal analysis was performed to further analyze the interaction/noninteraction of the polymers in the electrospun scaffolds. Moreover, photo-cross-linking of f-gelatin showed no new peaks.
TGA thermograms of blended electrospun scaffolds that were cross-linked are shown in Figure 3A. All electrospun samples demonstrated a characteristic three-stage weight loss pattern. The first stage corresponded to the loss of moisture from these samples, while the second and third stages corresponded to the thermal decomposition of gelatin and PCL, as also shown by our prior published studies.32,38 The loss of moisture in gelatin occurred over a temperature range of 21–90 °C and marked the first stage of weight loss. The first weight loss was correspondingly higher for the f-gelatin alone electrospun scaffolds and considerably lower in the blended electrospun scaffolds, which was apparent from the T–5% values as listed in Table 3. T–5% values represent the initial weight loss of the material used for analysis. The higher T–5% value represents a higher thermal stability of the material.7,12
Figure 3.
(A) TGA and (B) DSC of all electrospun scaffolds.
Table 3. Thermal Analysis of F-Gelatin-Based Electrospun Fibers.
| f-gelatin | f-gelatin and PCL(1:1) | coaxial f-gelatin > PCL | |
|---|---|---|---|
| Tmax1 (°C) | 276.5 | 223.03 | |
| Tmax2 (°C) | 278.33 | 376.38 | 380.56 |
| T–5% (°C) | 71.67 | 140.28 | 187.07 |
| denaturation temperature (°C) | 42.52 | 41.12 | 40.6 |
| melting temperature (°C) | 61.2 | 56.08 |
Tmax values are temperatures at which maximum mass rate change occurs, and two separate Tmax values corresponding to the degradation of f-gelatin and PCL were observed. With a different blending of PCL with f-gelatin, the Tmax2 values were found to differ correspondingly. The Tmax2 values correspond to β-sheet thermal decomposition in gelatin, which further proves that the addition of gelatin resulted in relatively easy crystallization and increased β-sheet content.7,38 The Tmax1 values correspond to the degradation initiated from chain scission of the ester linkage in PCL.7,38 Since the degradation steps correspond to the individual polymers, no interaction between the polymers in the electrospun scaffolds was observed through TGA.
Figure 3B represents the DSC of the electrospun scaffolds. Despite gelatin being derived from collagen, which involves rupture of the triple-helix structure by breaking of hydrogen bonds and a rearrangement of the triple-helix into a random configuration, renaturation is possible under certain conditions.12,38 Therefore, the characteristic endothermic peaks of gelatin have often been seen to occur at the denaturation temperature (TD).42 Miscible polymers have a single-phase blend for which a single glass transition, a single crystallization, and a single melting transition is observed.38 However, an immiscible blend typically shows two inflections or endotherms due to the glass transition and/or melting endotherms, although they are expected to deviate from those of the pure components.43 Hence, PCL and f-gelatin are thermodynamically immiscible polymers. Furthermore, f-gelatin and PCL (1:1) electrospun scaffolds exhibited a significantly higher Tm (melting temperature) when compared to the coaxial f-gelatin > PCL electrospun system.
An increased pressure and temperature when heat is transferred from the f-gelatin sheath to the PCL core led to the significant decrease in Tm of PCL in coaxial f-gelatin > PCL scaffolds.12 Since no third interactive peak was observed from DSC, it conclusively proved the noninteraction of the polymers after electrospinning and cross-linking. Thermal analysis also conclusively proves that the coalescence of fibers observed in the coaxial f-gelatin > PCL electrospun scaffolds was physical in nature alone with no chemical interactions.
3.3. Mechanical Parameters
Rheometric analysis was performed on the cross-linked electrospun scaffolds after 12 h of preswelling in 1× PBS (pH 7.4) and is presented in Figure 4. At 1% constant strain, the average elastic modulus was 3.46 ± 0.05 kPa for the f-gelatin electrospun scaffolds and 28.49 ± 0.26 kPa for the f-gelatin and PCL (1:1) scaffolds. The average elastic modulus of the coaxial f-gelatin > PCL scaffolds was 164 ± 3.85 kPa. The addition of PCL to the blended scaffolds was found to significantly improve the elastic modulus of the scaffolds (p = 0.02). In case of f-gelatin and PCL (1:1) electrospun scaffolds, the topical addition of PCL to f-gelatin polymer solution merely contributed to the enhancement of the elastic modulus of the resultant scaffold. However, for the coaxial f-gelatin > PCL scaffolds, a significantly higher elastic modulus was recorded. Hence, the coaxial f-gelatin > PCL electrospinning process significantly improved the mechanical stability of the fibrous scaffolds. Moreover, the physical coalescence of fibers at the junctions in coaxial f-gelatin > PCL electrospun scaffolds also led to their enhanced elastic modulus.7,12 The elastic modulus of the blended scaffolds fell within the range of the native ECM present in the human myocardium and can be potentially utilized as platforms or scaffolds for cardiac tissue engineering.37
Figure 4.
Elastic modulus of all electrospun scaffolds obtained through rheological analysis. F-Gelatin is labeled as F-GEL; blended f-gelatin and PCL (1:1) is labeled as 1:1, and coaxial f-gelatin > PCL electrospun scaffolds is labeled as COAX in the figure.
3.4. In Vitro Swelling and Dissolution Studies
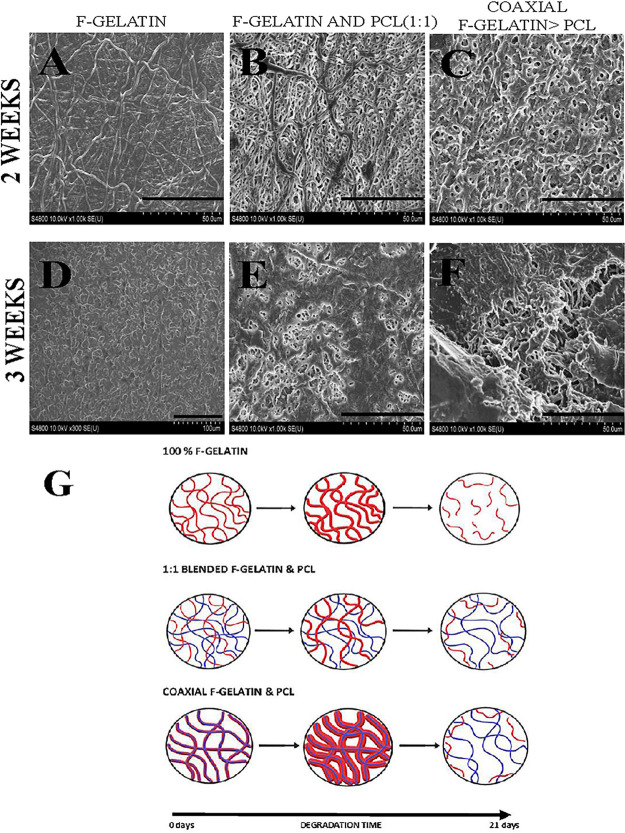
Figure 5 shows the SEM images of in vitro swelling of blended cross-linked fibers of f-gelatin, f-gelatin and PCL (1:1), and coaxial f-gelatin > PCL systems after 2 (Figure 5A–C) and 3 weeks (Figure 5D–F). Swelling of the fibers was detected in all electrospun scaffolds. The swelling pattern was significantly pronounced in pure f-gelatin electrospun scaffolds and least pronounced in the blended f-gelatin and PCL (1:1) system. The degree of swelling was proportional to the composition of f-gelatin present in the sample (Figure 5G). Correspondingly, changes in morphology of the fibers were increasingly pronounced with the presence and composition of f-gelatin. PCL being a hydrophobic polymer was found to undergo surface erosion dissolution, while gelatin present in the fibers corresponded to bulk dissolution of the fibers.7,12 The porosity and pore structure of the electrospun scaffolds are significantly affected by the swelling of f-gelatin after 2 weeks. The higher degree of swelling in f-gelatin electrospun scaffolds and coaxial f-gelatin > PCL scaffolds led to the complete occlusion of pores within 2 weeks. After 3 weeks, the high degree of swelling in f-gelatin electrospun scaffolds led to the disruption of its fibrous nature while in coaxial f-gelatin > PCL scaffolds the partial dissolution of the f-gelatin sheath exposed the internal PCL fibers in the scaffold. The blended f-gelatin and PCL (1:1) electrospun scaffolds clearly showed the chemical noninteraction of gelatin and PCL fibers during the study. The swelling of f-gelatin is clearly visible after 2 weeks and was found to be more pronounced after 3 weeks. The occlusion of pores was minimal in the blended f-gelatin and PCL (1:1) electrospun scaffolds and was highest in f-gelatin electrospun scaffolds. The hydrophilicity of the gelatin structure leads to bulk dissolution of the shell structure.12 The obtained results conclusively indicate that the increase in f-gelatin composition increased the biodegradability of the scaffold.
Figure 5.
(A–C) SEM images of in vitro dissolution of f-gelatin, f-gelatin and PCL (1:1), and coaxial f-gelatin > PCL electrospun scaffolds after 2 weeks (scale bar = 50 μm). (D–F) Scaffolds imaged using SEM after 3 weeks (scale bar = 50 μm). (G) Schematic representation of the proposed dissolution mechanism in blended electrospun scaffolds.
3.5. Biocompatibility
Figure 6A–C represents the adhesion of AC16 cardiomyocytes on all the electrospun scaffolds. All three sets of electrospun scaffold groups were found to support the adhesion of AC16 cardiomyocytes after only 24 h of culture. The percentage of adhered cells (Figure 6D) studied through PK26 cell-labeling using ImageJ software showed significantly better adhesion of cells on blended electrospun f-gelatin and PCL (1:1) (∼64%) and coaxial f-gelatin > PCL scaffolds (∼68%) than on f-gelatin electrospun scaffolds (∼39%). Although we did not report the biomarker characterization for gap junction analysis for the AC16 cardiomyocyte cells adhered onto the scaffolds, our previously published works have extensively highlighted the presence of Connexin 43 as a cardiac gap junction expressed by such cells atop f-gelatin-based scaffolds.36
Figure 6.
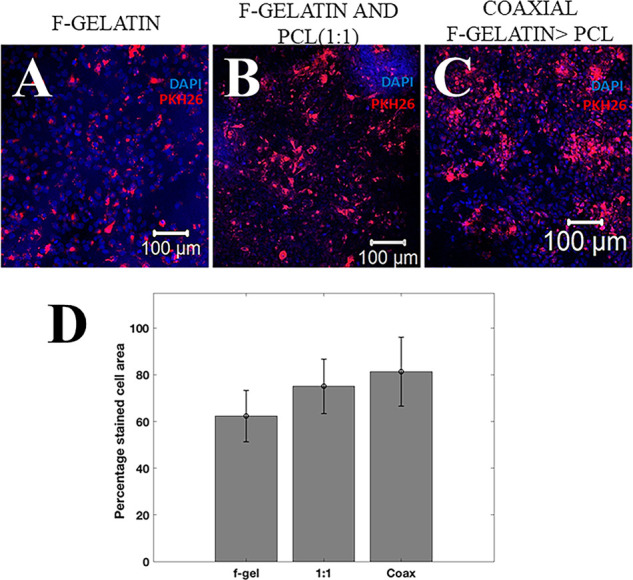
(A–C) Confocal microscopy images of AC16 cardiomyocytes stained with DAPI (blue) and PKH26 (red) on f-gelatin, f-gelatin and PCL (1:1), and coaxial f-gelatin > PCL electrospun scaffolds after 24 h (scale bar = 100 μm). (D) A quantitative estimate of the PKH26-stained cell area for all samples is shown. A significantly higher number of cells were present on the coaxial scaffolds (p < 0.05) in comparison with the other scaffolds.
The significant difference in elastic modulus was sensed by the cells and led to a higher adhesion of cells on the blended scaffolds with a higher elastic modulus.44 The exposure of RGD cell-binding sequence in f-gelatin enables the adhesion of the cardiomyocytes to the scaffolds.19 Moreover, the high surface to volume ratio created by the surface roughness of the electrospun fibrous scaffolds also provides an optimal substrate for the cells to adhere within 24 h.12
Figures 7A–F represents the FACS analysis of cardiomyocytes extracted from all the electrospun scaffolds. The cardiomyocytes were stained using CellTrace Violet (CTV) dye to track cell growth and proliferation using the concept of dye dilution after 1 and 7 days of culture. Since cell-doubling time for AC16 cardiomyocytes is ∼25 h, cell proliferation caused a reduction in intensity of the dye over successive generations, thereby permitting the analysis of several generations of proliferating cells within 7 days.45,46 In this experiment, positive controls included cells prestained with CTV (Supplementary Figure S2) and cultured for 48 h after which they were extracted. Negative controls (Supplementary Figure S2) included samples cultured using the exact conditions as the positive controls, without the addition of the CTV dye. The characteristic peaks from the negative controls were observed in the low intensity region, and peaks for the stained cells of the positive controls were observed in the high intensity region.
Figure 7.
FACS analysis of CTV cardiomyocytes extracted from cell culture plates after 1 day (A–C) and after 7-days (D–F). Unstained negative and stained positive controls are shown in Supplementary Figure S2 and were used to define and set the gate limits for the data shown in this figure.
Figure 7 panels A, B, and C represent the CTV prestained cells extracted from f-gelatin, blended f-gelatin and PCL (1:1), and coaxial f-gelatin > PCL electrospun scaffolds, respectively, on day 1. All sample runs shown in Figure 7 panels A, B, and C showed peaks (97.43–99.05%) in the positive control region (99.81%) (Supplementary Figure S2) signifying the adhesion of cells on the scaffolds. Since the time for proliferation was low within seeding until 24 h later, most of the cells exhibited a higher concentration of the dye in comparison with positive controls. Figure 7 panels D, E, and F represent the CTV prestained cells extracted from f-gelatin, blended f-gelatin and PCL (1:1), and coaxial f-gelatin > PCL electrospun scaffolds, respectively, on day 7. The intensity of the CTV dye significantly decreased by day 7 (23.89–49.62%), which clearly signified the dilution of dye with time, indicating proliferation of the prestained cardiomyocytes on the electrospun scaffolds. Among the three samples, dye dilution was maximized (24%) in the cells that proliferated atop the blended f-gelatin and PCL (1:1), followed by 42% on cells atop the coaxial f-gelatin > PCL electrospun scaffolds and 51% atop the cells on the f-gelatin scaffolds. This implies that the cells adhered and proliferated atop the f-gelatin blended scaffolds but also preferred an enhanced mechano-conductive scaffold such as the blended f-gelatin-PCL scaffolds in comparison with the pure f-gelatin scaffolds.
3.6. Cardiac Tissue Modeling
Since coaxial f-gelatin > PCL electrospun scaffolds exhibited significantly higher elastic modulus when compared with other scaffolds, Cellartis hiPSC-derived cardiomyocytes were seeded on them to study their biocompatibility. Figure 8A–C represents the adhesion of cells on the coaxial f-gelatin > PCL electrospun scaffolds after 3 days, thereby providing appropriate time for the cells to attach and adapt to the scaffold. The contractile function of the hiPSC-derived cardiomyocytes on the electrospun scaffolds was intact.
Figure 8.

(A–C) Confocal microscopy images of hiPSC-derived cardiomyocytes stained with DAPI and PKH26 on all the coaxially spun scaffolds after 72 h (scale bar = 200 μm). (D,E) Live/Dead assay results from hiPSC-derived cardiomyocytes grown on the coaxially spun scaffolds after 7 days.
More than 90% of cell viability was detected after 7 days of culture through the Live/Dead assay, as shown in Figure 8D,E.
4. Discussion
Electrospinning has witnessed a profound advancement in the field of tissue engineering and regenerative medicine.7 The versatility of the electrospinning process combined with the emergence of new biopolymers has been a major accelerator of this growth. The present study demonstrates the applicability of using an f-gelatin and PCL-based electrospun fibrous scaffolds geared toward cardiac tissue engineering applications. The f-gelatin consisted mainly of porcine gelatin, modified by the incorporation of a furfural group.28,37 Earlier studies by our group on this f-gelatin led to the development of a scaffold that can be rapidly cross-linked in the presence of visible light to maintain structural fidelity.28,37 However, the structural integrity and stiffness of the printed cross-linked structure improved only when blended with hyaluronic acid.37 Our long-term goal is to biofabricate multilayered, multicellular models, which can be used for mimicking in vivo cardiac tissue. Since the topographies of scaffolds have been shown to dictate cellular attachment, migration, proliferation, and differentiation, which are critical in engineering complex functional tissues with improved biocompatibility and functional performance, electrospinning of f-gelatin with PCL was chosen as the method to develop the needed platforms for cardiac tissue modeling.47
As a first step, we developed a novel visible light cross-linked electrospun scaffold based on f-gelatin and PCL, which would aid in providing ideal platforms for cardiac modeling applications. In comparison with f-gelatin 3D bioprinted gels reported by our group in prior studies, the f-gelatin electrospun scaffolds possessed improved mechanical properties as well as optimal swelling behavior.28,37 Electrospinning of polymer solutions requires the optimization of various parameters, i.e., concentration, viscosity, molecular weight, degree of entanglement, electrical conductivity, and surface tension.7,12,32,38 The hydrophobic synthetic polymer PCL has been electrospun using solvents such as dichloromethane and chloroform.48 Because of higher conductivity and solubility factors, HFP has been widely used to electrospin natural polymers.38 Moreover, HFP also reduces surface tension of the polymer solution, thereby enabling blending of natural polymers with synthetic polymers.38 Hence, HFP was chosen as the optimal solvent for electrospinning f-gelatin and blended f-gelatin and PCL fibers. The increase in diameter of coaxial f-gelatin > PCL fibers over f-gelatin and f-gelatin and PCL (1:1) blended fibers has been attributed to the presence of PCL core solution that caused a difference in charge relaxation time and viscosity of the coaxial f-gelatin > PCL spinning solution.49 The increase in fiber diameter corresponds to a decrease in surface area of the electrospun fibers, which might influence a decrease in adhesion of cells on the coaxial f-gelatin > PCL scaffolds.38 Thermal properties of the electrospun fibers were used to assess the miscibility and interaction of the polymers after electrospinning. However, no interactive peaks were exhibited by the scaffolds, leading to the conclusion that no interaction occurred between the polymers after electrospinning and cross-linking.
In comparison with other types of hybrid blended gelatin scaffolds, these scaffolds produced via a simple visible light cross-linking mechanism can maintain noninteraction between polymers, which will aid in maintaining the structural and functional properties of growth factors or other chemokines for releasing into a culture or in vivo, as desired because visible light cross-linking does not seem to alter the chemical functionality of the polymers.12,38 However, in vitro swelling studies performed on all the systems exhibited stark differences in the structural stability of the scaffolds over a 3 week period. Blended electrospun scaffolds were more structurally stable than f-gelatin electrospun scaffolds. The addition of hydrophobic PCL significantly improved the structural stability of the scaffolds. Moreover, the swelling and dissolution of gelatin in the blended scaffolds increased the pore diameter of the scaffolds, which thereby enabled the penetration of cells through the scaffolds. All electrospun scaffolds exhibited excellent biocompatibility by supporting the adhesion and proliferation of human AC16 cardiomyocytes over a period of 7 days. Cardiomyocytes prefer a hydrophobic surface for adhesion and proliferation.50 Moreover, an increased water uptake usually decreases resultant cell adhesion.12 However, the cell binding sequences in f-gelatin balanced the swelling and the hydrophilic surface properties of f-gelatin.12,38
We aim to fabricate cardiac wall tissue in vitro utilizing electrospinning, which can then be exploited in vitro for drug studies or probing into the underlying mechanisms involved in cardiac development and disease. The cross-linked electrospun scaffolds had elastic moduli ranging from 3 to 160 kPa, which were stable when exposed to long-term culture, implying they can be extremely effective for in vivo studies, as well. The human myocardium ranges in stiffness from 20 kPa (end of diastole) to 500 kPa (end of systole).51 Hence, the coaxial f-gelatin > PCL electrospun scaffold system, which possessed the highest elastic modulus (>150 kPa) among the three electrospun systems, was chosen as the potentially suitable candidate for cardiac platforms that could withstand the contractile forces of the native myocardium. Additionally in this study, we cultured human induced pluripotent stem cell-derived cardiomyocytes on the chosen coaxial f-gelatin > PCL electrospun scaffolds. The viability and contractile function of the hiPSC-cardiomyocytes were not affected by the coaxial f-gelatin > PCL electrospun platform, thereby confirming its potential as an ideal platform for cardiac tissue engineering applications.
5. Conclusion
In conclusion, we showed the applicability of f-gelatin as a novel biomaterial for electrospinning of scaffolds or platforms with cardiac tissue ECM-like mechanical properties. Although both synthetic and natural materials have been proposed to generate suitable tissue engineering grafts, the ideal material or scaffold for repair and regeneration of cardiac tissue has not yet been proposed.28 Coaxial f-gelatin > PCL electrospun scaffolds of PCL (core) and f-gelatin (sheath) exhibited improved structural and mechanical stability in comparison with electrospun scaffolds developed from f-gelatin alone and conventionally blended f-gelatin and PCL (1:1) scaffolds. The blending of f-gelatin with PCL significantly improved its mechanical and chemical structural stability. All electrospun scaffolds exhibited excellent biocompatibility by supporting the adhesion and growth of human AC16 cardiomyocytes and hiPSC cardiomyocytes, confirming the presence of the cell binding sequences in f-gelatin that were exposed in all scaffolds postelectrospinning and cross-linking.
The noninteraction of PCL with f-gelatin in the blended scaffolds will aid in the addition of drug molecules like growth factors and chemokines without any change in its structural and functional ability.52 Further studies may be aimed at testing the in vitro efficacy of the coaxial f-gelatin > PCL electrospun scaffolds with appropriately bound drug molecules and at developing a robust cardiac organoid platform for drug screening applications. In addition, since engineering of the heart muscle requires alignment because it is an anisotropic tissue, we will devise methods for electrospinning the fiber-based scaffolds in an aligned pattern.
We will also attempt to develop hierarchically structured scaffolds with piezoelectric or electro-conductive properties to mimic the native myocardium. This will enable us to generate a functional cardiac patch that can be used for drug cytotoxicity screening or exploring triggers for heart diseases in vitro.
Acknowledgments
The Joddar laboratory (IMSTEL) acknowledges NSF grants #1828268 and #1927628 and NIH SC1 grant #1SC1HL154511-01. We acknowledge the technical assistance received from Carla Loyola for confocal microscopy and Dr. Hugo Celio at UT Austin’s Texas Materials Institute for his help with the material characterization experiments. We are grateful to Ms. Blanca P. Ortega for the graphical content in Figure 5G.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00271.
Images of f-gelatin electrospun fibers (Figure S1) and FACS analysis of stained and unstained cardiomyocytes (Figure S2) (PDF)
Author Contributions
N.N. planned the experiments and contributed to the data analysis. R.E.K. performed FACS analysis and contributed to the data analysis. M.H.O., J.A., and Y.I. prepared the f-gelatin and characterized it for this study. D.A.R. performed the TEM analysis and contributed to the data analysis. N.N. and B.J. wrote the manuscript, and all authors have approved the final version for submission.
The authors declare no competing financial interest.
Notes
All data generated or analyzed during this study are included in this published article (and its Supporting Information files). The raw datasets for all the figures generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Supplementary Material
References
- Asaria P.; Elliott P.; Douglass M.; Obermeyer Z.; Soljak M.; Majeed A.; Ezzati M. Acute myocardial infarction hospital admissions and deaths in England: a national follow-back and follow-forward record-linkage study. Lancet Public Health 2017, 2 (4), e191–e201. 10.1016/S2468-2667(17)30032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja P.; Sdek P.; MacLellan W. R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007, 87 (2), 521–44. 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E. I.; Lucchino V.; Scaramuzzino L.; Scalise S.; Cuda G. Modeling Cardiac Disease Mechanisms Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Progress, Promises and Challenges. Int. J. Mol. Sci. 2020, 21 (12), 4354. 10.3390/ijms21124354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen J.; Migrino R. Q.; Nikkhah M. Three-dimensional microengineered models of human cardiac diseases. J. Biol. Eng. 2019, 13, 29. 10.1186/s13036-019-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acun A.; Vural D. C.; Zorlutuna P. A Tissue Engineered Model of Aging: Interdependence and Cooperative Effects in Failing Tissues. Sci. Rep 2017, 7 (1), 5051. 10.1038/s41598-017-05098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudou T.; Legant W. R.; Mu A.; Borochin M. A.; Thavandiran N.; Radisic M.; Zandstra P. W.; Epstein J. A.; Margulies K. B.; Chen C. S. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 2012, 18 (9–10), 910–9. 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah N.; Madhavi L.; Anitha R.; Srinivasan N. T.; Sivagnanam U. T. Electrospinning of poly (3-hydroxybutyric acid) and gelatin blended thin films: fabrication, characterization, and application in skin regeneration. Polym. Bull. 2013, 70 (8), 2337–2358. 10.1007/s00289-013-0956-6. [DOI] [PubMed] [Google Scholar]
- Majid Q. A.; Fricker A. T. R.; Gregory D. A.; Davidenko N.; Hernandez Cruz O.; Jabbour R. J.; Owen T. J.; Basnett P.; Lukasiewicz B.; Stevens M.; Best S.; Cameron R.; Sinha S.; Harding S. E.; Roy I. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution. Front. Cardiovasc. Med. 2020, 7 (192), 554597. 10.3389/fcvm.2020.554597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Li W.; Dong X.; Yuan X.; Midgley A. C.; Chang H.; Wang Y.; Wang H.; Wang K.; Ma P. X.; Wang H.; Kong D. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10 (1), 4620. 10.1038/s41467-019-12545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati S.; Kim S. J.; Shin Y. M.; Shin H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Convergence 2019, 6 (1), 36. 10.1186/s40580-019-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. P.; Leong K. W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (S4), 467–79. 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah N.; Madhavi L.; Anitha R.; Anandan C.; Srinivasan N. T.; Sivagnanam U. T. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33 (7), 4444–52. 10.1016/j.msec.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Negut I.; Dorcioman G.; Grumezescu V. Scaffolds for Wound Healing Applications. Polymers (Basel) 2020, 12 (9), 2010. 10.3390/polym12092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensini A.; Cristofolini L.; Zucchelli A.; Focarete M. L.; Gualandi C.; A D. E. M.; Kao A. P.; Roldo M.; Blunn G.; Tozzi G. Hierarchical electrospun tendon-ligament bioinspired scaffolds induce changes in fibroblasts morphology under static and dynamic conditions. J. Microsc 2020, 277 (3), 160–169. 10.1111/jmi.12827. [DOI] [PubMed] [Google Scholar]
- Ramalingam R.; Dhand C.; Leung C. M.; Ezhilarasu H.; Prasannan P.; Ong S. T.; Subramanian S.; Kamruddin M.; Lakshminarayanan R.; Ramakrishna S.; Verma N. K.; Arunachalam K. D. Poly-epsilon-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials 2019, 9 (3), 462. 10.3390/nano9030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizaw M.; Faglie A.; Pieper M.; Poudel S.; Chou S. F. The Role of Electrospun Fiber Scaffolds in Stem Cell Therapy for Skin Tissue Regeneration. Med. One 2019, 4, e190002 10.20900/mo.20190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh T. C.; Amanah A. Y.; Gluck J. M. Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering 2020, 7 (3), 105. 10.3390/bioengineering7030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats A.; Tolley N. S.; Bishop A. E.; Polak J. M. Embryonic stem cells and tissue engineering: delivering stem cells to the clinic. J. R Soc. Med. 2005, 98 (8), 346–50. 10.1177/014107680509800804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A. L.; Maresca M.; Ullrich W.; Doty S. B.; Butler W. T.; Prince C. W. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner 1993, 22 (2), 147–59. 10.1016/S0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- Angele P.; Kujat R.; Nerlich M.; Yoo J.; Goldberg V.; Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 1999, 5 (6), 545–54. 10.1089/ten.1999.5.545. [DOI] [PubMed] [Google Scholar]
- Ifkovits J. L.; Burdick J. A. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13 (10), 2369–85. 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- Ovsianikov A.; Deiwick A.; Van Vlierberghe S.; Dubruel P.; Moller L.; Drager G.; Chichkov B. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011, 12 (4), 851–858. 10.1021/bm1015305. [DOI] [PubMed] [Google Scholar]
- Sakai S.; Hirose K.; Taguchi K.; Ogushi Y.; Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30 (20), 3371–7. 10.1016/j.biomaterials.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Van Den Bulcke A. I.; Bogdanov B.; De Rooze N.; Schacht E. H.; Cornelissen M.; Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1 (1), 31–8. 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- Kuo W.-T.; Huang H.-Y.; Chou M.-J.; Wu M.-C.; Huang Y.-Y. Surface Modification of Gelatin Nanoparticles with Polyethylenimine as Gene Vector. J. Nanomater. 2011, 2011, 646538. 10.1155/2011/646538. [DOI] [Google Scholar]
- Lai J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci.: Mater. Med. 2010, 21 (6), 1899–1911. 10.1007/s10856-010-4035-3. [DOI] [PubMed] [Google Scholar]
- Son T. I.; Sakuragi M.; Takahashi S.; Obuse S.; Kang J.; Fujishiro M.; Matsushita H.; Gong J.; Shimizu S.; Tajima Y.; Yoshida Y.; Suzuki K.; Yamamoto T.; Nakamura M.; Ito Y. Visible light-induced crosslinkable gelatin. Acta Biomater 2010, 6 (10), 4005–10. 10.1016/j.actbio.2010.05.018. [DOI] [PubMed] [Google Scholar]
- AnilKumar S.; Allen S. C.; Tasnim N.; Akter T.; Park S.; Kumar A.; Chattopadhyay M.; Ito Y.; Suggs L. J.; Joddar B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J. Biomed Mater. Res. B Appl. Biomater 2019, 107 (2), 314–323. 10.1002/jbm.b.34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Ouyang H.; Lim C. T.; Ramakrishna S.; Huang Z. M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed Mater. Res. B Appl. Biomater 2005, 72B (1), 156–65. 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- Fu W.; Liu Z.; Feng B.; Hu R.; He X.; Wang H.; Yin M.; Huang H.; Zhang H.; Wang W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomedicine 2014, 9, 2335–44. 10.2147/IJN.S61375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilla P.; Bezuidenhout D.; Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials 2007, 28 (34), 5009–27. 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Johnson R.; Ding Y.; Nagiah N.; Monnet E.; Tan W. Coaxially-structured fibres with tailored material properties for vascular graft implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1–11. 10.1016/j.msec.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Feng B.; Zhang W.; Yan C.; Yao Q.; Shao C.; Yu F.; Li F.; Fu Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17 (5), 3717–3726. 10.3892/etm.2019.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musunuru K.; Sheikh F.; Gupta R. M.; Houser S. R.; Maher K. O.; Milan D. J.; Terzic A.; Wu J. C.; American Heart Association Council on Functional G.; Translational B.; Council on Cardiovascular Disease in the Y.; Council on C.; Stroke N. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement From the American Heart Association. Circ.: Genomic Precis. Med. 2018, 11 (1), e000043 10.1161/HCG.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah N.; Franchi F.; Peterson K.; Rodriguez-Porcel M. Stem Cell- Laden Coaxially Electrospun Fibrous Scaffold for Regenerative Engineering Applications. Current Protocols 2021, 1 (1), e13 10.1002/cpz1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury R.; Nagiah N.; Mudloff J. A.; Thakur V.; Chattopadhyay M.; Joddar B. 3D Bioprinted Spheroidal Droplets for Engineering the Heterocellular Coupling between Cardiomyocytes and Cardiac Fibroblasts. Cyborg and Bionic Systems 2021, 2021, 9864212. 10.34133/2021/9864212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil Kumar S.; Alonzo M.; Allen S. C.; Abelseth L.; Thakur V.; Akimoto J.; Ito Y.; Willerth S. M.; Suggs L.; Chattopadhyay M.; Joddar B. A Visible Light-Cross-Linkable, Fibrin-Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater Sci. Eng. 2019, 5 (9), 4551–4563. 10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiah N.; Johnson R.; Anderson R.; Elliott W.; Tan W. Highly Compliant Vascular Grafts with Gelatin-Sheathed Coaxially Structured Nanofibers. Langmuir 2015, 31 (47), 12993–3002. 10.1021/acs.langmuir.5b03177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y.; Yang X.; Ji Z.; Zhu L.; Ma N.; Chen D.; Jia X.; Tang J.; Cao Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5 (15), 8572–8578. 10.1021/acsomega.9b04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyonga J. H.; Cole C. G. B.; Duodu K. G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86 (3), 325–332. 10.1016/j.foodchem.2003.09.038. [DOI] [Google Scholar]
- Hashim D. M.; Man Y. B. C.; Norakasha R.; Shuhaimi M.; Salmah Y.; Syahariza Z. A. Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem. 2010, 118 (3), 856–860. 10.1016/j.foodchem.2009.05.049. [DOI] [Google Scholar]
- Zhang Y. Z.; Venugopal J.; Huang Z. M.; Lim C. T.; Ramakrishna S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47 (8), 2911–2917. 10.1016/j.polymer.2006.02.046. [DOI] [Google Scholar]
- Zhang Y Z; Feng Y; Huang Z-M; Ramakrishna S; Lim C T Fabrication of porous electrospun nanofibres. Nanotechnology 2006, 17, 901. 10.1088/0957-4484/17/3/047. [DOI] [Google Scholar]
- Jalali S.; Tafazzoli-Shadpour M.; Haghighipour N.; Omidvar R.; Safshekan F. Regulation of Endothelial Cell Adherence and Elastic Modulus by Substrate Stiffness. Cell Commun. Adhes 2015, 22 (2–6), 79–89. 10.1080/15419061.2016.1265949. [DOI] [PubMed] [Google Scholar]
- Begum J.; Day W.; Henderson C.; Purewal S.; Cerveira J.; Summers H.; Rees P.; Davies D.; Filby A. A method for evaluating the use of fluorescent dyes to track proliferation in cell lines by dye dilution. Cytometry A 2013, 83 (12), 1085–95. 10.1002/cyto.a.22403. [DOI] [PubMed] [Google Scholar]
- Filby A.; Perucha E.; Summers H.; Rees P.; Chana P.; Heck S.; Lord G. M.; Davies D. An imaging flow cytometric method for measuring cell division history and molecular symmetry during mitosis. Cytometry A 2011, 79 (7), 496–506. 10.1002/cyto.a.21091. [DOI] [PubMed] [Google Scholar]
- Manoukian O. S.; Matta R.; Letendre J.; Collins P.; Mazzocca A. D.; Kumbar S. G. Electrospun Nanofiber Scaffolds and Their Hydrogel Composites for the Engineering and Regeneration of Soft Tissues. Methods Mol. Biol. 2017, 1570, 261–278. 10.1007/978-1-4939-6840-4_18. [DOI] [PubMed] [Google Scholar]
- Baker S. R.; Banerjee S.; Bonin K.; Guthold M. Determining the mechanical properties of electrospun poly-ε-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Materials Science and Engineering: C 2016, 59, 203–212. 10.1016/j.msec.2015.09.102. [DOI] [PubMed] [Google Scholar]
- Yarin A. L. Coaxial electrospinning and emulsion electrospinning of core–shell fibers. Polym. Adv. Technol. 2011, 22, 310–317. 10.1002/pat.1781. [DOI] [Google Scholar]
- Zong X.; Bien H.; Chung C. Y.; Yin L.; Fang D.; Hsiao B. S.; Chu B.; Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005, 26 (26), 5330–8. 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Venugopal J. R.; Prabhakaran M. P.; Mukherjee S.; Ravichandran R.; Dan K.; Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J. R Soc. Interface 2012, 9 (66), 1–19. 10.1098/rsif.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi S.; Carotenuto F.; Rinaldi A.; Di Nardo P.; Manzari V.; Albertini M. C.; Araneo R.; Ramakrishna S.; Teodori L. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials 2020, 10 (9), 1781. 10.3390/nano10091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.