Abstract
Transgenic Leishmania infantum promastigotes, which constitutively express green fluorescent protein (GFP) in their cytoplasm, were used to monitor the effects of antileishmanial compounds in real time. The GFP-based assay provided a reliable measure of drug-induced inhibitory effects on protein expression, resulting in a dynamic picture of the responses of leishmanial promastigotes to the compounds tested.
Leishmaniasis is a major tropical and subtropical parasitic disease that has traditionally been treated with pentavalent antimonial compounds. Due to frequent relapses in leishmaniasis patients, alternative drugs such as amphotericin B, pentamidine, ketoconazole, miltefosine, and allopurinol have been used. Severe side effects, however, and failures in treatment of clinical cases are common (3, 8, 13, 18, 22). Therefore, more effective and less toxic therapeutic agents are needed, as are simple assays for in vitro screening of compounds for potential antileishmanial effects. The currently available screening assays have several shortcomings. Either they are labor-intensive and expensive or they require the use of radiolabeled precursors or metabolic substrates (2).
The green fluorescent protein (GFP) of the jellyfish Aequorea victoria has been introduced as a convenient reporter in many applications in eukaryotic organisms (4). This protein is intrinsically fluorescent, has a low toxicity, and allows easy imaging and quantification using fluorescence-activated cell sorting (FACS) or microscopy. Detection of GFP in living cells can be performed and is amenable to real-time analysis of molecular events (20, 24). Use of GFP also eliminates the need for fixation or cell permeabilization (4, 5, 12, 14). However, despite its general popularity as a reporter, GFP has yet to be widely exploited in antimicrobial screening assays (5). In our study, we have explored the suitability of transgenic Leishmania infantum promastigotes, which constitutively express GFP in their cytoplasm, as target cells for in vitro screening of antileishmanial drugs. The effects of the protein synthesis inhibitors cycloheximide and puromycin and the antileishmanial purine analogue allopurinol on cell viability and on GFP expression in these promastigotes were determined. In addition, we tested the efficacy of chloralin, a dinitroaniline shown to have high activity against L. infantum promastigotes in vitro (1, 15).
Promastigote forms of L. infantum strain p-229 (World Health Organization code MCAN/ES/89/IPZ229/1/89, zymodeme MON 1) were maintained at 27°C in 25-cm2 tissue culture flasks (T25; Corning) in liquid medium (a 1:1 mixture of SDM-79 and SM medium, pH 7.4) as described previously (11), supplemented with 10% heat-inactivated calf serum. Clonal lines of transgenic Leishmania p-229 promastigotes were obtained by electroporation of the expression vector pXG-GFP (kindly provided by S. Beverley, Washington University, St. Louis, Mo.) and selection in medium containing the antibiotic Geneticin (G418; Sigma) to a final concentration of 280 μg/ml (7, 12, 17).
Allopurinol (Sigma) was added from a 10-mg/ml stock solution dissolved in 0.1 N NaOH. Cycloheximide and puromycin (Sigma) were dissolved in water to form 10-mg/ml stock solutions. Chloralin was kindly provided by A. Armson (Murdoch University, Perth, Australia) and was dissolved in dimethyl sulfoxide to form a 100 mM stock solution.
Transgenic promastigotes (5 × 106 cells, logarithmic growth phase) were used to determine the GFP turnover by treating the cells with 100 μg of cycloheximide/ml as described previously (19). In a parallel assay, cells were treated with 800 μg of allopurinol/ml. At least 105 cells were collected at 24-h intervals for 4 days and analyzed by flow cytometry to quantify intracellular GFP fluorescence. The proportion of dead cells at these time intervals was also determined by the addition of 10 μg of propidium iodide (PI)/ml. Determination of PI exclusion is a standard method to assess cell viability in cell-sorting experiments (9, 10, 16). In a comparative series of experiments, the effects of 5 μg of cycloheximide/ml, 50 μg of puromycin/ml, and 2.7 μg of chloralin/ml (10 μM) on GFP expression and cell viability were determined after 24 h of exposure to the drugs.
The green fluorescence of GFP and the red fluorescence of PI were excited using an argon laser at a wavelength of 488 nm (FACS Calibor; Becton Dickinson, Heidelberg, Germany). At least 10,000 cells were analyzed per sample, and each experiment was repeated at least three times. Quantitative data analysis was performed using CELLQuest and WinMDI analysis software.
Live transgenic L. infantum parasites examined by fluorescence microscopy showed bright cytoplasmic GFP fluorescence. Total GFP fluorescence intensities were proportional to the number of cells and remained stable until late stationary phase. The growth kinetics were comparable to those of untransformed promastigotes, with a maximal cell concentration of 1.1 × 108 about 72 h postinoculation (data not shown).
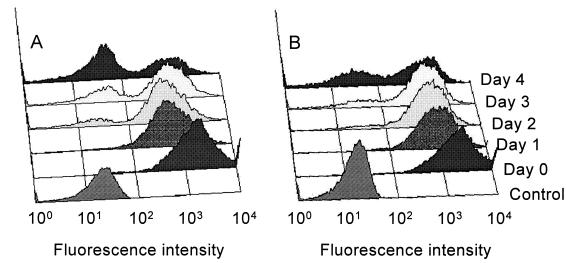
As a positive control for the effect of complete protein synthesis inhibition on cellular GFP fluorescence, transgenic cells were treated with 100 μg of cycloheximide/ml. FACS analysis showed a decrease in fluorescence of 57% after 96 h (Fig. 1A) compared to that of untreated parasites (∼1%, data not shown) and provided a rough estimate of approximately 80 h for the apparent half-life of GFP. Treatment with 800 μg of allopurinol/ml had a similar effect (Fig. 1B), with a 40% decrease in GFP fluorescence resulting. The number of PI-stained cells increased in parallel with the decrease in the number of GFP-positive cells, indicating a lethal effect of prolonged drug exposure. After 96 h, more than 60% of the promastigotes treated with 100 μg of cycloheximide/ml were dead (PI positive), compared to only 18.4% after treatment with 800 μg of allopurinol/ml.
FIG. 1.
Histograms of GFP fluorescence of L. infantum p-229 promastigotes exposed to 100 μg of cycloheximide/ml (A) or 800 μg of allopurinol/ml (B) for 4 days. The bottom lane represents unstained cells (control).
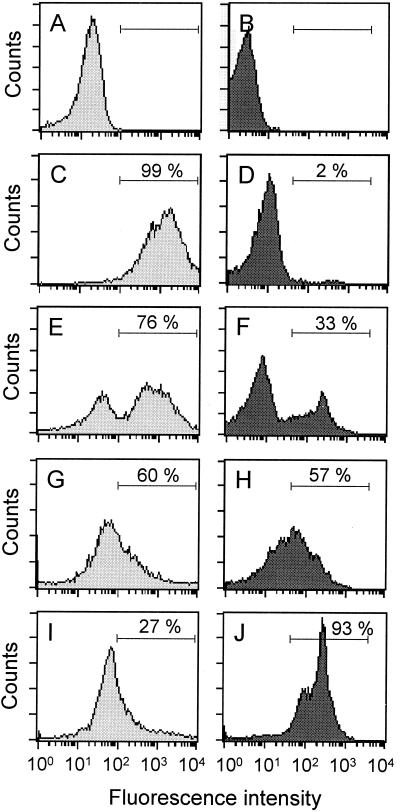
A series of histograms shows the GFP (Fig. 2A, C, E, G, and I) and PI (Fig. 2B, D, F, H, and J) fluorescence intensities after treatment with puromycin, cycloheximide, and chloralin. The numbers of GFP- and PI-positive cells after 24 h were determined by FACS analysis using the appropriate gating. Treatment with 5 μg of cycloheximide/ml or with 50 μg of puromycin/ml resulted in the inhibition of GFP expression in 24% (Fig. 2E) and 40% (Fig. 2G) of the promastigotes, respectively. Treatment with 2.7 μg of chloralin/ml (10 μM) showed the largest effect and resulted in a reduction in GFP fluorescence in 73% of the cells (Fig. 2I). The proportions of PI-stained dead cells after treatment with cycloheximide or puromycin were 33% (Fig. 2F) and 57% (Fig. 2H), respectively, whereas 93% of the cells died as a result of treatment with chloralin (Fig. 2J).
FIG. 2.
Histograms of GFP (A, C, E, G, and I) and PI (B, D, F, H, and J) fluorescence intensities of L. infantum p-229 promastigotes exposed to 5 μg of cycloheximide/ml (E and F), 50 μg of puromycin/ml (G and H), or 10 μM chloralin (I and J) for 24 h. The results for unstained control cells (A and B) and stained control cells (C and D) are also shown. A window was set (horizontal bars) to determine the proportions of GFP-and PI-positive cells, respectively, which are given as percentages.
In this study, we evaluated the use of intracellular GFP quantification for the assessment of drug action in transgenic L. infantum promastigotes. We have shown that even a stable form of GFP is affected by compounds that directly or indirectly interfere with protein synthesis. If protein synthesis is completely inhibited in a cell, the intracellular GFP levels will decrease according to the half-life of the protein. Even though we could easily detect decreased GFP levels in cells treated with allopurinol, a compound with a partial antileishmanial effect (3, 8, 15, 16), the sensitivity of the present assay would benefit greatly from a decreased half-life of GFP. Destabilizing signals which lead to an increased turnover of GFP have been introduced in other systems (6, 19, 21) and may also be adapted for use with Leishmania. Alternatively, our system may profit from a new generation of GFP molecules which change their emitted light from green to red as the age of the protein increases (23). The advantage of such improved systems would be a shortened time of incubation with the drug before an effect on gene expression could be accurately quantified (5, 14). The principal advantages of the GFP-based screening assays over traditional viability tests, such as [3H]thymidine incorporation, are speed, direct analysis of a sample in FACS without prior preparation, and a reliable and reproducible measure of the cells' status. Also, the cells are not killed during the procedure, which makes possible high-throughput screening in microtiter plates with repeated rounds of FACS quantification of GFP (5).
Staining of drug-treated cells with PI, a fluorescent dye incapable of crossing intact membranes of living cells, in combination with measurement of the fluorescence of GFP gives information on two different but possibly interconnected levels. Whereas GFP provides a measurement of actual protein synthesis, and can therefore detect cytostatic effects, PI staining gives direct information on cell viability, which is affected primarily by cytotoxic compounds. Treatment of transgenic promastigotes with 800 μg of allopurinol/ml decreased the GFP levels in 40% of the cells, but only 18.4% of the cells were dead (i.e., PI positive) after 96 h. These results are consistent with earlier findings that allopurinol has only a partial inhibitory effect on Leishmania in vitro (15, 16). Chloralin, on the other hand, had a profound effect on both cell viability and intracellular GFP levels. Similar results were obtained in previous studies by using either [3H]thymidine incorporation (1) or SYBR-14 or PI staining (15, 16) as a measurement of cell proliferation.
Our data have shown the usefulness of cytoplasmic GFP expression as a tool for monitoring drug-induced inhibitory effects in transgenic Leishmania parasites. The GFP-based assay provides a rapid, real-time assessment of changes in cellular protein expression over time, does not require additional preparation, and can be used to perform high-throughout screening.
Acknowledgments
We are indebted to Stephen Beverley (Washington University) for plasmid pXG-GFP.
This work was supported by the Foundation Research 3R, Switzerland (grant 53/96). A.B.H. is supported by grant 31-58912.99 from the Swiss National Science Foundation.
REFERENCES
- 1.Armson A, Kamau S W, Grimm F, Reynoldson J A, Best W M, MacDonald L M, Thompson R C A. A comparison of the effects of a benzimidazole and the dinitroanilines against Leishmania infantum. Acta Trop. 1999;73:303–311. doi: 10.1016/s0001-706x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 2.Callahan H L, Portal A C, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaliero T, Arnold P, Mathias A, Glaus T, Hofmann-Lehmann R, Deplazes P. Clinical, serologic and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J Vet Intern Med. 1999;13:330–334. doi: 10.1892/0891-6640(1999)013<0330:csapfu>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 5.Collins L A, Torero M N, Franzblau S G. Green fluorescent protein reporter microplate assay for high-throughout screening of compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:344–347. doi: 10.1128/aac.42.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corish P, Tyler-Smith C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 1999;12:1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 7.Cruz A, Coburn C M, Beverley S M. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denerolle P, Bourdoiseau G. Combination of allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis. J Vet Intern Med. 1999;13:413–415. doi: 10.1892/0891-6640(1999)013<0413:caaatv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Fogliene C, Meoni C, Davalli A M. Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol. 2001;115:223–229. doi: 10.1007/s004180100249. [DOI] [PubMed] [Google Scholar]
- 10.Garner D L, Johnson L A, Yue S T, Roth B L, Haugland R P. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J Androl. 1994;15:620–629. [PubMed] [Google Scholar]
- 11.Grimm F, Brun R, Jenni L. Promastigote infectivity in Leishmania infantum. Parasitol Res. 1991;77:185–191. doi: 10.1007/BF00930856. [DOI] [PubMed] [Google Scholar]
- 12.Ha D S, Schwarz J K, Turco S J, Beverley S M. Use of green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- 13.Jha T K, Sundar S, Thakur C P, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 14.Kain S R. Green fluorescent protein (GFP): applications in cell-based assays for drug discovery. Drug Discov Today. 1999;4:304–312. doi: 10.1016/s1359-6446(99)01330-6. [DOI] [PubMed] [Google Scholar]
- 15.Kamau S W, Nunez R, Grimm F. Flow cytometric analysis of the effect of allopurinol and the dinitroaniline compound (Chloralin) on the viability and proliferation of Leishmania infantum promastigotes. BMC Pharmacol. 2001;1:1–10. doi: 10.1186/1471-2210-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamau S W, Hurtado M, Müller-Doblies U U, Grimm F, Nunez R. Flow cytometric assessment of allopurinol susceptibility in Leishmania infantum promastigotes. Cytometry. 2000;40:353–360. [PubMed] [Google Scholar]
- 17.Kapler G M, Coburn C M, Beverley S M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Fichoux Y, Rousseau D, Ferrua B, Ruette S, Lelièvre A, Grousson D, Kubar J. Short- and long-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob Agents Chemother. 1998;42:654–658. doi: 10.1128/aac.42.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang C, Kain S R. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 20.Lippincott J, Li R. Nuclear envelope fission is linked to cytokinesis in budding yeast. Exp Cell Res. 2000;260:277–283. doi: 10.1006/excr.2000.5021. [DOI] [PubMed] [Google Scholar]
- 21.Mateus C, Avery S V. Destabilized green fluorescent protein for monitoring dynamic changes in yeast gene expression with flow cytometry. Yeast. 2000;16:1313–1323. doi: 10.1002/1097-0061(200010)16:14<1313::AID-YEA626>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Quellette M, Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993;9:150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 23.Terskikh A, Fradkov A, Ermakova G, Zaraisky A, Tan P, Kajava A V, Zhao X, Lukyanov S, Matz M, Kim S, Weissman I, Siebert P. “Fluorescent timer”: protein that changes color with time. Science. 2000;290:1585–1588. doi: 10.1126/science.290.5496.1585. [DOI] [PubMed] [Google Scholar]
- 24.Westphal M, Jungbluth A, Heidecker M, Muhlbauer B, Heizer C, Schwartz J M, Marriott G, Gerisch G. Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr Biol. 1997;7:176–183. doi: 10.1016/s0960-9822(97)70088-5. [DOI] [PubMed] [Google Scholar]