Abstract
Gene therapy is a critical constituent of treatment approaches for genetic diseases and has gained tremendous attention. Treating and preventing diseases at the genetic level using genetic materials such as DNA or RNAs could be a new avenue in medicine. However, delivering genes is always a challenge as these molecules are sensitive to various enzymes inside the body, often produce systemic toxicity, and suffer from off-targeting problems. In this regard, transdermal delivery has emerged as an appealing approach to enable a high efficiency and low toxicity of genetic medicines. This review systematically summarizes outstanding transdermal gene delivery methods for applications in skin cancer treatment, vaccination, wound healing, and other therapies.
Graphical abstract
Keywords: Transdermal delivery, Gene therapy, Microneedles, siRNA, miRNA, mRNA, DNA
Introduction
Gene therapy, one of the emerging technologies in the modern healthcare system, has attracted the great attention of researchers worldwide [1, 2]. Gene therapy involves transporting genetic material into target cells to prevent or cure diseases by compensating or correcting defective genes [3, 4]. As per the United States Food and Drug Administration (US-FDA) definition, gene therapy is a modification of genes to cure the disease that could work by several principles, including (i) inactivating a defective gene, (ii) replacing a defective gene, and (iii) introducing a new or modified gene [5]. Gene therapy has become the epicenter for future medicine with the advancement of gene vectors, breakthroughs in genome editing technology, and an upsurge of chimeric antigen T cell immunotherapy [6, 7]. The success of adeno-associated virus (AAV) mediated gene therapy to treat hemophilia [8] and Duchenne muscular dystrophy [9] has shown a bright future of gene therapies. In addition, ex vivo lentiviral and retroviral-based gene therapies are able to help modify hematopoietic cells and treat diseases such as beta-thalassemia and sickle-cell disease [10]. Non-viral vectors, including liposomes and lipid NPs [11], have been explored for gene therapy and shown promising results for siRNA, mRNA, and DNA delivery to treat various diseases [12, 13]. RNA-lipid NP-based gene therapies, especially those for COVID-19 vaccines from Pfizer/BioNTech and Moderna, are currently in the spotlight due to the pandemic and continue to dominate public and research interests [14]. These non-viral vectors stabilize the genetic materials, produce target-specific action, and enhance the therapeutic efficacy in vivo [15–17].
Genetic materials, however, are not favorable for oral delivery due to the presence of degrading enzymes, acidic pH, and mucus layer in the gastrointestinal (GI) tract. Possible alternative methods such as intramuscular (IM), intradermal, ocular, and interatrial administrations are under investigation for gene delivery [18]. While IM injections, intravenous (IV) injections, and transdermal delivery (TD) help to avoid the GI tract effects, TD is the only one that can minimize the requirement of trained personnel for administration, cause little to no pain and offer a minimally invasive method. Thus, TD offers an appealing administration pathway for gene delivery. In a pandemic with associated lockdowns and quarantines, TD becomes even more attractive as it could allow people to self-administer vaccines/drugs and access to medicines easily without the need of travelling to medical centers/facilities where there is a high risk of infection [19, 20].
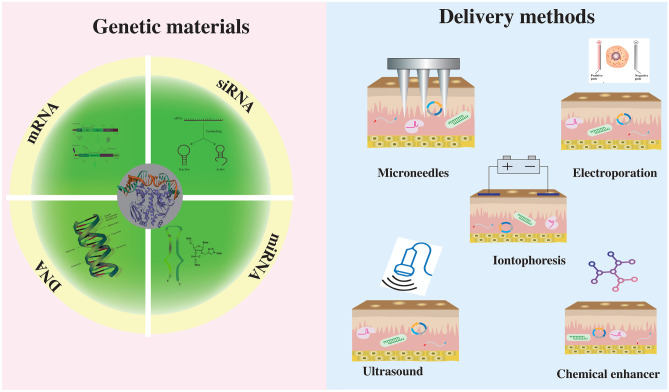
However, to perform TD, the medicine needs to bypass the stratum corneum (SC), the topmost layer of skin which provides the skin’s protection, and prevents the penetration of foreign molecules into the body [21]. Several methods have been developed to facilitate the drug delivery through SC [22, 23]. Among them, the outstanding approaches include microneedles, chemical enhancers, ultrasound, electroporation, and iontophoresis (Fig. 1). These TD systems can deliver different biomolecules, including protein antigens, siRNA, mRNA, and DNA, across the skin for different therapeutic effects [24–26]. As a significant challenge in gene therapy is how to effectively target specific cells, TD can deliver genetic materials in both localized and systemic manners to provide a targeted and cell-specific therapy [27–29]. In addition, the existence of many immune cells in the dermal layer of the skin makes TD appealing for gene immunotherapy. Some notable review articles have been published to discuss the use of TD for gene therapy, but they are all limited by either focusing on specific delivery techniques or lacking a broad description of current progresses in the field [29–32]. This review will (1) provide a broad overview of the current progresses in the transdermal gene therapy, (2) describe different modes and methods of the TD for gene therapy, and (3) discuss challenges with some possible future applications in this field.
Fig. 1.
Schematic of different transdermal gene therapy methods
Current methods of transdermal gene delivery
Transdermal/dermal microneedles
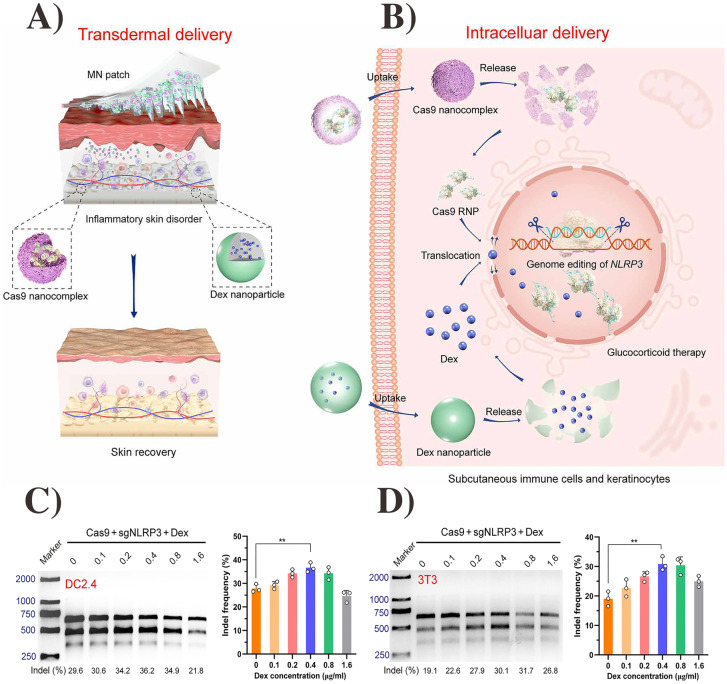
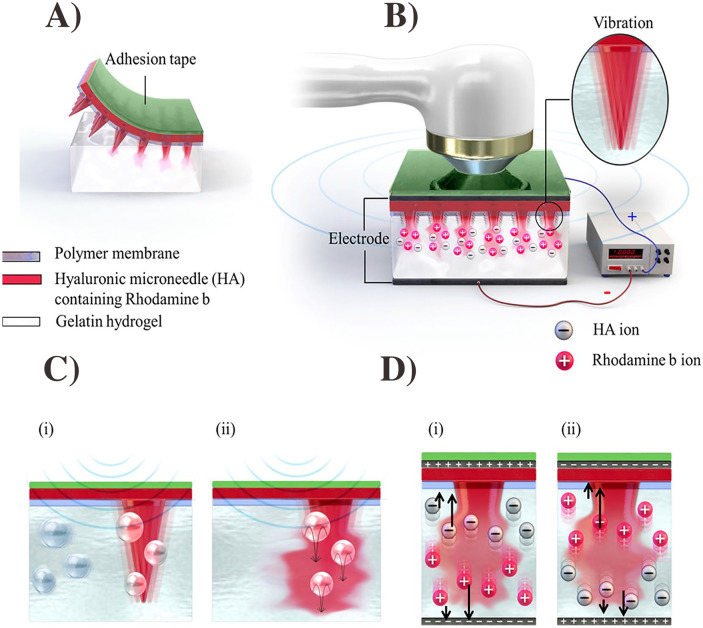
Microneedles (MNs) are micron-sized minimally invasive devices that penetrate through the SC layer and release the therapeutic agents by various mechanisms [33]. MNs are typically constructed from polymers, ceramics, steel, or silicon [34, 35]. MNs can be classified as biodegradable MNs, immediately dissolving MNs, hydrogel-forming MNs, bioresponsive MNs, and hollow MNs [36]. MNs have promising applications as transdermal carriers for immune/gene-therapy and continue to be an active area in biomedical engineering research [24, 29, 37]. In a notable work, Wan et al. investigated hyaluronic acid-based MNs that contained Cas9 ribonucleoprotein and dexamethasone loaded PLGA nanoparticles to genetically treat inflammatory skin disorders. The MNs quickly dissolved once inserted into the skin and produced their therapeutic effects in the inflammatory subcutaneous layer (Fig. 2). The MNs downregulated NLP3 expression and reduced the symptoms of inflammatory skin diseases by deactivating the immune response, resulting in a reduced glucocorticoid resistance in immune cells [38]. Besides this work, there are many other reports on the use of MNs to deliver genetic materials. In brief, Table 1 summarizes the current published works using transdermal MNs for gene/immunotherapy.
Fig. 2.
Schematic of transdermal microneedle patch delivery (A) and intracellular pathway (B) of genome editing agent (Cas9) nanocomplex and glucocorticoid nanoparticle (dexamethasone delivered from the patch) to treat the inflammatory skin diseases. T7 endonuclease I assay of indels (method to validate CRISPR reagents) introduced into the nod-like receptor family, pyrin domain-containing 3 (NLRP3) locus of DC (dendritic cell) 2.4 cells (C) and 3T3 cells (D) transfected with the two nanoparticles. The figure was
reproduced from ref. [38]. Copyright Science Advance 2021
Table 1.
MN-based transdermal gene therapy
| Gene/ protein | Type of MNs | MN materials | Fabrication method | Disease | Reference |
|---|---|---|---|---|---|
| P53 DNA | Dissolving MNs | Hyaluronic acid | Casting | Subcutaneous tumor | [39] |
| CD44-sd-siRNA | Coated MNs | Polyvinyl alcohol | Droplet borne air blowing | Skin disorders | [40] |
| siRNA-CBL3 | Dissolving MNs | Polyvinyl alcohol | Droplet borne air blowing | Skin disorders | [41] |
| Mesenchymal stem cell (MSC)-derived exosomes and a small molecular drug UK5099 | Degradable MNs | Hyaluronic acid/ Keratin | Micromolding | Hair growth | [42] |
| pDNA | Metal MNs | Silicon | Etching | Skin disorders | [43] |
| CCL22 and IL-2 Enriches Treg Homing | Dissolving MNs | Hyaluronic acid | Casting | Skin allografting | [44] |
| ovalbumin | Coated Metal MNs | Steel MNs | Etching | IgE-mediated airway allergy | [45] |
| anti-CTLA4 antibody | Degradable MNs | Hyaluronic acid/Dextran | Micromolding | Superficial tumor | [46] |
| DNA | Degradable MNs | Polycarbonate | Micromolding | Cancer | [47] |
| albumin-expressing plasmid OVA and immunostimulant-polyinosinic:polycytidylic acid | Dissolving MNs | Polypeptide copolymer | Casting | Cancer | [48] |
| Ovalbumin | Metal MNs | NR | Etching | Allergy | [49] |
MNs can be loaded with various drugs, biologics, and vaccines with tunable release profiles [50, 51]. In general, MNs are almost painless and can be self-administered, thus increasing the compliance for patients [52]. Despite the significant advantages of MNs, there is still a need to improve several aspects of this technology such as efficacy, fabrication scale, drug/biomolecule loading capacity, and drug/biomolecule stability before the MN technologies can be used in clinics.
Chemical enhancers for transdermal delivery
Chemical enhancers are molecules that enhance a drug flux through the skin barrier [53]. The purpose of chemical enhancers is to increase the permeability of the SC using chemical reactions [54, 55]. Chemical enhancers disrupt the stratum corneum lipid structure (lipid bilayer), protein structure (keratin), or the solvent nature of the SC [56].
An ideal chemical penetration enhancer (also termed as percutaneous absorption promoter or accelerant) has specific characteristics; it should be biocompatible, travels in one direction, offers reproducible results to remove the SC barrier, and enables cosmetic appearance after use. Unfortunately, there are not many chemicals or chemical mixtures that have all these qualities. Some chemical enhancers are hydrogel patches [57], designed to work in concert with other TD methods (e.g., iontophoresis). Other enhancers are ionic liquids (salts that exist as a liquid at room temperature) [55] and deep eutectic solvents (mixture of H-bond donors and acceptors with a lowered melting point below room temperature) [58]. They are made to increase dermal permeability of molecules with sizes of up to 150 kDa. With highly tunable chemical structures, they can be non-toxic and biodegradable [58]. There are hundreds of other potential chemical enhancers including amidated pectin hydrogels patches [59–61], which could be explored for gene therapy. These compounds can be classified on the basis of chemical and subsequent properties or on the basis of functional groups [62, 63]. One of the main benefits of chemical enhancers for TD is that they are incredibly adaptable and can be combined or mixed together to create even more potent enhancers.
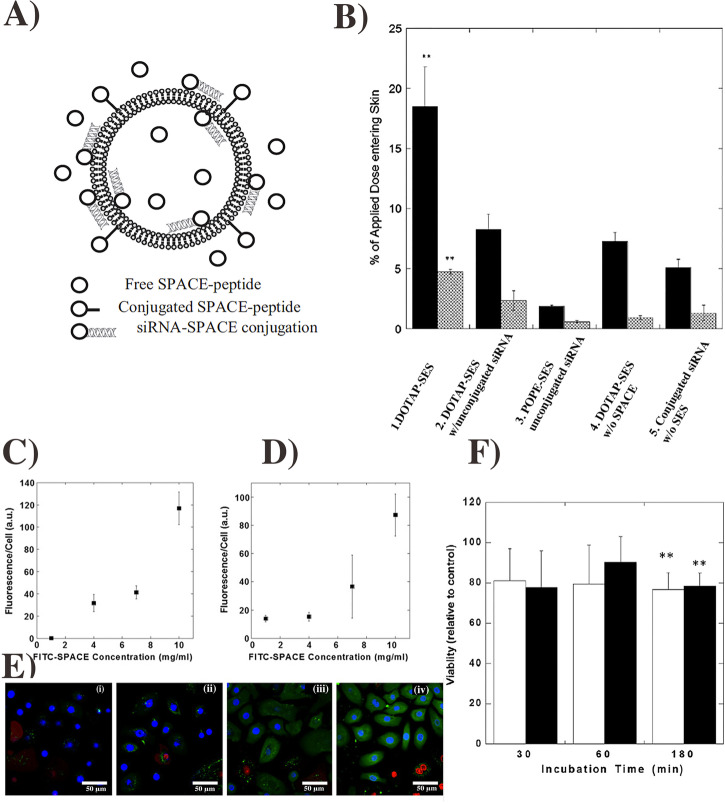
Chemical enhancers have been successfully reported for the delivery of genetic materials [64]. For example, Chen et al. reported TD of skin penetrating SPACE peptide (C-TGSTQHQ-CG, Disulfide Bridge 2–10) in combination with 1, 2-dioleoyl-3- trimethylammonium-propane (DOTAP)-based ethosomal system to treat the skin disorders (Fig. 3A−F). This chemical enhancer system significantly promotes the penetration of siRNA and knockdown of the GAPDH gene [65]. Furthermore, SPACE peptide also enhances the TD efficacy of other siRNA to treat skin diseases [66]. Yet, some concerns remain for the use of chemical enhancers. These include limited potency, irritant nature of potent enhancers, unpredictability in action, and complicated mechanisms, which need to be resolved to further develop this TD method [53].
Fig. 3.
Schematic of SPACE-peptide decorated cationic ethosomes for transdermal siRNA delivery (A). Skin permeability of peptide system under various conditions (B). Internalization of model drug by SPACE peptide after 30 min (C) and 120 min (D) incubation time. FITC internalization after 120-min incubation with various concentrations (E). Cell toxicity of SPACE peptide with 1 mg (open bar) and 10 mg (close bar) with keratinocytes for different time periods (F). The figure was
reproduced from ref. [65]. Copyright Elsevier 2014
Ultrasound
Besides diagnostic applications, Ultrasound (US) has been known to enhance therapeutics uptake into cells/organs [67]. Using high-frequency (0.7–1.6 MHz) to low-frequency (20–100 kHz) US, the method, so-called sonophoresis, has been investigated for transdermal drug delivery applications [68]. The principle behind sonophoresis is to create cavitation microbubbles toward the surface, leading to a microjet formed from the asymmetrical collapse of the bubble [69]. While not entirely understood, cavitation is considered the primary cause of increased skin permeability and drug penetration [68, 70, 71].
The US-mediated gene/vaccine therapy is a relatively new topic as few researchers have explored this field [72, 73]. The US-induced transdermal immunization can exploit the Langerhans immune cells of the skin, which have an essential role in inducing T cells to react against many foreign antigens [74, 75]. US-based transdermal drug delivery has been used to efficiently deliver small molecules, peptides, and genes [76–78]. For example, Manikkath et al. reported that peptide dendrimers in combination with ketoprofen could be delivered into the skin by using US treatment [79]. Sonophoresis has been proven to increase the skin permeability for topical delivery of stem cell factor gene, luciferase gene, and hepatocyte growth factor gene [80]. Ryu et al. investigated a synergistic effect of sonophoresis and MNs for siRNA and ovalbumin delivery. In this work, the combination increased the concentration of siRNA 7 times and ovalbumin delivered into the transdermal area by 15 times [81]. Another combination of sonophoresis with MN technology, known as sonophoretic-enhanced microneedle assay (SEMA), has been employed to deliver a larger molecular compound and increase the delivery rate of this compound via transdermal administration. For example, researchers showed that in vitro diffusion of bovine serum albumin (BSA) and calcein into explanted pig-skin via this combinatory use followed a zero-order release (i.e., nearly constant rate) with much higher skin-penetration dose than just using MNs alone or sonophoresis alone [82]. Moreover, Bok et al. have implemented the combination of US, MNs, and iontophoresis as a potent tool for TD gene therapy (Fig. 4A−D) [83]. A recent work suggests the benefit of using dual-frequency sonophoresis and the combination of sonophoresis with nanocarriers, which could be a new avenue in the field of TD [84].
Fig. 4.
Ultrasound-based transdermal therapies. Schematic of multi-transdermal techniques (MNs, ultrasound, and iontophoresis) for transdermal drug delivery (A-B). Dissolution mechanism by ultrasound (C) and predicted dissolution mechanism and ion direction (D). The figure was reproduced from ref. [83].
Copyright Nature publishing group 2020
However, to use sonophoresis in clinical settings, one would have to confirm the safety of this method. To date, a limited number of safety assessments have been done and harmful thermal effects of US on the skin are still relatively unexamined [78]. Other drawbacks also need to be considered for the use of sonophoresis; these include the efforts to tune US parameters, the complication of sonophoretic devices, and the cost of this therapy.
Iontophoresis
Iontophoresis is an active noninvasive drug delivery technique that can electrically increase the transport of charged and neutral molecules into or across biological membranes [85, 86]. To overcome the SC that has an extremely high electrical resistance (10 M-Ohm) and forms a significant barrier for iontophoresis-based drug delivery methods [87], iontophoresis uses a weak electrical current to transiently disrupt and increase the permeability of the SC [88–90]. The drug release rate can be programmed and controlled by changing the magnitude of the applied electrical current.
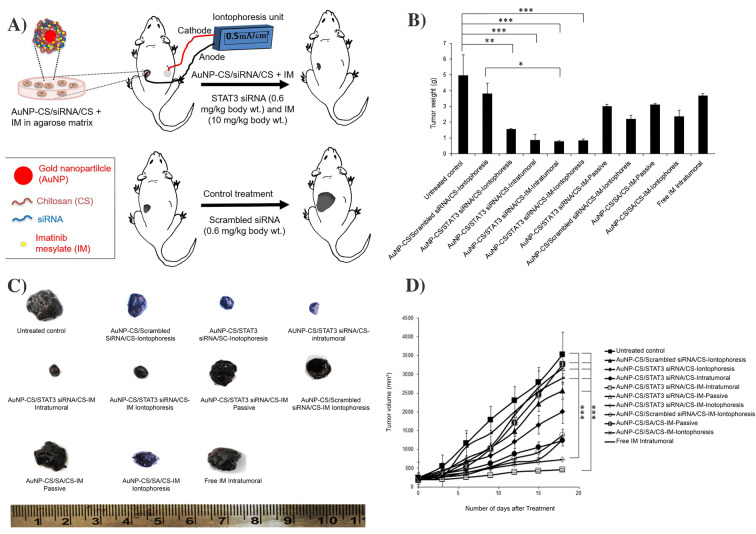
Recent advances have demonstrated that iontophoresis-based therapies could be cost-effective for TD delivery. As of right now, iontophoresis method is commercially available for fast-acting dermal anesthesia (LidoSiteTM, Vyteris Inc.) [91], anti-migraine drugs (ZecuityTM, NuPathe Inc.) [87], and postoperative pain relief (IonsysTM, Alza) [92]. Applications of iontophoresis are varied, but they have been growing popular among certain cancer therapies such as retinoblastoma, skin, and pancreatic cancers [88]. Recent works also show that this TD technique could deliver diabetic drugs such as insulin [93, 94]. In addition, studies in iontophoresis show the delivery of dexamethasone in phase 3 clinical trials [85]. For gene therapy, Labala et al. have reported iontophoresis-based TD of Au NPs containing STAT3 siRNA and Imatinib Mesylate to treat cancer (Fig. 5A). The effectiveness of this therapy was comparable with that of intratumoral administration, and in vivo study revealed a significant reduction in tumor volume/weight and suppression of STAT3 protein expression (Fig. 5B−D) [95].
Fig. 5.
Schematic of iontophoresis-based anti-STAT3 siRNA and imatinib mesylate delivery (A). STAT3 protein expression in mice after treatment (B). Quantified tumor weights after treatment with different formulations (C) and change in volume of tumor (D). The figure was
reproduced from ref. [95]. Copyright Elsevier 2017
However, some challenges need to be overcome for the clinical use of iontophoresis. Specifically, the pharmacokinetics of specific molecules delivered via this method need further in-depth investigations. Some molecules will stay in the receiving skin tissues for several days, but some drugs can travel throughout the body from this TD [96]. For example, iontophoretically applied drugs such as fentanyl and triptans systemically travel throughout the body [97]. In a pilot study, lidocaine, rapidly delivered by iontophoresis, showed similar results with an epinephrine/lidocaine cream applied to the skin for 60 min [96]. Side effects of iontophoresis are another issue and can manifest in multiple ways. One issue is electrical burns when wires, plates, or other metal components contact with the skin during operation. Another issue is the accidental overdose, which can be very harmful as some drugs have a narrow window for therapeutic effect [96].
Electroporation
Electroporation is a TD approach where high-voltage pulses are applied on the skin for a short duration to enhance the delivery of hydrophilic compounds and macromolecules into the skin (Fig. 6A) [98]. The electrophoresis works by sharply applying a threshold voltage of 75 V on the skin [99, 100] to increase the skin's permeability through aqueous pathways in the lipid bilayer membrane [101, 102]. Since the introduction of this technique in 1993, it has been demonstrated as an effective means for TD of polar molecules [103] and genetic materials, as summarized in Table 2.
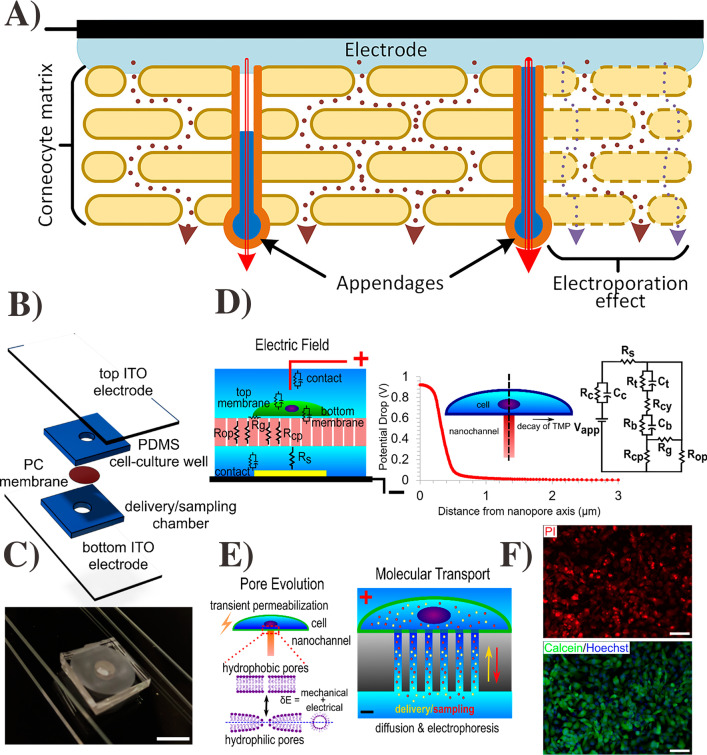
Fig. 6.
Schematic model of the skin–electrode interface, SC and its iconic pathways (A). The figure was
reproduced from ref. [102]. Copyright Plos One 2015. Schematic of electroporation device with distinct layers (B). Digital image of electroporation device sandwiched between two ITO electrodes (C). Schematic of concept of localize electroporation and asymmetric FEM stimulation of electric field along with single nano-channel underneath a cell (D). Pore evaluation model (left) and molecular transport model (right) (E). Electroporation-based delivery of PI into HT 1080 cells using a 10-V pulse (F). Delivery efficiency and viability of live and dead staining after 6-h electroporation. The figure was reproduced from ref. [111]. Copyright ACS publication 2019
Table 2.
Overview of recent gene therapy by using electroporation techniques
| Therapy |
Electroporation conditions (Voltage, pulse duration) |
Gene | Test model | Reference |
|---|---|---|---|---|
| Gene expression study | 20-50 V, 2.5–5 ms | recombinant Cas9 nuclease and synthetic dual guide RNA | Zebra fish | [114] |
| Tumor therapy | 400 V, 10 ms | pDNA encoding with interleukin -12 (IL-12) | Mouse | [115] |
| Gene knockdown study | 50 V, 25 ms | short hairpin RNA | Sea Anemone | [116] |
| Vaccination | 670 V, 10 ms | Plasmid DNA containing PRRSV antigen | Mouse | [117] |
| Vaccination | 62.5 V, 40 ms | Plasmid DNA containing C179 and S139 | Mouse | [118] |
| Vaccination |
70 V, 10 ms 50 V. 10 ms |
Cy5-Si RNA | Mouse | [119] |
| Would healing and vaccination |
700 V, 100 μs + 200 V, 400 μs 1600 V,100 μs + |
pDNA encoding with hCAP-18/LL37 and ovalbumin | Mouse | [120] |
| Would healing and tumor treatment |
570 V, 100 μs 60 V, 150 ms |
pDNA with IL-12, shRNA against endoglin, shRNA | Mouse | [121] |
| Vaccination | 1600 V, 100 μs | pDNA encoding IL-12 with collagen receptor | Mouse | [122] |
|
Vaccination Tumor treatment |
700 V, 100 μs 200 V, 400 ms 200 V, 200 ms |
pDNA encoding with ovalbumin, luciferase, gp160 against HIV, P1A against P815 mastocytoma | Mouse | [104] |
Notably, Vandermeulen et al. reported on the use of electroporation to deliver gp160[TN1] [sp2] as an HIV vaccine antigen [104] and showed different immune responses from different administration sites when performing electroporation. The muscular site induced the highest humoral immune response against the antigen. In another study, a small clinical trial conducted on 15 patients showed a good patient compliance, good safety profile, and an increased T cell activity from vaccines delivered by electroporation [105].
In electroporation, factors such as amplitude, shape, duration, size of electrode, frequency of electric pulses, spacing, and orientation can significantly influence the efficacy of the techniques [106–110]. In addition, cell membrane tension plays a vital role on the uniformity and transportation of drugs, especially the large biomolecules (Fig. 6B−F) [111]. Therefore, the application of electroporation technique requires careful optimization on all these parameters to be effective and avoid complications which can cause cell damage, muscle contraction, skin sensation, pain, and impairment of the skin barrier's function [112, 113].
Transdermal delivery of specific genetic materials
Genes or genetic materials such as DNA and RNAs are basic units of heredity. RNA is the result of the DNA transcription process, and later on, RNA is directly involved in translation to synthesize proteins. Besides the three primary forms of RNA, messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), other RNAs such as small nuclear RNA (snRNA), transfer-messenger RNA (tmRNA), ribozymes, double-stranded RNA (dsRNA), circular RNA (circRNA), and regulatory RNA also play significant roles in protein transcription [123–125]. For gene delivery, regulatory RNA or small non-protein coding RNAs such as siRNA and miRNA regulate genes through different mechanisms. These include binding to mRNA or protein targets, modifying proteins, and modulating gene expression [126]. More popular than regulatory RNAs, mRNA has become a significant genetic material nowadays because of its use for vaccines, including the ones against SARS-COVID-2 virus. In the next sections, we will provide a thorough review on the TD of different important genetic materials including mRNA, siRNA, miRNA, and DNA.
Transdermal delivery of mRNA
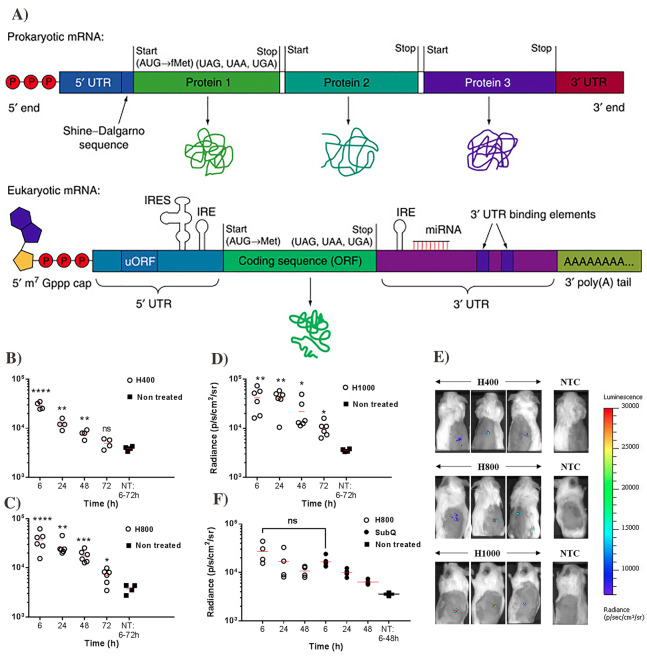
In a eukaryotic cell, a mature mRNA consists of five elements that include: 5 prime Cap (5’ Cap), coding region, poly-A tail, and the two untranslated regions, 5 prime (5’ UTR) and 3 prime (3’UTR) (Fig. 7A) [127]. 5′ Cap results from the mRNA capping process by modifying nucleotide on the 5’ end of the primary transcript on mRNA and the 5′ end with a guanine nucleotide is linked to mRNA by a 5′ to 5′ triphosphate linkage. Also, at the 7th position, guanine nucleotide will be methylated by methyltransferase, which is why the cap is known as a 7-methyl guanylate cap (m7G). Thus, the 5′ cap plays a primary role in producing, regulating mature mRNA, serving as a ribosomal recognition sign, and ensuring mRNA stability during the translation process [128].
Fig. 7.
Schematic of prokaryotic and eukaryotic mRNA (A). The figure was
adopted from Ref. [127]. Copyright Elsevier 2016. Transfection efficiency and expression of 5 μg luciferase mRNA via MNs with different heights:H400 (B), H800 (C), and H1000 (D) in the mice with different time points. Fluorescence images after 6-h transfection (E). Luciferase expression in the mice by MNs and subcutaneous injection (F). The figure was reproduced from ref. [146]. Copyright Nature publication 2018
The untranslated region (UTR) refers to the 5' UTR, which is on the 5 prime (also referred to as a leader RNA, transcript leader), or the 3' UTR which is on the three prime (also called trailer sequence). These regions usually do not contain information to synthesize proteins, but they do regulate gene expressions by influencing mRNA stability [129], translation efficiency [130, 131], or modulation of the transport of mRNAs out of the nucleus and subcellular localization [132]. The 5' UTR is upstream from the initiation coding sequence. This coding sequence is sometimes burdened with AUG codons (Start codon), and their function is to control protein expression through their complex secondary structure. However, in a particular mRNA, some regions of the 5′ untranslated regions form proteins that eventually govern the translation process of the main coding regions, especially on specific proteins such as PDGF2, FGF-2, TGF-beta, and VEGF during embryonic development [130]. 3′ UTR's prominent role is to aid in gene regulation so that the proper proteins are made at the right time. 3'UTR has binding sites for regulatory RNAs or proteins to attach that may hamper translation processes or degrade transcript.
In the translation process, mRNA serves as a mediator between DNA and protein by bringing information copied from DNA in the form of triplets of nucleotides (codons) to ribosomes and tRNA to synthesize proteins. Each codon only translates to one amino acid; however, an amino acid can result from various codons. That explains why only 20 amino acids are represented while there are 64 possible codons in the genetic code. In addition, there is one start codon and three stop codons, which signal ribosomes to either begin or terminate the translation process. In addition, mRNA also contains information for coding regions that can control the rate of translation and the timing of that translation.
mRNA has enormous potential to revolutionize the field of vaccination and protein therapy due to its ability to control over the rate and timing of the translation into various proteins and antigens. However, mRNA therapy encounters many challenges related to the nature of mRNA. First of all, mRNA with a molecular weight of 300–5000 kDa is generally larger than other oligonucleotides. Together with a highly negative charge density, this may affect the permeation of mRNA across the cell membranes [133]. Secondly, mRNA is intrinsically unstable since it is significantly prone to degradation by extracellular ribonucleases (i.e., 5’ exonucleases, 3’ exonucleases, and endonucleases), which are abundantly present in body fluid [134]. The approximate plasma half-life of mRNA is 7 h, and the reported absorption is very low [135]. Thus, a suitable delivery system and an optimal administration route could enhance the therapeutic effect of mRNA. Different formulation strategies have been developed to improve mRNA delivery via the use of molecular stabilization [136], self-amplifying mRNA [137], and viral vectors [138]. Notably, polymeric- or lipid-based particles have gained popularity with the recently implemented mRNA SARS-COV-19 vaccines [13, 139, 140].
As mRNA is inherently immunostimulatory, intradermally administered mRNA-based vaccines are considered advantageous in inducing cellular and humoral immune responses [141, 142]. Skin is an ideal site for immunogen delivery because it accommodates various antigen-presenting cells [143]. mRNA is recognized by various cell surfaces, endosomal and cytosolic innate immune receptors, which produce adjuvant effects to elicit dendritic cells maturation and robust T and B cell immune responses [144].
For TD of mRNA, MNs could be a potential tool to provide reliable targeted localization, which could be challenging to achieve using conventional hypodermic needles [145]. In this direction, Koh et al. have reported a proof-of-concept in vitro study on the fabrication, characterization, and therapeutic evaluation of a dissolvable MNs patch containing mRNA [146]. These MN-based RNA patches, made of mRNA mixed in low molecular weight polyvinylpyrrolidone (PVP) solution, could mediate transgene expression of mRNA encoding luciferase for up to 72 h with transfection efficiency and kinetics comparable to subcutaneous injection (Fig. 7B−F). Moreover, administration of mRNA in solid dosage form by using MNs helps increase mRNA stability/shelf life and avoid complications of using mRNA in a liquid dosage form [34]. Golombek et al. have evaluated the exogenous delivery of synthetic mRNA by using hollow MNs. In this work, an ex vivo porcine skin model has been used to demonstrate a high level of secreting humanized Gaussia luciferase (hGLuc) protein encoded by the mRNA via the TD [147].
Besides MNs, other TD techniques such as electroporation [148, 149] and the use of gene guns [150, 151] have been successfully implemented for transdermal mRNA therapy. Direct delivery of mRNA into the skin offers a localized treatment while avoiding unspecific uptake, rapid elimination, and clearance from the bloodstream via the filtration at liver, spleen, and kidneys [152]. Significant interest has been developed in formulating thermostable mRNA therapeutics [153, 154]. mRNA-based vaccines have emerged as promising alternatives to traditional vaccines due to their high potency, safety, rapid development, and potential for low-cost manufacturing [155]. Therefore, using TD to easily deliver mRNA-vaccines could play a significant role in the global immunization process for pandemics [156].
Transdermal delivery of siRNA
Small interfering RNA (siRNA) is double-stranded with 20–27 length of base pairs and also known as silencing RNA or short-interfering RNA. The critical function of siRNA is to silence genes by interacting with a target gene with complementary nucleotides to degrade mRNA after the transcription process, thus hampering the protein synthesis [157]. siRNA consists of a guide strand and a passenger strand, which can bind to RNA-induced silencing complex protein. After attaching to the complex protein, the guide strand starts searching for target mRNA and cuts mRNA into small pieces by using the cleavage enzyme argonaute-2 [158–162]. Since siRNAs can clear out mRNAs and prevent them from translating into proteins, these genetic materials are considered a highly potential therapeutic treatment, especially for breaking down mutated or harmful genes that cause cancers, autoimmune diseases, and genetic disorders [163].
siRNA is primarily found in eukaryotic organisms [164]. Initially, miRNA was thought to only be endogenously generated, while the siRNA was thought to be exogenously generated [164, 165]. Recently, it has been suggested that due to their similar structures and functions, siRNA and miRNA have similar origins. It appears that they are about the same size (miRNA is ~20 nucleotides while siRNA is 20–30 nucleotides) and are also negatively charged [166]. Furthermore, both are dependent on the same two protein families [164].
siRNA's degradation speed is relatively high compared to mRNA; fluorescence resonance energy transfer (FRET) analysis on the 3' and 5' ends of the siRNA indicates that it lasts about 3.5 h in cytosol [167]. As such, a careful and delicate modification process is needed for the in vivo delivery of siRNA [168]. Chemical modification can increase stability of genetic materials, but the nature of siRNA biochemical structure limits such a chemical manipulation. Therefore, it was proposed to develop precursor prodrugs for siRNA (small-interfering ribonucleic neutrals) that can be metabolized in the body to form the desired siRNA nucleotide molecules [168].
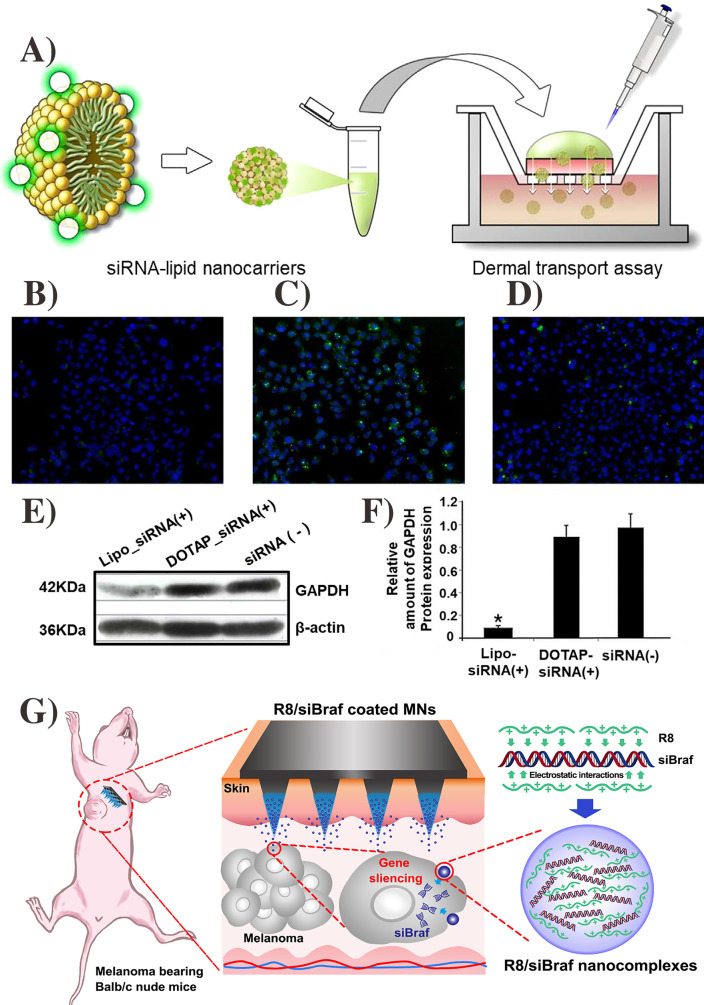
As siRNA is inherently immunostimulatory, transdermal administration of siRNA for immune therapy is advantageous for treating various cancers at different stages such as ovarian, prostate, skin, and liver cancers [169, 170]. Chemical enhancers like liposomes have been explored for transdermal siRNA delivery [171–173] as these molecules are effective for nucleotide stability [169, 174]. For example, Lee et al. reported lipid nanocarriers (liposomes) as a chemical enhancer to deliver the siRNA for skin disorder treatment (Fig. 8A). This nanosystem enhances the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at the cellular level (Fig. 8B−F) [175]. In addition, other transdermal methods such as MNs [176], electroporation [177, 178], and iontophoresis [179] can deliver siRNA to treat various diseases. For example, in the research of siRNA-based cancer therapy, Ruan et al. delivered BARF siRNA gene by using MNs coated with cell-penetrating peptide octa-arginine (R8) (Fig. 8G). This strategy effectively inhibited the tumor growth and silenced the BARF gene in vivo [180]. Overall, the advances of TD techniques like MNs could play an essential role in siRNA-based therapies.
Fig. 8.
Schematic of FTIC attached lipid nanocarriers for transdermal delivery (A). Comparison of DOTAP-NC and lipofectamine effect on GAPDH knockdown in HeLa cells after 6 h, control; only free siRNA (B), positive control; lipo-siRNA (C); DOTAP-NC-siRNA (D). GAPDH gene expression using by western blotting (E) and relative gene expression (F). The figure was
reproduced from ref. [175]. Copyright Wiley–VCH 2020. Schematic of R8/siBARF coated MNs for tumor therapy (G). The figure was reproduced from ref. [180]. Copyright Elsevier 2018
Transdermal delivery of miRNA
Micro-RNAs (miRNAs) are small noncoding, single-stranded, short RNAs with 21–25 nucleotides in length. miRNAs’ functions involve regulating gene expression by binding on the 3′ UTR of target mRNAs and causing mRNA degradation, which leads to translational suppression [181]. However, reports have proven that miRNA can also bind to other parts of mRNA such as promoters, coding region, or 5′ UTR and can trigger translation depending on the circumstances [182]. Due to their translational suppression traits, they are also found to have great therapeutic potential [183]. Their general function is to regulate gene expression in multiple ways, resulting in diverse cell proliferation, metastasis, and apoptosis [184]. They are also very water-soluble, and due to the low intestinal absorption, they are poor candidates for oral administration [184, 185]. miRNA binds to complementary portions of mRNA called the “seed” (nucleotides 2–7 of the mRNA), and during the mRNA transcription, that strand of complementary mRNA is not transcribed [185, 186]. miRNA is fairly stable but has varying half-life, which were reported from 15 min to 24 h [187].
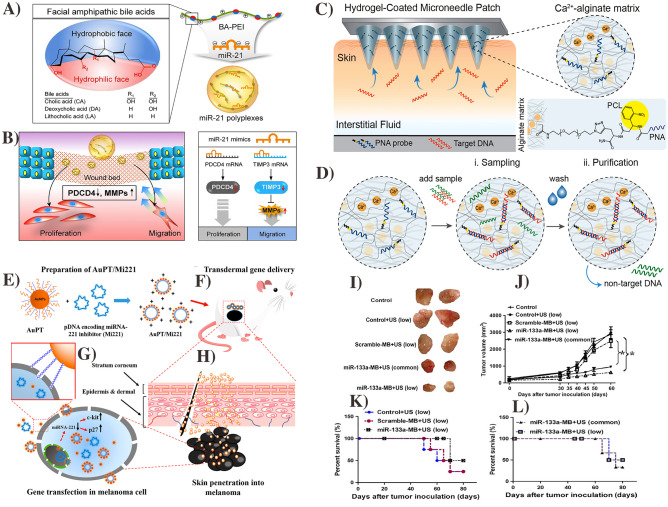
miRNA can be used to detect and even treat various cancers [188, 189] especially, skin cancer [190]. However, there are significant challenges of transdermal miRNA delivery such as off-target problems, low stability, and undesired activation of an immune response from the nucleotide. A number of methods have been employed involving the use of lipid nanoparticles (e.g., liposomes) and biodegradable polymers [184]. miRNA-21 can be used to stimulate re-epithelization for wound repairing processes, but the lack of a proper delivery system hampers its applications. To deliver the miRNA-21, Wang et al. reported a nanocarrier system containing bile acid conjugated polyethyleneimines (BA-PEI) (Fig. 9A). This system increased the rate and quality of wound healing by enhancing the re-epithelialization and collagen synthesis (Fig. 9B) [191]. miRNA could also be a biomarker for cancers and other skin disorders via transdermal routes. For example, Sulaiman et al. fabricated the alginate-peptide nucleic acid-coated MNs for sampling, isolation, and detection of miRNA-based biomarkers for early-stage detection of skin cancer (Fig. 9C, D) [192]. Niu et al. fabricated a TD system that contained an antisense sequence of miRNA-221 (which is abnormally expressed in several types of melanomas) and relies on a functional peptide conjugated cationic Au NPs as a chemical enhancer to treat the skin cancer (Fig. 9E−H). This approach produced effective transfection in the cells and could be a promising tool for TD gene therapy [27]. In addition, Ji et al. reported anti-miRNA-133 (anti-miR133) delivery and its effect on breast tumor reduction by using a transdermal ultrasound-facilitated delivery technique. The results showed that tumor size was reduced after miRNA therapy with no cytotoxic effect on cells or other body organs (Fig. 9I−L) [193].
Fig. 9.
Transdermal miRNA-based gene therapy. Schematic of miRNA-21 mimic BA-PEI nano-carrier for wound healing (A) and effect of miRNA-21 in wound healing (B). The figure was reproduced from [191].
Copyright Theranostic publication. MNs functionalized with be spoke peptide nucleic acid probe with covalently bound to alginate hydrogel via photo-cleavable linker (C). Protocol for MN-based sampling of target biomarker and purification to remove the non-target sequence (D). The figure was reproduced from ref. [192]. Copyright ACS publications 2019. Schematic of transdermal delivery of pDNA encoding miRNA-221 inhibitor gene by NPs for skin cancer treatment (E–H). The figure was reproduced from ref. [27]. Copyright ACS publication 2017. The miRNA-1331 delivered by ultrasound with low frequency suppressed the tumor growth and improved the survival rate. Tumor images after therapy (I), tumor growth during therapy (J), Survival rate with scramble-miRNA-MB (K), and survival rate with low frequency ultrasound (L). The figure was reproduced from ref. [193]. Copyright Wiley VCH 2016
Transdermal delivery of DNA
Plasmid DNA is one of the important methods for gene therapy to establish the functional protein in vivo. DNA-based gene therapy, especially immunotherapy/vaccination, has been extensively explored in various animal models [194, 195]. Furthermore, the good biocompatibility of plasmid DNA, long shelf-life, and cost-effectiveness attract researchers to develop a DNA-based therapy for various diseases [196]. DNA-based gene therapy can be delivered by using viral and non-viral systems or simply using naked DNA. However, due to the DNA sensitivity toward serum nuclease, naked DNA is not suitable for systemic administration [197]. On the other hand, viral vector-based gene therapy has several advantages, such as a rapid transcription and a high transfection rate. Despite several advantages of DNA for gene therapy, the clinical applications of DNA are still limited by the low capacity to incorporate foreign DNA sequence to the cell genome, the poor target specificity, the toxic inflammatory effect, the viral wild-type mutation, and the undesired immune over-reaction [197, 198].
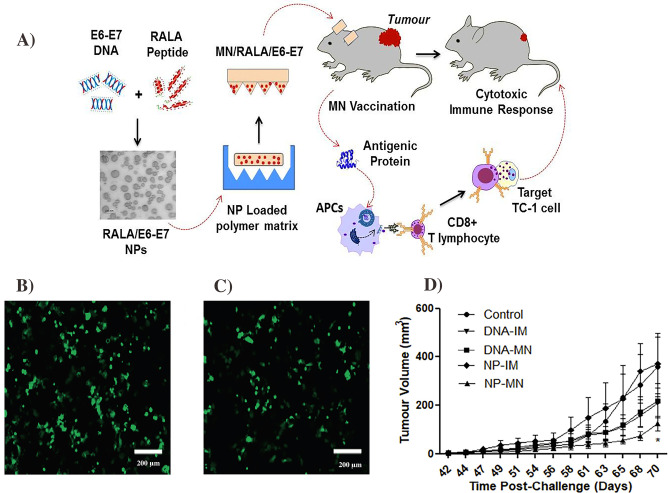
TD of DNA therapeutics has been widely investigated for various applications. The most common TD techniques are to use gene guns [199], MNs [200], electroporation [201], and chemical enhancers [202]. Ali et al. reported a DNA-based smart therapeutic transdermal system against cervical cancer (Fig. 10A). This system contained peptide-condensed DNA in NPs, which were further encapsulated into the MNs. The mice vaccinated by the DNA-NPs MNs exhibited high expressions of E6/E7-specific IgGs, more TFN-g, and T-cell medicated TC-1 cytotoxicity than the mice, which received intramuscular (IM) NPs [203]. In addition, Surer et al. were able to use steel MNs to effectively deliver the DNA and proteins into the cell (Fig. 10B, C) [204]. In a similar approach, Cole et al. employed the cationic RALA/pDNA NPs encapsulated inside dissolvable MNs to treat prostate cancer. These MNs were able to elicit tumor-specific immune response both ex vivo and in vivo (Fig. 10D) [205]. Overall, MNs and other TD techniques are attractive to promote the efficacy of DNA therapies.
Fig. 10.
Schematic of MN-based DNA delivery to prevent the cervical cancer (A). The figure was
reproduced from ref. [203]. Copy right Elsevier 2017. Fluorescence images of cell EGFP gene expression in COS-7 cells transfected with DNA release from coated MNs over 0–3 h (B-C) and 36–47 h using Lipofectamine 2000. The figure was reproduced from ref. [204]. Copyright ACS publication. Average tumor volume of control and immunized mice (D). The figure was reproduced from ref. [205]. Copyright Elsevier 2019
Conclusion
Here, we have provided an overall review on transdermal gene therapy using different delivery methods including MNs, chemical enhancers, ultrasound, electroporation, and iontophoresis to treat and prevent different diseases. Each technique for the delivery of popular genetic materials including mRNA, siRNA, miRNA, and DNA has been discussed with positive and negative aspects.
Transdermal gene delivery addresses the challenges of systemic gene delivery such as the first pass metabolism in oral delivery, the enzymatic degradation in blood circulation, and the poor target specificity of systemic administrations. Skin is a suitable site for gene therapy as it possesses a large population of lymphocytes, keratinocytes, and dendritic and T cells. Furthermore, the skin is the most abundant and accessible tissue in the human body, thus offering a significant pathway for gene delivery. Despite several advantages of transdermal gene therapies, general challenges associated with TD gene therapy include: (1) the non-effective intracellular and nuclear gene expression, (2) the instability of genes during their transportation within skin layers and the cytoplasm environment, and (3) the lack of control and achievement of desired quantitative outcomes (e.g., how many proteins to be expressed, and genes will be silenced) along with some other limiting factors like poor gene delivery by carriers, and short lifetime of genes in circulation [206–208].
Each transdermal approach for gene delivery has its own pros and cons. TD MNs can offer a controllable gene delivery with a defined quantity of genes to be delivered into the skin/body. Yet, they require an extensive manufacturing process, and stability of loaded genes/vaccines in the MNs, which could be challenging to be scaled up for industrial use. Chemical enhancers such as peptides, liposomes, and NPs can be manufactured at a large scale, but they are less effective than MNs to deliver drugs through the skin. As such, a combination of different TD techniques could be useful for gene therapy to overcome the aforementioned challenges. Indeed, the combination of ultrasound, iontophoresis, and MNs has shown a promising strategy for gene delivery [83]. In the future, researchers should perform additional investigations on the long-term safety of different transdermal techniques and further explore the mechanism/science of different transdermal gene delivery approaches as well as their combinations.
Despite such challenges, the field of transdermal gene therapy is still highly attractive due to many significant advantages. TD can help to avoid physiological barriers (such as the GI tract) to effectively deliver genetic materials into the body, trigger a significant immune response in immune therapeutic or vaccine applications, and enable a high patient compliance/adherence via a convenient, easy-to-use (also painless in many cases such as the use of MN patches) administration process. By December 2021, more than 27 gene medicines had been approved by US-FDA and regulatory agencies in many other countries. Several gene therapies are in the third stage of clinical trials [6]. Collectively, the birth and development of transdermal gene therapies could bring about significant treatments and prevention for many dangerous diseases, offering a significant impact on modern medicine.
Authors' contributions
Parbeen Singh and I’jaaz Muhammad have contributed equally.
Declarations
Consent for publication
The authors agree for submission of the review article.
Conflicts of interest/Competing interests
TDN has a conflict of interest with PiezoBioMembrane Inc. and SingleTimeMicroneedles Inc.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parbeen Singh and I’jaaz Muhammad contributed equally to the work
References
- 1.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372). 10.1126/science.aan4672. [DOI] [PubMed]
- 2.Bulaklak K, Gersbach CA. The once and future gene therapy. Nat Commun. 2020;11(1):5820. doi: 10.1038/s41467-020-19505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65(2):e26865. doi: 10.1002/pbc.26865. [DOI] [PubMed] [Google Scholar]
- 4.Wolf DP, Mitalipov PA, Mitalipov SM. Principles of and strategies for germline gene therapy. Nat Med. 2019;25(6):890–897. doi: 10.1038/s41591-019-0473-8. [DOI] [PubMed] [Google Scholar]
- 5.FDA. What is gene therapy. 2019 a.
- 6.Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502. doi: 10.1016/j.biotechadv.2019.107502. [DOI] [PubMed] [Google Scholar]
- 7.Braendstrup P, Levine BL, Ruella M. The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy. 2020;22(2):57–69. doi: 10.1016/j.jcyt.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasi KJ, Rangarajan S, Mitchell N, Lester W, Symington E, Madan B, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N Engl J Med. 2020;382(1):29–40. doi: 10.1056/NEJMoa1908490. [DOI] [PubMed] [Google Scholar]
- 9.Mendell JR, Sahenk Z, Lehman K, Nease C, Lowes LP, Miller NF, et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy: A nonrandomized controlled trial. JAMA Neurol. 2020;77(9):1122–1131. doi: 10.1001/jamaneurol.2020.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2018;378(16):1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 11.Vhora I, Lalani R, Bhatt P, Patil S, Misra A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int J Pharm. 2019;563:324–336. doi: 10.1016/j.ijpharm.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–363. doi: 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021;601:120586. doi: 10.1016/j.ijpharm.2021.120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu Abed OS. Gene therapy avenues and COVID-19 vaccines. Genes Immun. 2021;22(2):120–124. doi: 10.1038/s41435-021-00136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zylberberg C, Gaskill K, Pasley S, Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24(8):441–452. doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Zhang L, Zhu W, Guo R, Sun H, Chen X, et al. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol Ther - Methods Clin Dev. 2020;18:751–764. doi: 10.1016/j.omtm.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano. 2021 doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]
- 18.Foldvari M, Chen DW, Nafissi N, Calderon D, Narsineni L, Rafiee A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J Control Release. 2016;240:165–190. doi: 10.1016/j.jconrel.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Kretchy IA, Asiedu-Danso M, Kretchy J-P. Medication management and adherence during the COVID-19 pandemic: perspectives and experiences from low-and middle-income countries. Res Social Adm Pharm. 2021;17(1):2023–2026. doi: 10.1016/j.sapharm.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legido-Quigley H, Asgari N, Teo YY, Leung GM, Oshitani H, Fukuda K, et al. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet. 2020;395(10227):848–850. doi: 10.1016/S0140-6736(20)30551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias PM. Stratum corneum defensive functions: an integrated view. J Investig Dermatol. 2005;125(2):183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 22.Lalani R, Misra A, Amrutiya J, Patel H, Bhatt P, Patel V. Challenges in dermal delivery of therapeutic antimicrobial protein and peptides. Curr Drug Metab. 2017;18(5):426–436. doi: 10.2174/1389200218666170222151217. [DOI] [PubMed] [Google Scholar]
- 23.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Li H, Shi D, Liu Z, Yuan W. Microneedles as a delivery system for gene therapy. Front Pharmacol. 2016;7:137. doi: 10.3389/fphar.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczepanik M, Majewska-Szczepanik M. Transdermal immunotherapy: Past, present and future. Pharmacol Rep. 2016;68(4):773–781. doi: 10.1016/j.pharep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Jiang T, Xu G, Chen G, Zheng Y, He B, Gu Z. Progress in transdermal drug delivery systems for cancer therapy. Nano Res. 2020;13(7):1810–1824. doi: 10.1007/s12274-020-2664-5. [DOI] [Google Scholar]
- 27.Niu J, Chu Y, Huang YF, Chong YS, Jiang ZH, Mao ZW, et al. Transdermal gene delivery by functional peptide-conjugated cationic gold nanoparticle reverses the progression and metastasis of cutaneous melanoma. ACS Appl Mater Interfaces. 2017;9(11):9388–9401. doi: 10.1021/acsami.6b16378. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Hu D, Xu H, Patra HK, Liu X, Zhou Z, et al. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials. 2021;264:120410. doi: 10.1016/j.biomaterials.2020.120410. [DOI] [PubMed] [Google Scholar]
- 29.McCaffrey J, Donnelly RF, McCarthy HO. Microneedles: an innovative platform for gene delivery. Drug Deliv Transl Res. 2015;5(4):424–437. doi: 10.1007/s13346-015-0243-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen X. Current and future technological advances in transdermal gene delivery. Adv Drug Deliv Rev. 2018;127:85–105. doi: 10.1016/j.addr.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki R, Oda Y, Utoguchi N, Maruyama K. Progress in the development of ultrasound-mediated gene delivery systems utilizing nano- and microbubbles. J Control Release. 2011;149(1):36–41. doi: 10.1016/j.jconrel.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Mali S. Delivery systems for gene therapy. Indian J Hum Genet. 2013;19(1):3–8. doi: 10.4103/0971-6866.112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh P, Carrier A, Chen Y, Lin S, Wang J, Cui S, et al. Polymeric microneedles for controlled transdermal drug delivery. J Control Release. 2019;315:97–113. doi: 10.1016/j.jconrel.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7(3):90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32. doi: 10.1016/j.mser.2016.03.001. [DOI] [Google Scholar]
- 36.Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 37.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 38.Wan T, Pan Q, Ping Y. Microneedle-assisted genome editing: A transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci Adv. 2021;7(11):eabe2888. doi: 10.1126/sciadv.abe2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q, Li X, Zhang P, Wang Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J Mater Chem B. 2020;8(19):4331–4339. doi: 10.1039/D0TB00105H. [DOI] [PubMed] [Google Scholar]
- 40.Lara MF, González-González E, Speaker TJ, Hickerson RP, Leake D, Milstone LM, et al. Inhibition of CD44 gene expression in human skin models, using self-delivery short interfering RNA administered by dissolvable microneedle arrays. Hum Gene Ther. 2012;23(8):816–823. doi: 10.1089/hum.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Gonzalez E, Speaker TJ, Hickerson RP, Spitler R, Flores MA, Leake D, et al. Silencing of reporter gene expression in skin using siRNAs and expression of plasmid DNA delivered by a soluble protrusion array device (PAD) Mol Ther. 2010;18(9):1667–1674. doi: 10.1038/mt.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13(4):4354–4360. doi: 10.1021/acsnano.8b09573. [DOI] [PubMed] [Google Scholar]
- 43.Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, et al. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–877. doi: 10.1111/j.1365-2133.2004.05921.x. [DOI] [PubMed] [Google Scholar]
- 44.Puigmal N, Dosta P, Solhjou Z, Yatim K, Ramírez C, Choi JY, et al. Microneedle-Based Local Delivery of CCL22 and IL-2 Enriches Treg Homing to the Skin Allograft and Enables Temporal Monitoring of Immunotherapy Efficacy. Adv Funct Mater. 2021: 2100128. 10.1002/adfm.202100128.
- 45.Shakya AK, Lee CH, Gill HS. Microneedle-mediated allergen-specific immunotherapy for the treatment of airway allergy in mice. Mol Pharm. 2020;17(8):3033–3042. doi: 10.1021/acs.molpharmaceut.0c00447. [DOI] [PubMed] [Google Scholar]
- 46.Yang P, Lu C, Qin W, Chen M, Quan G, Liu H, et al. Construction of a core-shell microneedle system to achieve targeted co-delivery of checkpoint inhibitors for melanoma immunotherapy. Acta Biomater. 2020;104:147–157. doi: 10.1016/j.actbio.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 47.Duong HTT, Yin Y, Thambi T, Nguyen TL, Giang Phan VH, Lee MS, et al. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24. doi: 10.1016/j.biomaterials.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Duong HTT, Yin Y, Thambi T, Kim BS, Jeong JH, Lee DS. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J Mater Chem B. 2020;8(6):1171–1181. doi: 10.1039/C9TB02175B. [DOI] [PubMed] [Google Scholar]
- 49.Shakya AK, Lee CH, Uddin MJ, Gill HS. Assessment of Th1/Th2 Bias of sting agonists coated on microneedles for possible use in skin allergen immunotherapy. Mol Pharm. 2018;15(11):5437–5443. doi: 10.1021/acs.molpharmaceut.8b00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran KTM, Gavitt TD, Farrell NJ, Curry EJ, Mara AB, Patel A, et al. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat Biomed Eng. 2020 doi: 10.1038/s41551-020-00650-4. [DOI] [PubMed] [Google Scholar]
- 51.Kathuria H, Lim D, Cai J, Chung BG, Kang L. Microneedles with tunable dissolution rate. ACS Biomater Sci Eng. 2020;6(9):5061–5068. doi: 10.1021/acsbiomaterials.0c00759. [DOI] [PubMed] [Google Scholar]
- 52.Cemeroglu AP, Can A, Davis AT, Cemeroglu O, Kleis L, Daniel MS, et al. Fear of needles in children with type 1 diabetes mellitus on multiple daily injections and continuous subcutaneous insulin infusion 1. Endocr Pract. 2015;21(1):46–53. doi: 10.4158/EP14252.OR. [DOI] [PubMed] [Google Scholar]
- 53.Kováčik A, Kopečná M, Vávrová K. Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opin Drug Deliv. 2020;17(2):145–155. doi: 10.1080/17425247.2020.1713087. [DOI] [PubMed] [Google Scholar]
- 54.Voshavar C, Kumar Vemula P, Marepally S. Topical and transdermal delivery with chemical enhancers and nanoparticles. Imaging Technologies and Transdermal Delivery in Skin Disorders. 2019:169–200. https://onlinelibrary.wiley.com/doi/abs/10.1002/9783527814633.ch8.
- 55.Sidat Z, Marimuthu T, Kumar P, du Toit LC, Kondiah PPD, Choonara YE, et al. Ionic liquids as potential and synergistic permeation enhancers for transdermal drug delivery. Pharmaceutics. 2019;11(2). 10.3390/pharmaceutics11020096. [DOI] [PMC free article] [PubMed]
- 56.Haque T, Talukder MMU. Chemical enhancer: a simplistic way to modulate barrier function of the stratum corneum. Adv Pharm Bull. 2018;8(2):169. doi: 10.15171/apb.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teaima MH, Mohamed MAA, Abd El Rehem RT, Tayel SA, El-Nabarawi MA, Fouad SA. Enhanced transdermal delivery of bisoprolol hemifumarate via combined effect of iontophoresis and chemical enhancers: ex vivo permeation/in vivo pharmacokinetic studies. Pharmaceutics. 2021;13(5):682. doi: 10.3390/pharmaceutics13050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi QM, Duffy M, Curreri AM, Balkaran JP, Tanner EE, Mitragotri S. Comparison of ionic liquids and chemical permeation enhancers for transdermal drug delivery. Adv Func Mater. 2020;30(45):2004257. doi: 10.1002/adfm.202004257. [DOI] [Google Scholar]
- 59.Hadebe SI, Ngubane PS, Serumula MR, Musabayane CT. Transdermal delivery of insulin by amidated pectin hydrogel matrix patch in streptozotocin-induced diabetic rats: effects on some selected metabolic parameters. PLoS ONE. 2014;9(7):e101461-e. doi: 10.1371/journal.pone.0101461. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Amirian J, Zeng Y, Shekh MI, Sharma G, Stadler FJ, Song J, et al. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohyd Polym. 2021;251:117005. doi: 10.1016/j.carbpol.2020.117005. [DOI] [PubMed] [Google Scholar]
- 61.Munjeri O, Collett JH, Fell JT. Amidated pectin hydrogel beads for colonic drug delivery-an in vitro study. Drug Deliv. 1997;4(3):207–211. doi: 10.3109/10717549709051894. [DOI] [Google Scholar]
- 62.Vasyuchenko EP, Orekhov PS, Armeev GA, Bozdaganyan ME. CPE-DB: An open database of chemical penetration enhancers. Pharmaceutics. 2021;13(1):66. doi: 10.3390/pharmaceutics13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thong H-Y, Zhai H, Maibach HI. Percutaneous penetration enhancers: an overview. Skin Pharmacol Physiol. 2007;20(6):272–282. doi: 10.1159/000107575. [DOI] [PubMed] [Google Scholar]
- 64.Zheng Y, Tai W. Insight into the siRNA transmembrane delivery-From cholesterol conjugating to tagging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1606. doi: 10.1002/wnan.1606. [DOI] [PubMed] [Google Scholar]
- 65.Chen M, Zakrewsky M, Gupta V, Anselmo AC, Slee DH, Muraski JA, et al. Topical delivery of siRNA into skin using SPACE-peptide carriers. J Control Release. 2014;179:33–41. doi: 10.1016/j.jconrel.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci U S A. 2011;108(38):15816–15821. doi: 10.1073/pnas.1016152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Azagury A, Khoury L, Enden G, Kost J. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127–143. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152(3):330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crum LA, Mason TJ, Reisse JL, Suslick KS. Sonochemistry and sonoluminescence. Springer Science & Business Media; 1998.
- 70.Park D, Park H, Seo J, Lee S. Sonophoresis in transdermal drug deliverys. Ultrasonics. 2014;54(1):56–65. doi: 10.1016/j.ultras.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Daftardar S, Neupane R, Boddu SHS, Renukuntla J, Tiwari AK. Advances in ultrasound mediated transdermal drug delivery. Curr Pharm Des. 2019;25(4):413–423. doi: 10.2174/1381612825666190211163948. [DOI] [PubMed] [Google Scholar]
- 72.Chowdhury SM, Abou-Elkacem L, Lee T, Dahl J, Lutz AM. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J Control Release. 2020;326:75–90. doi: 10.1016/j.jconrel.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Mullick Chowdhury S, Lee T, Willmann JK. Ultrasound-guided drug delivery in cancer. Ultrasonography. 2017;36(3):171–184. doi: 10.14366/usg.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bickham K, Goodman K, Paludan C, Nikiforow S, Tsang ML, Steinman RM, et al. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J Exp Med. 2003;198(11):1653–1663. doi: 10.1084/jem.20030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lavon I, Kost J. Ultrasound and transdermal drug delivery. Drug Discovery Today. 2004;9(15):670–676. doi: 10.1016/S1359-6446(04)03170-8. [DOI] [PubMed] [Google Scholar]
- 76.Seto JE, Polat BE, Lopez RFV, Blankschtein D, Langer R. Effects of ultrasound and sodium lauryl sulfate on the transdermal delivery of hydrophilic permeants: Comparative in vitro studies with full-thickness and split-thickness pig and human skin. J Control Release. 2010;145(1):26–32. doi: 10.1016/j.jconrel.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mutalik S, Nayak UY, Kalra R, Kumar A, Kulkarni RV, Parekh HS. Sonophoresis-mediated permeation and retention of peptide dendrimers across human epidermis. Skin Res Technol. 2012;18(1):101–107. doi: 10.1111/j.1600-0846.2011.00539.x. [DOI] [PubMed] [Google Scholar]
- 78.Newman CM, Lawrie A, Brisken AF, Cumberland DC. Ultrasound gene therapy: On the road from concept to reality. Echocardiography. 2001;18(4):339–347. doi: 10.1046/j.1540-8175.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 79.Manikkath J, Hegde AR, Kalthur G, Parekh HS, Mutalik S. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int J Pharm. 2017;521(1–2):110–119. doi: 10.1016/j.ijpharm.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Bez M, Foiret J, Shapiro G, Pelled G, Ferrara KW, Gazit D. Nonviral ultrasound-mediated gene delivery in small and large animal models. Nat Protoc. 2019;14(4):1015–1026. doi: 10.1038/s41596-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryu YC, Kim DI, Kim SH, Wang H-MD, Hwang BH. Synergistic transdermal delivery of biomacromolecules using sonophoresis after microneedle treatment. Biotechnol Bioprocess Eng. 2018;23(3):286–292. doi: 10.1007/s12257-018-0070-6. [DOI] [Google Scholar]
- 82.Chen B, Wei J, Iliescu C. Sonophoretic enhanced microneedles array (SEMA)—Improving the efficiency of transdermal drug delivery. Sens Actuators B Chem. 2010;145(1):54–60. doi: 10.1016/j.snb.2009.11.013. [DOI] [Google Scholar]
- 83.Bok M, Zhao Z-J, Jeon S, Jeong J-H, Lim E. Ultrasonically and iontophoretically enhanced drug-delivery system based on dissolving microneedle patches. Sci Rep. 2020;10(1). 10.1038/s41598-020-58822-w. [DOI] [PMC free article] [PubMed]
- 84.Seah BC-Q, Teo BM. Recent advances in ultrasound. Int J Nanomedicine. 2018;13:7749–7763. doi: 10.2147/IJN.S174759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gratieri T, Santer V, Kalia YN. Basic principles and current status of transcorneal and transscleral iontophoresis. Expert Opin Drug Deliv. 2017;14(9):1091–1102. doi: 10.1080/17425247.2017.1266334. [DOI] [PubMed] [Google Scholar]
- 86.Kanikkannan N. Iontophoresis-based transdermal delivery systems. BioDrugs. 2002;16(5):339–347. doi: 10.2165/00063030-200216050-00003. [DOI] [PubMed] [Google Scholar]
- 87.Kusama S, Sato K, Matsui Y, Kimura N, Abe H, Yoshida S, et al. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-20948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byrne JD, Yeh JJ, DeSimone JM. Use of iontophoresis for the treatment of cancer. J Control Release. 2018;284:144–151. doi: 10.1016/j.jconrel.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 89.Gratieri T, Gelfuso GM, Lopez RFV. Basic principles and applications of iontophoresis for cutaneous penetration of drugs. Quim Nova. 2008;31(6):1490–1498. doi: 10.1590/S0100-40422008000600040. [DOI] [Google Scholar]
- 90.Baspinar Y, Borchert H-H. Penetration and release studies of positively and negatively charged nanoemulsions—is there a benefit of the positive charge? Int J Pharm. 2012;430(1–2):247–252. doi: 10.1016/j.ijpharm.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 91.Pasero C. Lidocaine iontophoresis for dermal procedure analgesia. J Perianesth Nurs. 2006;21(1):48–52. doi: 10.1016/j.jopan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 92.Poplawski S, Johnson M, Philips P, Eberhart LH, Koch T, Itri LM. Use of fentanyl iontophoretic transdermal system (ITS)(IONSYS®) in the management of patients with acute postoperative pain: A case series. Pain Ther. 2016;5(2):237–248. doi: 10.1007/s40122-016-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fonte P, Araújo F, Reis S, Sarmento B. Oral insulin delivery: how far are we? J Diabetes Sci Technol. 2013;7(2):520–531. doi: 10.1177/193229681300700228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banerjee A, Chen R, Arafin S, Mitragotri S. Intestinal iontophoresis from mucoadhesive patches: a strategy for oral delivery. J Control Release. 2019;297:71–78. doi: 10.1016/j.jconrel.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 95.Labala S, Jose A, Chawla SR, Khan MS, Bhatnagar S, Kulkarni OP, et al. Effective melanoma cancer suppression by iontophoretic co-delivery of STAT3 siRNA and imatinib using gold nanoparticles. Int J Pharm. 2017;525(2):407–417. doi: 10.1016/j.ijpharm.2017.03.087. [DOI] [PubMed] [Google Scholar]
- 96.Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol. 2014;77(1):63–71. doi: 10.1111/bcp.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chelly JE, Grass J, Houseman TW, Minkowitz H, Pue A. The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: a multicenter, placebo-controlled trial. Anesth Analg. 2004;98(2):427–433. doi: 10.1213/01.ane.0000093314.13848.7e. [DOI] [PubMed] [Google Scholar]
- 98.Charoo NA, Rahman Z, Repka MA, Murthy SN. Electroporation: an avenue for transdermal drug delivery. Curr Drug Deliv. 2010;7(2):125–136. doi: 10.2174/156720110791011765. [DOI] [PubMed] [Google Scholar]
- 99.Pliquett UF, Zewert TE, Chen T, Langer R, Weaver JC. Imaging of fluorescent molecule and small ion transport through human stratum corneum during high voltage pulsing: localized transport regions are involved. Biophys Chem. 1996;58(1):185–204. doi: 10.1016/0301-4622(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 100.Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, et al. A review on electroporation-based intracellular delivery. Molecules. 2018;23(11). 10.3390/molecules23113044. [DOI] [PMC free article] [PubMed]
- 101.Preat V, Prausnitz MR. Enhanced drug delivery using high-voltage pulses. Adv Drug Deliv Rev. 1999;35:1–2. [Google Scholar]
- 102.Vargas Luna JL, Krenn M, Cortés Ramírez JA, Mayr W. Dynamic impedance model of the skin-electrode interface for transcutaneous electrical stimulation. PLoS ONE. 2015;10(5):e0125609. doi: 10.1371/journal.pone.0125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong Z, Chang L. Recent electroporation-based systems for intracellular molecule delivery. Nanotechnol Precis Eng. 2021;4(4):045001. doi: 10.1063/10.0005649. [DOI] [Google Scholar]
- 104.Vandermeulen G, Vanvarenberg K, De Beuckelaer A, De Koker S, Lambricht L, Uyttenhove C, et al. The site of administration influences both the type and the magnitude of the immune response induced by DNA vaccine electroporation. Vaccine. 2015;33(28):3179–3185. doi: 10.1016/j.vaccine.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Eriksson F, Tötterman T, Maltais A-K, Pisa P, Yachnin J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine. 2013;31(37):3843–3848. doi: 10.1016/j.vaccine.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 106.Todorovic V, Kamensek U, Sersa G, Cemazar M. Changing electrode orientation, but not pulse polarity, increases the efficacy of gene electrotransfer to tumors in vivo. Bioelectrochemistry (Amsterdam, Netherlands) 2014;100:119–127. doi: 10.1016/j.bioelechem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Donate A, Heller R. Assessment of delivery parameters with the multi-electrode array for development of a DNA vaccine against Bacillus anthracis. Bioelectrochemistry (Amsterdam, Netherlands) 2013;94:1–6. doi: 10.1016/j.bioelechem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zorec B, Becker S, Reberšek M, Miklavčič D, Pavšelj N. Skin electroporation for transdermal drug delivery: the influence of the order of different square wave electric pulses. Int J Pharm. 2013;457(1):214–223. doi: 10.1016/j.ijpharm.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 109.Gothelf A, Mahmood F, Dagnaes-Hansen F, Gehl J. Efficacy of transgene expression in porcine skin as a function of electrode choice. Bioelectrochemistry (Amsterdam, Netherlands) 2011;82(2):95–102. doi: 10.1016/j.bioelechem.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Ferraro B, Heller LC, Cruz YL, Guo S, Donate A, Heller R. Evaluation of delivery conditions for cutaneous plasmid electrotransfer using a multielectrode array. Gene Ther. 2011;18(5):496–500. doi: 10.1038/gt.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mukherjee P, Nathamgari SSP, Kessler JA, Espinosa HD. Combined numerical and experimental investigation of localized electroporation-based cell transfection and sampling. ACS Nano. 2018;12(12):12118–12128. doi: 10.1021/acsnano.8b05473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Denet AR, Vanbever R, Préat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 113.Ita K. Perspectives on transdermal electroporation. Pharmaceutics. 2016;8(1):9. doi: 10.3390/pharmaceutics8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang C, Ren Z, Gong Z. Transgenic expression and genome editing by electroporation of zebrafish embryos. Mar Biotechnol (NY) 2020;22(5):644–650. doi: 10.1007/s10126-020-09985-0. [DOI] [PubMed] [Google Scholar]
- 115.Mukhopadhyay A, Wright J, Shirley S, Canton DA, Burkart C, Connolly RJ, et al. Characterization of abscopal effects of intratumoral electroporation-mediated IL-12 gene therapy. Gene Ther. 2019;26(1–2):1–15. doi: 10.1038/s41434-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karabulut A, He S, Chen CY, McKinney SA, Gibson MC. Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone, Nematostella vectensis. Dev Biol. 2019;448(1):7–15. doi: 10.1016/j.ydbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 117.Bernelin-Cottet C, Urien C, McCaffrey J, Collins D, Donadei A, McDaid D, et al. Electroporation of a nanoparticle-associated DNA vaccine induces higher inflammation and immunity compared to its delivery with microneedle patches in pigs. J Control Release. 2019;308:14–28. doi: 10.1016/j.jconrel.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 118.Andrews CD, Luo Y, Sun M, Yu J, Goff AJ, Glass PJ, et al. In vivo production of monoclonal antibodies by gene transfer via electroporation protects against lethal influenza and Ebola infections. Mol Ther Methods Clin Dev. 2017;7:74–82. doi: 10.1016/j.omtm.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang D, Zhao D, Wang X, Li C, Yang T, Du L, et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics. 2018;8(9):2361–2376. doi: 10.7150/thno.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kos S, Vanvarenberg K, Dolinsek T, Cemazar M, Jelenc J, Préat V, et al. Gene electrotransfer into skin using noninvasive multi-electrode array for vaccination and wound healing. Bioelectrochemistry (Amsterdam, Netherlands) 2017;114:33–41. doi: 10.1016/j.bioelechem.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 121.Kos S, Blagus T, Cemazar M, Lampreht Tratar U, Stimac M, Prosen L, et al. Electrotransfer parameters as a tool for controlled and targeted gene expression in skin. Mol Ther - Nucleic Acids. 2016;5:e356. doi: 10.1038/mtna.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kos S, Tesic N, Kamensek U, Blagus T, Cemazar M, Kranjc S, et al. Improved specificity of gene electrotransfer to skin using pDNA under the control of collagen tissue-specific promoter. J Membr Biol. 2015;248(5):919–928. doi: 10.1007/s00232-015-9799-4. [DOI] [PubMed] [Google Scholar]
- 123.Park YW, Wilusz J, Katze MG. Regulation of eukaryotic protein synthesis: Selective influenza viral mRNA translation is mediated by the cellular RNA-binding protein GRSF-1. Proc Natl Acad Sci. 1999;96(12):6694. doi: 10.1073/pnas.96.12.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qadir MI, Bukhat S, Rasul S, Manzoor H, Manzoor M. RNA therapeutics: Identification of novel targets leading to drug discovery. J Cell Biochem. 2020;121(2):898–929. doi: 10.1002/jcb.29364. [DOI] [PubMed] [Google Scholar]
- 125.Dammes N, Peer D. Paving the road for RNA therapeutics. Trends Pharmacol Sci. 2020;41(10):755–775. doi: 10.1016/j.tips.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;14(suppl_1):R121–R132. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- 127.Goss DJ, Domashevskiy AV. Messenger RNA (mRNA): The link between DNA and protein. In: Bradshaw RA, Stahl PD, editors. Encyclopedia of Cell Biology. Waltham: Academic Press; 2016. pp. 341–345. [Google Scholar]
- 128.Ibba ML, Ciccone G, Esposito CL, Catuogno S, Giangrande PH. Advances in mRNA non-viral delivery approaches. Adv Drug Deliv Rev. 2021;177:113930. doi: 10.1016/j.addr.2021.113930. [DOI] [PubMed] [Google Scholar]
- 129.Bashirullah A, Cooperstock RL, Lipshitz HD. Spatial and temporal control of RNA stability. Proc Natl Acad Sci. 2001;98(13):7025–7028. doi: 10.1073/pnas.111145698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Der Velden AW, Thomas AA. The role of the 5′ untranslated region of an mRNA in translation regulation during development. Int J Biochem Cell Biol. 1999;31(1):87–106. doi: 10.1016/S1357-2725(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 131.Kurosaki T, Popp MW, Maquat LE. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat Rev Mol Cell Biol. 2019;20(7):406–420. doi: 10.1038/s41580-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jansen R-P. mRNA localization: message on the move. Nat Rev Mol Cell Biol. 2001;2(4):247–256. doi: 10.1038/35067016. [DOI] [PubMed] [Google Scholar]
- 133.Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;19(5):281. doi: 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- 134.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136(4):763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 135.Sharova LV, Sharov AA, Nedorezov T, Piao Y, Shaik N, Ko MS. Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res. 2009;16(1):45–58. doi: 10.1093/dnares/dsn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jia L, Mao Y, Ji Q, Dersh D, Yewdell JW, Qian S-B. Decoding mRNA translatability and stability from the 5′ UTR. Nat Struct Mol Biol. 2020;27(9):814–821. doi: 10.1038/s41594-020-0465-x. [DOI] [PubMed] [Google Scholar]
- 137.Blakney AK, Ip S, Geall AJ. An update on self-amplifying mRNA vaccine development. Vaccines. 2021;9(2):97. doi: 10.3390/vaccines9020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luthra P, Sun D, Silverman RH, He B. Activation of IFN-β expression by a viral mRNA through RNase L and MDA5. Proc Natl Acad Sci. 2011;108(5):2118–2123. doi: 10.1073/pnas.1012409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X, Zhao W, Nguyen GN, Zhang C, Zeng C, Yan J, et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci Adv. 2020;6(34):eabc2315. doi: 10.1126/sciadv.abc2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2(10):1–17. doi: 10.1038/natrevmats.2017.56. [DOI] [Google Scholar]
- 141.McNamara MA, Nair SK, Holl EK. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015. [DOI] [PMC free article] [PubMed]
- 142.Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020;65:14–20. doi: 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 143.Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534. doi: 10.3389/fimmu.2015.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen N, Xia P, Li S, Zhang T, Wang TT, Zhu J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 2017;69(5):297–304. doi: 10.1002/iub.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest. 1994;106(5):1463–1465. doi: 10.1378/chest.106.5.1463. [DOI] [PubMed] [Google Scholar]
- 146.Koh KJ, Liu Y, Lim SH, Loh XJ, Kang L, Lim CY, et al. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch) Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-30290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Golombek S, Pilz M, Steinle H, Kochba E, Levin Y, Lunter D, et al. Intradermal delivery of synthetic mRNA using hollow microneedles for efficient and rapid production of exogenous proteins in skin. Mol Ther Nucleic Acids. 2018;11:382–392. doi: 10.1016/j.omtn.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 2001;8(23):1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- 149.Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep. 2015;5(1):11315. doi: 10.1038/srep11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peking P, Koller U, Hainzl S, Kitzmueller S, Kocher T, Mayr E, et al. A gene gun-mediated nonviral RNA trans-splicing strategy for Col7a1 repair. Mol Ther-Nucleic Acids. 2016;5:e287. doi: 10.1038/mtna.2016.3. [DOI] [PubMed] [Google Scholar]
- 151.Sun X, Zeng L, Huang Y. Transcutaneous delivery of DNA/mRNA for cancer therapeutic vaccination. J Gene Med. 2019;21(7):e3089. doi: 10.1002/jgm.3089. [DOI] [PubMed] [Google Scholar]
- 152.Islam MA, Reesor EKG, Xu Y, Zope HR, Zetter BR, Shi J. Biomaterials for mRNA delivery. Biomater Sci. 2015;3(12):1519–1533. doi: 10.1039/C5BM00198F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao P, Hou X, Yan J, Du S, Xue Y, Li W, et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater. 2020;5(2):358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mai Y, Guo J, Zhao Y, Ma S, Hou Y, Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354:104143. doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 155.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discovery. 2018;17(4):261. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crommelin DJ, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tatiparti K, Sau S, Kashaw SK, Iyer AK. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials (Basel). 2017;7(4). 10.3390/nano7040077. [DOI] [PMC free article] [PubMed]
- 158.Li J, Xue S, Mao Z-W. Nanoparticle delivery systems for siRNA-based therapeutics. J Mater Chem B. 2016;4(41):6620–6639. doi: 10.1039/C6TB01462C. [DOI] [PubMed] [Google Scholar]
- 159.Sashital DG, Doudna JA. Structural insights into RNA interference. Curr Opin Struct Biol. 2010;20(1):90–97. doi: 10.1016/j.sbi.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 161.Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110(5):563–574. doi: 10.1016/S0092-8674(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 162.de Fougerolles A, Vornlocher H-P, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discovery. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13(9):819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 164.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miller AD. Delivery of RNAi therapeutics: work in progress. Expert Rev Med Devices. 2013;10(6):781–811. doi: 10.1586/17434440.2013.855471. [DOI] [PubMed] [Google Scholar]
- 166.Khvorova A, Osborn MF, Hassler MR. Taking charge of siRNA delivery. Nat Biotechnol. 2014;32(12):1197–1198. doi: 10.1038/nbt.3091. [DOI] [PubMed] [Google Scholar]
- 167.Shin S, Kwon H-M, Yoon K-S, Kim D-E, Hah SS. FRET-based probing to gain direct information on siRNA sustainability in live cells: asymmetric degradation of siRNA strands. Mol BioSyst. 2011;7(7):2110–2113. doi: 10.1039/c1mb05054k. [DOI] [PubMed] [Google Scholar]
- 168.Meade BR, Gogoi K, Hamil AS, Palm-Apergi C, Van Den Berg A, Hagopian JC, et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat Biotechnol. 2014;32(12):1256. doi: 10.1038/nbt.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13(2):48. [PMC free article] [PubMed] [Google Scholar]
- 170.Pai S, Lin Y, Macaes B, Meneshian A, Hung C-F, Wu TC. Prospects of RNA interference therapy for cancer. Gene Ther. 2006;13(6):464–477. doi: 10.1038/sj.gt.3302694. [DOI] [PubMed] [Google Scholar]
- 171.Lin C-M, Huang K, Zeng Y, Chen X-C, Wang S, Li Y. A simple, noninvasive and efficient method for transdermal delivery of siRNA. Arch Dermatol Res. 2012;304(2):139–144. doi: 10.1007/s00403-011-1181-5. [DOI] [PubMed] [Google Scholar]
- 172.Zheng D, Giljohann DA, Chen DL, Massich MD, Wang X-Q, Iordanov H, et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci. 2012;109(30):11975. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jose A, Labala S, Ninave KM, Gade SK, Venuganti VVK. Effective skin cancer treatment by topical co-delivery of curcumin and STAT3 siRNA using cationic liposomes. AAPS PharmSciTech. 2018;19(1):166–175. doi: 10.1208/s12249-017-0833-y. [DOI] [PubMed] [Google Scholar]
- 174.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee K, Min D, Choi Y, Kim J, Yoon S, Jang J, et al. Study and evaluation of the potential of lipid Nanocarriers for transdermal delivery of siRNA. Biotechnol J. 2020;15(12). 10.1002/biot.202000079. [DOI] [PubMed]
- 176.Deng Y, Chen J, Zhao Y, Yan X, Zhang L, Choy K, et al. Transdermal delivery of siRNA through microneedle array. Sci Rep. 2016;6(1):1–8. doi: 10.1038/srep21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wei Z, Zhao D, Li X, Wu M, Wang W, Huang H, et al. A laminar flow electroporation system for efficient DNA and siRNA delivery. Anal Chem. 2011;83(15):5881–5887. doi: 10.1021/ac200625b. [DOI] [PubMed] [Google Scholar]
- 178.Liu H, Huang L, Mao M, Ding J, Wu G, Fan W, et al. Viral Protein-Pseudotyped and siRNA-Electroporated Extracellular Vesicles for Cancer Immunotherapy. Adv Func Mater. 2020;30(52):2006515. doi: 10.1002/adfm.202006515. [DOI] [Google Scholar]
- 179.Labala S, Jose A, Venuganti VVK. Transcutaneous iontophoretic delivery of STAT3 siRNA using layer-by-layer chitosan coated gold nanoparticles to treat melanoma. Colloids Surf B. 2016;146:188–197. doi: 10.1016/j.colsurfb.2016.05.076. [DOI] [PubMed] [Google Scholar]
- 180.Ruan W, Zhai Y, Yu K, Wu C, Xu Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553(1–2):298–309. doi: 10.1016/j.ijpharm.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 181.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 182.Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA. 2012;3(3):311–330. doi: 10.1002/wrna.121. [DOI] [PubMed] [Google Scholar]
- 183.Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Adv Drug Deliv Rev. 2018;129:308–318. doi: 10.1016/j.addr.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 184.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther-Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ganju A, Khan S, Hafeez BB, Behrman SW, Yallapu MM, Chauhan SC, et al. miRNA nanotherapeutics for cancer. Drug Discov Today. 2017;22(2):424–432. doi: 10.1016/j.drudis.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chipman LB, Pasquinelli AE. miRNA targeting: growing beyond the seed. Trends Genet. 2019;35(3):215–222. doi: 10.1016/j.tig.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Marzi MJ, Ghini F, Cerruti B, De Pretis S, Bonetti P, Giacomelli C, et al. Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome Res. 2016;26(4):554–565. doi: 10.1101/gr.198788.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Asakura K, Kadota T, Matsuzaki J, Yoshida Y, Yamamoto Y, Nakagawa K, et al. A miRNA-based diagnostic model predicts resectable lung cancer in humans with high accuracy. Commun Biol. 2020;3(1):1–9. doi: 10.1038/s42003-020-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pottoo FH, Javed MN, Rahman JU, Abu-Izneid T, Khan FA. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin Cancer Biol: Elsevier; 2021. p. 391–8. [DOI] [PubMed]
- 190.Singh A, Gupta A, Chowdhary M, Brahmbhatt HD. Integrated analysis of miRNA‐mRNA networks reveals a strong anti‐skin cancer signature in vitiligo epidermis. Exp Dermatol. 2021. [DOI] [PubMed]
- 191.Wang SY, Kim H, Kwak G, Jo SD, Cho D, Yang Y, et al. Development of microRNA-21 mimic nanocarriers for the treatment of cutaneous wounds. Theranostics. 2020;10(7):3240–3253. doi: 10.7150/thno.39870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Al Sulaiman D, Chang JYH, Bennett NR, Topouzi H, Higgins CA, Irvine DJ, et al. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano. 2019;13(8):9620–9628. doi: 10.1021/acsnano.9b04783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ji Y, Han Z, Shao L, Zhao Y. Evaluation of in vivo antitumor effects of low-frequency ultrasound-mediated miRNA-133a microbubble delivery in breast cancer. Cancer Med. 2016;5(9):2534–2543. doi: 10.1002/cam4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 195.Zahm CD, Colluru VT, McNeel DG. DNA vaccines for prostate cancer. Pharmacol Ther. 2017;174:27–42. doi: 10.1016/j.pharmthera.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hobernik D, Bros M. DNA vaccines—how far from clinical use?. Int J Mol Sci. 2018;19(11). 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed]
- 197.Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 2004;57(1):1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 198.Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28(3):709–722. doi: 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee P-W, Peng S-F, Su C-J, Mi F-L, Chen H-L, Wei M-C, et al. The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials. 2008;29(6):742–751. doi: 10.1016/j.biomaterials.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 200.Zhi D, Yang T, Zhang T, Yang M, Zhang S, Donnelly RF. Microneedles for gene and drug delivery in skin cancer therapy. J Control Release. 2021;335:158–177. doi: 10.1016/j.jconrel.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 201.Brown DW, Bahrami AJ, Canton DA, Mukhopadhyay A, Campbell JS, Pierce RH, et al. Development of an adaptive electroporation system for intratumoral plasmid DNA delivery. Bioelectrochemistry (Amsterdam, Netherlands) 2018;122:191–198. doi: 10.1016/j.bioelechem.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 202.Li H, Liu Q, Crielaard BJ, de Vries JW, Loznik M, Meng Z, et al. Fast, efficient, and targeted liposome delivery mediated by DNA hybridization. Adv Healthcare Mater. 2019;8(14):1900389. doi: 10.1002/adhm.201900389. [DOI] [PubMed] [Google Scholar]
- 203.Ali AA, McCrudden CM, McCaffrey J, McBride JW, Cole G, Dunne NJ, et al. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed Nanotechnol Biol Med. 2017;13(3):921–932. doi: 10.1016/j.nano.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 204.Saurer EM, Flessner RM, Sullivan SP, Prausnitz MR, Lynn DM. Layer-by-layer assembly of DNA- and protein-containing films on microneedles for drug delivery to the skin. Biomacromol. 2010;11(11):3136–3143. doi: 10.1021/bm1009443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cole G, Ali AA, McErlean E, Mulholland EJ, Short A, McCrudden CM, et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019;96:480–490. doi: 10.1016/j.actbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 206.Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4(2):43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Koehler DR, Hitt MM, Hu J. Challenges and strategies for cystic fibrosis lung gene therapy. Mol Ther. 2001;4(2):84–91. doi: 10.1006/mthe.2001.0435. [DOI] [PubMed] [Google Scholar]
- 208.Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D, Chawla K, et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]