Abstract
Herpes simplex virus (HSV) reactivation from latency was investigated. Reactivation of thymidine kinase-negative HSV, which is defective for reactivation, was greatly enhanced by thymidine (TdR). The reactivation-enhancing effect of TdR was blocked by dipyridamole (DPM), a known nucleoside transport inhibitor. DPM also inhibited wild-type HSV reactivation, suggesting potential antiviral use.
In experimental models of herpes simplex virus (HSV) infection, expression of viral thymidine kinase (TK) has been shown to be important for viral latency. This was first suggested in studies of mice infected with TK-negative HSV. It was shown that in cases of acute infection, HSV replicated well in ocular tissues but not in trigeminal ganglia (TG) and that HSV reactivated poorly in ganglia during latency. Subsequently it was shown that establishment of latency was intact, i.e., latency-associated transcript (LAT) was readily detected in ganglion neurons, but reactivation was impaired (4, 5, 16). The defect of reactivation was explored in studies which showed that TK-negative HSV could in fact readily reactivate in ganglia if explant medium was supplemented with thymidine (TdR) (17). The present study extended this observation in three ways. First, it was shown that if a nucleoside transport inhibitor is added along with supplemental TdR, the effect of TdR on enhancing TK-negative HSV reactivation is blocked. This suggests the specific roles of TdR and phosphorylation by TK in the reactivation process. Second, it is shown that the nucleoside transport inhibitor also blocks wild-type HSV reactivation from latency. Lastly, supplemental TdR decreased the dipyridamole (DPM) block of wild-type HSV reactivation.
Latent infection of TG and lumbar dorsal root ganglia (DRG) was established in randomly bred CD-1 mice (Charles River Laboratories, Wilmington, Mass.) by standard methods. In brief, mice were anesthetized (methoxyflurane), and corneal inoculation (5 μl) or footpad inoculation (25 μl) was performed (17). Inoculation was performed with either TK-positive wild-type HSV type 1 (HSV-1; strain KOS, 5 × 108 PFU/ml) or with mutant TK-negative HSV-1 (dlsactk, 4 × 108 PFU/ml). The titers of the viruses were determined on Vero cells using standard methods. The KOS virus had been used previously (16, 17). It readily established latency (i.e., LAT expression) and reactivated from latency in explants with a frequency of 90 to 100%. The dlsactk mutant strain was kindly provided by D. Coen (Harvard Medical School, Boston, Mass.). It was shown to express less than 1% of parental TK activity (4, 7). LAT expression during latency in mice inoculated with dlsactk was similar to that in mice with the TK-positive KOS strain, but reactivation from latency occurred at a frequency of 0 to 10% (4, 7, 17).
After 28 to 30 days, mice were anesthetized (methoxyflurane) and exsanguinated by cardiac puncture. HSV inoculation of mice, as well as housing and eventual sacrifice, were done in accordance with institutional and federal guidelines. TG and DRG (from lumbar vertebrae 4 and 5) were removed and washed in balanced saline solution. TG were cut into five or six pieces, and DRG were bisected. Ganglion fragments were cultivated at 37°C in medium consisting of medium 199 without tryptose phosphate broth and containing 2% dialyzed calf serum (Gibco BRL, Gaithersburg, Md.). In some instances, explant medium was supplemented with TdR and/or DPM (Sigma, St. Louis, Mo.). After explantation for 5 days, ganglia were homogenized and tested for reactivated infectious HSV on Vero cell monolayers (17). Results are presented in terms of individual TG and DRG pairs from lumbar vertebrae 4 and 5 being positive or negative for HSV reactivation.
Initially we investigated TK-negative HSV to supplement prior results which had demonstrated that although the dlsactk mutant virus reactivated poorly from latently infected ganglia, reactivation was greatly enhanced by the addition of TdR to the explant medium. This was demonstrated for three different TK-negative mutants (17). In Table 1 it is shown that dlsactk reactivation did not occur when explant medium did not contain supplemental TdR but that reactivation occurred at a frequency of 100% when explant medium contained supplemental TdR (100 μM). In ganglia latently infected with TK-negative HSV, supplemental TdR may have facilitated the synthesis of TdR nucleotides by means of very low levels of viral TK that might have been present, but synthesis was more likely facilitated by means of cellular TK.
TABLE 1.
TdR-enhanced reactivation of TK-negative HSV (dlsactk) and DPM inhibition of this effect
| Supplemental TdR in medium (concn) | No. of ganglia reactivated/no. tested (% positive) in medium with DPM at indicated concn (μM)
|
||
|---|---|---|---|
| 0 | 25 | 50 | |
| No | 0/10 (0) | NDa | ND |
| Yes (100 μM) | 10/10 (100) | 10/18 (56) | 0/19 (0) |
ND, not done.
However, when the explant medium contained DPM in addition to 100 μM TdR, reactivation was inhibited (Table 1). DPM is a known nucleoside transport inhibitor (6, 11, 12). Blocking of the reactivation-enhancing effect of TdR, probably by inhibition of TdR transport into latently infected neurons, supports the existence of specific roles for TdR and phosphorylation by TK in facilitating HSV reactivation. This conclusion is supported by the observation that other nucleosides were minimally effective in enhancing reactivation of TK-negative HSV mutants (17).
With evidence that DPM blocked the effect of TdR on TK-defective HSV reactivation, we investigated the effect of DPM on reactivation of wild-type HSV in explant culture. Results with TG and DRG explanted in standard medium without supplemental TdR are shown in Table 2. Reactivation of HSV was inhibited in both tissues by DPM in a dose-dependent manner. Reactivation of HSV was inhibited in DRG somewhat more so than in TG. This was probably due to a greater HSV latency load in the latter, although it has been noted that HSV latency may otherwise differ somewhat between these tissues (13). Lastly, the effect of TdR on the DPM-mediated inhibition of wild-type HSV reactivation was evaluated. Supplemental TdR in the explant medium partially reversed the blocking effect of DPM (Table 3). Nucleoside transport in mammalian cells is mediated by multiple transport mechanisms, and some transporters are less sensitive to DPM than are others (2, 9). Excess TdR apparently circumvented the inhibition of reactivation by DPM, although specific mechanisms of inhibition remain to be determined.
TABLE 2.
DPM inhibition of wild-type HSV (strain KOS) reactivation
| Tissue tested | No. of ganglia reactivated/no. tested (% positive) in medium with DPM at indicated concn (μM)
|
||
|---|---|---|---|
| 0 | 25 | 50 | |
| DRG | 10/10 (100) | 1/12 (8) | 0/12 (0) |
| TG | 10/10 (100) | 6/12 (50) | 1/12 (8) |
TABLE 3.
Decrease of the DPM inhibitory effect on wild-type HSV (strain KOS) reactivation by TdRa
| Tissue tested | No. of ganglia reactivated/no. tested (% positive) in medium with TdR at indicated concn (μM)
|
|||
|---|---|---|---|---|
| 0 | 50 | 100 | 200 | |
| DRG | 0/10 (0) | 4/11 (36) | 6/11 (54) | 6/11 (54) |
| TG | 3/10 (30) | 6/11 (54) | 8/11 (73) | 7/11 (64) |
DPM was added to the medium at a concentration of 25 μM.
DPM has been used occasionally in antiviral studies (18), including investigations of HSV (14). In the latter study, it was not shown to be a potent antiviral. However, that study investigated the effect of DPM on HSV replication in cell culture, a situation which differs markedly from reactivation from latency. First, the molecular state of the virus differs, and second, the amount of virus present probably differs. It is suggested that DPM may be effective in blocking HSV reactivation from latency because during reactivation the HSV genome is in a particularly vulnerable state or perhaps simply because only a small amount of virus is present.
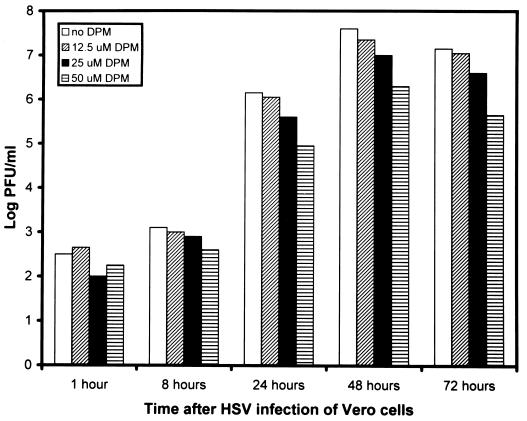
The possibility of a toxic effect of DPM on latently infected neurons as a means of explaining inhibition of HSV reactivation cannot be completely excluded. This was investigated in part in a viral growth study (Fig. 1). Only a slight antiviral effect of DPM in a dose-dependent pattern was noted, and significant cellular toxicity was unlikely. In addition, the 25 μM DPM concentration used was similar to that used in other HSV studies, where there was no apparent cellular toxicity with 20 μM DPM (14). It does remain possible that DPM is particularly toxic to neurons and that destruction of neurons led to reactivation in ganglia.
FIG. 1.
Effect of DPM on HSV replication. HSV KOS at a multiplicity of infection of 0.01 was added to confluent Vero cell monolayers in 35-mm-diameter plates. After adsorption for 1 h at 37°C, medium with or without DPM was added, and the plates were incubated at 37°C. Plates were freeze-thawed at the times indicated, and supernatant fluids were titrated on Vero cell monolayers. The results are the average of two independent studies.
However, the results in Table 3 show that reactivatable virus was present, albeit when supplemental TdR was added, indicating that at least some latently infected neurons survived DPM treatment. Lastly, DPM has been clinically used as an antiplatelet agent (6, 11, 12) and neurotoxicity has not been noted.
Although it is a nucleoside transport inhibitor, DPM has been shown to not inhibit transport into cells of nucleoside analogue antivirals such as acyclovir (8), zidovudine (1, 3), and lamivudine (3, 10). These observations and the reported DPM inhibition of transport of nucleosides such as TdR and deoxycytidine, which compete with the antivirals for kinase-mediated phosphorylation, have suggested mechanisms by which DPM may potentiate the antiviral effect of the dideoxynucleoside drugs (15).
There is widespread clinical experience with DPM as an antiplatelet agent; the mechanism of action may be inhibition of nucleoside transport, particularly of adenosine transport, into platelets (6, 11, 12). The antiviral results obtained in the present study suggest that DPM may also be clinically useful for the inhibition of HSV reactivation from latency.
Acknowledgments
This work was supported by National Institutes of Health grant NS20684 to Richard B. Tenser.
The secretarial assistance of Tracy Monette is gratefully acknowledged.
REFERENCES
- 1.Betageri G V, Szebeni J, Hung K, Patel S S, Wahl L M, Corcoran M, Weinstein J N. Effect of dipyridamole on transport and phosphorylation of thymidine and 3′-azido-3′-deoxythymidine in human monocyte/macrophages. Biochem Pharmacol. 1990;40:867–870. doi: 10.1016/0006-2952(90)90328-i. [DOI] [PubMed] [Google Scholar]
- 2.Cass C E, Young J D, Baldwin S A. Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem Cell Biol. 1998;76:761–770. doi: 10.1139/bcb-76-5-761. [DOI] [PubMed] [Google Scholar]
- 3.Chan T C, Boon G D, Shaffer L, Redmond R. Antiviral nucleoside toxicity in canine bone marrow progenitor cells and its relationship to drug permeation. Eur J Haematol. 1992;49:71–76. doi: 10.1111/j.1600-0609.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 4.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efstathiou S, Kemp S, Darby G, Minson A C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989;70:869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald G A. Dipyridamole. N Engl J Med. 1987;316:1247–1257. doi: 10.1056/NEJM198705143162005. [DOI] [PubMed] [Google Scholar]
- 7.Kosz-Vnenchak M, Jacobson J, Coen D M, Knipe D M. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J Virol. 1993;67:5383–5393. doi: 10.1128/jvi.67.9.5383-5393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahony W B, Domin B A, McConnell R T, Zimmerman T P. Acyclovir transport into human erythrocytes. J Biol Chem. 1988;263:9285–9291. [PubMed] [Google Scholar]
- 9.Pastor-Anglada M, Felipe A, Casado F J. Transport and mode of action of nucleoside derivatives used in chemical and antiviral therapies. Trends Pharmacol Sci. 1998;19:424–430. doi: 10.1016/s0165-6147(98)01253-x. [DOI] [PubMed] [Google Scholar]
- 10.Patel S S, Szebeni J, Wahl L M, Weinstein J N. Differential inhibition of 2′-deoxycytidine salvage as a possible mechanism for potentiation of the anti-human immunodeficiency virus activity of 2′,3′-dideoxycytidine by dipyridamole. Antimicrob Agents Chemother. 1991;35:1250–1253. doi: 10.1128/aac.35.6.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson A R, Lau E Y, Dahlig E, Cass C E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzothioinosine? Mol Pharmacol. 1980;18:40–44. [PubMed] [Google Scholar]
- 12.Plagemann P G W, Wohlhueter R M. Effects of nucleoside transport inhibitors on the salvage and toxicity of adenosine and deoxyadenosine in L1210 and P388 mouse leukemia. Cancer Res. 1985;45:6418–6424. [PubMed] [Google Scholar]
- 13.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snoeck R, Andrei G, Balzarini J, De Clercq E. Dipyridamole potentiates the activity of various acyclic nucleoside phosphonates against varicella-zoster virus, herpes simplex virus and human cytomegalovirus. Antivir Chem Chemother. 1994;5:312–321. [Google Scholar]
- 15.Szebeni J, Wahl S M, Popovic M, Wahl L M, Gartner S, Fine R L, Skaleric U, Friedman R M, Weinstein J N. Dipyridamole potentiates the inhibition by 3′-azido-3′ deoxythymidine and other dideoxynucleosides of human immunodeficiency virus replication in monocyte-macrophages. Proc Natl Acad Sci USA. 1989;86:3842–3846. doi: 10.1073/pnas.86.10.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenser R B, Hay K A, Edris W A. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989;63:2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenser R B, Gaydos A, Hay K A. Reactivation of thymidine kinase-defective herpes simplex virus is enhanced by nucleoside. J Virol. 1996;70:1271–1276. doi: 10.1128/jvi.70.2.1271-1276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonew E, Indulen M K, Dzeguze D R. Antiviral activity of dipyridamole and its derivatives against influenza virus A. Acta Virol. 1982;26:125–129. [PubMed] [Google Scholar]