Abstract
The endometrial scratch procedure is an IVF ‘add-on’ sometimes provided prior to the first IVF cycle. A 2019 systematic review concluded that there was insufficient evidence to show whether endometrial scratch has a significant effect on pregnancy outcomes (including live birth rate, LBR) when undertaken prior to the first IVF cycle. Further evidence was published following this review, including the Endometrial Scratch Trial (ISRCTN23800982). The objective of the current review was to synthesize and critically appraise the evidence for the clinical effectiveness and safety of the endometrial scratch procedure in women undergoing their first IVF cycle. Databases searched include MEDLINE, Embase, CINAHL and ClinicalTrials.gov. Eligible randomized controlled trials included women undergoing IVF for the first time that reported the effectiveness and/or safety of the endometrial scratch procedure; 12 studies were included. Meta-analysis showed no evidence of a significant effect of the endometrial scratch on LBR (10 trials, odds ratio [OR] 1.17, 95% confidence interval [CI] 0.76–1.79) or other pregnancy outcomes. This review confirms that there is a lack of evidence that endometrial scratch improves pregnancy outcomes, including LBR, for women undergoing their first IVF cycle. Clinicians are recommended not to perform this procedure in individuals undergoing their first cycle of IVF.
Keywords: Endometrial scratch, First cycle, Induced endometrial trauma, IVF, Live birth rate, Systematic review
Introduction
Endometrial scratch is a procedure that has been rapidly adopted into routine clinical practice at a rate that far exceeds the rate of production of good-quality evidence (Lensen et al., 2016). While the procedure was initially adopted for women suffering from recurrent implantation failure during IVF treatment, based on evidence from the initial study by Barash et al. (2003), it then rapidly spread to other populations of women and other types of treatments.
Among these groups are women undergoing their first IVF cycle, where endometrial scratch had started to be offered despite the lack of evidence (Lensen et al., 2016). Indeed, there have since been many studies, but these were mostly subject to significant confounding factors or not designed or powered to address this particular group (Vitagliano et al., 2019).
A systematic review on this topic, focusing on randomized controlled trials (RCT) and the effectiveness of the endometrial scratch procedure in women undergoing their first IVF cycle, was published by Vitagliano et al. (2019). The review included seven RCTs and concluded that there was no evidence that the endometrial scratch followed by IVF compared with IVF alone increased the success of treatment, with a relative risk/risk ratio (RR) of live birth (or ongoing pregnancy if live birth rate [LBR] was not reported) of 0.99 (95% confidence interval [CI] 0.57–1.73, P = 0.97) (Vitagliano et al., 2019). Secondary outcomes (miscarriage, multiple pregnancy and ectopic pregnancy) were also not significantly altered by undertaking the endometrial scratch. Notably, the small sample sizes of the included studies resulted in uncertainty around the effects of endometrial scratch in women undergoing their first IVF cycle, so a positive effect could not be ruled out. In addition, the trials included were at either a high or unclear risk of bias, making it difficult to draw reliable conclusions. Consequently, the authors concluded that a robust and definitive RCT is required to assess the effect of endometrial scratch on the chances of success of the first IVF cycle (Vitagliano et al., 2019).
The current authors have recently published evidence from a large definitive multicentre RCT in the UK (the Endometrial Scratch Trial) that focused only on women undergoing their first IVF cycle, with or without intracytoplasmic sperm injection (ICSI) (Metwally et al., 2021). This trial found no evidence for any significant benefit from the endometrial scratch. Given the large number of other studies in the literature, some with similar and some with conflicting findings, and given that often a meta-analysis of all published literature rather than a single RCT is important in propagating a certain research finding and implementing change in practice, the current meta-analysis was performed to synthesize the effect of endometrial scratch in increasing success rates of pregnancy outcomes in women undergoing first-time IVF treatment with or without ICSI.
Materials and methods
The review was conducted, and this manuscript written, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Page et al., 2021).
Protocol registration
The systematic review was registered with PROSPERO (CRD42018111139, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID = 111139) on 18 October 2018.
Study selection
Only RCTs examining the clinical effect or safety of endometrial scratch in women undergoing their first IVF cycle with or without ICSI, compared with treatment as usual (IVF/ICSI without the use of endometrial scratch), were eligible for inclusion. Studies that included participants undergoing intrauterine insemination (IUI) or ovulation induction (or other treatments not classed as IVF) and/or their second or subsequent IVF cycle, were excluded from this review, unless separate outcome data could be extracted for a subset of women who had undergone their first IVF cycle. All forms of endometrial scratch were included, regardless of the timing of the procedure during the cycle, but procedures defined as a mock transfer, where the aim of the procedure was not to scratch the endometrium but to test embryo transfer techniques, were excluded.
Reports published as abstracts only were excluded if insufficient methodological details were reported to allow extraction of study characteristics. Those published in languages other that English were also excluded, unless an English language abstract with sufficient methodological details existed.
Outcome measures
The following clinical and safety outcome measures were considered, which were included regardless of the definition or timing of assessments: (i) primary: LBR; (ii) secondary: implantation rate, clinical pregnancy rate, ongoing pregnancy rate; miscarriage rate; ectopic pregnancy rate; pain related to the procedure; adverse and serious adverse event rates.
Search methods for identification of studies
Data sources and search period
The following electronic databases were searched without language restrictions on 10 January 2020 (apart from ClinicalTrials.gov, which was searched on 21 September 2020): (i) MEDLINE via Ovid from 1948 to present (see Appendix in the Supplementary Material); (ii) Embase (Ovid) from 1980 to present; (iii) Cochrane Database of Systematic Reviews from 2005 to present; (iv) ClinicalTrials.gov (http://www.clinicaltrials.gov/); (v) Cumulative Index to Nursing and Allied Health Literature (CINAHL) from 1981 to present; (vi) CENTRAL via the Cochrane Register of Studies Online from 1898 to present.
Language restrictions were applied after the search was undertaken.
ClinicalTrials.gov was searched using combinations of keywords: ’endometrial biopsy’ and ‘infertility’, ‘endometrial biopsy’ and ‘subfertility’, ‘endometrial hysteroscopy’ and ‘infertility’, ‘endometrial hysteroscopy’ and ‘subfertility’.
The reference lists of all retrieved articles, relevant journals and conference proceedings were also searched by hand. In addition, authors were contacted to seek data clarification and to obtain additional information on missing data.
Selection of studies and data extraction
Titles, abstracts and full-text articles were screened independently by two reviewers (JH and LR). Any disagreements regarding eligibility were resolved through discussion with RC.
Data were extracted from the studies by one researcher (JH) and all data checked by RC. Data extracted included the outcomes, study characteristics (e.g. country where research was conducted, number of trial arms, description of trial arms, control condition(s), timing of endometrial scratch procedure in menstrual cycle, device used for endometrial scratch) and participant characteristics (e.g. average age of trial population, average duration of infertility and egg source). Where further information was needed, the authors were contacted.
Quality assessment strategy
The methodological quality of the included RCTs was assessed using the Cochrane Collaboration risk of bias assessment criteria at an outcome level (Sterne et al., 2019). The risk of bias was assessed for each reported outcome. The assessment was undertaken independently by two reviewers (either PK, RC, AP or JH). Discrepancies were resolved by a third reviewer who had not been involved in the previous assessments of that study (RC or JH). Studies were graded with an overall risk of bias of ‘high’, ‘low’ or ‘unclear’.
Data analysis
Statistical analysis was conducted according the guidelines outlined by the Cochrane Collaboration (Higgins et al., 2021). For each included RCT, summaries on the number of events and the denominator were recorded for binary outcomes and meta-analysis performed using RevMan (Review Manager [RevMan]. Version 5.3, The Cochrane Collaboration, 2014). Study-specific treatment effects as measured by odds ratios (OR) and RR were combined to produce pooled OR or RR with 95% CI, where appropriate using the Mantel–Haenszel method, which performs relatively well in several settings (Piaget–Rossel and Taffé, 2019). A random-effects model was used when between-study heterogeneity was viewed as substantial; a fixed-effects model was used when there was no evidence of significant heterogeneity. Heterogeneity between studies was assessed using chi-squared and I2 statistics (Higgins and Thompson, 2002; Higgins et al., 2003). For example, the I2 statistic quantifies the percentage of total variation in treatment effects estimates attributable to between-study heterogeneity; a value of >50% indicates evidence of significant heterogeneity of treatment effects between studies (Deeks et al., 2021). A subgroup analysis was conducted for two trials that reported ‘early’ miscarriages (prior to 12 weeks) (Izquierdo Rodriguez et al., 2020; Maged, 2018). Only one trial separately reported ‘late’ miscarriages (occurring between 12 and 24 weeks) (Izquierdo Rodriguez et al., 2020), with all other trials reporting miscarriages up to 24 weeks (which also included early miscarriages); therefore, due to the heterogeneity of this outcome, the late miscarriage subgroup was not included in the current analysis.
Where there was evidence of significant heterogeneity, a random-effects model was used in addition to an exploration of the causes of heterogeneity, followed by a sensitivity analysis where appropriate. Meta-analyses are presented in forest plots. Some outcomes (pain scores, adverse events) were narratively assessed due to a small number of studies reporting these outcomes, and/or heterogeneity in the definition of outcomes.
Results
Screening and study eligibility
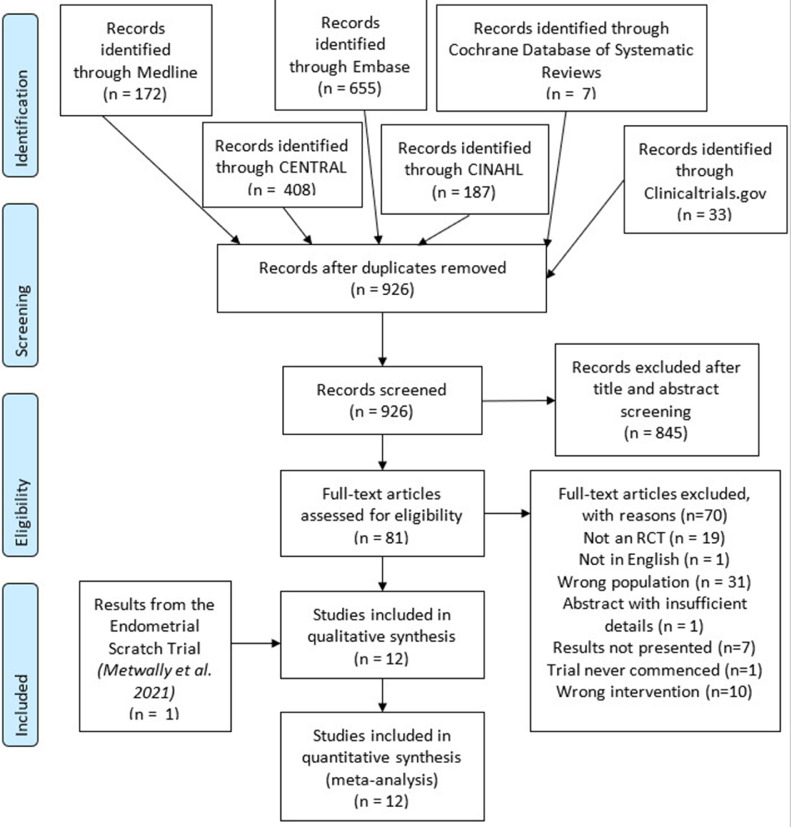
Searches identified a total of 1462 records. When needed, authors were contacted regarding missing data and to help assess eligibility. Of the 14 authors that were contacted, eight were not available. One author confirmed that the trial was not eligible for inclusion as recruitment to the trial had not begun (Checa, 2013). The authors of four trials that included participants undergoing their first IVF cycle but did not present their outcomes separately provided data and were included in the review (Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Mackens et al., 2020; Nastri et al., 2013). Polanski et al. (2014), authors of a study that included women undergoing an unselected number of previous IVF cycles, could not be contacted to obtain data for first-cycle participants only. However, unpublished data received directly from the authors in a recent systematic review, were used (Vitagliano et al., 2019). A risk of bias assessment could not be conducted for this study due to a lack of methodological details described in the abstract. After screening, 11 RCT were eligible for inclusion in the review (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014). Also included were the results of the recently conducted Endometrial Scratch Trial, published after the searches were undertaken, thus bringing the total to 12 RCTs (Metwally et al., 2021) published between 2010 and 2021. Details of the literature search and study selection can be seen in Figure 1.
Figure 1.
PRISMA flow chart. CENTRAL = Cochrane Register of Controlled Trials; CINAHL = Cumulative Index to Nursing and Allied Health Literature; RCT = randomized controlled trial.
Characteristics of included trials
Table 1 summarizes the characteristics of the 12 included RCT comprising 3382 participants undergoing their first IVF/ICSI cycle.
Table 1.
Characteristics of included trials
| Author (trial registration number) | Centre characteristics | Intervention / control | Inclusion criteria | No. of participants randomized undergoing first IVF cycle (total) | IVF/ICSI | Instrument used | Timing of ES | Outcomes |
|---|---|---|---|---|---|---|---|---|
|
Karimzade et al., 2010 (NCT00846183) |
Single centre, Iran, June 2008 to January 2009 | ES versus usual care | Age <38 years, BMI > 19 and <30 kg/m2, day 3 FSH < 12 mIU/ml Four to 14 oocytes retrieved |
156 | IVF with or without ICSI | Novak curette | During IVF Day of oocyte retrieval |
CPR, OPR, IR, MAE |
|
Yeung et al., 2014 (NCT01977976) |
Single centre, China, March 2011 to October 2013 | ES versus usual care | Excluded donor eggs | 209 | IVF with or without ICSI | Pipelle | Prior to IVF 7 days post LH surge / day 21 of cycle preceding IVF |
LBR, OPR, CPR, IR, MR, MPR, QP, MAE |
|
Mahran et al., 2016 (ISRCTN61316186) |
Multicentre (two centres), Egypt, June 2012 to September 2014 | ES versus usual care | 20–40 years, FSH ≤12 mIU/ml and two or more good-quality embryos transferred | 418 | IVF with or without ICSI | Pipelle | Prior to IVF Day 21 to 24 of cycle preceding IVF |
LBR, CPR, IR, MR, MPR, QP, MAE, EPR |
|
Maged et al., 2018 (NCT02660125) |
Single centre, Egypt, January 2016 to March 2017 | ES versus usual care | Age <40 years and FSH <10 mIU/ml | 300 | ICSI | Pipelle | Prior to IVF Luteal phase |
CPR, IR, MR, MPR, MAE |
|
Liu et al., 2017 (ChiCTR-IOR-17011506) |
Single centre, China, February 2012 to November 2014 | ES (proliferative phase) / ES (luteal phase) versus sham procedure (proliferative phase) / sham procedure (luteal phase) | Age ≤40 years, FSH <12 mIU/ml | 142 | IVF with or without ICSI | Pipelle | Prior to IVF Proliferative phase (day 10 to 12) or luteal phase (day 7 to 9) of preceding cycle |
LBR, CPR, IR, MR, EPR, MPR, QP, MAE |
|
Eskew et al., 2019 (Clinical trials registration unknown) |
Single centre, USA, September 2013 to July 2017 | ES versus usual care | Age 18–43 years | 66 | IVF | Pipelle | Prior to IVF 7–13 days post LH surge in cycle preceding IVF |
LBR, CPR, MR |
|
Lensen et al., 2019 (ACTRN12614000 626662) |
Multicentre (13 centres), New Zealand, Belgium, Sweden and UK | ES versus usual care | Age >18 years | 626 | IVF with or without ICSI | Pipelle | Prior to IVF Day 3 of the preceding cycle to day 3 of the IVF cycle |
LBR, OPR, CPR, MR, EPR, MPR, SBR, NP, MAE, NAE |
|
Mackens et al., 2020 (NCT02061228) |
Single centre, Belgium, April 2014 to October 2017 | ES versus usual care | Age ≥18 and <40 years, BMI ≤35 or ≥18 kg/m2 and excluded donor eggs | 148 | IVF with or without ICSI | Pipelle | During IVF Days 6 to 7 of ovarian stimulation |
LBR, CPR, MR, EPR, QP, MAE |
|
Izquierdo Rodriguez et al., 2020 (NCT03108157) |
Single centre, Spain, January 2017 to October 2018 | ES versus usual care | Only donor eggs | 140 | ICSI | Endometrial biopsy catheter | Prior to IVF Luteal phase: 5–10 days before the start of the period |
LBR, CPR, OPR, IR, MR, MPR, EPR, NAE |
|
Nastri et al., 2013 (NCT01132144) |
Single centre, Brazil, June 2010 to March 2012 | ES versus sham procedure | Age <38 years | 18 | IVF with or without ICSI | Pipelle | Prior to IVF 7–14 days prior to planned start of ovarian stimulation |
LBR, CPR, MR, NP, NAE |
|
Metwally et al., 2021 (ISRCTN23800982) |
Multicentre (16 sites), UK, July 2016 to October 2018 | Two arms, ES versus usual care | Age 18 to 37 years, BMI ≤35 kg/m2, FSH <10 mIU/ml | 1048 | IVF with or without ICSI | Pipelle | Prior to IVF Mid-luteal phase defined as 5–7 days before the expected next period, or 7–9 days after a positive ovulation test |
LBR, CPR, IR, SBR, NP, MAE, PTR, MR, EPR, NAE |
|
Polanski et al., 2014 (NCT01882842) |
Single centre, UK, February 2013 to June 2015 | Two arms: ES, usual care | Age <49 years | 111 | IVF with or without ICSI | Pipelle or Wallace endometrial sampler | Prior to IVF 7–9 days post LH surge in the cycle preceding IVF |
LBR, MPR, CPR |
BMI = body mass index; CPR = clinical pregnancy rate; EPR = ectopic pregnancy rate; ES = endometrial scratch; ICSI = intracytoplasmic sperm injection; IR = implantation rate; LBR = live birth rate; MAE = maternal adverse events; MBR = multiple birth rate; MPR = multiple pregnancy rate; MR = miscarriage rate; NAE = neonatal adverse events; NP = numerical pain score; OPR = ongoing pregnancy rate; PTR = preterm delivery rate; QP = qualitative pain score; SBR = stillbirth rate.
Nature of trials and geographical coverage
Only three of the 12 studies were multicentre RCTs (Lensen et al., 2019; Mahran et al., 2016; Metwally et al., 2021), with other studies involving a single centre. All 12 studies were individually randomized and were conducted across ten countries: Iran, Egypt (two studies), China (two studies), USA, Belgium, Spain, Brazil and the UK (two studies). One RCT was undertaken multinationally across five countries (Lensen et al., 2019). The total number of participants included in each trial who were undergoing their first IVF cycle ranged from 18 to 1048.
Eleven studies were two-arm RCTs (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014) and one was a four-arm RCT (Liu et al., 2017). Nine of the two-arm RCT compared the endometrial scratch procedure to usual care and two trials included a comparator involving a sham procedure (Liu et al., 2017; Nastri et al., 2013). The four-arm trial compared endometrial scratch at two different time points with a sham procedure undertaken at the same two different time points in the menstrual cycle – proliferative and luteal (Liu et al., 2017).
Recruitment to three of the trials was prematurely ended due to an unplanned futility analysis showing no differences in clinical pregnancy rates between intervention and control groups in one trial (Eskew et al., 2019), a planned interim analysis identifying higher miscarriage rates in the endometrial scratch arm in another trial (Mackens et al., 2020), and identifying a significant benefit of the endometrial scratch during a planned interim analysis (Nastri et al., 2013).
Characterization of the endometrial scratch procedure and timing
The method of undertaking endometrial scratch was largely similar across studies. Most used a Pipelle sampler to invoke injury, except for one trial that used an embryo transfer catheter (Izquierdo Rodriguez et al., 2020), one that used a Novak curette (Karimzade et al., 2010), and another that used either a Pipelle or Wallace endometrial sampler (Polanski et al., 2014). However, there was substantial variation in the timing of when endometrial scratch was performed across trials. Two trials undertook endometrial scratch during the IVF cycle, either on the day of egg collection (Karimzade et al., 2010), or during ovarian stimulation (Mackens et al., 2020). Ten trials undertook endometrial scratch in the menstrual cycle prior to IVF, with seven within the luteal phase (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Polanski et al., 2014; Yeung et al., 2014), and one during the early or mid-luteal phase (Nastri et al., 2013). Lensen et al. (2019) undertook endometrial scratch at any point between day 3 of the menstrual cycle prior to endometrial scratch and day 3 of the cycle in which IVF was being undertaken. However, this trial reported that the median time (interquartile range, IQR) between endometrial scratch and embryo transfer was 35 days (22–39), and therefore it is likely that most women received endometrial scratch in the menstrual cycle prior to IVF. Liu et al. (2017) undertook endometrial scratch either in the proliferative or luteal phases of the menstrual cycle, therefore in this review the two time points of delivery of endometrial scratch, or the sham procedure, were combined, so that, for each outcome, there was one rate for the endometrial scratch arm (both proliferative and luteal), and another for the sham arm (both proliferative and luteal). Two trials provided hysteroscopy to all trial participants, prior to IVF (Mahran et al., 2016; Yeung et al., 2014).
Participant eligibility
Trials used different participant eligibility criteria. Nine trials had age restrictions with an upper limit of between 35 and 49 years (Eskew et al., 2019; Karimzade et al., 2010; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014). Three trials restricted the body mass index (BMI) to an upper limit ranging from 30 to 35 kg/m2 (Karimzade et al., 2010; Mackens et al., 2020; Metwally et al., 2021). Five trials selected women that were deemed to have a good ovarian reserve, by allowing only those with a certain concentration of FSH to participate, with a maximum concentration of 10 IU/ml in two studies (Maged et al., 2018; Metwally et al., 2021) and 12 IU/ml in three studies (Karimzade et al., 2010; Liu et al., 2017; Mahran et al., 2016). At eligibility screening, two studies set requirements for the number of oocytes collected or embryos transferred: two or more embryos transferred in one study (Mahran et al., 2016), and four to 14 oocytes collected in another (Karimzade et al., 2010). Only one trial stipulated that the embryos transferred had to be of a certain quality, with Mahran et al. (2016) stating that the two or more embryos transferred had to be ‘good’ quality. However, the exact method of grading embryos or defining a good-quality embryo was not reported. Two trials excluded women receiving donor eggs (Mackens et al., 2020; Yeung et al., 2014), while one study only included those receiving donor eggs (Izquierdo Rodriguez et al., 2020).
Trial outcomes
Ten trials reported LBR (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014). Clinical pregnancy rates were reported in all trials. However, this was defined inconsistently, with marked variation in the time point at which this outcome was assessed: at 4 weeks post embryo transfer in two trials (Maged et al., 2018; Mahran et al., 2016); 5 weeks in one trial (Karimzade et al., 2010); 6 weeks in four trials (Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Yeung et al., 2014); 7 weeks (Mackens et al., 2020) and 8 weeks (Metwally et al., 2021); and not defined in three trials (Eskew et al., 2019; Nastri et al., 2013; Polanski et al., 2014). Ongoing pregnancy rates were reported in four trials, which were assessed at 12 weeks (Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019) and 20 weeks (Yeung et al., 2014) post embryo transfer.
Implantation rates were reported in seven studies (Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Liu et al., 2017; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Yeung et al., 2014). Six studies defined this similarly as the number of gestational sacs divided by the number of embryos transferred, while in Metwally et al. (2021) this was defined as the number of gestational sacs divided by the number of participants randomized to each arm (under intention-to-treat principles). Therefore, in order to include the current trial in this meta-analysis, this outcome was recalculated using the number of embryos transferred as the denominator. Miscarriage rates per clinical pregnancy were reported in 11 trials (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014), with the time point of data collection differing between 12 and 24 weeks of gestation, but unclear in two trials (Lensen et al., 2019; Mackens et al., 2020). A subjective assessment of pain of the endometrial scratch procedure on a numerical rating scale was reported in three trials (Lensen et al., 2019; Metwally et al., 2021; Nastri et al., 2013), with four studies providing qualitative reports of pain (Liu et al., 2017; Mackens et al., 2020; Mahran et al., 2016; Yeung et al., 2014). Eight trials reported adverse events and/or complications in the participating women (Karimzade et al., 2010; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Yeung et al., 2014), and four trials reported such events in the baby or neonate (Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Metwally et al., 2021; Nastri et al., 2013).
Risk of bias assessment
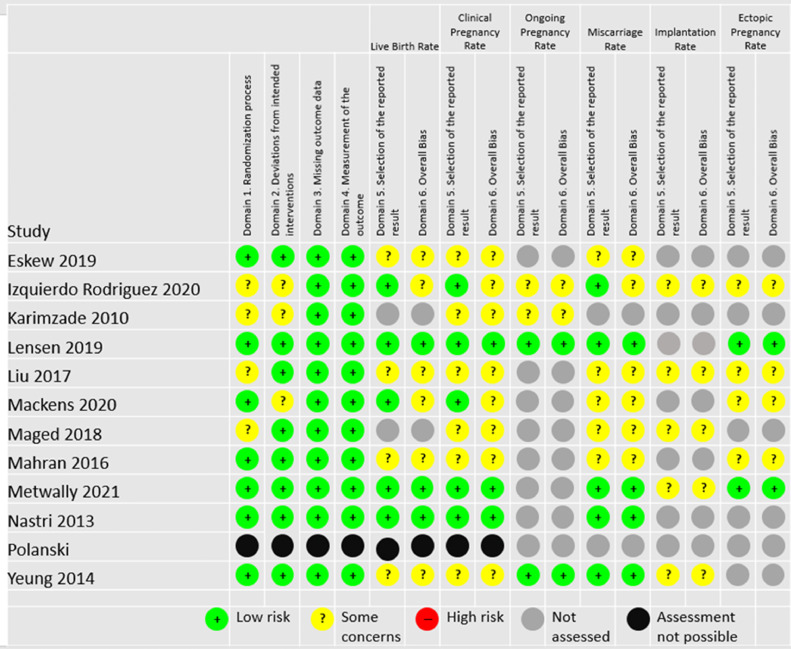
It was not possible to conduct a risk of bias assessment for Polanski et al. (2014) as the author did not respond to a request for essential missing information. The only information available was from a recent review, which used a previous version of the risk of bias tool and therefore the authors’ assessments could not be considered in this review (Polanski et al., 2014). For other included studies, Figure 2 summarizes the assessment of the risk of bias.
Figure 2.
Risk of bias assessment.
Domains 1 to 3 were consistent across all outcomes per study. Four trials were assessed as ‘some concerns’ in Domain 1 (allocation concealment) (Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Liu et al., 2017; Maged et al., 2018). Nine trials used a computerized system to undertake randomization (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019; Mackens et al., 2020; Maged et al., 2018; Metwally et al., 2021; Nastri et al., 2013; Yeung et al., 2014), one trial used sealed envelopes (Mahran et al., 2016), while another used a table of random numbers (Liu et al., 2017).
Domain 2 considered the risk of bias due to deviations from the intended interventions. Three trials were considered to have some concerns of bias for this domain (Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Mackens et al., 2020). The differing dropout rate between the endometrial scratch group (8.5%) and the control group (2.2%) in Izquierdo Rodriguez et al. (2020) resulted in this assessment. Similarly, in Karimzade et al. (2010), there were four patients excluded from the analysis in the endometrial scratch arm only.
Domain 3 considered the risk of bias due to missing outcome data. The rate of missing data was low across all trials, therefore all were considered to be at low risk of bias for this domain.
All studies were judged to be at low risk of bias for Domain 4, ‘measurement of the outcome’.
Given the nature of the outcomes being assessed, patient knowledge of the intervention was unlikely to have affected the analysis. Therefore, even though only three of the 12 included studies involved some form of blinding (Eskew et al., 2019; Liu et al., 2017; Nastri et al., 2013), this is not considered to affect the patient outcomes. There was also no blinding in most of the included trials, however it is unlikely that a participant's or clinician's knowledge of the intervention could have biased outcome assessment due to the included trials using objective outcome measures unlikely to be influenced by placebo effect.
Domain 5 assessed the risk of bias in selection of the reported result. For all outcomes, studies where the outcome was not specified prior to the start of the trial (no protocol, trials registry, or a retrospectively added trials registry), the timing of the outcome was not specified, or the outcome specification in the paper did not match the protocol/registry, were considered to have some concerns.
Only Lensen et al. (2019) and Nastri et al. (2013) were considered to have a low risk of bias across all assessments. Metwally et al. (2021) was denoted to have ‘some concerns’ for the implantation rate outcome only, as this was originally reported under intention-to-treat principles using the number of randomized women as the denominator in each arm; this was recalculated using the number of embryos transferred for the purposes of this meta-analysis (Metwally et al., 2021). All other studies had at least one outcome considered to have some bias concerns.
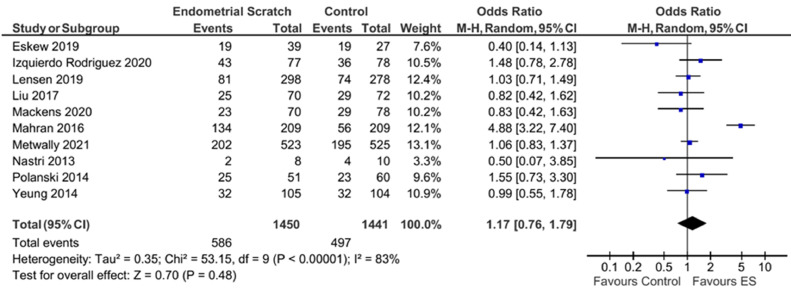
Live birth rate (LBR)
Pooled analysis of the ten trials that reported LBR showed no evidence for a significant effect for endometrial scratch on the LBR (Figure 3, OR 1.17; 95% CI 0.76–1.79; P = 0.48) (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014). There was a substantial between-study heterogeneity in treatment effects (I2 = 83%).
Figure 3.
Forest plot showing the effect of endometrial scratch on live birth rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
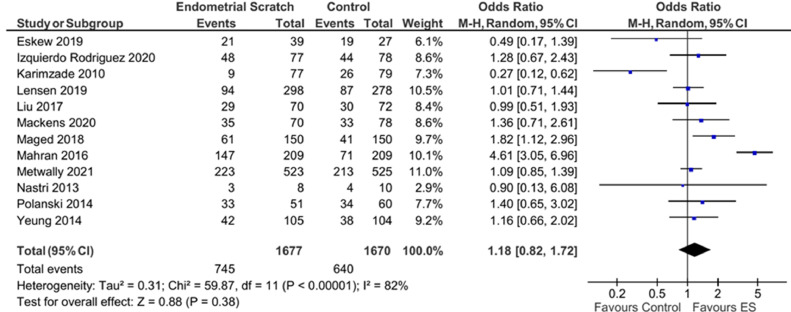
Clinical pregnancy rate (CPR)
Pooled analysis of the 12 trials that reported CPR showed no evidence for a significant effect for endometrial scratch on the CPR (Figure 4, OR 1.18; 95% CI 0.82–1.72; P = 0.38) (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Metwally et al., 2021; Nastri et al., 2013; Polanski et al., 2014; Yeung et al., 2014). Results showed evidence for significant heterogeneity between the included studies (I2 = 82%).
Figure 4.
Forest plot showing the effect of endometrial scratch on clinical pregnancy rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
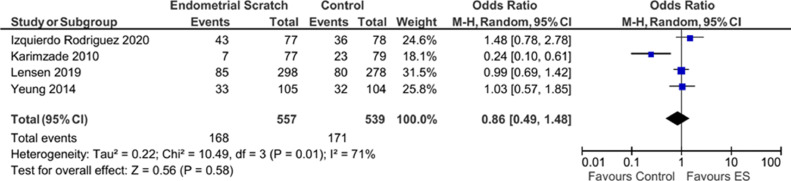
Ongoing pregnancy rate (OPR)
Pooled analysis of the four studies that reported on OPR (Izquierdo Rodriguez et al., 2020; Karimzade et al., 2010; Lensen et al., 2019; Yeung et al., 2014) showed no evidence for a significant effect for endometrial scratch on the OPR (Figure 5, OR 0.86; 95% CI 0.49–1.48; P = 0.58). Results showed evidence for significant heterogeneity between the included studies (I2 = 71%).
Figure 5.
Forest plot showing the effect of endometrial scratch on ongoing pregnancy rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
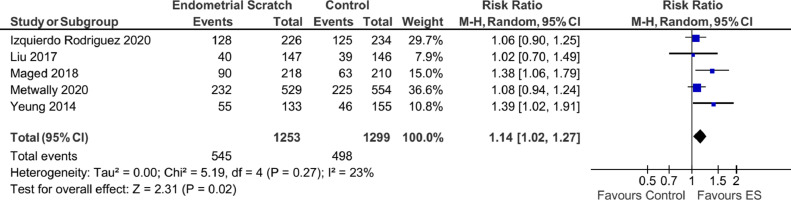
Implantation rate
Two trials (Karimzade et al., 2010; Mahran et al., 2016) where the implantation rate was only reported as a percentage without the absolute numbers were excluded from this analysis. Consequently, five trials (Izquierdo Rodriguez et al., 2020; Liu et al., 2017; Maged et al., 2018; Metwally et al., 2021; Yeung et al., 2014) were included and the overall effect showed evidence to support a significant improvement in the implantation rate attributed to the use of endometrial scratch (Figure 6, OR 1.14; 95% CI 1.02–1.27; P = 0.02), with moderate evidence of heterogeneity (I2 = 23%).
Figure 6.
Forest plot showing the effect of endometrial scratch on implantation rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
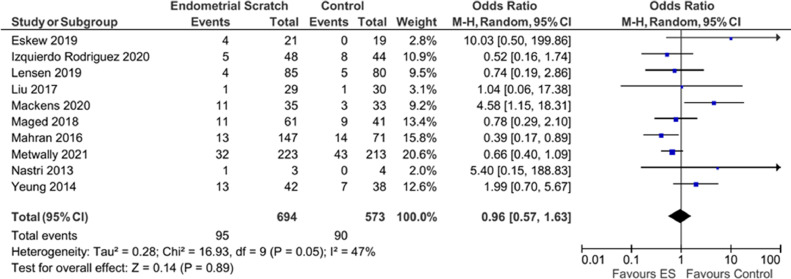
Miscarriage rate
Analysis of ten trials reporting on this outcome (Eskew et al., 2019; Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Maged et al., 2018; Mahran et al., 2016; Metwally et al., 2021; Nastri et al., 2013; Yeung et al., 2014) did not show evidence for a significant effect of endometrial scratch on reducing the miscarriage rate (Figure 7, OR 0.96; 95% CI 0.57–1.63; P = 0.89). Results showed evidence for moderately significant heterogeneity (I2 = 47%).
Figure 7.
Forest plot showing the effect of endometrial scratch on miscarriage rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
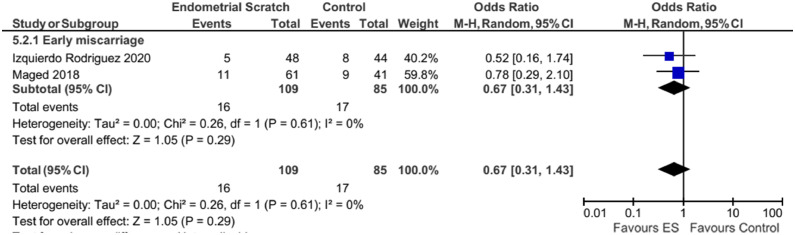
Subgroup analysis
A subgroup analysis was performed on ‘early’ miscarriages (before 12 weeks), which was reported by two studies (Izquierdo Rodriguez et al., 2020; Maged, 2018). Results remained unchanged as there was no evidence for a significant effect for endometrial scratch on the early miscarriage rate, with no evidence of significant heterogeneity between the included studies (I2 = 0%), but with significant uncertainty in this result (Figure 8, early: OR 0.67; 95% CI 0.31–1.43; P = 0.29).
Figure 8.
Forest plot showing the effect of endometrial scratch on early miscarriage rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
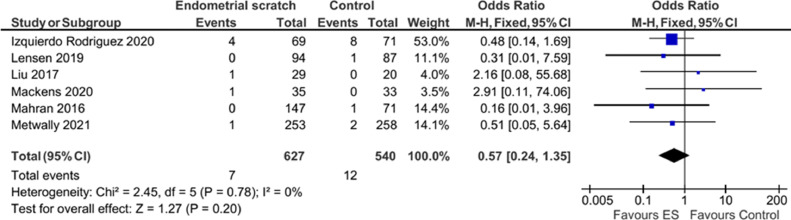
Ectopic pregnancy rate (EPR)
Pooled analysis of the six studies that reported ectopic pregnancy (Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Liu et al., 2017; Mackens et al., 2020; Mahran et al., 2016; Metwally et al., 2021), showed no evidence for a significant effect for endometrial scratch on this outcome, with high uncertainty (Figure 9, OR 0.57; 95% CI 0.24–1.35; P = 0.20). There was no evidence of significant heterogeneity between studies (I2 = 0%), but there was high uncertainty in this estimate.
Figure 9.
Forest plot showing the effect of endometrial scratch on ectopic pregnancy rate. CI = confidence interval; df = degrees of freedom; ES = endometrial scratch; M–H = Mantel–Haenszel.
Pain
It was not possible to perform a meta-analysis on the effect of endometrial scratch procedure on the pain experienced by participants as only three trials reported a quantitative outcome used to assess pain (Lensen et al., 2019; Metwally et al., 2021; Nastri et al., 2013). Two of the trials reported similar pain levels (on a rating scale of 0 to 10, mean ± SD) directly post procedure (4.1 ± 2.4) and during the procedure (4.2 ± 2.5) (Lensen et al., 2019; Metwally et al., 2021), whereas the third reported slightly higher pain levels (6.42 ± 2.35) directly after the procedure (Nastri et al., 2013). Other trials reported pain qualitatively, with four trials reporting a low proportion of participants that reported severe pain post procedure (0/150 in Yeung et al. (2014), 3/209 (1.4%) in Mahran et al. (2016), 0/70 in Liu et al. (2017), 2/70 (2.9%) in Mackens et al. (2020)).
Adverse events
Maternal
Of the eight trials that reported adverse events, seven appeared to have collected adverse events in the endometrial scratch arm only, although information was often not presented in the reports. Four trials reported that there were ‘no complications’ recorded (Karimzade et al., 2010; Liu et al., 2017; Maged et al., 2018; Yeung et al., 2014) and one trial reported ‘minimal’ spotting for a few days after endometrial scratch (number of participants not reported) (Mahran et al., 2016).
One trial reported that four participants (1.3%) experienced fainting, two (0.7%) reported excessive pain and one (0.3%) reported excessive bleeding (Lensen et al., 2019). Another trial reported that three participants (5%) experienced bleeding (Mackens et al., 2020). Metwally et al. (2021) was the only study to report safety events in both trial arms, and found comparable/similar incidences of adverse events between groups.
Neonatal
Four trials reported adverse events in the baby or neonate (Izquierdo Rodriguez et al., 2020; Lensen et al., 2019; Metwally et al., 2021; Nastri et al., 2013). The incidences of adverse events in participants undergoing the first or subsequent cycle of IVF in these trials was low, with no ‘major’ fetal malformations reported in one trial (Nastri et al., 2013), rare congenital abnormalities (endometrial scratch arm: 3 [1.6%], treatment as usual [TAU] arm: 0 [0%]), fetal growth restriction (endometrial scratch: 10 [5.2%], TAU: 8 [4.3%]) and neonatal death (endometrial scratch: 0 [0%], TAU: 1 [0.5%]) in another trial (Lensen et al., 2019) and rare placentation abnormalities (endometrial scratch: 1 [1.0%], TAU: 0 [0%]) and intrauterine growth restriction (endometrial scratch: 0 [0%], TAU: 1 [1%]) in the final trial (Izquierdo Rodriguez et al., 2020). Metwally et al. (2021) was the only trial to present accessible data separately for those babies born to participants undergoing their first IVF cycle, reporting no neonatal deaths in both arms, no severe congenital abnormalities in the endometrial scratch arm and low numbers of congenital abnormalities in both trial arms.
Sensitivity analysis
A sensitivity analysis was performed after exclusion of studies that were thought to be contributing to the heterogeneity, to explore the impact on the conclusions. Four such studies were identified for exclusion from the analyses (Karimzade et al., 2010; Mackens et al., 2020; Mahran et al., 2016; Polanski et al., 2014).
Mahran et al. (2016) appeared to be a clear outlier both statistically and methodologically. Firstly, this was the only trial to require participants to have two good-quality embryos transferred to be eligible to participate; including such a stipulation prior to randomization may have resulted in a participant population not comparable to other trials included in this review. Secondly, all participants received hysteroscopy prior to IVF, a procedure which in theory could have an effect similar to endometrial scratch, thus exposing the endometrium to two rather than one event of controlled endometrial trauma. Finally, an average of three embryos were transferred, while all other trials included in this review averaged one or two embryos transferred.
Polanski et al. (2014) included women who had undergone an unselected number of previous IVF cycles and could not be contacted to obtain data for first-cycle participants only. However, unpublished data pertaining to the participants that had undergone their first IVF cycle presented in a recent systematic review were used (Vitagliano et al., 2019). A risk of bias assessment therefore could not be conducted for this study due to a lack of methodological details described in the abstract. Mackens et al. (2020) and Karemizade et al. (2010) were the only studies to undertake endometrial scratch during the IVF cycle, while the other trials undertook endometrial scratch in the menstrual cycle prior to endometrial scratch.
In summary, exclusion of these four studies eliminated or resulted in significant reduction in heterogeneity, but conclusions on the effect of endometrial scratch on LBR (Supplementary Figure 1, OR 1.01; 95% CI 0.91–1.31), CPR (Supplementary Figure 2, OR 1.12; 95% CI 0.96–1.32), OPR (Supplementary Figure 3, OR 1.08; 95% CI 0.82–1.42, miscarriage rate (Supplementary Figure 4, OR 0.87; 95% CI 0.62–1.22) and EPR (Supplementary Figure 5, OR 0.56; 95% CI 0.23–1.42) remained consistent and unchanged.
Discussion
This systematic review and meta-analysis of RCTs in women undergoing the endometrial scratch procedure prior to their first cycle of IVF has identified that there is no evidence of a significant effect on LBR, CPR, OPR, EPR or miscarriage rate. These conclusions were consistent following sensitivity analyses excluding four studies that were contributing to significant heterogeneity. Uncertainty was high for some analyses, specifically miscarriage and ectopic pregnancy rates. A meta-analysis of implantation rates across five studies showed a significant positive effect of undertaking endometrial scratch. However, due to the sensitivity of implantation rate to the number of embryos transferred, and artificial inflation of a sample size when more than one embryo is transferred per woman, these results are unreliable and should be interpreted with extreme caution (Griesinger, 2016). Only six trials reported ectopic pregnancy rates, and for those trials that did, there were very few events reported, and surprisingly smaller trials reported more events than larger trials. This is difficult to explain, but may be attributable to trials using different definitions for this outcome. Seven trials reported pain post endometrial scratch; four of these trials reported a low proportion of participants experiencing ‘severe’ pain post endometrial scratch. Three trials reported similar moderate post endometrial scratch pain ratings. The procedure appears to be safe, with studies reporting very rare complications post endometrial scratch. None of the included trials were deemed to be at a high risk of bias.
Numerous systematic reviews have been undertaken to assess the clinical effectiveness of the endometrial scratch procedure in the first IVF cycle. However, previous reviews have been unable to conclude the effect of endometrial scratch on the first cycle of IVF, due to a lack of definitive, high-quality evidence (Nastri et al., 2012; Potdar et al., 2012; Vitagliano et al., 2019). Following the completion of the Endometrial Scratch Trial, the results of which are included in this review, it can now be concluded that endometrial scratch should not be undertaken prior to the first cycle of IVF.
The ease and simplicity of the endometrial scratch procedure has perhaps been the main reason for its rapid adoption into routine clinical practice, initially without the support of good-quality research. The main problem that has afflicted previous studies is the inclusion of different and heterogeneous populations of participants and clinical practices. This has led to heterogeneity and a lack of reliability of evidence when addressing any one specific population. The current study focuses only on the population of women undergoing their first IVF cycle, with or without ICSI.
There have been several key studies published over the last 7 years that are relevant to women undergoing first-time IVF treatment. These include the studies by Yeung et al. (2014), Lensen et al. (2019) and the most recent study from this author group (Metwally et al., 2021). The first of these studies (Yeung et al., 2014) was conducted in an unselected population of women undergoing IVF, of whom nearly 70% were having their first IVF cycle and subgroup analysis of this group (n = 209 of n = 300 individuals included in this trial) similarly found no difference in OPR between groups (Yeung et al., 2014). However, in this study a mixture of protocols was used and there were no restrictions regarding age or day of embryo transfer, with most patients receiving two embryo transfers. Similarly, the study by Lensen et al. (2019) combined a mixture of patients with different prognostic potential, with two main subgroups, the first being women with recurrent implantation failure and the second women who had had a maximum of one previous cycle. The latter group, although providing some useful reflections regarding women having endometrial scratch in the absence of recurrent implantation failure, cannot be used as a substitute for a well-designed study powered to just the initial IVF cycle. This was the aim in a recently published study (Metwally et al., 2021), which was the first large multicentre RCT that was powered only to a population of potential good responders due to undergo their first IVF cycle, and accounted for many of the sources of heterogeneity that may have affected previous studies. Results showed that in this particular population, endometrial scratch had no clinical benefit although it was tolerable and safe. However, it cannot be ignored that the ease of use of endometrial scratch combined with the promises made by results of other studies regarding the ability of this procedure to revolutionize success rates, coupled with the numerous plausible physiological hypotheses that have been put forward regarding how this procedure may act to improve implantation, present a barrier to changing the current pattern of practice. This meta-analysis, which combines the study by the current authors with all relevant previous studies, helps to conclusively settle the debate regarding the use of this procedure in the first cycle of IVF, and at the same time it identifies the main problems with the previous literature, which are highlighted in a systematic and robust way.
The main strength of this study is identifying the largest sources of bias and heterogeneity in the literature. This was particularly useful when examining outcomes that were associated with a significant level of statistical heterogeneity. Consequently, the analysis was repeated after excluding the main studies that were identified as potential sources of heterogeneity (Karimzade et al., 2010; Mackens et al., 2020; Mahran et al., 2016; Polanski et al., 2014). This approach successfully identified the studies that were causing marked heterogeneity in treatment effects across studies, as shown by the significant decrease in statistical markers of heterogeneity after exclusion of these studies. However, the sensitivity analysis did not alter the overall findings and there remained no evidence for a significant improvement in fertility outcomes with the use of endometrial scratch.
Some outcomes were difficult to assess, in particular ectopic pregnancy rates, where half of the studies reported this outcome, with smaller trials reporting more events. Pain scores were also difficult to assess; those that did report this outcome did so differently. Adverse events were often not recorded in the control arms of studies, limiting the comparisons that can be made to ‘usual’ IVF treatment. Due to time constraints, it was not possible to contact all authors to collect information to aid assessment of the risk of bias. For one trial included in the review (Polanski et al., 2014), it was not possible to obtain the full-text article or correspond with the author in order to obtain data, and therefore, data for this trial were extracted from a recent systematic review (Vitagliano et al., 2019). As a result, a risk of bias assessment for this trial could not be undertaken. Key outcomes (e.g. miscarriage rate) were variably defined across the included trials; consensus regarding the definition of key fertility outcomes should be reached in order to ensure the results of future trials can be combined within meta-analyses.
The findings of this systematic review and meta-analysis conclusively confirm that there is no evidence that induced endometrial trauma improves IVF outcomes, including live birth and pregnancy rates, for women undergoing their first IVF cycle. It is therefore recommended that the endometrial scratch procedure is not undertaken in this population of women undergoing their first IVF cycle. Despite uncertainty over the effect of endometrial scratch on miscarriage and ectopic pregnancy outcomes, it is recommended that further research is not undertaken, due to endometrial scratch not having a significant effect on LBR.
Acknowledgments
Data availability
The data supporting this systematic review and meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available upon reasonable request to the corresponding author.
Acknowledgements
We would like to thank the authors that kindly provided data on request. Thanks to Louisa Robinson for assisting with the screening of journal articles, and Pavithra Kumar and Anna Packham for helping with risk of bias assessments.
This study was funded by the National Institute for Health Research (NIHR) [Health Technology Assessment Programme (project reference 14/08/45)]. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funder did not have any role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Biography

Mostafa Metwally is a Consultant Gynaecologist in the NHS. His main areas of interest are reproductive medicine, fertility treatment and reproductive surgery. He was the Chief Investigator of the recently completed Endometrial Scratch Trial, an NIHR-funded trial to assess the effectiveness of endometrial scratch prior to first-cycle IVF.
Key message.
There is no evidence that the endometrial scratch procedure, undertaken prior to the first cycle of IVF, improves pregnancy outcomes, including LBR. Clinicians are recommended not to perform this procedure in individuals undergoing their first cycle of IVF.
Alt-text: Unlabelled box
Declaration: The authors report no financial or commercial conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.rbmo.2021.11.021.
Appendix. Supplementary materials
References
- Barash A., Dekel N., Fieldust S., Segal I., Schechtman E., Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003;79:1317–1322. doi: 10.1016/S0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Checa, M.A., 2013. Endometrial scratching in in vitro fertilization cycles with oocyte donation.https://clinicaltrials.gov/show/NCT01842178 (accessed 3.12.21).
- Deeks, J.J., Higgins J.P.T., Altman, D.G. on behalf of the Cochrane Statistical Methods Group, 2021. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. https://training.cochrane.org/handbook/current/chapter-10 (accessed 8.24.21).
- Eskew A.M., Reschke L.D., Woolfolk C., Schulte M.B., Boots C.E., Broughton D.E., Jimenez P.T., Omurtag K.R., Keller S.L., Ratts V.S., Odem R.R., Jungheim E.S. Effect of endometrial mechanical stimulation in an unselected population undergoing in vitro fertilization: futility analysis of a double-blind randomized controlled trial. J. Assist. Reprod. Genet. 2019;36:299–305. doi: 10.1007/s10815-018-1356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger G. Beware of the ‘implantation rate’! Why the outcome parameter ‘implantation rate’ should be abandoned from infertility research. Hum. Reprod. 2016 doi: 10.1093/humrep/dev322. [DOI] [PubMed] [Google Scholar]
- Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V. (eds), 2021. Cochrane Handbook for Systematic Reviews of Interventions version 6.2https://training.cochrane.org/handbook (accessed 7.5.21).
- Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003 doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo Rodriguez A., de la Fuente Bitaine L., Spies K., Lora D., Galindo A. Endometrial scratching effect on clinical pregnancy rates in patients undergoing egg donor in vitro fertilization cycles: the ENDOSCRATCH Randomized Clinical Trial ( NCT03108157) Reprod. Sci. 2020:1–10. doi: 10.1007/s43032-020-00204-8. [DOI] [PubMed] [Google Scholar]
- Karimzade M.A., Oskouian H., Ahmadi S., Oskouian L. Local injury to the endometrium on the day of oocyte retrieval has a negative impact on implantation in assisted reproductive cycles: a randomized controlled trial. Arch. Gynecol. Obstet. 2010;281:499–503. doi: 10.1007/s00404-009-1166-1. [DOI] [PubMed] [Google Scholar]
- Lensen S., Osavlyuk D., Armstrong S., Stadelmann C., Hennes A., Napier E., Wilkinson J., Sadler L., Gupta D., Strandell A., Bergh C., Vigneswaran K., Teh W.T., Hamoda H., Webber L., Wakeman S.A., Searle L., Bhide P., McDowell S., Peeraer K., Khalaf Y., Farquhar C. A randomized trial of endometrial scratching before in vitro fertilization. N. Engl. J. Med. 2019;380:325–334. doi: 10.1056/NEJMoa1808737. [DOI] [PubMed] [Google Scholar]
- Lensen S., Sadler L., Farquhar C. Endometrial scratching for subfertility: everyone's doing it. Hum. Reprod. 2016;31:1241–1244. doi: 10.1093/humrep/dew053. [DOI] [PubMed] [Google Scholar]
- Liu W., Tal R., Chao H., Liu M., Liu Y. Effect of local endometrial injury in proliferative vs. luteal phase on IVF outcomes in unselected subfertile women undergoing in vitro fertilization. Reprod. Biol. Endocrinol. 2017;15:75. doi: 10.1186/S12958-017-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackens S., Racca A., Van de Velde H., Drakopoulos P., Tournaye H., Stoop D., Blockeel C., Santos-Ribeiro S. Follicular-phase endometrial scratching: a truncated randomized controlled trial. Hum. Reprod. 2020;35:1090–1098. doi: 10.1093/humrep/deaa018. [DOI] [PubMed] [Google Scholar]
- Maged, A., 2018. Effect of endometrial injury in couples with unexplained infertility.https://clinicaltrials.gov/ct2/show/NCT03398993 (accessed 3.12.21).
- Maged A.M., Rashwan H., AbdelAziz S., Ramadan W., Mostafa W.A.I., Metwally A.A., Katta M. Randomized controlled trial of the effect of endometrial injury on implantation and clinical pregnancy rates during the first ICSI cycle. Int. J. Gynecol. Obstet. 2018;140:211–216. doi: 10.1002/ijgo.12355. [DOI] [PubMed] [Google Scholar]
- Mahran A., Ibrahim M., Bahaa H. The effect of endometrial injury on first cycle IVF/ICSI outcome: a randomized controlled trial. Int. J. Reprod. Biomed. 2016;14:193–198. [PMC free article] [PubMed] [Google Scholar]
- Metwally M., Chatters R., Dimairo M., Walters S., Pye C., White D., Bhide P., Chater T., Cheong Y., Choudhary M., Child T., Drakeley A., Evbuomwan I., Gelbaya T., Grace J., Harris P., Laird S., da Silva S.M., Mohiyiddeen L., Pemberton K., Raine-Fenning N., Rajkhowa M., Young T., Cohen J. A randomised controlled trial to assess the clinical effectiveness and safety of the endometrial scratch procedure prior to first-time IVF, with or without ICSI. Hum. Reprod. 2021;36:1841–1853. doi: 10.1093/humrep/deab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastri C.O., Ferriani R.A., Raine-Fenning N., Martins W.P. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: a randomized controlled trial. Ultrasound Obstet. Gynecol. 2013;42:375–382. doi: 10.1002/uog.12539. [DOI] [PubMed] [Google Scholar]
- Nastri C.O., Gibreel A., Raine-Fenning N., Maheshwari A., Ferriani R.A., Bhattacharya S., Martins W.P. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst. Rev. 2012;7 doi: 10.1002/14651858.CD009517.pub2. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget-Rossel R., Taffé P. Meta-analysis of rare events under the assumption of a homogeneous treatment effect. Biometrical J. 2019;61:1557–1574. doi: 10.1002/bimj.201800381. [DOI] [PubMed] [Google Scholar]
- Polanski, L., Gov, N.R.-F.-C., 2014, U., 2014. A study to assess if scratching the lining of the womb prior to IVF treatment increases the chances of pregnancy [WWW Document].
- Potdar N., Gelbaya T., Nardo L.G. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta-analysis. Reprod. Biomed. Online. 2012;25:561–571. doi: 10.1016/j.rbmo.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/BMJ.L4898. [DOI] [PubMed] [Google Scholar]
- Vitagliano A., Andrisani A., Alviggi C., Vitale S.G., Valenti G., Sapia F., Favilli A., Martins W.P., Raine-Ferring N., Polanski L., Ambrosini G. Endometrial scratching for infertile women undergoing a first embryo transfer: a systematic review and meta-analysis of published and unpublished data from randomized controlled trials. Fertil. Steril. 2019;111 doi: 10.1016/j.fertnstert.2018.12.008. 734–746.E2. [DOI] [PubMed] [Google Scholar]
- Yeung T.W.Y., Chai J., Li R.H.W., Lee V.C.Y., Ho P.C., Ng E.H.Y. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum. Reprod. 2014;29:2474–2481. doi: 10.1093/humrep/deu213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this systematic review and meta-analysis are from previously reported studies and datasets, which have been cited. The processed data are available upon reasonable request to the corresponding author.