Abstract
In order to explore the influence of different chemical demineralizations on coal combustion characteristics and combustion kinetics, five coals subjected to different chemical demineralization processes were investigated via thermogravimetric analysis. The ash contents of clean coal was reduced to 0.1–1.55% after different chemical demineralizations. The ignition temperature of coal decreased by 12–69 °C, and the peak temperature decreased by 7–62 °C. The burnout temperature of clean coal increased by 63 °C after demineralization by NaOH. The adsorption of noncombustible NaOH into the porous structure of TaiXi-3 caused an increase in burnout temperature. Alkali-soluble minerals were proven to have a negative effect on the combustion performance of coal, while acid-soluble minerals had the opposite effect. The combustion kinetics of five kinds of coals at a heating rate of 10 °C/min was investigated. The activation energy of coal obviously changes before and after demineralization (58.39–91.39 kJ mol–1). The activation energy of clean coal is obviously lower than that of raw coal.
1. Introduction
As an important fossil energy, coal has accounted for more than 30% of the primary energy for a long time. More than 80% of coal is used for power generation. China’s energy consumption is also dominated by coal, which is largely used for combustion to generate electricity.1 In some cases, many of the environmental and technical problems that may be associated with the use of coal may originate from mineral matter.2 Therefore, judging the slagging and ash accumulation behavior of coal in the whole combustion process solely on the basis of the elemental composition of coal ash is difficult.3 In recent years, the importance of understanding and predicting the behavior of minerals in coal during combustion has gradually increased.2 Hence, understanding combustion characteristics is helpful in the design and maintenance of boilers to maximize combustion efficiency and reduce the emission of carbon particles.4−6
As important components of coal, minerals have an important effect on coal combustion. Researchers have previously explored the influence of different forms of minerals on coal combustion characteristics.3 Subsequently, minerals in coal have been concluded to exert an important influence on combustion characteristics. In the UK, Spears et al. explored the role of clay minerals in coal combustion and found that clay minerals have a negative effect on the calorific value of coal and increase the cost related to ash treatment.7 Liu et al. investigated the effects of quartz, pyrite, calcite, gypsum, and kaolinite on the calorific value of coal through thermogravimetric analysis (TGA-DTA) and showed that minerals reduce the calorific values by varying degrees.8 Chen et al. investigated the effect of water-soluble sodium in Xinjiang high-sodium coal on the combustion performance. They found that water-soluble sodium is not conducive to reductions in ignition and burnout temperatures or the improvement in the combustion characteristics of high-sodium coal. However, organic sodium in coal has a positive effect on combustion.9 Minerals have a considerable influence on coal combustion characteristics. Other minerals must be removed from coal before investigating the influence of one or two minerals on coal combustion characteristics to eliminate their influence on coal combustion characteristics. Given that the physical and chemical properties of minerals in coals differ, different purification methods can retain specific minerals. This effect facilitates studying the influence of minerals on coal combustion performance.
Many factors affect coal combustion characteristics, and various indexes have been applied to evaluate coal combustion performance. Research performed in the early stages of exploring coal combustion characteristics mostly focused on the relationship between single indexes (such as volatile matter,10 calorific value,11 combustion efficiency,12 and activation energy13) and coal combustibility. In recent years, ignition temperature, maximum combustion rate, combustion characteristic index, combustion rate intensity index, and ignition index have also attracted great attention. TG-DTG, the monitoring of sample weight loss as a function of temperature, has been shown to be an effective tool for studying coal combustion behavior.4,14−17 It is the easiest and most cost-effective technology for exploring coal combustion behavior.18,19 TG-DTG analysis can be used to identify various important changes in coal combustion properties due to coal cleaning.20−23 By using this method, most workers have studied the combustion processes of coal char,24−26 biomass char,27,28 brown coals,29 soot, and wastes.30 Most of the current works have focused on the influence of added minerals on the combustion characteristics of coal. Studies on the influence of the coal sample’s own minerals on the combustion characteristics have been limited.
The aim of this work is to investigate the influence of self-contained minerals in coal on the combustion characteristics and to obtain combustion characteristic parameters via TG-DTG. The combustion behaviors of coals with different types of minerals were investigated in an air atmosphere. According to the difference in chemical properties between the studied minerals and other minerals, different impurity removal reagents were used, and other minerals were removed while the studied minerals were retained. The effects of different types of minerals on coal combustion characteristics were compared through TG-DTG. Furthermore, different combustion parameters, such as combustion performance, combustion rate intensity, ignition, and burnout indexes were calculated on the basis of thermogravimetric curves, and the combustion characteristics of coal were evaluated. The results provided insightful information on the evaluation of coal combustion.
2. Experimental Section
2.1. Materials
Anthracite coal (TaiXi) samples were provided by Ningxia Shengchuan Carbon Materials Co. Ltd., Ningxia Province, China. The TaiXi coal samples were ground and screened to select a size fraction of −74 μm by following a standard coal sampling method (No. GB474-2008). The size of −74 μm was chosen because it is used in pulverized fuel power plants.4 The differential size distributions and cumulative size distributions of the raw coal samples are shown in Figure 1. The particle size of raw coal samples was between 1 and 100 μm. The particle size distribution was relatively wide and conformed with the normal distribution. The D10, D50, and D90 values of anthracite were 9.9, 34.2 μ and 74.0 μm, respectively. Table 1 gives the ultimate analysis, sulfur content, and proximate analyses of raw coal in accordance with Chinese standards (GB/T 476-2008, GB/T 214-2008, and GB/T 212-2008). Two main uncertainty factors in the process of coal proximate analysis were analyzed to reduce the uncertainty of data: uneven sampling and weighing error. Even if the coal used in the experiment were evenly mixed, uneven sampling could still lead to fluctuations in ash, volatile, moisture, and fixed carbon contents, thus resulting in data uncertainty. The coning and quartering method is a good uniform sampling method. In proximate analysis, the coning and quartering method was adopted for sampling, and three sets of parallel tests were performed for each index to reduce the uncertainty of the results due to uneven sampling and ultimately ensure the accuracy of the results. At the same time, each sample was weighed three times, and each weighing fluctuation was less than 0.3%, thus avoiding the excessive error caused by weighing equipment. The ash of TaiXi-0 was prepared via air oxidation at 800 °C in a muffle furnace, and its chemical composition and phase composition are given in Table 2 and Figure 2, respectively. Figure 2 shows that the ash contained SiO2, Fe2O3, CaSO4, MgFe2O4, TiO2, and aluminosilicate. Table 2 and Figure 2 show that the main elements were consistent.
Figure 1.

Differential size distribution and cumulative size distribution of raw coal.
Table 1. Ultimate Analysis, Proximate Analysis, and the Heating Value of Raw Coal (TaiXi-0)a.
| ultimate
analysis (wt %) |
proximate
analysis (wt %) |
heating
value (MJ/kg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sample | C | H | Ob | N | St | FCad | Aad | Vad | Mad | HHV | LHV |
| TaiXi-0 | 86.56 | 2.77 | 9.27 | 1.13 | 0.27 | 86.24 | 3.40 | 9.32 | 1.04 | 33.08 | 33.04 |
Definitions: t, sum of all forms of sulfur; ad, air-dry basis; M, moisture; V, volatile matter; A, ash; FC, fixed carbon.
Calculated by difference.
Table 2. Chemical Composition of Ash in TaiXi-0 (wt %).
| TaiXi-0 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Si | Al | Fe | Ca | S | Mg | Na | other | |
| content | 29.48 | 20.00 | 15.58 | 11.21 | 11.02 | 4.49 | 2.70 | 2.66 | 2.86 |
Figure 2.
Phase composition of ash in raw coal.
2.2. Experimental Setup and Procedure
In this study, the effect of different types of mineral matter on the combustion characteristics of coal was investigated. HCl, NaOH, HNO3, and HF were used to remove minerals from coal to obtain clean coal containing different types of minerals. A total of 15 g of coal was mixed with 150 mL of 13.56 mol/L HF and reacted at 180 °C for 6 h in a hydrothermal reactor. Subsequently, under atmospheric pressure, the sample was reacted with 300 mL of 6.0 mol/L HCl at 60 °C for 4 h. The sample was then filtered, washed with distilled water, and postdried to obtain No. 1 clean coal (TaiXi-1). Under atmospheric pressure, 15 g of coal was mixed with 300 mL of 4.0 mol/L HNO3 and reacted at 70 °C for 4 h. The sample was filtered, washed with distilled water, and postdried to obtain No. 2 clean coal (TaiXi-2). A total of 15 g of coal was mixed with 150 mL of 7.5 mol/L NaOH and reacted in a hydrothermal reactor at 210 °C for 10 h. The sample was filtered, washed with distilled water, and postdried to obtain No. 3 clean coal (TaiXi-3). A total of 15 g of coal was mixed with 150 mL of 7.5 mol/L NaOH and reacted in a hydrothermal reactor at 210 °C for 10 h. Then, under atmospheric pressure, the sample was reacted with 225 mL of 6.0 mol/L HNO3 at 70 °C for 4 h. Subsequently, the coal was filtered, washed with distilled water, and postdried to obtain No. 4 clean coal (TaiXi-4). The raw coal was designated as No. 0 (TaiXi-0). The proximate analyses of raw coal and four kinds of purified coal (TaiXi-1–4) are given in Table 3. The ultimate composition of purified coal is shown in Table 4.
Table 3. Proximate Analysis of Raw Coal and Four Kinds of Purified Coal (TaiXi-1–4).
| proximate
analysis (wt %) |
||||||
|---|---|---|---|---|---|---|
| sample | FCad | Aad | Vad | Mad | fuel ratio (FCad/Vad) | Aad/FCad (%) |
| TaiXi-0 | 86.24 ± 0.26 | 3.40 ± 0.12 | 9.32 ± 0.08 | 1.04 ± 0.06 | 9.25 | 3.94 |
| TaiXi-1 | 83.25 ± 0.21 | 0.10 ± 0.13 | 13.20 ± 0.06 | 3.45 ± 0.02 | 6.31 | 0.12 |
| TaiXi-2 | 84.26 ± 0.12 | 1.49 ± 0.03 | 11.23 ± 0.07 | 3.02 ± 0.02 | 7.50 | 1.77 |
| TaiXi-3 | 76.01 ± 0.20 | 1.55 ± 0.12 | 18.64 ± 0.06 | 3.80 ± 0.04 | 4.08 | 2.04 |
| TaiXi-4 | 84.10 ± 0.18 | 0.45 ± 0.09 | 12.33 ± 0.06 | 3.12 ± 0.03 | 6.82 | 0.54 |
Table 4. Ultimate Composition of Purified Coal (TaiXi-1–4).
| ultimate analysis (wt %) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sample | C | O | Al | Fe | Ca | S | Si | H | N | Na | other |
| TaiXi-1 | 83.18 | 11.24 | 0.02 | 0.02 | 0.03 | 0.38 | 0.01 | 3.96 | 1.16 | <0.01 | <0.01 |
| TaiXi-2 | 84.28 | 8.69 | 0.30 | <0.01 | <0.01 | 0.31 | 1.02 | 3.32 | 2.03 | 0.06 | <0.03 |
| TaiXi-3 | 76.17 | 14.59 | 0.61 | 0.12 | 0.30 | 0.07 | 0.45 | 5.58 | 1.03 | 0.86 | <0.01 |
| TaiXi-4 | 84.05 | 10.37 | 0.23 | <0.01 | 0.28 | 0.33 | <0.01 | 2.87 | 1.87 | <0.01 | <0.01 |
The combustion characteristics of coal were obtained via TG-DTG. In our research, TG-DTG experiments were performed by using a Waters Corp. SDT-650 differential thermogravimetric analyzer (room temperature to 1500 °C) with a sensitivity of 10–6 g. During combustion, 12 ± 0.5 mg of coal was evenly distributed in an Al2O3 crucible. The temperature was raised from room temperature to 1273 K at a rate of 10 K/min in an air atmosphere (VN2:VO2 = 78%:21%) with a flow rate of 100 mL/min. A Fourier transform infrared spectrometer (FT-IR, Thermo Fisher Nicolet Is5) was utilized to detect the effects of different chemical demineralization processes on coal surface functional groups to investigate the influence of surface functional groups on coal combustion performance. Surface functional groups were identified with sample/KBr pellets and FT-IR by adding 256 scans in the 400–4000 cm–1 spectral range at 4 cm–1 resolution. Furthermore, ash phase compositions were characterized via X-ray diffraction (XRD, Rigaku TTR-III, Cu Kα radiation, λ = 1.54 Å). X-ray fluorescence (XRF-1800, Shimadzu Corporation, Japan) was used to detect the contents of the main elements in ash.
2.3. Evaluation of Combustion Characteristics
Several specific combustion parameters were obtained by analyzing the data points of the TG-DTG curve to determine the influence of different chemical demineralization processes on coal combustion reactivity. Several basic combustion parameters, namely ignition temperature Ti (°C), peak temperature Tp (°C), and burnout temperature Tf (°C),31−35 were identified from the TG-DTG curves. Ti is defined as the temperature at which coal shows a weight loss of 1%/min, signifying that the coal sample has started burning, and is determined via the TG-DTG tangent method.31,36,37Tf is defined as the temperature that corresponds to the mass loss of coal that exceeds 99%, which indicates that the coal has stopped burning.19,32Tp is the temperature that corresponds to the peak of the DTG profile and at which the weight loss rate of coal is at the maximum.19,31 Additionally, five indexes of interest, namely, the ignition index (Di), burnout index (Df), combustion performance index (S), index of intensity (Hf), and flammability index (C), were investigated31,37−39 during combustion. These indexes are defined by eqs 1–5, respectively
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
where DTGmax is the maximum combustion rate (mg min–1), ti is the ignition time (min), tp is the corresponding time of DTGmax (min), tf is the burnout time (min), Δt1/2 is the time range of DTG/DTGmax = 0.5 (min), DTGmean is the mean combustion rate (mg min–1); ΔT1/2 is the temperature range of DTG/DTGmax = 0.5 (K), and Tp is the peak temperature (K).
3. Results and Discussion
3.1. Effect of Different Chemical Demineralization Processes on Ti, Tp, and Tf
The coal mass, volatile content, fixed carbon content, mineral content, particle size, and oxygen concentration influence the ignition temperature.40 The method for evaluating ignition temperature is not our aim. On the basis of comparison, we used the same burnoff procedure for raw coal and coal with different chemical demineralization processes and observed the influence of different chemical demineralization processes on the ignition temperature. Figure 3 shows the nonisothermal combustion behavior of TaiXi-0–4 obtained through a TG-DTG analysis. As observed in Figure 3, for all coal samples, 1–4% mass loss occurs due to the removal of moisture and is complete at approximately 100 °C. After 150 °C, TaiXi-0–4 exhibit a mass gain of approximately 1%. After demineralization, a large number of pore structures are formed, and the surface area is increased, which is conducive to oxygen adsorption.19,40Table 5 gives the surface areas of coal cleaned by acid and base. Table 5 shows that the surface area of TaiXi coal increases to different degrees after acid and base demineralization. The surface areas of TaiXi-0–4 are 18.03, 21.9, 23.41, 22.41, and 21.49 m2/g, respectively. The adsorption of oxygen on the coal surface facilitates early ignition and combustion.19 As the temperature is increased to beyond 400 °C, TaiXi-0, TaiXi-1, and TaiXi-3 undergo a sharp mass decrease due to rapid coal combustion. Notably, the mass change of TaiXi-0, TaiXi-1, and TaiXi-3 from 100 to 400 °C is not obvious. However, the masses of TaiXi-2 and TaiXi-4 decrease continuously with an increase in temperature. An important factor related to the mass loss is that TaiXi-2 and TaiXi-4 were leached with HNO3. This observation may be due to the saturation of active sites in the coal structure by adsorbed oxygen and the formation of nitrate compounds during HNO3 leaching.41 Steel and Patrick42 reported that the carbonaceous matrix is affected during HNO3 leaching even with low HNO3 concentrations (0.03 mol/L). H2SO4 is formed through the reaction between HNO3 and FeS2 and reacts with HNO3 to form NO2+, as shown in the following reactions:
| 6 |
| 7 |
| 8 |
Figure 3.
TG-DTG curves of coal with different chemical demineralizations (TaiXi-0–4).
Table 5. Temperature Interval, Surface Area, Mass Loss (ML), and Mass Loss Rate (R) for Each Region Separately.
| sample | reaction region (°C) | surface area(m2/g) | ML (%) | R (%/min) |
|---|---|---|---|---|
| TaiXi-0 | 334–757 | 18.03 | 97.9 | 1.32 |
| TaiXi-1 | 314–738 | 21.94 | 99.9 | 1.41 |
| TaiXi-2 | 125–741 | 23.41 | 98.5 | 1.37 |
| TaiXi-3 | 312–820 | 22.41 | 98.4 | 1.23 |
| TaiXi-4 | 130–725 | 21.49 | 99.5 | 1.42 |
NO2+, a powerful nitrating agent, reacts with the carbonaceous matrix. The changes in the functional groups of coal leached with HNO3 can be seen through a comparison of FT-IR spectra, which is given in Figure 4. Comparing the five coal samples reveals that HNO3-leached coals (TaiXi-2 and TaiXi-4) have the characteristic infrared absorption peaks of C–N and −NO2 at 1320 and 1521 cm–1, respectively. The FT-IR results provide evidence for the changes in the carbonaceous matrix of the coal, and −NO2 is incorporated into TaiXi-2 and TaiXi-4 that have been demineralized by nitric HNO3. The above results confirm that HNO3, as a powerful oxidant and nitrating agent, reacts with the carbonaceous matrix during leaching. The generated nitrogen compounds are relatively unstable at high temperatures, resulting in the continuous reduction in coal mass.43 Comparing the DTG curves of the five coal samples in Figure 3 shows that the DTG peak has shifted to a low temperature after chemical demineralization. This behavior indicates that chemical demineralization improves coal reactivity. Rubieira et al. explored the combustion behavior of HNO3-leached clean coal and showed that, in accordance with our results, clean coal has a higher reactivity and improved combustion efficiency.44Table 5 gives the temperature intervals, ignition temperatures, peak temperaturess, mass losses (MLs), mass loss rates (R), and burn-out temperatures for each region separately.
Figure 4.

FT-IR images of TaiXi-0–4.
In the process of thermogravimetry testing, the data collection interval is 0.1 s but the drawing process is continuous. As a result, the temperature at the intersection in Figure 3 may not be the temperature that was actually collected by the instrument (TG-DTG). Simply using the temperature at the intersection point to represent Ti, Tp, and Tf causes great uncertainty. The temperature within ±1.0 s is usually selected at the intersection point to obtain reliable data. The uncertainty of the combustion parameter temperatures obtained via mathematical statistics is reduced. The Ti values of TaiXi-0–4 are presented in Figure 5. Overall, Figure 5 illustrates that the Ti values of the clean coal samples obtained via different chemical demineralization processes are different. As shown in Figure 5, clean coal has a lower ignition temperature in comparison to raw coal. Chemical demineralization is proven to significantly reduce the Ti value of coal (ΔTi = 12–69 °C). Additionally, TaiXi-3 has the lowest ignition temperature (Ti = 468 °C). Notably, Table 1 depicts that clean coal has the highest the volatile matter content of 18.64% (fuel ratio 4.08) after NaOH treatment. Volatiles and tar are released during pyrolysis and adsorb on the surfaces of coal for preferential combustion. The combustion of fixed carbon can be heated and prompted by the volatiles and preferential tar combustion.45 As the volatile matter in TaiXi-3 is released, the porosity increases and the reactive surface enlarges. High porosity and large reactive surfaces indicate that additional oxygen is adsorbed during combustion. This effect reduces combustion resistance and ignition temperature. Previous reports have demonstrated that clay minerals and quartz have negative effects on coal combustion and that the carbon–oxygen reaction is inhibited.7,46 CaO, Fe2O3, and FeS2 catalysts have positive effects on reducing the ignition temperature of coal.32,40,47 The ash phase composition of coals obtained through different chemical demineralization processes is given in Figure 6. Figure 6 shows that the ash of TaiXi-2 and TaiXi-4 does not contain Fe2O3, which indicates that HNO3 removes Fe2O3 and FeS2. Therefore, the catalytic effect of iron oxide and iron sulfide on coal combustion is eliminated. As can be seen from Figure 4, under the strong oxidation of HNO3, the carbon matrix reacts with HNO3, and it burns preferentially in the combustion process, which improves the combustion performance of coal. Fe2O3 and FeS2 in TaiXi-1 and TaiXi-3 are retained and aluminosilicates are removed, thus eliminating the negative influence on the combustion process. It can be seen from Table 3 that the impurity content in TaiXi-1 is only 0.1%, so that even if Fe2O3 and FeS2 are retained, their effect on the ignition temperature is not obvious. The volatile content of TaiXi-3 was increased to 18.64% after treatment with NaOH, which was beneficial to the combustion of coal. Figure 6 shows that the ash phase of TaiXi-3 is composed of NaWO3, K6Fe2O5, MgO·Fe2O3, CaFeO3, and a small amount of Al0.5Si0.75O2.25. TaiXi-3 has the lowest TI value under the action of low fuel ratio and internal mineral catalysis (Fe2O3 and FeS2).
Figure 5.

Ignition temperature of coal with different chemical demineralizations.
Figure 6.
Phase composition of ash with different chemical demineralization processes (TaiXi-1–4).
Figure 7 presents the Tp values of TaiXi-0–4. As can be observed from Figure 7, the clean coal samples obtained through different chemical demineralization processes have lower peak temperatures in comparison to raw coal. TaiXi-3 has the lowest peak temperature (Tp = 563 °C). Table 3 shows that chemical demineralization has improved the fuel ratio of coal. A low fuel ratio is indicative of high volatile matter content. Volatile matter is preferentially released and burnt during coal combustion. The heat released by volatile matter combustion preheats the fixed carbon and promotes the combustion of the fixed carbon, thus reducing the peak temperature of clean coal combustion. The peak temperatures of TaiXi-0–4 lack obvious differences (ΔTp = 12, 7, 17 °C), indicating a weak correlation between the peak temperature and the mineral content of coal. The peak temperature of coal is closely related to volatile matter content, and the volatile content has a negative correlation with peak temperature. Kizgut et al. explored the effect of chemical demineralization on the thermal behavior of bituminous coals.41 They found a clear increase in the reactivity of chemically demineralized coal in comparison with that of raw coal.
Figure 7.

Peak temperature of coal with different chemical demineralizations.
The Tf values of coal subjected to different chemical demineralization processes and raw coal were obtained from the TG-DTG curves and are shown in Figure 8. The burnout temperature of coal samples, except for that of TaiXi-3, after chemical demineralization is lower than that of raw coal (ΔTf = 16–63 °C). This result is generally in accordance with previously reported findings showing that demineralized coals have lower burnout temperatures in comparison to untreated coals.4,7Table 3 summarizes the important parameters of the five coal samples. As observed in Table 3, TaiXi-3 coal has the lowest fixed carbon content after NaOH treatment. Given that TaiXi-3 has the lowest fixed carbon content (76.01%) and the highest volatile content (18.64%), it has the lowest fuel ratio (fuel ratio 4.08) among the five coals. A low fuel ratio is indicative of a low calorific value. Notably, volatiles are released during pyrolysis and preferentially combusted. The combustion of fixed carbon can be heated and prompted by volatile combustion. However, as can be seen from Figure 3, TaiXi-3 has a high amount of volatile matter that begins to escape and burn at approximately 425 °C. This process ends at approximately 465 °C. When the volatile matter escapes in a concentrated manner, the combustion of fixed carbon becomes difficult. TaiXi-3 has the highest Aad/FCad index among the four kinds of chemically demineralized coals. At similar fixed carbon contents, the burnout temperatures of TaiXi-1, TaiXi-2, and TaiXi-4 are negatively correlated with the Aad/FCad index. Zou et al. explored the effect of catalysts on the combustion reactivity of anthracite. They reported that minerals suppress anthracite burnout at low doses.32 The adsorption of noncombustible NaOH into the porous structure of TaiXi-3 during NaOH demineralization causes an increase in burnout temperature.19
Figure 8.

Burnout temperature of coal with different chemical demineralizations.
3.2. Combustion Kinetic Analysis
Duo to the presence of the numerous complex components and their parallel and consecutive reactions,6,23 it is a great challenge to explore the nonisothermal kinetics of mass loss during coal combustion. An Arrhenius-type kinetic model was used to analyze the TG-DTG data. Figure 3 shows that the DTG curves of five coals obtained at a constant rate of heating (10 °C/min) are single bell-shaped peaks with no shoulders in the range from ignition temperature to burnout temperature. This indicates that the combustion process of the five coals is a single-step process. This is in accordance with the method of recognition of single-step kinetics in the literature.48 Currently, the Coats–Redfern method is used to investigate the combustion kinetics of coal. The Coats–Redfern method is used on the combustion zone between the ignition and the burnout temperature.6 In this method, the coal combustion process is approximated as a first-order kinetic reaction; the reaction mechanism function is
| 9 |
On substitution of eq 9 into the basic reaction kinetic equation, we obtain
| 10 |
Integration and use of the Coats–Redfern method to organize gives
| 11 |
The extent of conversion of coal combusted was defined by
| 12 |
where m0 is the initial mass, mt is the mass after combustion (t), and mf is the mass after complete combustion.
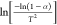 as the ordinate (Y), 1/T as the
abscissa (X) gave a straight line
with the slope equaling −E/R. Finally, the activation energy of different coal combustion is
obtained.
as the ordinate (Y), 1/T as the
abscissa (X) gave a straight line
with the slope equaling −E/R. Finally, the activation energy of different coal combustion is
obtained.
Figure 9 shows the Arrhenius curves of TaiXi-0–4. The correlation coefficients were in the range of 0.9848–0.9972. The activation energies of the five coals are calculated using Coats–Redfern kinetic methods and given in Table 6 (heating rate 10 °C/min). Table 6 shows that the activation energies of the coal sample before and after removal of minerals vary in the range of 58.39–91.39 kJ mol–1. The results show that the activation energy of clean coal after the chemical demineralization process is obviously lower than that of raw coal. The removal of minerals has a positive effect on the combustibility of coal, and this result is consistent with the above research results.
Figure 9.

Arrhenius curves of TaiXi-0–4.
Table 6. Activation Energies (kJ mol–1) of Coal Samples (TG/DTG).
| sample |
|||||
|---|---|---|---|---|---|
| TaiXi-0 | TaiXi-1 | TaiXi-2 | TaiXi-3 | TaiXi-4 | |
| activation energy | 91.39 | 84.30 | 76.00 | 66.78 | 58.39 |
3.3. Effect of Different Chemical Demineralization Processes on Combustion Performance Indexes
Generally, ignition, peak, and burnout temperatures are used to evaluate the combustion parameters of coal. Di, Df, S, Hf, and C are used to evaluate coal combustion performance to further explore the influence of different chemical demineralization processes on the coal combustion performance. In addition to the ignition temperature, Di is an important parameter that is used to evaluate the ignition performance of coal. A high Di index reflects a good ignition performance of the coal. Df is an important index for characterizing the combustibility of combustibles in coal. In this work, Df is introduced to describe the burnout characteristics of coal. S, a highly representative comprehensive combustion characteristic index, is used to characterize the overall combustion characteristics of coal to comprehensively evaluate the combustion of coal. Notably, a high S value is indicative of good coal combustion performance. Hf describes the rate and the intensity of combustion. A low Hf value reflects a good combustion performance.31,49,50 Furthermore, the flammability index C is adopted to further evaluate the combustion stability of coal. The combustion characteristic indexes of coal obtained from combustion studies carried out via TG-DTG are shown in Table 7.
Table 7. Combustion Characteristic Indexes of Coal with Different Chemical Demineralizations.
| sample | 10–4Di | 10–6Df | 10–10S | Hf | 10–6C |
|---|---|---|---|---|---|
| TaiXi-0 | 2.15 | 3.16 | 5.78 | 6.52 | 1.09 |
| TaiXi-1 | 2.30 | 3.41 | 5.03 | 6.26 | 1.03 |
| TaiXi-2 | 2.37 | 2.91 | 4.44 | 6.48 | 1.01 |
| TaiXi-3 | 2.58 | 2.54 | 2.42 | 6.11 | 0.97 |
| TaiXi-4 | 2.44 | 2.75 | 3.68 | 6.41 | 0.93 |
As shown in Table 7, TaiXi-3 has the highest Di value, indicating that it has the best the ignition performance among the five coal samples. Notably, TaiXi-3 has the lowest ignition temperature among the five coal samples (Figure 5). The variation in Di is similar to that in ignition temperature, indicating that the ignition temperature determines the Di value to a certain extent. This relationship is generally in accordance with previous reports.38,39 A high volatile content in coal contributes to the release of volatile matter. The rapid oxidation and volatilization rates of volatile matter are associated with the low ignition temperature of coal.51 Nevertheless, in the present study, the change in ignition temperature with volatile matter content is inconsistent with that reported in the aforementioned literature. This observation may be due to the fact that the ash contents and mineral types of TaiXi coal have changed after different chemical demineralization processes. In particular, during demineralization, the carbonaceous matrix in the coal reacts with the reagents and the surface functional groups change. Table 7 shows that the Df value of TaiXi-1 is greater than those of TaiXi-0 and TaiXi-2–4. As observed from Tables 3 and 7, Taixi-1 has the lowest ash content and the highest Df value. These results indicate that the burnout performance of TaiXi-1 is superior to that of other coals. Interestingly, a low ash content is not associated with a high Df value. TaiXi-0 has an ash content of 3.4% and a Df value of 3.16 × 10–6, which is second to that of TaiXi-1 coal. Therefore, the minerals in raw coal have been proven to improve the burnout performance of coal. The minerals may affect burnout performance by acting as oxygen carriers that promote oxygen transfer to the inside of coal.40 As observed from Figure 8 and Table 7, TaiXi-3 has the highest burnout temperature (820 °C) and the lowest burnout index (2.54 × 10–6), indicating the worst burnout performance. NaOH is adsorbed into the porous structure of TaiXi-3 during NaOH demineralization, thus causing a decrement in burnout index.19
As observed from Table 7, TaiXi-0 has the highest comprehensive combustion characteristic index (S = 5.78 × 10–10), likely because chemical demineralization reduces the comprehensive combustion performance of coal. Among the TaiXi coal samples obtained through chemical demineralization, TaiXi-1 has the highest comprehensive combustion characteristic index (S = 5.03 × 10–10). In particular, the comprehensive combustion characteristic index of coal leached by NaOH is lower than that of other coal samples. The comprehensive combustion characteristic indexes of TaiXi-3 and TaiXi-4 are 2.42 × 10–10 and 3.68 × 10–10, respectively. As was mentioned above, NaOH is adsorbed into the porous structure of coal and the combustion of coal is hindered. TaiXi-4 is obtained from TaiXi-3 by leaching with HNO3. The partial removal of NaOH eliminates the negative effect of NaOH on combustibility. As observed from Table 7, the Hf value of TaiXi-0 is 6.52 and that of TaiXi-3 is 6.11. This result indicates that the combustion of TaiXi-0 is the most stable and that of TaiXi-3 is the most intense. The same phenomenon is observed in the industrial analysis of the five kinds of coal. Table 3 shows that the volatile contents of TaiXi-0–4 are 9.32%, 13.20%, 11.23%, 18.67%, and 12.33%, respectively. Notably, the volatile content and Hf are negatively correlated; that is, Hf decreases with an increase in volatile content. Volatiles are released during pyrolysis and adsorbed on the surfaces of coal for preferential combustion. The combustion of fixed carbon can be heated and prompted by the preferential combustion of volatiles.45 As volatile matter is released, the porosity increases and the reactive surface area expands. High porosity and large reactive surfaces indicate that additional oxygen is adsorbed during combustion, which reduces the resistance of oxygen transport. Meanwhile, ash covers carbon surfaces in coal during combustion, which hinders the rapid combustion of coal.31,52C is adopted to evaluate the combustion stability of coal. The C indexes are shown in Table 7. The C indexes of the five kinds of coal are between 0.93 × 10–6 and 1.09 × 10–6, among which the C index of raw coal is the highest, indicating that the combustion stability of raw coal is the best, followed by those of TaiXi-1 and TaiXi-2. Chemical demineralization can reduce the combustion stability of TaiXi coal. The combustion stabilities of TaiXi-2 and TaiXi-4 leached by a strong oxidant (HNO3) are lower than those of the samples treated via the other two methods. Table 3 shows that TaiXi-3 has the highest volatile matter content of 18.64% (fuel ratio 4.08). Volatile matter escapes rapidly during heating and combustion, and the combustion intensifies, which reduces the combustion stability of coal. In addition to the high volatile content of TaiXi-4 coal, HNO3 leaching also affects combustion stability. HNO3 reacts with the carbonaceous matrix during leaching, and the generated nitrogen compounds are relatively unstable at high temperature. When nitrogen oxides are decomposed and escape rapidly, the combustion stability of coal is decreased.
4. Conclusions
TG-DTG, FT-IR, and XRD techniques were used to explore the effects of different chemical demineralization methods on coal combustion performance. Several specific combustion parameters were obtained by analyzing the data points of the TG-DTG curve to determine the influence of different chemical demineralization methods on coal combustion reactivity. The main conclusions are as follows.
-
(1)
The ignition performance and peak temperature of clean coal have significantly improved after different chemical demineralization processes. The ignition temperature of clean coal has decreased by 12–69 °C, and the peak temperature has decreased by 7–62 °C. In comparison with those of raw coal, the ignition temperature and peak temperature of clean coal (TaiXi-3) have decreased by 69 and 62 °C, respectively, after NaOH demineralization.
-
(2)
Chemical demineralization improves the burnout performance of coal. In particular, the burnout temperature of clean coal (TaiXi-3) after NaOH demineralization has increased by 63 °C. The adsorption of noncombustible NaOH into the porous structure of TaiXi-3 causes an increase in burnout temperature.
-
(3)
When aluminosilicate is removed from coal, the reactivity of coal increases. In contrast to TaiXi-2 and TaiXi-3, aluminosilicate has a negative influence on coal combustion performance. TaiXi-1 does not have the best combustion performance despite the removal of its aluminosilicate and acid-soluble mineral content. This behavior indicates that acid-soluble minerals in coal have a positive influence on coal combustion performance.
-
(4)
An Arrhenius-type kinetic model was used to analyze the TG-DTG data. The activation energies of the five coals were calculated using Coats–Redfern kinetic methods, and the activation energies of the coal sample before and after removal of minerals vary in the range of 58.39–91.39 kJ mol–1. The activation energy of clean coal is obviously lower than that of raw coal. The demineralization process has a positive effect on the combustibility of coal.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 52174338 and 51904349) and the Natural Science Foundation of Hunan Province, China (No. 2021JJ30796).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00522.
Surface areas and pore widths of coal cleaned by acid and base (PDF)
Author Contributions
L.T. carried out the conceptualization, methodology, formal analysis, writing-original draft, writing-review, and editing. J.X. carried out the supervision, validation, writing-review editing, and funding acquisition. Q.M. carried out the supervision, formal analysis, simulation, writing-review, and editing. Z.Z. carried out the investigation, validation, data analysis, writing-review, and editing. Z.Y. carried out the validation, methodology, simulation, writing-review, and editing. X.Z. carried out the validation, writing-review, and editing. S.Y. carried out the investigation, validation, data analysis, writing-review, and editing. Q.Z. carried out the validation, conceptualization, methodology, funding acquisition, writing-original draft, writing-review, and editing. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Qi H.; Sun R.; Peng J.; Meng X.; Cao Z.; Wang Z.; Ren X.; Yuan M.; Zhang L.; Ding S. Experimental investigation on the ignition and combustion characteristics of pyrolyzed char and bituminous coal blends. Fuel 2020, 281, 118732. 10.1016/j.fuel.2020.118732. [DOI] [Google Scholar]
- Ward C. R.; Dai S. Minerals and Trace Elements in Coal. Int. J. Coal. Geol. 2012, 94, 1–2. 10.1016/j.coal.2012.01.016. [DOI] [Google Scholar]
- Chen F.; Wang J.; Zhao B.; Li X.; Tao Q. Effect of mineral form in coal on combustion characteristics and fusibility of coal ash. J. Fuel. Chem. Technol. 2015, 43 (1), 27–33. [Google Scholar]
- Crelling J. C.; Hippo E. J.; Woerner B. A.; West D. P. Combustion characteristics of selected whole coals and macerals. Fuel 1992, 71 (2), 151–158. 10.1016/0016-2361(92)90003-7. [DOI] [Google Scholar]
- Kok M. V. Simultaneous thermogravimetry-calorimetry study on the combustion of coal samples: Effect of heating rate. Energy Convers. Manage. 2012, 53 (1), 40–44. 10.1016/j.enconman.2011.08.005. [DOI] [Google Scholar]
- Kok M. V. Temperature-controlled combustion and kinetics of different rank coal samples. J. Therm. Anal. Calorim. 2005, 79 (1), 175–180. 10.1007/s10973-004-0581-6. [DOI] [Google Scholar]
- Spears D. A. Role of clay minerals in UK coal combustion. Appl. Clay. Sci. 2000, 16, 87–95. 10.1016/S0169-1317(99)00048-4. [DOI] [Google Scholar]
- Liu H.; Wang W.; Zhang R.; Li G. The Impact of Mineral Matter on the Calorific Value of Coal. Coal 2009, 18 (4), 12–14. [Google Scholar]
- Chen C.; Zhang S.; Liu D.; Guo X.; Dong A.; Xiong S.; Shi D.; Lu J. Existence form of sodium in high sodium coals from Xinjiang and its effect on combustion process. J. Fuel. Chem. Technol. 2013, 41 (7), 832–838. [Google Scholar]
- Chen J.; Sun X. Determination of the Devolatilization Index and Combustion Characteristic Index of Pulverized Coals. Power. Eng. 1987, (5), 13–18. [Google Scholar]
- Wynter C. I.; May L.; Oliver F. W.; Hall J. A.; Hoffman E. J.; Kumar A.; Christopher L. Correlation of Coal Calorific Value and Sulphur Content with 57Fe Mössbauer Spectral Absorption. Hyperfine Interact. 2004, 153, 147–152. 10.1023/B:HYPE.0000024719.48753.af. [DOI] [Google Scholar]
- Man C.; Zhu J.; Ouyang Z.; Liu J.; Lyu Q. Experimental study on combustion characteristics of pulverized coal preheated in a circulating fluidized bed. Fuel Process. Technol. 2018, 172, 72–78. 10.1016/j.fuproc.2017.12.009. [DOI] [Google Scholar]
- Zhang H.; Chen X.; Ding H.; Gu X.; Xu Y. Generalized Algorithm for Estmiating Kinetic Properties in Combustion from Original TG Data. J. Combust. Sci. Technol. 2005, 11 (4), 319–322. [Google Scholar]
- Zhang T. Research on Combustion Property with PCI. Heilongjiang Metallurgy 2010, 30 (1), 14–16. [Google Scholar]
- Zhang H.; Zhang J.; Xu R.; Wang G.; Xu T.; Tang Q. Combustion Characteristics and Mechanism Analysis of Blended Coal of Semi Coke and Bitunminous Coal. China Metallurgy 2016, 26 (2), 7–12. [Google Scholar]
- Namkung H.; Park J. H.; Lee Y. J.; Song G. S.; Choi J. W.; Kim J. G.; Park J. S.; Um B. H.; Song K. H.; Park S. J. Characteristics of novel synthetic fuels using coal and sewage sludge impregnated bioliquid applying for a coal combustion system. Fuel Process. Technol. 2017, 167, 153–161. 10.1016/j.fuproc.2017.06.030. [DOI] [Google Scholar]
- Jayaraman K.; Kok M. V.; Gokalp I. Pyrolysis, Combustion and Gasification Studies of Different Sized Coal Particles using TGA-MS. Appl. Therm. Eng. 2017, 125, 1446–1455. 10.1016/j.applthermaleng.2017.07.128. [DOI] [Google Scholar]
- Magdziarz A.; Wilk M. Thermogravimetric study of biomass, sewage sludge and coal combustion. Energy Convers. Manage. 2013, 75, 425–430. 10.1016/j.enconman.2013.06.016. [DOI] [Google Scholar]
- Ken B. S.; Aich S.; Saxena V. K.; Nandi B. K. Combustion behavior of KOH desulphurized coals assessed by TGA-DTG. Energy Source. Part A 2018, 40 (20), 2458–2466. 10.1080/15567036.2018.1502844. [DOI] [Google Scholar]
- Altun N. E.; Kök M. V.; Hicyilmaz C. Effect of Different Binders on the Combustion Properties of Lignite. Part II. Effect on kinetics. J. Therm. Anal. Calorim. 2001, 65, 797–804. 10.1023/A:1011967914611. [DOI] [Google Scholar]
- Altun N. E.; Hicyilmaz C.; Kok M. V. Effect of Different Binders on the Combustion Properties of Lignite Part I. Effect on thermal properties. J. Therm. Anal. Calorim. 2001, 65, 787–795. 10.1023/A:1011915829632. [DOI] [Google Scholar]
- Gundogar A. S.; Kok M. V. Thermal characterization, combustion and kinetics of different origin crude oils. Fuel 2014, 123, 59–65. 10.1016/j.fuel.2014.01.058. [DOI] [Google Scholar]
- Jayaraman K.; Kok M. V.; Gokalp I. Combustion mechanism and model free kinetics of different origin coal samples: Thermal analysis approach. Energy 2020, 204, 117905. 10.1016/j.energy.2020.117905. [DOI] [Google Scholar]
- Wang G.; Zhang J.; Hou X.; Shao J.; Geng W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour. Technol. 2015, 177, 66–73. 10.1016/j.biortech.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Wu S.; Gu J.; Zhang X.; Wu Y.; Gao J. Variation of carbon crystalline structures and CO2 gasification reactivity of Shenfu coal chars at elevated temperatures. Energy Fuel. 2008, 22 (1), 199–206. 10.1021/ef700371r. [DOI] [Google Scholar]
- Xiang Y.; Wang Y.; Zhang J.; Huang J.; Wu J. Study on Combustion Kinetics of Partial Gasified Coal Char by Using Distributed Activation Energy Model. J. Combust. Sci. Technol. 2003, 9 (6), 566–570. [Google Scholar]
- Cai Z.; Ma X.; Fang S.; Yu Z.; Lin Y. Thermogravimetric analysis of the co-combustion of eucalyptus residues and paper mill sludge. Appl. Therm. Eng. 2016, 106, 938–943. 10.1016/j.applthermaleng.2016.06.088. [DOI] [Google Scholar]
- Jaroenkhasemmeesuk C.; Tippayawong N. Thermal degradation kinetics of sawdust under intermediate heating rates. Appl. Therm. Eng. 2016, 103, 170–176. 10.1016/j.applthermaleng.2015.08.114. [DOI] [Google Scholar]
- Ozbas K. E.; Hicyilmaz C.; Kok M. V.; Bilgen S. Effect of cleaning process on combustion characteristics of lignite. Fuel Process. Technol. 2000, 64 (1), 211–220. 10.1016/S0378-3820(00)00064-3. [DOI] [Google Scholar]
- Liu G.; Liao Y.; Guo S.; Ma X.; Zeng C.; Wu J. Thermal behavior and kinetics of municipal solid waste during pyrolysis and combustion process. Appl. Therm. Eng. 2016, 98, 400–408. 10.1016/j.applthermaleng.2015.12.067. [DOI] [Google Scholar]
- Zhang Y.; Cuo Y.; Cheng F.; Yan K.; Cao Y. Investigation of combustion characteristics and kinetics of coal gangue with different feedstock properties by thermogravimetric analysis. Thermochim. Acta 2015, 614, 137–148. 10.1016/j.tca.2015.06.018. [DOI] [Google Scholar]
- Zou C.; Zhao J.; Li X.; Shi R. Effects of catalysts on combustion reactivity of anthracite and coal char with low combustibility at low/high heating rate. J. Therm. Anal. Calorim. 2016, 126 (3), 1469–1480. 10.1007/s10973-016-5806-y. [DOI] [Google Scholar]
- Aich S.; Nandi B. K.; Bhattacharya S. Effect of weathering on physico-chemical properties and combustion behavior of an Indian thermal coal. Int. J. Coal. Sci. Technol. 2019, 6 (1), 51–62. 10.1007/s40789-018-0235-0. [DOI] [Google Scholar]
- Pinchuk V. A.; Sharabura T. A.; Kuzmin A. V. The effect of water phase content in coal-water fuel on regularities of the fuel ignition and combustion. Fuel Process. Technol. 2019, 191, 129–137. 10.1016/j.fuproc.2019.04.011. [DOI] [Google Scholar]
- Dong M. R.; Luo F. S.; Huang M.; Li S. S.; Lu J. D. Study on the ignition characteristics and alkali release of single coal particles with additional different forms of potassium. Fuel Process. Technol. 2020, 203, 106385. 10.1016/j.fuproc.2020.106385. [DOI] [Google Scholar]
- Li X. G.; Ma B. G.; Xu L.; Hu Z. W.; Wang X. G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim. Acta 2006, 441 (1), 79–83. 10.1016/j.tca.2005.11.044. [DOI] [Google Scholar]
- Ma B. G.; Li X. G.; Xu L.; Wang K.; Wang X. G. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Thermochim. Acta 2006, 445 (1), 19–22. 10.1016/j.tca.2006.03.021. [DOI] [Google Scholar]
- Ebrahimi-Kahrizsangi R.; Abbasi M. H. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. T. Nonferr. Metal. Soc. 2008, 18 (1), 217. 10.1016/S1003-6326(08)60039-4. [DOI] [Google Scholar]
- Liu J.; Chen J.; Sun S.; Kuo J.; Chang K.; Huang S.; Huang L.; Chen N.; Ren J.; Liu Z. Analysis of the co-combustion of sewage sludge and spent coffee grounds. Acta. Sci. Circumst. 2016, 36 (10), 3784–3794. [Google Scholar]
- Li X. G.; Ma B. G.; Xu L.; Luo Z. T.; Wang K. Catalytic Effect of Metallic Oxides on Combustion Behavior of High Ash Coal. Energy Fuel. 2007, 21 (5), 2669–2672. 10.1021/ef070054v. [DOI] [Google Scholar]
- Kizgut S.; Baris K.; Yilmaz S. Effect of chemical demineralization on thermal behavior of bituminous coals. J. Therm. Anal. Calorim. 2006, 86 (2), 483–488. 10.1007/s10973-005-7329-9. [DOI] [Google Scholar]
- Steel K. M.; Patrick J. W. The production of ultra clean coal by sequential leaching with HF followed by HNO3. Fuel 2003, 82, 1917–1920. 10.1016/S0016-2361(03)00149-2. [DOI] [Google Scholar]
- Pietrzak R.; Wachowska H. Thermal analysis of oxidised coals. Thermochim. Acta 2004, 419 (1), 247–251. 10.1016/j.tca.2004.02.014. [DOI] [Google Scholar]
- Rubiera F; Arenillas A; Pevida C; Garcıa R; Pis J.J; Steel K.M; Patrick J.W Coal structure and reactivity changes induced by chemical demineralisation. Fuel Process. Technol. 2002, 79 (3), 273–279. 10.1016/S0378-3820(02)00185-6. [DOI] [Google Scholar]
- Zhao D.; Zhang J.; Wang G.; Conejo A. N.; Xu R.; Wang H.; Zhong J. Structure Characteristics and Combustibility of Carbonaceous Materials from Blast Furnace Flue Dust. Appl. Thermal Eng. 2016, 108, 1168–1177. 10.1016/j.applthermaleng.2016.08.020. [DOI] [Google Scholar]
- Mccollor D. P.; Jones M. L.; Benson S. A.; Young B. C. Promotion of char oxidation by inorganic constituents. Symposium on Combustion 1989, 22 (1), 59–67. 10.1016/S0082-0784(89)80011-6. [DOI] [Google Scholar]
- Zhang S.; Chen Z. D.; Chen X. J.; Gong X. Z. Effects of ash/K2CO3/Fe2O3 on ignition temperature and combustion rate of demineralized anthracite. J. Fuel Chem. Technol. 2014, 42 (2), 166–174. 10.1016/S1872-5813(14)60013-X. [DOI] [Google Scholar]
- Vyazovkin S.; Burnham A. K.; Favergeon L.; Koga N.; Moukhina E.; Pérez-Maqueda L. A.; Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. 10.1016/j.tca.2020.178597. [DOI] [Google Scholar]
- Gao Z.; Fang L.; Zhou J.; Yan W. Research on The Combustion Performance of Blended Coal in Thermal-Balance. Power Eng. 2002, 22, 1764–1767. [Google Scholar]
- Niu S. L.; Lu C. M.; Han K. H.; Zhao J. L. Thermogravimetric analysis of combustion characteristics and kinetic parameters of pulverized coals in oxy-fuel atmosphere. J. Thermal Anal. Calorim. 2009, 98, 267–274. 10.1007/s10973-009-0133-1. [DOI] [Google Scholar]
- Zhang L.; Binner E.; Qiao Y.; Li C. Z. In situ diagnostics of Victorian brown coal combustion in O2/N2 and O2/CO2 mixtures in drop-tube furnace. Fuel 2010, 89 (10), 2703–2712. 10.1016/j.fuel.2010.04.020. [DOI] [Google Scholar]
- Öner C.; Altun Ş. Improved combustion of asphaltite coals in a rotating head combustor with various air supply arrangements. Energy Fuel. 2014, 28, 2971–2976. 10.1021/ef500196c. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






