Abstract
Introduction
Intestinal ultrasound [IUS] is useful for assessment of inflammation, complications, and treatment follow-up in inflammatory bowel disease [IBD] patients. We aimed to study outcomes and impact on disease management for point-of-care [POC] IUS in IBD patients.
Methods
Two patient cohorts undergoing POC IUS [January 2016–July 2018 and October 2019–December 2019] were included retrospectively. Disease management after IUS was analysed and IUS outcomes were compared with symptoms, biomarkers, and additional imaging within 8 weeks from IUS. To study differences in use of IUS over time, cohorts were compared.
Results
In total, 345 examinations (280 in Crohn’s disease [CD]/65 in ulcerative colitis [UC]) were performed. Present inflammation on IUS was comparable between symptomatic and asymptomatic CD [67.6% vs 60.5%; p = 0.291]. In 60%, IUS had impact on disease management with change in medication in 47.8%. Additional endoscopy/magnetic resonance imaging [MRI] was planned after 32.8% examinations, showing good correlation with IUS in 86.3% [ρ = 0.70, p <0.0001] and 80.0% [ρ = 0.75, p <0.0001] of cases, respectively. Faecal calprotectin was higher in active versus inactive disease on IUS [664 µg/g vs 79 µg/g; p <0.001]. Over the years, IUS was performed more frequently to monitor treatment response and the use of MRI was reduced within the cohort.
Conclusions
POC IUS affects clinical decision making and could detect preclinical relapse in CD patients, with potential to reduce additional endoscopy or MRI. In addition, the paradigm expands towards monitoring treatment and close follow-up for IUS. Based on our results, we propose a POC IUS algorithm for follow-up of IBD patients.
Keywords: IBD, imaging, intestinal ultrasound, monitoring, point-of-care
1. Introduction
Inflammatory bowel disease [IBD] is a common denominator for the chronic inflammatory conditions ulcerative colitis [UC] and Crohn’s disease [CD]. The chronic and relapsing pattern causes long-term bowel damage and complications such as stenosis and perforating disease in CD patients.1 Therefore, complete and objective control of inflammation is the preferred treatment target leading to superior long-term outcomes.2,3 Currently, this ‘treat to target’ concept is the ultimate strategy in the treatment of IBD patients.1,4
To adequately control inflammation, close monitoring of the disease is crucial.5 Clinical symptoms such as abdominal pain, diarrhoea, and rectal blood loss as well as non-invasive biomarkers such as C-reactive protein [CRP] and faecal calprotectin [FCP] are useful to guide clinical decision making, but are not always sensitive and accurate and lack information on disease severity and extent.6–8
Diagnostic modalities for monitoring IBD patients include endoscopy, magnetic resonance imaging [MRI], computed tomography [CT], and intestinal ultrasound [IUS]. Endoscopy is considered the reference standard for assessment of mucosal disease activity.9 However, it is impossible to frequently implement this technique due to burden for the patient, costs, and waiting lists.9,10 MRI is useful for the assessment of complications and small bowel inflammation. However, implementation is also limited by costs and waiting lists.11 CT scans are quick but generally only recommended in the acute setting due to radiation exposure. Since IUS is non-invasive, accurate, reliable, and cheap, it is a suitable tool for frequent assessment of the bowel, especially in a point-of-care [POC] setting.12–17 Additionally, it has been shown that patients prefer IUS over other modalities.18
POC medicine is defined as medical testing at or near the site of patient care, with fast results which facilitate rapid clinical decision making. POC tests for CRP and FCP are already widely available, but lack the ability to objectify complications, location, severity, and extent of disease activity.19,20 On the contrary, IUS has the potential to identify these and thereby guide immediate decision making. Whereas studies have shown good accuracy and reliability of IUS, there are limited data on the impact of IUS outcomes on daily clinical decision making.21,22
In this retrospective study, we studied a large cohort of IBD patients who were evaluated with POC IUS in a real-world outpatient setting, and aimed to provide insight on the impact of POC IUS in daily clinical practice. Additionally, we compared IUS outcomes with symptoms, biomarkers, and additional imaging or endoscopy. Furthermore, we highlighted the potential of POC IUS to reduce the need and cost of additional imaging and to avoid treatment delay. Our findings may serve as a basis for future prospective studies and for the optimal implementation of POC IUS in IBD patients.
2. Methods
2.1. Patient population and study design
IBD patients who were evaluated with POC IUS at the outpatient IBD clinic of the Amsterdam University Medical Center were included consecutively from implementation in our clinic [January 2016] up to reaching 250 patients in July 2018. To compare evolution of POC IUS in clinical practice over time, we collected a second cohort retrospectively before the COVID pandemic between October 2019 and December 2019. All patients were identified using the IUS outpatient lists in the electronic health cohort. All data were retrieved from electronic patient records. Exclusion criteria were: no formal IUS report available; under 18 years of age; or no confirmed IBD diagnosis at the moment of data collection.
2.2. Patient, biomarker, and treatment data
The following baseline data were collected: age at IUS, gender, age at diagnosis, disease phenotype [Montreal classification], medication use, previous surgery, clinical symptoms, and biochemical markers [FCP and CRP]. Data on clinical symptoms were collected and scored at data collection as follows: general well-being good vs bad, presence/absence of abdominal pain, diarrhoea, rectal blood loss, urgency, bloating, loss of appetite, and extra-intestinal manifestations. Patients were considered symptomatic when they had at least one symptom. FCP and CRP values were collected and compared with IUS when they were available within 4 weeks from IUS. FCP values >50 µg/g and CRP values >5 mg/L were considered as elevated, reflecting active inflammation. The following data on disease management after IUS were collected: medication started, stopped or adjusted; additional endoscopy or imaging planned; endoscopic dilation planned; surgery planned; continuation without change.
2.3. IUS examinations
All examinations were performed by investigators specifically trained in IUS (SB [>200 IUS in 2016], FV [>500 IUS in October 2019], KG [>500 IUS in October 2019], MJ [>200 IUS in October 2019]). All IUS examinations at our clinic are performed by systematically scanning the bowel from the ileum through all segments of the colon and the rectum. Additionally, a sweep of the remaining small bowel is performed. Patients were not fasting. IUS parameters as mentioned below were noted in a standardised report. All the examinations were performed with a Philips EPIQ 5G machine with C5-1, L12-5, and L18-4 transducers or with a Hitachi Noblus machine with C5 and L13 transducers. At colour Doppler, the velocity was adjusted for slow flow detection with a maximum velocity scale of 5–7 cm/s.
2.4. IUS parameters
IUS data were collected by assessing IUS reports and the reason for IUS was documented. Bowel wall thickness [BWT], colour Doppler signal [CDS], presence of fatty-wrapping, loss of colonic haustrations, loss of wall layer stratification, presence of reactive lymph nodes, and absence of small bowel motility were scored per segment [terminal ileum, ascending colon, transverse colon, descending colon, sigmoid, and rectum]. Presence of disease activity was scored per segment and confirmed when BWT >2.0 and 3.0 mm for the small bowel and colon, respectively, and a second parameter was pathological [Supplementary Table 1, available as Supplementary data at ECCO-JCC online]. In addition, presence of complications was documented. Quality of the images was also documented and scored as good [proper evaluation], moderate [sufficient but incomplete evaluation], poor [hard to draw conclusions], and very poor [no conclusions possible]. Furthermore, reasons for poor acquisition were collected [i.e., artefacts, reduced quality due to bowel gas, abdominal fat, complex anatomy due to surgery, etc.]. Uncertainty was scored by evaluation of the IUS report stating that the ultrasonographer was uncertain due bowel gas, minor findings, abdominal fat, or complex anatomy. Inconclusive was scored as poor image quality. The IUS results were considered adequate when a maximum of one bowel segment [excluding the rectum] could not be assessed.
2.5. Additional imaging
The following data on endoscopy and MRI performed after IUS were collected by assessing reports: presence of inflammation, location of inflammation, presence of complications, and location of complications per segment. When additional imaging was performed within 8 weeks of IUS and the results of IUS and additional imaging were considered adequate, the outcomes were compared. All endoscopies were performed by an accredited gastroenterologist, according to local protocol. Endoscopic disease activity was defined as an eMayo score ≥1 and a Simple Endoscopic Score for CD [SES-CD] score ≥3 in at least one segment. Findings were considered comparable when disease activity, complications, or normal findings were detected in the same locations in the bowel. Since this was a retrospective study, we did not compare disease severity between IUS, endoscopy, and MRI.
2.6. Statistical analysis
Descriptive statistics were used to study the population. Differences in proportions were tested with the chi square test. Differences in not-normally distributed continuous variables were tested using a Mann–Whitney U test, and correlation was computed using Spearman’s correlation coefficient. A value of 0.00–0.10 was considered as negligible correlation, 0.10–0.39 as weak correlation, 0.40–0.69 as moderate correlation, 0.70–0.89 as strong correlation, and 0.90–1.00 as very strong correlation.23 Agreement between dichotomous variables was also tested with Cohen’s kappa statistics. A value of 0.0–0.20 was considered as slight agreement, 0.21–0.4 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement and 0.81–1.0 as almost perfect agreement.24,25 A p-value <0.05 was considered statistically significant. All analyses were performed with SPSS 25.0 software [IBM Corporation, Armonk, NY, USA].
2.7. Ethical approval and patient consent
This study was approved by the ethical committee of the Academic Medical Center Amsterdam. All data were anonymously extracted from the patient records and retrieving informed consent was therefore not necessary.
3. Results
3.1. Patient population
We studied 301 patients with confirmed IBD, in whom 345 IUS examinations were performed. Of these, 242 had CD and 59 UC [280 CD and 65 UC examinations, respectively]. Cohort characteristics are described in Table 1.
Table 1.
Cohort characteristics
| IUS examinations | n = 345 |
|---|---|
| CD patients | n = 242 |
| Examinations in CD patients; n | n = 280 |
| Male; n [%] | 92 [38.0%] |
| Age at IUS; median [range], years | 37 [27–52] |
| Disease duration at time of IUS in median years [IQR] | 10 [4–19] |
| Montreal classification in CD patients | |
| A1 [<16 years] | 45 [18.6%] |
| A2 [17–40 years] | 164 [67.8%] |
| A3 [>40 years] | 33 [13.6%] |
| L1 [ileum] | 106 [43.8%] |
| L2 [colon] | 36 [14.9%] |
| L3 [ileocolonic] | 99 [40.9%] |
| + L4 [upper GI] | 1 [5.8%] |
| L4 only | 1 [0.4%] |
| B1 [non stricturing, non-penetrating] | 138 [57.0%] |
| B2 [stricturing] | 58 [24.0%] |
| B3 [penetrating] | 46 [19.0%] |
| P [perianal disease] | 66 [27.3%] |
| Previous surgical resection at time of IUS | |
| ICR and ileal re-resections | 113 [40.4%] |
| [partial] colonic resection | 31 [11.1%] |
| Medication use at time of IUS | 112 [40.0%] |
| Biologics [infliximab, adalimumab, vedolizumab, ustekinumab] | 64 [22.9%] |
| Immunomodulators [thiopurines/methotrexate] | 48 [17.1%] |
| Corticosteroids [oral/topical] 5-ASA [oral/topical] | 10 [3.6%] |
| UC patients | n = 59 |
| IUS examinations in UC patients; n | 65 |
| Male; n [%] | 22 [37.3%] |
| Age at IUS; median [range], years | 40 [27–51] |
| Disease duration at time of IUS in median years [IQR] | 7 [5–13] |
| Disease extent | |
| E1 [proctitis] | 5 [8.5%] |
| E2 [left-sided] | 19 [32.2%] |
| E3 [pancolitis] | 35 [59.3%] |
| Previous surgical resection at IUS | 0 [0%] |
| Medication use at time of IUS | 25 [38.5%] |
| Biologics | 17 [26.2%] |
| Immunomodulators | 13 [20.0%] |
| Corticosteroids [oral/topical] | 38 [58.5%] |
| 5-ASA Tofacitinib |
3 [4.6%] |
CD, Crohn’s disease; IUS, intestinal ultrasound; IQR, interquartile range; GI, gastrointestinal; 5-ASA, 5-aminosalicylate; UC, ulcerative colitis; ICR, ileocaecal resection.
3.2. Intestinal ultrasound
The indications for IUS are shown in Table 2, the most common being symptoms of active disease and/or elevated FCP. Of 345 IUS examinations, 190 [55.1%] showed active disease and 113 [32.8%] showed no signs of inflammation. In 37 [10.7%] examinations, presence of inflammation was uncertain, and in five [1.4%] the examinations were inconclusive. A total of 73 complications were detected in CD patients [i.e., strictures, abscesses, phlegmons, and fistulas]. The results of the IUS examinations are summarised in Table 3. Uncertainty and low image quality were explained by a variety of reasons [i.e., minor findings, bowel gas, abdominal fat, complex surgical history], as shown in Table 4.
Table 2.
Indications for IUS [patients could have more than one indication].
| Indication | n [%] |
|---|---|
| Symptoms of active disease | 198 [57.4%] |
| Suspicion of stricture/abscess | 85 [24.6%] |
| Elevated FCP | 130 [37.7%] |
| Elevated CRP | 115 [33.3%] |
| Monitoring treatment response | 40 [11.6%] |
IUS, intesrinal ultrasound; FCP, faecal calprotectin; CRP, C-reactive protein.
Table 3.
Summary of IUS findings.
| 3a. Disease activity | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD [n = 280] | Inflammation | No inflammation | Uncertain | Inconclusive | ||||
| Overall | 161 [57.5%] | 83 [29.6%] | 31 [11.1%] | 5 [1.8%] | ||||
| Ileum | 118 [42.1%] | 128 [45.7%] | 23 [8.2%] | 11 [3.9%] | ||||
| Ascending colon | 33 [11.8%] | 233 [83.2%] | 3 [1.1%] | 11 [3.9%] | ||||
| Transverse colon | 26 [9.3%] | 239 [85.4%] | 3 [1.1%] | 12 [4.3%] | ||||
| Descending colon | 32 [11.4%] | 235 [83.9%] | 6 [2.1%] | 7 [2.5%] | ||||
| Sigmoid | 34 [12.1%] | 226 [80.7%] | 12 [4.3%] | 8 [2.9%] | ||||
| Rectum | 7 [2.5%] | 124 [44.3%] | 8 [2.9%] | 141 [50.4%] | ||||
| Ileocolonic anastomosis [n = 113] | 48 [17.1%] | 55 [48.7%] | 8 [7.1%] | 3 [2.7%] | ||||
| Proximal small bowel | 9 [3.2%] | 265 [94.6%] | 0 [0%] | 6 [2.1%] | ||||
| Ileum affected length | ||||||||
| Length cm | 0–5 | 5–10 | 10–15 | 15–20 | 20–25 | 25–30 | >30 | Unknown |
| n = 118 | 14 | 28 | 22 | 8 | 8 | 8 | 3 | 27a |
| % | 11.9% | 23.7% | 18.6% | 6.8% | 6.8% | 6.8% | 2.5% | 22.9% |
| UC [n = 65] | ||||||||
| Overall | 29 [44.6%] | 30 [46.2%] | 6 [9.2%] | 0 [0%] | ||||
| Ascending colon | 6 [9.2%] | 58 [89.2%] | 1 [1.5%] | 0 [0%] | ||||
| Transverse colon | 7 [10.8%] | 58 [89.2%] | 0 [0%] | 0 [0%] | ||||
| Descending colon | 22 [33.8%] | 42 [64.6%] | 1 [1.5%] | 0 [0%] | ||||
| Sigmoid | 28 [43.1%] | 33 [50.8%] | 3 [4.6%] | 1 [1.5%] | ||||
| Rectum | 25 [38.5%] | 19 [29.2%] | 4 [6.2%] | 17 [26.2%] | ||||
| 3b. Complications in CD | ||||||||
| Present | Absent | Uncertain | Inconclusive | |||||
| Stricture | 48 [17.1%] | 209 [74.7%] | 22 [7.9%] | 6 [2.1%] | ||||
| Prestenotic dilation | 29 [10.4%] | 236 [84.3%] | 8 [2.9%] | 7 [2.5%] | ||||
| Phlegmon | 12 [4.3%] | 260 [92.9%] | 1 [0.4%] | 7 [2.5%] | ||||
| Abscess | 6 [2.1%] | 264 [94.3%] | 3 [1.1%] | 7 [2.5%] | ||||
| Fistula | 7 [2.5%] | 261 [93.3%] | 6 [1.8%] | 6 [1.8%] |
Uncertain = doubt regarding disease activity due to various reasons [i.e., suboptimal images, minor findings]. Inconclusive = no conclusions possible due to poor image quality
IUS, intestinal ultrasound; CD, Crohn’s disease; UC, ulcerative colitis.
aReasons for unknown length not shown
Table 4.
Image quality and reasons for uncertainty.
| Image quality | |
|---|---|
| Good | 243 [70.4%] |
| Moderate | 68 [19.7%] |
| Poor | 29 [8.4%] |
| Very poor | 5 [1.4%] |
| Reasons for uncertainty [patients could have more than one reason] | |
| Minor findings | 28 [8.1%] |
| Bowel gas | 31 [9.0%] |
| Abdominal fat | 34 [9.9%] |
| Complex surgical history | 8 [2.3%] |
3.3. Disease management after IUS
Disease management after IUS is shown in Table 5. In 207/345 [60%] cases, the treatment plan was changed [i.e., medication, imaging, surgery]. Medication use was changed in 99/207 [47.8%] cases and in 122/207 [58.9%] cases, additional imaging or endoscopy was planned after IUS. In 77/207 [37.2%] cases, additional evaluation was performed because this was considered necessary by the treating gastroenterologist [i.e., more information needed or IUS insufficient]. Surgery was performed 16 times after IUS. Reasons for additional evaluation are shown in Table 6.
Table 5.
Treatment decisions after IUS.
| CD examinations [n = 280] | UC examinations [n = 65] | |
|---|---|---|
| No change | 106 [37.9%] | 32 [49.2%] |
| Imaging n = 122 | 104 | 18 |
| Endoscopy | ||
| Total | 73[26.1%] | 16 [24.6%] |
| Dilation stricture | 13 [12.3%] | - |
| MRI | 23 [8.6%] | 1 [3.1%] |
| CT scan | 8 [3.5%] | 1 [3.1%] |
| Medication change n = 99 | ||
| Biologics | ||
| Start | 25 [8.9%] | 3 [4.6%] |
| Dose intensification | 4 [1.4%] | 3 [4.6%] |
| Dose de-escalation | 1 [0.4%] | - |
| Stop | 1 [0.4%] | - |
| Immunomodulators | ||
| Start | 23 [8.2%] | 3 [4.6%] |
| Stop | 2 [0.7%] | 1 [1.5%] |
| Tofacitinib | - | 1 [1.5%] |
| Stop | ||
| Corticosteroids [oral/topical] | ||
| Start | 9 [3.2%] | 4 [6.2%] |
| Stop | 1 [0.5%] | - |
| Budesonide | ||
| Start | 8 [2.9%] | 1 [1.5%] |
| Stop | - | 1 [1.5%] |
| 5-ASA | 0 [0%] | 6 [9.2%] |
| Inclusion in clinical trial | 1 [0.5%] | 1 [1.5%] |
| Surgical resection | ||
| Total | 16 [5.7%] | - |
IUS, intestinal ultrasound; CD, Crohn’s disease; UC, ulcerative colitis; MRI, magnetic resonance imaging; CT, computed tomography; 5-ASA, 5-aminosalicylate
Table 6.
Reasons for additional endoscopy, MRI or CT.
| N = 122 | |
|---|---|
| IUS insufficient/additional evaluation deemed necessary | 77 |
| Baseline evaluation before starting or follow-up of biologic treatment | 13 |
| Stricture dilation | 14 |
| Evaluation of treatment response | 3 |
| Extensive complications | 4 |
| Inclusion in clinical trial | 8 |
| Melaena | 1 |
| Suspicion of malignancy | 2 |
IUS, intestinal ultrasound; MRI, magnetic resonance imaging; CT, computed tomography.
3.4. IUS versus clinical symptoms
In total, 254/345 [73.6%] patients who underwent IUS were symptomatic. IUS examinations with uncertain or inconclusive outcome were excluded from comparison with clinical symptoms [n = 42]. IUS showed inflammation and/or complications in 145/222 [65.2%] symptomatic patients. In comparison, IUS showed inflammation and/or complications in 44/81 [54.3%] asymptomatic patients [p = 0.080]. In 117/173 [67.6%] symptomatic CD patients, IUS showed inflammation and/or complications. Conversely, IUS showed inflammation and/or complications in 43/71 [60.5%] asymptomatic CD patients [p = 0.291]. In 28/49 [57.1%] symptomatic UC patients, IUS showed active disease. In comparison, IUS showed active disease in 1/10 [10.0%] asymptomatic UC patients [p = 0.007].
3.5. IUS versus biomarkers
FCP measurements were available within 1 month of IUS in 229/345 [66.4%] cases, and the median time between FCP measurement and IUS was 7 days (interquartile range [IQR] 1–16). FCP levels were compared with those IUS examinations with certain outcome [n = 195]. The median FCP level was 664 µg/g [IQR 278–1800] and 75 µg/g [IQR 22–351] in all IBD patients who had IUS examinations showing active or inactive disease, respectively [p <0.001]. In CD patients, the median FCP level was 517 µg/g [IQR 224–1706] versus 79 µg/g [IQR 25–276] [p <0.001], and for UC patients the median FCP level was 1720 µg/g [IQR 400–3304] versus 75 µg/g [IQR 18–772] in IUS examinations showing active or inactive disease [p <0.001]. IUS showed active disease in 110/155 [71.0%] cases with FCP >50 µg/g versus 6/40 [15.0%] cases with FCP <50 µg/g [p <0.001]. The same comparisons were made for an FCP cut-off of 150 µg/g and of 250 µg/g, based on previous work.26,27 IUS showed active disease in 101/137 [73.7%] cases with FCP >150 µg/g versus 14/58 [24.1%] cases with FCP <150 µg/g [p <0.001], and IUS showed active disease in 90/113 [79.6%%] cases with FCP >250 µg/g versus 26/82 [31.7%] cases with FCP <250 µg/g [p <0.001].
CRP measurements were available within 1 month of IUS in 275 [79.7%] cases, and the median time between CRP measurement and IUS was 5 days [IQR 0–17]. CRP levels were compared with IUS examinations with certain outcome [n = 259]. When comparing active disease or complications versus inactive disease on IUS, the median CRP level in was 5.5 mg/L [IQR 1.9–20.1] versus 2.1 mg/L [IQR 0.8–5.5] [p <0.001]. In CD patients, the median CRP level was 6.7 mg/L [IQR 1.8–20.5] versus 1.9 mg/L [IQR 0.7–3.7] [p <0.001], and in UC patients the median CRP level was 3.6 mg/L [IQR 1.4–20.8] versus 1.8 [IQR 0.6–6.4] [p = 0.076]. IUS showed disease activity or complications in 86/111 [77.5%] cases with CRP level >5 mg/L versus 76/148 [51.4%] in cases with CRP level <5 mg/L [p <0.001].
3.6. Endoscopy after IUS
Endoscopy was planned following IUS in 89 cases and was performed within 8 weeks after IUS in 65 cases. The median time between IUS and endoscopy was 4 weeks [IQR 1–6]. Of these, 51 IUS examinations and endoscopies had a certain outcome, which were analysed further. Overall, presence or absence of disease activity was comparable between IUS and endoscopy 44 out of 51 times [86.3%] [p <0.001] and showed strong correlation [ρ = 0.70, p <0.0001]. The kappa agreement was substantial [κ = 0.61; p <0.001]. In 36/41 [87.8%] cases, both IUS and endoscopy showed active disease. In 5/13 [38.5%] cases, endoscopy showed active disease whereas IUS did not. Of these, 2/5 had rectal disease and 1/5 had minor findings on IUS, not considered as active disease. Of the remaining two with normal IUS, one had ileitis and one had left-sided Crohn’s colitis [SES-CD 4] on endoscopy. Faecal calprotectin levels were elevated in all these five patients[4/5 FCP >150 µg/g and 1/5 FCP >50 µg/g]. In 2/10 [20.0%] cases, IUS showed active colonic disease whereas endoscopy did not. Both patients [one CD and one UC] were treated with corticosteroids for several weeks before endoscopy was performed. In 12/12 cases with a stricture on IUS, this stricture was also seen on endoscopy. However, endoscopy identified a stricture which was not seen on IUS in four cases. Full comparison between IUS and endoscopy is shown in Table 7.
Table 7.
Comparison of IUS findings versus endoscopy and when IUS and additional imaging were adequate and performed within 2 months.
| IUS versus endoscopy | |||||
|---|---|---|---|---|---|
| n = 51 | Comparable findings | IUS active | IUS inconclusive | Endoscopy active | Endoscopy inconclusive |
| Overall | 44/51 [86.3%] | 38/51 [74.5%] | - | 41/51 [80.4%] | - |
| TI | 37/38 [97.4%] | 19/51 [37.3%] | 2/51 [3.9%] | 20/51 [39.2%] | 11/51 [21.6%] |
| AC | 38/43 [88.4%] | 3/51 [5.9%] | - | 4/51 [7.8%] | 8/51 [15.7%] |
| TC | 36/45 [80.0%] | 10/51 [19.6%] | - | 9/51 [17.6%] | 6/51 [11.8%] |
| DC | 44/51 [86.3%] | 15/51 [29.4%] | - | 14/51 [27.5%] | - |
| SC | 44/50 [88.0%] | 17/51 [33.3%] | - | 16/51 [31.4%] | - |
| Rectum | 26/40 [65.0%] | 10/51 [19.6%] | 11/51 [21.6%] | 21/51 [41.2%] | - |
| Stricture | 47/51 [92.2%] | 12/51 [23.5%] | - | 16/51 [31.4%] | - |
| n = 15 | Comparable findings | IUS present | MRI present | ||
| Disease activity | |||||
| Overall | 12 [80.0%] | 9 [60%] | 12 [80.0%] | ||
| Ileal disease | 12 [80.0%] | 8 [53.3%] | 11 [73.3%] | ||
| Proximal small bowel disease | 9 [60.0%] | 1 [6.7%] | 4 [26.7%] | ||
| Complications | |||||
| Stricture | 12 [80.0%] | 5 [33.3%] | 8 [53.3%] | ||
| Intra-abdominal abscess | 15 [100%] | 0 [0%] | 0 [0%] | ||
| Intra-abdominal fistula | 12 [80.0%] | 1 [6.7%] | 1 [6.7%] |
Overall = presence/absence of disease activity for endoscopy and presence/absence of disease activity and/or complications for MRI. IUS inconclusive = segment not investigated due to various reasons. Endoscopy inconclusive = not investigated due to various reasons [i.e., sigmoidoscopy, technical difficulties, etc.].
AC, ascending colon; DC, descending colon; IUS, intestinal ultrasound; MRI, magnetic resonance imaging; SC, sigmoid colon; TC, transverse colon; TI, terminal ileum.
3.7. MRI after IUS
MRI was planned after IUS in 24 cases of which 19 were conducted within 8 weeks. The median time between MRI was 4 weeks [IQR 3–7]. In 15 cases, the IUS results were considered adequate [see below] and were analysed further. Overall, assessment of disease activity and strictures was comparable between IUS and MRI in 12/15 [80.0%] [p <0.001] cases and showed strong correlation [ρ = 0.75, p <0.0001]. The agreement was moderate [κ = 0.47; p = 0.032]. In 15 IUS and MRI examinations with certain outcome [i.e., adequate image quality and no doubt] and MRI performed within 8 weeks, 9/12 [75.0%] IUS examinations showed active disease comparable to MRI versus 3/12 [25.0%] IUS examinations that did not show active disease where MRI showed active disease [p = 0.018]. Full comparison between IUS and MRI is shown in Table 7.
3.8. Certain versus uncertain IUS
Differences between patients with certain [n = 303] or uncertain [n = 42] IUS outcomes were analysed. Image quality in IUS with uncertain outcome was poor or very poor in 12/42 [28.6%] cases versus 22/303 [7.3%] in patients with certain outcome [p <0.001]. The most frequent reasons for poor IUS quality were abdominal fat and presence of bowel gas. The proportion of symptomatic patients was comparable between cases with certain versus uncertain IUS outcome (222/303 [73.3%] versus 32/42 [76.3%]; p = 0.687). In addition, no significant difference was observed between CD and UC patients [data not shown]. Median FCP level was not significantly different between patients with uncertain versus certain IUS outcome (181 µg/g [IQR 47–600] versus 350 µg/g [IQR 97–1351] [p = 0.134]). The median CRP level in patients with uncertain IUS outcome was 2.7 mg/L [IQR 1.1–5.4] versus 3.3 mg/L [IQR 1.1–11.7] in patients with certain IUS outcome [p = 0.374]. Endoscopy was planned in 17/42 [40.5%] cases with uncertain IUS outcome versus 72/303 [23.8%] cases with certain IUS outcome [p = 0.020]. When endoscopy was performed within 8 weeks after IUS, endoscopy showed active disease in 7/10 [70%] cases with uncertain IUS outcome versus 41/51 [80.4%] cases with certain IUS outcome [p = 0.624]. In 2/53 [3.8%] cases with certain IUS outcome, the endoscopy outcome was inconclusive [i.e., due to faecal contamination, pain,or technical difficulties.]. Additional MRI was planned in 5/42 [11.9%] cases with uncertain IUS outcome versus 19/303 [6.3%] with certain IUS outcome [p = 0.179]. When MRI was performed within 8 weeks after IUS, MRI showed active disease in 0/4 [0%] cases with uncertain IUS outcome.
3.9. IUS from 2016–2018 versus IUS in 2019
Characteristics of the two cohorts were compared. Totals of 250 and 95 IUS examinations were performed in the first and second cohort, respectively. For the same period in 2016 [October–December] and 2019 [October–December], 40 and 95 IUS examinations were performed, respectively. Distributions of age, gender, disease duration, age at disease onset, and Montreal classification were not different between cohorts. In addition, presence of clinical symptoms was equally distributed in both cohorts. In the first cohort, FCP was more frequently ≥50 µg/g [83.7% vs 53.7%, p <0.0001] and ≥250 µg/g [57.1% vs 40.0%, p = 0.004] compared with the second cohort. Other biochemical parameters were not significantly different between cohorts.
In the first cohort, confirmation of active inflammation [63% vs 43%, p = 0.001] and complications [30.2% vs 11.1%, p <0.0001] was more often an indication to request IUS than in the second cohort. In addition, IUS showed active disease more often in the first cohort as opposed to the second cohort [70% vs 55.8%, p = 0.017]. In the second cohort, monitoring treatment response was more often an indication to perform IUS when compared with the first cohort [25.0% vs 6.4%, p <0.0001]. Furthermore, patients in the first cohort were treated less with corticosteroids [9.6% vs 39%, p <0.0001] and biologics [35.2% vs 51.6%, p = 0.004], whereas use of thiopurines and methotrexate was similar between the cohorts. Disease management after IUS was comparable, except for a more frequent decision to continue current treatment in the second cohort [36.4% vs 49.5%, p = 0.019].
There were no differences in certainty of the IUS conclusion, visibility of segments, and detection of complications. Furthermore, the results of subsequent endoscopy or MRI and their correlation with IUS did not differ between the groups. However, we did perform MRI more frequently in the first cohort when compared with the second cohort [8.4% vs 2.1%, p = 0.024]. There was no difference in amount of performed endoscopies between the cohorts.
3.10. Proposal of a POC IUS algorithm
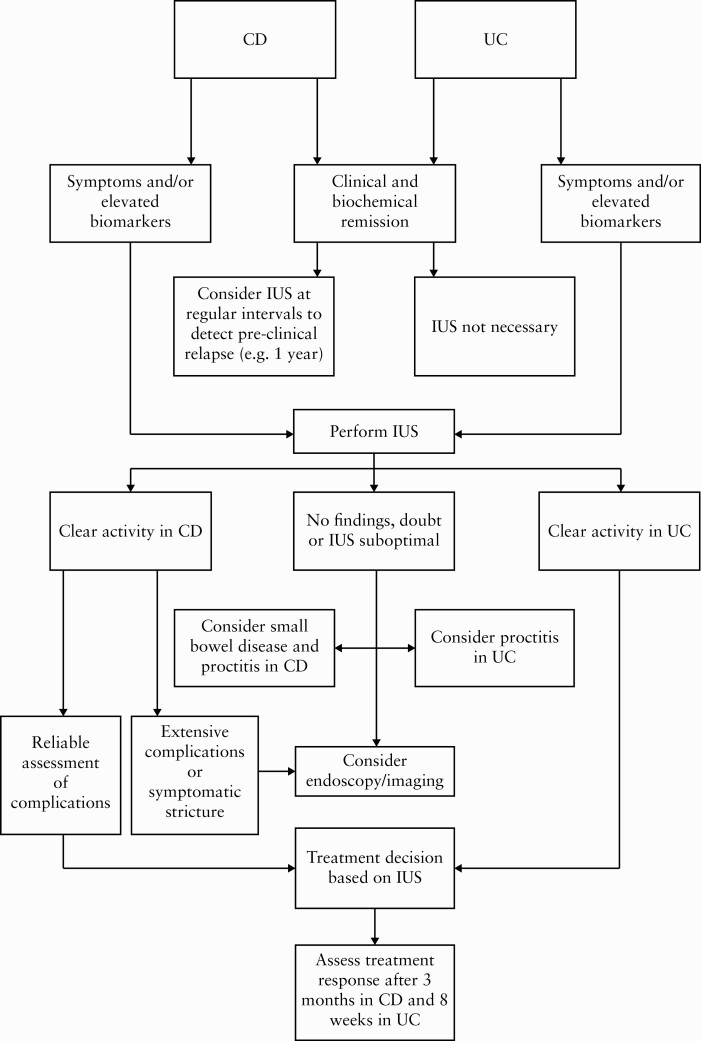
In Figure 1 we propose a POC IUS algorithm, based on the results of this study and previous studies.
Figure 1.
Proposal of a point-of-care intestinal ultrasound algorithm. CD, Crohn’s disease; UC, ulcerative colitis; IUS, intestinal ultrasound.
4. Discussion
In this study we describe the validity of POC IUS and its impact on disease management in a large real-world cohort of IBD patients. In our cohort, IUS revealed presence of disease activity in more than half of patients, leading to change in medication in almost half of these patients. This is in line with previous work.21 In most cases, treatment was initiated or upscaled as opposed to downscaling or stopping treatment.
When comparing IUS findings with clinical symptoms, we observed a large proportion of asymptomatic CD patients with signs of inflammation and/or complications on IUS. In CD patients, symptoms show poor correlation with biochemical or endoscopic disease activity and complications.21,28,29 Therefore, IUS had an important role in the detection of preclinical or subclinical relapse in CD patients, especially when combined with biochemical markers. Conversely, clinical symptoms are more reliable in UC patients, as shown by our data and in previous studies.30–32 Therefore, presence of disease activity on IUS in UC patients, together with clinical disease activity or elevated biochemical markers, could be sufficient to guide disease management. Additionally, IUS is sufficient in most UC patients with symptoms and/or elevated biomarkers to confirm disease activity and to determine disease extent, as also suggested previously.33
Endoscopy did not provide additional information regarding presence of inflammation in 86.3% of cases when the IUS results were considered adequate. As such, IUS has significant cost-, time- and burden-saving potential. Several studies have shown high accuracy, sensitivity, and specificity for IUS when compared with other modalities in both CD and UC patients.17,33–40 In our cohort, we also showed comparable detection of inflammation for IUS and endoscopy. However, it is important to note that we did not compare disease severity between the two modalities and that we did not include IUS examinations with uncertain outcome in the comparison.
In our cohort, we found endoscopically active disease in 5/13 patients despite ‘normal’ IUS. Two of these patients had rectal disease, and in the other three IUS did not detect ileal or colonic disease. Additionally, in 7/10 [70%] patients with uncertain outcome on IUS, active disease was observed with endoscopy. Hence, a certain proportion of patients will benefit from additional investigation even when IUS does not indicate disease activity. Clinical disease activity and/or elevated biomarkers could guide the decision to perform additional evaluation in these cases.
In approximately 25% of CD patients a complication was found, most often a stricture with or without prestenotic dilation. Since many CD patients develop strictures over time, POC IUS could play an important role in early detection of strictures and guiding decision making in these patients. In our cohort, IUS guided disease management such as referring patients for endoscopic dilation and/or surgery. Although data are lacking on identifying with IUS strictures which are most suitable for endoscopic dilation, anti-inflammatory treatment, or surgery, IUS has the potential to guide disease management, and ongoing studies are focusing on this [Netherlands Trial Register: NL9105].
IUS is less accurate when assessing the proximal small bowel or the rectum.33 In our study, MRI showed small bowel disease more often than IUS in a small proportion of patients. Taylor et al. found similar results when MRI and IUS were compared in small bowel CD.38 Furthermore, proctitis was more frequently shown with endoscopy than with IUS. This is in line with previous work, showing that IUS is generally not feasible for assessment of the rectum.33 However, since IUS was comparable with endoscopy for assessment of the rectum in 65% of cases, it could be useful in some cases, especially in patients with established proctitis and in combination with FCP. However, a recent study found perineal ultrasound to be a more accurate but non-invasive alternative to assess the rectum when compared with endoscopy.41
Furthermore, we have studied the implementation of IUS over time in clinical practice. When we started using IUS in our clinic in 2016, all examinations were performed by one physician, predominantly to confirm active disease or diagnose complications in patients with clinical symptoms or elevated biomarkers. In recent years, the paradigm for IUS has expanded towards monitoring treatment response and reassuring regarding quiescent disease. This has resulted in an increased demand for IUS examinations, and more physicians who were trained at our clinic are now performing IUS. Concurrently, MRI was performed less frequently in the second cohort. In this cohort more patients received therapy with probably milder disease activity, and hence fewer MRI requests. However, the increase of IUS in the second cohort might also be a valid reason for the decline in MRI. Although future research should confirm this statement, we show that with sufficient expertise, IUS could be used as first non-invasive choice in a POC setting.
Overall, our findings indicate that POC IUS has the potential to reduce the need for endoscopy and MRI, with the latter already occurring in clinical practice. It seems that additional evaluation should mainly be considered when the results of IUS are uncertain, in case of suspicion of small bowel disease or proctitis, or in cases of extensive complications such as multiple strictures and complex fistulising disease. Indeed, in our cohort, endoscopy was more often planned when the IUS outcome was uncertain. Other indications for endoscopy include stricture dilation or screening for malignancy. It has been postulated that endoscopy or MRI should also be considered for decisions such as starting and monitoring treatment with biologics. This too may be subject for debate, since studies have shown that IUS can be reliably used for follow-up of biologic treatment.1,12,14,42–44 More studies on this topic are expected in the future.
Other studies that investigated the implementation of POC IUS are limited. Novak et al. studied POC IUS in 49 CD patients by comparing POC IUS with regular care in a blinded study.21 They found that POC IUS changed clinical management in 60% of patients, which is similar to our findings. Additionally, they showed that many asymptomatic CD patients had signs of active disease on IUS, also in concordance with our findings. Shatanantan et al. compared POC IUS in 74 IBD patients with ileocolonoscopy in a blinded study, and found high sensitivity and specificity of POC IUS for detection of disease activity in both UC and CD.22 A third study found also a high correlation for IUS and endoscopic disease activity in CD patients.45 In our cohort we found similar findings. We studied a large real-world cohort and further demonstrated the impact of IUS on clinical decision making. Furthermore, we performed a detailed analysis of IUS outcomes and reasons for uncertainty in daily clinical practice. With regards to uncertainty: bowel gas, ,and mild inflammation all contributed to poor image quality or uncertainty. In these patients, endoscopy or other cross-sectional imaging techniques are more suitable to detect inflammation. However, endoscopy was performed only in a small number of patients with uncertain IUS outcome; thus controlled studies are needed to elucidate the role of additional endoscopy in patients with uncertain outcomes at IUS, as inflammation might be limited or absent. On the contrary, an unsuccessful endoscopy could be an additional reason for IUS to objectify disease activity, predominantly in the terminal ilem [TI] or proximal small bowel.45
To illustrate the use of POC IUS in daily clinical practice, we propose an algorithm which may have the potential to reduce unnecessary additional evaluation in the future and to reduce delay in disease management. In a recent review, Allocca et al. also proposed a POC IUS algorithm based on the available literature.46 This algorithm is mostly comparable with our suggestion. However, we defined a somewhat different strategy between UC and CD patients, since absence of symptoms is more reliable in UC patients. Additionally, we propose when additional imaging should be performed after IUS, such as when the IUS outcome is uncertain. We also propose to assess treatment response at different time points in CD and UC patients, since recent studies suggest that UC patients respond to treatment earlier than CD patients.14,47 However, prospective studies are needed to optimise the implementation of POC IUS for the monitoring of IBD patients. In particular, the best timing for assessment of treatment response and IUS evaluation in patients without symptoms and normal biomarkers is unknown. From a logistic point of view it would also be challenging to frequently perform scheduled IUS in every IBD patient in a large clinic with a large cohort of patients. This emphasises the need for proper risk assessment. In our algorithm we propose to perform POC IUS once a year in CD patients. However, it is plausible that predicted disease progression should also be taken into account when determining the frequency of scheduled IUS.
Our study has several limitations. First, this was a retrospective observational cohort study. Therefore, comparisons with symptoms, biochemical markers, and other imaging modalities were probably less reliable than in a prospective controlled setting. We could not properly account for time, change of symptoms, and change of medication between IUS and additional imaging. Additionally, we did not compare severity between IUS and endoscopy or MRI, as endoscopy/MRI might show already improvement after starting any treatment based on IUS findings. Also, there is a considerable risk for selection bias between the first and second cohort, as IUS became more standard care over time. Hence, reasons for IUS could have changed accordingly. In addition, we did not have a control group receiving no IUS and therefore we could not determine the absolute effect on disease management for IUS. However, we were able to study a large cohort of patients. Additionally, real-world data are more representative of the clinical situation, which could also be considered a strength. For instance, our data may reliably show the incidence of problems that may arise when performing IUS, such as poor image quality and unreliable results due to bowel gas, abdominal fat, or mild disease activity. Regardless, we show a clear impact of IUS in clinical disease management in a real-world cohort.
In conclusion, POC IUS significantly affects disease management in the follow-up of IBD patients and has the potential to reduce the need for additional endoscopy and MRI. We have proposed an algorithm for implementation of POC IUS. Prospective studies are needed to study the optimal implementation and timing of POC IUS in close monitoring and treatment follow-up in daily clinical care.
Supplementary Material
Contributor Information
S Bots, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
F De Voogd, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
M De Jong, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
V Ligtvoet, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
M Löwenberg, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
M Duijvestein, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
C Y Ponsioen, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
G D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
K B Gecse, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Location AMC, Amsterdam, The Netherlands.
Funding
Dr. Falk Pharma Benelux B.V. financially supported implementation of IUS at our clinic.
Conflict of Interest
SB has served as speaker for Abbvie, Merck, Sharp & Dome, Takeda, Jansen Cilag, Pfizer, and Tillotts. FV received speaker fees or honoraria from AbbVie and Janssen. MJ received speaker fees from Biogen. VML has served as speaker and/or principal investigator for Abbvie, Covidien, Dr. Falk, Ferring Pharmaceuticals, Merck Sharp & Dohme, Receptos, Takeda, Tillotts; and Tramedico; he has received research grants from AbbVie, Merck Sharp & Dohme, Achmea Healthcare and ZonMW. MD received advisory fees from Echo Pharma and Robarts Clinical Trials Inc., speaker fees from Janssen, Merck, Pfizer, Takeda, and Tillotts Pharma, and non-financial support from Dr Falk Pharma. CP has received a research grant from Takeda, served as advisor for Takeda, Pliant, and Shire, and received speaker fees from Tillotts and Pfizer. GD has served as adviser for Abbvie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Ferring, Dr. Falk Pharma, Engene, Galapagos, Gilead, Glaxo Smith Kline, Hospira, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Novonordisk, Pfizer, Prometheus Laboratories/Nestle, Protagonist, Receptos, Robarts Clinical Trials Inc., Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor, and received speaker fees from Abbvie, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Shire, Millenium/Takeda, Tillotts, and Vifor. KG has received grants from Pfizer and Celltrion, consultancy fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, Immunic Therapeutics, Janssen Pharmaceuticals, Novartis, Pfizer, Samsung Bioepis, and Takeda, and speaker’s honoraria from Celltrion, Ferring, Janssen Pharmaceuticals, Novartis, Pfizer Inc, Samsung Bioepis, Takeda, and Tillotts.
Author Contributions
SB: study design, study selection, data acquisition, data interpretation, writing first draft of the manuscript and final approval of the manuscript. FV: study design, study selection, data acquisition, data interpretation, writing first draft of the manuscript and final approval of the manuscript. MJ: data acquisition, data interpretation, revising and final approval of the manuscript. VL: data acquisition, revising and final approval of the manuscript. ML: revising the manuscript and final approval of the manuscript. MD: revising the manuscript and final approval of the manuscript. CP: revising the manuscript and final approval of the manuscript. GD: revising the manuscript and final approval of the manuscript. KG: study design, revising the manuscript, and final approval of the manuscript.
References
- 1. Maaser C, Sturm A, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 2. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 5. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in Inflammatory Bowel Disease. J Crohns Colitis 2019;13:144. –64. [DOI] [PubMed] [Google Scholar]
- 6. Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohns Colitis 2012;6:412–8. [DOI] [PubMed] [Google Scholar]
- 7. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82. [DOI] [PubMed] [Google Scholar]
- 8. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000;119:15–22. [DOI] [PubMed] [Google Scholar]
- 9. Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: Role in diagnosis, management, and treatment. World J Gastroenterol 2018;24:4014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharara AI, El Reda ZD, Harb AH, et al. The burden of bowel preparations in patients undergoing elective colonoscopy. United European Gastroenterol J 2016;4:314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 2014;146:374–82.e1. [DOI] [PubMed] [Google Scholar]
- 12. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017;15:535. –42. [DOI] [PubMed] [Google Scholar]
- 13. Novak KL, Nylund K, Maaser C, et al. Expert Consensus on Optimal Acquisition and Development of the International Bowel Ultrasound Segmental Activity Score [IBUS-SAS]: A reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s Disease. J Crohns Colitis 2021;15:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maaser C, Petersen F, Helwig U, et al. ; German IBD Study Group and the TRUST&UC study group.. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020;69:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Voogd F, Wilkens R, Gecse K, et al. A reliability study: strong inter-observer agreement of an expert panel for intestinal ultrasound in ulcerative colitis. J Crohns Colitis 2021;15:1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Voogd FAE, Verstockt B, Maaser C, Gecse KB. Point-of-care intestinal ultrasonography in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:209–10. [DOI] [PubMed] [Google Scholar]
- 17. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011;34:125–45. [DOI] [PubMed] [Google Scholar]
- 18. Miles A, Bhatnagar G, Halligan S, et al. ; METRIC investigators. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: patient acceptability and perceived burden. Eur Radiol 2019;29:1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delefortrie Q, Schatt P, Grimmelprez A, et al. Comparison of the Liaison® Calprotectin kit with a well established point of care test [Quantum Blue - Bühlmann-Alere®] in terms of analytical performances and ability to detect relapses amongst a Crohn population in follow-up. Clin Biochem 2016;49:268–73. [DOI] [PubMed] [Google Scholar]
- 20. Rogler G, Aldeguer X, Kruis W, et al. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: expert clinical opinion. J Crohns Colitis 2013;7:670–7. [DOI] [PubMed] [Google Scholar]
- 21. Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn’s disease: impact on clinical decision making. J Crohns Colitis 2015;9:795–801. [DOI] [PubMed] [Google Scholar]
- 22. Sathananthan D, Rajagopalan A, Van De Ven L, et al. Point-of-care gastrointestinal ultrasound in inflammatory bowel disease: An accurate alternative for disease monitoring. JGH Open 2020;4:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. [DOI] [PubMed] [Google Scholar]
- 24. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 25. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med [Zagreb] 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 26. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE] Initiative of the International Organization for the Study of IBD [IOIBD]: Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 27. D’Haens G, Ferrante M, Vermeire S, et al. Faecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 28. Zittan E, Kabakchiev B, Kelly OB, et al. Development of the Harvey-Bradshaw Index-pro [HBI-PRO] score to assess endoscopic disease activity in Crohn’s Disease. J Crohns Colitis 2017;11:543–8. [DOI] [PubMed] [Google Scholar]
- 29. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002;122:512–30. [DOI] [PubMed] [Google Scholar]
- 30. D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007;132:763–86. [DOI] [PubMed] [Google Scholar]
- 31. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith RL, Taylor KM, Friedman AB, Gibson RN, Gibson PR. Systematic review: Clinical utility of gastrointestinal ultrasound in the diagnosis, assessment and management of patients with ulcerative colitis. J Crohns Colitis 2020;14:465–79. [DOI] [PubMed] [Google Scholar]
- 34. Bots S, Nylund K, Löwenberg M, Gecse K, Gilja OH, D’Haens G. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018;12:920–9. [DOI] [PubMed] [Google Scholar]
- 35. Goodsall TM, Nguyen TM, Parker CE, et al. Systematic review: Gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis 2021;15:125–42. [DOI] [PubMed] [Google Scholar]
- 36. Allocca M, Fiorino G, Bonovas S, et al. Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis 2018;12:1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bots S, Nylund K, Löwenberg M, Gecse K, D’Haens G. Intestinal ultrasound to assess disease activity in ulcerative colitis: Development of a novel UC-Ultrasound index. J Crohns Colitis 2021;15:1264. –71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taylor SA, Mallett S, Bhatnagar G, et al. ; METRIC study investigators. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease [METRIC]: a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sasaki T, Kunisaki R, Kinoshita H, et al. Use of color Doppler ultrasonography for evaluating vascularity of small intestinal lesions in Crohn’s disease: correlation with endoscopic and surgical macroscopic findings. Scand J Gastroenterol 2014;49:295–301. [DOI] [PubMed] [Google Scholar]
- 40. Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J Crohns Colitis 2018;12:1280–7. [DOI] [PubMed] [Google Scholar]
- 41. Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther 2020;51:1373–83. [DOI] [PubMed] [Google Scholar]
- 42. Moreno N, Ripollés T, Paredes JM, et al. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis 2014;8:1079–87. [DOI] [PubMed] [Google Scholar]
- 43. Albshesh A, Ungar B, Ben-Horin S, Eliakim R, Kopylov U, Carter D. Terminal ileum thickness during maintenance therapy is a predictive marker of the outcome of infliximab therapy in Crohn Disease. Inflamm Bowel Dis 2020;26:1619–25. [DOI] [PubMed] [Google Scholar]
- 44. Parente F, Molteni M, Marino B, et al. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig Dis 2009;27:285–90. [DOI] [PubMed] [Google Scholar]
- 45. Wilkens R, Novak KL, Lebeuf-Taylor E, Wilson SR. Impact of intestinal ultrasound on classification and management of Crohn’s disease patients with inconclusive colonoscopy. Can J Gastroenterol Hepatol 2016;2016:8745972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Allocca M, Furfaro F, Fiorino G, Peyrin-Biroulet L, Danese S. Point-of-care ultrasound in inflammatory bowel disease. J Crohns Colitis 2021;15:143–51. [DOI] [PubMed] [Google Scholar]
- 47. Kucharzik T, Wittig BM, Helwig U, et al. ; TRUST study group. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017;15:535–42.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.