Abstract
The formation of chromatin not only compacts the eukaryotic genome into the nucleus but also provides a mechanism for the regulation of all DNA templated processes. Spatial and temporal modulation of chromatin structure is critical in such regulation and involves fine-tuned functioning of the basic subunit of chromatin, the nucleosome. It has become apparent that the nucleosome is an inherently dynamic system, but characterization of these dynamics at the atomic level has remained challenging. NMR spectroscopy is a powerful tool for investigating the conformational ensemble and dynamics of proteins and protein complexes, and recent advances have made the study of large systems possible. Here we review recent studies which utilize NMR spectroscopy to uncover the atomic level conformation and dynamics of the nucleosome and provide a better understanding of the importance of these dynamics in key regulatory events.
Introduction
The eukaryotic genome is packaged into the cell nucleus in the form of chromatin, the basic subunit of which is the nucleosome. Each nucleosome contains a core histone octamer, consisting of one (H3-H4)2 tetramer and two H2A-H2B dimers, wrapped by ~147 base pairs (bp) of DNA1,2. Protruding from this core are the N-termini of all eight histones and the C-termini of H2A, together referred to as the histone tails3. Nucleosomes are separated by a variable length (~10-90 bp) of DNA also known as linker DNA4 (see Figure 1). Importantly, all these chromatin components are quite dynamic and greatly contribute to the regulation of various DNA templated processes. Chromatin structure is known to be modulated by many factors, including ATP-dependent remodeling of the nucleosomes, chemical modification of the DNA and histones, and incorporation of linker histones on the linker DNA.
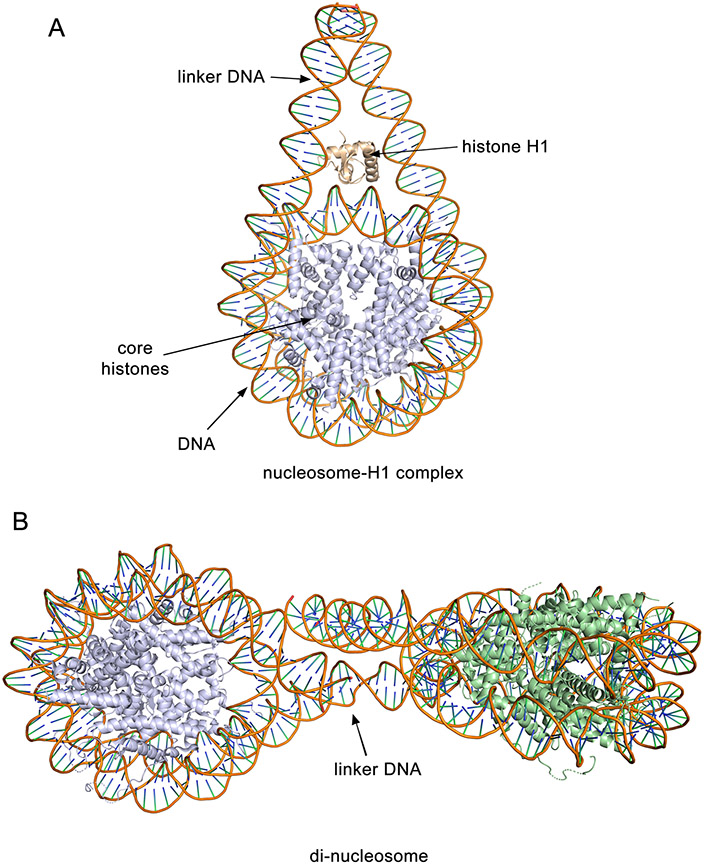
Figure 1.
Crystal structures of the nucleosome. a) The nucleosome in complex with histone H1. Octamer of histones H2A, H2B, H3 and H4 as well as DNA, wrapped around the histone core, are shown in a ribbon diagram and colored light blue and orange, respectively. Histone H1 is shown in wheat. PDB ID: 5nl0. b) Di-nucleosome with coloring the same as in (a) for one nucleosome with the octamer shown in green for the second nucleosome. PDB ID: 1ZBB.
Given the fundamental importance of chromatin, there has been much effort invested in gaining a structural understanding of individual nucleosomes and nucleosome arrays5. In particular, X-ray crystallography, and more recently cryo-electron microscopy (cryo-EM) have been extensively utilized. These studies provide tremendous insight into the nucleosome structure and the mechanism of chromatin compaction but afford a largely static view of these systems. Many studies suggest that the nucleosome is in fact a dynamic unit, sampling a complex conformational ensemble that is functionally important6,7. Yet, characterization of the nucleosome conformational dynamics has been challenging due to its size and complexity.
In the past ten years pioneering studies have demonstrated that nuclear magnetic resonance (NMR) spectroscopy is uniquely suited for investigating the conformation and dynamics of the nucleosome and nucleosome arrays. Advances in solution NMR spectroscopy are allowing for unprecedented insight into the conformational dynamics of both the histone core and histone tails8. Furthermore, solid state NMR techniques provide additional tools to examine compacted nucleosomes and nucleosome arrays9. Here we review what these studies are revealing about the conformational landscape of the nucleosome in a variety of chromatin environments and discuss how various factors modulate nucleosomal ensembles.
The nucleosome as a dynamic complex
The dynamic nature of the histone tails was evident even from early studies of nucleosomes. In nearly all X-ray crystal and cryo-EM structures of the nucleosome the tails, though present, do not resolve. This suggests substantial conformational heterogeneity, which is further supported by a number of biochemical studies (reviewed in 3,10).
It has recently become apparent that the core also experiences conformational fluctuations. The stability of the nucleosome core is due to extensive contacts within the octamer as well as between the octamer and the DNA (see Figure 1). These contacts are well resolved in the crystal structures of the nucleosome (e.g.1). However, the nucleosome conformation seen in these structures potentially represents just one conformational state of the nucleosome. Though the crystal form is likely the most populated state, studies of the nucleosome in solution using single molecule, atomic force microscopy, and small angle X-ray scattering reveal that the nucleosome can at least transiently sample alternative conformational states at low population (substates)6,7,11,12. This includes DNA unwrapping (breathing)13, as well as opening of the octamer through transient destabilization of dimer/tetramer14, and transient DNA looping/bulging15,16 (see Figure 2). Incorporation of histone variants or histone modifications and different DNA sequences have been proposed to shift the population of these states. This, in turn, could facilitate or amend the action of histone modifying enzymes, ATP-dependent remodelers, and RNA polymerase.
Figure 2.
Dynamics of the octamer core and histone H3 tails. (Top) The nucleosome core particle shown left with H2A/H2B dimers shown in dark blue and the H3/H4 tetramer shown in light blue, DNA is shown in gray. Octamer opening and DNA breathing are shown to the right. (Bottom) Models of the different conformational ensembles that the H3 tails adopt in the 147bp-nucleosome core particle (NCP), nucleosome with linker DNA, hexasome, and chromatasome. Coloring is the same as in top with the linker histone H1 shown in wheat.
Though now apparent, nucleosome dynamics remain difficult to characterize, especially at an atomic level. In principle, NMR spectroscopy is a fantastic approach for investigating conformational changes of a system, but at >200 kDa in size the nucleosome pushes the limits of traditional solution state NMR approaches. However, the modular nature of the nucleosome, combined with advances in NMR methodology17 have made it possible to study the solution state conformational dynamics of the nucleosome at the atomic level. In addition, solid state approaches have allowed for characterization of the nucleosome in compact environments mimicking the chromatin state. As sample preparation and spectroscopic approaches have been thoroughly reviewed recently8, we will only briefly touch on those here and instead will focus on the recent findings that are advancing our knowledge of the conformational ensembles adopted by the nucleosome, the challenges still present, and the many opportunities for further progress in this area.
Conformational dynamics of the core
Though NMR spectroscopy had been utilized in some early studies of the nucleosome, the Bai and Kay groups really pioneered higher resolution analysis18. Taking advantage of recently developed methyl-TROSY approaches for study of large molecular weight systems (see Table 1), they performed the first solution state chemical shift assignment of the ILV side-chain methyl groups throughout all four histones within the nucleosome, providing residue level probes of the core histone octamer. Using this approach, these groups were able to determine the molecular mechanism of HMGN2 binding to the nucleosome core through chemical shift perturbations (CSPs) and paramagnetic relaxation enhancement (PRE) experiments, which allow for mapping of the amino acids involved in complex formation (see Table 1). This not only demonstrated the applicability of NMR to probe the nucleosome core, but also paved the way for additional studies based on their assignments.
Table 1:
Glossary of NMR terms
| Chemical shift | Reports on the chemical environment of an atom, and thus can be interpreted with respect to structural state. |
| Chemical shift perturbation (CSP) | A change in the chemical shift that can report on a variety of factors such as ligand binding or conformational change. |
| Chemical shift assignments | The assignment of NMR signals to specific amino acids. |
| Methyl TROSY NMR | An approach used for high molecular weight systems, involves looking at side-chain methyl groups. |
| ILV labeling | A method for isotropic enrichment of the methyl groups in isoleucine, leucine, and valine side-chains. Used in methyl-TROSY experiments |
| Uniform labeling | A method for uniform isotopic enrichment of 15N and/or 13C. Traditionally used for lower molecular weight systems. |
| Paramagnetic enhancement experiment | An experiment that uses a paramagnetic probe to induce relaxation of signals in a distance dependent manner. Can be used to determine the proximity of a paramagnetic-labeled residue within a complex. |
| Spin relaxation experiments/parameters | A set of experiments/parameters that report on (or can be interpreted with respect to) the amplitude and timescale of residue motions. Under certain conditions these can also report on the chemical shift and population of substates sampled through these motions. |
| Residue specific rotational correlation times (τC) | Reports on the timescale of motion of residues |
| Order parameter | Reports on the amplitude of dynamic of residues |
| 15N{1H} heteronuclear NOE (hetNOE) | Reports on the flexibility of residues on timescales less than the overall correlation time. |
In addition to mapping binding pockets, NMR is an excellent tool for investigating conformational dynamics and identifying low populational conformational states. A recent study demonstrated that the elusive octamer dynamics are detectable by NMR19. Single and double mutations in the (H3-H4)2 tetramer, known to destabilize the nucleosome, were incorporated. Chemical shift analysis revealed that these mutations do not perturb the average overall structure of the tetramer or the H2A-H2B dimer. However, spin relaxation experiments, which report on conformational fluctuations (see Table 1), showed increased dynamics on the μs-ms timescale of residues at the dimer-tetramer interface. This analysis was consistent with these residues adopting a low population substate not seen in wild-type. Notably, comparison of the substates for the single and double mutants through extracted chemical shifts demonstrated that both adopt the same state but that the double mutant has twice the population. Although the exact structure of the substate cannot be assessed here, the location of CSPs and increase in dynamics is consistent with a transient octamer opening event (see Figure 2).
Solid state data on a 145bp-nucleosome precipitated with Mg2+ or arrays of nucleosomes (separated by 15bp of linker DNA) identified hotspots of dynamics in histone H4 and histone H320,21. Determined order parameters from dipolar based spectra revealed that the C-terminal region of histone H4 has large amplitude motions on the ns-μs timescale, as compared to the rest of the H4 core. Regions with increased dynamics on the μs-ms timescale were also observed in these C-terminal residues as well as near the N-terminal tail and in core residues near the entry/exit DNA (super helical location ±0.5)20. Similar analysis of H3 showed that several residues in the N-terminal half of the core domain of H3 (i.e. αN, loop N, and loop 1) also have increased dynamics on the μs-ms timescales as compared to the rest of the H3 core residues. Together, these H3 and H4 residues form distinct clusters revealing dynamical networks that extend from the core to the DNA, and towards the histone tails21.
Two studies found that the core dynamics are altered by DNA sequence of the nucleosome. Although the majority of studies utilize the Widom 601 sequence, more recently a comparison was made with nucleosomes formed with the 5s rRNA DNA sequence19 and telomeric DNA21. In both cases, the majority of residues in the core (observing H3 and H4 in telomeric and H2B in 5s rRNA) have similar chemical shifts, indicating an overall similar structure of these histones in the most highly populated state. However, an increase in the population of a stable substate was observed for the 5s rRNA nucleosome19. In addition, there was an increase in the flexibility of residues in the H3-H4 dynamical networks noted above 21.
Collectively these studies demonstrate that NMR spectroscopy is sensitive to dynamics of the histone octamer, can identify low-population conformational states, and has promise to uncover how changes in the conformational ensemble are coupled to function. Especially exciting is the realization of dynamical networks by which post-translational modifications, binding, and differences in DNA sequence may manifest.
Conformational dynamics of the histone tails
Closely following assignment of the ILV residues in the histone octamer core, the Bai group published the backbone assignments of all the histone tails in the nucleosome context22. The fact that the tails could be observed with traditional isotope enrichment strategies (see Table 1) supported a high level of dynamics and is also consistent with the lack of resolution in the crystal structures. Chemical shift analysis revealed that all the tails are intrinsically disordered. One set of peaks for the set of tails points to symmetry in their conformation and fast exchange between all NMR-visible conformational states. The exception to this was the H2A N-terminal tail residues R3, K5, and G7 for which two sets of peaks were observed. In addition, signals for the basic patch of the H4 tail (K16–K23) were missing, suggesting that these residues adopt a more stable conformation and are in contact with the nucleosome core, making them invisible with this labeling approach. The H3 and H4 tail dynamics were also assessed by groundbreaking solid-state NMR analysis performed in the Jaroniec group23. These allowed for monitoring the H3 and H4 tails in nucleosome arrays under various states, including extended, compact, or highly condensed. Intriguingly, these studies indicated that even in the highly condensed state of the nucleosomes, the H3 and H4 tails are unstructured and dynamic, much like they behave in solution. These analyses also recapitulated the restriction of the H4 tail basic patch.
While these initial reports pointed to the intrinsic disorder and dynamics of the histone tails, further analysis revealed a much more complex conformational landscape than was at first apparent. To define the conformation of the H3 tail, chemical shift analysis was performed on the tail in multiple states; the peptide state, bound to DNA, in the context of a 187bp-nucleosome, as well as in the context of the 187bp-nucleosome bound to the linker histone H124. Together these data support that the H3 tail associates with linker DNA in the context of the nucleosome (see Figure 2). Chemical shift analysis of a 147bp-nucleosome core particle lacking any linker DNA agreed that the H3 tails interact with nucleosomal DNA independent of the presence of linker DNA25 (see Figure 2). A DNA-bound state was also found through chemical shift analysis of sedimented mono-nucleosomes in solid state NMR26. Most recently, chemical shift analysis of the H3 tail in arrays of nucleosomes with varying linker lengths revealed that the tails are insensitive to the linker length greater than 15bp, indicating that they are also insensitive to the relative orientation of nucleosomes27. Importantly, the DNA-bound conformation was shown to inhibit the modification of the H3 tail within the mono-nucleosome as compared to peptide24, and decrease the accessibility of the H3 tail to reader domains compared to free peptides by up to a factor of ~100, indicating the functional impact of the chromatin context 25,28.
Measured 15N spin relaxation parameters for the H3 tail in several chromatin relevant contexts confirmed that the tail is quite dynamic on the ns timescale24,27,29. Calculation of residue specific rotational correlation times (τC) from measured relaxation rates, which reports on residue level dynamics, indicated that the tail dynamics are substantially restricted within the nucleosome as compared to free peptide due to the interaction with DNA24. Interestingly, the H3 tails become increasingly more restricted when moving from the 147bp-nucleosome to the 187bp-nucleosome with linker DNA, to nucleosome arrays24,27. Stabilization of the tail in the array as compared to 187bp-nucleosome was suggested to be due to stabilization of the linker DNA itself27, which is further corroborated by a reduction of dynamics of the H3 tail upon H1 binding (which also stabilizes the linker DNA)24. In addition to the chromatin context dependence of the H3 tail dynamics, relaxation parameters also demonstrated non-uniform dynamics across the tail in a sequence dependent manner, with greatest restriction observed at Arg/Lys residues and the least at Thr, Gly, Gly elements24,27,29. Measured R2 relaxation rates on the H4 tail in 147bp-nucleosomes show similar restriction in the context of the nucleosome as compared to peptide and similar non-uniform dynamics across the tail, with greatest restriction at Arg/Lys residues30. Notably, in nucleosomes reconstituted with telomeric DNA, the H3 tails, and to a lesser extent the H4 tails, were shown to have altered conformational ensembles and increased flexibility21.
Chemical shift analysis of the H2A and H2B N-terminal tails and H2A C-terminal tails also indicate that these tails are bound to DNA within the nucleosome, both in solution and sedimented26,31. Analysis of the chemical shifts in the 145bp-nucleosome core particle or 193bp-nucleosome revealed that the presence of linker DNA had no effect on the conformational ensemble of the H2A and H2B N-terminal tails. However, distinct chemical shifts were observed for the H2A C-terminal tails in the presence of linker DNA, supporting a distinct conformational ensemble. Notably, doublets were observed for both the H2A and H2B N-terminal tails revealing that the tails adopt two distinct and stable conformational ensembles as they bind the DNA. From this analysis alone, it is not possible to determine if each tail is adopting a distinct conformational ensemble, or if both tails identically adopt two distinct conformational ensembles. Interestingly, upon increasing salt concentrations these doublets are seen to collapse into a single peak.
Collectively, these findings suggest that the histone tails are unstructured and highly dynamic in the nucleosome. However, their interaction with linker and/or nucleosomal DNA somewhat restrict their motions and have a dramatic effect on their accessibility. Overall, the histone tails can be described as adopting high-affinity fuzzy complexes with DNA in the chromatin context30,32-34. These complexes are unique, depend on the tail sequence, positioning, and chromatin context (see Figure 2) and likely mediate functional interactions involving the histone tails.
Effect of modifications on core and histone tail dynamics
One of the major regulators of chromatin is post-translational modifications (PTMs) of the histone proteins and chemical modifications of DNA, together known as the epigenome. While many of these are known to be read out by reader domains, the effect of these modifications on the nucleosome itself are largely unknown. While a number of modified nucleosomes have been crystallized, they produce essentially the same structure, which is likely stabilized by crystal contacts and, as discussed above, the tails are not resolved. However, NMR spectroscopy provides an outstanding opportunity to develop an understanding of how individual and sets of modifications alter the conformational ensemble and dynamics of the nucleosome.
An immediate implication of the histone tails adopting a high-affinity fuzzy complex with DNA is that their modification may directly alter the conformational ensemble and thus histone tail accessibility and chromatin structure32. Indeed modifications (or mutations) that alter the charge of tail residues have been found to alter the conformational dynamics of the H3 tail. Addition of a neutralizing acetyl group to H3K14 in the context of the 187bp-nucleosome led to an increase in the conformational dynamics as measured by a decrease in the residue level τC for residues 3–26 of the H3 tail, though with little effect on residues 27–3624. The dual acetylation of H3K14 and phosphorylation of H3S10 (adding negative charge and bulk) increased the dynamics further for residues 3–26, but again with little effect on residues 27–36. Dual phosphorylation of H3S10 and H3S28 led to an increase in the dynamics of residues 3–31 with smaller effects on residues 32–36. Interestingly, mutation of H3S28 to E within the 187bp-nucleosome without any additional modification/mutation to the N-terminal portion of the H3 tail led to changes in chemical shift and a decrease in residue level τC values for the majority of the tail35. Importantly, these effects were only seen in the context of the nucleosome, with none of these modifications leading to a change in the dynamics of the tail peptide. The presence of charge altering modifications on the histone tail can attenuate the association of the tail with DNA and promote binding of reader domains or additional modification24,25,36. Through the generation of asymmetrically modified nucleosomes the effect of H3S10 phosphorylation on accessibility of the H3 tail was shown to be largely in cis when comparing the two H3 tails37.
As noted above, the H4 tail has two distinct dynamical regions, with the first 15 residues experiencing fast dynamics and the basic patch (residues 16–23) adopting a more stable conformation. In one study, H4K16 was mutated to Q to mimic acetylation, which resulted in the appearance of backbone amide resonances for residues 16–19 and was consistent with an increase in conformational dynamics. A more recent study found that tetra-acetylation at H4K5/K8/K12/K16 caused chemical shift perturbations and increased intensity of signals for residues 2–15 as well as appearance of additional resonances in the H4 tail38. However, intriguingly, the acetylation of the H4 tail also led to chemical shift perturbations in backbone amide resonances of the H3 tail, supporting an altered conformational ensemble and demonstrating that histone tail modifications can have in-trans effects, potentially altering the DNA surface available for binding.
Changes in the conformational ensemble and dynamics of H4 have also been detected upon mono-methylation of H4K2039. Solid state NMR analysis of the H4 tails within nucleosome arrays revealed that the unmodified or tri-methylated H4K20 tail yields a doublet for H4V21 suggesting two distinct conformations of the H4 tail. In contrast, one of the doublet peaks disappears upon mono-methylation, indicating that one of these states is lost and that mono-methylation of H4K20 leads to a distinct conformational ensemble of the tail. This correlated with unfolding of the nucleosome arrays containing mono-methylated H4K20 but not unmodified H4K20 or tri-methylated H4K20. In addition, peak intensity analysis showed an increase in the dynamics of residues in the core of H4.
Recently, the Kay laboratory developed a method to enzymatically install labelled methyl groups at C5 on cytosines and N6 on adenines. While this approach should allow for future investigation of the nucleosomal DNA itself (see challenges below), it has already provided a powerful tool for testing the effect of these epigenetic modifications on histone dynamics, which were shown to have no effect on the structure or ps-ns dynamics of H2B core residues40.
Together these studies have begun revealing the complex effects of histone modifications on the conformational ensemble and dynamics of the nucleosome and nucleosome arrays. It is especially notable that while some modifications appear to have very localized effects, others have much broader impacts that propagate to other regions of the nucleosome or even throughout a nucleosome array. Continued studies are likely to transform our understanding of the histone code.
The effect of binding on nucleosome dynamics
Another mechanism for modulating chromatin structure involves the binding of additional factors. Advances in cryo-electron microscopy have led to several structures of nucleosome complexes determined in recent years. However, the use of NMR spectroscopy to explore nucleosome binding by proteins has a number of advantages. First, changes to the conformational dynamics of the nucleosome, which remain largely elusive in the static structures are accessible by NMR. Second, the dynamic regions, which do not resolve in the static structures, are ‘visible’ by NMR. Finally, weaker binding events, which often cannot be captured in static structures (whether it be inherently weak binding, or testing mutants) are suitable to study by NMR.
Binding to modified histone tails was thought to be an important factor in proper localization of nuclear proteins to chromatin. However, the majority of histone tail binding studies have been carried out with histone tail peptides, rather than with the nucleosome. The use of the nucleosome is critical for understanding not only multivalent interactions but also the impact on the conformational ensemble and dynamics of the nucleosome. In one of the first studies to explore nucleosome binding using NMR were performed on the PWWP domain of PSIP and the Tudor domain of PHF1 41,42. Through chemical shift analysis, these reader domains were found to be stabilized through contacts with both the tri-methylated at H3K36 tail and DNA within the nucleosome. Notably, chemical shift perturbations and a decrease in peak intensity were observed only around H3K36 indicating binding and dynamic stabilization of this region of the tail, without any effect on the conformational ensemble or dynamics of the remainder of the H3 taic41. Multivalent binding to the nucleosome ubiquitylated at H2AK13 or H2AK15 was also detected for the RNF169 UDM2 region 43,44. Chemical shift perturbations in ILV-labelled ubiquitin or ILV-labelled H2A-H2B ubiquitylated nucleosomes revealed the association of RNF169 with both the ubiquitin moiety and the acidic patch on the H2A-H2B dimer21,22. Spin relaxation experiments of the ubiquitin moiety showed that it is quite dynamic in the nucleosome, but localized chemical shift perturbations and peak broadening as compared to un-conjugated ubiquitin, also indicated that it makes at least transient contacts with the nucleosome surface (near or on the DNA). A further decrease in the dynamics of ubiquitin upon binding of RNF169 pointed to stabilization of the surface-bound conformation.
Association of the Snf2h chromatin remodeler led to substantial chemical shift perturbations and peak broadening in ILV methyl groups in the core of H4 and H2A. Many of these groups were buried and away from the known binding site, supporting that Snf2h promotes plasticity of the octamer, which was shown to be critical for remodeling activity45. Increased dynamics of the histone tails have also been observed29,46. The FACT histone chaperone is known to mediate unwrapping of one side of the nucleosome by 33bp (such that the core is only wrapped by 112bp), and the phosphorylated acidic intrinsically disordered (pAID) region of FACT binds to the exposed DNA binding region of the octamer. Chemical shift analysis of the H3 tails in the pAID-bound partially unwrapped nucleosome showed two sets of peaks for the two tails, one overlapping with the 145bp-nucleosomal H3 tail, and the second shifted to a new position 46. This suggests that the usually conformationally symmetric H3 tails become asymmetric upon pAID association, and that the pAID adjacent tail adopts a unique conformational ensemble. Measured 15N{1H} heteronuclear NOE (hetNOE) values on both tails, which report on the flexibility of each residue (see Table 1), revealed that the pAID adjacent H3 tail has increased flexibility on the ps-ns timescale, which resulted in increased accessibility for acetylation.
Altered nucleosome composition leads to a similar phenomenon as was observed with the FACT pAID. Removal of one (H2A-H2B) dimer forms the asymmetric hexasome particle in which ~30-40 bp of DNA are unwrapped from one side of the core. Further removal of the second (H2A-H2B) dimer leads to the tetrasome and unwrapping of the other side. Chemical shift analysis and measurement of hetNOEs on the H3 tails in the nucleosome, hexasome, and tetrasome demonstrated that in the tetrasome the H3 tails adopt identical conformational ensembles but are in a unique conformation compared to the nucleosome29. In addition, the tetrasomal tails have increased flexibility as compared to the nucleosomal tails. In the hexasome, two sets of peaks are observed for the two tails pointing to asymmetry similar to the FACT-bound nucleosome, with one tail adopting the tetrasomal conformational ensemble and one tail adopting the nucleosomal conformational ensemble (see Figure 2). Again, similar to FACT, the increased dynamics in the tetrasomal tail is associated with increased accessibility. Both studies suggest that it is the loss of DNA density around the H3 tail upon DNA unwrapping that alters the conformational ensemble of the H3 tail and causes increased accessibility.
Finally, several studies have investigated the effect of linker histone binding on the nucleosome conformational ensemble. Linker histones contain a globular domain that binds at the nucleosome dyad, and a highly basic C-terminal tail that binds to the linker DNA. Binding of the Drosophila H1 globular domain as well as human full-length H1.0, H1.4, and H1.10 induces changes in the chemical shift and peak intensity of backbone amides in the H2A C-terminal tails 47,48. Decrease of the peak intensity by one-half was interpreted that one of the tails had become substantially stabilized while the other remained dynamic. This was further supported by asymmetric appearance of density corresponding to the H2A tail in cryo-EM structures. Chemical shift analysis confirmed that the conformational ensemble of the H3 tails is affected by H1 binding. The globular domain alone does not induce any perturbations in the H3 tail. However, the presence of the H1 C-terminal tail leads to chemical shift perturbations in backbone amide peaks for the H3 tail, supporting a distinct conformational ensemble, with the greatest effects observed around residues K23–K27. A decrease in peak intensity and increase in residue specific τC reveals that this ensemble is less dynamic, again with the largest effects seen for the residues closer to the core24,48. Notably, a cryo-EM structure indicates a stabilization of the tails on the nucleosomal DNA and suggests that H1 C-terminal tail binding causes a shift in the population of the tails from the linker DNA to the nucleosomal DNA (see Figure 2).
Challenges
Arguably, one of the major challenges in NMR studies of the nucleosome is the difficulty and expense often required for sample preparation. This is especially true when generating the nucleosomes containing histone modifications. While PTM analogues have proven useful, they are limiting when investigating ligand binding as often they are not recognized by proteins as well as the natural modification. In addition, cysteine-based analogues are extremely limiting when wanting to install patterns of modifications. Similarly, characterization of the nucleosomal DNA has been hampered by restrictions on labeling. While uniform labeling is achievable in E. Coli, the resultant overlap in spectra of large DNAs, such as in the nucleosome, precludes gaining meaningful data, but again, installation of methyl groups at cytosine and adenine provides feasible labeling for probing the DNA.
Another major challenge relates to the interpretation of the resultant chemical shifts in terms of building an actual picture of the conformational ensembles of the nucleosomes. Certainly, trends are starting to emerge for the most studied H3 tails, which have now been measured in multiple chromatin contexts, but building an accurate conformational ensemble remains elusive. In addition, salt concentration has certainly been shown to have an effect on chemical shifts, especially on that of the tails. Thus, different buffers and salt concentrations often make comparison of chemical shifts between different studies very difficult.
Opportunities
There is a seemingly endless list of interesting questions yet to be addressed for which NMR spectroscopy is ideal. A plethora of histone modifications remain to be investigated for effects on the conformational ensemble and dynamics of the nucleosome. There are fewer restrictions on the requirements for strong positioning and stability of nucleosomes in the NMR tube as compared to crystallography, and thus characterization of the effect of different DNA sequences is possible as well. In fact, differences in the dynamics of the histone core with two different sequences have already been uncovered. The effect of the tails on the association of the octamer with these sequences can also be investigated.
There is a huge opportunity to combine NMR studies and MD simulations to build a picture of the conformational ensembles of the nucleosome. These are highly complementary techniques as many NMR parameters can be easily computed from MD trajectories allowing for a direct point of comparison. Historically however, the dynamics of the nucleosome have been observed to be over stabilized in simulations of the nucleosome, especially the tails, making this challenging. Two recent studies have made substantial progress on this front, uncovering that the water models utilized in the simulations are critical30,49. In one study of the histone H4 tails in the context of the nucleosome adjusting the water model led to 15N spin relaxation parameters that matched well between simulation and experiment. With this good correlation, it was possible to dissect the nature of the interactions between the H4 tail and DNA. The resulting fuzzy complex was found to be predominantly stabilized by quickly forming and dissolving salt bridges involving charged residues in the tail and phosphate backbone of the DNA. Finally, a very powerful combination of NMR, MD simulations, and cryo-EM is emerging as an impactful approach to build a full “picture” of the conformational ensemble of nucleosomes and nucleosome complexes43,48. Though often lower resolution, cryo-EM can still provide substantial insight into larger changes in the conformational ensemble. This can be supplemented by the residue level information obtained by NMR spectroscopy and a structural characterization of the system at atomic level provided by the MD trajectories.
Funding
Work in the Musselman lab is funded by the National Institutes of Health (NIH; R35GM128705). Work in the Kutateladze lab is funded by HL151334, CA252707, GM125195, GM135671 and AG067664.
Keywords
- Chromatin
A complex of DNA and histone proteins found in the nucleus of eukaryotic cells.
- Nucleosome
The basic subunit of chromatin consisting of an octameric histone core wrapped by ~147 base-pairs of DNA.
- Histone Core
The folded octameric complex of histones H2A, H2B, H3, and H4 found within the wrap of DNA in a nucleosome.
- Histone tail
The N-termini of histones H2A, H2B, H3, and H4 as well as the C-termini of H2A that protrude from the nucleosome core.
- Octamer opening
A dynamic motion of the octamer within the nucleosome in which there is opening between the core histones.
- DNA breathing and looping
Breathing is a dynamic motion of the DNA within the nucleosome in which the entry/exit DNA partially unwraps from the histone core. Looping is a dynamic motion in which a central segment of DNA loops off the histone core.
- Fuzzy Complex
A dynamic complex arising when both substrate and ligand contain multiple non-distinct binding sites which exchange quickly between each other.
- Histone post-translational modification
Small chemical groups or small proteins that are covalently linked to histone residue sidechains after translation.
- Conformational ensemble
A description of the possible structural states of a macromolecule or macromolecular complex.
- Conformational dynamics
The molecular motions undergone during transitions between conformational or structural states.
REFERENCES
- (1).Luger K, Mäder AW, Richmond RK, Sargent DF, and Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260. [DOI] [PubMed] [Google Scholar]
- (2).Cutter AR, and Hayes JJ (2015) A brief review of nucleosome structure. Febs Lett 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hansen JC, Tse C, and Wolffe AP (1998) Structure and Function of the Core Histone N-Termini: More Than Meets the Eye †. Biochemistry-us 37, 17637–17641. [DOI] [PubMed] [Google Scholar]
- (4).Grigoryev SA (2018) Chromatin Higher-Order Folding: A Perspective with Linker DNA Angles. Biophys J 114, 2290–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhou K, Gaullier G, and Luger K (2018) Nucleosome structure and dynamics are coming of age. Nature structural & molecular biology 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Luger K, Dechassa ML, and Tremethick DJ (2012) New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nature Reviews Molecular Cell Biology 13, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Andrews AJ, and Luger K (2011) Nucleosome Structure(s) and Stability: Variations on a Theme. Annual review of biophysics 40, 99–117. [DOI] [PubMed] [Google Scholar]
- (8).van Emmerik C, and van Ingen H. (2019) Unspinning chromatin: Revealing the dynamic nucleosome landscape by NMR. Prog Nucl Mag Res Sp 110, 1–19. [DOI] [PubMed] [Google Scholar]
- (9).Ackermann BE, and Debelouchina GT (2021) Emerging Contributions of Solid-State NMR Spectroscopy to Chromatin Structural Biology. Frontiers Mol Biosci 8, 741581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zheng C, and Hayes JJ (2003) Structures and interactions of the core histone tail domains. Biopolymers 68, 539–546. [DOI] [PubMed] [Google Scholar]
- (11).Killian JL, Li M, Sheinin MY, and Wang MD (2012) Recent advances in single molecule studies of nucleosomes. Current opinion in structural biology 22, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Fierz B, and Poirier MG (2019) Biophysics of Chromatin Dynamics. Annual review of biophysics. [DOI] [PubMed] [Google Scholar]
- (13).Li G, Levitus M, Bustamante C, and Widom J (2005) Rapid spontaneous accessibility of nucleosomal DNA. Nat Struct Mol Biol 12, 46–53. [DOI] [PubMed] [Google Scholar]
- (14).Böhm V, Hieb AR, Andrews AJ, Gansen A, Rocker A, Tóth K, Luger K, and Langowski J (2011) Nucleosome accessibility governed by the dimer/tetramer interface. Nucleic Acids Res 39, 3093–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chakraborty K, Kang M, and Loverde SM (2018) Molecular Mechanism for the Role of the H2A and H2B Histone Tails in Nucleosome Repositioning. J Phys Chem B 122, 11827–11840. [DOI] [PubMed] [Google Scholar]
- (16).Kulić IM, and Schiessel H (2003) Nucleosome Repositioning via Loop Formation. Biophys J 84, 3197–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Rosenzweig R, and Kay LE (2014) Bringing Dynamic Molecular Machines into Focus by Methyl-TROSY NMR. Annu Rev Biochem 83, 291–315. [DOI] [PubMed] [Google Scholar]
- (18).Kato H, van Ingen H, Zhou B-R, Feng H, Bustin M, Kay LE, and Bai Y (2011) Architecture of the high mobility group nucleosomal protein 2-nucleosome complex as revealed by methyl-based NMR. Proc National Acad Sci 108, 12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kitevski-LeBlanc JL, Yuwen T, Dyer PN, Rudolph J, Luger K, and Kay LE (2018) Investigating the Dynamics of Destabilized Nucleosomes Using Methyl-TROSY NMR. J Am Chem Soc 140, 4774–4777. [DOI] [PubMed] [Google Scholar]
- (20).Shi X, Prasanna C, Nagashima T, Yamazaki T, Pervushin K, and Nordenskiöld L (2018) Structure and Dynamics in the Nucleosome Revealed by Solid-State NMR. Angew Chem-ger Edit 130, 9882–9886. [DOI] [PubMed] [Google Scholar]
- (21).Shi X, Prasanna C, Soman A, Pervushin K, and Nordenskiöld L (2020) Dynamic networks observed in the nucleosome core particles couple the histone globular domains with DNA. Commun Biology 3, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhou B-R, Feng H, Ghirlando R, Kato H, Gruschus J, and Bai Y (2012) Histone H4 K16Q mutation, an acetylation mimic, causes structural disorder of its N-terminal basic patch in the nucleosome. Journal of molecular biology 421, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gao M, Nadaud PS, Bernier MW, North JA, Hammel PC, Poirier MG, and Jaroniec CP (2013) Histone H3 and H4 N-Terminal Tails in Nucleosome Arrays at Cellular Concentrations Probed by Magic Angle Spinning NMR Spectroscopy. J Am Chem Soc 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Stützer A, Liokatis S, Kiesel A, Schwarzer D, Sprangers R, Söding J, Selenko P, and Fischle W (2016) Modulations of DNA Contacts by Linker Histones and Post-translational Modifications Determine the Mobility and Modifiability of Nucleosomal H3 Tails. Molecular Cell 61, 247–259. [DOI] [PubMed] [Google Scholar]
- (25).Morrison EA, Bowerman S, Sylvers KL, Wereszczynski J, and Musselman CA (2018) The conformation of the histone H3 tail inhibits association of the BPTF PHD finger with the nucleosome. Elife 7, e31481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Xiang S, le Paige UB, Horn V, Houben K, Baldus M, and van Ingen H. (2018) Site-Specific Studies of Nucleosome Interactions by Solid-State NMR Spectroscopy. Angewandte Chemie Int Ed Engl 57, 4571–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Zandian M, Salguero NG, Shannon MD, Purusottam RN, Theint T, Poirier MG, and Jaroniec CP (2021) Conformational Dynamics of Histone H3 Tails in Chromatin. J Phys Chem Lett 6174–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Gatchalian J, Wang X, Ikebe J, Cox KL, Tencer AH, Zhang Y, Burge NL, Di L, Gibson MD, Musselman CA, Poirier MG, et al. , (2017) Accessibility of the histone H3 tail in the nucleosome for binding of paired readers. Nat Commun 8, 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Morrison EA, Baweja L, Poirier MG, Wereszczynski J, and Musselman CA (2021) Nucleosome composition regulates the histone H3 tail conformational ensemble and accessibility. Nucleic Acids Res 49, gkab246-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rabdano SO, Shannon MD, Izmailov SA, Salguero NG, Zandian M, Purusottam RN, Poirier MG, Skrynnikov NR, and Jaroniec CP (2021) Histone H4 Tails in Nucleosomes: a Fuzzy Interaction with DNA. Angewandte Chemie Int Ed 60, 6480–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ohtomo H, Kurita J, Sakuraba S, Li Z, Arimura Y, Wakamori M, Tsunaka Y, Umehara T, Kurumizaka H, Kono H, et al. , (2021) The N-terminal Tails of Histones H2A and H2B Adopt Two Distinct Conformations in the Nucleosome with Contact and Reduced Contact to DNA. J Mol Biol 433, 167110. [DOI] [PubMed] [Google Scholar]
- (32).Ghoneim M, Fuchs HA, and Musselman CA (2021) Histone Tail Conformations: A Fuzzy Affair with DNA. Trends Biochem Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Tompa P, and Fuxreiter M (2008) Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci 33, 2–8. [DOI] [PubMed] [Google Scholar]
- (34).Olsen JG, Teilum K, and Kragelund BB (2017) Behaviour of intrinsically disordered proteins in protein-protein complexes with an emphasis on fuzziness. Cell Mol Life Sci Cmls 74, 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Pelaz DA, Yerkesh Z, Kirchgäßner S, Mahler H, Kharchenko V, Azhibek D, Jaremko M, Mootz HD, Jaremko Ł, Schwarzer D, et al. , (2020) Examining histone modification crosstalk using immobilized libraries established from ligation-ready nucleosomes. Chem Sci 11, 9218–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Tencer AH, Cox KL, Di L, Bridgers JB, Lyu J, Wang X, Sims JK, Weaver TM, Allen HF, Zhang Y, et al. , (2017) Covalent Modifications of Histone H3K9 Promote Binding of CHD3. Cell Reports 21, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Liokatis S, Klingberg R, Tan S, and Schwarzer D (2016) Differentially Isotope-Labeled Nucleosomes to Study Asymmetric Histone Modification Crosstalk by Time-Resolved NMR Spectroscopy. Angewandte Chemie (International ed. in English). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Furukawa A, Wakamori M, Arimura Y, Ohtomo H, Tsunaka Y, Kurumizaka H, Umehara T, and Nishimura Y (2020) Acetylated histone H4 tail enhances histone H3 tail acetylation by altering their mutual dynamics in the nucleosome. P Natl Acad Sci Usa 202010506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Shoaib M, Chen Q, Shi X, Nair N, Prasanna C, Yang R, Walter D, Frederiksen KS, Einarsson H, Svensson JP, et al. , (2021) Histone H4 lysine 20 mono-methylation directly facilitates chromatin openness and promotes transcription of housekeeping genes. Nat Commun 12, 4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Abramov G, Velyvis A, Rennella E, Wong LE, and Kay LE (2020) A methyl-TROSY approach for NMR studies of high-molecular-weight DNA with application to the nucleosome core particle. P Natl Acad Sci Usa 202004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).van Nuland R, van Schaik FM, Simonis M, van Heesch S, Cuppen E, Boelens R, Timmers HM, and van Ingen H (2013) Nucleosomal DNA binding drives the recognition of H3K36-methylated nucleosomes by the PSIP1-PWWP domain. Epigenetics & chromatin 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Musselman CA, Gibson MD, Hartwick EW, North JA, Gatchalian J, Poirier MG, and Kutateladze TG (2013) Binding of PHF1 Tudor to H3K36me3 enhances nucleosome accessibility. Nat Commun 4, 2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kitevski-LeBlanc J, Fradet-Turcotte A, Kukic P, Wilson MD, Portella G, Yuwen T, Panier S, Duan S, Canny MD, van Ingen H et al. , (2017) The RNF168 paralog RNF169 defines a new class of ubiquitylated-histone reader involved in the response to DNA damage. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Hu Q, Botuyan MV, Cui G, Zhao D, and Mer G (2017) Mechanisms of Ubiquitin-Nucleosome Recognition and Regulation of 53BP1 Chromatin Recruitment by RNF168/169 and RAD18. Mol Cell 66, 473–487.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Sinha KK, Gross JD, and Narlikar GJ (2017) Distortion of histone octamer core promotes nucleosome mobilization by a chromatin remodeler. Science 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Tsunaka Y, Ohtomo H, Morikawa K, and Nishimura Y (2020) Partial replacement of nucleosomal DNA with human FACT induces dynamic exposure and acetylation of histone H3 N-terminal tails. Iscience 23, 101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zhou B-R, Feng H, Kato H, Dai L, Yang Y, Zhou Y, and Bai Y (2013) Structural insights into the histone H1-nucleosome complex. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zhou B-R, Feng H, Kale S, Fox T, Khant H, Val N. de, Ghirlando R, Panchenko AR, and Bai Y (2020) Distinct Structures and Dynamics of Chromatosomes with Different Human Linker Histone Isoforms. Mol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Peng Y, Li S, Onufriev A, Landsman D, and Panchenko AR (2021) Binding of regulatory proteins to nucleosomes is modulated by dynamic histone tails. Nat Commun 12, 5280. [DOI] [PMC free article] [PubMed] [Google Scholar]