Abstract

Oxygen reduction reaction (ORR) is the main reaction at the cathode of a fuel cell that utilizes Pt/C as the benchmark catalyst. Due to sluggish activity, high cost, rare abundance, and durability issues, Pt/C must be replaced by nonprecious, stable, and easily synthesizable materials. This work involves the synthesis of novel, simple, low-cost, and environmentally friendly phenolphthalein-bearing cobalt(II) phthalocyanine polymer, poly(CoIITPpPc) dyad, as an efficient catalyst for ORR. The results of analytical characterizations reveal the formation of the poly(CoIITPpPc) polymer in the pure state. To further enhance the catalytic response of poly(CoIITPpPc), a hybrid composite is prepared using poly(CoIITPpPc) and multiwalled carbon nanotubes (MWCNTs) that increase the surface area and conductivity. The poly(CoIITPpPc) and hybrid composite are separately deposited on the electrode surfaces. The electron microscopy images confirm the uniform distribution of the poly(CoIITPpPc) molecules on the electrode surface and MWCNTs. The poly(CoIITPpPc) and hybrid composite electrodes are evaluated for ORR, and the hybrid composite exhibits better onset potential at 0.803 V versus reversible hydrogen reference electrode for ORR according to linear sweep voltammograms (LSVs). The obtained data are superior compared to those of other carbon-based redox-active materials reported previously and nearer to those of the benchmark catalyst (Pt/C). The rotating disc electrode measurement of the hybrid composite electrode confirms the total number of electrons involved in ORR to be four. Furthermore, the hybrid composite electrode exhibits an excellent stability for 100 LSV scans. The synergistic effect of poly(CoIITPpPc) and MWCNTs leads to the surprisingly high ORR activity due to the improved surface area, conductivity, and interfacial confined surface.
1. Introduction
Industries, automobiles, agriculture, mining, and other sectors demand an enormous amount of energy for their routine activity.1−3 The major requirement of energy is fulfilled by fossil fuels, which are the major cause of greenhouse effect and global warming because of their combustion. Also, fossil fuels are being gradually depleted due to their extensive utilization. Among various alternative energy sources, fuel cells are promising because of their high-energy output, continuous supply of energy, and zero emission footprints.4 The wide commercialization of fuel cells is still limited due to some challenges associated with the cost of the components and the catalysts as well as the durability of the catalysts. Pt/C is used as a catalyst in fuel cells for the oxygen reduction reaction (ORR) at the cathode, which is sluggish, expensive, and less durable. Hence, a wide variety of alloys, metal oxides, nanoparticles, conducting polymers, composites, and so forth have been explored as possible alternatives for Pt/C in fuel cells.5 However, the development of state-of-the-art catalysts with low cost, high efficiency, and high durability in comparison to Pt/C is still challenging.
Organic molecules play an instrumental role in the designing of efficient catalysts because even a smaller change in substitution can dramatically change their properties.6,7 One of those organic molecules, which is widely employed as a catalyst, is phthalocyanine (Pc). The Pcs have a structural similarity to chlorophyll and hemoglobin with an 18-π electron-conjugated system. The Pcs are thermally and electrochemically stable and redox active in nature. These organic molecules are extensively used for potential applications in nonlinear optics, supramolecular chemistry, electrocatalysis, energy storage, charge transfer process, data storage devices, and so forth.8−10 The Pcs have been studied as efficient ORR catalysts to replace expensive Pt/C catalysts. The efficiency of the electrocatalytic reduction of O2 depends on the metal center and substituents attached to the Pc ring.11−13 The characteristic properties of the Pcs can be modulated by attaching various functional groups. Among different substituents, lactone rings provide an extended conjugation and improve the conductivity and redox activity, leading to high catalytic activity.
Phenolphthalein (C20H14O4; H2In, PhPh, H2PP, or PP) molecule is employed in the acid–base titration as an indicator. PP is a colorless solid at <pH = 8 and exists in the form of dianion (PP–2) at >8.14 Phenolphthalein and its derivatives have received much interest because of the benzene-fused lactone ring. The attachment of phenolphthalein to the framework of Pcs is expected to improve the physicochemical properties, conductivity, and stability due to the ring expansion and extension of conjugation.
Furthermore, the surface area along with conductivity plays an important role in catalysis. The surface area and conductivity of Pcs can be improved by involving various carbonaceous materials. The carbon atoms can form a strong covalent bond with other atoms and also with themselves in different hybridizations (sp, sp2, and sp3).15 The unique properties of the carbon allotropes, including physical properties, hardness, electrical conductivity, thermal conductivity, and so forth, are also important in organic and physical chemistry. Multiwalled carbon nanotubes (MWCNTs) are the most extensively studied carbon allotrope. Highly porous nature with the hexagonal honeycomb structure of MWCNTs gives unique properties of the electrical conductor.16 The porous structure of MWCNTs can accommodate a large number of Pcs molecules, allowing the designing of a novel hybrid composite with enhanced surface area, high conductivity, and active sites. This study aims at synthesizing a novel cobalt phenolphthalein phthalocyanine, poly(Co(II)TPpPc), and studying the effect of MWCNTs on enhancing the ORR activity of poly(Co(II)TPpPc). The electrocatalytically active hybrid composite—poly(Co(II)TPpPc)-MWCNTs is expected to have an improved electron transfer and enhanced ORR activity.
2. Experimental Section
2.1. Materials
Phenolphthalein (C20H14O4, 98%), methanol (CH3OH, 99.8%), ethanol (C2H5OH, 99.5%), acetone (CH3COCH3, 99.5%), 4-nitrophthalonitrile (C8H3N3O2, 99.0%), potassium carbonate (K2CO3, 99.0%), cobalt(II) chloride hexahydrate (CoCl2•6H2O, 98%), amyl alcohol (C5H12O, 99%), DBU (C9H16N2, 98%), n-hexane (C6H14, 95%), ethyl acetate (C4H8O2, 99.8%), dimethyl formamide (DMF: C3H7NO, 99.8%), dimethyl sulfoxide (DMSO: C2H6OS, 99.9%), tetrabutylammonium perchlorate [TBAP: (C4H9)4N(ClO4), 99.0%], hydrochloric acid (HCl, 37%), sulfuric acid (H2SO4, 97%), isopropyl alcohol (IPA, 99.0%), and potassium ferricyanide (K4[Fe(CN)6], 97%) were purchased from Sigma-Aldrich, Pt/C (20%) was purchased from Thermo Fisher Scientific, and MWCNTs were purchased from AkzoNobel. Other chemical reagents used to prepare solutions were obtained from Merck. Double-distilled water was used throughout the experiments.
2.2. Synthesis
The synthesis of phenolphthalein (1)-substituted phthalocyanine polymer involves the displacement reaction of nitro group of 4-nitrophthalonitrile (4-NPN) (2) with the hydroxyl moiety of the phenolphthalein analogue. The target polymer was synthesized according to the following synthetic protocol.
2.2.1. Synthesis of 3,3′-bis-[4-(3,4-Dicyano-phenoxy)-phenyl]-3H-isobenzofuran-1-one (3)
First, 2.077 g (0.012 mol) of phenolphthalein (1) and 2.0 g (0.006 mol) of 4-NPN (2) were mixed with 25 mL of dimethyl formamide (DMF) under vigorous stirring at 30 °C, and then, 5.0 g (0.036 mol) of anhydrous K2CO3 was slowly added for 2 h. Furthermore, the reaction was continued for 24 h under a N2 atmosphere, and then, 100 mL of water was added to the obtained crude and mixed for 15 min. The resulting precipitate (3) was washed with cold water and re-crystallized with CHCl3. Solubility: CHCl3, CH2Cl2, (CH2)4O, DMF, and (CH3)2CO. Yield: 3.4 g (86%). mp 242 °C. Molecular formula: C36H18N4O4; molecular weight: 570.525 g•mol–1. Chemical composition: 74.99% C, 3.78% H, 10.02% N, and 11.20% O (calculated composition) and 75.28% C, 3.66% H, and 9.54% N (analyzed composition). FTIR (KBr pellet): 731, 843, 967, 1082, 1178, 1255, 1284, 1422, 1489, 1563, 1589, 1743, 2231, and 3079 cm–1. 1H NMR (CDCl3), ppm: d 8.03 (dd, J = 8.03 Hz, J = 8.06 Hz, 2H), 7.83 (d, J = 7.83 Hz, 1H), 7.81 (d, J = 7.3 Hz, 1H), 7.97 (d, J = 0.6 Hz, 2H), 7.76 (t, J = 7.3 Hz, 2H), 7.47 (d, J = 6.0 Hz, 4H), 7.44 (dd, J = 7.2 Hz, J = 0.8 Hz, 2H), 7.19 (d, J = 7.9 Hz, 4H). Mass (m/z): 571.16 (M+1).
2.2.2. Synthesis of Cobalt(II) Tetra {β-[2,10,16,24-(phenolphthaleinyl)phthalocyaninato]} Polymer (4), poly(Co(II)TPpPc)
A well-ground mixture of 0.5 g (0.87 mmol) of precursor (3), 0.06 g (0.22 mmol) of cobalt chloride, and 0.002 g (0.01 mmol) of 1,8-diazabicyclo[5.4.0] undec-7-ene (DBU) was placed in a 100 mL RB flask containing 15 mL of n-pentanol, and the reaction mixture was slowly heated at a rate of 3–4 °C•min–1 to 140 °C with stirring and then refluxed for 24 h. After the completion of the reaction, the resulting product was allowed to cool down and then the crude product was transferred into 20 mL of ethanol and stirred. Then, the residue was washed with 10 mL of hot ethanol twice and excess water. Pure green-colored product was finally obtained after drying in a vacuum desiccator over anhydrous phosphorous pentoxide. Solubility: DMF, DMSO, and concentrated H2SO4. Yield: 0.41 g (78%). Molecular formula: (C144H72N16O16Co)n; molecular mass of monomer: 2341 g•mol–1. Chemical composition: 73.84% C, 3.12% H, 9.59% N, 10.96% O, and 2.52% Co (calculated composition) and 74.23% C, 3.16% H, 9.29% N, and 2.65% N (analyzed composition). UV–vis (DMSO): 333, 605, and 670 nm. FTIR (KBr pellet): 749, 850, 908, 1092, 1171, 1240, 1386, 1470, 1505, 1613, 1774, 2859, 2928, and 3430 cm–1. Mass (MALDI-TOF) (m/z): 2342.6 (M+2).
2.2.3. Preparation of the Poly(Co(II)TPpPc)-MWCNT Hybrid Composite
The hybrid composite catalyst was prepared by mixing poly(Co(II)TPpPc) (4.0 mg) and commercially available MWCNTs (1.0 mg) in the ratio of 80:20, and the mixture was ground well using a mortar and a pestle and sonicated with ethanol for 20 min. The resulting hybrid mixture was filtered, washed with water, and dried in an oven at 110 °C. Furthermore, this hybrid composite was used to prepare a catalyst ink.
2.3. Characterization
The melting point of the sample was determined using a melting point apparatus from Sisco instruments (model no. 70818209, India). The elemental analysis of the synthesized compounds was performed using a CHN&S elemental analyzer (Vario ELIII CHNS). The UV–vis absorption spectrum of the sample was measured in a quartz cuvette using an Ocean optics spectrometer with flame (FLAME-S-UV-VIS-ES, serial no. FLS04808) in the wavelength range of 280–850 nm using 1 mg of poly(Co(II)TPpPc) in 5 mL of DMSO. Fourier-transform infrared spectroscopy (FT-IR) analysis was performed using a Spectrum Two FT-IR spectrometer (PerkinElmer) with a resolution of 1 cm–1 using the KBr pellet method. The 1H NMR spectrum was recorded on a Bruker AM 400 MHz spectrometer with tetramethyl silane as the internal standard. The mass spectra (70 eV, electron impact mode) were measured using a Finnigan MAT instrument (Agilent). The thermal stability of the synthesized phthalocyanine was analyzed using a STA 6000 Simultaneous Thermal Analyzer (PerkinElmer) in the temperature range of 50–700 °C at a heating rate of 10 °C•min–1 under air flow (30 mL•min–1). The X-ray diffraction (XRD) pattern of the sample was measured using a Bruker D8 Advance X-ray diffractometer. Transmission electron microscopy (TEM) images were taken using a Talos F200S (Thermo Fisher Scientific), and X-ray photoelectron spectroscopy (XPS) analysis were conducted using a SPECSMXPS system.
2.4. Electrochemical Characterization
Glassy carbon electrode (GCE) with a 3 mm diameter was polished on a micro-cloth pad using an Al2O3 powder with a grain size of 0.05 μm, sonicated in ethanol for about 15 min, washed with water, and dried in vacuum. The catalyst ink was prepared by dispersing 5 mg of poly(CoTPpPc) in 0.5 mL of isopropyl alcohol, and 5 μL of Nafion (5%) was added to protect the active catalyst layer against leaching out from the electrode surface. Nafion binder forms a protective/permeable layer that resists the chemical attack and forms an ionic conductive film between liquid electrolyte and the active catalyst molecules, creating a strong bond with active catalysts. Hence, it protects the catalyst layer against leaching while performing the experiment.17 Afterward, the catalyst ink was homogenized under sonication for 20 min, and 5 μL of catalyst ink was deposited on the surface of the pre-cleaned GCE by drop-casting and dried under ambient conditions. The catalyst ink of the hybrid composite was prepared by dispersing 5 mg of synthesized hybrid composite mixture [poly(CoTPpPc)-MWCNTs] in 0.5 mL of isopropyl alcohol, adding 5 μL of Nafion (5 wt %), and homogenizing under sonication for 20 min. Afterward, 5 μL of poly(Co(II)TPpPc)-MWCNTs ink was deposited on the surface of the pre-cleaned GCE by drop-casting and dried under ambient conditions. For comparison, the standard Pt/C ink was prepared by dispersing 2.0 mg of commercial 20% Pt/C in 0.2 mL of isopropyl alcohol, adding 5 μL of Nafion (5 wt %), and homogenizing under sonication for 20 min. Then, 5 μL of Pt/C ink was deposited on the surface of the pre-cleaned GCE by drop-casting and dried under ambient conditions.
All electrochemical measurements were conducted using an electrochemical workstation (potentiostat CHI6005E, CH Instruments, Inc.) controlled by the electrochemical software. A standard three-electrode configuration was used for the electrochemical characterizations with a modified glassy carbon electrode as the working electrode, a platinum wire as the counter electrode, and Ag/AgCl (3 M KCl) as the reference electrode. The cyclic voltammograms (CVs) were measured from +1.35 to +0.15 V (vs RHE) at a scan rate of 10 mV•s–1. All potentials in this study were converted and represented versus reversible hydrogen reference electrode (RHE) at an ambient temperature. All cyclic voltammetry measurements were conducted in N2- or O2-saturated 0.1 M KOH electrolytes at a scan rate of 10 mV•s–1. The rotating disc electrode (RDE) characterization was performed using a RRDE-3A Rotating Ring Disk Electrode Apparatus Ver. 2.0 (ALS Co., Ltd.) using an RDE having a 3 mm diameter in O2-saturated 0.1 M KOH electrolyte with different rotations from 100 to 4900 rpm. The capacitive current of the working electrode for ORR was removed by subtracting the current value of the electrode in N2-saturated KOH electrolyte. Accordingly, the net Faradic current was retrieved for the interpretation of ORR activity of the samples.
3. Results and Discussion
The synthesis of novel tetraphenolphthalein cobalt(II) phthalocyanine polymer involves cyclotetramerization and hydrolysis process, and its synthesis path is schematically represented in Figure 1. Precursor (3) was synthesized according to the synthesis procedure previously reported elsewhere.18 The phenolphthalein (1) molecule has a fused lactone ring with two hydroxyl groups, and it reacts with 2 mol of 4-nitrophthalonitrile (2). The hydroxyl groups of the phenolphthalein molecule provide a position for the substitution of the 4-nitrophthalonitrile moiety. The displacement reaction takes place in dry DMF, where the nitro group is displaced, and the reaction is catalyzed with mild base K2CO3 under an inert atmosphere at room temperature. The completion of the reaction was monitored by thin-layer chromatography and FTIR. Then, the precursor (3) was treated with CoCl2 in the presence of DBU using n-pentanol as solvent during refluxing for 24 h. Here, the basic catalyst DBU promotes the cyclization, tetramerization, and metallation reaction to form novel tetra phenolphthalein cobalt(II) phthalocyanine polymer—poly(Co(II)TPpPc). It has been reported that the −CN functional group induces the ligation, chelating, ring closure, cyclotetramerization, and coordination of the central metal ion and stabilizes aromaticity under controlled conditions in high boiling solvent to form the polymeric complex. The crude compound was washed with ethanol and hot water several times. Then, the compound was treated with 10% HCl and 4% NaOH aqueous solutions simultaneously. Furthermore, it was thoroughly washed with water until the filtrate was free from chloride. Finally, the polymeric complex was treated with a mixture of hexane and acetone (1:1 volume ratio) and dried at 100 °C for 1 h to obtain pure poly(Co(II)TPpPc).
Figure 1.

Synthesis route of the poly(Co(II)TPpPc) complex: (i) K2CO3, DMF, stirring for 72 h under N2 and (ii) CoCl2•4H2O, n-pentanol, DBU, refluxing for 24 h at 140 °C.
The obtained green-colored poly(Co(II)TPpPc) is soluble in concentrated H2SO4, DMSO, and DMF. The poly(Co(II)TPpPc) was characterized by various analytical and spectroscopic techniques. The UV–visible spectral analysis infers the confined electronic transition from the ground state to the excited state. The UV–visible spectrum of poly(Co(II)TPpPc) is shown in Figure 2a, indicating two characteristic absorption bands at 333 and 670 nm. The first absorption band in the ultraviolet region, that is, at lower wavelength, is called as the B-band (Soret band). The B-band observed at 333 nm is mainly because of the electronic transition from n → π*, that is, the transition from the deeper levels of π-orbitals to the lowest unoccupied molecular orbitals (LUMOs). The lone pair electrons of the O and N atoms of Pc undergo transition to the π* antibonding orbital after absorbing UV light. Another important characteristic band observed in the visible region at longer wavelength (670 nm) is called as the Q-band. The Q-band of poly(Co(II)TPpPc) corresponds to the π–π* transition from the highest occupied molecular orbital (HOMO) to the LUMO of the Pc macrocycle. The π electrons of the aromatic macrocycle undergo transition to higher energy π* antibonding orbital after absorbing visible light. The synthesized polymeric complex has an intense dark-green color, and the intense coloration of polymeric Pc is mainly due to the strong absorption of the Q-band and its higher energy vibronic satellite. A small shoulder observed at about 605 nm is associated with oligomers present in poly[Co(II)TPpPc] and aggregation of phthalocyanine units.19,20
Figure 2.
(a)UV–vis spectrum of poly(Co(II)TPpPc), (b) FTIR spectra of (i) precursor (3) and (ii) poly(Co(II)TPpPc), (c) TG curve, and (d) XRD pattern of poly(Co(II)TPpPc).
The FTIR spectra of precursor (3) and poly[Co(II)TPpPc] are shown in Figure 2b. For precursor (3), the absorption bands observed at 731, 1082, and 1489 cm–1 are attributed to the out-of-plane and in-plane of C–H and C=C stretching vibrations, respectively. The intensive absorption band noted at 2231 cm–1 corresponds to the nitrile (−C≡N) group, indicating the successful formation of the nitrile group-containing oxy-bridged ligand. The absorption band at 1740 cm–1 is ascribed to the C=O group. The disappearance of the absorption band for the nitrile group and the appearance of characteristic absorption bands of the Pc skeletal at 1171, 1092, 908, 850, and 749 cm–1 confirm the successful formation of poly(Co(II)TPpPc).21 Furthermore, the absorption bands in the FTIR spectrum of poly(Co(II)TPpPc) are less intense and broader in nature, indicating its polymeric nature.
The thermal stability of poly(Co(II)TPpPc) was analyzed in the temperature range from 20 to 700 °C. The TG curve shown in Figure 2c reveals that water molecules/moisture content and other volatile matters, such as ethanol, methanol, and so forth, are removed at the temperature below 120 °C. It is difficult to find the residual solvents or volatile solvents present in the Pc compound by gas chromatography as it is not soluble in low boiling organic solvents. The poly(Co(II)TPpPc) is stable upto 330 °C and gradually undergoes decomposition to yield stable metal oxide beyond 330 °C. The estimated weight of Co in stable metal oxide formed after 500 °C is equivalent to the theoretical weight of Co present in the poly(Co(II)TPpPc). Clearly, the poly(Co(II)TPpPc) exhibits higher thermal stability than the monomeric parent Pc due to the extended conjugation and delocalization of electrons. The XRD pattern of poly(Co(II)TPpPc) is shown in Figure 2d, confirming its amorphous nature.22
The purity and structure of precursor (3) were analyzed by 1H NMR. The proton NMR spectrum of precursor (3) is shown in Figure S1. The chemical shift values and the corresponding protons of the ligand in different chemical environments are presented in the synthesis part. The absence of a sharp peak at about 9.65 ppm for −OH proton in phenolphthalein compound indicates the successful conversion and formation of precursor (3).
The synthesized precursor (3) and monomer of poly(Co(II)TPpPc) have the theoretical molecular weights of 570.525 and 2341.14 amu, respectively. Figure S2 shows the mass spectrum of precursor (3) with a peak at m/z = 571.16, which is assignable to molecular ion M+1. The mass spectrum of poly(Co(II)TPpPc) in Figure S3 shows a peak at m/z = 2342.63, which corresponds to the monomeric Pc of poly(Co(II)TPpPc) and can be accounted for the M+2 monomeric molecular ion. The peaks noted in the mass spectra clearly confirm the formation of precursor (3) and poly(Co(II)TPpPc).
X-ray photoelectron spectroscopy (XPS) measurements were conducted to investigate the surface composition and their chemical states in poly(Co(II)TPpPc) and poly(Co(II)TPpPc)-MWCNT modified electrodes. The XPS survey spectra of pristine and hybrid composite material in Figure 3a show peaks at 284.8, 400.1, 533.2, and 781.8 eV, which are related to carbon (C 1s), nitrogen (N 1s), oxygen (O 1s), and cobalt (Co 2p). The narrow-scan C 1s and N 1s XPS spectra of poly(Co(II)TPpPc) and poly(Co(II)TPpPc)-MWCNT hybrid composite are shown in Figure 3b,e,c,f, respectively. The deconvolution of the high-resolution spectra of different peaks was performed using XPSpeak 4.1 and Origin software. The C 1s XPS spectrum is deconvoluted into four superimposed peaks in the pristine Pc and hybrid composite. The peak at 284.8 eV is ascribed to aromatic (sp2) carbon atoms (C=C, C–C), and peaks at 286.3 and 288.9 eV are assigned to C=N (Pyrrol) and C=O, respectively. The peak at higher binding energy of 290.6 eV may be due to the π–π* interaction of aromatic rings of MWCNT and poly(Co(II)TPpPc).23 The nitrogen (N 1s) spectrum (Figure 3c,f) showed peaks at 400.1 eV related to the −C–N=C bond and 401.2 eV indicates the N bonded with carbon and cobalt metal. The Co 2p XPS spectra of poly(Co(II)TPpPc) and poly(Co(II)TPpPc)-MWCNT hybrid composite in Figure 3d show two main broad peaks that are ascribed for Co(2p1/2 and 2p3/2), respectively. This infers that cobalt is in its +2 oxidation state in both the samples. The O 1s XPS spectrum(Figure 3g,h) of Pc and composite material exhibit two peaks at 535 and 533.6 eV, which can be attributed to C–O and C=O, respectively. The parity between the poly(CoTPpPc) and MWCNTs in the hybrid catalyst is due to the π-electron interaction and affinity between the two components. The composites prepared via the chemical method have shown slight positive increase/shifting in binding energy due to the enhancement in electron density and formation of axial bond/axial interaction between cobalt ion in Pc and conducting material (CNT).24 However, the XPS spectra of the designed poly(Co(II)TPpPc)-MWCNT hybrid composite did not show any shift in the binding energy because of physical interaction indicating no axial bonding formation between Pc and MWCNTs. In general, the physical mixing of polymeric Pc with MWCNT leads to a physical interaction rather than chemical interaction and bond formation.
Figure 3.
XPS spectra of poly(Co(II)TPpPc) and poly(Co(II)TPpPc)-MWCNT hybrid composite: survey scan (a), and C 1s (b,e), N 1s (c,f), Co 2p (d), and O 1s (g,h) XPS spectra.
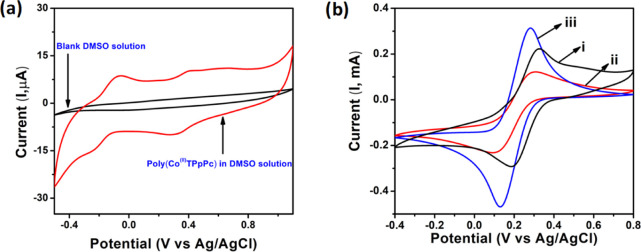
The CV exhibiting the electrochemical behavior of poly(Co(II)TPpPc) in DMSO containing 10 mM tetra butyl ammonium phosphate (TBAP) is shown in Figure 4a. The electrolytic solution was purged with N2 gas for 20 min to remove the dissolved oxygen prior to the measurement. As shown, the bare GCE does not have any peaks in the studied potential window, whereas two reversible pair of peaks are noticed for poly(Co(II)TPpPc), suggesting that the poly(Co(II)TPpPc) complex is electro-active in nature.25 The redox pair appeared at −0.054 to −0.22 V (ΔE = 166 mV) can be possibly attributed to the redox nature of Pc (Pc–2/Pc–1), and another redox pair at +0.41 to +0.29 V (ΔE = 120 mV) can be accounted for the redox property of Co (Co+2/Co+1) in poly(Co(II)TPpPc). The poly(Co(II)TPpPc) was immobilized on the GCE surface and characterized. The CV of the modified electrode in DMSO shows weak redox peaks at the same potential applied for Pc in solution. The peaks in CV clearly indicate the modification of the GCE with poly(Co(II)TPpPc).
Figure 4.
(a) CVs of blank (black line) and poly(CoTPpPc) (red line) in DMSO containing 0.1 M TBAP at GCE(scan rate is 10 mV•s–1 in N2) and (b) CVs for of the ferro/ferri cyanide system at (i) bare GCE, (ii) poly(CoTPpPc)/GCE, and (iii) poly(CoTPpPc)-MWCNTs/GCE.
The CVs for the ferro/ferri cyanide system help in understanding the promoted electron transfer at the interface of the modified electrodes.26 The charge transfer kinetics of the modified electrodes were analyzed in a phosphate buffer solution (pH = 7) containing 1.0 mM K4[Fe(CN)6] redox probe. Figure 4b shows the CVs for the redox probe on bare GCE, poly(Co(II)TPpPc)/GCE, and poly(Co(II)TPpPc)-MWCNTs/GCE electrodes. Having a characteristic redox behavior, the bare GCE shows highly resolved reversible peaks for the redox probe with a peak separation, ΔEp = 120 mV (ΔEp = Epc – Epa). The modification of the GCE surface with poly(Co(II)TPpPc) leads to a larger separation of redox peak potentials for the ferro/ferri cyanide system (ΔE = 189 mV). This is associated with a decline in the current density compared to the pristine GCE. The larger peak potential separation and lower current density confirm that electron transfer kinetics is slower at poly(Co(II)TPpPc) electrode due to the insulating behavior of Pc, implying that the film slightly blocks electron transfer. Meantime, the poly(Co(II)TPpPc)-MWCNT hybrid composite electrode shows a smaller peak potential separation (ΔE = 155 mV) with an increase in the current density, indicating that the modified hybrid composite electrode behaves as a conducting medium and mediates the facile electron transfer between the electrolyte and electrode. The slight increase in the peak potential separation for the ferro/ferri cyanide system at the hybrid composite is related to the thicker and complex nature of the fabricated film.
The morphology and surface characteristics of pure MWCNTs, poly(Co(II)TPpPc), and poly(Co(II)TPpPc)-MWCNT hybrid composite on the carbon surface were examined by means of scanning electron microscopy (SEM). The SEM image in Figure 5a shows the distribution and coverage of carbon substrate in an irregular manner with tube-like structures of MWCNTs. The SEM image of poly(Co(II)TPpPc) in Figure 5b shows highly aggregated web-like clustered structure of polymeric phthalocyanine with uniform distribution. The SEM image of poly(Co(II)TPpPc)-MWCNT hybrid composite in Figure 5c exhibits a cage-like arrangement with a random distribution of MWCNTs in poly(Co(II)TPpPc) on the carbon substrate. Figure 5d presents the energy-dispersive spectrometry (EDS) spectrum of poly(Co(II)TPpPc)-MWCNT hybrid composite on the carbon surface. The EDS results are consistent with the elemental composition, and the composition in terms of quantitative element ratio is inserted as inset in Figure 4d. The quantitative element ratio observed for poly(Co(II)TPpPc)-MWCNTs confirms the successful modification of the surface. Furthermore, the structural characteristics and morphology of the pure poly(Co(II)TPpPc)-MWCNTs were confirmed from the TEM image shown in Figure 5e,f. The TEM image of poly(Co(II)TPpPc)-MWCNTs shown in Figure 5f reveals the uniform congregation of interlayer of polymeric Pc and tubular-shaped MWCNTs. The good interaction and parity between the poly(Co(II)TPpPc) and MWCNTs might be due to the π–π interaction of the conjugated rings of poly(Co(II)TPpPc) with the MWCNT backbone.
Figure 5.
SEM images of (a) pure MWCNTs and (b) poly(Co(II)TPpPc), and (c,d) SEM image and EDS spectra of poly(Co(II)TPpPc)-MWCNTs. TEM images of (e) MWCNTs and (f) poly(Co(II)TPpPc)-MWCNT hybrid composite.
Different hybrid composites of poly(Co(II)TPpPc) with carbon black (CB), Vulcan carbon (VC), and MWCNTs were prepared and immobilized on the electrode, and their ORR activity was evaluated. Linear sweep voltammograms (LSVs) of these hybrid composites were measured in O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1. As shown in Figure 6a, the LSV of bare GCE shows an insignificant response for ORR compared to all hybrid composite electrodes. The onset potential is the potential where all the thermodynamic and kinetic barriers become downhill. The starting point of potential at which there is a sharp increase in reduction current is considered as ORR onset potential. The poly(Co(II)TPpPc)-CB and poly(Co(II)TPpPc)-VC hybrid composite electrodes exhibit a nearly identical onset potential (0.74 V); however, the poly(Co(II)TPpPc)-VC electrode has an enhanced reduction current density than the poly(Co(II)TPpPc)-CB electrode. On the other hand, the poly(Co(II)TPpPc)-MWCNT hybrid composite electrode exhibits a surprisingly higher positive onset potential than the poly(Co(II)TPpPc)-CB and poly(Co(II)TPpPc)-VC hybrid composite electrodes. This confirms that the MWCNTs, in comparison to CB and VC, have a better combined effect with poly(Co(II)TPpPc) to boost the ORR activity because of their high electrical conductivity, large surface area, extensive hexagonal structure, and lightweight.27−29
Figure 6.
LSVs of (a) bare GCE, poly(Co(II)TPpPc)-MWCNTs, poly(Co(II)TPpPc)-CB, and poly(Co(II)TPpPc)-VC measured in O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1. ORR activity of bare GCE, poly(Co(II)TPpPc), MWCNTs, and poly(Co(II)TPpPc)-MWCNTs evaluated in (b) N2-saturated and (c) O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1.
The ORR activity of the poly(Co(II)TPpPc)-MWCNTs hybrid composite electrode was evaluated using LSV in 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1. Initially, the LSV curves were recorded in the inert condition by purging N2 for 20 min prior to the measurement. Then, the electrolyte was purged with O2 for 20 min and LSV response was measured. The LSV curves of GCE, poly(Co(II)TPpPc)/GCE, MWCNTs/GCE, and poly(Co(II)TPpPc)-MWCNTs/GCE electrodes measured in N2 are shown in Figure 6b. The LSV curves measured in the N2-saturated electrolyte show only a small background current for all fabricated electrodes. In contrast, the LSV curves, measured in the O2-saturated electrolyte, of poly(Co(II)TPpPc)/GCE, MWCNTs/GCE, and poly(Co(II)TPpPc)-MWCNTs/GCE show a higher reduction current with a shift in the onset potential to higher positive potential (Figure 6c). Particularly, the LSV curve of poly(Co(II)TPpPc)-MWCNTs/GCE exhibits a maximum positive shift in the onset potential (0.802 V vs RHE) for ORR with a higher current density (−2.87 mA•cm–2) compared with poly(Co(II)TPpPc)/GCE (0.612 V vs RHE) and MWCNTs/GCE (0.713 V vs RHE) with a current density of ∼−0.5 mA•cm–2, indicating that the poly(Co(II)TPpPc)-MWCNT hybrid composite is an efficient catalyst for ORR.
Furthermore, the CVs of poly(Co(II)TPpPc)-MWCNTs/GCE measured in N2- and O2-saturated 0.1 M KOH electrolytes at a scan rate of 10 mV•s–1 are shown in Figure 7a. The hybrid composite electrode exhibits a superior response with a higher onset potential of 0.803 V versus RHE and a current density of −2.92 mA•cm–2 in O2-saturated electrolyte, which is consistent with the LSV results. The superior onset potential of poly(Co(II)TPpPc)-MWCNTs/GCE observed for ORR in the O2-saturated electrolyte is due to the collusive entanglement of poly(Co(II)TPpPc) and MWCNTs. This is one of the better onset potential value reported so far for ORR compared with other organic-based electrodes reported previously (Table 1).
Figure 7.
CVs of (a) poly(Co(II)TPpPc)-MWCNTs/GCE measured in N2- and O2-saturated electrolytes and (b) poly(Co(II)TPpPc)-MWCNTs/GCE and Pt/C/GCE in O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1.
Table 1. Comparison of Electrochemical Parameters of ORR.
| catalyst | electrolyte | onset potential (V vs RHE) (V) | refs |
|---|---|---|---|
| polyelectrolyte-functionalized graphene | 0.1 M KOH | +0.74 | (36) |
| FeCo–Nx embedded graphene | 0.5 M H2SO4 | +0.65 | (38) |
| rGO-NiPc | 0.1 M HClO4 | +0.25 | (37) |
| Electropolymerized films of vinyl terpidine complexes of Fe, Ni, Co | Na2B4O7/HCl | +0.4 | (39) |
| CoPc/CNTs | 0.5 M H2SO4 | +0.72 | (12) |
| cobalt oxide embedded CoPc | pH 7 PBS buffer | +0.655 | (13) |
| Co(tetra butyl)Pc/MWCNTs | 0.1 M NaOH | +0.739 | (28) |
| rGO/CuPc | 0.1 M KOH | +0.72 | (43) |
| Co2P-40/GCD | 0.1 M KOH | +0.80 | (44) |
| CNx/GCD | 0.1 M KClO4 | +0.78 | (45) |
| N-CNTFePc | 0.1 M KOH | +0.93 | (30) |
| FePc-GO | 0.1 M KOH | +0.95 | (31) |
| cK-FePc2Ph | 0.1 M KOH | +0.88 | (32) |
| Poly(Co(II)TpPc)-MWCNTs | 0.1 M KOH | +0.803 | This work |
It can be noted from Figure 7b that the poly(Co(II)TPpPc)-MWCNTs/GCE exhibits an onset potential that is nearer to that of the benchmark catalyst Pt/C with a higher current density compared with that of the commercial Pt/C catalyst. However, the half-wave potential of the poly(Co(II)TPpPc)+MWCNTs hybrid electrode is 0.672 V versus RHE, which is slightly lower than that of 20% commercial Pt/C electrode having a half-wave potential of 0.832 V versus RHE for ORR. The advantage of these hybrid materials is their cost-effective synthesis and stability. Furthermore, the half-wave potential and onset potential can be improved by tuning the properties of Pc. The enhancement in the catalytic activity of poly(Co(II)TPpPc)-MWCNT hybrid composite interms of onset potential and current density for ORR can be attributed to the synergistic effect of poly(Co(II)TPpPc) and MWCNTs. The conjugated and delocalized polymeric Pc molecule has a strong and efficient π–π interaction with MWCNTs which provides a large surface area. MWCNTs induce lesser migration of charges and improve the catalytic reactivity of the catalyst for the analyte. Furthermore, the electron-extracting effect of the ketonic group of poly(Co(II)TPpPc) has the ability to reduce the electron density on cobalt and minimize the energy gap between the donor poly(Co(II)TPpPc) and acceptor O2, leading to an efficient oxygen reduction.27 Furthermore, the methodology involved in the fabrication of the proposed electrode is simpler compared to other electrodes in the literature.30−32
The cost of the developed composite material is about $20–30 per gram, which can be dramatically decreased with a large-scale production. In contrast, the cost of Pt/C is $300 per gram. Hence, the poly(Co(II)TPpPc)-MWCNT hybrid composite can be a potential alternative for ORR to replace commercially available expensive Pt/C.
The effective diffusion of oxygen in the electrolyte is one of the important parameters influencing the limitation of current density and onset potential during ORR in the LSV measurements.33−35 In addition, the diffusion of oxygen also depends on oxygen flow rate, conditions of inlet, and gas tightness. The influence of O2 diffusion on ORR was investigated by performing LSV measurements using the poly(Co(II)TPpPc)-MWCNT hybrid composite in 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1 by varying the inlet oxygen concentration with time. A minimum current density of −0.6 mA•cm–2 was observed at a less positive onset potential of 0.73 V in the LSV curve measured without oxygen supply (Figure S4a). After purging the electrolyte with O2 for 20 min, the same electrode shows a higher current density of −1.28 mA•cm–2 at a more positive onset potential of 0.802 V.
For the poly(Co(II)TPpPc)-MWCNT electrode, the 3D-trajectory image made for the LSV curves measured with a time-dependent oxygen supply is shown in Figure S4b. The current density was improved and onset potential increased with the increase in the oxygen supply to the electrolyte. Both values were increased until the electrolyte was saturated with O2, and then, the current density and onset potential became stable and no further change was observed. This implies that the dissolution and diffusion of O2 in the electrolyte have a significant influence in ORR to generate a higher current density and a positive onset potential.
The electron transfer kinetics involved in the ORR helps to understand the efficiency and mechanism of ORR.36,37 Hence, the reaction kinetics of ORR over poly(Co(II)TPpPc)-MWCNT hybrid composite was assessed by the rotating glassy carbon disc electrode (GC-RDE) measurement at different rotations per minute (100–4900 rpm) in O2-saturated 0.1 M KOH electrolyte. As shown in Figure 8a, the current density of the polarization curves was increased with the increase in the rotational speed due to the improved mass transfer, and less time was required for the dissolution of oxygen at the surface of the hybrid composite electrode.
Figure 8.
(a) GC-RDE measurements for ORR of poly(Co(II)TPpPc)-MWCNT hybrid composite electrode conducted in O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV•s–1 by applying different rotations per minute. (b) Koutechý–Levich plots for ORR of the poly(Co(II)TPpPc)-MWCNT hybrid composite electrode, and the inset graph indicates the number of electrons involved at various potentials (vs RHE).
The Koutechý–Levich plots in Figure 8b was obtained by extracting the current density at different electrode potentials of the GC-RDE polarization curves. The plots exhibit a good linearity without much variation in the slope values at different potentials. That is, the slope values are relatively stable in the potential range from 1.35 to 0.15 V with respect to RHE, implying that the number of electrons transferred during the ORR is identical. The good linearity and close similarity of the polarization curves suggest that the reaction proceeds via the first-order reaction kinetics which mainly depends on the concentration of the dissolved oxygen.38,39 The number of electrons involved per O2 molecule during the ORR over poly(Co(II)TPpPc)-MWCNT hybrid composite electrode was calculated by the Koutechý–Levich equation.40,41
| 1 |
| 2 |
where j is the measured current density, jK is the kinetic current density, ω is the electrode rotating rate in rpm, n is the number of electrons transferred per molecular oxygen (O2), D0 is the diffusion coefficient of O2 in 0.1 M KOH (1.9 × 10–5 cm2•s–1), C0 is the bulk concentration of O2 (1.38 × 10–6 mol•cm–3), F is the Faraday constant (96485 C•mol–1), and v is the kinetic viscosity (0.01 cm2•s–1).
In general, the ORR at the electrode surface proceeds either via the four-electron pathway, where O2 is directly converted into H2O, or two-electron process, where dissolved O2 is converted into H2O2 first before being reduced to H2O.42 The number of electrons transferred during the ORR at poly(Co(II)TPpPc)-MWCNTs was calculated using the slope values of Koutechý–Levich plots and found to be 3.903, 3.850, 3.904, 3.934, 3.945, and 3.983 at 0.2, 0.25, 0.3, 0.35, 0.4, and 0.5 V versus RHE, respectively (inset of Figure 7b). The slope values estimated at different potentials confirm that four electrons were released during the ORR. As expected, the Co ions acted as the active centers for adsorption process, and the feasible four-electron transfer mechanism for the ORR at poly(Co(II)TPpPc)-MWCNTs is expressed in eqs 3–7, where the active site is indicated with *. The four-electron pathway for ORR at the poly(Co(II)TPpPc)-MWCNT hybrid composite electrode is similar to that of commercial Pt/C.43−45 In literature, the ORR catalytic activity at the cobalt phthalocyanine based-electrodes proceeds via a two-electron reduction process, resulting in the formation of a peroxide anion.46,47 Nevertheless, the results from the present work infer that the poly(Co(II)TPpPc)-MWCNT hybrid composite follows a stable four-electron transfer kinetics as cobalt is used as an active center. The density functional theory simulations from the literature,48 suggest that the possible mechanism for ORR at CoN4 embedded carbon material is as follows: O2 displaces the OH– species and directly gets chemisorbed on the Co2+ active site via the inner sphere electron transfer mechanism. This chemisorbed O2 undergoes direct dissociation and hydrogenation to form OOH on the surface of Co. The hydrogenation of OOH leads to the production of coadsorbed oxygen and water instead of forming H2O2. The coadsorbed oxygen is at the top site of Co and it is hydrogenated into OH and finally forms H2O and this water will get easily desorbed from the active site of the catalyst. Cobalt active sites (*) are probably the only active site for the oxygen reduction process here. Because the free energies for the formation of intermediates such as *OOH, *OH, and *O adsorbed on the Co active sites are much lower than the adsorption toward the carbon and nitrogen sites, it implies that the binding capacity is much simpler on Co atoms rather than other sites. The enhancement of ORR catalytic activity may be due to the π–π* interaction between the conjugated polymeric Pc molecules and MWCNTs. Moreover, also the porosity of MWCNTs favored the incorporation of CoPc on the surface of MWCNTs, leading to an increased number of available active metal sites. Thus, the hybrid composite may induce a lesser migration of charges than pristine Pc. Therefore, the impressive electrocatalytic activity of CoPc-MWCNTs is due to the Co active sites and the higher electrical conductivity and surface area induced by MWCNTs.
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
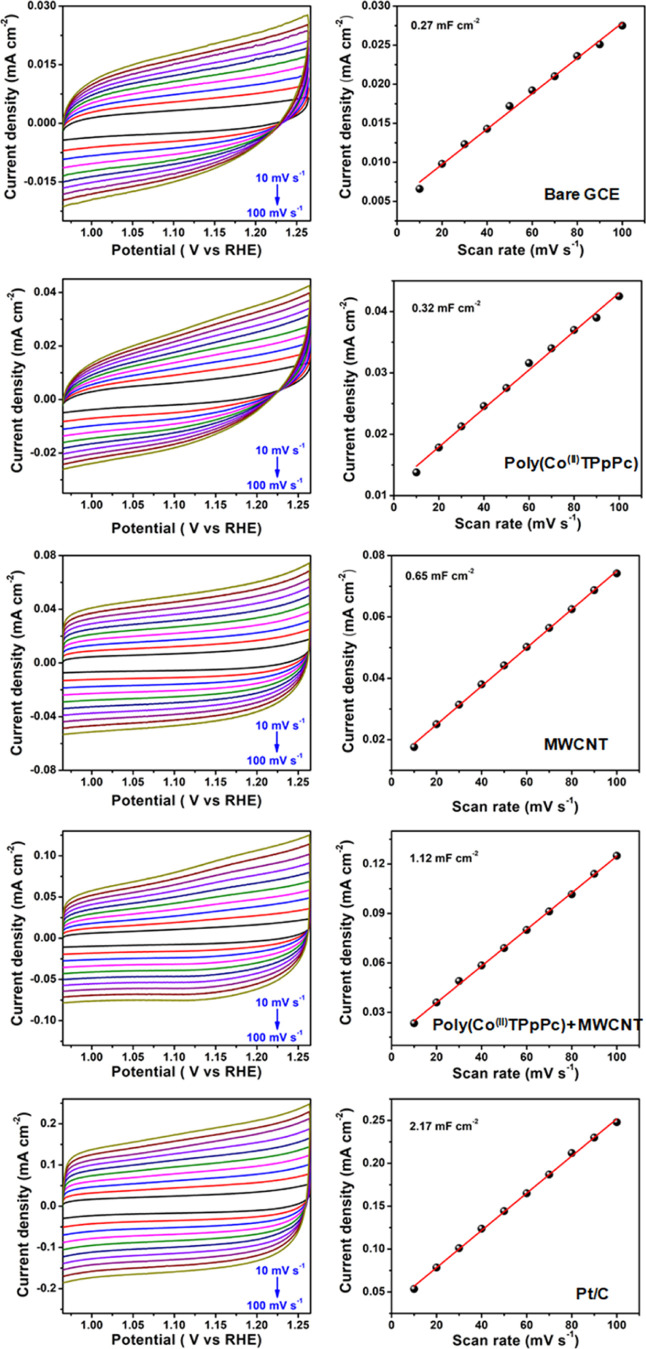
The significantly enhanced electrocatalytic activity for ORR of the hybrid composite electrode can be explained using the active electrochemical surface area (ECSA) of different electrodes. The ECSA was determined using cyclic voltammetry by scanning the potential in the non-Faradaic region. The CVs provide information about the double layer capacitance (Cdl) of the active catalyst. As shown in Figure 9, the bare GCE, poly(Co(II)TPpPc), MWCNTs, poly(Co(II)TPpPc)-MWCNTs, and Pt/C electrodes exhibit the double layer capacitance of 0.27, 0.32, 0.65, 1.42, and 2.17 mF•cm–2, respectively. Clearly, the poly(Co(II)TPpPc)-MWCNTs hybrid composite electrode shows a higher Cdl value compared with bare GCE, MWCNTs, and poly(Co(II)TPpPc) electrodes, suggesting that the hybrid composite electrode provides a higher active surface area for the ORR than other electrodes. The comparatively smaller ECSA value is observed for the poly(Co(II)TPpPc)-MWCNTs compared with commercial Pt/C possibly due to the bulkiness and slight aggregation of the polymeric Pc.47
Figure 9.
CVs of different electrodes at various scan rates in the non-Faradaic region in 0.1 M KOH solution.
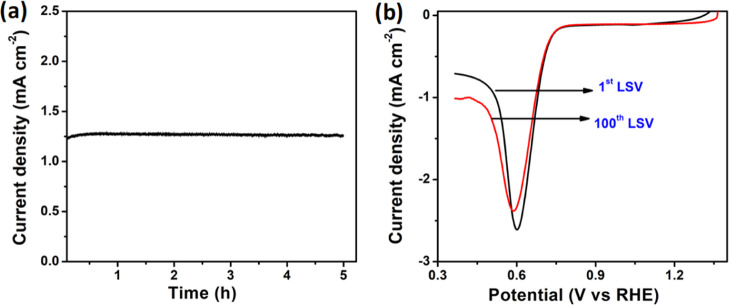
The stability of the electrode catalyst is an important parameter in ORR for designing and commercialization.47,49 The long-term stability of the poly(Co(II)TPpPc)-MWCNT composite electrode was evaluated by chronoamperometry at the potential of 0.7 V versus RHE and scanning100 LSV curves in O2-saturated 0.1 M KOH. The fabricated poly(Co(II)TPpPc)-MWCNT hybrid electrode showed higher stability for more than 5 h on a continuous operation (Figure 10a). Furthermore, the durability tests were carried out by LSV cycling, and the 1st and 100th LSV curves in Figure 10b for ORR at the poly(Co(II)TPpPc)-MWCNT electrode showed no significant change in the onset potential. Nevertheless, a slight decrease in the current density was noticed in the chronoamperometric (I–t) curve and LSV curve presumably due to the detachment of a small amount of active catalyst coated on the surface of the electrode.50 The obtained results reveal that the poly(Co(II)TPpPc)-MWCNTs hybrid composite electrode is highly stable for ORR in 0.1 M KOH.
Figure 10.
(a) Chronoamperometric response for ORR activity at 0.7 V versus RHE and (b) 1st and 100th LSV of poly(Co(II)TPpPc)-MWCNTs hybrid composite electrode in O2-saturated 0.1 M KOH at a scan rate of 10 mV•s–1.
4. Conclusions
In this work, a novel phenolphthalein-embedded cobalt phthalocyanine polymer [poly(Co(II)TPpPc)] was synthesized, and its high purity was confirmed by various analytical techniques. Various hybrid composites were prepared using poly(Co(II)TPpPc) with different carbon materials (carbon black, Vulcan carbon, and MWCNTs) to evaluate their efficiency for ORR in alkaline media. The electrode fabricated using a poly(Co(II)TPpPc)-MWCNT hybrid composite exhibited better ORR performance in alkaline media as well as a straightforward, rapid, and reproducible ORR performance. The poly(Co(II)TPpPc)-MWCNT hybrid composite electrode also showed a better onset potential (0.803 V) for ORR, and the ORR mechanism involving an appropriate four-electron transfer pathway was confirmed. The MWCNT significantly influenced the catalytic activity of poly(Co(II)TPpPc) due to its high conductivity, large surface area, and presence of active pores. Furthermore, the poly(Co(II)TPpPc)-MWCNT hybrid composite electrode showed an onset potential closer to that of Pt/C with higher current density and stability than Pt/C. The fabricated hybrid catalyst can be applied as a cathode catalyst in fuel cells, oxygen sensors, and metal-air batteries due to its low cost and high stability in comparison to commercial Pt/C.
Acknowledgments
We would like to acknowledge CSIR EMR-II grant no. 01(2915)/17/EMR-II), DST-SERB, Govt. of India grant no. DST-FIST (SR/FST/CSI-003/2016), India-Uzbekistan Collaborative Grant (nos. INT/Uzbek/P-21 and UZB-Ind-2021-91), and VGST–KFIST grant no. KSTePS/VGST-KFIST (L1)/2017/267) (GRD no. 555). We acknowledge Dr. Uday Deshpande, UGC-DAE, CSR, Indore for his support and help in carrying out the XPS measurements. G.K. thanks Dept. of OBC, Govt. of Karnataka for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01157.
1H NMR spectrum of precursor (3), mass spectra of the precursor (3) and poly(CoIITPpPc), LSVs of poly(Co(II)TPpPc)-MWCNTs measured with time-dependent O2 supply, and 3D-trajectory image (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cséfalvay E.; Horváth I. T. Sustainability Assessment of Renewable Energy in the USA, Canada, the European Union, China, and the Russian Federation. ACS Sustain. Chem. Eng. 2018, 6, 8868–8874. 10.1021/acssuschemeng.8b01213. [DOI] [Google Scholar]
- Elishav O.; Mosevitzky Lis B.; Miller E. M.; Arent D. J.; Valera-Medina A.; Dana A.; Shter G. E.; Grader G. S. Progress and Prospective of Nitrogen-Based Alternative Fuels. Chem. Rev. 2020, 120, 5352–5436. 10.1021/acs.chemrev.9b00538. [DOI] [PubMed] [Google Scholar]
- Semieniuk G.; Taylor L.; Rezai A.; Foley D. K. Plausible energy demand patterns in a growing global economy with climate policy. Nat. Clim. Change 2021, 11, 313–318. 10.1038/s41558-020-00975-7. [DOI] [Google Scholar]
- Staffell I.; Scamman D.; Abad A.; Balcombe P.; Dodds P. E.; Ekins P.; Shah N.; Ward K. R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. 10.1039/c8ee01157e. [DOI] [Google Scholar]
- Firouzjaie H. A.; Mustain W. E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234. 10.1021/acscatal.9b03892. [DOI] [Google Scholar]
- Poizot P.; Gaubicher J.; Renault S.; Dubois L.; Liang Y.; Yao Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. 10.1021/acs.chemrev.9b00482. [DOI] [PubMed] [Google Scholar]
- Xu C.; Puente-Santiago A. R.; Rodríguez-Padrón D.; Muñoz-Batista M. J.; Ahsan M. A.; Noveron J. C.; Luque R. Nature-inspired hierarchical materials for sensing and energy storage applications. Chem. Soc. Rev. 2021, 50, 4856–4871. 10.1039/c8cs00652k. [DOI] [PubMed] [Google Scholar]
- Sorokin A. B. Phthalocyanine Metal Complexes in Catalysis. Chem. Rev. 2013, 113, 8152–8191. 10.1021/cr4000072. [DOI] [PubMed] [Google Scholar]
- Sudhakara S. M.; Devendrachari M. C.; Kotresh H. M. N.; Khan F. Phthalocyanine pendented polyaniline via amide linkage for an electrochemical sensing of H2O2. Microchem. J. 2021, 161, 105781. 10.1016/j.microc.2020.105781. [DOI] [Google Scholar]
- Aralekallu S.; Sajjan V. A.; Palanna M.; Prabhu K. C. P.; Hojamberdiev M. Ni foam supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2020, 449, 227516. [Google Scholar]
- Park J. S.; Chang D. W. Iron Phthalocyanine/Graphene Composites as Promising Electrocatalysts for the Oxygen Reduction Reaction. Energies 2020, 13, 4073. 10.3390/en13164073. [DOI] [Google Scholar]
- Ramavathu L. N.; Maniam K. K.; Gopalram K.; Chetty R. Effect of pyrolysis temperature on cobalt phthalocyanine supported on carbon nanotubes for oxygen reduction reaction. J. Appl. Electrochem. 2012, 42, 945–951. 10.1007/s10800-012-0481-6. [DOI] [Google Scholar]
- Ahmed J.; Kim H. J.; Kim S. Embedded cobalt oxide nano particles on carbon could potentially improve oxygen reduction activity of cobalt phthalocyanine and its application in microbial fuel cells. RSC Adv. 2014, 4, 44065–44072. 10.1039/c4ra05940a. [DOI] [Google Scholar]
- Wittke G. Reactions of Phenolphthalein at Various pH Values. J. Chem. Educ. 1983, 60, 239. 10.1021/ed060p239. [DOI] [Google Scholar]
- Georgakilas V.; Perman J. A.; Tucek J.; Zboril R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. 10.1021/cr500304f. [DOI] [PubMed] [Google Scholar]
- Hirsch A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. 10.1038/nmat2885. [DOI] [PubMed] [Google Scholar]
- Chlistunoff J.; Sansiñena J.-M. On the use of Nafion in electrochemical studies of carbon supported oxygen reduction catalysts in aqueous media. J. Electroanal. Chem. 2016, 780, 134–146. 10.1016/j.jelechem.2016.09.014. [DOI] [Google Scholar]
- Altun S.; Altındal A.; Özkaya A.; Bulut M.; Bekaroğlu Ö. Synthesis, characterization, electrochemical and CO2 sensing properties of novel mono and ball-type phthalocyanines with four phenolphthalein units. Tetrahedron Lett. 2008, 49, 4483–4486. 10.1016/j.tetlet.2008.05.065. [DOI] [Google Scholar]
- Giddaerappa; Nemakal M.; Mohammed I.; Koodlur Sannegowda L. Mannich reaction derived phthalocyanine polymer for electrochemical detection of salicylic acid. Inorg. Chim. Acta 2020, 512, 119895. 10.1016/j.ica.2020.119895. [DOI] [Google Scholar]
- Nemakal M.; Giddaerappa S.; Shantharaja V. A.; Sajjan V. A. Novel amide coupled phthalocyanines: Synthesis and structure-property relationship for electrocatalysis and sensing of hydroquinone. J. Electroanal. Chem. 2021, 898, 115657. 10.1016/j.jelechem.2021.115657. [DOI] [Google Scholar]
- Seoudi R.; El-Bahy G. S.; El Sayed Z. A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. 10.1016/j.molstruc.2005.06.003. [DOI] [Google Scholar]
- Sajjan V. A.; Aralekallu S.; Nemakal M.; Palanna M.; Prabhu C. P. K.; Sannegowda L. K. Nanomolar detection of lead using electrochemical methods based on a novel phthalocyanine. Inorg. Chim. Acta 2020, 506, 119564. 10.1016/j.ica.2020.119564. [DOI] [Google Scholar]
- Parnell C. M.; Chhetri B. P.; Mitchell T. B.; Watanabe F.; Kannarpady G.; RanguMagar A. B.; Zhou H.; Alghazali K. M.; Biris A. S.; Ghosh A. Simultaneous Electrochemical Deposition of Cobalt Complex and Poly(pyrrole) Thin Films for Supercapacitor Electrodes. Sci. Rep. 2019, 9, 5650. 10.1038/s41598-019-41969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Chen J.; Guo R.; Xu J.; Fang X.; Han Y.-F. Cobalt Phthalocyanine Coordinated to Pyridine-Functionalized Carbon Nanotubes with Enhanced CO2 Electroreduction. Appl. Catal., B 2019, 251, 112–118. 10.1016/j.apcatb.2019.03.047. [DOI] [Google Scholar]
- Nombona N.; Nyokong T. The synthesis, cyclic voltammetry and spectro electrochemical studies of Co(II) phthalocyanines tetra-substituted at the α and β positions with phenylthio groups. Dyes Pigm. 2009, 80, 130–135. 10.1016/j.dyepig.2008.06.002. [DOI] [Google Scholar]
- Manjunatha N.; Imadadulla M.; Lokesh K. S.; Reddy K. R. Synthesis and electropolymerization of tetra-[β-(2-benzimidazole)] and tetra-[β-(2-(1-(4- aminophenyl))benzimidazole)] embedded cobalt phthalocyanine and their supercapacitance behavior. Dyes Pigm. 2018, 153, 213–224. 10.1016/j.dyepig.2018.01.042. [DOI] [Google Scholar]
- Li C.; Huang T.; Huang Z.; Sun J.; Zong C.; Yang J.; Deng W.; Dai F. A sulfonated cobalt phthalocyanine/carbon nanotube hybrid as a bifunctional oxygen electrocatalyst. Dalton Trans. 2019, 48, 17258–17265. 10.1039/c9dt03360b. [DOI] [PubMed] [Google Scholar]
- Morozan A.; Campidelli S.; Filoramo A.; Jousselme B.; Palacin S. Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 2011, 49, 4839–4847. 10.1016/j.carbon.2011.07.004. [DOI] [Google Scholar]
- Luo C.; Xie H.; Wang Q.; Luo G.; Liu C. A Review of the Application and Performance of Carbon Nanotubes in Fuel Cells. J. Nanomater. 2015, 2015, 560392. 10.1155/2015/560392. [DOI] [Google Scholar]
- Morais R. G.; Rey-Raap N.; Figueiredo J. L.; Pereira M. F. R. Highly electroactive N-Fe hydrothermal carbons and carbon nanotubes for the oxygen reduction reaction. J. Energy Chem. 2020, 50, 260–270. 10.1016/j.jechem.2020.03.039. [DOI] [Google Scholar]
- Wan W.; Triana C. A.; Lan J.; Li J.; Allen C. S.; Zhao Y.; Iannuzzi M.; Patzke G. R. Bifunctional Single Atom Electrocatalysts: Coordination–Performance Correlations and Reaction Pathways. ACS Nano 2020, 14, 13279–13293. 10.1021/acsnano.0c05088. [DOI] [PubMed] [Google Scholar]
- Valverde-González A.; Guan L. Z.; Ferrer M. L.; Iglesias M.; Maya E. M. Iron Phthalocyanine Knitted Polymers as Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 32681–32688. 10.1021/acsami.0c07412. [DOI] [PubMed] [Google Scholar]
- Garcia A. C.; Koper M. T. M. Effect of Saturating the Electrolyte with Oxygen on the Activity for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 9359–9363. 10.1021/acscatal.8b01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G.; Xu S.; Liu L.; Zheng C. Z.; Dou J.; Wang F.; Fu X.; Liao W.; Wang H. Effect of Experimental Operations on the Limiting Current Density of Oxygen Reduction Reaction Evaluated by Rotating Disk Electrode. ChemElectroChem 2020, 7, 1107–1114. 10.1002/celc.201902085. [DOI] [Google Scholar]
- Perry S. C.; Denuault G. The oxygen reduction reaction (ORR) on reduced metals: evidence for a unique relationship between the coverage of adsorbed oxygen species and adsorption energy. Phys. Chem. Chem. Phys. 2016, 18, 10218–10223. 10.1039/c6cp00106h. [DOI] [PubMed] [Google Scholar]
- Wang S.; Yu D.; Dai L.; Chang D. W.; Baek J.-B. Polyelectrolyte-Functionalized Graphene as Metal-Free Electrocatalysts for Oxygen Reduction. ACS Nano 2011, 5, 6202–6209. 10.1021/nn200879h. [DOI] [PubMed] [Google Scholar]
- Sonkar P. K.; Ganesan V.; Gupta R.; Yadav D. K.; Yadav M. Nickel phthalocyanine integrated graphene architecture as bifunctional electrocatalyst for CO2 and O2 reductions. J. Electroanal. Chem. 2018, 826, 1–9. 10.1016/j.jelechem.2018.08.020. [DOI] [Google Scholar]
- Fu X.; Liu Y.; Cao X.; Jin J.; Liu Q.; Zhang J. FeCo-Nx embedded graphene as high-performance catalysts for oxygen reduction reaction. Appl. Catal., B 2013, 130–131, 143–151. 10.1016/j.apcatb.2012.10.028. [DOI] [Google Scholar]
- Arana C.; Keshavarz M.; Potts K. T.; Abruña H. D. Electrocatalytic reduction of CO2 and O2 with electropolymerized films of vinyl-terpyridine complexes of Fe, Ni and Co. Inorg. Chim. Acta 1994, 225, 285–295. 10.1016/0020-1693(94)04059-1. [DOI] [Google Scholar]
- Jiang Y.; Lu Y.; Lv X.; Han D.; Zhang Q.; Niu L.; Chen W. Enhanced Catalytic Performance of Pt-Free Iron Phthalocyanine by Graphene Support for Efficient Oxygen Reduction Reaction. ACS Catal. 2013, 3, 1263–1271. 10.1021/cs4001927. [DOI] [Google Scholar]
- Bard A. J.; Faulkner L. R.. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Willey: New York, 2001; pp 235–237. [Google Scholar]
- Ge X.; Sumboja A.; Wuu D.; An T.; Li B.; Goh F. W. T.; Hor T. S. A.; Zong Y.; Liu Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. 10.1021/acscatal.5b00524. [DOI] [Google Scholar]
- Mukherjee M.; Samanta M.; Sarkar S.; Das G. P.; Chattopadhyay K. K. Graphene wrapped organic nanotube: A promising material for Oxygen Reduction Reaction. Mater. Lett. 2019, 248, 8–11. 10.1016/j.matlet.2019.03.132. [DOI] [Google Scholar]
- Parra-Puerto A.; Ng K. L.; Fahy K.; Goode A. E.; Ryan M. P.; Kucernak A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. 10.1021/acscatal.9b03359. [DOI] [Google Scholar]
- Mamtani K.; Jain D.; Dogu D.; Gustin V.; Gunduz S.; Co A. C.; Ozkan U. S. Insights into Oxygen Reduction Reaction (ORR) and Oxygen Evolution Reaction (OER) Active Sites for Nitrogen-doped Carbon Nanostructures (CNx) in Acidic Media. Appl. Catal., B 2018, 220, 88–97. 10.1016/j.apcatb.2017.07.086. [DOI] [Google Scholar]
- Tasso T. T.; Furuyama T.; Kobayashi N. Absorption and Electrochemical Properties of Cobalt and Iron Phthalocyanines and Their Quaternized Derivatives: Aggregation Equilibrium and Oxygen Reduction Electrocatalysis. Inorg. Chem. 2013, 52, 9206–9215. 10.1021/ic4002048. [DOI] [PubMed] [Google Scholar]
- Prabhu C. P. K.; Aralekallu S.; Sajjan V. A.; Palanna M.; Kumar S.; Sannegowda L. K. Non-precious cobalt phthalocyanine-embedded iron ore electrocatalysts for hydrogen evolution reactions. Sustain. Energy Fuels 2021, 5, 1448–1457. 10.1039/d0se01829e. [DOI] [Google Scholar]
- Zhang X.; Lu Z.; Yang Z. The mechanism of oxygen reduction reaction on CoN4 embedded graphene: A combined kinetic and atomistic thermodynamic study. Int. J. Hydrogen Energy 2016, 41, 21212–21220. 10.1016/j.ijhydene.2016.08.011. [DOI] [Google Scholar]
- Chen R.; Li H.; Chu D.; Wang G. Unraveling Oxygen Reduction Reaction Mechanisms on Carbon-Supported Fe-Phthalocyanine and Co-Phthalocyanine Catalysts in Alkaline Solutions. J. Phys. Chem. C 2009, 113, 20689–20697. 10.1021/jp906408y. [DOI] [Google Scholar]
- Hadimane S.; Aralekallu S.; Prabhu C. P.K.; Hojamberdiev M.; Sannegowda L. K. Bioinspired Precious-Metal-Free N4 Macrocycle as an Electrocatalyst for the Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 10826–10834. 10.1021/acsaem.1c01796. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.