Abstract
A facile and highly efficient method for the preparation of 2-(3-oxoindolin-2-ylidene)acetonitriles from 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitriles is described. The featured transformation operates via nucleophilic intramolecular cyclization and involves oxidation of the aniline moiety. Overall, this modification allowed for the improvement of yields and expansion of the reaction scope.
Introduction
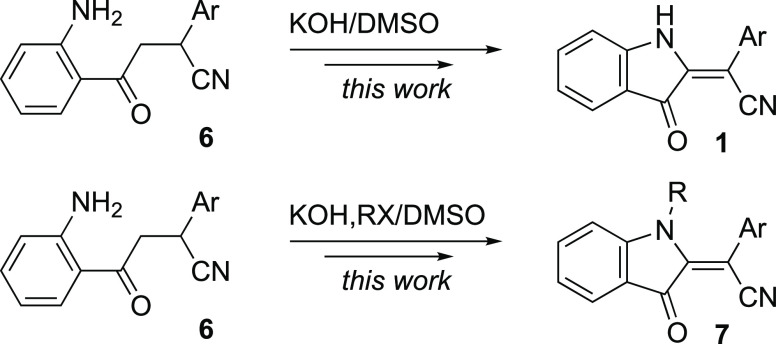
2-Alkylideneindolin-3-one natural products and their synthetic derivatives are important synthons actively used in drug discovery and development. This heterocyclic motif possesses numerous important biological activities. Bis-indole indirubin, the main component of “Tyrian purple” dye, is a known active ingredient of a traditional Chinese herbal medicine.1 Synthetic analogues of this dye displayed a highly selective pharmacological profile in glycogen synthase kinase and cyclin-dependent kinase inhibition.2−7 Moreover, alkylideneindolinones were shown to induce apoptosis of human cancer cells. They are also being explored as prospective therapeutics for the treatment of several neurodegenerative conditions.2−7 2-Alkylideneindolin-3-one derivatives bearing a single indoline moiety or two remotely positioned indoline subunits are found in nature and are endowed with important biological activities as well.8−15 2-Alkylideneindolin-3-ones bearing a conjugated nitrile function (i.e., 2-(3-oxoindolin-2-ylidene)acetonitriles 1) were used as advanced precursors in the synthesis of pyridazino[4,3-b]indoles 2, which possess strong inhibitory activity against Mycobacterium tuberculosis (Scheme 1).16 Recently, we have communicated on the unexpected formation of 2-alkylideneindolin-3-ones taking place upon treatment of ortho-nitrochalcones with potassium cyanide and acetic acid in methanol.17 It was shown that conjugate addition of KCN to chalcone 3 triggers intramolecular attack of nucleophilic enole moiety in 4, leading to the formation of intermediate cyclic nitronate 5 (Scheme 2). Upon addition of AcOH, the emerald-green compound could be reduced into an orange-red conjugated nitrile 1 (Scheme 2).17 However, this attractive and simple method had serious limitations, permitting access only to indolines with non-substituted nitrogen atoms. Herein, we report on the development of a more general and efficient synthetic method to address these issues. This method takes advantage of a one-pot oxidative cyclization of ortho-aminochalcones 6, combined with optional in situ alkylation of the aniline moiety. Such an approach provides expeditious access to both 2-(3-oxoindolin-2-ylidene)acetonitriles 1 and their N-alkylated derivatives 7 (Scheme 3).
Scheme 1. 2-(3-Oxoindolin-2-ylidene)acetonitriles 1 as Precursors in the Preparation of Antimycobacterial Drugs.
Scheme 2. Synthesis of 2-(3-Oxoindolin-2-ylidene)acetonitriles 1 via the Reductive Cyclization of Nitrochanchones 3.
Scheme 3. Featured Method for the Preparation of 2-(3-Oxoindolin-2-ylidene)acetonitriles 1.
Results and Discussion
The indoline nitrogen in acetonitrile 1 is originated from the nitro group when obtained via the original method.17 Since this involves reduction of the N–O bond in cyclic nitronate 5, it is hardly possible to derivatize it directly into anything but an N–H bond, especially under acidic conditions. Therefore, installation of other substituents at this position to access derivatives 7 would involve additional steps in the synthetic sequence. We envisioned an alternative approach to N-alkylated products 7 that would involve a base-assisted 5-exo-trig conjugate addition of aniline functionality in 4-oxobut-2-enenitrile 8 (Scheme 4). The latter would be obtained by the oxidation of precursor 6, which potentially could be combined with base-assisted alkylation of the primary aniline function (Scheme 4). Compound 6 should be routinely available via hydrocyanation of ortho-aminochalcones 9, as described in our recent report.18
Scheme 4. Retrosynthetic Analysis of the Featured Method.
With this idea in mind, we attempted the oxidative cyclization of 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitrile 6aa into 1aa in the presence of KOH (2 equiv). The first test reaction was performed in the absence of any oxidant (in argon atmosphere). Expectedly, this led to no conversion of product, and the starting material was recovered unchanged (Table 1, entry 1). Next, the same transformation was carried in acetonitrile in the presence of strong oxidants, such as potassium permanganate or DDQ. These reactions resulted in decomposition of the starting material to form heavy tars (entries 2 and 3). Evidently, the employment of strong oxidants proved to be detrimental; therefore, we decided to test milder ones. Test reactions involving oxidation with urea complex and hydrogen peroxide in acetonitrile rendered good results (entry 4). Also, oxidation with activated carbon under oxygen atmosphere19 looked very promising (entry 5). In contrast, oxidation with (bis(trifluoroacetoxy)iodo)benzene led to the decomposition of the starting materials without formation of the target product (entry 6). Cu(II) and SeO2 proved inefficient as oxidants, as the reactions provided marginal yields or did not proceed at all (entries 7–9) We also tested oxidations with dimethyl sulfoxide (DMSO) in N,N-dimethylformamide (DMF) (entry 10) or water (entry 11), which both provided good yields of 1aa. The best results, however, were obtained when oxidation with DMSO was performed without any diluents. The reaction under these conditions was complete in 40 min, affording an 80% yield of 1aa (entry 12). In the presence of NaOH as a base, the reaction was less efficient affording only 52% yield of the target material (entry 13). Finally, oxidation with diphenylsulfoxide in acetonitrile also occurred, although it proceeded sluggishly and provided lower yields (entry 14). It should be mentioned that this transformation may be combined with the hydrocyanation of ortho-aminochalcone 9aa. Treatment with potassium cyanide and potassium hydroxide-assisted cyclization can be performed as a two-step cascade process, and the final quenching with acetic acid generates 1aa in moderate yields (Scheme 5). While substantiating the principal possibility to perform these two steps in a one-pot fashion, it proved more convenient and practical to do the sequence stepwise, as this conventional approach provides better overall yields and simplifies the purification of the final product.
Table 1. Optimization of Reaction Condition toward the Formation of 2-(3-Oxoindolin-2-ylidene)acetonitrile 1aa.
| solvent | oxidant | time, min | yield %a | |
|---|---|---|---|---|
| 1 | DMF | none | 40 | NR |
| 2 | MeCN | KMnO4 | 5 | Dec. |
| 3 | MeCN | DDQ | 5 | Dec. |
| 4 | MeCN | H2O2/urea | 300 | 60 |
| 5 | DMF | O2/C | 180 | 64 |
| 6 | MeCN | PhI(TFA)2 | 10 | Dec. |
| 7 | MeCN | CuSO4 | 300 | NR |
| 8 | MeCN | Cu(OAc)2 | 720 | 15 |
| 9 | MeCN | SeO2 | 300 | NR |
| 10 | DMF | DMSO | 180 | 67 |
| 11 | water | DMSO | 120 | 65 |
| 12 | DMSO | DMSO | 40 | 80 |
| 13 | DMSO | DMSO | 60 | 52b |
| 14 | MeCN | Ph2SO | 300 | 65 |
All of the test reactions were performed in 0.5 mmol scale in 5 mL vials under argon atmosphere at r.t. KOH (4 equiv) was used as a base unless specified otherwise. Quenching with AcOH (200 mg for 30 min at RT) was performed in one-pot fashion. NMR yields are provided.
NaOH was used instead of KOH.
Scheme 5. Direct Synthesis of 2-(3-Oxoindolin-2-ylidene)acetonitrile 1aa from ortho-Aminochalcone 9aa.
With the optimized conditions in hand, we performed these transformations in a preparative scale (up to 2.00 mmol) and managed to obtain comparably high isolated yields of 1aa (77%) (Scheme 6). The reaction demonstrated good tolerance and compatibility with a variety of substituents, including methoxyarenes (1ae, 1af, 1ca), halogenated arenes (1ag, 1ah, 1ai, 1aj, 1ak, 1ba), and hetarenes (1al) (Scheme 6).
Scheme 6. Preparation of 2-(3-Oxoindolin-2-ylidene)acetonitriles 1 from 4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitriles 6.

Next, we explored the possibility to apply this approach for the preparation of N-substituted indole derivatives 7. Initial attempts involved reactions of N-substituted precursors 10.
Unexpectedly, these reactions took a different route entailing hydrolytic cleavage of the cyano group. Thus, upon treatment with KOH in DMSO under standard reaction conditions, N-methylated derivatives 10da and 10de supplied 2-aryloyl-3-hydroxyindoles in yields of 61 and 77%, respectively. “Normal” product 7da was obtained in low yields with the reaction of compound 10da only, while analogous product 7de was not detected at all (Scheme 7). N-Benzylated precursor 10ea reacted in a similar manner affording ketone 11ea as the sole isolable product, albeit in moderate yields (Scheme 7). It also should be pointed out that we failed to engage N-tosylated (10fa) and N-acylated (10ga) 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitriles into reactions involving the formation of N-protected indolinone products 7. Instead, base-assisted deprotection of the aniline moiety occurred, after which the reaction took the normal course, affording in both cases sole product 1aa in good yields (Scheme 7). This process could be combined with the in situ alkylation of the primary amino group in 4-oxo-butanenitriles 6 with methyl iodide or benzyl bromide in DMSO in the presence of excess KOH followed by quenching with acetic acid. The corresponding 2-acylindoles 11 were formed in this case as sole products in moderate yields (Scheme 7). The same reaction could be performed employing isolated 1aa as a starting material, which upon methylation with MeI afforded ketone 11da in 58% yield. This suggests that 1aa could be an intermediate in the transformation of 6aa into 11da.
Scheme 7. Reactivity of 4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitriles 8 with Protected Aniline Moiety.

It seems impossible to access products 7 via direct oxidative cyclization of N-substituted precursors 8 due to detrimental side processes, but in situ alkylation works. Accordingly, we decided to invest in a design of synthetic approach involving the featured oxidative cyclization 6 → 1 and subsequent alkylation of the indoline moiety 1 → 7. To validate this approach, 4-(2-aminophenyl)-2-phenyl-4-oxobutyronitrile (6aa) was treated with KOH in DMSO according to the standard procedure, then the reaction mixture was quenched with methyl iodide. Gratifyingly, methylated target product 7da was obtained in 58% yield. Furthermore, a slightly modified procedure involving a final quench with dimethyl sulfate produced 7da in greater yields (88%) (Scheme 8). One-pot alkylation with in situ methylation of halogenated precursors 6ag and 6ai proceeded in the presence of dimethyl sulfate with comparable efficiency affording N-methylated products 7dg and 7di, respectively (Scheme 8). Also, benzylation with BnBr could be incorporated in the one-pot protocol to convert 6aa into N-benzylated product 7ea (Scheme 8). In a similar manner, allylation and propargylation could be performed in the presence of corresponding organic halides yielding N-allyl (7ha) and N-propargyl (7ia) derivatives, respectively (Scheme 8). The formation of an indoline-3-one moiety and successful incorporation of an N-allyl substituent into the structure of compound 7ha was unambiguously confirmed by single-crystal X-ray diffraction (CCDC # 2126910, see the Supporting Information for details).
Scheme 8. One-Pot Approach for the Preparation of N-Alkylated 2-(3-oxoindolin-2-ylidene)acetonitriles 7 from 4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitriles 6.
Mechanistic rationale elucidating the formation of 2-(3-oxoindolin-2-ylidene)acetonitriles 1 is shown in Scheme 9. It is believed that the process begins with base-assisted deprotonation of the α-CH bond in ketone 6 to afford enolate 12, which attacks a molecule of DMSO providing dimethylvinylsulfanol 13. The acidic α-CH bond next to cyano function in this structure could also be deprotonated with KOH, followed by the elimination of dimethylsulfide and water to afford cyanochalcone 14. In our recent report, we demonstrated similar oxidations of cyanoketones lacking ortho-aniline functionality.20
Scheme 9. Mechanistic Rationale for the Formation of Products 1.
In the presence of such ortho-amino group, subsequent nucleophilic 5-exo-trig cyclization would render indoline-3-one 15, which could then be deprotonated at the α-CH bond of the cyano group. The resulting cyclic enolate 16 could again react with DMSO providing dimethylvinylsulfanol 17. The following deprotonation α-CH bond next to cyano function followed by the elimination of dimethylsulfide and water produces product 1 (Scheme 9). In a similar manner, secondary aniline 10 would afford N-substituted indoline 7; however, there is a drastic difference in the postreaction behavior. The N–H bond in indoline-3-one 1 in the presence of excess base could be deprotonated, which dramatically decreases its electrophilicity. As a result, anionic species 18 is relatively persistent and could survive before being quenched with protic acid or SN2-active organyl halide to afford products 1 or 7, respectively (Scheme 10). Compound 7 generated from secondary aniline 10, in the presence of excess base, cannot be deactivated via deprotonation. Consequently, it would suffer from the nucleophilic attack of hydroxide across the conjugated C=C bond to afford enolate 19. Subsequent elimination of the cyano group would provide enol 20, further tautomerizing into thermodynamically more stable form 11 (Scheme 10).
Scheme 10. Mechanistic Rationale for the Formation of Products 11.
Finally, it was decided to explore how this methodology could be used to streamline the approach toward antimycobacterial pyridazino[4,3-b]indoles structures 2, originally investigated by Velezheva.16 Oxidative cyclization of 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitriles 6 was carried out as usual, but during the acidic quenching, hydrazide hydrate was added to the reaction mixture, which was boiled at reflux for 4 h. Gratifyingly, this one-pot sequence led to the formation of the desired molecules 2 in good yields (Scheme 11).
Scheme 11. One-Pot Synthesis of Antimycobacterial Pyridazino[4,3-b]indoles 2.
Conclusions
An improved synthetic approach toward 2-(3-oxoindolin-2-ylidene)acetonitriles 1 was developed. This method involves base-assisted cyclization of 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitriles 6 accompanied by oxidation with DMSO. It was shown that this reaction proceeds smoothly only for primary aniline derivatives 6. Starting materials 10 possessing a secondary aniline group participated in a side reaction involving cleavage of the cyano group. However, an alternative access toward N-substituted indoline-3-ones 7 was also designed, employing in situ alkylation of indolines 1. This methodology was used to design an expeditious one-pot approach toward pyridazino[4,3-b]indoles 2 with known antimycobacterial activities. Synthetic studies toward more complex polycyclic scaffolds taking advantage of this newly developed method are currently underway in our laboratories.
Experimental Part
General
1H and 13C{1H} NMR spectra were recorded on a Bruker Avance-III spectrometer (400 or 100 MHz, respectively) equipped with BBO probe in CDCl3 or DMSO-d6 using TMS as an internal standard. High-resolution mass spectra were registered with a Bruker Maxis spectrometer (electrospray ionization, in MeCN solution, using HCO2Na–HCO2H for calibration). Melting points were measured with a Stuart smp30 apparatus. All reactions were performed in oven-dried 3 mL Weaton microreactors equipped with magnetic spin-vane and Mininert valve, employing magnetic stirring. Reaction progress and purity of isolated compounds were controlled by thin-layer chromatography (TLC) on Silufol UV-254 plates, eluting with a 4:1 hexanes/EtOAc mixture. All 4-(2-aminophenyl)-4-oxo-2-arylbutanenitriles (6), except for compound 6ak, were synthesized according to the procedure published in our recent report.18 All other reagents and solvents were purchased from commercial vendors and used as received.
4-Bromo-2′-aminochalcone (9ak)
This compound was prepared by the procedure described in the literature21 employing 2′-aminoacetophenone (405 mg, 3 mmol) and 4-bromobenzaldehyde (552 mg, 3.00 mmol). The title compound was obtained as yellow solid, mp 101.7–102.8 °C (EtOH), lit21 mp 94–96 °C (EtOH), Rf 0.44 (EtOAc/hexane, 1:4). 1H NMR (400 MHz, CDCl3) δ 7.84 (dd, J = 8.4, 1.5 Hz, 1H), 7.66 (d, J = 15.5 Hz, 1H), 7.60 (d, J = 15.5 Hz, 1H), 7.56–7.52 (m, 2H), 7.51–7.47 (m, 2H), 7.30 (ddd, J = 8.5, 7.1, 1.5 Hz, 1H), 6.74–6.66 (m, 2H), 6.34 (s, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 191.5, 151.2, 141.6, 134.6, 134.4, 132.3 (2C), 131.1, 129.8 (2C), 124.4, 123.8, 119.0, 117.5, 116.0; FTIR (film, NaCl, cm–1): 3427, 3326, 2926, 1636, 1581, 1542, 1482, 1446, 1390, 1333, 1207, 1164, 1070, 1005; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C15H12BrNNaO 323.9994; found 323.9990 (1.4 ppm).
(E)-1-(2-Amino-5-nitrophenyl)-3-phenylprop-2-en-1-one (9ja)
1-(2-Amino-5-nitrophenyl)ethan-1-one22 (540 mg, 3.00 mmol), benzaldehyde (318 mg, 3.00 mmol), and ethanol (15 mL) were placed in a 50 mL beaker. Then, 300 μL of 40% KOH was added and the mixture was left overnight, after which the formed precipitate was filtered off. The title compound was obtained as a yellow solid, mp 154.6–156.2 °C (EtOH), Rf 0.56 (EtOAc/hexane, 1:2). Yield 579 mg (2.16 mmol, 72%). 1H NMR (400 MHz, DMSO-d6) δ 8.92 (d, J = 2.6 Hz, 1H), 8.46 (br. s, 2H), 8.12 (dd, J = 9.3, 2.6 Hz, 1H), 8.01 (d, J = 15.4 Hz, 1H), 7.94–7.88 (m, 2H), 7.71 (d, J = 15.4 Hz, 1H), 7.47 (dd, J = 5.0, 1.9 Hz, 3H), 6.93 (d, J = 9.3 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 190.3, 156.4, 143.8, 135.1, 134.7, 130.6, 129.1 (2C), 129.0, 129.0, 129.0 (2C), 122.6, 117.2, 115.7; FTIR (film, NaCl, cm–1): 3419, 3296, 1645, 1616, 1588, 1494, 1474, 1308, 1260, 1206, 1115; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C15H12N2NaO3 291.0740; found 291.0738 (0.8 ppm).
4-(2-Aminophenyl)-2-(4-bromophenyl)-4-oxobutanenitrile (6ak)
This compound was prepared via a method previously described in the literature.18 A 25 mL round-bottom flask was charged with 4-bromo-2′-aminochalcone (9ak) (1.34 g, 2.00 mmol), acetic acid (120 mg, 114 μL, 2.00 mmol), and DMSO (6 mL). The mixture was vigorously stirred, and a solution of KCN (260 mg, 4.00 mmol) in water (0.5 mL) was added dropwise. Then, the reaction vessel was equipped with a reflux condenser, and the mixture was stirred at 50 °C for 1 h, while the reaction progress was monitored by TLC. Upon complete conversion, the mixture was diluted with water (30 mL) and extracted with dichloromethane (4 × 15 mL). Combined organic extracts were washed with water (4 × 15 mL), concentrated in vacuum, and purified by preparative column chromatography on silica gel eluting with 1:4 EtOAc/hexane. The title compound was obtained as a yellow solid, mp 105.1–106.0 °C (EtOH), Rf 0.24 (EtOAc/hexane, 1:4). Yield 315 mg (0.96 mmol, 48%). 1H NMR (400 MHz, CDCl3) δ 7.57 (dd, J = 8.2, 1.5 Hz, 1H), 7.56–7.48 (m, 2H), 7.34–7.26 (m, 3H), 6.66 (dd, J = 8.4, 1.1 Hz, 1H), 6.62 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 6.29 (s, 2H), 4.51 (dd, J = 7.9, 6.1 Hz, 1H), 3.68 (dd, J = 17.5, 7.9 Hz, 1H), 3.46 (dd, J = 17.6, 6.1 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 195.7, 150.8, 135.3, 134.7, 132.5 (2C), 130.5, 129.4 (2C), 122.5, 120.6, 117.7, 116.7, 116.1, 44.7, 31.7; FTIR (film, NaCl, cm–1): 3470, 3355, 2930, 2246, 1643, 1612, 1571, 1547, 1489, 1453, 1248, 1171, 1072; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H13BrN2NaO 351.0103; found 351.0095 (2.4 ppm).
4-(2-Amino-5-nitrophenyl)-4-oxo-2-phenylbutanenitrile (6ja)
This compound was prepared in analogy to the method described for 6ak employing (E)-1-(2-amino-5-nitrophenyl)-3-phenylprop-2-en-1-one (9ja) (536 mg, 2 mmol). The title compound was obtained as a yellow solid, mp 183.9–186.0 °C (EtOH), Rf 0.44 (EtOAc/hexane, 1:2). Yield 572 mg (1.94 mmol, 97%). 1H NMR (400 MHz, DMSO-d6) δ 8.66 (d, J = 2.6 Hz, 1H), 8.36 (s, 2H), 8.09 (dd, J = 9.4, 2.6 Hz, 1H), 7.60–7.48 (m, 2H), 7.46–7.39 (m, 2H), 7.39–7.33 (m, 1H), 6.90 (d, J = 9.4 Hz, 1H), 4.56 (dd, J = 9.1, 5.0 Hz, 1H), 4.09 (dd, J = 18.2, 9.2 Hz, 1H), 3.80 (dd, J = 18.2, 5.1 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 197.2, 155.6, 135.8, 134.9, 129.4, 129.1, 128.9 (2C), 128.0, 127.9 (2C), 121.5, 117.4, 113.8, 43.0, 31.2; FTIR (film, NaCl, cm–1): 3464, 3356, 2242, 1657, 1616, 1558, 1484, 1310, 1274, 1264, 1246, 1109; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H13N3NaO3 318.0849; found 318.0848 (0.4 ppm).
4-(2-(Methylamino)phenyl)-2-phenyl-4-oxobutyronitrile (10da)
4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (1.25 g, 5.00 mmol), MeCN (3 mL), Me2SO4 (1.26 g, 0.95 mL, 10.0 mmol), and K2CO3 (1.38 g, 10.0 mmol) were placed in a 25 mL round-bottom flask equipped with a magnetic stirring bar and allowed to reflux to 1.5 h. After consumption of the starting material, the resulting solution was poured out into a separating funnel filled with water (50 mL), NaHCO3 (0.84 g, 10 mmol), and CH2Cl2 (50 mL). The aqueous layer was discarded and the organic layer washed with saturated NaHCO3 solution (2 × 30 mL). Next, the solution was concentrated in vacuo and purified by column chromatography eluting with benzene. The title compound was isolated as an orange oil, Rf 0.40 (EtOAc/hexane, 1:4). Yield 726 mg (2.75 mmol, 55%). 1H NMR (400 MHz, DMSO-d6) δ 8.77 (s, 1H), 7.61 (dd, J = 8.2, 1.6 Hz, 1H), 7.47–7.29 (m, 6H), 6.71 (dd, J = 8.6, 1.1 Hz, 1H), 6.56 (ddd, J = 8.2, 7.0, 1.1 Hz, 1H), 4.51 (dd, J = 8.6, 5.6 Hz, 1H), 3.71 (dd, J = 17.5, 8.6 Hz, 1H), 3.49 (dd, J = 17.5, 5.6 Hz, 1H), 2.93 (d, J = 5.1 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 196.1, 152.4, 135.8, 135.7, 131.2, 129.3 (2C), 128.4, 127.6 (2C), 121.1, 116.2, 114.2, 111.8, 44.8, 32.3, 29.5; FTIR (film, NaCl, cm–1): 3340, 2926, 2246, 1631, 1569, 1520, 1424, 1251, 1202, 1166; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H16N2NaO 287.1151; found 287.1155 (1.3 ppm).
2-(4-Methoxyphenyl)-4-(2-(methylamino)phenyl)-4-oxobutanenitrile (10de)
This compound was prepared via the published method used for the preparation of 10da employing 4-(2-aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanenitrile18 (6ae) (1.40 g, 5.00 mmol) and Me2SO4 (1.26 g, 0.95 mL, 10.0 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:4. The title compound was obtained as a yellow oil, Rf 0.35 (EtOAc/hexane, 1:4). Yield 779 mg (2.65 mmol, 53%). 1H NMR (400 MHz, CDCl3) δ 8.76 (br. s, 1H), 7.62 (d, J = 8.1 Hz, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.33 (d, J = 8.6 Hz, 2H), 6.91 (d, J = 8.5 Hz, 2H), 6.70 (d, J = 8.6 Hz, 1H), 6.56 (t, J = 7.6 Hz, 1H), 4.46 (dd, J = 8.4, 5.8 Hz, 1H), 3.81 (s, 3H), 3.67 (dd, J = 17.4, 8.3 Hz, 1H), 3.46 (dd, J = 17.4, 5.8 Hz, 1H), 2.92 (d, J = 5.1 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 196.2, 159.5, 152.4, 135.8, 131.2, 128.8 (2C), 127.5, 121.4, 116.2, 114.7 (2C), 114.2, 111.7, 55.5, 44.8, 31.5, 29.4; FTIR (film, NaCl, cm–1): 3350, 2940, 2246, 1634, 1566, 1513, 1424, 1248, 1173, 1029; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C18H18N2NaO2 317.1260; found 317.1258 (0.7 ppm).
4-(2-(Benzylamino)phenyl)-4-oxo-2-phenylbutanenitrile (10ea)
This compound was prepared via the published method used for the preparation of 6da employing 4-(2-aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanenitrile18 (6ae) (1.4 g, 5 mmol) and benzyl bromide (1.71 g, 1.19 mL, 10.0 mmol). Eluent for preparative column chromatography: benzene. The title compound was obtained as an orange solid, mp 121.2–122.2 °C (EtOH), Rf 0.44 (EtOAc/hexane, 1:4). Yield 1.02 g (3.00 mmol, 60%). 1H NMR (400 MHz, DMSO-d6) δ 9.27 (t, J = 5.9 Hz, 1H), 7.65 (dd, J = 8.2, 1.6 Hz, 1H), 7.48–7.27 (m, 11H), 6.68 (d, J = 8.6 Hz, 1H), 6.58 (t, J = 7.6 Hz, 1H), 4.53 (dd, J = 8.7, 5.5 Hz, 1H), 4.48 (d, J = 5.7 Hz, 2H), 3.75 (dd, J = 17.5, 8.7 Hz, 1H), 3.50 (dd, J = 17.5, 5.5 Hz, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 196.3, 151.4, 138.5, 135.8, 135.7, 131.3, 129.4 (2C), 128.9 (2C), 128.4, 127.6 (2C), 127.4, 127.1 (2C), 121.1, 116.5, 114.8, 112.7, 46.9, 45.0, 32.4; FTIR (film, NaCl, cm–1): 3326, 2241, 1631, 1571, 1518, 1453, 1255, 1195, 1055; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C23H20N2NaO 363.1468; found 363.1457 (3.0 ppm).
N-(2-(3-Cyano-3-phenylpropanoyl)phenyl)acetamide (10fa)
4-(2-Aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (1.00 g, 4.00 mmol), CH2Cl2 (4 mL), and Et3N (444 mg, 317 μL, 4.40 mmol) were placed in a 25 mL round-bottom flask equipped with a magnetic stirring bar. Then, AcCl (377 mg, 343 μL, 4.80 mmol) was added maintaining the reaction mixture temperature below 30 °C and stirred for 3 h. After consumption of starting material, the resulting solution was partitioned between water (50 mL) and CH2Cl2 (50 mL). The aqueous layer was discarded, and the organic layer washed with saturated NaHCO3 solution (2 × 30 mL). Next, the solution was concentrated in vacuo and purified by column chromatography (eluting with ethyl acetate/hexane 1:2 v/v) or by recrystallization from benzene. The title compound was obtained as a colorless solid, m.p. = 113.7–115.6 (benzene), Rf 0.44 (EtOAc/Hex, 1:2). Yield 1.121 g (3.84 mmol, 96%). 1H NMR (400 MHz, CDCl3) δ 11.46 (s, 1H), 8.82–8.68 (m, 1H), 7.78 (dd, J = 8.2, 1.6 Hz, 1H), 7.56 (ddd, J = 8.6, 7.3, 1.5 Hz, 1H), 7.48–7.30 (m, 5H), 7.12–7.00 (m, 1H), 4.47 (dd, J = 8.9, 5.2 Hz, 1H), 3.79 (dd, J = 18.0, 8.8 Hz, 1H), 3.54 (dd, J = 18.0, 5.3 Hz, 1H), 2.25 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 198.8, 169.6, 141.5, 136.0, 134.9, 130.4, 129.5 (2C), 128.7, 127.5 (2C), 122.5, 121.1, 120.6, 120.5, 45.8, 32.2, 25.7; FTIR (film, NaCl, cm–1): 3258, 2964, 2251, 1694, 1660, 1585, 1537, 1448, 1354, 1293, 1243, 1190; HRMS (ESI TOF) calc’d for C18H16N2NaO2 (M + Na)+ 315.1104, found 315.1100 (1.3 ppm).
(E)-2-(3-Oxoindolin-2-ylidene)-2-phenylacetonitrile (1aa)
Typical Procedure A for the Synthesis of (E)-2-(3-Oxoindolin-2-ylidene)-2-arylacetonitriles 1
4-(2-Aminophenyl)-4-oxobutyronitrile18 (6aa) (125 mg, 0.50 mmol), DMSO (0.4 mL), and KOH (112 mg, 2.00 mmol) were mixed in a 5 mL round-bottom flask and stirred at room temperature for 30–40 min. Saturated green color of the reaction mixture quickly developed, which may indicate peroxidation. Next, AcOH (0.2 mL) was added and the mixture was stirred for another 30 min, during which the reaction product usually precipitated. The reaction was diluted with 60 mL of CH2Cl2 and washed with saturated NaHCO3 solution (3 × 10 mL). Next, the solution was concentrated and the residual oil was purified by column chromatography (eluting with ethyl acetate/hexane 1:2 v/v) or by recrystallization from ethanol. Yield 95 mg (0.39 mmol, 77%). The same compound was prepared starting from N-(2-(3-cyano-3-phenylpropanoyl)phenyl)acetamide (10fa) (146 mg, 0.50 mmol) with yield 79 mg (0.32 mmol, 64%). Alternatively, this compound was prepared starting from N-(2-(3-cyano-3-phenylpropanoyl)phenyl)-4-methylbenzenesulfonamide18 (10ga) (202 mg, 0.50 mmol) in a yield of 83 mg (0.34 mmol, 67%). All physical data were identical to those previously described.17Rf 0.65 (EtOAc/Hex, 1:1). 1H NMR (400 MHz, DMSO-d6) δ 10.49 (br. s, 1H), 7.68–7.61 (m, 3H), 7.57 (t, J = 7.6 Hz, 3H), 7.48 (t, J = 7.3 Hz, 1H), 7.09 (d, J = 8.0 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.2, 152.5, 142.5, 137.4, 132.1, 129.3 (2C), 129.1, 128.8 (2C), 124.9, 121.4, 119.5, 118.0, 112.7, 88.8; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H10N2NaO 269.0685; found 269.0693 (−3.0 ppm).
Typical Procedure B for the Synthesis of (E)-2-(3-Oxoindolin-2-ylidene)-2-arylacetonitriles 1 (Scale-Up Procedure)
4-(2-Aminophenyl)-4-oxobutyronitrile18 (6aa) (0.50 g, 2.00 mmol), DMSO (1.6 mL), and KOH (448 mg, 8.00 mmol) were mixed in a 5 mL round-bottom flask and left for 30–40 min under stirring at room temperature. A saturated green color of the reaction mixture quickly developed, which may indicate peroxidation. Next, AcOH (0.8 mL) was added and the mixture was stirred for another 30 min, during which the reaction product usually precipitated. The reaction was diluted with CH2Cl2 (240 mL) and washed with saturated solution of NaHCO3 (3 × 40 mL). Next, the solution was concentrated and purified by column chromatography (eluent ethyl acetate:hexane 1:2 v/v) or by recrystallization from alcohol. Yield 389 mg (1.58 mmol, 79%). The obtained sample is identical to that obtained by typical procedure A.
(E)-2-(3-Oxoindolin-2-ylidene)-2-(p-tolyl)acetonitrile (1ab)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-4-oxo-2-(p-tolyl)butanenitrile18 (6ab) (132 mg, 0.5 mmol). Eluent for preparative column chromatography:EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.46 (EtOAc/hexane, 1:4). Yield 117 mg (0.45 mmol, 90%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.43 (s, 1H), 7.64 (d, J = 7.6 Hz, 1H), 7.62–7.45 (m, 3H), 7.38 (d, J = 7.8 Hz, 2H), 7.09 (d, J = 8.1 Hz, 1H), 7.01 (t, J = 7.4 Hz, 1H), 2.38 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.2, 152.5, 142.1, 139.0, 137.4, 130.0 (2C), 129.2, 128.7 (2C), 124.8, 121.4, 119.5, 118.0, 112.7, 89.2, 20.9; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H12N2NaO 283.0842; found 283.0845 (−1.0 ppm).
(E)-2-(4-Ethylphenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ac)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-ethylphenyl)-4-oxobutanenitrile18 (6ac) (139 mg, 0.50 mmol). Eluent for preparative column chromatography:EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.56 (EtOAc/Hex, 1:2). Yield 116 mg (0.42 mmol, 85%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.45 (br. s, 1H), 7.64 (dt, J = 7.6, 0.9 Hz, 1H), 7.62–7.53 (m, 3H), 7.44–7.38 (m, 2H), 7.09 (d, J = 8.0 Hz, 1H), 7.01 (td, J = 7.5, 0.9 Hz, 1H), 2.68 (q, J = 7.6 Hz, 2H), 1.22 (t, J = 7.6 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.1, 152.5, 145.2, 142.1, 137.4, 129.4, 128.8 (2C), 128.8 (2C), 124.8, 121.4, 119.5, 118.0, 112.7, 89.2, 28.0, 15.4; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C18H14N2NaO 297.0998; found 297.0994 (1.5 ppm).
(E)-2-(4-Isopropylphenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ad)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-isopropylphenyl)-4-oxobutanenitrile18 (6ad) (146 mg, 0.50 mmol). Eluent for preparative column chromatography:EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.47 (EtOAc/Hex, 1:2). Yield 83 mg (0.29 mmol, 58%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H), 7.65 (d, J = 7.3 Hz, 1H), 7.62–7.51 (m, 3H), 7.47–7.40 (m, 2H), 7.10 (d, J = 8.0 Hz, 1H), 7.02 (td, J = 7.4, 0.7 Hz, 1H), 3.04–2.86 (m, 1H), 1.25 (d, J = 6.9 Hz, 6H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.6, 152.9, 150.2, 142.6, 137.8, 130.0 (2C), 129.3 (2C), 127.8, 127.8, 125.3, 121.8, 120.0, 118.4, 113.2, 89.6, 33.8, 24.1; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C19H16N2NaO 311.1555; found 311.1148 (2.2 ppm).
(E)-2-(4-Methoxyphenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ae)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanenitrile18 (6ae) (140 mg, 0.50 mmol). Eluent for preparative column chromatography:EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.43 (EtOAc/Hex, 1:2). Yield 106 mg (0.39 mmol, 77%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) 10.40 (s, 1H), 7.78–7.43 (m, 4H), 7.21–7.05 (m, 3H), 7.01 (t, J = 7.4 Hz, 1H), 3.84 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.5, 160.3, 152.9, 142.0, 137.7, 130.0 (2C), 125.2, 124.5, 121.7, 120.1, 118.5, 115.3 (2C), 113.2, 89.9, 55.9; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H12N2NaO2 299.0791; found 299.0784 (2.3 ppm).
(E)-2-(2-Methoxyphenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1af)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(2-methoxyphenyl)-4-oxobutanenitrile18 (6af) (140 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as an orange solid, mp 261.5–263.1 °C (EtOH), Rf 0.28 (EtOAc/hexane, 1:2). Yield 60 mg (0.21 mmol, 43%). 1H NMR (400 MHz, DMSO-d6) δ 10.01 (s, 1H), 7.63 (d, J = 7.6 Hz, 1H), 7.58–7.46 (m, 2H), 7.43 (d, J = 7.5 Hz, 1H), 7.20 (d, J = 8.4 Hz, 1H), 7.11 (t, J = 7.5 Hz, 1H), 6.99 (t, J = 8.2 Hz, 2H), 3.85 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.4, 157.3, 152.7, 143.8, 138.0, 131.6, 131.3, 125.3, 121.6, 121.5, 120.4, 119.9, 118.2, 112.8, 112.7, 86.3, 56.2; FTIR (film, NaCl, cm–1): 3268, 2212, 1701, 1605, 1484, 1462, 1330, 1212, 1142; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H12N2NaO 299.0791; found 299.0801 (3.5 ppm).
(E)-2-(4-Fluorophenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ag)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-fluorophenyl)-4-oxobutanenitrile18 (6ag) (134 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.54 (EtOAc/hexane, 1:4). Yield 94 mg (0.36 mmol, 71%). All physical data identical to previously described.171H NMR (400 MHz, DMSO-d6) δ 10.49 (s, 1H), 7.79–7.62 (m, 3H), 7.58 (t, J = 7.8 Hz, 1H), 7.48–7.33 (m, 2H), 7.08 (d, J = 8.0 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.6, 162.6 (d, J = 247.9 Hz), 152.9, 143.1, 138.0, 131.7 (d, J = 8.7 Hz, 2C), 128.9 (d, J = 3.2 Hz), 125.4, 122.0, 120.0, 118.4, 116.8 (d, J = 22.0 Hz, 2C), 113.1, 88.3; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9FN2NaO 287.0591; found 287.0600 (−3.0 ppm).
(E)-2-(2-Fluorophenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ah)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(2-fluorophenyl)-4-oxobutanenitrile18 (6ah) (134 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.63 (EtOAc/hexane, 1:1). Yield 110 mg (0.42 mmol, 83%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.41 (s, 1H), 7.69–7.52 (m, 4H), 7.48–7.31 (m, 2H), 7.02 (t, J = 7.6 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.4, 159.8 (d, J = 249.7 Hz), 152.7, 144.7, 138.3, 132.3 (d, J = 8.5 Hz), 131.9 (d, J = 2.5 Hz), 125.8 (d, J = 3.4 Hz), 125. 6, 122.1, 119.9 (d, J = 14.7 Hz), 119.8, 117.1 (d, J = 20.9 Hz), 117.0, 112.9, 82.4; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9FN2NaO 287.0591; found 287.0583 (2.8 ppm).
(E)-2-(4-Chlorophenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ai)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-chlorophenyl)-4-oxobutanenitrile18 (6ai) (142 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.22 (EtOAc/hexane, 1:2). Yield 115 mg (0.41 mmol, 82%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H), 7.70–7.55 (m, 6H), 7.07 (d, J = 8.0 Hz, 1H), 7.03 (t, J = 7.4 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.2, 152.4, 142.9, 137.6, 133.7, 131.0, 130.8 (2C), 129.4 (2C), 125.0, 121.7, 119.5, 117.8, 112.7, 87.5; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9ClN2NaO 303.0296; found 303.0292 (1.1 ppm).
(E)-2-(3-Chlorophenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1aj)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(3-chlorophenyl)-4-oxobutanenitrile18 (6aj) (142 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.57 (EtOAc/hexane, 1:2). Yield 67 mg (0.24 mmol, 48%). All physical data were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 7.72–7.62 (m, 2H), 7.64–7.51 (m, 4H), 7.09 (d, J = 8.0 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.2, 152.4, 143.1, 137.6, 134.2, 133.9, 131.1, 129.0, 128.5, 127.6, 125.0, 121.7, 119.4, 117.7, 112.7, 87.0; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9ClN2NaO 303.0296; found 303.0292 (1.2 ppm).
(E)-2-(4-Bromophenyl)-2-(3-oxoindolin-2-ylidene)acetonitrile (1ak)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-2-(4-bromophenyl)-4-oxobutanenitrile18 (6ak) (164 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.66 (EtOAc/hexane, 1:2). Yield 141 mg (0.44 mmol, 87%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.55 (s, 1H), 7.76 (d, J = 8.1 Hz, 2H), 7.65 (d, J = 7.6 Hz, 1H), 7.58 (d, J = 8.0 Hz, 3H), 7.07 (d, J = 8.0 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.2, 152.4, 142.9, 137.6, 132.3 (2C), 131.4, 130.9 (2C), 125.0, 122.4, 121.6, 119.4, 117.7, 112.7, 87.5; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9N2NaO 346.9790; found 346.9788 (0.8 ppm).
(E)-2-(3-Oxoindolin-2-ylidene)-2-(pyridin-2-yl)acetonitrile (1al)
This compound was prepared by typical procedure A employing 4-(2-aminophenyl)-4-oxo-2-(pyridin-2-yl)butanenitrile18 (6al) (146 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.36 (EtOAc/Hex, 1:2). Yield 104 mg (0.42 mmol, 84%). All physical properties were identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 11.68 (s, 1H), 8.76 (d, J = 4.9 Hz, 1H), 7.99 (t, J = 7.8 Hz, 1H), 7.80 (d, J = 8.1 Hz, 1H), 7.65 (d, J = 7.6 Hz, 1H), 7.60 (t, J = 7.7 Hz, 1H), 7.49–7.31 (m, 2H), 7.05 (t, J = 7.4 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 185.6, 152.8, 152.3, 149.6, 143.6, 138.4, 138.0, 125.4, 123.1, 122.9, 122.6, 119.6, 117.0, 113.9, 85.4; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C15H9N3NaO 270.0638; found 270.0645 (−2.7 ppm).
(E)-2-(5-Bromo-3-oxoindolin-2-ylidene)-2-phenylacetonitrile (1ba)
This compound was prepared by typical procedure A employing 4-(2-amino-5-bromophenyl)-4-oxo-2-phenylbutanenitrile18 (6ba) (164 mg, 00.5 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a dark red solid, mp 261.5–263.1 °C (EtOH), Rf 0.5 (EtOAc/hexane, 1:2). Yield 142 mg (0.44 mmol, 88%). Yield mg (4.32 mmol, 72%). All physical data were identical to those previously described.161H NMR (400 MHz, DMSO-d6) δ 10.61 (s, 1H), 7.79 (d, J = 2.1 Hz, 1H), 7.72 (dd, J = 8.6, 2.1 Hz, 1H), 7.67–7.62 (m, 2H), 7.61–7.55 (m, 2H), 7.53–7.47 (m, 1H), 7.06 (d, J = 8.5 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 183.4, 151.8, 142.7, 139.9, 132.3, 129.9 (2C), 129.8, 129.3 (2C), 127.5, 121.8, 118.2, 115.3, 113.5, 90.4; FTIR (film, NaCl, cm–1): 3321, 2202, 1708, 1600, 1465, 1316, 1272, 1217, 1166, 1120; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9BrN2NaO 346.9790; found 346.9787 (0.9 ppm).
(E)-2-(5,6-Dimethoxy-3-oxoindolin-2-ylidene)-2-phenylacetonitrile (1ca)
This compound was prepared by typical procedure A employing 4-(2-amino-4,5-dimethoxyphenyl)-4-oxo-2-phenylbutanenitrile18 (6ca) (155 mg, 0.50 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, Rf 0.47 (EtOAc/hexane, 1:1). Yield 69 mg (0.23 mmol, 45%). All physical data identical to those previously described.171H NMR (400 MHz, DMSO-d6) δ 10.12 (s, 1H), 7.62 (d, J = 7.1 Hz, 2H), 7.55 (t, J = 7.6 Hz, 2H), 7.47 (t, J = 7.3 Hz, 1H), 7.07 (s, 1H), 6.62 (s, 1H), 3.85 (s, 3H), 3.75 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 181.9, 157.8, 150.2, 144.9, 143.9, 132.2, 129.3 (2C), 129.0, 128.8 (2C), 118.0, 110.4, 105.8, 95.9, 88.5, 56.1, 55.9; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C18H14N2NaO3 329.0897; found 329.0887 (2.8 ppm).
(E)-2-(3-Oxoindolin-2-ylidene)-2-(p-tolyl)acetonitrile (1ja)
This compound was prepared by typical procedure A employing 4-(2-amino-5-nitrophenyl)-4-oxo-2-phenylbutanenitrile (6ja) (148 mg, 0.5 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:1. The title compound was obtained as a yellow solid, mp 269.1–271.6 °C (EtOH), Rf 0.41 (EtOAc/hexane, 1:1). Yield 62 mg (0.21 mmol, 42%). 1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.43 (dd, J = 8.8, 2.4 Hz, 1H), 8.39 (d, J = 2.3 Hz, 1H), 7.71–7.64 (m, 2H), 7.63–7.57 (m, 2H), 7.57–7.50 (m, 1H), 7.23 (d, J = 8.9 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 182.8, 156.0, 142.8, 141.4, 132.4, 131.5, 129.8, 129.5 (2C), 129.0 (2C), 120.8, 119.7, 117.4, 113.1, 92.4; FTIR (film, NaCl, cm–1): 3272, 3101, 3065, 2215, 1709, 1628, 1606, 1590, 1521, 1474, 1455, 1344, 1318, 1250, 1208, 1134; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H9N3NaO3 314.0536; found 314.0534 (0.8 ppm).
(E)-2-(1-Methyl-3-oxoindolin-2-ylidene)-2-phenylacetonitrile (7da)
Typical Procedure C for the Synthesis of (E)-2-(1-Alkyl-3-oxoindolin-2-ylidene)-2-arylacetonitriles 7 from 4-(2-Aminophenyl)-4-oxo-2-arylbutanenitriles 6
4-(2-Aminophenyl)-2-phenyl-4-oxobutyronitrile18 (6aa) (125 mg, 0.50 mmol), DMSO (0.2 mL), and KOH (56 mg, 1.0 mmol) were mixed in a 5 mL round-bottom flask and left for 30–40 min under stirring at room temperature. Next, an alkylating agent was added and the mixture was left for another 5 min (TLC control), after which the reaction was immediately diluted with 60 mL of CH2Cl2 and washed (3 × 10 mL) with saturated NaHCO3 solution. Next, the solution was evaporated and purified by column chromatography (eluent ethyl acetate:hexane 1: 2 v/v) or by recrystallization from ethyl alcohol. When Me2SO4 (126 mg, 95 μL, 1.00 mmol) was used, the yield was 114 mg (0.44 mmol, 88%). When MeI (142 mg, 62 μL, 1.00 mmol) was used, the yield was 75 mg (0.29 mmol, 58%). All physical data were identical to those previously described.16 The title compound was obtained as a red solid, mp 149.0–151.2 °C (MeOH), Rf 0.33 (EtOAc/hexane, 1:2). Yield mg (4.32 mmol, 72%). 1H NMR (400 MHz, DMSO-d6) δ 7.74–7.60 (m, 2H), 7.57–7.42 (m, 5H), 7.20 (d, J = 8.1 Hz, 1H), 7.09 (t, J = 7.4 Hz, 1H), 2.81 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.0, 154.2, 144.4, 137.5, 132.4, 130.1 (2C), 129.1, 128.7 (2C), 124.6, 121.9, 119.6, 118.8, 110.8, 89.3, 33.7; FTIR (film, NaCl, cm–1): 2983, 2193, 1708, 1617, 1581, 1475, 1361, 1328, 1248, 1198, 1164, 1130, 1104, 1026, 973; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H12N2NaO 283.0842; found 283.0833 (3.0 ppm).
Typical Procedure D for the Synthesis of (E)-2-(1-Alkyl-3-oxoindolin-2-ylidene)-2-arylacetonitriles 7 from 4-(2-(Methylamino)phenyl)-4-oxo-2-arylbutanenitriles 10
4-(2-(Methylamino)phenyl)-2-phenyl-4-oxobutyronitrile18 (10da) (125 mg, 0.50 mmol), DMSO (0.4 mL), and KOH (112 mg, 2 mmol) were mixed in a 5 mL round-bottom flask and left for 10 min under stirring at room temperature. AcOH (0.2 mL) was added and, then, the reaction was diluted with H2O (20 mL) and extracted with CH2Cl2 (4 × 15 mL). Next, the solution was evaporated and purified by column chromatography (eluting with ethyl acetate/hexane 1:2 v/v) or by recrystallization from alcohol. Yield 20 mg (0.08 mmol, 15%). The obtained sample is identical to that obtained via typical procedure C.
Typical Procedure E for the Synthesis of (E)-2-(1-Alkyl-3-oxoindolin-2-ylidene)-2-arylacetonitriles 7 from 4-(2-Aminophenyl)-4-oxo-2-arylbutanenitriles 6 (Scale-Up Procedure)
4-(2-Aminophenyl)-2-phenyl-4-oxobutyronitrile18 (6aa) (500 mg, 2.00 mmol), DMSO (0.8 mL), and KOH (224 mg, 4.00 mmol) were mixed in a 25 mL round-bottom flask and left for 30–40 min under stirring at room temperature. Next, Me2SO4 (504 mg, 380 μL, 4.00 mmol) was added and the mixture was left for another 5 min (TLC control), after which the reaction was immediately diluted with 240 mL of CH2Cl2 and washed (3 × 40 mL) with saturated NaHCO3 solution. Next, the solution was evaporated and purified by column chromatography (eluting with ethyl acetate/hexane 1:2 v/v) or by recrystallization from ethyl alcohol. Yield 442 mg (1.70 mmol, 85%). The isolated sample was identical to that obtained via typical procedure C.
(E)-2-(4-Fluorophenyl)-2-(1-methyl-3-oxoindolin-2-ylidene)acetonitrile (7dg)
This compound was prepared by typical procedure C employing 4-(2-aminophenyl)-2-(4-fluorophenyl)-4-oxobutanenitrile18 (6ag) (134 mg, 0.50 mmol) and Me2SO4 (126 mg, 95 μL, 1.00 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, mp 170.8–174.1 °C (EtOH), Rf 0.67 (EtOAc/hexane, 1:1). Yield 107 mg (0.39 mmol, 77%). 1H NMR (400 MHz, DMSO-d6) δ 7.73–7.62 (m, 2H), 7.61–7.54 (m, 2H), 7.40–7.32 (m, 2H), 7.22 (d, J = 8.1 Hz, 1H), 7.10 (t, J = 7.4 Hz, 1H), 2.82 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.1, 162.3 (d, J = 247.5 Hz), 154.2, 144.7, 132.5 (d, J = 8.7 Hz), 132.4, 128.9 (d, J = 3.3 Hz, 2C), 124.7, 122.1, 119.6, 118. 9, 115.9 (d, J = 22.0 Hz, 2C), 111.0, 88.2, 33.8; FTIR (film, NaCl, cm–1): 3081, 2199, 1707, 1626, 1572, 1502, 1475, 1330, 1242, 1127; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H11FN2NaO 301.0748; found 301.0749 (−0.3 ppm).
(E)-2-(4-Chlorophenyl)-2-(1-methyl-3-oxoindolin-2-ylidene)acetonitrile (7di)
This compound was prepared by typical procedure C employing 4-(2-aminophenyl)-2-(4-chlorophenyl)-4-oxobutanenitrile18 (6ai) (142 mg, 0.50 mmol) and Me2SO4 (126 mg, 95 μL, 1.00 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, mp 172.4–174.5 °C (EtOH), Rf 0.44 (EtOAc/hexane, 1:1). Yield 98 mg (0.34 mmol, 67%). 1H NMR (400 MHz, DMSO-d6) δ 7.73–7.61 (m, 2H), 7.59 (d, J = 8.3 Hz, 2H), 7.54 (d, J = 8.6 Hz, 2H), 7.23 (d, J = 8.1 Hz, 1H), 7.11 (t, J = 7.5 Hz, 1H), 2.84 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.1, 154.2, 144.8, 137.7, 133.8, 132.0 (2C), 131.5, 128.8 (2C), 124.7, 122.1, 119.6, 118.7, 111.0, 87.8, 34.0; FTIR (film, NaCl, cm–1): 3061, 2194, 1701, 1612, 1586, 1470, 1362, 1322, 1126; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H11ClN2NaO 317.0452; found 317.0454 (−0.7 ppm).
(E)-2-(1-Benzyl-3-oxoindolin-2-ylidene)-2-phenylacetonitrile (7ea)
This compound was prepared by typical procedure C employing 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (125 mg, 0.5 mmol) and benzyl bromide (94 mg, 65 μL, 0.55 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, mp 166.9–169.0 °C (EtOH), Rf 0.53 (EtOAc/hexane, 1:2). Yield 129 mg (0.39 mmol, 77%). All physical data were identical to those previously described.161H NMR (400 MHz, DMSO-d6) δ 7.76 (dd, J = 7.6, 1.3 Hz, 1H), 7.62 (ddd, J = 8.5, 7.3, 1.4 Hz, 1H), 7.44–7.30 (m, 5H), 7.21–7.14 (m, 4H), 7.12 (t, J = 7.4 Hz, 1H), 6.83–6.73 (m, 2H), 4.65 (s, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 183.9, 153.7, 143.5, 137.7, 135.3, 132.0, 129.9 (2C), 129.5, 128.7 (2C), 128.5 (2C), 127.3, 125.9 (2C), 125.0, 122.3, 119.9, 118.8, 111.5, 90.8, 47.5; FTIR (film, NaCl, cm–1): 3036, 2188, 1735, 1713, 1622, 1583, 1467, 1342, 1241, 1181, 1111; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C23H16N2NaO 359.1155; found 359.1147 (2.2 ppm).
(E)-2-(1-Allyl-3-oxoindolin-2-ylidene)-2-phenylacetonitrile (7ha)
This compound was prepared by typical procedure C 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (125 mg, 0.50 mmol) and allyl bromide (61 mg, 43 μL, 0.55 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, mp 139.6–143.8 °C (EtOH), Rf 0.50 (EtOAc/hexane, 1:2). Yield 92 mg (0.32 mmol, 64%). 1H NMR (400 MHz, DMSO-d6) δ 7.73 (d, J = 7.6 Hz, 1H), 7.70–7.60 (m, 1H), 7.55–7.46 (m, 5H), 7.20 (d, J = 8.2 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 5.58–5.31 (m, 1H), 5.03 (dt, J = 10.3, 1.4 Hz, 1H), 4.86 (dt, J = 17.1, 1.3 Hz, 1H), 3.96 (d, J = 4.8 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 184.0, 153.5, 143.5, 137.6, 132.3, 131.1, 129.9 (2C), 129.6, 128.9 (2C), 124.9, 122.2, 119.9, 118.8, 117.5, 111.6, 90.5, 46.6; FTIR (film, NaCl, cm–1): 3082, 2178, 1703, 1582, 1472, 1443, 1346, 1193, 1105; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C19H14N2NaO 309.0998; found 309.1004 (−1.8 ppm).
(E)-2-(3-Oxo-1-(prop-2-yn-1-yl)indolin-2-ylidene)-2-phenylacetonitrile (7ia)
This compound was prepared by typical procedure C employing 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (125 mg, 0.50 mmol) and propargyl bromide (65 mg, 42 μL, 0.55 mmol). Eluent for preparative column chromatography: EtOAc/hexane, 1:2. The title compound was obtained as a red solid, mp 169.7–171.4 °C (EtOH), Rf 0.47 (EtOAc/hexane, 1:2). Yield 88 mg (0.31 mmol, 62%). 1H NMR (400 MHz, DMSO-d6) δ 7.83–7.68 (m, 2H), 7.62–7.47 (m, 5H), 7.39 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.4 Hz, 1H), 4.14 (d, J = 2.4 Hz, 2H), 3.23 (d, J = 2.6 Hz, 1H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 183.7, 153.2, 143.4, 137.8, 131.8, 129.9 (2C), 129.9, 129.1 (2C), 125.0, 122.9, 120.5, 118.3, 112.0, 92.3, 77.2, 75.7, 35.0; FTIR (film, NaCl, cm–1): 3264, 2195, 1707, 1590, 1477, 1435, 1336, 1186, 1111; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C19H12N2NaO 307.0842; found 307.0838 (1.3 ppm).
1-Methyl-2-benzoyl-3-hydroxyindole (11da)
Typical Procedure F for the Preparation of 2-Aryloyl-3-hydroxyindoles 11 from 4-(2-Aminophenyl)-4-oxobutyronitriles 6
4-(2-Aminophenyl)-2-phenyl-4-oxobutyronitrile18 (6aa) (125 mg, 0.50 mmol), DMSO (0.4 mL), and KOH (112 mg) were mixed in a 5 mL round-bottom flask and stirred at room temperature for 30–40 min. Then, MeI (142 mg, 62 μL, 1.00 mmol) was added and the mixture was stirred for another 30–40 min (TLC control). The reaction was diluted with H2O (20 mL) and extracted with CH2Cl2 (4 × 15 mL). Next, the solution was evaporated and purified by column chromatography (eluting with ethyl acetate/hexane 1:3 v/v). Yield 70 mg (0.28 mmol, 56%). All physical data were identical to those previously described.23 The title compound was obtained as a yellow amorphous solid, Rf 0.25 (EtOAc/hexane, 1:2). 1H NMR (400 MHz, CDCl3) δ 10.76 (s, 1H), 7.83 (dt, J = 8.1, 1.0 Hz, 1H), 7.76–7.72 (m, 2H), 7.61–7.55 (m, 1H), 7.55–7.49 (m, 2H), 7.45 (ddd, J = 8.3, 6.9, 1.2 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 3.31 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 188.9, 153.8, 140.9, 139.1, 131.9, 129.1, 128.7 (2C), 128.6 (2C), 121.6, 121.3, 120.0, 117.3, 110.9, 33.9; FTIR (film, NaCl, cm–1): 1701, 1612, 1494, 1470, 1453, 1427, 1371, 1323, 1294, 1253, 1094; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C16H13NNaO2 274.0838; found 274.0834 (1.6 ppm).
Typical Procedure G for the Preparation of 2-Aryloyl-3-hydroxyindoles 11 from N-Alkylated 4-(2-aminophenyl)-4-oxobutyronitriles 10
4-(2-(Methylamino)phenyl)-2-phenyl-4-oxobutyronitrile18 (10da) (125 mg, 0.05 mmol), DMSO (0.4 mL), and KOH (112 mg) were mixed in a 5 mL round-bottom flask and stirred at room temperature for 30–40 min. Next, AcOH (0.2 mL) was added and the mixture was stirred for another 30 min. The reaction was diluted with H2O (20 mL) and extracted with CH2Cl2 (4 × 15 mL). Next, the solution was evaporated and purified by column chromatography (eluting with ethyl acetate/hexane 1:3 v/v). Yield 77 mg (0.31 mmol, 61%). The isolated sample was identical to that obtained via typical procedure F. In a single case, we managed to isolate an intermediate product (7da) by quenching the reaction after 5 min. Yield 20 mg (0.08 mmol, 15%).
Typical Procedure H for the Preparation of 2-Aryloyl-3-hydroxyindoles 11 from 4-(2-Aminophenyl)-4-oxobutyronitriles 6 (Scale-Up Procedure)
4-(2-Aminophenyl)-2-phenyl-4-oxobutyronitrile18 (6aa) (500 mg, 2.00 mmol), DMSO (1.6 mL), and KOH (448 mg, 8.00 mmol) were mixed in a 25 mL round-bottom flask and left for 30–40 min under stirring at room temperature. Then, MeI (568 mg, 248 μL, 4.00 mmol) was added and the mixture was left for another 30–40 min (TLC control). The reaction was diluted with H2O (80 mL) and extracted with CH2Cl2 (4 × 60 mL). Next, the solution was evaporated and purified by column chromatography (eluting with ethyl acetate/hexane 1:3 v/v). Yield 296 mg (1.18 mmol, 59%). The isolated sample was identical to that obtained via typical procedure F.
1-Methyl-2-(4-methoxybenzoyl)-3-hydroxyindole (11de)
This compound was prepared via typical procedure F employing 4-(2-aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanenitrile18 (6ae) (140 mg, 0.50 mmol) and benzyl bromide (94 mg, 65 μL, 0.55 mmol) with yield 101 mg (0.36 mmol, 72%). Alternatively, this compound was prepared by typical procedure G employing 2-(4-methoxyphenyl)-4-(2-(methylamino)phenyl)-4-oxobutanenitrile (10de) (147 mg, 0.50 mmol) with yield 108 mg (0.39 mmol, 77%). Eluent for preparative column chromatography:EtOAc/hexane, 1:4. The titled compound was obtained as a brown amorphous solid, Rf 0.32 (EtOAc/hexane, 1:4). 1H NMR (400 MHz, DMSO-d6) δ 10.46 (s, 1H), 7.82 (dt, J = 8.0, 1.0 Hz, 1H), 7.80–7.74 (m, 2H), 7.45 (ddd, J = 8.3, 6.9, 1.2 Hz, 1H), 7.26 (d, J = 8.5 Hz, 1H), 7.13 (ddd, J = 7.9, 7.0, 0.8 Hz, 1H), 7.06–6.99 (m, 2H), 3.90 (s, 3H), 3.38 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 187.8, 162.9, 153.2, 141.0, 131.5, 131.3 (2C), 128.8, 121.8, 121.2, 120.1, 117.8, 114.0 (2C), 111.1, 55.6, 34.2; FTIR (film, NaCl, cm–1): 1725, 1680, 1595, 1513, 1465, 1427, 1304, 1253, 1173, 1108, 1029; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H15NNaO3 304.0944; found 304.0945 (−0.2 ppm).
1-Benzyl-2-benzoyl-3-hydroxyindole (11ea)
This compound was prepared by typical procedure F employing 4-(2-aminophenyl)-4-oxo-2-phenylbutanenitrile18 (6aa) (125 mg, 0.50 mmol) and benzyl bromide (94 mg, 65 μL, 0.55 mmol) with yield 77 mg (0.24 mmol, 47%). Alternatively, this compound was prepared by typical procedure G employing 4-(2-(benzylamino)phenyl)-4-oxo-2-phenylbutanenitrile (10ea) (170 mg, 0.50 mmol) with yield 78 mg (0.24 mmol, 48%). Eluent for preparative column chromatography: EtOAc/hexane, 1:3. The title compound was obtained as a yellow amorphous solid, Rf 0.29 (EtOAc/hexane, 1:2). 1H NMR (400 MHz, CDCl3) δ 10.72 (s, 1H), 7.88 (dt, J = 8.1, 1.0 Hz, 1H), 7.68–7.62 (m, 2H), 7.57–7.52 (m, 1H), 7.42 (ddd, J = 8.6, 6.9, 1.3 Hz, 3H), 7.28–7.24 (m, 1H), 7.14 (tt, J = 7.7, 6.2 Hz, 4H), 6.63–6.57 (m, 2H), 5.04 (s, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 189.2, 154.3, 140.6, 139.0, 137.2, 131.8, 129.2, 128.6 (2C), 128.5 (2C), 128.4 (2C), 127.5, 126.2 (2C), 121.4, 120.4, 120.3, 117.9, 111.7, 49.2; FTIR (film, NaCl, cm–1): 3065, 1737, 1619, 1590, 1574, 1523, 1494, 1448, 1342, 1287, 1183, 1120, 1019; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C22H17NNaO2 350.1151; found 350.1156 (−1.3 ppm).
4-Phenyl-5H-pyridazino[4,3-b]indol-3-amine (2aa)
Typical Procedure I for the Preparation of 5H-Pyridazino[4,3-b]indol-3-amines 23 from 4-(2-Aminophenyl)-4-oxobutyronitriles 6
This procedure is a modified one-pot version of the method reported in the literature.15 4-(2-Aminophenyl)-2-phenyl-4-oxobutyronitrile18 (6aa) (250 mg, 1.00 mmol), DMSO (0.8 mL), and KOH (224 mg) were mixed in a 25 mL round-bottom flask and stirred at room temperature for 30–40 min. Then, acetic acid (8.5 mL) and hydrazine hydrate (98%, 4 mL) were added and the reaction mixture was heated under reflux for 4 h. After this period, the solution was allowed to cool down and then poured down into 100 mL of crushed ice. The mixture was extracted with CH2Cl2 (4 × 15 mL). Next, the solution was evaporated and purified by column chromatography (eluting with acetone). Additional purification can be achieved by recrystallization from EtOH of i-PrOH. The title compound was obtained as a white solid, mp 306.5–308.9 °C (i-PrOH), lit15 mp 308–310 °C, Rf 0.34 (acetone). Yield 148 mg (0.57 mmol, 57%). 1H NMR spectra was identical to that reported in the literature.151H NMR (400 MHz, DMSO-d6) δ 11.00 (s, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.65–7.56 (m, 4H), 7.55–7.48 (m, 1H), 7.43 (ddd, J = 8.2, 6.9, 1.3 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.23 (ddd, J = 8.0, 6.9, 1.3 Hz, 1H), 5.72 (s, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 155.6, 142.7, 141.8, 134.3, 131.9, 129.6 (2C), 129.4 (2C), 128.5, 127.7, 120.8, 120.5, 119.5, 111.7, 103.8; FTIR (film, NaCl, cm–1): 3483, 3277, 3118, 1624, 1540, 1459, 1441, 1383, 1356, 1230, 1244, 1160, 1145; HRMS (ESI TOF) m/z: (M + H)+ calc’d for C16H13N4 261.1135; found 261.1131 (1.4 ppm).
4-(p-Tolyl)-5H-pyridazino[4,3-b]indol-3-amine (2ab)
This compound was prepared via typical procedure I employing 4-(2-aminophenyl)-4-oxo-2-(p-tolyl)butanenitrile18 (6ab) (264 mg, 1.00 mmol). The title compound was obtained as a white solid, mp 313.7–316.0 °C (i-PrOH), lit15 mp 210–212 °C (EtOH), Rf 0.34 (acetone). Yield 151 mg (0.55 mmol, 55%). 1H NMR spectra were identical to those reported in the literature.151H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.63–7.32 (m, 6H), 7.22 (t, J = 7.3 Hz, 1H), 5.69 (s, 2H), 2.42 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 155.7, 142.6, 141.8, 137.8, 134.4, 130.1 (2C), 129.3 (2C), 128.8, 127.7, 120.8, 120.4, 119.5, 111.7, 103.9, 21.0.; FTIR (film, NaCl, cm–1): 3460, 3289, 3110, 1612, 1536, 1457, 1379, 1352, 1302, 1242, 1151; HRMS (ESI TOF) m/z: (M + Na)+ calc’d for C17H15N4 275.1291; found 275.1292 (−0.2 ppm).
4-(4-Methoxyphenyl)-5H-pyridazino[4,3-b]indol-3-amine (2ae)
This compound was prepared via typical procedure I employing 4-(2-aminophenyl)-2-(4-methoxyphenyl)-4-oxobutanenitrile18 (6ae) (280 mg, 1.00 mmol). The title compound was obtained as a white solid, mp 293.1–294.6 °C (EtOH), lit15 mp 289–290 °C, Rf 0.28 (acetone). Yield 180 mg (0.62 mmol, 62%). 1H NMR spectra was identical to those reported in the literature.151H NMR (400 MHz, DMSO-d6) δ 10.96 (s, 1H), 8.14 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 8.3 Hz, 2H), 7.42 (t, J = 7.5 Hz, 1H), 7.37 (d, J = 7.9 Hz, 1H), 7.21 (t, J = 7.4 Hz, 1H), 7.17 (d, J = 8.3 Hz, 2H), 5.68 (s, 2H), 3.85 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 159.3, 155.9, 142.6, 141.8, 134.5, 130.7 (2C), 127.6, 123.7, 120.9, 120.4, 119.5, 115.0 (2C), 111.7, 103.8, 55.3; FTIR (film, NaCl, cm–1): 3337, 3142, 3023, 2967, 1771, 1762, 1505, 1430, 1356, 1252, 1154, 1108; HRMS (ESI TOF) m/z: (M + H)+ calc’d for C17H15N4O 291.1240; found 291.1236 (1.7 ppm).
Acknowledgments
Synthetic studies performed in the frame of this project were supported by grants from the Russian Science Foundation (Grant number 21-73-10029, https://rscf.ru/project/21-73-10029/).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01238.
Spectral data: 1H, 13C{1H} NMR, and HRMS spectral charts and X-ray crystallography data (PDF)
Accession Codes
Crystallography data CCDC number 2126910.
The authors declare no competing financial interest.
Supplementary Material
References
- Cooksey C. J. Tyrian purple: 6,6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–739. 10.3390/60900736. [DOI] [Google Scholar]
- Hoessel R.; Leclerc S.; Endicott J. A.; Nobel M. E.; Lawrie A.; Tunnah P.; Leost M.; Damiens E.; Marie D.; Marko D.; Niederberger E.; Tang W.; Eisenbrand G.; Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- Leclerc S.; Garnier M.; Hoessel R.; Marko D.; Bibb J. A.; Snyder G. L.; Greengard P.; Biernat J.; Wu Y.-Z.; Mandelkow E.-M.; Eisenbrand G.; Meijer L. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors?. J. Biol. Chem. 2001, 276, 251–260. 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- Meijer L.; Skaltsounis A.-L.; Magiatis P.; Polychronopoulos P.; Knockaert M.; Leost M.; Ryan X. P.; Vonica C. A.; Brivanlou A.; Dajani R.; Crovace C.; Tarricone C.; Musacchio A.; Roe S. M.; Pearl L.; Greengard P. GSK-3-Selective Inhibitors Derived from Tyrian Purple Indirubins. Chem. Biol. 2003, 10, 1255–1266. 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Polychronopoulos P.; Magiatis P.; Skaltsounis A.-L.; Myrianthopoulos V.; Mikros E.; Tarricone A.; Musacchio A.; Roe S. M.; Pearl L.; Leost M.; Greengard P.; Meijer L. Structural Basis for the Synthesis of Indirubins as Potent and Selective Inhibitors of Glycogen Synthase Kinase-3 and Cyclin-Dependent Kinases. J. Med. Chem. 2004, 47, 935–946. 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- Seidler J.; McGovern S. L.; Doman T. N.; Shoichet B. K. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J. Med. Chem. 2003, 46, 4477–4486. 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhou G.-B.; Liu P.; Song J.-H.; Liang Y.; Yan X.-J.; Xu F.; Wang B.-S.; Mao J.-H.; Shen Z.-X.; Chen S.-J.; Chen Z. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 4826–4831. 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gein V. L.; Tatarinov V. V.; Rassudikhina N. A.; Vakhrin M. I.; Voronina E. V. Synthesis and antimicrobial activity of 2-aroylmethylene-6-hydroxy-2,3-dihydroindol-3-ones. Pharm. Chem. J. 2011, 45, 231–232. 10.1007/s11094-011-0602-2. [DOI] [Google Scholar]
- Lack N. A.; Axerio-Cilies P.; Tavassoli P.; Han F. G.; Chan K. H.; Feau C.; LeBlanc E.; Guns E. T.; Guy R. K.; Rennie P. S.; Cherkasov A. Targeting the Binding Function 3 (BF3) Site of the Human Androgen Receptor through Virtual Screening. J. Med. Chem. 2011, 54, 8563–8573. 10.1021/jm201098n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.-Q.; Mao S.-C.; Zhang H.-Y.; Yu X.-Q.; Feng M.-T.; Wang B.; Feng L.-H.; Guo Y.-W. Racemosins A and B, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. 10.1016/j.fitote.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Medvedev A. E.; Ivanov A. S.; Kamyshanskaya N. S.; Kirkel A. Z.; Moskvitina T. A.; Gorkin V. Z.; Li N. Y.; Marshakov V. Y. Interaction of indole derivatives with monoamine oxidase A and B. Studies on the structure-inhibitory activity relationship. Biochem. Mol. Biol. Int. 1995, 36, 113–122. [PubMed] [Google Scholar]
- Medvedev A. E.; Ivanov A. S.; Veselovsky A. V.; Skvortsov V. S.; Archakov A. I. QSAR analysis of indole analogues as monoamine oxidase inhibitors. J. Chem. Inf. Comput. Sci. 1996, 36, 664–671. 10.1021/ci950126t. [DOI] [PubMed] [Google Scholar]
- Ornano L.; Donno Y.; Sanna C.; Ballero M.; Serafini M.; Bianco A. Phytochemical study of Caulerpa racemosa (Forsk.) J. Agarth, an invading alga in the habitat of La Maddalena Archipelago. Nat. Prod. Res. 2014, 28, 1795–1799. 10.1080/14786419.2014.945928. [DOI] [PubMed] [Google Scholar]
- Roaiah H. M.; Ahmed K. M.; Fawzy N. M.; Wietrzyk J.; Pawlik A.; Ali M. M.; Soliman A. M. Synthesis of novel acetamide derivatives and evaluation of their antiproliferative potency against different cancer cell lines. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 129–136. [Google Scholar]
- Letribot B.; Delatouche R.; Rouillard H.; Bonnet A.; Chérouvrier J.-R.; Domon L.; Besson T.; Thiéry V. Synthesis of 2-Mercapto-(2-Oxoindolin-3-Ylidene)Acetonitriles from 3-(4-Chloro-5H-1,2,3-Dithiazol-5-Ylidene)Indolin-2-ones. Molecules 2018, 23, 1390. 10.3390/molecules23061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velezheva V. S.; Brennan P. J.; Marshakov V. Y.; Gusev D. V.; Lisichkina I. N.; Peregudov A. S.; Tchernousova L. N.; Smirnova T. G.; Andreevskaya S. N.; Medvedev A. E. Novel Pyridazino[4,3-b]indoles with Dual Inhibitory Activity against Mycobacterium tuberculosis and Monoamine Oxidase. J. Med. Chem. 2004, 47, 3455–3461. 10.1021/jm030479g. [DOI] [PubMed] [Google Scholar]
- Aksenov N. A.; Aksenov D. A.; Arutiunov N. A.; Aksenova D. S.; Aksenov A. V.; Rubin M. Unexpected cyclization of ortho-nitrochalcones into 2-alkylideneindolin-3-ones. RSC Adv. 2020, 10, 18440–18450. 10.1039/D0RA03520C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov N. A.; Aksenov D. A.; Skomorokhov A. A.; Prityko L. A.; Aksenov A. V.; Griaznov G. D.; Rubin M. Synthesis of 2-(1H-Indol-2-yl)acetamides via Bronsted Acid-Assisted Cyclization Cascade. J. Org. Chem. 2020, 85, 12128–12146. 10.1021/acs.joc.0c01344. [DOI] [PubMed] [Google Scholar]
- Kawashita Y.; Nakamichi N.; Kawabata H.; Hayashi M. Direct and Practical Synthesis of 2-Arylbenzoxazoles Promoted by Activated Carbon. Org. Lett. 2003, 5, 3713–3715. 10.1021/ol035393w. [DOI] [PubMed] [Google Scholar]
- Aksenov N. A.; Aksenov D. A.; Kurenkov I. A.; Aksenov A. V.; Skomorokhov A. A.; Prityko L. A.; Rubin M. Preparation of 3,5-diarylsubstituted 5-hydroxy-1,5-dihydro-2H-pyrrol-2-ones via base-assisted cyclization of 3-cyanoketones. RSC Adv. 2021, 11, 16236–16245. 10.1039/D1RA02279B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata R. P.; Antoniolli G.; Lancellotti M.; Kawano D. F.; Guimaraes Barbosa E.; Almeida W. P. Synthesis and biological evaluation of 2′-Aminochalcone: A multi-target approach to find drug candidates to treat Alzheimer’s disease. Bioorg. Chem. 2020, 103, 104201 10.1016/j.bioorg.2020.104201. [DOI] [PubMed] [Google Scholar]
- Leonard N. J.; Boyd S. N. Jr. Cinnolines. I. Synthesis of aminoacetophenones and aminopropiophenones. J. Org. Chem. 1946, 11, 405–418. 10.1021/jo01174a018. [DOI] [PubMed] [Google Scholar]
- Guo J.; Chen Z.-S.; Chen W.-S.; Zhao X.; Ji K. Gold(I)-Catalyzed Oxidative Amination of β-Amino-ynones to Quaternary Ammonium-olate Salts: The Benefit of a P,N-Bidentate Ligand. Org. Lett. 2021, 23, 8873–8877. 10.1021/acs.orglett.1c03382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.