Abstract
An efficient and novel photoinduced trifluoromethylation employing CF3Br as a trifluoromethyl source is described. With commercially accessible fac-Ir(III)(ppy)3 as the catalyst, radical trifluoromethylation between O-silyl enol ether and CF3Br occurs successfully. This method provides various α-CF3-substituted ketones with a broad substrate scope in good yields under mild reaction conditions.
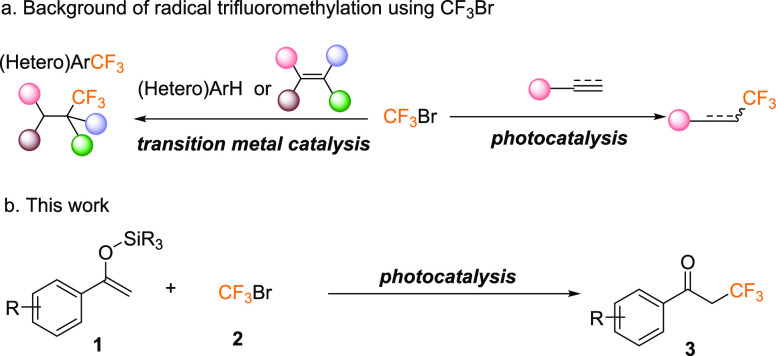
Introduction
CF3-containing molecules are of great academic and industrial importance with widespread applications in pharmaceuticals, agrochemicals, and functional materials.1 As such, the strong demand for CF3-containing molecules has nourished various synthetic methods.2
Specifically, radical trifluoromethylation has recently received considerable attention along with the rapid development of mild methods to generate trifluoromethyl radical species.3 Diverse trifluoromethylation reagents including Togni reagents,4 Umemoto reagents,5 Ruppert–Prakash reagents (Me3SiCF3),6 Langlois reagent (CF3SO2Na),7 CF3SO2Cl,8 CF3I,9 and CF3Br10 have been developed as radical trifluoromethyl sources successively. Among these reagents, CF3Br is the only one that combines the advantages of low cost and high atom economy.
CF3Br is a stable, nontoxic, and easily available industrial product. Until now, CF3Br has been mainly used as an extinguishant11 as well as a primary starting material of several prominent trifluoromethylation reagents in the fluoride chemistry.12 In a sharp contrast, only a handful of studies on trifluoromethylation directly using CF3Br as the CF3 source have been reported. This can be mainly attributed to the following reasons: first, low solubility and gas–liquid mass transfer efficiency seriously hinder the development of CF3Br as the CF3 source. Second, CF3Br has a high reduction potential (−1.7 V vs SCE) so that it can only be reduced under strong reduction conditions.13 More recently, two ingenious catalytic strategies that allow CF3Br to participate directly in radical trifluoromethylation have been developed (Scheme 1a): (i) Outstanding studies on transition metal catalytic trifluoromethylation by Beller’s,10a Wang’s10c and Li’s10d groups have shown that judicious selection of metals and ligands together with the assistance of other reaction conditions enables reduction of CF3Br to CF3 radicals, thus triggering radical trifluoromethylation. (ii) Visible-light-induced hydrotrifluoromethylation of alkenes and alkynes by using CF3Br as the trifluoromethyl reagent was reported by Professor Zhang’s group in 2018.10b This method not only provides an easy access to convert CF3Br to CF3 radicals but also offers a new opportunity for the application of CF3Br in a green, safe, and efficient way.
Scheme 1. Radical Trifluoromethylation by Using CF3Br.
Inspired by the high efficiency of photocatalysis, we envisioned that more trifluoromethylation reactions between CF3Br and radical receptors would occur through the photocatalytic process. Among numerous radical receptors, O-silyl enol ethers can be easily accessible from the corresponding carbonyl compounds and show great reactivity for CF3 radicals. Furthermore, the products α-CF3-substituted ketones are important fragments of marketed drugs such as Alpelisib and Elexacaftor. Therefore, we would like to investigate the photoinduced trifluoromethylation of O-silyl enol ether with CF3Br as the trifluoromethyl source to produce α-CF3-carbonyl compounds and get more detail insights into the mechanism for the production of trifluoromethyl radicals from CF3Br under visible light irradiation (Scheme 1b).
Results and Discussion
In our initial studies, we performed the trifluoromethylation of 1-phenyl-1-trimethylsiloxyethylene with CF3Br in the presence of 1 mol % fac-Ir(III)(ppy)3 and 3 equiv of diisopropyl ethyl amine (DIPEA) in THF under 10 W blue-LED irradiation (460 nm) (Table 1, entry 1). The reaction gave two dimer products 4a and 4b as well as a large amount of acetophenone 5 without desired product 3a (Scheme 2a). Then, DIPEA was removed from the reaction system, and the desired product 3a was obtained only in trace amounts (Table 1, entry 2). Subsequently, substrates with different trialkylsilyl groups were screened (Table 1, entries 3–4). To our delight, the bulky triisopropylsilyl (TIPS) group could effectively increase the yield to 25%. The reason is the bulky TIPS group can prevent the photoinduced formation of acetophenone from O-silyl enol ether (Scheme 2b).14 To prevent the formation of acetophenone, several aprotic solvents were screened in which MeCN afforded the best result (50% yield, Table 1, entries 5–9). Meanwhile, diverse light sources were also evaluated, but 10 W blue LED remained the best option (Table 1, entries 10 and 12). Afterward, the concentration of CF3Br, the loading of the photocatalyst, and the volume of the flask were investigated (Table 1, entries 13–17), and we finally increased the yield of 3a to 85% (1.5 atm CF3Br, 1 mol % fac-Ir(III)(ppy)3, with 50 mL Schlenk flasks). In addition, no product was observed in the absence of the photocatalyst or light (Table 1, entries 18 and 19). For more detailed data, refer to the Supporting Information.
Table 1. Optimization of Reaction Conditionsa.
| entry | SiR3 | additive | cat. (mol %) | light source | solvent | yieldb (%) |
|---|---|---|---|---|---|---|
| 1 | TMS | DIPEA | 1% | 10 W blue LED | THF | 0 |
| 2 | TMS | 1% | 10 W blue LED | THF | trace | |
| 3 | TES | 1% | 10 W blue LED | THF | 13% | |
| 4 | TIPS | 1% | 10 W blue LED | THF | 25% | |
| 5 | TIPS | 1% | 10 W blue LED | dioxane | trace | |
| 6 | TIPS | 1% | 10 W blue LED | DMF | 5% | |
| 7 | TIPS | 1% | 10 W blue LED | DMSO | 5% | |
| 8 | TIPS | 1% | 10 W blue LED | hexane | N.D. | |
| 9 | TIPS | 1% | 10 W blue LED | CH3CN | 50 | |
| 10 | TIPS | 1% | 5 W blue LED | CH3CN | 37 | |
| 11 | TIPS | 1% | 15 W blue LED | CH3CN | 50 | |
| 12 | TIPS | 1% | 10 W white LED | CH3CN | 25 | |
| 13c | TIPS | 1% | 10 W blue LED | CH3CN | 69 | |
| 14c | TIPS | 2% | 10 W blue LED | CH3CN | 64 | |
| 15c | TIPS | 3% | 10 W blue LED | CH3CN | 60 | |
| 16c,d | TIPS | 1% | 10 W blue LED | CH3CN | 80 | |
| 17c,e | TIPS | 1% | 10 W blue LED | CH3CN | 85 | |
| 18c,e | TIPS | 10 W blue LED | CH3CN | N.D. | ||
| 19c,e | TIPS | 1% | CH3CN | N.D. |
Reaction conditions: 1a (0.5 mmol, 1.0 equiv), CF3Br (1.0 atm), photocatalyst (0.005 mmol, 1 mol %) in a 20 mL quartz tube, room temperature, 10 W blue LED.
Isolated yield.
Replace the air in the container with CF3Br three times and then fill it with CF3Br (1.5 atm).
25 mL flask.
50 mL Schlenk flask. N.D.: not detected.
Scheme 2. Formation of Byproducts.
The optimized condition was then applied to the reactions of various O-silyl enol ethers derived from aryl methyl ketones to examine the generality of this transformation. As summarized in Scheme 3, the aryl silyl enol ethers with electron-donating substituents on their phenyl moiety were all suitable to produce the corresponding products 3a–3i in 53–85% yields. The substrates with weak electron-withdrawing groups such as F, Cl, Br, and −CO2Me on the phenyl rings provided the products 3j–3o in slightly lower yields. When strong electron-withdrawing groups such as nitro and cyano groups were attached to the phenyl rings, there were no products observed. O-Silyl enol ethers bearing α-naphthyl, benzothienyl, and thienyl were also successfully transformed into corresponding products 3p–3r in 45–72% yields. Yet, α-pyridyl silyl enol ether failed to give the product. The substrates containing druglike scaffolds, such as clofibrate and ibuprofen derivatives, were also amenable for this process, providing 3s and 3t with 33 and 74% yields, respectively.
Scheme 3. Substrate Scope.
Reaction conditions: 1a (0.5 mmol, 1.0 equiv), CF3Br (1.5 atm), photocatalyst (0.0025 mmol, 0.5 mol %), in a 50 mL Schlenk flask, room temperature, and 10 W blue LED (460 nm). N.D.: not detected.
Afterward, O-silyl enol ethers derived from other aryl alkyl ketones, indanones, and aliphatic ketones were explored under the optimized reaction condition (Scheme 4). A large number of O-silyl enol ethers from aryl alkyl ketones were trifluoromethylated efficiently and afforded the corresponding products in 27–77% yields (7a–7h). O-Silyl enol ethers derived from indanones with electron-donating and electron-withdrawing substitutions on the aryl ring could also work smoothly, achieving 7i–7p in 41–63% yields. As for aliphatic O-silyl enol ethers, only trace products can be monitored from 19F NMR, but they could not be separated (7q–7s).
Scheme 4. Substrate Scope.
Reaction conditions: 1a (0.5 mmol, 1.0 equiv), CF3Br (1.5 atm), photocatalyst (0.0025 mmol, 0.5 mol %), in a 50 mL Schlenk flask, room temperature, and 10 W blue LED (460 nm). N.D.: not detected.
To gain an insight into the reaction mechanism, several control experiments were performed. The addition of a radical-trapping reagent TEMPO (1 mmol) to the reaction system led to a suppression of the formation of 3a, while TEMPO-CF3 adduct 6 was detected by 19F NMR in 12.5% yield (Figure 1a). When TEMPO reacted with CF3Br without substrate 1a, TEMPO-CF3 adduct 6 could also be detected by 19F NMR in 35% yield (Figure 1b). These experiments indicated that the CF3 radical was produced from CF3Br under the reaction conditions, and then, it participated in the subsequent reaction. Further fluorescence quenching experiments of the excited fac-Ir(III)(ppy)3* with different concentrations of 1a and CF3Br also indicated that the excited fac-Ir(III)(ppy)3* could be quenched by CF3Br (Figure 1c). Moreover, the detected dimer products 4a and 4b showed the existence of radical intermediate A (Scheme 5).
Figure 1.
Mechanistic studies. (a,b) Radical-trapping experiments. (c) Fluorescence quenching experiments.
Scheme 5. Proposed Reaction Mechanism.
On the basis of the above results and the literature,10b the possible reaction mechanism is proposed in Scheme 5. Under the blue LED irradiation, the photocatalyst fac-Ir(III)(ppy)3 was excited to fac-Ir(III)(ppy)3*, which was oxidatively quenched by CF3Br and resulted in CF3 radicals and bromine ions. Meanwhile, fac-Ir(III)(ppy)3* is oxidized to fac-Ir(IV)(ppy)3. The CF3 radical attacked the terminal carbon on the C=C bond of O-silyl enol ether to form intermediate A, which was then oxidized by fac-Ir(IV)(ppy)3 to generate intermediate B. After desilylation of the intermediate B, the α-trifluoromethylketone product 3 was produced. If DIPEA is present in the reaction system, fac-Ir(IV)(ppy)3 would be reduced first by DIPEA to give fac-Ir(III)(ppy)3, and the intermediate A is left to couple with itself to form dimer products 4a and 4b.
Conclusions
In summary, the successful use of CF3Br as a trifluoromethyl source in photoinduced trifluoromethylation of O-silyl enol ethers has been achieved under mild and practical conditions. Enabled by the photocatalysis, the C–Br bond of CF3Br can be cleaved to deliver CF3 radicals, which provides facile access to utilize CF3Br as the trifluoromethyl source. Moreover, this method provides a series of α-CF3-substituted ketones, which have great potential applications in the pharmaceutical and agrochemical fields.
Experimental Section
Materials and Methods
Reagents were obtained from commercial suppliers and used without further purification unless otherwise noted. O-Silyl enol ethers 1a–t and 6a–s were synthsized according to the lierature.15 NMR experiments were carried out in deuterated chloroform (CDCl3). 1H NMR, 13C{1H} NMR, and 19F NMR spectra were recorded at 400 or 600 MHz, 100 or 150 MHz, and 376 MHz spectrometers, respectively. High-resolution mass spectra were recorded on a Micro TOF-QII mass instrument (ESI).
General Procedure for Synthesis of α-CF3-Substituted Ketones
O-Silyl enol ethers 1 (0.5 mmol), fac-Ir(III)(ppy)3 (0.5 mol %), and CH3CN (3 mL) were added into a 50 mL Schlenk flask. The mixture was degassed with CF3Br gas (1.5 atm observed from a barometer). The reaction mixture was stirred at room temperature under a blue LED lamp (hν = 460 nm) for 7–36 h. The reaction mixture was then concentrated in vacuo, and the residue was purified by silica gel chromatography with PE/DCM (10:1 to 1:1) as the eluent to afford pure product 3.
Note:It is worth mentioning that some of the products are a bit volatile.
Acknowledgments
We are thankful for financial support from the National Natural Science Foundation of China (Grant Nos. 22061037 and 21662030), State Key Laboratory of Applied Organic Chemistry, Lanzhou University, and Shanghai Sinofluoro Chemicals Co., Ltd.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01241.
Copies of 1H NMR, 13C and 19F NMR spectra; mass spectra; radical-trapping experiments; and fluorescence quenching experiments (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Inoue M.; Sumii Y.; Shibata N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ogawa Y.; Tokunaga E.; Kobayashi O.; Hirai K.; Shibata N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467–101520. 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For some selected reviews, see; a Tomashenko O. A.; Grushin V. V. Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev. 2011, 111, 4475–4521. 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]; b Chu L.; Qing F.-L. Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)trimethylsilane as a Nucleophilic CF3 Source. Acc. Chem. Res. 2014, 47, 1513–1522. 10.1021/ar4003202. [DOI] [PubMed] [Google Scholar]; c Merino E.; Nevado C. Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev. 2014, 43, 6598–6608. 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xu X.-H.; Matsuzaki K.; Shibata N. Synthetic Methods for Compounds Having CF3-S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. 10.1021/cr500193b. [DOI] [PubMed] [Google Scholar]
- For some selected reviews, see; a Studer A. A “Renaissance” in Radical Trifluoromethylation. Angew. Chem., Int. Ed. 2012, 51, 8950–8958. 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]; b Pan X.; Xia H.; Wu J. Recent advances in photoinduced trifluoromethylation and difluoroalkylation. Org. Chem. Front. 2016, 3, 1163–1185. 10.1039/C6QO00153J. [DOI] [Google Scholar]; c Barata-Vallejo S.; Postigo A. New Visible-Light-Triggered Photocatalytic Trifluoromethylation Reactions of Carbon-Carbon Multiple Bonds and (Hetero)Aromatic Compounds. Chem. – Eur. J. 2020, 26, 11065–11084. 10.1002/chem.202000856. [DOI] [PubMed] [Google Scholar]; d Xiao H.; Zhang Z.; Fang Y.; Zhu L.; Li C. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. 10.1039/D1CS00200G. [DOI] [PubMed] [Google Scholar]
- For some selected examples, see; a Charpentier J.; Früh N.; Togni A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650–682. 10.1021/cr500223h. [DOI] [PubMed] [Google Scholar]; b Wang X.; Studer A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 2017, 50, 1712–1724. 10.1021/acs.accounts.7b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang B.; Peng Q.; Guo D.; Wang J. NHC-Catalyzed Radical Trifluoromethylation Enabled by Togni Reagent. Org. Lett. 2020, 22, 443–447. 10.1021/acs.orglett.9b04203. [DOI] [PubMed] [Google Scholar]; d Zhang Z.-Q.; Xu Y.-H.; Dai J.-C.; Li Y.; Sheng J.; Wang X.-S. Copper-Catalyzed Trifluoromethylation/Cyclization of Alkynes for Synthesis of Dioxodibenzothiazepines. Org. Lett. 2021, 23, 2194–2198. 10.1021/acs.orglett.1c00344. [DOI] [PubMed] [Google Scholar]
- For some selected examples, see; a Umemoto T. Electrophilic Perfluoroalkylating Agents. Chem. Rev. 1996, 96, 1757–1778. 10.1021/cr941149u. [DOI] [PubMed] [Google Scholar]; b Yasu Y.; Koike T.; Akita M. Three-component Oxytrifluoromethylation of Alkenes: Highly Efficient and Regioselective Difunctionalization of C=C Bonds Mediated by Photoredox Catalysts. Angew. Chem., Int. Ed. 2012, 51, 9567–9571. 10.1002/anie.201205071. [DOI] [PubMed] [Google Scholar]; c Mizuta S.; Verhoog S.; Engle K. M.; Khotavivattana T.; O’Duill M.; Wheelhouse K.; Rassias G.; Médebielle M.; Gouverneur V. Catalytic Hydrotrifluoromethylation of Unactivated Alkenes. J. Am. Chem. Soc. 2013, 135, 2505–2508. 10.1021/ja401022x. [DOI] [PubMed] [Google Scholar]; d Nadiveedhi M. R.; Cirandur S. R.; Akondi S. M. Visible-light-promoted photocatalyst- and additive-free intermolecular trifluoromethyl-thio(seleno)cyanation of alkenes. Green Chem. 2020, 22, 5589–5593. 10.1039/D0GC01726D. [DOI] [Google Scholar]
- For some selected examples, see; a Ye Y.; Lee S. H.; Sanford M. S. Silver-Mediated Trifluoromethylation of Arenes Using TMSCF3. Org. Lett. 2011, 13, 5464–5467. 10.1021/ol202174a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu X.; Chu L.; Qing F.-L. Silver-Catalyzed Hydrotrifluoromethylation of Unactivated Alkenes with CF3SiMe3. Angew. Chem., Int. Ed. 2013, 52, 2198–2202. 10.1002/anie.201208971. [DOI] [PubMed] [Google Scholar]; c Wang Y.-F.; Lonca G. H.; Chiba S. PhI(OAc)2-Mediated Radical Trifluoromethylation of Vinyl Azides with Me3SiCF3. Angew. Chem., Int. Ed. 2014, 53, 1067–1071. 10.1002/anie.201307846. [DOI] [PubMed] [Google Scholar]; d Wang Y.-F.; Lonca G. H.; Le Runigo M.; Chiba S. Synthesis of Polyfluoroalkyl Aza-Polycyclic Aromatic Hydrocarbons Enabled by Addition of Perfluoroalkyl Radicals onto Vinyl Azides. Org. Lett. 2014, 16, 4272–4275. 10.1021/ol501997n. [DOI] [PubMed] [Google Scholar]; e Yu W.; Xu X.-H.; Qing F.-L. Silver-Mediated Oxidative Fluorotrifluoromethylation of Unactivated Alkenes. Adv. Synth. Catal. 2015, 357, 2039–2044. 10.1002/adsc.201500027. [DOI] [Google Scholar]
- For some selected examples, see; a Guyon H.; Chachignon H.; Cahard D. CF3SO2X (X = Na, Cl) as reagents for trifluoromethylation, trifluoromethylsulfenyl-, −sulfinyl- and -sulfonylation. Part 1: Use of CF3SO2Na. Beilstein J. Org. Chem. 2017, 13, 2764–2799. 10.3762/bjoc.13.272. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tang L.; Yang Z.; Chang X.; Jiao J.; Ma X.; Rao W.; Zhou Q.; Zheng L. K2S2O8-Mediated Selective Trifluoromethylacylation and Trifluoromethylarylation of Alkenes under Transition-Metal-Free Conditions: Synthetic Scope and Mechanistic Studies. Org. Lett. 2018, 20, 6520–6525. 10.1021/acs.orglett.8b02846. [DOI] [PubMed] [Google Scholar]; c Zhao L.; Li P.; Zhang H.; Wang L. Photoinduced synthesis of α-trifluoromethylated ketones through the oxidative trifluoromethylation of styrenes using CF3SO2Na as a trifluoromethyl reagent without an external photoredox catalyst. Org. Chem. Front. 2019, 6, 87–93. 10.1039/C8QO01079J. [DOI] [Google Scholar]; d Li B.; Zeng W.; Wang L.; Geng Z.; Loh T.-P.; Xie P. Visible-Light-Induced Trifluoromethylation of Allylic Alcohols. Org. Lett. 2021, 23, 5235–5240. 10.1021/acs.orglett.1c01767. [DOI] [PubMed] [Google Scholar]
- For some recent examples, see; a Han H. S.; Lee Y. J.; Jung Y.-S.; Han S. B. Stereoselective Photoredox-Catalyzed Chlorotrifluoromethylation of Alkynes: Synthesis of Tetrasubstituted Alkenes. Org. Lett. 2017, 19, 1962–1965. 10.1021/acs.orglett.7b00470. [DOI] [PubMed] [Google Scholar]; b Chen L.; Wu L.; Duan W.; Wang T.; Li L.; Zhang K.; Zhu J.; Peng Z.; Xiong F. Photoredox-Catalyzed Cascade Radical Cyclization of Ester Arylpropiolates with CF3SO2Cl To Construct 3-Trifluoromethyl Coumarin Derivatives. J. Org. Chem. 2018, 83, 8607–8614. 10.1021/acs.joc.8b00581. [DOI] [PubMed] [Google Scholar]; c Lin S.; Cui J.; Chen Y.; Li Y. Copper-Catalyzed Direct Cycloaddition of Imidazoles and Alkenes to Trifluoromethylated Tricyclic Imidazoles. J. Org. Chem. 2021, 86, 15768–15776. 10.1021/acs.joc.1c01832. [DOI] [PubMed] [Google Scholar]
- For some selected examples, see; a Nagib D. A.; Scott M. E.; MacMillan D. W. C. Enantioselective α-Trifluoromethylation of Aldehydes via Photoredox Organocatalysis. J. Am. Chem. Soc. 2009, 131, 10875–10877. 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ye Y.; Sanford M. S. Merging Visible-Light Photocatalysis and Transition-Metal Catalysis in the Copper-Catalyzed Trifluoromethylation of Boronic Acids with CF3I. J. Am. Chem. Soc. 2012, 134, 9034–9037. 10.1021/ja301553c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Straathof N. J. W.; Tegelbeckers B. J. P.; Hessel V.; Wang X.; Nöl T. A mild and fast photocatalytic trifluoromethylation of thiols in batch and continuous-flow. Chem. Sci. 2014, 5, 4768–4773. 10.1039/C4SC01982B. [DOI] [Google Scholar]; d Silvi M.; Aggarwal V. K. Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters. J. Am. Chem. Soc. 2019, 141, 9511–9515. 10.1021/jacs.9b03653. [DOI] [PubMed] [Google Scholar]
- a Natte K.; Jagadeesh R. V.; He L.; Rabeah J.; Chen J.; Taeschler C.; Ellinger S.; Zaragoza F.; Neumann H.; Brückner A.; Beller M. Palladium-Catalyzed Trifluoromethylation of (Hetero)Arenes with CF3Br. Angew. Chem., Int. Ed. 2016, 55, 2782–2786. 10.1002/anie.201511131. [DOI] [PubMed] [Google Scholar]; b Ren Y.-Y.; Zheng X.; Zhang X. Bromotrifluoromethane: A Useful Reagent for Hydrotrifluoromethylation of Alkenes and Alkynes. Synlett 2018, 29, 1028–1032. 10.1055/s-0036-1591944. [DOI] [Google Scholar]; c Zhang K.-F.; Bian K.-J.; Li C.; Sheng J.; Li Y.; Wang X.-S. Nickel-Catalyzed Carbofluoroalkylation of 1,3-Enynes to Access Structurally Diverse Fluoroalkylated Allenes. Angew. Chem., Int. Ed. 2019, 58, 5069–5074. 10.1002/anie.201813184. [DOI] [PubMed] [Google Scholar]; d Li Q.; Fan W.; Peng D.; Meng B.; Wang S.; Huang R.; Liu S.; Li S. Cobalt-Tertiary-Amine-Mediated Hydroxytrifluoromethylation of Alkenes with CF3Br and Atmospheric Oxygen. ACS Catal. 2020, 10, 4012–4018. 10.1021/acscatal.0c00498. [DOI] [Google Scholar]; e Peng D.; Fan W.; Zhao X.; Chen W.; Wen Y.; Zhang L.; Li S. Zinc-Brønsted acid mediated practical hydrotrifluoromethylation of alkenes with CF3Br. Org. Chem. Front. 2021, 8, 6356–6363. 10.1039/D1QO01073E. [DOI] [Google Scholar]
- Siegemund G.; Schwertfeger W.; Feiring A.; Smart B.; Behr F.; Vogel H.; McKusick B.; Kirsch P.. Fluorine Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Caron S. Where Does the Fluorine Come From ? A Review on the Challenges Associated with the Synthesis of Organofluorine Compounds. Org. Process Res. Dev. 2020, 24, 470–480. 10.1021/acs.oprd.0c00030. [DOI] [Google Scholar]
- a Francèse C.; Tordeux M.; Wakselman C. Reactions of bromotrifluoromethane with acid derivatives in the presence of zinc. Tetrahedron Lett. 1988, 29, 1029–1030. 10.1016/0040-4039(88)85326-7. [DOI] [Google Scholar]; b Tordeux M.; Francese C.; Wakselman C. Reactions of trifluoromethyl bromide and related halides: part 9. Comparison between additions to carbonyl compounds, enamines, and sulphur dioxide in the presence of zinc. J. Chem. Soc., Perkin Trans. 1990, 1, 1951–1957. 10.1039/p19900001951. [DOI] [Google Scholar]; c Grobe J.; Hegge J. Facile Aluminum Induced Synthesis of (Trifluoromethyl)trimethylsilane. Synlett 1995, 1995, 641–642. 10.1055/s-1995-5009. [DOI] [Google Scholar]
- a Heidbreder A.; Mattay J. PET-Oxidative Cyclization of Unsaturated Enol Silyl Ethers. Tetrahedron Lett. 1992, 33, 1973–1976. 10.1016/0040-4039(92)88117-N. [DOI] [Google Scholar]; b Ackermann L.; Heidbreder A.; Wurche F.; Klärner F.-G.; Mattay J. Mechanistic studies on PET-oxidative cyclization of unsaturated silyl enol ethers: dependence of the regioselectivity on alcohol addition and pressure effects. J. Chem. Soc., Perkin Trans. 1999, 2, 863–870. 10.1039/a807683i. [DOI] [Google Scholar]
- a Yu J.-Q.; Wu H.-C.; Corey E. J. Pd(OH)2/C-Mediated Selective Oxidation of Silyl Enol Ethers by tert-Butylhydroperoxide, a Useful Method for the Conversion of Ketones to α,β-Enones or β-Silyloxy-α,β-enones. Org. Lett. 2005, 7, 1415–1417. 10.1021/ol050284y. [DOI] [PubMed] [Google Scholar]; b Holmbo S. D.; Godfrey N. A.; Hirner J. J.; Pronin S. V. A Catalytic Intermolecular Formal Ene Reaction between Ketone-Derived Silyl Enol Ethers and Alkynes. J. Am. Chem. Soc. 2016, 138, 12316–12319. 10.1021/jacs.6b06847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.