Abstract
Purpose
Sorafenib inhibits Raf kinase and vascular endothelial growth factor (VEGF) receptor. Bevacizumab is a monoclonal antibody targeted against VEGF. We hypothesized that the complementary inhibition of VEGF signaling would have synergistic therapeutic effects.
Patients and Methods
Patients had advanced solid tumors, Eastern Cooperative Oncology Group performance status of 0 to 1, and good end-organ function. A phase I dose-escaiation trial of sorafenib and bevacizumab was initiated at below-recommended single-agent doses because of possible overlapping toxicity: sorafenib 200 mg orally twice daily and bevacizumab intravenously at 5 mg/kg (dose level [DL] 1) or 10 mg/kg (DL2) every 2 weeks. Additional patients were enrolled at the maximum-tolerated dose (MTD).
Results
Thirty-nine patients were treated. DL1 was the MTD and administered in cohort 2 (N = 27). Dose-limiting toxicity in DL2 was grade 3 proteinuria and thrombocytopenia. Adverse events included hypertension, hand-foot syndrome, diarrhea, transaminitis, and fatigue. Partial responses (PRs) were seen in six (43%) of 13 patients with ovarian cancer (response duration range, 4 to 22 + months) and one of three patients with renal cell cancer (response duration, 14 months). PR or disease stabilization ≥ 4 months (median, 6 months; range, 4 to 22+ months) was seen in 22 (59%) of 37 assessable patients. The majority (74%) required sorafenib dose reduction to 200 mg/d at a median of four cycles (range, one to 12 cycles).
Conclusion
Combination therapy with sorafenib and bevacizumab has promising clinical activity, especially in patients with ovarian cancer. The rapidity and frequency of sorafenib dose reductions indicates that sorafenib at 200 mg twice daily with bevacizumab 5 mg/kg every 2 weeks may not be tolerable long term, and alternate sorafenib dosing schedules should be explored.
INTRODUCTION
Small-molecule signal transduction inhibitors (STIs) with oral bioavailability have demonstrated single-agent clinical activity in tumors with documented molecular defects in dominant biochemical pathways. Signaling pathway targets inhibited by STIs include the bcr-abl fusion protein in chronic myelogenous leukemia and c-kit or epidermal growth factor receptor mutations in gastrointestinal stromal tumors and non–small-cell lung cancer.1–3 Combinatorial strategies using signal inhibitory agents with related targets have the potential for induced biochemical and clinical synergism,4 with the expectation that therapeutic interruption of pathways in series (vertical inhibition) may be successful using lower doses of complementary agents that intersect the pathway at multiple sites.
Sorafenib is a STI against Raf kinase and vascular endothelial growth factor (VEGF) receptor-2.5 Raf kinase is a downstream modulator of the VEGF signaling pathway. Clinical benefits observed with sorafenib alone have resulted in United States Food and Drug Administration approval for its use in advanced renal cell carcinoma (RCC).6 Bevacizumab, a VEGF-neutralizing monoclonal antibody, has been shown to prolong progression-free survival (PFS) for patients with RCC when administered at doses of 10 to 15 mg/kg every 2 to 3 weeks.7 Bevacizumab also demonstrated single-agent activity in relapsed epithelial ovarian cancer (EOC), with a response rate of 17%.8 The addition of bevacizumab to chemotherapy has resulted in improved survival in phase III studies in patients with metastatic colorectal and non–small-cell lung cancers.9,10 We hypothesized that sorafenib and bevacizumab would act in series in the VEGF pathway (Fig 1), inhibiting ligand-receptor interaction, receptor activation, and propagation of downstream signals that both produce VEGF and increase endothelial cell proliferation, survival, and vascular remodeling.11 Here we report the first trial targeting VEGF signaling in series with combination therapy and demonstrate increased anti-VEGF–associated toxicities with promising clinical activity.
Fig 1.
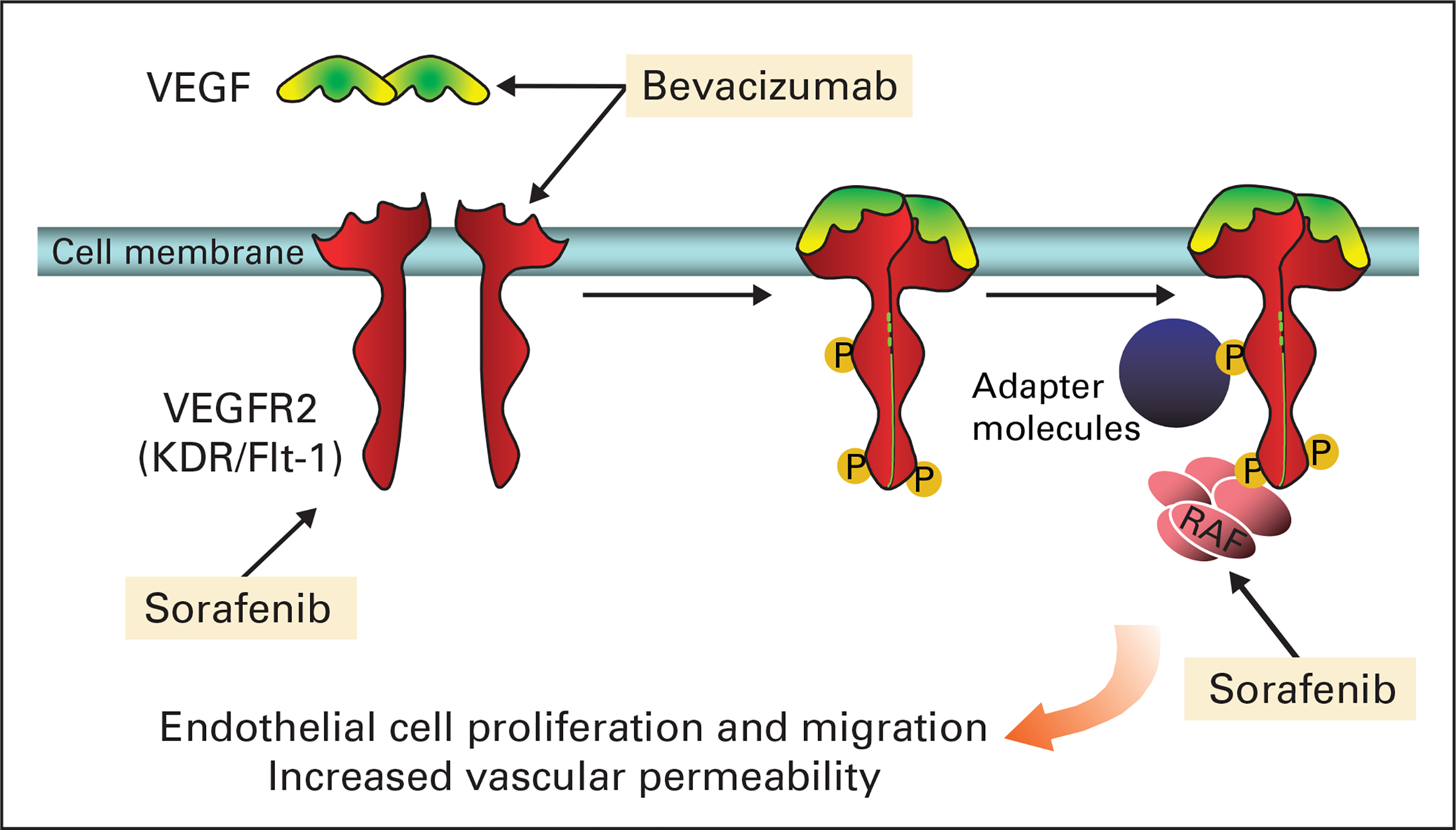
Targeting the vascular endothelial growth factor (VEGF) pathway. Sorafenib and bevacizumab cooperate to dampen the signaling of the VEGF pathway in series. Bevacizumab binds free VEGF, whereas sorafenib targets the VEGF-2 receptor as well as Raf kinase, which is a downstream effector of the VEGF receptor.
PATIENTS AND METHODS
Eligibility
This study was approved by the institutional review board of the National Cancer Institute. Written informed consent was obtained from all patients before enrollment. Eligibility requirements were as follows: patients were required to have solid tumors without curative treatment options, no treatment for at least 4 weeks, Eastern Cooperative Oncology Group performance status of 0 to 1, leukocyte count ≥ 3,000/μL, absolute neutrophil count more than 1,200/μL, platelet count ≥ 100,000/μL, serum creatinine ≤ 1.5 mg/dL, transaminases ≤ 2.5 × upper limit of normal, bilirubin ≤ 1.5 mg/dL, and normal amylase and lipase. Coagulation parameters within 1.25 × upper limit of normal and (corrected) blood pressure (BP) of ≤ 140/90 mmHg were required. Preexisting treatment-related toxicity must have recovered to grade 1 or better. Patients with brain metastases, cardiac arrhythmias requiring treatment, active infection, hemoptysis, recent thrombosis, or bleeding diatheses were excluded. Patients could not have been previously treated with either experimental agent.
Treatment Plan and Dose Modifications
This phase I study of daily oral sorafenib and every 2 weeks intravenous bevacizumab escalated each drug as shown in Table 1. There was a 6-week accrual pause between dose levels to monitor for delayed toxicity. A history, physical examination, and urinalysis were performed before each bevacizumab dose. Bevacizumab was held if urine protein was 2+ by dipstick or if 24-hour urine protein exceeded 1 gm/24 hours for the initial dose or 2 gm/24 hours for subsequent doses. Patients were enrolled in cohort 2, an expanded group at the maximum-tolerated dose (MTD), to assess outcome and translational studies. Methods for sample size determination, pharmacokinetics (plasma and serum), and measurement of plasma VEGF concentrations are presented in the Appendix (online only). The clinical schema for cohort 2 patients consisted of single-agent randomization for one 4-week cycle followed by treatment with the combination for all subsequent cycles.
Table 1.
Dose Levels
| Dose Level | No. of Patients | Sorafenib (mg bid) | Bevacizumab (mg/kg q 2 weeks) | Range of Cycles |
|---|---|---|---|---|
|
| ||||
| 1 | Cohort 1: 6 Cohort 2: 27 |
200 | 5 | 1–22+ |
| 2 | 6 | 200 | 10 | 3–6 |
| 3 | 0 | 400 | 10 | (did not accrue) |
Abbreviations: bid, twice per day; q, every.
Dose-Limiting Toxicity and Dose Modifications
Dose-limiting toxicity (DLT) was defined as any recurrent grade 2 or any grade 3 to 4 event related to study medications occurring within the first 6 weeks of treatment as delimited by the Common Terminology Criteria for Adverse Events (version 3) with the following exception12: patients with a history of hypertension requiring therapy were allowed one additional antihypertensive drug, whereas up to two agents could be introduced to previously normotensive patients. Dose levels were expanded to six patients if a DLT was observed. If two or more patients were found to have DLT, the MTD was considered to be exceeded. Documented grade 2 or 3 toxicity required a hold in therapy until resolution to grade 1. Patients had their dose reduced by one level for grade 3 toxicity or recurrent grade 2 toxicitys treatment was discontinued for grade 4 toxicity. Patients underwent BP measurement daily for the first 4 weeks; BP in excess of 160/100 mmHg required intervention, with stability below that level for 3 days.
Patient Monitoring and Response Assessment
Pretreatment assessments were made within 2 weeks of therapy initiation and included history and physical examination, laboratory studies, ECG, chest radiograph, and noninvasive imaging to demonstrate tumor burden. Patients were seen in clinic every 2 weeks for the first 8 weeks of treatment, then monthly. Reassessment imaging was performed every 8 weeks and evaluated by the reference radiologist without knowledge of the patient’s clinical status. CA-125 or carcinoembryonic antigen (as appropriate) were drawn at baseline and monthly but were not used for outcome determination; the effects of these agents on tumor marker concentrations are not known. Tumor effects were characterized using Response Evaluation Criteria In Solid Tumors.13
RESULTS
Patient Accrual
Thirty-nine patients were accrued between November 2004 and September 20071 (Table 2). As of September 1, 2007, 250+ cycles of therapy have been administered (median, four cycles; range, 1 to 22+ cycles). Tumor types represented included EOC (n = 13), melanoma (n = 7), RCC (n = 3), and sarcoma (n = 5).
Table 2.
Patient Characteristics
| Characteristic | Value | |
|---|---|---|
|
| ||
| Age, years | ||
| Median | 58 | |
| Range | 30–76 | |
| No. of patients | 39 | |
| ECOG performance status, n | ||
| 0 | 5 | |
| 1 | 34 | |
| Sex, n | ||
| Female | 26 | |
| Male | 13 | |
| Previous anticancer treatments | ||
| Total | ||
| Median | 5 | |
| Range | 0–15 | |
| Chemotherapy | ||
| Median | 4 | |
| Range | 1–12 | |
| Radiation therapy | ||
| Median | 1 | |
| Range | 1–6 | |
| Immunotherapy | ||
| Median | 1.5 | |
| Range | 1–4 | |
| Hormonal therapy | ||
| Median | 1 | |
| Range | 1–3 | |
| Targeted therapy | ||
| Median | 1.5 | |
| Range | 1–2 | |
| Vaccine therapy | ||
| Median | 1 | |
| Range | 1–2 | |
| Tumor type | ||
| Ovarian cancer | 13 | |
| Melanoma (ocular, visceral, cutaneous) | 7 | |
| Renal cell cancer | 3 | |
| Sarcoma | 5 | |
| Colon cancer | 2 | |
| Other* | 9 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Includes mesothelioma, follicular thyroid cancer, carcinoma of unknown primary, adenoid cystic breast cancer, cervical cancer, adrenal cancer, fallopian tube cancer, and endometrioid endometrial cancer.
Toxicity
Doses administered were initiated below approved single-agent dose because of concerns of potentiating toxicity. Unexpected severity of toxicity was seen, preventing full dose escalation (Table 3). A DLT of recurrent grade 2 hand-foot syndrome (HFS) was noted in dose level (DL) 1. This patient with refractory EOC had extensive abdominal and serosal involvement at presentation and also developed an abscess at the site of abdominal subcutaneous tumor 2 weeks into treatment. The abscess was caused by a grade 2 enterocutaneous fistula occurring at a site of marked and rapid tumor reduction. Sorafenib dose was reduced to 200 mg/d and the patient had a continuing partial remission (PR) until withdrawal owing to travel weariness at 21 months Grade 3 toxicities in DL1 were delayed.
Table 3.
Grade 2 to 5 Toxicity by Maximum Grade per Patient (N = 39)*
| Toxicity Grade (No. of patients) |
||||||
|---|---|---|---|---|---|---|
| Grade 2 |
Grade 3 |
Grade 4 |
||||
| Toxicity | DL1 | DL2 | DL1 | DL2 | DL1 | DL2 |
|
| ||||||
| Diarrhea | 1 | 1 | 4 | 1 | 0 | 0 |
| Fatigue | 10 | 2 | 3 | 0 | 0 | 0 |
| Fistula | 1 | 1 | 0 | 0 | 0 | 0 |
| Hand-foot syndrome | 18† | 4 | 0 | 1 | 0 | 0 |
| Hypertension | 12 | 1 | 8 | 4 | 1 | 0 |
| Perforation | 0 | 0 | 1 | 0 | 0 | 0 |
| Proteinuria | 3 | 1 | 0 | 2‡ | 0 | 0 |
| Thrombocytopenia | 1 | 0 | 0 | 1‡ | 0 | 0 |
| Thrombosis | 0 | 0 | 2 | 0 | 1 | 0 |
| Transaminitis | 9 | 0 | 3 | 0 | 1 | 0 |
Abbreviations: DL1, dose level 1; DL2, dose level 2.
Cohort 2 (translational) patients enrolled on DL1 dosage (n = 24).
Recurrent grade 2 hand-foot syndrome was the dose-limiting toxicity in DL1.
Dose-limiting toxicity in DL2.
DLT of grade 3 thrombocytopenia and proteinuria (4.8 and 5.2 gm/24 hours) were observed in DL2. Grade 3 proteinuria resolved within 3 and 6 weeks, respectively, and the patients continued treatment after bevacizumab dose reduction. Grade 3 thrombocytopenia, nadir 46,000/μL, occurred in the patient with the greatest proteinuria and resolved after 21 days. Neither thrombocytopenia nor proteinuria recurred after bevacizumab dose reduction. DL1 is the dose level onto which patients in cohort 2 were enrolled.
Dose modifications of sorafenib occurred on both dose levels Five of the six patients treated at DL1 had sorafenib dose reduction by the fourth cycle of therapy (median, 2.5 cycles); none required reduction of bevacizumab. Sorafenib dose reduction occurred in all six patients treated at DL2 by the mid fourth cycle (median, three cycles). There was no difference in time to dose reduction for patients in cohort 2, who received single-agent therapy for 1 month, compared with the dose-finding cohort in which both drugs were administered concurrently. Overall, 29 (74%) of 39 patients required sorafenib reduction to a single daily dose of 200 mg at a median of four cycles (range, one to 12 cycles). Common other causes of dose reductions were HFS (n = 7), anorexia/fatigue/weight loss (n = 6), and hypertension (n = 4). Four of six patients treated at DL2 required dose reduction of bevacizumab within the first 8 weeks for hypertension (n = 2) and proteinuria (n = 2). No differences were seen for number extent, or timing of cumulative hypertension and HFS between the two cohorts of patients.
Hypertension was an expected adverse event for both agents. Twenty-six (67%) of 39 patients developed hypertension and required institution or modification of an antihypertensive regimen. Treatment was discontinued for two patients with hypertension uncontrolled on maximally allowable treatment after two and six cycles. Both patients had preexisting hypertension.
We observed HFS in 25 (76%) of 30 patients treated at DL1 and six (100%) of six patients treated at DL2. Patients experienced skin redness, scaling, and pain, occurring most commonly in hands and feet. Use of emollients provided some relief, as well as addition of pyridoxine in doses up to 400 mg administered twice per day. Temporary cessation of sorafenib administration was associated with a rapid symptom improvement, allowing reinstitution of drug within 3 to 5 days. Reduction to a single dose of 200 mg was associated with reduced HFS severity. All patients but one were able to continue with infrequent dose interruption.
Two patients with EOC developed fistulae in areas of rapid tumor regression; the first is discussed above. The second patient (DL2) developed a grade 2 enterovaginal fistula 2 weeks into therapy; treatment was discontinued at 4 months because of a persistent bacteria infection in her left gluteal muscle that was later shown to be caused by an enterocutaneous fistula at a site of tumor regression. She had a confirmed PR before therapy discontinuation. Neither fistula event was dose limiting; both were associated with rapid disease regression, were managed conservatively, and both patients had confirmed PRs. A patient with melanoma (cohort 2) underwent emergency surgery for a perforated appendix 4 months into study. Pathology was inconclusive in determining causality: infection, tumor, and/or investigationa drugs. One study death occurred after treatment was discontinued for a patients in cohort 2 with liposarcoma metastatic to lung. Treatment was halted after four cycles when cavitation of a pulmonary hilar mass was seen; 1 week later, the patient experienced massive hemoptysis and died.
Clinical and Tumor Response
All patients entered onto the study had progressive disease at the time of enrollment. PR or stable disease for ≥ 4 months was seen in 22 (59%) of 37 assessable patients (Table 4). Confirmed and sustained PR was seen in six (46%) of 13 patients with EOC (4, 10+, 16+, 19, 21, and 22+ months). CA-125 concentrations decreased concomitantly with measured tumor changes in five of six patients with EOC achieving PRs. One patient with sarcomatoid RCC achieved a PR lasting 14 months. No patient with a PR experienced disease progression while undergoing therapy.
Table 4.
Clinical Outcomes
| Dose Level 1* |
Dose Level 2 |
||||
|---|---|---|---|---|---|
| Tumor Type | No. of Patients | Best Response† | Time on Study (months) | Best Response | Time on Study (months) |
|
| |||||
| Ovary | 13 | PR | 10†, 16†, 19‡, 21‡, 22† | PR | 4‡ |
| SD | 4, 5, 5 | SD | 4, 5 | ||
| Renal cell | 3 | PR | 14‡ | SD | 4‡ |
| SD | 8 | ||||
| Melanoma | 7 | SD | 2‡, 4, 4, 4‡, 6‡ | SD | 4 |
| Colon | 2 | SD | 6 | SD | 6 |
| Sarcoma | 5 | SD | 2†, 4‡, 4, 15 | ||
| Other† | 9 | SD | 3, 4, 5‡, 5, 6, 20† | ||
Abbreviations: PR, partial response; SD, stable disease.
Cohort 2 (translational) patients enrolled on DL1 dosage (N = 27).
All other patients had progressive disease at or before first restaging (n = 5) or are too early to be assessed (n = 2).
Patients were removed from study for toxicity/safety or patient choice.
Pharmacokinetic and Cytokine Studies
Blood was drawn for pharmacokinetic studies for the 24 patients enrolled in the translational cohort and randomly assigned to single-agent treatment for the first cycle (Appendix). No difference in sorafenib or bevacizumab concentrations were demonstrated between single-agent and dual-drug therapy; analysis of bevacizumab pharmacokinetics is limited because bevacizumab had not reached steadystate by the time of introduction of sorafenib. The majority of bevacizumab concentrations fell into a predicted 10th to 90th percentile range for bevacizumab administered at 5 mg/kg every 2 weeks, as determined by population modeling. Plasma VEGF concentrations were measured before treatment and monthly. A consistent increase in plasma VEGF concentration was obseived from on-study through cycle 4, incorporating all tested patients. The most prominent changes were noted between baseline and cycle 1 of the arm with bevacizumab monotherapy (median change, 414 μg/mL; range, −74 to 766 μg/mL; P = .006 by two-tailed Wilcoxon signed rank test) and between baseline and cycle 2 of the same arm (median change, 540 μg/mL; range, 24 to 965 μg/mL; P = .004).
DISCUSSION
Inhibition of angiogenesis has emerged as an important therapeutic strategy.14 Several classes of agents have been developed to exploit this multistep process.15 The specificity of the agents is varied, with highly specific antiligand monoclonal antibodies and others with more promiscuous activity, such as some STIs.16,17 We hypothesized that use of STIs in series would yield activity at or below defined single-agent doses and postulated that bevacizumab and sorafenib would have supra-additive effects against the VEGF pathway. Interruption of VEGF-2 receptor to Raf-kinase downstream signaling would be expected to yield reduced VEGF production and inhibition of vascular proliferation and survival pathways through dysregulation of both feed-forward and feed-back loops.19,20 This marks the first reported study of combination therapy with two anti-VEGF targeted agents applied in series. Our findings confirmed that this combination resulted in an unexpected degree of toxicity at the lower combined doses, with some clinical activity in ovarian cancer and other solid tumors.
Our trial design increased the dose of each agent sequentially. We were unable to escalate either agent to its full single-agent dose when sorafenib was combined with bevacizumab. The MTD as defined by the protocol was sorafenib 200 mg twice daily with every other week bevacizumab at 5 mg/kg. However, even with both drugs being administered at doses lower than recommended for single-agent treatment, 29 (74%) of 39 patients required sorafenib dose reduction to 200 mg daily, with median time to dose reduction of three cycles. Eleven of 12 patients who remained on the study for 6 months or more had to have their dose reduced to sorafenib 200 mg once daily. The biologic and clinical activity of this low dose of sorafenib, which is one quarter of the recommended single-agent dose, has not been determined. However, the patients did not seem to lose clinical benefit after sorafenib reduction to this dose when given with bevacizumab. All seven patients with PRs had dose reduction of sorafenib to 200 mg daily occurring between two and 11 cycles; they stabilized or continued to have tumor shrinkage up to 22 months. Taken together, these findings indicate that sorafenib given at 200 mg twice daily is not tolerable over time and that alternative dosing schedules should be explored. We are currently investigating a schedule in which sorafenib is taken twice daily for 5 of 7 days; our phase II study of this combination in ovarian cancer is using that dosing scheme.
The dose of bevacizumab used is also half or less of the dose exposure in other solid tumor studies.7 We were unable to escalate bevacizumab to 10 mg/kg every 2 weeks because of massive proteinuria (two patients) and thrombocytopenia (one patient). Delayed toxicities in this dose level included hypertension (five patients) and exacerbation of sorafenib-associated toxicities (four patients). Sorafenib toxicities worsened when bevacizumab was escalated to 10 mg/kg every 2 weeks in DL2. This suggests that addition of the second agent may have further interrupted the vascular signaling pathways in a dynamic fashion that resulted in cross-over toxicity. Pharmacokinetic analysis did not show altered sorafenib concentrations in the presence of bevacizumab. Treatment with both agents did demonstrate a trend toward increasing VEGF levels. In the single-agent study, sorafenib 400 mg twice daily improved PFS for patients with RCC,6 and Bukowski et al21 reported increasing VEGF concentrations with single-agent sorafenib treatment in RCC. They showed that high on-study VEGF concentration was an independent predictor of PFS. We were unable to perform such an analysis, as only four of the patients with a response were in the translational cohort that included cytokine analysis. These end points have been incorporated into our recently initiated phase II study of this combination in ovarian cancer.
Hypertension has been observed with both agents. Patients receiving fall-dose single-agent sorafenib (400 mg twice daily) were reported to have systolic BP increase of 20 mmHg (80%), but only two of the 20 patients required antihypertensives.22 Multiple studies with bevacizumab have reported grade 3 hypertension in up to 25% of patients.23 The etiology of the hypertension remains unknown but may involve decreased levels of the VEGF-stimulated vasodilator, nitric oxide, or endothelial dysfunction with capillary rarefaction.23,24 In our study, 26 (67%) of 39 patients experienced grades 1 to 4 hypertension, with four patients requiring dose reductions of sorafenib for hypertension; two had to discontinue treatment because of inability to control hypertension within the protocol guidelines.
HFS has been reported to occur with single-agent sorafenib treatment, with frequencies ranging from 25% to 50%.25,26 We report a 79% incidence of grade 1 to 3 HFS that was manageable with symptomatic treatment and dose reduction; only one patient had grade 3 HFS, and one other patient had to discontinue treatment because of recurrent grade 2 HFS. Reduction of sorafenib dose to 200 mg once daily decreased the severity of hypertension and HFS, allowing patients to remain on therapy.
Some clinical benefit was observed in 31 of 37 assessable patients. These patients were heavily pretreated with cytotoxic agents, with a median of five previous treatments, and all had documented progressive disease before study. Six (46%) of the 13 patients with ovarian cancer had PR, and three patients had disease stabilization for at least 4 months. A phase II study of single-agent bevacizumab (15 mg/kg every 3 weeks) in patients with relapsed ovarian cancer with two or fewer prior regimens yielded a 21 % response rate, with 40% of patients progression-free at 6 months; a second phase II trial of bevacizumab alone in platinum-resistant patients with EOC with three or fewer prior regimens demonstrated a response rate of 16%, with a median PFS of 4.4 months.8,27 Our results show a promising response rate in heavily pretreated patients with EOC, providing the impetus for our ongoing phase II study of this combination. Two of seven patients with melanoma had stable disease lasting 4 to 6 months with tumor reduction that did not meet Response Evaluation Criteria In Solid Tumors response criteria; both of these patients had treatment discontinuation for toxicity (appendiceal perforation in one patient and hypertension in the other patient). The one patient with peritoneal mesothelioma received therapy for 20 months and had a persistent 68% reduction in CA-125. Five patients had sarcomas; one patient with leiomyosarcoma received treatment for 14 months. Sosman et al28 are evaluating these two drugs in a phase I/II trial in RCC and have seen partial responses in four of 14 assessable patients at last report. Further study of this drug combination might also be considered in melanoma, sarcoma, and mesothelioma.
This study demonstrates preliminary evidence for potential clinical benefit of this combination of STIs, albeit with therapy-limiting toxicities. The combination of the low-dose sorafenib and bevacizumab was at least supra-additive in adverse events with a greater intensity, rapidity, and frequency of side effects. It should be considered experimental and only used in a clinical trial setting pending phase II studies and further toxicity assessment. This combination holds promise for clinical benefit in multiple tumor types, especially ovarian cancer. Combinations such as this may produce additive, synergistic, or antagonistic toxicity or antitumor activity. Hainsworth et al18 reported a 25% response rate and 1-year PFS of 43% in patients with metastatic RCC treated with full-dose erlotinib and bevacizumab. On the basis of our results, it may not be necessary or advisable to institute therapy with these agents at the single-agent MTDs in combination, even in the absence of anticipated pharmacodynamic interactions. Rational combinatorial strategies with targeted STIs may result in enhanced activity and/or toxicity in patients with cancer. Our study was not designed to test this hypothesis. Our findings point toward the importance of understanding the downstream signaling effects of agents to better predict toxicity and adjust clinical monitoring.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
Presented in part at 42nd Annual Meeting of the American Society of Clinical Oncology, June 2–6, 2006 Atlanta, GA.
Clinical Trials repository link available on JCO.org.
REFERENCES
- 1.Druker BJ, Talpaz M, Resta DJ, et al. : Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031–1037, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, von Mehren M, Blanke CD, et al. ; Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472–480, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. : Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Petricoin EF, Zoon KC, Kohn EC, et al. : Clinical proteomics: Translating benchside promise into bedside reality. Nat Rev Drug Discov 1:683–695, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Carter C, Tang L, et al. : BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109 2004 [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. : Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Haworth L, Sherry RM, et al. : A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burger RA, Sill M, Monk BJ, et al. : Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group (GOG) study. J Clin Oncol 25:5165–5171, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. : Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, et al. : Paclitaxelcarboplatin alone or with bevacizumab for non-small-cell lung cancer.N Engl JMed 355:2542–2550,2006 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9:669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE) v. 3.0. http://ctep.info.nih.gov/reporting/ctc.html
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treament in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Insttute of Canada. J Natl Cancer Inst 92:205–216, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Jain RK: Antiangiogenic therapy for cancer: Current and emerging concepts. Oncology (Williston Park) 19:7–16, 2005 [PubMed] [Google Scholar]
- 15.Wanebo HJ, Argiris A, Bergsland E, et al. : Targeting growth factors and angiogenesis; using small molecules in malignancy. Cancer Metastasis Rev 25:279–292, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Buchdunger E, Zimmermann J, Mett H, et al. : Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res 56:100–104, 1996 [PubMed] [Google Scholar]
- 17.Wilhelm S, Chien DS: BAY 43–9006: Preclinical data. Curr Pharm Des 8:2255–2257,2002 [DOI] [PubMed] [Google Scholar]
- 18.Hainsworth JD, Sosman JA, Spigel DR, et al. : Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib.J Clin Oncol 23:7889–7896, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Akula SM, Ford PW, Whitman AG, et al. : B-Raf-dependent expression of vascular endothelial growth factor-A in Kaposi sarcoma-associated herpesvirus-infected human B cells. Blood 105: 4516–4522,2005 [DOI] [PubMed] [Google Scholar]
- 20.Okajima E, Thorgeirsson UP: Different regulation of vascular endothelial growth factor expression by the ERK and p38 kinase pathways in v-ras, v-raf, and v-myc transformed cells. Biochem Biophys Res Commun 270:108–111,2000 [DOI] [PubMed] [Google Scholar]
- 21.Bukowski RM, Eisen T, Szczylik C, et al. : Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: Survival and biomarker analysis. J Clin Oncol 25:240s, 2007. (suppl; abstract 5023) [Google Scholar]
- 22.Veronese ML, Mosenkis A, Flaherty KT, et al. : Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol 24:1363–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Saif MW, Mehra R: Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 5:553–566, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Morere JF, Des Guetz G, Mourad B, et al. : Mechanism of bevacizumab-induced arterial hypertension: Relation with skin capillary rarefaction in patients treated for metastatic colorectal cancer. J Clin Oncol 25:152s, 2007. (suppl; abstr 3557) [Google Scholar]
- 25.Awada A, Hendlisz A, Gil T, et al. : Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer 92:1855–1861,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strumberg D, Richly H, Hilger RA, et al. : Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23:965–972, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Cannistra SA, Matulonis UA, Penson RT, et al. : Phase II trial of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25:5180–5186, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sosman JA, Flaherty K, Atkins MB, et al. : A phase I/II trial of sorafenib (S) with bevacizumab (B) in metastatic renal cell cancer (mRCC) patients (Pts). J Clin Oncol 24:128s, 2006. (suppl; abstr 3031) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


