Abstract
Bacterial oxidation of 14CH2Br2 and 14CH3Br was measured in freshwater, estuarine, seawater, and hypersaline-alkaline samples. In general, bacteria from the various sites oxidized similar amounts of 14CH2Br2 and comparatively less 14CH3Br. Bacterial oxidation of 14CH3Br was rapid in freshwater samples compared to bacterial oxidation of 14CH3Br in more saline waters. Freshwater was also the only site in which methyl fluoride-sensitive bacteria (e.g., methanotrophs or nitrifiers) governed brominated methane oxidation. Half-life calculations indicated that bacterial oxidation of CH2Br2 was potentially significant in all of the waters tested. In contrast, only in freshwater was bacterial oxidation of CH3Br as fast as chemical removal. The values calculated for more saline sites suggested that bacterial oxidation of CH3Br was relatively slow compared to chemical and physical loss mechanisms. However, enrichment cultures demonstrated that bacteria in seawater can rapidly oxidize brominated methanes. Two distinct cultures of nonmethanotrophic methylotrophs were recovered; one of these cultures was able to utilize CH2Br2 as a sole carbon source, and the other was able to utilize CH3Br as a sole carbon source.
The brominated methanes, methyl bromide (CH3Br) and dibromomethane (CH2Br2), deliver bromine to the atmosphere from marine and terrestrial sources. Bromine is a catalyst for stratospheric ozone destruction, and ozone depletion could have notable consequences for biology and the climate (44). The potential of CH3Br to deplete stratospheric ozone has led to restrictions on the use of this compound as a fumigant and to its scheduled phase-out (44). The atmospheric lifetime of CH2Br2 (0.29 year) (45) is shorter than that of CH3Br (∼0.8 year) (38), making it less likely to deliver bromine to the stratosphere. However, shorter-lived compounds can reach altitudes higher than might be expected from their atmospheric lifetimes (20). Indeed, CH2Br2 has been detected near the tropical tropopause (36) and in the lower stratosphere (21), indicating that it could participate in depleting stratospheric ozone.
The atmospheric burden of brominated methanes is controlled by a balance of sources and sinks. The sources of brominated methanes are both natural (10, 41) and anthropogenic (27, 44), and the sinks are both chemical (16, 19) and biotic (5, 9, 19). Recognition of a bacterial sink in soil led to reduced estimates of the CH3Br atmospheric lifetime and ozone depletion potential (38). The potential importance of bacterial sinks in bromine biogeochemistry has motivated recent investigations of CH2Br2 and CH3Br biodegradation in saline (9, 19, 40) and hypersaline (5) environments, although investigations of estuarine and freshwater sites still appear to be lacking. Brominated methane degradation has also been reported for pure cultures of methanotrophs (9, 31), ammonia oxidizers (18), and methylotrophs (4, 37). The mechanisms, microbial ecology, and environmental impact of bacterial sinks have yet to be fully assessed.
The pattern of brominated methane degradation may provide insight into the mechanisms behind the process. Certain enzymes, such as the methane monooxygenase, can dehalogenate both CH2Br2 and CH3Br (1), while other enzymes dehalogenate only dihalomethanes (22). In this study we directly compared bacterial oxidation of 14CH2Br2 and 14CH3Br in samples from a variety of aquatic environments and used an inhibitor, methyl fluoride (CH3F) (30), to determine whether bacteria, such as methane or ammonia oxidizers, play a role in brominated methane oxidation in natural waters. Measurements of oxidized products provided the first direct evidence that bacterial oxidation of brominated methanes occurs in fresh, estuarine, and marine waters. The environmental implications of bacterial oxidation of CH2Br2 and CH3Br were explored by comparing rate constants to the rate constants for chemical consumption and volatilization.
MATERIALS AND METHODS
Field samples.
Waters having different salinities and pH values were collected from a number of sites in central and northern California (Table 1). Surface water samples were collected by hand; the samples of Mono Lake water were collected at a depth of either 1 or 5 m and the samples of San Francisco Bay water were collected at a depth of 1 m with a Nisken bottle. Samples were collected in 1996, 1997, and 1998 during all seasons. Most of the San Francisco Bay water samples were collected from San Pablo Bay; the only exceptions were the samples obtained during January 1997 and April 1998, which were collected from central San Francisco Bay due to low salinity caused by heavy rain. The water was filtered on site to remove organisms that were larger than 80 μm (larger than 120 μm for Mono Lake water) and were stored in polyethylene bottles at 4°C for several hours or up to 1 week.
TABLE 1.
Description of sampling sites
| Site | Water type | Salinity (g/liter) | pH | Collection temp (°C) | Avg initial no. of bacteria/ml (no. of samples) |
|---|---|---|---|---|---|
| Searsville Lake | Freshwater | NDa | 7.6–8.3 | 15–23 | 3 × 106 (3) |
| San Francisco Bay | Estuarine | 6–19b | 7.4–7.8 | 11–21 | 2 × 106 (5) |
| Monterey Bay, Moss Beach, Mendocino | Coastal seawater | 29–34 | 7.3–8.2 | 10–16 | 1 × 106 (10) |
| Mono Lake | Hypersaline, alkaline | 74–77 | 9.8 | 3–21 | 1 × 107 (3) |
ND, not detectable with a refractometer.
The sample containing 6 g of salt per liter was obtained during a period of heavy rain in January 1997.
Radiolabeling experiments.
Oxidation of 14CH2Br2 and 14CH3Br was measured by using a modification of the method of Connell et al. (5). Triplicate live and control samples (6 or 8 ml) were incubated without headspace in Glass-pak syringes (Becton Dickinson). The syringe tips were adapted with Teflon-silicone septa sealed with silicone to allow injection of radiolabel and removal of samples over time. The syringes were incubated statically without headspace in the dark at room temperature (23°C). Killed controls were either double filtered (pore size, 0.2 μm), autoclaved, or killed with 4% formaldehyde. Inhibited controls were used in some experiments. CH3F was added from a saturated solution to a final concentration of 9% (vol/vol) in order to inhibit methane and ammonia oxidation. Chloramphenicol (20 μg/ml) and cycloheximide (483 μg/ml) were used to inhibit prokaryotic and eukaryotic protein synthesis, respectively. The effect of adding 100 μM unlabeled CH3Br, CH3OH, CH3Cl, or trimethylamine (TMA) on the rates of 14CH3Br oxidation was also assessed with seawater samples.
Aliquots (1 ml) of water were removed from syringes at different times and centrifuged with a solution consisting of 20 μl of 1 M NaOH, 0.5 ml of 1 M SrCl2 · 6H2O, and 100 μl of 1 M Na2CO3. The Na2CO3 was added to ensure adequate pellet formation. Each resulting pellet was rinsed twice with 1 ml of ethanol, suspended in a dilute NaOH solution (pH ∼11.7), and collected in ScintiVerse II counting cocktail (Fisher Scientific). The 14C in the pellet, the 14C in the supernatant, and the 14C in the ethanol rinses were collected separately and counted with a liquid scintillation spectrophotometer (model LS 6000SC; Beckman).
The concentrations of each labeled brominated methane (nanocuries per liter) were multiplied by the specific activity of the compound to calculate the total brominated methane concentrations (nanomolar). 14CH2Br2 (>99% pure; Dupont NEN) was added to syringes from an aqueous stock solution that had a specific activity of 49.7 mCi/mmol. A typical final concentration was 2.4 nCi/ml (49 nM total CH2Br2). 14CH3Br (99.9% pure; Dupont NEN) was added from ethanolic stock solutions that had specific activities of 54.9 and 49.0 mCi/mmol. A typical final concentration was 16 nCi/ml (∼300 nM total CH3Br). The final concentrations of ethanol typically ranged from 0.14 to 8.6 mM depending on the 14CH3Br stock solution and the concentration used. The oxidation rates were comparable for similar 14CH3Br concentrations; therefore, ethanol concentrations in this range did not appear to affect 14CH3Br oxidation rates.
The levels of recovery of 14CH2Br2 in the supernatants ranged from 94 to 100% after processing. Chemical degradation of 14CH2Br2 was not significant on the time scale of these experiments (25). The initial levels of recovery of 14CH3Br in the supernatants were >95% before processing but typically 80 to 90% after the supernatant and pellet were separated, presumably because of losses due to volatilization. The supernatant values were corrected for the amounts lost in processing. The levels of recovery of counts in the pellet fraction were approximately 100% based on the recovery of 14C-labeled bicarbonate (0.0934 nCi/ml; specific activity, 54.4 mCi/mmol; Dupont NEN). The pellet was designated Σ14CO2 to indicate that it could contain labeled cellular material, as well as carbonate products from biological oxidation which were precipitated as SrCO3(s).
Measurement of bacterial density and growth.
Bacterial density was determined by using acridine orange direct counts (AODC) (15). Sterile sodium citrate (0.1 M, pH 6.6) was added dropwise during filtration of samples from Searsville Lake and Mono Lake to remove background fluorescence (12). The coefficients of variation (CV) (CV = standard deviation/average) of AODC measurements were ∼5 to 30%. Bacterial densities were determined for samples taken at the beginning of each experiment. In addition, time courses for cell growth were determined in some experiments. The incubations were conducted by using unlabeled brominated methane in parallel with radiolabel experiments. The dissolved oxygen concentration was monitored during some syringe incubations by using an electrode (model 10M-4 oxygen meter; Microelectrodes, Inc.).
Calculations.
In all of the cases tested, first-order kinetics (e.g., the rate was proportional to the concentration) were observed for the brominated methane concentrations typically used during incubations. However, a straight line generally fit time courses as well as or better than an exponential curve fit due to small changes in concentration over time and the limited number of data points. Thus, oxidation rates were calculated by linear regression over the linear portion of the time course of Σ14CO2 production. All rates are given below in terms of bacterial oxidation. These rates were calculated by determining the difference between the regression values of live and control samples. The CV for the rates (CV = standard error/slope) were typically 3 to 9% for CH2Br2 and 8 to 19% for CH3Br.
Reaction rates (nanomoles/liter day−1) and half-lives were calculated by using the following equations:
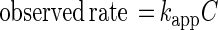 |
1 |
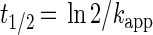 |
2 |
where kapp is the apparent first-order rate constant (day−1), C is the applied brominated methane concentration (nanomolar), and t1/2 is the half-life due to bacterial oxidation (days). Using these equations allowed us to directly compare our values to rate constants provided by other researchers (5, 19). However, the numbers of bacteria differed in the various types of water, and oxidation rates are expected to be a function of bacterial number. To normalize for differences in bacterial number, the rate constants for each experiment were divided by the initial bacterial density. Thus, the reaction rates (nanomoles/liter day−1 per cell per milliliter) and half-lives could be calculated by using the following equations:
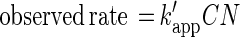 |
3 |
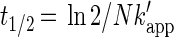 |
4 |
where k′app is the normalized apparent rate constant (nanomoles/liter day−1 per cell per milliliter), C is the applied brominated methane concentration (nanomolar), N is the density of brominated methane-oxidizing bacteria (cells per milliliter), and t1/2 is the half-life due to bacterial oxidation (days). Equations 2 and 4 are equivalent (N cancels). However, equation 4 is more robust because normalized apparent rate constants account for different bacterial densities in natural waters. The equations given above assume that there is no threshold for bacterial uptake. The total bacterial numbers obtained from AODC measurements taken at the start of each incubation were used as a proxy for N. Note that the actual number of oxidizers is an unknown subset of the total population.
Enrichment cultures.
Enrichment cultures were started by amending seawater samples (50 ml in 160-ml vials) with KH2PO4 (0.02 g/liter), NH4Cl (0.5 g/liter), vitamins (including vitamin B12) (1 ml/liter) (33), and CH2Br2 (10 μM) or CH3Br (50 μM). Cultures received several spikes of brominated methane (a total of ∼500 μM brominated methane) and were then transferred to a defined medium. Serial dilution was used for purification because growth on agar plates was not obtained. Degradation was observed at 10−8 dilutions, but microscopic observation showed that there were at least two bacterial morphologies, indicating that the cultures were not pure. The CH2Br2 culture was maintained on a basal medium adapted from the medium of Visscher and Taylor (42) and supplemented with NaCl (35 g/liter), vitamins (including vitamin B12) (33), trace metal solution SL-10 (43), and CH2Br2 as the sole carbon source, and bicarbonate was added as the buffer after autoclaving in order to achieve a final pH of 7.0 to 7.3. The CH3Br culture was cultivated on a basal medium adapted from the medium of Doronina et al. (7) and supplemented with NaCl (10 g/liter), vitamins (including vitamin B12) (33), trace metal solution SL-10 (43), and CH3Br as the sole carbon source. The enrichment cultures were maintained in serum vials capped with Teflon-butyl rubber septa. The cultures were tested for their ability to degrade CH3Br, CH2Br2, and CH4. The concentrations of these gases were measured by headspace injection (200 μl) into a gas chromatograph equipped with flame ionization detection (model HNU gas chromtograph; 60/80 Carbopack B column [4 ft by 0.20 in.]); the detector temperature was 250°C, and the oven temperatures were 60°C for CH4, 70°C for CH3Br, and 175°C for CH2Br2. Bromide ion concentrations were determined by ion chromatography (30). CH2Br2 was added to cultures as a liquid (99% pure; Chem Service), and CH3Br was added as a gas (99.9% pure; Matheson).
RESULTS
Brominated methane oxidation in natural samples.
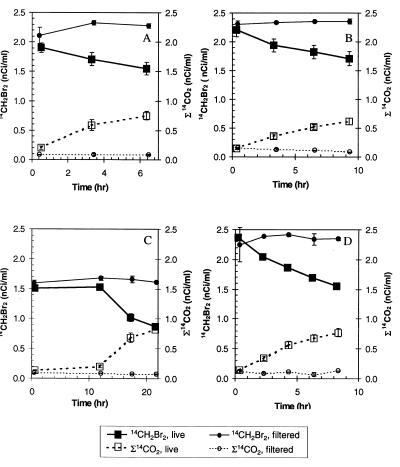
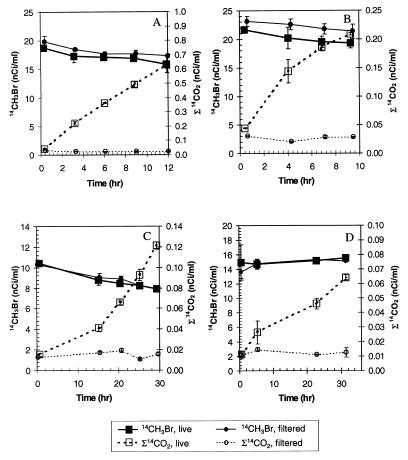
Biological oxidation of 14CH2Br2 and 14CH3Br to Σ14CO2 was measured in samples from freshwater, estuarine, coastal seawater, and hypersaline-alkaline sites. No oxidation occurred in filtered controls (Fig. 1 and 2) or in samples in which bacterial activity was eliminated by formaldehyde treatment or by autoclaving (data not shown). Bacteria from the different sites oxidized similar amounts of 14CH2Br2 (Fig. 1) and tended to oxidize less 14CH3Br (Fig. 2). Freshwater bacteria oxidized the greatest amounts of 14CH3Br (Fig. 2), although the Mono Lake samples contained the highest initial cell numbers (Table 1). Oxidation of CH2Br2 and CH3Br proceeded without a lag in fresh, estuarine, and hypersaline samples. A several-hour lag period (generally >10 and <24 h) was observed for production of Σ14CO2 in coastal seawater samples (Fig. 1 and 2B).
FIG. 1.
Time course of bacterial oxidation of 14CH2Br2 and simultaneous formation of oxidized products in freshwater (A), estuarine water (B), coastal seawater (C), and hypersaline-alkaline water from Mono Lake (D). The error bars represent ± standard deviation of the mean obtained with three replicate syringes.
FIG. 2.
Time course of bacterial oxidation of 14CH3Br and simultaneous formation of oxidized products in freshwater (A), estuarine water (B), coastal seawater (C), and hypersaline-alkaline water from Mono Lake (D). The error bars represent ±1 standard deviation of the mean obtained with three replicate syringes.
Effects of inhibitors and additions.
Oxidation of 14CH2Br2 and 14CH3Br in freshwater samples was strongly inhibited by CH3F (Table 2). 14CH2Br2 oxidation in Mono Lake samples and 14CH3Br oxidation in San Francisco Bay samples were slightly inhibited by CH3F (Table 2). CH3F did not affect brominated methane oxidation in other experiments. Therefore, freshwater samples were the only samples in which bacteria, such as methane or ammonia oxidizers, predominately mediated brominated methane oxidation. The eukaryotic inhibitor cycloheximide was tested with Mono Lake samples and had no significant effect on 14CH2Br2 or 14CH3Br oxidation (data not shown); therefore, eukaryotes were not significantly involved in oxidation at that site. It has been shown previously that cycloheximide does not affect CH2Br2 oxidation in seawater (9). Oxidation in unamended samples occasionally slowed during incubation (Fig. 1 and 2), suggesting that activity sometimes became limited. The dissolved oxygen concentration remained >0.4 mM when it was measured, suggesting that oxygen was not the limiting factor (34).
TABLE 2.
Effect of CH3F on bacterial oxidation of 14CH2Br2 and 14CH3Br
| Water type | Amt of dibromomethane oxidized (nmol liter−1 day−1)
|
Amt of methyl bromide oxidized (nmol liter−1 day−1)
|
||||
|---|---|---|---|---|---|---|
| Without CH3F | With CH3F | % Inhibition | Without CH3F | With CH3F | % Inhibition | |
| Freshwater | 9.5 ± 0.8 | 1.7 ± 0.5 | 82 | 12 ± 0.8 | 0 | 100 |
| Estuarine | 5.2 ± 0.3 | 5.3 ± 0.4a | 0 | 9.1 ± 0.8 | 7.5 ± 0.3 | 19 |
| Seawater | 16 ± 1.2 | 17 ± 1.3 | 0 | 0.11 ± 0.02 | 0.14 ± 0.05 | 0 |
| Seawater | 21 ± 0.9 | 20 ± 2.2 | 0 | |||
| Hypersaline-alkaline | 27 ± 1.2 | 21 ± 1.8 | 22 | 0.45 ± 0.05 | 0.44 ± 0.07 | 0 |
Samples were taken during a period of heavy rain.
The supernatant in 14CH3Br experiments may have contained products of 14CH3Br hydrolysis (14CH3OH) or halide exchange (14CH3Cl) (19), raising the possibility that Σ14CO2 was formed indirectly from bacterial oxidation of these chemical degradation products. However, previous studies performed with Mono Lake water (5) and agricultural soils (29) indicated that Σ14CO2 resulted from direct bacterial oxidation of 14CH3Br and not from oxidation of chemical degradation products. We obtained similar results with seawater samples. Addition of unlabeled CH3OH or CH3Cl (100 μM) had no significant effect on Σ14CO2 production (Table 3). In contrast, addition of unlabeled CH3Br (100 μM) resulted in a significant decrease in the rate of Σ14CO2 production (Table 3), as expected from isotope dilution. In a separate experiment, the rate of Σ14CO2 production was significantly decreased by adding only 2 μM unlabeled CH3Br (data not shown). These results indicate that the bacteria directly oxidized methyl bromide, which is consistent with previous findings (5, 29). Also consistent with previous results obtained with Mono Lake water (5) is the finding that adding TMA to seawater increased 14CH3Br oxidation rates (Table 3).
TABLE 3.
Effect of adding unlabeled C1 compounds on bacterial oxidation of 14CH3Br in seawater samplesa
| Addition | Amt of 14CH3Br added (nCi) | Amt of 14ΣCO2 produced (nCi/day) |
|---|---|---|
| None | 164 | 0.43 ± 0.07b |
| CH3Br (100 μM) | 164 | 0.25 ± 0.04 |
| None | 85 | 0.14 ± 0.01 |
| CH3OH (100 μM) | 85 | 0.16 ± 0.01 |
| None | 135 | 0.24 ± 0.02 |
| CH3Cl (100 μM) | 135 | 0.25 ± 0.02 |
| TMA (100 μM) | 135 | 0.34 ± 0.05 |
The data are from three separate experiments.
Average ± standard error.
Apparent rate constants.
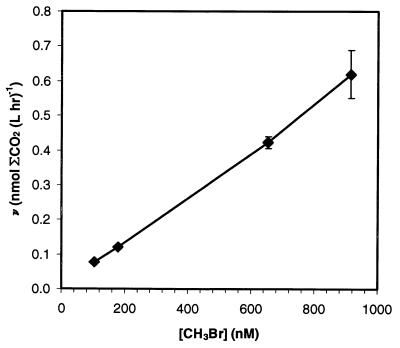
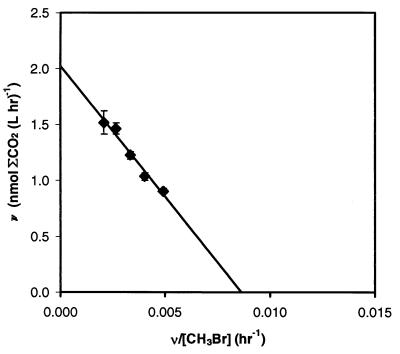
Apparent rate constants (Tables 4 and 5) were calculated from oxidation rates by assuming that first-order kinetics occurred with respect to brominated methane concentration. First-order kinetics were shown to occur for CH2Br2 in seawater and for CH3Br concentrations typically used for seawater, estuarine water, and freshwater samples. In seawater samples, the rates of 14CH2Br2 oxidation were proportional to concentration over the range tested (35 to 455 nM CH2Br2) (data not shown), and the rates of 14CH3Br oxidation were also proportional to concentration over the range tested (125 to 1,035 nM CH3Br) (Fig. 3). In estuarine samples, the oxidation rates were proportional to concentration at concentrations between 160 and 1,337 nM CH3Br but declined at higher concentrations (data not shown), presumably due to toxicity from the ethanol in the 14CH3Br stock solution. Although true saturation was not reached, the apparent half-saturation constant (app Km) for estuarine water was determined to be >2,000 nM CH3Br. Oxidation of 14CH3Br in Searsville Lake followed Michaelis-Menten kinetics, as demonstrated by a linear Eadie-Hofstee plot (Fig. 4). The app Km was 234 ± 32 nM CH3Br. The maximum velocity (Vmax) was 48 ± 2 nmol of CH3Br liter−1 day−1.
TABLE 4.
Apparent first-order rate constants for bacterial oxidation of 14CH2Br2 and 14CH3Bra
| Water type |
kapp (day−1) for:
|
|
|---|---|---|
| Dibromomethane | Methyl bromide | |
| Freshwater | 0.30 ± 0.15 (2/2)b | 0.20 ± 0.18 (3/3) |
| Estuarine | 0.37 ± 0.31 (2/2) | 0.019 ± 0.013 (10/4) |
| Coastal seawater | 0.44 ± 0.10 (7/5) | 0.0084 ± 0.006 (10/6) |
| Hypersaline-alkaline | 0.58 ± 0.19 (4/3) | 0.0023 ± 0.0017 (5/3) |
Values were determined by using equation 1.
Average ± standard deviation. The values in parentheses are number of experiments/number of separately collected water samples.
TABLE 5.
Normalized apparent rate constants for oxidation of 14CH2Br2 and 14CH3Bra
| Water type |
k′app (108 day−1 per bacterium−1) per ml)
|
|
|---|---|---|
| Dibromomethane | Methyl bromide | |
| Freshwater | 8.1 ± 0.81 (2/2)b | 5.1 ± 2.2 (3/3) |
| Estuarine | 39 ± 44 (2/2) | 1.3 ± 0.7 (10/4) |
| Coastal seawater | 72 ± 30 (7/5) | 0.56 ± 0.33 (10/6) |
| Hypersaline-alkaline | 6.2 ± 1.7 (2/2) | 0.022 ± 0.016 (3/3) |
Values were determined by using equation 3.
Average ± standard deviation. The values in parentheses are number of experiments/number of separately collected water samples. Individual rate constants were normalized by initial bacterial density for each experiment. The errors in AODC measurements ranged from ∼5 to 30%.
FIG. 3.
Rates of ΣCO2 production versus CH3Br concentrations in seawater samples. The rates were calculated by linear regression after the lag period. The error bars represent ±1 standard error of the regression for the rate.
FIG. 4.
Eadie-Hofstee plot of CH3Br oxidation in Searsville Lake samples. The error bars represent ±1 standard error of the regression for the rate.
Bacterial oxidation of 14CH3Br tended to be slower as the salinity of the water increased. This pattern was seen in the rate constants calculated with equation 1 (Table 4) and with equation 3 (Table 5). The rate constants for 14CH2Br2 calculated from equation 1 varied little (Table 4) and thus were greater in marine and estuarine waters when they were normalized to the initial cell number (Table 5). In freshwater, the rate constants for CH2Br2 and CH3Br were similar (Tables 4 and 5) and resulted in half-lives of 2 days for CH2Br2 and 5 days for CH3Br (Table 6). In samples from more saline sites, the rate constants for CH2Br2 oxidation were 20- to 280-fold higher than the rate constants for CH3Br oxidation (Tables 4 and 5). For example, the kapp values for estuarine water corresponded to a half-life of 2 days for CH2Br2 and a half-life of 36 days for CH3Br (Table 6). The kapp values for seawater also gave a 2-day half-life for CH2Br2, but the half-life of CH3Br due to biological oxidation of CH3Br was 82 days. In Mono Lake samples, the half-life of CH2Br2 was only 1 day, but the half-life of CH3Br was 298 days.
TABLE 6.
Half-lives of dibromomethane and methyl bromide due to bacterial oxidation, chemical consumption, and volatilization
| Water | Half-lives (days) of:
|
|||||
|---|---|---|---|---|---|---|
| Dibromomethane
|
Methyl bromide
|
|||||
| Bacteriala | Chemicalb | Voltili-zationc | Bacteriala | Chemicald | Voltili-zationc | |
| Freshwater | 2 | 5 | ∼30 | |||
| Estuarine | 2 | 36 | 8–15 | |||
| Seawater | 2 | 82 | ∼5 | |||
| Hypersaline-alkaline | 1 | 298 | ∼3 | |||
| All waters | 4 × 104 | ∼1–16 | ∼1–16 | |||
Values were determined by using equation 2 and the kapp values in Table 4.
Data from reference 25.
Values were determined by using the following expression: ln 2/KL, where K is the transfer velocity (1 to 4.2 m day−1) (seasonal wind speeds, 0 to 20 m/s) and L is the mixed-layer depth (4 to 6 m for Searsville Lake, 2 to 40 m for San Francisco Bay, 11 to 23 m for Mono Lake, and 2 to 100 m for coastal seawater) (3, 14, 23, 28) (see also the following website: http://sfbay.wr.usgs.gov/access/wqdata).
Our CH3Br kapp value for seawater (Table 4) was approximately sevenfold lower than the biological rate constant of King and Saltzman (19) for seawater samples incubated at 21°C. In their experiments, King and Saltzman used 13CH3Br at concentrations of 50 to 800 pM, values which are up to 103-fold lower than the 14CH3Br concentrations used here. Our somewhat lower apparent rate constant suggests that the 14CH3Br concentrations used in this study did not artificially increase the numbers of CH3Br-degrading bacteria. Our CH3Br kapp value for Mono Lake water (Table 4) was two- to fourfold lower than the values reported by Connell et al. (5) based on in situ measurements. Connell et al. used 10-fold-higher 14CH3Br concentrations than the concentrations used here, suggesting that the 14CH3Br concentrations which we used were also not inhibitory.
Bacterial growth and brominated methane oxidation.
Differences in oxidation rates appeared to reflect environmental variations at a sampling site over time. For example, brominated methane oxidation rates and bacterial numbers declined during a period of heavy rain and flooding in 1997. During the height of this flood event, 14CH3Br oxidation was not detected in seawater (data not shown). In contrast, the oxidation rates and bacterial numbers were not significantly affected by storing collected water at 4°C in the dark for more than 1 week (data not shown).
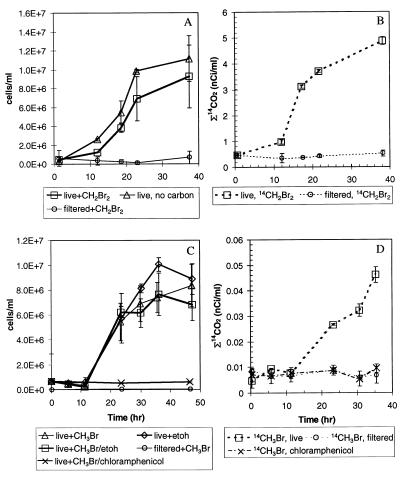
Time courses of AODC measurements were used to monitor changes in bacterial number during syringe incubations. Only total cell numbers could be measured; numbers of brominated methane-degrading bacteria per se could not be measured. The total cell numbers did not increase significantly during incubation of freshwater samples and increased only slightly (1.5- to 3-fold) during incubation of estuarine water samples. The increases in cell number varied from 1.5- to 19-fold for seawater and Mono Lake samples. Figures 5A and C show the results of two separate experiments in which order-of-magnitude increases in cell number were measured during seawater incubation, as expected from bottle effects (8). Cell growth was similar in syringes that were acid washed and heat treated to remove organic material (data not shown), indicating that the syringes did not provide a significant source of substrate. Cell growth in seawater was similar whether brominated methane (Fig. 5A), ethanol (Fig. 5C), or methanol (data not shown) was added to syringes or not. No growth or oxidation occurred in filter-sterilized controls amended with 14CH2Br2 or 14CH3Br (Fig. 5).
FIG. 5.
Time courses of cell growth in the presence and absence of CH2Br2 for live and filtered seawater samples (A) with parallel 14CH2Br2 oxidation (B) and time courses of cell growth in the presence and absence of CH3BR, ethanol (etoh), and chloramphenicol for live and filterd seawater samples (C) with parallel 14CH3Br oxidation (D).
The total cell number was independent of the brominated methane concentration (Fig. 5); therefore, Monod type equations did not describe brominated methane oxidation. A nonlinear least-squares regression analysis of cell number and substrate concentration after the lag verified that degradation was best estimated by assuming that the cell number remained constant (data not shown). Thus, the use of equation 3 was supported even in the worst-case scenario of cell growth (Fig. 5). However, a lag in cell growth (Fig. 5A and C) did accompany oxidation of 14CH2Br2 (Fig. 5B) and 14CH3Br (Fig. 5D) in seawater samples, and addition of chloramphenicol to seawater samples inhibited both cell growth and oxidation of 14CH3Br (Fig. 5C and D). In addition, chloramphenicol added to estuarine water inhibited both the increase in cell number and the oxidation rate by a factor of about 2 (data not shown), indicating that blocking protein synthesis could affect brominated methane oxidation.
Mass balance of brominated methanes.
Syringes were tested for their ability to retain radiolabeled brominated methanes. Two types of syringes, Glass-pak (Becton Dickinson) and all-glass (Baxter) syringes, were tested by using either stopcocks or Teflon-silicone septa. The most reliable performance was obtained with Glass-pak syringes sealed with septa whose septum puncture was resealed with silicone after each sampling. Mass balance of radiolabel was achieved for all incubations of 14CH2Br2 (Fig. 1). The rates of loss of radiolabel from 14CH3Br were 3 to 15% per day, but they were higher (3 to 34% per day) if the septa were not resealed with silicone. These losses and the error associated with measuring 14CH3Br in the supernatant made it difficult to distinguish live samples from control samples without measuring ΣCO2 production (Fig. 2). The loss of 14CH3Br from syringes was less pronounced when Mono Lake water or culture medium was used. To investigate this phenomenon, either the pH or the salinity (1.5, 3, or 7.5% NaCl) of filtered Searsville Lake samples was increased. The water pH was increased from 8 to 10 by adding NaOH or 50 mM NaCO3. Only the addition of NaCO3 halted the loss of radiolabel from syringes (the rate of loss was 24% per day at pH 8, compared with 0.76% per day at pH 10). The increased carbonate alkalinity caused by the addition of NaCO3 should have increased the hydrolysis of 14CH3Br to 14CH3OH due to buffer catalysis (32). Methanol is less volatile than CH3Br, and enhanced production of CH3OH was observed in Mono Lake water (5), which may have resulted in less loss of radiolabel from syringes.
Oxidation of brominated methanes by enrichment cultures.
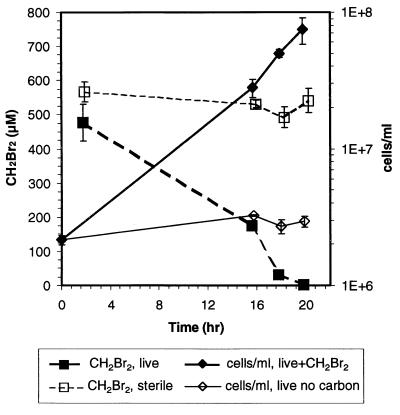
An enrichment culture designated EBr2 was obtained from seawater, and this culture could degrade 500 μM CH2Br2 when it was provided as the sole carbon source in a defined medium. Concentrations of CH2Br2 greater than 3.6 mM halted cell growth and degradation activity. The culture degraded CH2Br2 with concomitant cell growth (Fig. 6) and stoichiometric release of free bromide ion (data not shown). In addition, the culture oxidized 14CH2Br2 to Σ14CO2 (data not shown). Cell growth was observed only with samples that received CH2Br2 (Fig. 6). The cell number increased exponentially during CH2Br2 degradation, resulting in a growth yield of 6 × 106 cells ml−1 μmol−1 and a specific growth rate of 0.18 h−1 (Fig. 6). The specific growth rate was similar to that reported for a dichloromethane utilizer, strain DM11, growing on CH2Br2 (37). However, unlike DM11, our enrichment culture did not degrade CH2Cl2 (25 or 300 μM). Our culture also did not consume CH3Br (30 or 300 μM) or CH4 (1 or 10%), nor was it inhibited by CH3F. This culture grew on methylated amines (data not shown).
FIG. 6.
Time course of CH2Br2 degradation (dashed lines) and concomitant exponential growth (solid lines) for enrichment culture EBr2 that was incubated in serum vials and received CH2Br2 as the sole carbon source in a defined medium. The error bars represent ±1 standard deviation of the mean obtained with three replicate samples.
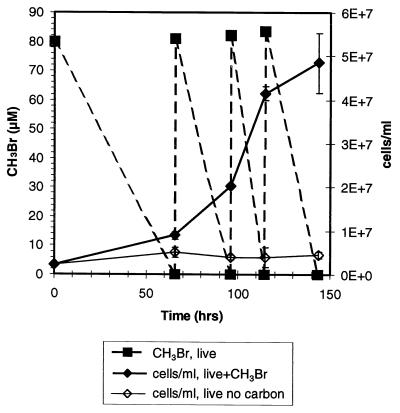
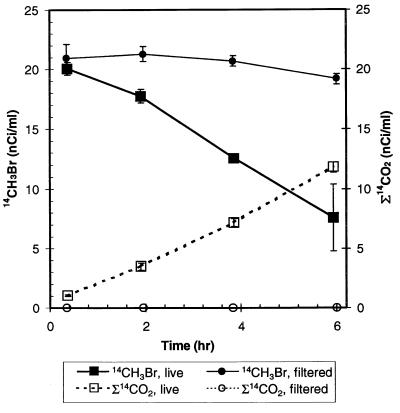
CH3Br-degrading enrichment cultures were established from seawater and were maintained by 1:10 dilution in aged seawater amended with nutrients and vitamins. Cell growth was observed only in seawater transfers that received CH3Br (Fig. 7). After several transfers, an enrichment culture (designated EBr1) was grown in a defined medium supplemented with 50 ml of aged seawater per liter. Under these conditions, the culture quickly oxidized 14CH3Br to Σ14CO2 in syringe experiments performed like the experiments with natural waters (Fig. 8), and the kapp was 2.3 day−1 for 370 nM applied CH3Br. The mass of 14CH3Br lost was fully recovered as oxidized product, and the cell density remained constant over the course of the incubation (∼4.5 × 106 cells/ml). This enrichment culture did not consume CH2Br2 (20 or 600 μM) or CH4 (1%), nor was it inhibited by CH3F. The culture grew on methylated amines (data not shown).
FIG. 7.
Cell growth in seawater for live samples of an enrichment culture that received multiple additions of CH3Br gas (dashed line) and samples that did not receive CH3Br.
FIG. 8.
Oxidation of 14CH3Br to Σ14CO2 by enrichment culture EBr1 grown in a defined medium supplemented with 50 ml of seawater liter−1. Incubation was performed in syringes with no headspace, just like experiments performed with natural samples.
DISCUSSION
Bacteria were able to oxidize 14CH2Br2 and 14CH3Br in natural waters whose salinities varied from ∼0 to 77 g/liter (Table 1 and Fig. 1 and 2). Freshwater was distinguished from other water types on the basis of relatively high 14CH3Br oxidation rates (Fig. 2 and Tables 4 and 5). Freshwater was also the only water type in which oxidation of 14CH3Br and 14CH2Br2 was governed primarily by CH3F-sensitive bacteria (Table 2), such as methane or ammonia oxidizers. Cooxidation in freshwater probably resulted in the relatively high rates of 14CH3Br oxidation. The difference in app Km values for CH3Br in Searsville Lake (234 nM) and San Francisco Bay (>2,000 nM) also demonstrated that the bacteria that oxidized brominated methanes in freshwater had a distinct physiology compared to the bacteria at the other sites.
In contrast to freshwater samples, CH3F caused only slight or no inhibition in samples from other sites (Table 2); therefore, methane or ammonia oxidizers played only a limited role at these sites. Lack of CH3F inhibition was observed previously for 14CH3Br oxidation in Mono Lake (5) and for CH2Br2 degradation in seawater (9). Methyl fluoride does not inhibit other aspects of C1 metabolism, such as methanol or formate oxidation (30); thus, other types of methylotrophs could be responsible for bacterial oxidation in such waters. For example, oxidation of 14CH3Br in seawater samples was not inhibited by CH3F (Table 2), and TMA added to seawater stimulated oxidation of 14CH3Br (Table 3). Similar results were obtained with TMA in Mono Lake water (5) and with CH3OH in agricultural soils (29). In addition, enrichment cultures EBr1 and EBr2 were not inhibited by CH3F, and both of these cultures consumed brominated methanes that were present as sole carbon sources (Fig. 6 and 8) or when they were growing on methylated amines (data not shown). Recovery of these separate enrichment cultures, one of which was able to utilize CH2Br2 and one of which was able to utilize CH3Br, suggests that the bacteria that oxidize 14CH2Br2 and the bacteria that oxidize 14CH3Br in natural seawater samples may be distinct.
The app Km for CH3Br in Searsville Lake samples was substantially lower than the Ki measured for CH3Br for an ammonia oxidizer (500 μM) (18) and was considerably lower than the app Km for CH4 measured in freshwater lake samples (5 to 10 μM) (11). However, the app Km values for Searsville Lake and San Francisco Bay samples were much higher than the ambient CH3Br concentrations (13, 19), making it unlikely that ambient levels could provide enough energy for CH3Br-oxidizing bacteria to maintain themselves (6). However, higher concentrations than the concentrations indicated by steady-state measurements may be available to bacteria, particularly bacteria in the vicinity of brominated methane sources, because production and consumption occur simultaneously in aquatic systems (6). Known aquatic sources of brominated methanes include macroalgae (10, 26) and phytoplankton (41). Terrestrial plants also have the capacity to biohalogenate (35), suggesting that submerged freshwater and estuarine plants may also be sources of brominated methanes.
It is also likely that bacteria oxidize brominated methanes in nature while supporting themselves on other C1 compounds. For example, bacteria can oxidize atmospheric concentrations of CH4 with no threshold as long as they are provided with other suitable C1 compounds to sustain themselves (2, 17). In the freshwater lake, there should have been enough CH4 (28) to support methanotrophic cooxidation of brominated methanes. In the other systems, where methanotrophs were not primarily involved (Table 2), methylated amines may have been used to maintain the brominated methane-degrading population. For example, adding TMA stimulated 14CH3Br oxidation in seawater (Table 3) and in Mono Lake samples (5). In addition, both EBr1 and EBr2 could consume brominated methanes while they grew on methylated amines (data not shown). Furthermore, a facultatively methylotrophic bacterium isolated from agricultural soil grows on CH3Br, as well as methylated amines (4).
In addition to removal by bacterial oxidation, brominated methanes may be removed from the water column by chemical degradation. CH2Br2 is chemically stable in water and has a half-life due to hydrolysis on the order of hundreds of years (25). Bacterial oxidation of CH2Br2 thus appears to be faster than chemical consumption for all of the waters tested (Table 6). In contrast, CH3Br undergoes significant chemical degradation, particularly in more saline waters (16, 19). Bacterial oxidation of CH3Br appears to be as fast as chemical consumption only in freshwater (Table 6).
Brominated methanes may also be removed from the water column by volatilization. Volatilization from a body of water depends on the transfer velocity and the mixed-layer depth (14). The half-lives of CH2Br2 and CH3Br due to volatilization from the different sites examined are on the order of 1 to 16 days depending on the conditions (Table 6). When this rough estimate was used, bacterial oxidation of CH2Br2 had the potential to compete with volatilization for all of the natural waters sampled in this study (Table 6). This result is consistent with the conclusions of Goodwin et al. (9) for coastal seawater. In Searsville Lake, the half-life of CH3Br due to bacterial oxidation was comparable to the half-life due to volatilization (Table 6). Therefore, bacteria may help regulate the flux of brominated methanes in this lake. Bacterial oxidation of CH3Br appears to be slower than volatilization in San Francisco Bay, coastal seawater, and Mono Lake (Table 6).
Oxidation of CH3Br in natural seawater samples was relatively slow compared to oxidation of CH3Br in less saline samples (Tables 4 and 5). However, enrichment culture EBr1 demonstrated that marine bacteria can grow on CH3Br (Fig. 7) and can rapidly oxidize 14CH3Br in syringe experiments (Fig. 8). The slow turnover observed in natural samples may have been due to a low abundance of CH3Br-degrading bacteria in the environment. For example, the kapp for 14CH3Br oxidation in seawater was about 300 times lower than the kapp for EBr1 (2.3 day−1) (Fig. 8). The numbers of cells in the EBr1 culture were similar to the final numbers of cells in seawater samples (∼6 × 106 cells ml−1), but the predominant bacteria in EBr1 should have been CH3Br-degrading bacteria, unlike in natural samples. Bacteria of the type found in the EBr1 culture could thus account for the oxidation rates observed in seawater samples if they constituted only ∼0.3% of the final sample population. If bacteria of the type found in EBr1 had been present at a similar ratio in the initial sample population (before growth), they would have been present at a concentration of about 103 cells/ml. At that cell density, the initial oxidation rates would have been below the limit of detection of the method used in these experiments, which is consistent with the observed lag (Fig. 2B) and with the results of chloramphenicol amendment experiments (Fig. 5C and D).
The effect of chloramphenicol addition on CH3Br oxidation in estuarine and seawater (Fig. 5D) samples raises the possibility that enzyme induction and/or cell growth could cause kapp values to be overestimated in some cases. Overestimation of kapp values due to optimization of the degrading population seems to be most likely for seawater and Mono Lake water samples because the lower oxidation rates and the lag for seawater samples necessitated relatively long incubation times (>24 h) (Fig. 1 and 2). Any correction for cell growth would have lowered the apparent rate constants and would have supported the dominance of abiotic consumption and volatilization over biological oxidation of CH3Br (Table 6).
Lobert et al. (23) demonstrated that large regions of the open ocean are undersaturated with respect to CH3Br, and recent models have implicated a significant biological sink for CH3Br (24). Our results confirm that bacterial oxidation of CH3Br does occur in coastal seawater and that carbonate products are produced. However, the reaction rate (Table 2) obtained in our experiments suggests that biological removal is not greater than chemical consumption. Our reaction rate was almost 10 times lower than that of King and Saltzman (at 21°C) (19), which may reflect geographical differences in microbial density and composition. This difference underscores the need to experimentally assess bacterial oxidation of CH3Br in different ocean regions and cautions against extrapolating results based on nearshore, urban environments to the open ocean.
ACKNOWLEDGMENTS
This work was supported by a National Research Council postdoctoral associateship and by NASA Upper Atmosphere Research Program grant 5188-AU-0080.
We thank B. Jellison, D. Heil, and C. Culbertson for supplying Mono Lake water, T. Connell Hancock, G. Hancock, and D. Hayward for providing coastal seawater, N. Chiariello for providing access to Searsville Lake, and the Moss Beach Marine Preserve and the Long Marine Laboratory for providing access to seawater.
REFERENCES
- 1.Bartnicki E W, Castro C E. Biodehalogenation: rapid oxidative metabolism of mono- and polyhalomethanes by Methylosinus trichosporium OB-3b. Environ Toxicol Chem. 1994;13:241–245. [Google Scholar]
- 2.Benstead J, King G M, Williams H G. Methanol promotes atmospheric methane oxidation by methanotrophic cultures and soils. Appl Environ Microbiol. 1988;64:1091–1098. doi: 10.1128/aem.64.3.1091-1098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broecker W S, Peng T H. Gas exchange rates between air and sea. Tellus. 1974;26:19–35. [Google Scholar]
- 4.Connell T L, Costello A M, Lidstrom M E, Oremland R S. Strain IMB-1: a novel bacterium for the removal of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1998;64:2899–2905. doi: 10.1128/aem.64.8.2899-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell T L, Joye S B, Miller L G, Oremland R S. Bacterial oxidation of methyl bromide in Mono Lake, California. Environ Sci Technol. 1997;31:1489–1495. [Google Scholar]
- 6.Conrad R. Capacity of aerobic microorganisms to utilize and grow on atmospheric trace gases (H2,CO, CH4) In: Klug M J, Reddy C R, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 461–467. [Google Scholar]
- 7.Doronina N V, Krauzova V I, Trotsenko Y A. Methylophaga limanica sp. nov., a new species of moderately halophilic, aerobic, methylotrophic bacteria. Microbiology (Engl Transl Mikrobiologiya) 1997;66:434–439. [Google Scholar]
- 8.Ferguson R L, Buckley E N, Palumbo A V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984;47:49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin K D, Lidstrom M E, Oremland R S. Marine bacterial degradation of brominated methanes. Environ Sci Technol. 1997;31:3188–3192. [Google Scholar]
- 10.Goodwin K D, North W J, Lidstrom M E. Production of bromoform and dibromomethane by giant kelp: factors affecting release and comparison to anthropogenic bromine sources. Limnol Oceanogr. 1997;42:1725–1734. [Google Scholar]
- 11.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey R W. A fluorochrome-staining technique for counting bacteria in saline, organically enriched, alkaline lakes. Limnol Oceanogr. 1987;32:993–995. [Google Scholar]
- 13.Haung, W., and R. Gammon. Personal communication.
- 14.Helz G R, Hsu R Y. Volatile chloro- and bromocarbons in coastal waters. Limnol Oceanogr. 1978;23:858–869. [Google Scholar]
- 15.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffers P M, Wolfe N L. On the degradation of methyl bromide in sea water. Geophys Res Lett. 1996;23:1773–1776. [Google Scholar]
- 17.Jensen S, Priemé A, Bakken L. Methanol improves methane uptake in starved methanotrophic microorganisms. Appl Environ Microbiol. 1998;64:1143–1146. doi: 10.1128/aem.64.3.1143-1146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keener W K, Arp D J. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol. 1993;59:2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King D B, Saltzman E S. Removal of methyl bromide in coastal seawater: chemical and biological rates. J Geophys Res. 1997;102:18715–18721. [Google Scholar]
- 20.Kley D, Crutzen P J, Smit H G J, Vomel H, Oltmans S J, Grassl H, Ramanathan V. Observations of near-zero ozone concentrations over the convective Pacific: effects on air chemistry. Science. 1996;274:230–232. [Google Scholar]
- 21.Kourtidis K, Borchers R, Fabian P. Dibromomethane (CH2Br2) measurements at the upper troposphere and lower stratosphere. Geophys Res Lett. 1996;23:2581–2583. [Google Scholar]
- 22.Leisinger T, Bader R. Microbial dehalogenation of synthetic organohalogen compounds: hydrolytic dehalogenases. Chimia. 1993;47:116–121. [Google Scholar]
- 23.Lobert J M, Butler J H, Montzka S A, Geller L S, Myers R C, Elkins J W. A net sink for atmospheric CH3Br in the east Pacific Ocean. Science. 1995;267:1002–1005. doi: 10.1126/science.267.5200.1002. [DOI] [PubMed] [Google Scholar]
- 24.Lobert J M, Yvon-Lewis S A, Butler J H, Montzka S A, Myers R C. Undersaturation of CH3Br in the southern ocean. Geophys Res Lett. 1997;24:171–172. [Google Scholar]
- 25.Mabey W, Mill T. Critical review of hydrolysis of organic compounds in water under environmental conditions. J Phys Chem Ref Data. 1978;7:383–409. [Google Scholar]
- 26.Manley S L, Goodwin K, North W J. Laboratory production of bromoform, methylene bromide and methyl iodide by macroalgae and distribution in nearshore southern California waters. Limnol Oceanogr. 1992;37:1652–1659. [Google Scholar]
- 27.Mellouki A, Talukdar R K, Schmoltner A, Gierczak T, Mills M J, Solomon S, Ravishankara A R. Atmospheric lifetimes and ozone depletion potentials of methyl bromide (CH3Br) and dibromomethane (CH2Br2) Geophys Res Lett. 1992;19:2059–2062. [Google Scholar]
- 28.Miller L G, Oremland R S. Methane efflux from the pelagic regions of four lakes. Glob Biogeochem Cycles. 1988;2:269–277. [Google Scholar]
- 29.Miller L G, Connell T L, Guidetti J R, Oremland R S. Bacterial oxidation of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1997;63:4346–4354. doi: 10.1128/aem.63.11.4346-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oremland R S, Culbertson C W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992;58:2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oremland R S, Miller L G, Culbertson C W, Connell T L, Jahnke L L. Degradation of methyl bromide by methanotrophic bacteria in cell suspensions and soils. Appl Environ Microbiol. 1994;60:3640–3646. doi: 10.1128/aem.60.10.3640-3646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perdue E M, Wolfe N L. Prediction of buffer catalysis in field and laboratory studies of pollutant hydrolysis reactions. Environ Sci Technol. 1983;17:635–642. doi: 10.1021/es00117a003. [DOI] [PubMed] [Google Scholar]
- 33.Phenning N. Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol. 1978;28:283–288. [Google Scholar]
- 34.Rheinheimer G. Aquatic microbiology. 4th ed. Chichester, United Kingdom: John Wiley & Sons; 1991. p. 144. [Google Scholar]
- 35.Saini H S, Attieh J M, Hanson A D. Biosynthesis of halomethanes and methanethiol by higher plants via a novel methyltransferase reaction. Plant Cell Environ. 1995;18:1027–1033. [Google Scholar]
- 36.Schauffler S M, Heidt L E, Pollock W H, Gilpin T M, Vedder J F, Solomon S, Lueb R A, Atlas E L. Measurements of halogenated organic compounds near the tropical tropopause. Geophys Res Lett. 1993;20:2567–2570. [Google Scholar]
- 37.Scholtz R, Wackett L P, Egli C, Cook A M, Leisinger T. Dichloromethane dehalogenase with improved catalytic activity isolated from a fast-growing dichloromethane-utilizing bacterium. J Bacteriol. 1988;170:5698–5704. doi: 10.1128/jb.170.12.5698-5704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shorter J H, Kolb C E, Crill P M, Kerwin R A, Talbot R W, Hines M E, Harriss R C. Rapid degradation of atmospheric methyl bromide in soils. Nature. 1995;377:717–719. [Google Scholar]
- 39.Singh H B, Salas L J, Stiles R E. Methyl halides in and over the eastern Pacific (40 N - 32 S) J Geophys Res. 1983;88:3684–3690. [Google Scholar]
- 40.Tanhua T, Fogelqvist E, Bastürk Ö. Reduction of volatile halocarbons in anoxic seawater, results from a study in the Black Sea. Mar Chem. 1996;54:159–170. [Google Scholar]
- 41.Tokarczyk R, Moore R M. Production of volatile organohalogens by phytoplankton cultures. Geophys Res Lett. 1994;21:285–288. [Google Scholar]
- 42.Visscher P T, Taylor B F. Demethylation of dimethylsulfoniopropionate to 3-mercaptopropionate by an aerobic marine bacterium. Appl Environ Microbiol. 1994;60:4617–4619. doi: 10.1128/aem.60.12.4617-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widdel F, Kohring G-W, Mayer F. Studies on the dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov., sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol. 1983;134:286–294. [Google Scholar]
- 44.World Meteorological Organization. Scientific assessment of ozone depletion: 1994. World Meteorological Organization Global Ozone Research and Monitoring Project-Report no. 37. Geneva, Switzerland: World Meteorological Organization; 1995. [Google Scholar]
- 45.Zhang D Q, Zhong J X, Qiu L X. Kinetics of the reaction of hydroxyl radicals with CH2Br2 and its implication in the atmosphere. J Atmos Chem. 1997;27:209–215. [Google Scholar]