Abstract
The growth and culturability of Campylobacter jejuni NCTC 11351 and other campylobacters were examined in media having different osmolalities at a range of temperatures (4, 25, and 42°C). The medium osmolalities used ranged from the osmolality of full-strength nutrient medium (modified campylobacter broth having an osmolality of around 254 mosmol) down to 96 mosmol. The following two methods were used to produce media having different osmolalities: dilution of the nutrient medium with distilled water and reformulation of the medium such that the concentrations of various osmolytes were altered while the nutrient content of the medium was unchanged. The results obtained with the two experimental methods were similar, indicating that there was an osmotic threshold effect, such that none of the campylobacters examined (C. jejuni NCTC 11351 and ATCC 33291, Campylobacter lari, and Campylobacter coli) grew in media having osmolalities around 130 mosmol and at temperatures below at 42°C. Conversely, growth occurred in media having osmolalities of around 175 mosmol and above. Osmolar concentrations can be expressed in terms of osmolarity or osmolality. Osmolality is easier to evaluate, is the more commonly used term, and was used in the current study. In nutrient media having low osmolalities (i.e., 130 mosmol and below), the number of CFUs per milliliter declined rapidly regardless of the temperature, and no cells were recovered after 24 h. However, at nongrowth temperatures (25 and 4°C) in higher-osmolality media (175 mosmol and above) a significant population was recovered throughout the experiment (up to 96 h). In low-osmolality nutrient media, the cellular morphology was principally coccoid, while in the early stages of growth in full-strength media the morphology was predominantly rodlike. We propose that the formation of coccoid cells in these experiments was the result of osmotic stress in low-osmolality media. This osmotic effect was apparent regardless of the osmolyte used to reformulate the medium (NaCl, KCl, Na2SO4, NH4Cl, and glucose were used).
Campylobacter jejuni is recognized as a leading cause of acute bacterial gastroenteritis in humans. This organism is a microaerophile which grows optimally at 42°C, and complex media are required for recovery of C. jejuni from clinical and environmental sources (3, 36). C. jejuni is recognized as a leading food-borne pathogen (27, 29, 34, 35). Several studies have found that the rate of isolation of C. jejuni from stools of hospitalized, diarrheic patients is comparable to the rate of isolation of Salmonella spp. During the last 10 years, marked increases in the incidence of human enteric campylobacteriosis have been reported in many countries (34). Campylobacter species cause a wide spectrum of infections besides human diarrheal disease, including reproductive disorders in domestic animals and opportunistic infections in immunocompromised human patients (6, 7, 12).
A variety of foods, including raw milk, poultry, and pork, have been implicated as vehicles for transmission of campylobacters to susceptible individuals. Foods of animal origin are of particular concern, because many animals harbor C. jejuni as part of the normal intestinal flora; for example, C. jejuni is frequently associated with chicken products (10, 20, 29). A model for predicting the survival of C. jejuni in various foods has been developed (13).
Several morphological forms of C. jejuni are found in cultures (15). Rod-shaped forms (26), including spiral, S-shaped, and characteristically curved cells, predominate in fresh young cultures, whereas nonculturable, coccoid forms occur mainly in old cultures. In addition, rods transform to coccoid forms when conditions are unfavorable for growth. The transition from spiral to coccoid forms is influenced by many factors, such as bacterial strain, temperature, pH, medium, etc. (8). A number of studies have shown that it is difficult to recover certain coccoid forms of C. jejuni by conventional culture techniques (5, 26), and although forms are nonculturable, they may be viable (32).
When enteric and pathogenic bacteria are released from their hosts into natural environments (9, 16, 23), they are often challenged by various environmental stresses, such as nutrient starvation, osmotic shock, temperature variation, oxidative stress, etc. (8, 28, 30, 31, 33). Greater understanding of the effects of environmental stresses on C. jejuni is urgently required. As a fastidious pathogen, C. jejuni normally grows only in vivo, and transmission to a new host often involves a period of exposure to a hostile external environment. How C. jejuni cells cope with such exposure and the possible role of the so-called viable nonculturable form are poorly understood at this time. New information concerning the influence of environmental factors on the physiology of C. jejuni cells should help increase our understanding of how these organisms survive and retain infectivity in natural environments and may also help in the development of improved methods for the resuscitation and recovery of environmentally stressed cells. A number of environmental factors have been examined previously, but there have been no reports on the behavior of C. jejuni in nutrient media having low osmolalities.
In this study we examined the responses of several Campylobacter species to exposure to media having osmolalities ranging from the osmolality of distilled water (2 mosmol) to the osmolality of the standard growth medium used for Campylobacter spp. (i.e., full-strength broth) (251 to 257 mosmol). Osmolality is defined as the number of moles of solute present per kilogram of solvent (14). In all of our experiments, the test medium was incubated in a microaerobic environment. Medium osmolality was altered both by diluting whole broth and, more rigorously (since diluting whole broth could not exclude the possibility that nutrient dilution had an effect on the cultures), by altering the concentrations of a number of salts (NaCl, NH4Cl, KCl) or glucose (which is not metabolized by Campylobacter species) while maintaining the concentrations of nutrient sources in the medium. The osmolalities of all of the media formulated were analyzed by using a freezing point depression osmometer, and culture responses were characterized by determining changes in the number of CFUs and by light and electron microscopy observation.
In addition, since environmental temperature may also have a significant effect on growth and survival in such environments, we examined temperatures ranging from a refrigeration temperature through a high ambient temperature (25°C) to the normal growth temperature (42°C).
This paper describes how adjusting the osmolality of the medium by simple dilution or by using osmolytes affects the growth and culturability of C. jejuni and other Campylobacter species.
MATERIALS AND METHODS
Test strain.
The test strain of C. jejuni NCTC 11351, Campylobacter coli NCTC 11350, and Campylobacter lari 11352 were obtained from the Central Public Health Laboratory, London, England, as freeze-dried ampoules. C. jejuni ATCC 33291 was obtained from Unipath, Basingstoke, United Kingdom, in cultiloops. The strains were maintained in brucella broth containing 15% glycerol at −70°C. Brucella broth (Difco Laboratories, Detroit, Mich.) contained (per liter) 10 g of Bacto Tryptone (Difco), 10 g of Bacto Peptamin (Difco), 1 g of Bacto Dextrose (Difco), 2 g of Bacto Yeast Extract (Difco), 5 g of sodium chloride, and 0.1 g of sodium bisulfite. Culturable colonies were prepared by culturing organisms on modified charcoal-cefoperazone-deoxycholate agar (CCDA) plates (Unipath) at 42°C for 48 h in a microaerobic environment generated with CampyPak gas generators (Unipath) generating 5% O2, 10% CO2, and 85% N2.
Preparation of inocula.
Inocula were prepared by transferring well-isolated 48-h-old colonies of the Campylobacter spp. from streaked CCDA plates into culture tubes containing 5.5 ml of modified campylobacter broth (MCB); the cultures in the tubes were then incubated for 18 h at 42°C in a microaerobic atmosphere before they were used as inocula. MCB contained (per liter) 10 g of Bacto Tryptone (Unipath), 10 g of Bacto Peptone (Difco), 2 g of Bacto Yeast Extract (Difco), and 5.0 g of NaCl.
Preparation of test medium.
In one set of experiments the medium osmolality and nutrient content were altered by diluting MCB with distilled water; 5.5-ml portions of test media having different strengths were prepared in 12-ml glass tubes with caps which permitted free gaseous exchange with the environment in the incubation jar (1). The tubes were incubated under microaerobic conditions (5% O2, 10% CO2, and 85% N2) by using CampyPak gas generators (Unipath) in a sealed jar at temperatures of 4, 25, and 42°C. Samples were taken every 12 h for 96 h.
In a second set of experiments the osmolality of MCB was varied by altering the concentrations of specific osmolytes. Media with osmolalities of 251 to 257, 168 to 174, 127 to 133, 111 to 117, and 91 to 97 mosmol were prepared, and sterile distilled water with an osmolality of 2 to 3 mosmol was also used. Osmolality was varied by altering the amount of salt or glucose used in the medium. As determined by both calculations and osmolality measurements, the required osmotic values of the medium were obtained. The approximate osmotic values were intended to be close to the osmotic values of the media generated by dilution.
Osmolality measurements.
The actual osmolal concentrations of the media were determined with a freezing point osmometer (model 3D3; Advanced Instruments Inc., Norwood, Mass.). Known standards having osmolalities of 100, 500, and 900 mosmol, supplied by the osmometer manufacturer, were used to calibrate the instrument.
Plate counts.
Serial dilutions of the broth cultures were made in diluents having the same osmolalities as the test media, and triplicate 0.1-ml aliquots of the cultures were spread plated onto CCDA plates. The plates were incubated at 42°C under microaerobic (CampyPak) conditions for 48 h, and the CFUs were counted.
Statistical analysis.
All of the experiments in this study were performed in triplicate. The effects of different osmolalities of the media on the growth and survival of the organisms were calculated with 95% confidence intervals by analysis of variance. The statistical calculations were performed with the software package Microcal Origin 5.0 (Microcal Software, Inc.).
Electron microscopy.
Test media were centrifuged (10 min, 10,000 × g, 4°C), and the supernatants were discarded. The pellets were washed with phosphate-buffered saline twice and fixed with 2.5% glutaraldehyde (Sigma-Aldrich). Cells were then filtered onto 0.2-μm-pore-size Isopore GTTP membrane filters (Millipore). The cells were dehydrated once in 50, 70, 80, and 90% ethanol and twice in 100% ethanol, treated with 100% isoamyl acetate, and critical point dried. Finally, cells were sputter coated with 15-nm Au particles and viewed with a model JSM-T200 scanning electron microscope (JEOL).
RESULTS
Simple dilution of the culture medium had a dramatic effect on growth. To investigate this effect, C. jejuni was cultured in a range of dilutions of MCB, and the osmolalities of full-strength and diluted media were recorded.
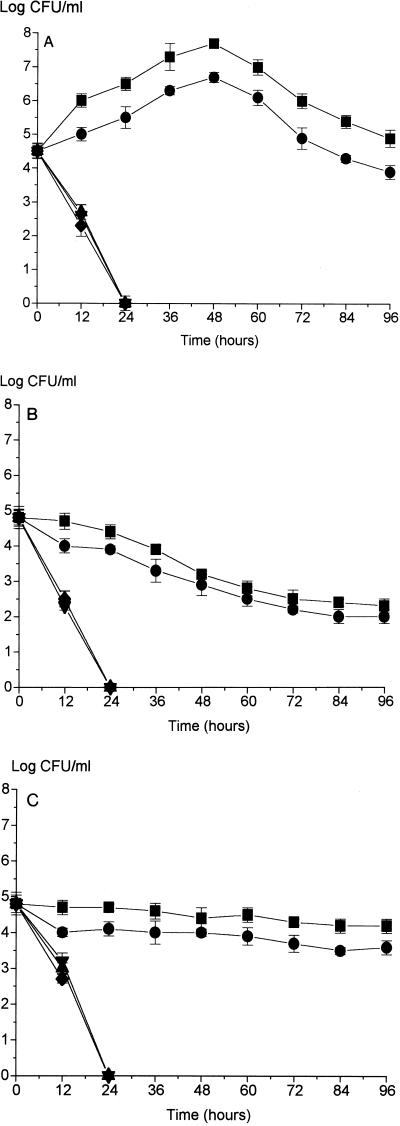
In order to examine the effect of temperature on cultures, experiments were carried out at the normal growth temperature (42°C), at a high ambient temperature (25°C), and at a refrigeration temperature (4°C); both 25 and 4°C are temperatures at which C. jejuni does not grow. The culture response was evaluated in terms of CFU plate counts and morphology for 96 h. The data obtained at 42, 25, and 4°C are shown in Fig. 1A, B, and C, respectively.
FIG. 1.
Growth of C. jejuni NCTC 11351 at 42°C (A), 25°C (B), and 4°C (C) in media having differing osmolalities. The basal medium used was MCB, and media having differing osmolalities were produced by diluting MCB. Symbols: ■, 257 to 263 mosmol; •, 172 to 178 mosmol; ▴, 122 to 128 mosmol; ▾, 114 to 117 mosmol; ⧫, 93 to 99 mosmol.
In normal-osmolality media (i.e., media having osmolalities of 172 to 178 mosmol and above) growth occurred in the first 48 h and then there was a decline in the number of recoverable cells (Fig. 1A). In half-strength medium (122 to 128 mosmol) and in media having lower osmolalities there was no apparent growth, and a rapid decline in the CFU count occurred from time zero.
As expected, growth did not occur at 25°C even in full-strength and three-quarter-strength media (Fig. 1B). However, the rates of decline in the CFU counts in normal-osmolality media were markedly lower (P ≤ 0.05) than the rates of decline in media having osmolalities of ∼122 to 128 mosmol and below. In the latter media, no colony-forming cells were recovered after 24 h (similar to the results obtained at 42°C). The patterns of decline were very similar in all media having osmolalities that were less than approximately 122 to 128 mosmol (half-strength and below). By 72 h the rate of decline had decreased in the normal-osmolality media.
At 4°C (Fig. 1C), there was only a slight decline in the number of CFUs per milliliter over the experimental time period in normal-osmolality media, while in half-strength media and below the decline in the CFU counts was significantly greater (P ≤ 0.05) and followed the pattern observed at higher temperatures.
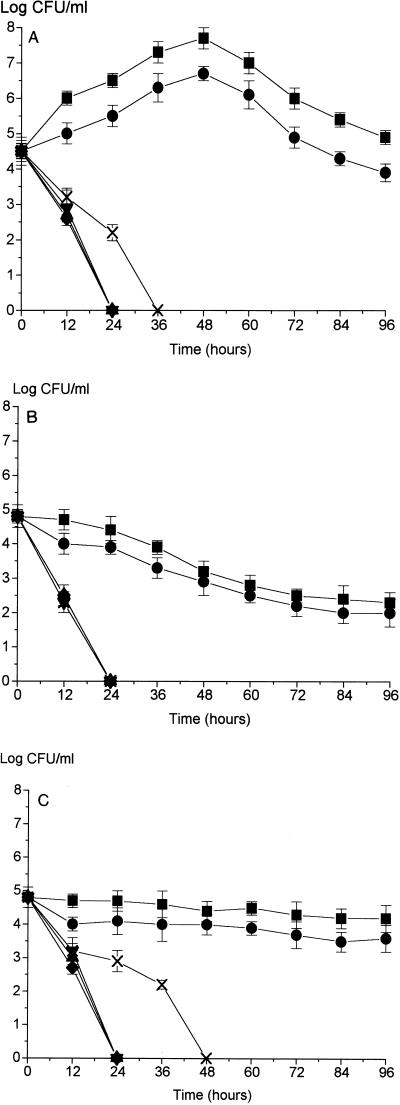
In a second series of experiments, media having differing osmolalities were produced by varying the amounts of NaCl added to a basic nutrient medium. This produced a range of media having osmolalities broadly similar to those shown in Fig. 1A to C. The data obtained at 42, 25, and 4°C in this series of experiments are shown in Fig. 2A, B, and C, respectively.
FIG. 2.
Growth of C. jejuni NCTC 11351 at 42°C (A), 25°C (B), and 4°C (C) in media having differing osmolalities. The basal medium used was MCB, and media having differing osmolalities were produced by altering the amount of NaCl in MCB. Symbols: ■, 251 to 257 mosmol; •, 168 to 174 mosmol; ▴, 127 to 133 mosmol; ▾, 111 to 114 mosmol; ⧫, 91 to 97 mosmol; ×, sterile distilled water (2 to 3 mosmol).
At 42°C (Fig. 2A) a distinct pattern was apparent. Growth of the culture in normal-strength media (≥168 mosmol) was followed by a decline, but growth was not apparent and a rapid decline in culturability occurred in media having low osmolalities (≤127 mosmol). The pattern observed was very similar to the pattern observed in the dilution series experiments. For comparison, the culture response in distilled water was also examined. The number of CFUs per milliliter declined in distilled water, but they declined less rapidly (P ≤ 0.05) than the number of CFUs per milliliter declined in nutrient media having low osmolalities (the culturability was zero after 36 h in distilled water and after 24 h in the nutrient media).
Figures 2B and C show responses by the cultures to medium osmolality that are broadly similar to the responses observed in the dilution series experiments (Fig. 1B and C). Greater maintenance of culturability (P ≤ 0.05) occurred in normal-osmolality media than in nutrient media having low osmolalities. Again, the increased culturability of C. jejuni NCTC 11351 in distilled water at 4°C compared to the culturability in low-osmolality nutrient media was clear (Fig. 2C); the times until there were no recoverable CFUs were 48 h in distilled water and 24 h in the low-osmolality nutrient media.
In additional tests we established that the critical medium osmolality for the test strain used (C. jejuni NCTC 11351) when it was grown at 42°C was 143 to 146 mosmol. At this value culturability was maintained, and very slight growth (as determined by CFU plate counts) occurred over a 24-h period at 42°C under microaerobic conditions.
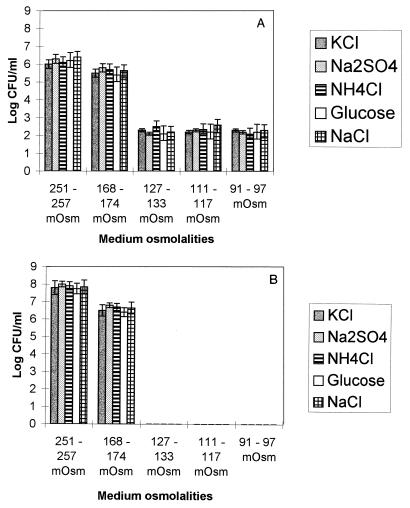
In order to determine if the nature of the osmolyte affected the culture response, media were prepared with different osmolytes, including a number of salts (KCl, Na2SO4, and NH4Cl) and a sugar (glucose).
The results of this experiment are shown in Fig. 3; medium prepared with NaCl was used for comparative purposes. There was a clear pattern of growth in media having normal osmolalities, and there were rapid declines in culturability in low-osmolality nutrient media, regardless (P ≤ 0.05) of the osmolyte used in the medium.
FIG. 3.
CFU plate counts for C. jejuni NCTC 11351 at 42°C in MCB having differing osmolalities after 12 h (A) and 24 h (B). At each osmolality, MCB was reformulated by altering the amount of one osmolyte (salt or glucose). The CFU counts were zero after 24 h in media having osmolalities of 127 to 133 mosmol or less.
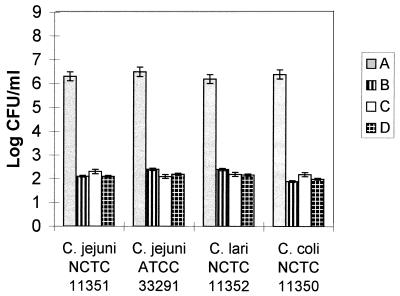
To examine whether this behavior was a characteristic of strain NCTC 11351 of C. jejuni, the responses of another C. jejuni strain (ATCC 33291) and two other Campylobacter species (C. lari and C. coli) were determined in normal- and low-osmolality nutrient media at an incubation temperature of 42°C. The numbers of CFUs per milliliter after 12 h of incubation in nutrient media having normal and low osmolalities and prepared with KCl for the various species are shown in Fig. 4. Whereas good growth of all four strains occurred in normal-osmolality media, the CFU counts decreased below the inoculum levels in media having osmolalities of ≤127 mosmol after 12 h of incubation, and no CFUs were recovered after 24 h (data not shown). The same result (P ≤ 0.05) was obtained with all four strains when NH4Cl or glucose was used as the osmolyte (data not shown).
FIG. 4.
CFU plate counts for different Campylobacter spp. after 12 h of incubation in MCB having differing osmolalities (osmalality was adjusted by using KCl as the osmolyte). A, 251 to 257 mosmol; B, 127 to 133 mosmol; C, 111 to 117 mosmol; D, 91 to 97 mosmol.
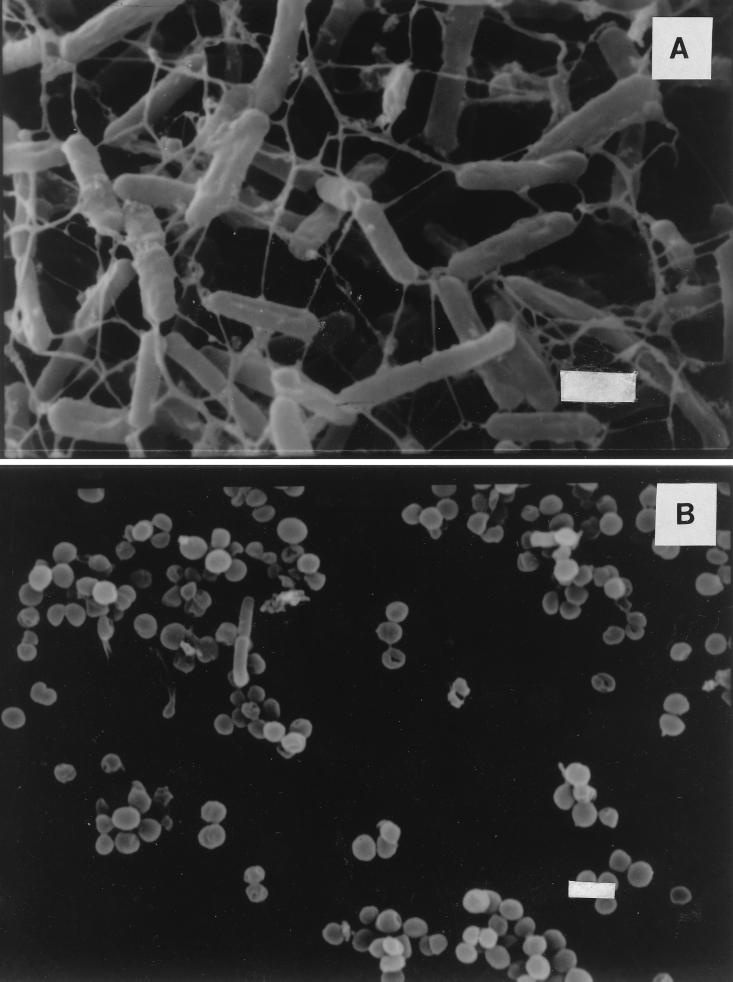
The morphology of C. jejuni NCTC 11351 cultures in media having different osmolalities was examined by scanning electron microscopy. Figure 5A shows the predominantly rodlike appearance of cells in full-strength nutrient medium after 18 h of incubation, while Fig. 5B shows the typical coccoid appearance of C. jejuni cells in nutrient media having low osmolalities after 18 h.
FIG. 5.
Scanning electron micrographs of C. jejuni NCTC 11351 in a medium having an osmolality between 251 and 257 mosmol, showing predominantly rodlike cells (A), and in a medium having an osmolality of 111 to 117 mosmol, showing predominantly coccoid cells (B), after 18 h of incubation at 42°C. Bars = 1 μm.
DISCUSSION
In this study, we used two methods to alter medium osmolality, simple dilution and medium reformulation. The latter method was used to eliminate the possibility that the data resulting from medium dilution might have been due to a nutritional effect instead of an osmotic effect. The results produced by the two experimental regimens were very similar and were consistent with the existence of an osmotic threshold for growth of C. jejuni at 42°C. The results established that for the main strain used (C. jejuni NCTC 11351) the critical medium osmolality was 143 to 146 mosmol at 42°C under microaerophilic growth conditions. To our knowledge, this is the first report of the impact of medium osmolality on C. jejuni at a range of temperatures.
Previous research on the effect of NaCl on C. jejuni has been concerned mainly with inhibitory effects of high salt concentrations, such as those used in food preservation. Abram and Potter (1) found that the survival of this organism in foods held at refrigeration temperatures decreased as the sodium chloride concentration was increased from 0 to 2%, while there was a more rapid decline in numbers at room temperature. More recent predictive microbiology work, performed to develop the Food Micromodel, has examined the inhibitory effects of high salt concentrations on the growth of Campylobacter spp. (2).
We also found in this study that culturability (as determined by CFU plate counts) was maintained better in media having normal osmolalities than in low-osmolality media at lower temperatures, especially 4°C (Fig. 1C and 2C). This is consistent with the findings of Rollins and Colwell (32), who studied the viable but nonculturable state of C. jejuni and postulated that at higher temperatures (37 and 25°C) use of the available substrates was more rapid and occurred at the expense of other functions, leading to a more rapid decline in culturability. The transition to the unculturable state was said to be logarithmic at these higher temperatures.
It has been reported previously (17) that aging of rodlike or spiral C. jejuni cells in either nutrient-poor or nutrient-rich environments results in the formation of nonculturable coccoid cells at 4, 12, and 25°C after different periods of time, with the cells incubated at 4°C in nutrient-deficient media remaining culturable the longest. The fatty acid composition of cocci formed at 4°C was remarkably similar to the fatty acid composition of spiral cells, and it was suggested that the cocci could well play a role in the contamination cycle of C. jejuni (17).
In the present study, we observed that at 4°C and medium osmolalities of 254 and 171 mosmol cells could still be recovered after 4 days, whereas at medium osmolalities of 130 mosmol and below there was a rapid decline in the culturability of the organism. The low osmotic values also caused a change in morphology from a rod shape to a coccoid shape (Fig. 5A and B), which may have been a stress response (32).
It is interesting that Rollins and Colwell (32) recovered C. jejuni over a period of time in stream water, but in the present study cells could not be recovered after 48 h when they were inoculated into sterile distilled water (osmolality, 2 ± 1 mosmol) in a microaerobic environment at 4°C. Humphrey (18) also reported that when water samples were inoculated with C. jejuni, there was a rapid decline in the number of viable cells when the cultures were kept at refrigeration temperatures (1 to 4°C).
The rapid decline in culturability in water samples in our experiments might have been due to exposure to microaerophilic conditions, which, while commonly used to grow Campylobacter species, may not be suitable for survival of Campylobacter species in water. Buswell et al. (11) also reported that there are variations in the survival of C. jejuni strains in water. They noted that in an anaerobic environment, C. jejuni strains survived poorly. It has also been suggested that C. jejuni is capnophilic rather than microaerophilic (24) since growth can occur in moist air containing 10% CO2. Variation in the gas atmospheres used to recover campylobacters and variation in the gaseous conditions to which they were exposed in liquid systems may help explain the variations in the results obtained in previous studies.
In order to examine the possibility that the results obtained in this study were specifically caused by NaCl, we performed experiments in which the osmolality of the medium was altered by using a number of different osmolytes (Fig. 3A and B). The results were similar to the results obtained in media prepared with NaCl, confirming that for the osmolytes examined, the type of osmolyte had little or no impact on growth in normal-osmolality media (half-strength media and above) or maintenance of culturability in low-osmolality nutrient media (half-strength media and below), again indicating that the effects observed were the result of an osmotic effect rather than the concentrations of specific ionic species.
Our experiments showed (Fig. 4) that the osmotic threshold effect is not restricted to C. jejuni NCTC 11351, since C. jejuni ATCC 33291 and the two other Campylobacter species examined (C. coli and C. lari) also failed to grow in low-osmolality media and, as observed with C. jejuni NCTC 11351, no CFUs were recovered after 24 h in these media. Overall, the responses to the osmolytes and temperatures tested were very similar for the different Campylobacter strains tested.
It should be noted that although in the present study exposure of the test species to nutrient media having low osmolalities (i.e., half-strength media and below) led to a complete lack of culturability within 24 h, this should not be considered necessarily equivalent to complete culture death at all temperatures. It is possible that exposure to such media may have subjected the organisms to an osmotic stress, making them increasingly difficult to recover as the duration of the stress increased. The coccoid morphology shown in Fig. 5B, which is typical of the morphology of cultures in low-osmolality nutrient media, may well be indicative of a viable, nonculturable form.
Rapid declines in the culturability of C. jejuni could be the result of losing cytoplasmic contents due to exposure to low-osmolality nutrient media. Moran and Upton (25) reported that cytoplasmic contents leaked from C. jejuni cells during morphological transformation from rod-shaped or spiral cells to coccoid, nonculturable cells. These authors reported that coccoid forms represented a degenerate cell form. The loss of cytoplasmic contents by bacterial cells was said to indicate both damage to the cell membrane and degradation of cellular components (28). Baker and Park (4) reported that formation of spheres in Vibrio sp. strain NCTC 4176 cultures correlated with changes in the peptidoglycan of the cell wall. Moran and Upton (25) described changes in the peptidoglycan of the cell wall of coccoid forms of C. jejuni. Based on these reports, it is likely that at low medium osmolalities coccoid morphology of C. jejuni is an indication that some cellular degradation has occurred.
It is recognized that much remains to be learned about the physiology of Campylobacter species. Factors that influence the survival of these organisms in the environment and the recovery of campylobacters in vivo are not well-understood, and workers have referred to a so-called viable but nonculturable form (19, 21, 22). The results presented in this study indicate that Campylobacter species are particularly poor at osmoadaptation to low-osmolality nutrient conditions. Further studies are required to explain this unusual feature and to determine the significance of this characteristic in the infection cycle of these important pathogens.
ACKNOWLEDGMENT
A.R. acknowledges the Universiti Putra Malaysia for receipt of a scholarship.
REFERENCES
- 1.Abram D D, Potter N N. Survival of Campylobacter jejuni at different temperatures in broth, beef, chicken and cod supplemented with sodium chloride. J Food Prot. 1984;47:795–800. doi: 10.4315/0362-028X-47.10.795. [DOI] [PubMed] [Google Scholar]
- 2.Advisory Committee on the Microbiological Safety of Foods. Interim report on Campylobacter. London, United Kingdom: Her Majesty’s Stationery Office; 1993. [Google Scholar]
- 3.Baggerman W I, Koster T. A comparison of enrichment and membrane filtration methods for isolation of Campylobacter from fresh and frozen foods. Food Microbiol. 1992;9:87–94. [Google Scholar]
- 4.Baker D A, Park R W. Changes in morphology and cell wall structure that occur during growth of Vibrio sp. NCTC 4716 in batch culture. J Gen Microbiol. 1975;86:12–28. doi: 10.1099/00221287-86-1-12. [DOI] [PubMed] [Google Scholar]
- 5.Beumer R R, De Vries J, Rombous F M. Campylobacter jejuni nonculturable coccoid cells. Int J Food Microbiol. 1992;15:153–163. doi: 10.1016/0168-1605(92)90144-r. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M J. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176:S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 7.Bolton F J, Wareing D R A, Sails A D. Comparison of a novel microaerobic system with three other gas-generating systems for the recovery of Campylobacter species from human faecal samples. Eur J Clin Microbiol. 1997;16:839–842. doi: 10.1007/BF01700415. [DOI] [PubMed] [Google Scholar]
- 8.Boucher S N, Slater E R, Chaberlain A H L, Adams M R. Production and viability of coccoid forms of Campylobacter jejuni. J Appl Bacteriol. 1994;77:303–307. doi: 10.1111/j.1365-2672.1994.tb03078.x. [DOI] [PubMed] [Google Scholar]
- 9.Brenhorvd O, Kapperud G, Langeland G. Survey of thermotolerant Campylobacter spp. and Yersinia spp. in three surface water sources in Norway. Int J Food Microbiol. 1992;15:327–338. doi: 10.1016/0168-1605(92)90066-c. [DOI] [PubMed] [Google Scholar]
- 10.Bryan F L, Doyle M P. Health risks and consequences of Salmonella and Campylobacter jejuni in raw poultry. J Food Prot. 1995;58:326–344. doi: 10.4315/0362-028X-58.3.326. [DOI] [PubMed] [Google Scholar]
- 11.Buswell C M, Herlihy Y M, Lawrence L M, McGuiggan J T M, Marsh P D, Keevil C W, Leach S A. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and rRNA staining. Appl Environ Microbiol. 1998;64:733–741. doi: 10.1128/aem.64.2.733-741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butzler, J. P. 1982. Campylobacter enteritis. Infection 10(Suppl.):S67–S69. [DOI] [PubMed]
- 13.Curtis L M, Patrick M, Blackburn C de W. Survival of Campylobacter jejuni in foods and comparison with a predictive model. Lett Appl Microbiol. 1995;21:194–197. doi: 10.1111/j.1472-765x.1995.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 14.Dufour D R. Osmometry. The rational basis for use of an underappreciated diagnostic tool. New York, N.Y: American Association for Clinical Chemistry Meeting; 1993. [Google Scholar]
- 15.Griffiths P L. Morphological changes of Campylobacter jejuni growing in liquid culture. Lett Appl Microbiol. 1993;17:152–155. doi: 10.1111/j.1472-765x.1993.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanninen M L, Niskanen M, Korhonen L. Water as a reservoir for Campylobacter jejuni infection in cows studied by serotyping and pulsed-field gel electrophoresis (PFGE) J Vet Med. 1998;45:37–42. doi: 10.1111/j.1439-0450.1998.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 17.Hazelger W C, Janse J D, Koenraad P M F J, Beumer R R, Rombouts F M, Abee T. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl Environ Microbiol. 1995;61:2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphrey T J. Techniques for the optimum recovery of cold injured Campylobacter jejuni from milk and water. J Appl Bacteriol. 1986;61:125–132. doi: 10.1111/j.1365-2672.1986.tb04265.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones D M, Sutcliffe E M, Curry A. Recovery of viable but nonculturable Campylobacter jejuni. J Gen Microbiol. 1991;137:2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- 20.Lander K P. Agriculture, Campylobacter. Luxembourg, Luxembourg: Office for Official Publications of the European Communities; 1985. [Google Scholar]
- 21.McKay A M. Viable but nonculturable forms of potentially pathogenic bacteria in water. Lett Appl Microbiol. 1992;14:129–135. [Google Scholar]
- 22.Medema G J, Schets F M, Van de Giessen A W, Havelaar A H. Lack of colonization of 1 day old chicks by viable, nonculturable Campylobacter jejuni. J Appl Bacteriol. 1992;72:512–516. doi: 10.1111/j.1365-2672.1992.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 23.Mentzing L O. Waterborne outbreaks of Campylobacter enteritis in Central Sweden. Lancet. 1981;ii:352–353. doi: 10.1016/s0140-6736(81)90658-9. [DOI] [PubMed] [Google Scholar]
- 24.Mihowich M F, Brooks B W, Joshi S H, Yamazaki H. A liquid medium for growth of the thermophilic campylobacters in a carbon dioxide air mixture as studied by polymyxin-cloth enzyme immunoassay. Food Microbiol. 1998;15:119–125. [Google Scholar]
- 25.Moran A P, Upton M E. A comparative study of the rod and coccoid forms of Campylobacter jejuni ATCC 29428. J Appl Bacteriol. 1986;60:103–110. doi: 10.1111/j.1365-2672.1986.tb03366.x. [DOI] [PubMed] [Google Scholar]
- 26.Moran A P, Upton M E. Factors affecting production of coccoid forms by Campylobacter jejuni on solid media during incubation. J Appl Bacteriol. 1987;62:527–537. doi: 10.1111/j.1365-2672.1987.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 27.Notermans S, Hoogenboom-Verdegaal A. Existing and emerging foodborne diseases. Int J Food Microbiol. 1992;15:197–205. doi: 10.1016/0168-1605(92)90049-9. [DOI] [PubMed] [Google Scholar]
- 28.Palumbo S A. Heat injury and repair in Campylobacter jejuni. Appl Environ Microbiol. 1984;48:477–480. doi: 10.1128/aem.48.3.477-480.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penner J L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988;1:157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rautelin H, Koota K, Essen R, Jahkola M, Siitonen A, Kosunen T U. Waterborne Campylobacter jejuni epidemic in a Finnish hospital for rheumatic diseases. Scand J Infect Dis. 1990;22:321–326. doi: 10.3109/00365549009027054. [DOI] [PubMed] [Google Scholar]
- 31.Rogol M, Sechter I, Falk H, Shtark Y, Alfi S, Greenberg Z, Mizrachi R. Waterborne outbreak of Campylobacter enteritis. Eur J Clin Microbiol. 1983;2:588–590. doi: 10.1007/BF02016571. [DOI] [PubMed] [Google Scholar]
- 32.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha S K, Saha S, Sanyal S C. Recovery of injured Campylobacter jejuni cells after animal passage. Appl Environ Microbiol. 1991;57:3388–3389. doi: 10.1128/aem.57.11.3388-3389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 36.Tran T T. A blood-free enrichment medium for growing Campylobacter spp. under aerobic conditions. Lett Appl Microbiol. 1998;26:145–148. doi: 10.1046/j.1472-765x.1998.00295.x. [DOI] [PubMed] [Google Scholar]