Abstract
Carbonic anhydrases IX and CAXII (CAIX/CAXII) are transmembrane zinc metalloproteins that catalyze a very basic but crucial physiological reaction: the conversion of carbon dioxide into bicarbonate with a release of the proton. CA, especially CAIX and CAXII isoforms gained the attention of many researchers interested in anticancer drug design due to pivotal functions of enzymes in the cancer cell metastasis and response to hypoxia, and their expression restricted to malignant cells. This offers an opportunity to develop new targeted therapies with fewer side effects. Continuous efforts led to the discovery of a series of diverse compounds with the most abundant sulphonamide derivatives. Here we review current knowledge considering small molecule and antibody-based targeting of CAIX/CAXII in cancer.
Keywords: Cancer, carbonic anhydrase, inhibitor, sulphonamides
Graphical Abstract
Introduction
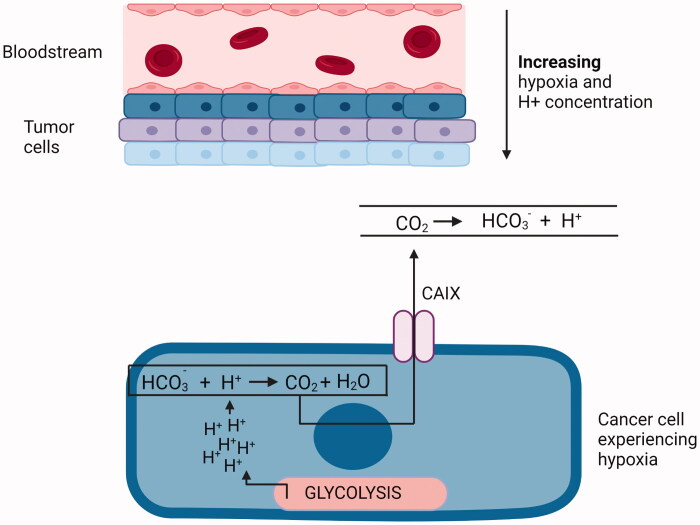
Carbonic anhydrases (CAs; EC 4.2.1.1) constitute a class of zinc metalloproteins that catalyze a very basic but crucial physiological reaction: the conversion of carbon dioxide into bicarbonate with a release of proton1. CAs are involved in the transfer of CO2 and protons through biological membranes, including intercellular, intracellular, and extracellular spaces (Figure 1). In addition to pH regulation and ion transport, CAs are involved in the bone resorption and the secretion of gastric, cerebrospinal fluid, and pancreatic juice2. Carbonic anhydrase IX and XII (CAIX and CAXII) are among the 16 isoforms of the carbonic anhydrases family that are expressed in the human body3,4.
Figure 1.
Cancer cells experiencing hypoxia undergo glycolytic switch and rely mainly on glycolysis as the source of energy. Protons generated during this process lead to progressive acidification of the intracellular environment. To maintain optimal pH inside the cell CAIX expression is increased. This enzyme converts CO2 and H2O to HCO3− and H+ regulating intracellular pH4. Created with BioRender.com
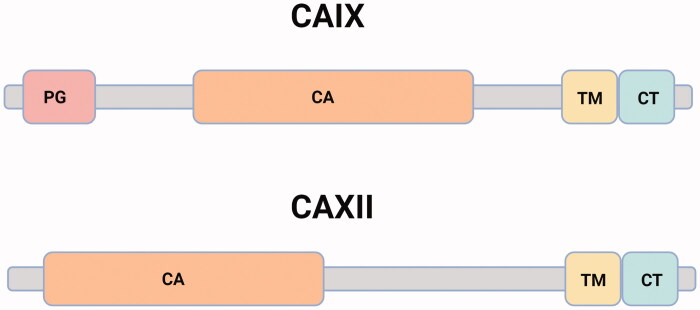
The CAIX protein is encoded by the CA9 gene located in chromosomal locus 9p12–13 and comprises 11 exons that code for different structural domains of the protein5,6. The CAXII protein is encoded by the CA12 gene located in chromosomal locus 15q22 and comprises 13 exons, but the mature protein lacks the proteoglycan-like (PG) region7. In the structure of membrane-associated CAs four main domains can be distinguished: topological extracellular, helical transmembrane, cytoplasmic domains, and proteoglycan-like region (Figure 2)8,9.
Figure 2.
Schematic domain organization of CAIX and CAXII. CAIX: The proteoglycan-like domain, PG (residues 53–111, pink); the catalytic domain, CA (residues 137–391, orange); the transmembrane segment, TM (residues) 415–433, yellow) and the intracellular C-terminal domain, CT (residues 434–459, blue)8,10. CAXII is shorter in length than CAIX and lacks the PG. It consists of 4 distinct domains including a signal peptide (not shown here), N terminus extracellular CA domain (1–269), a TM domain (270–296), and a CT domain (297–325)9,11. Created with BioRender.com
The CAIX works as a dimeric molecule composed of two monomeric proteins liked with a disulfide bond between cysteine residues of the two CAIX monomers, while the structure of CAXII is stabilized by 19 hydrogen-bonded interactions in the dimer interface. The details can be found elsewhere12. In all CAs, the catalytic domains exhibit a three-dimensional fold, which is predominately composed of beta-strands. Histidine residues in the catalytic domain of the enzyme coordinate with Zn2+ in a tetrahedral manner1,12. The histidine residue of the enzyme's active site is stabilized by a hydrophilic region adjacent to it. The CO2 molecule is nucleophilically attacked by a Zn-bound OH in the first stage of CA catalysis resulting in the formation of HCO3-. HCO3 ion is displaced by a water molecule and released into solution. Because of a highly conserved proton transfer event, the Zn-bound water regenerates back to OH-. The efficacy of these enzymes is determined by the rate at which proton shuttles during the two steps of the catalytic mechanism3. PG-like domain and intracellular tail have been found to be crucial in modifying CAIX's catalytic activity. CAIX remains active at low pH values that would kill most enzymes. This is attributed to the presence the PG-like domain13. On the other hand, the extracellular catalytic domain's function was found by mutagenesis of a cluster of basic amino acids in the intracellular tail, suggesting that the cytoplasmic tail is involved in inside-out signaling14. The intracellular tail also has three phosphorylation sites: threonine 443 (443T), serine 448 (448S), and tyrosine 449 (449Y). 449Y is involved in epidermal growth factor (EFGR)-induced signaling to RAC-alpha serine/threonine-protein kinase (AKT). In contrast in hypoxic conditions, cyclic adenosine monophosphate (cAMP)-mediated activation of cAMP-dependent protein kinase catalytic subunit alpha (PKA) leads to PKA-induced phosphorylation on 443T of CAIX, leading to enhanced enzymatic activity. Dephosphorylation of 448S appears to be required for full CAIX activation15.
Carbonic anhydrases in cancer
The process of tumor growth and metastasis is a complex interplay between abilities acquired by the cancer cells due to genetic and epigenetic alterations and microenvironment which is subject to various modifications16,17. Moreover, quickly proliferating tumor cells experience harsh conditions including limited access to oxygen and nutrient supply18,19. Thus the metabolism of such cells needs to adapt to the new setting. Hypoxia (the condition in which the supply of oxygen to tumor cells is not sufficient enough to fulfill the usual demand of cells) is a critical component of the tumor microenvironment that has a significant impact on tumor phenotype and cancer progression20. Because cancer cells have limited access to oxygen, they must rely heavily on lactate, which is formed during anaerobic respiration21,22. Overexpression of monocarboxylate transporter 1 and 4 (MCT1, MCT4) and glucose transporters (GLUT1-GLUT3), was observed in cancer cells and contribute to cell survival in stress conditions with an accompanied general shift toward the glycolytic metabolism. MCTs are responsible for the transfer of monocarboxylic acids (such as lactate, pyruvate, and ketone bodies) into and out of cells via the plasma and mitochondrial membranes. The solute carrier (SLC) 16A family consists of 14 members with a similar structure. As a proton-linked monocarboxylate transporter, only four isoforms (MCT1–MCT4) have been identified and functionally characterized. The primary function of these proteins is to regulate the efflux of lactate and protons as byproducts of glycolysis from the intracellular to extracellular space to maintain physiological pHi and, as a result, contribute to extracellular acidosis. On the other hand, GLUT1-3 controls the uptake of glucose by the cells that is further converted to pyruvate, generating 2 ATP per glucose and proton. In the presence of diminished oxidative phosphorylation, this allows the prolonged, albeit less efficient, production of energy23,24.
Five major protein families regulate cellular cytoplasmic pH. MCT transporters, sodium hydrogen ion exchangers (NHE), and vacuolar-type H+-adenosine triphosphatases (V-ATPases) directly transport H+ across membranes. The intake of bicarbonate, which is utilized to titrate intracellular H+, also works as a mechanism of pH regulation. The SLC4 and SLC26 gene families of sodium-coupled bicarbonate transporters and the chloride–bicarbonate exchangers transporters are therefore crucial for the maintenance of optimal pH conditions. Finally, carbonic anhydrases regulate pH by catalyzing the reversible hydration of CO2. These were extensively reviewed by other authors23–27.
Moreover, some tumor cells are highly reliant on glutamine absorption and glutaminolysis. Glutamine is a critical supply of reduced nitrogen for biosynthetic pathways, as well as carbon for the tricarboxylic acid (TCA) cycle, glutathione synthesis, and nucleotide and lipid synthesis via reductive carboxylation. The cancer cell's origin tissue, genetic makeup, and microenvironment can all influence glutamine metabolism. Although mammalian cells can synthesis glutamine de novo using glutamine synthetase (GLUL), some cancer cells require exogenous glutamine, which is catabolized in mitochondria via glutaminase GLS. GLS appears to be involved in the development of some malignancies in vivo28,29. The expression of MCT1 and other genes involved in glucose or lactate transport and their metabolism is regulated by hypoxia-inducible transcription factor (HIF)28–32. HIF plays a crucial role in the response to hypoxic conditions. HIF-1α subunit is sensitive to oxygen and is often missing in well-oxygenated cells as a result of negative regulation by the von Hippel–Lindau tumor suppressor (VHL), which drives it to proteasome degradation. Hypoxic conditions force the inactivation of VHL that tigers the activation of HIF1α. In such conditions, HIF1α is stabilized, enters the nucleus, and forms a dimer with the HIF-1β subunit that is constitutively synthesized in the cell. As a result, the active transcription complex is formed, which interacts with transcriptional co-factors to carry out its functions. In turn, the HIF1 transcription complex induces the expression of many target genes with HIF-responsive elements (HREs) in their regulatory regions including the C-X-C chemokine receptor type 4 receptor (CXCR4) or vascular endothelial growth factor (VEGF), further promoting cell survival and metastasis. Furthermore, HIF acts as a versatile transcription factor that activates the expression of various genes involved in anaerobic metabolisms, angiogenesis, and metastasis33–36. Both HIF expression and activation are regulated by various signaling pathways involved in the oncogenic transformation of the cell including epidermal growth factor receptor (EGFR)37–40, mitogen-activated protein kinase (MAPK)41,42(p3),43, and phosphoinositide 3-kinase (PI3K) pathways44,45. One of the examples include protein kinase A (PKA)-mediated phosphorylation of the carbonic anhydrase intercellular domain in hypoxic conditions due to the rise in cyclic AMP (cAMP) levels46.
The increased production and storage of acidic metabolites, mainly lactate, carbon dioxide (CO2), and protons (H+), that occurs as a result of cancer cells' metabolic rewiring in hypoxia force changes in the expression of enzymes and transporters that act in concert to provide effective pH regulation to offset the potentially damaging repercussions of an increasingly hypoxic and acidic tumor microenvironment47. HIF-mediated expression of CAIX is one of the major adaptations to hypoxic conditions48,49, while CAXII is not induced by the protein50. Moreover, CAIX can be induced by processes that are not dependent on hypoxia, such as lactate and the redox-mediated stabilization of HIF-151,52. Not only does HIF contribute to the expression of CAs. Enzyme expression may be regulated by other transcription factors such as transcription factor AP-1/AP-2 (AP-1/AP-2), protein C-ets-1 (ETS-1), transcription factor SP1/SP3 (SP1/SP3), and epigenetically controlled via regulation of methylation status of the regulatory sequences of CAS9 gene or by posttranscriptional mechanisms through splicing and mRNA stabilization8,53. It is worth mentioning that in renal cell malignancies, inactivation of the pVHL tumor suppressor protein, which negatively regulates HIF stability, results in a constitutive increase in the expression of the CA9 gene54.
The maintenance of optimal pH conditions inside the cell is a firm strategy protecting cancer cells from apoptotic cell death55. When malignant melanoma cells (MM) were compared to MeT-5A mesothelial cells, CAIX expression was found to be considerably greater in the former56. This was further amplified by hypoxia (1% O2) and accompanied by elevated catalytic Fe2+ levels. Incubation of cells with S4 and SLC-0111 CAIX inhibitors resulted in diminished survival and migration of MM cells, as well as overexpression of transferrin receptor protein 1 (TFRC), citrate hydro-lyases (IREB1/2), and solute carrier family 40 protein (SLC40A1), and downregulation of ferritin heavy chain/ferritin light chain (FTH/FTL) expression. This expressional pattern was identical to that observed in cells that had been exposed to erastin a ferroptosis inducing agent. Increased autophagy and mitochondrial fission following CAIX inhibition were found which was associated with higher catalytic Fe2+ in both mitochondria and lysosomes. These findings were also accompanied by increased peroxides, mitochondrial O2, and lipid peroxidation. Deferoxamine, ferrostatin-1, and Z-VAD-FMK (ferroptosis inhibitors) were all shown to greatly reduce the final cell death, indicating that it could be a combination of ferroptosis and apoptosis that occurred in the exposed cells. The current study demonstrated for the first time an intimate relationship between iron metabolism and hypoxia and redox regulation, in which CAIX plays a crucial role (at least in MM tumor cells, which are associated with iron overload and high malignancy). Furthermore, this relationship was not detected in mesothelial cells that were not cancerous (MeT-5A). In MM cells the occurring cell death caused by CAIX inhibition under hypoxic conditions may be the mix of, three separate forms of controlled cell death (autophagy, ferroptosis, and apoptosis)56. Furthermore, it is commonly accepted that tumor cells prefer a higher alkaline pHi (intracellular pH) than normal cells57,58. pHi/pHe (ratio between intracellular and extracellular pH) ratio changes of 0.1–0.2 pH units can have catastrophic repercussions for important biological processes such as cancer cell proliferation, migration, survival, and metastasis. Given that, CA may contribute to the production and maintenance of an alkaline pHi that is favorable for tumor growth. The enzymes may play a role in the formation of an increasingly acidic extracellular space, which promotes tumor cell invasion15,59,60. For example, it was found that CAIX appears to be able to interact with matrix metalloproteinase 14 (MMP14) facilitating collagen degradation. This is achieved due to the activity of CA providing the H+ that the protease requires for catalytic activity61. Suppression of CAIX activity or deletion of its catalytic domain results in a reduction of these processes62. Additionally, hypoxia and extracellular acidosis are pivotal factors that influence cancer cell dedifferentiation and the development of stem cell characteristics in esophageal63 and breast cancer64,65. It has been shown that the expression of CAIX is associated with stemness, including the expression of the stem cell marker CD4460,65,66. Furthermore, CAIX has the potential to alter the binding of E-cadherin to the cytoskeleton and therefore impact cell adhesion67. It also may affect the activity of RHO-GTPase and thus regulate the transforming protein RHO/RHO-associated protein kinase (RHO/ROCK) signaling pathway involved in the acquisition of metastatic potential of cells68. It has been demonstrated that the glycoprotein CD44 is found at the invadopodia of MDA-MB-231 cells. CD44 promotes the activity of NHE1, the most prevalent isoform of the Na+/H+ exchanger (NHE) family that is present in all mammalian cells. The activity of the RHOA effector ROCK1 is activated, which results in cell invasion. In addition, the RhoA–ROCK–PI3K pathway and Ras-related C3 botulinum toxin substrate 1 (RAC1) are both engaged in CD44-mediated cell invasion69,70. CAIX may also act as an adhesion molecule. Its PG-like domain helps form focal adhesion connections during cell attachment and spreading on solid surfaces. By competing with β-catenin for E-cadherin CAIX can disrupt intercellular adhesions. It is unclear whether CAIX functions simply via mechanosensitive adhesion forces or possibly via pH control mechanisms. However, current research shows that acidosis can reduce or promote cancer cell adhesion depending on the timing of exposure67,71–73. The proximity-dependent biotin identification (BioID) method has recently been used to screen CAIX-interacting proteins61. These investigations revealed a complex CAIX interactome in cancer cells that regulates pH, transport of metabolic intermediates, cell migration, and invasion and identified two key membrane protein classes linked with CAIX, namely metabolic transport proteins and cell adhesion/migration/invasion proteins61,74,75. CAIX functions with bicarbonate transporters (including electroneutral bicarbonate transporter NBCn1 (SLC4A7)) to bring in HCO3- produced in a CAIX-mediated hydration reaction of CO2, and amino acid transporters (including the large neutral amino acid transporter 1 (LAT1) and CD98 heavy chain (CD98hc)) to transport in essential amino acids and glutamine (via interaction with glutamine transporter SLC1A5 and sodium-coupled neutral amino acid transporter 2 (SNAT2) facilitating the uptake of aminoacid by SLC1A5), which cancer cells employ as an alternative metabolic energy source and biosynthetic precursor (Figure 3). CAIX also creates unique interactions with collagen- and laminin-binding integrins at the leading edge of migrating cells76.
Figure 3.
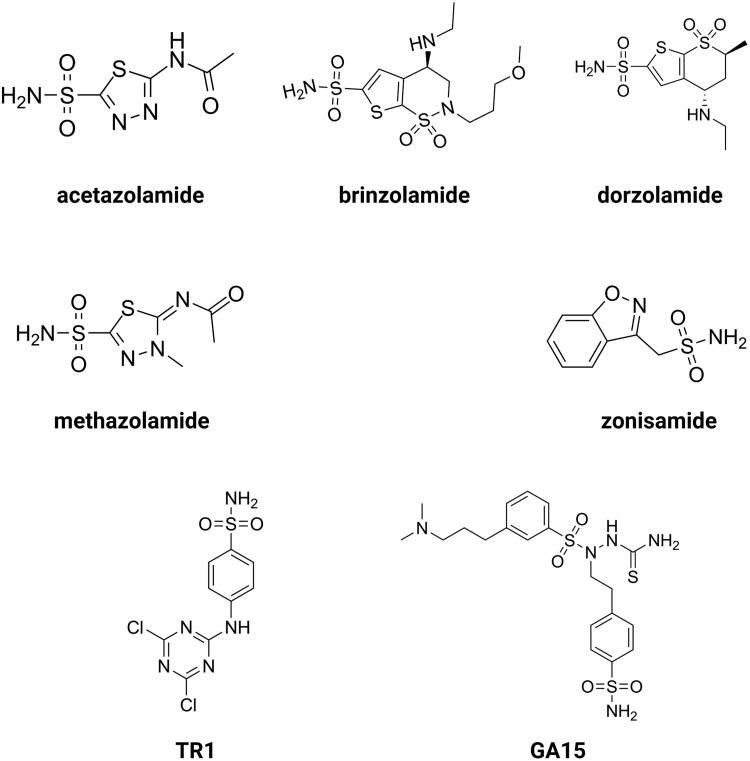
Chemical structures of acetazolamide (AZ), brinzolamide, dorzolamide, methazolamide, zonisamide, TR1 and GA15. Created with BioRender.com
Effects of CAIX knock-down/out
In solid tumors, the substantial data on CAIX's physiological activity hints at a fundamental function that may go beyond pH regulation. The physiological functions of CAIX also vary between primary tumor types. This shows that inhibiting CA activity in hypoxic tumors may not disturb the tumor microenvironment. Thus, removing CAIX expression from hypoxic tumors may be more advantageous than just inhibiting its function10.
CAIX is necessary for matrigel and type 1 collagen invasion by tumor cells. CAIX's PG and CT domains also play a role in invasion. The CAIX interactome shows that CAIX interacts with numerous metabolic cell surface transporters, which may be important for hypoxia metabolic response. CAIX interacts with integrins, integrin-associated proteins (CD98hc), and MMP14 on the cell surface. Invadopodia and pseudopodia are known to be composed of integrins and MMP14, and colocalization studies show that CAIX preferentially localizes to these areas. CAIX colocalizes with the integrin α2/β1 and MMP14, but not with F-actin or focal adhesion kinase (FAK) at focal adhesions. The intracellular CT domain of carbonic anhydrase interacts with MMP14 and modulates CAIX catalysis, which is necessary for CAIX-mediated invasion. Single domain truncation mutants of the enzyme reduce CAIX function significantly compared to the wild-type protein, but the residual functional activity exists. Given the extensive CAIX interactome revealed utilizing the BioID technique, simultaneous disruption of many CAIX domains may be required to completely block the enzymatic function of CAIX. On the contrary, deletion of the CT domain but not the PG domain lowers both CAIX catalytic activity and invasion through collagen I (a significant component of tumor stroma). A small regions inside CAIX's CT domain appears to be crucial in mediating its connection with MMP14. This region contains three putative phosphorylation residues, mutations of which diminish or abolish the connection with MMP14, indicating possible phosphorylation–dephosphorylation-mediated regulation between these two proteins. The findings imply that increased co-expression of CAIX and MMP14 may promote invasion and metastasis by activating MMP14 within invasive structures like invadopodia61.
To therapeutically reduce CAIX expression in tumors, RNA interference (RNAi) or short hairpin RNA (shRNA)-mediated expression silencing technology can be employed. RNAi technique was used in multiple research to modulate CAIX expression in many cancer models. For example, siRNA has been utilized to modify CAIX expression in liver, breast, kidney, brain, and prostate cancer. CAIX knockdown reduces primary tumor development and proliferation in breast cancer models and enhances the effects of hexokinase inhibitors10. Several targets including CAIX, MCT1, and anionic amino acid transporter light chain, xc- system (xCT) were identified as essential for tumor initiation and growth in a patient-derived pancreatic tumor model. CAIX was overexpressed in tumor-initiating cells. Moreover, CAIX expression was required for tumor initiation because shRNA knockdown prevented cell growth in vivo77. Zandberga et al. investigated the global gene expression in two breast cancer cell lines, MCF7 and MDA-MB-231. It was estimated that CAIX knock-down had only a minor impact on the overall transcriptional response to hypoxia, but it prevented hypoxia-induced upregulation of stanniocalcin-1 (STC1), a secreted glycoprotein that has been shown to promote tumor progression and metastasis in breast cancer. STC1 expression is linked to poor survival in basal-type breast cancer but not in luminal A or receptor tyrosine kinase (HER2) positive subtypes. The association is also strong in a cohort of basal-type breast cancer patients with cellular tumor antigen p53 (TP53) mutations, indicating a possible STC1-TP53 collaboration in highly aggressive breast cancer subgroups. These findings suggest that CAIX inhibitors may partially suppress STC1 expression in breast cancer cells and identify a subgroup of patients who may benefit from CAIX directed treatment78. In another study Doyen et al. studied the effects of CAIX, and CAXII knock-down combined with ionizing radiation. CAIX's pHi-regulating activity protected irradiated fibroblasts in an acidic environment with the reduction in the number of cells in radiosensitive phases of the cell cycle. Radiation of human colon carcinoma LS174Tr spheroids silenced for either CAIX or both CAIX/CAXII increased cell death by 50 and 75% due to disruption of CA-mediated pHi regulation. Finally, coupled CAIX/CAXII silencing and irradiation slowed LS174Tr tumor development. These findings emphasize the potential role of anti-cancer strategy combining radiation with CA targeting79. Similarly, RNAi-mediated knockdown of CAIX enhanced the radiosensitivity of the nasopharyngeal carcinoma cell line (CNE-2). Ionizing radiation together with lipofectamine 2000 treatment reduced CAIX expression and viability, decreasing cell proliferation, colony formation, and increasing the number of CNE-2 cells trapped in the G2/M phase of the cell cycle. Radiation sensitivity increased in hypoxic CNE-2 cells following CAIX inhibition. These findings point to CAIX as a potential therapeutic target for radioresistant human nasopharyngeal carcinoma80(p2).
CAIX expression is limited in normal adult tissues, hence genetic disruption of CAIX expression in mice may lead to modest symptoms. The targeted gene disruption of CAIX caused gastric hyperplasia of the glandular epithelium, with many cysts, in homozygous mice. Animals developed normally without alterations in their gastric pH, acid secretion, and serum gastrin levels. These findings reveal that, while CAIX deficiency is not a tumor-promoting factor in and of itself, it does cause glandular atrophy in the body mucosa, which is regarded to be a preneoplastic alteration in the stomach81(p9). Importantly, CAIX absence did not increase tumorigenicity, even though old CAIX null animals may develop degenerative brain diseases82. Li et al. found that claudin-18A2 a tight junction protein was downregulated in CAIX KO mice stomachs, followed by the increase in inflammatory mediators (interleukin 1, inducible nitric oxide synthase), and inflammation-induced parietal cell loss with hypergastrinemia, which promoted foveolar hyperplasia as a consequence of loss of stomach acid secretion83.
Targeting CAIX with small molecule inhibitors
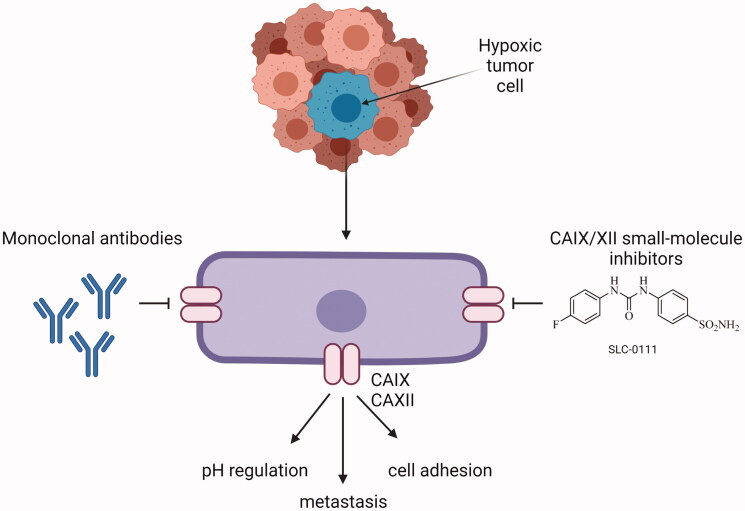
CAIX has received a lot of attention in anticancer drug design since it is expressed at a low level in normal tissues84. In contrast, several tumor tissues have shown increased expression of CAIX85–89. It has been found that CAIX may contribute to both chemo- and radiotherapy resistance and inhibiting the enzyme may increase the efficiency of the treatment55,90. Studies suggest that the enhanced anticancer effect of combinatory treatment with radiation and sulphonamide-bearing inhibitors may result from changes in the tumor microenvironment55. The antitumor effects of CAIX inhibition are thought to be mediated by the crucial role of the enzyme in pH regulation. However, recent research has revealed that CAIX interacts with several signaling pathways involved in the cellular response to radiation4. Moreover, inhibition of CAIX activity in hypoxic cancer cells may constitute an important advantage in selective targeting of these cells without causing an effect in normal cells55. The pH-regulating function of CAIX is critical for the survival and protection of tumor cells against the toxic effects of drugs or radiation, which is consistent with the concept that extracellular acidosis can negatively affect drug uptake and radiation damage. Moreover, the reduction of extracellular acidosis may enhance the intake of basic drugs such as doxorubicin and influence the therapeutic outcome8,91.
The development of novel agents that limit the enzymatic activity of CAIX by binding to or near its active site, and therefore impairing the catalytic function of CAIX, are at the forefront of drug development research. The second approach involves the design of specific monoclonal antibodies and their use in tumors with a high expression of the enzyme8. By far, the most frequently represented and therapeutically employed carbonic anhydrase inhibitors (CAIs) are sulphonamide-based compounds and their isosteres (sulfamides or sulfamates)92. Sulphonamides bind to zinc ion leading to the displacement of bound water/hydroxide molecule at the same time maintaining the tetrahedral conformation around the zinc ion93,94. CAIs can be designed to have physiochemical features that make them impermeable to the plasma membrane, hence reducing the likelihood of them blocking off-target cytosolic CAs. Charged, membrane-impermeable derivatives of CAIX and XII are favored in the targeted design of molecules because they selectively inhibit just these and not the cytosolic/mitochondrial CAs94. This approach of a drug design technique that incorporates site-specific targeting of CAIX rather than relying solely on variances in inhibition profiles to create effective drugs is more feasible. At this point, various agents have been developed that have low membrane permeability. Such compounds make use of bulky chemical moieties, such as albumin-acetazolamide, or charged moieties, such as fluorescently labeled sulfonamides or cationic sulfonamide derivatives. In that way, one can design high molecular weight molecules that are just too bulky to pass the plasma membrane, or via addition cationic moieties make the agents impermeable across membranes due to electrostatic repulsions10. A relatively recent generation of CAIs includes glycoconjugated sulfonamides that have been demonstrated to exhibit membrane impermeability as well as isoform-specific inhibition of CAIX. This class of CAIs include benzene sulfonamides, sulfonamides, or cyclic secondary sulfonamides that have been attached to a mono- or disaccharide tail. It is believed that the substantial molecular weight of these molecules, as well as the inclusion of a sugar moiety, prevent them from permeating across the plasma membrane. The inclusion of a sugar moiety permits these CAIs to retain their water solubility and as a consequence maintain high bioavailability with excellent inhibitory profile, and selectivity for CAIX over CAII in some situations exceeding 1000-fold. The use of carbohydrate, notably a monosaccharide, may however mistakenly lead to its interaction with glucose transporters10. Moreover, CA inhibitors may be designed as prodrugs95. De Simone and colleagues created prodrug CAIs that were specifically tailored to target CAIX. These chemicals that operate as passive molecules by unmasking themselves in the hypoxic niche are based on a sulfonamide derivative that has a chemical moiety of dithiodi-aliphatic/aromatic acyl halides reducible in the presence of an acidic environment. Furthermore, such molecules possess a disulfide-linkage bond that can be broken down by the tumor microenvironment components to create thiol derivatives that possess substantial anticancer activity. The most promising of these compounds is 4-(2-mercaptophenylcarboxamido) benzenesulfonamide. Its affinity for CAIX increases 60-fold following activation of the prodrug. In contrast, active prodrugs require esterases to be activated. These include coumarins and thiocoumarins. More intriguingly, when compared to other CAIs, these compounds have the most isoform-selective CA inhibitory profile, resulting in the hydrolyzed product directly interacting with CAIX specific residues present in the hydrophobic and hydrophilic pockets of the enzyme. Furthermore, these compounds do not interact with the catalytic zinc when they are in hydrolyzed form10. Construction of CAIX-directed immunoliposomes constitute another approach of CA targeting. These therapeuticals consist of anti-cancer drug conjugated to a liposome carrier. Once the antibody region of the immunoliposome binds to CAIX, the carrier is released and the anticancer agent can be taken up by the tumor cell. Current applications include the delivery of liposomes containing docetaxel to the lung carcinoma cells. It was demonstrated that a CAIX-directed immunolipsome system was capable of facilitating tumor selective administration of the therapeutic drug96,97. Different classes of sulfonamides and similar compounds have shown efficacy in inhibiting CAIX in vitro, and some of these agents have demonstrated anticancer activity in vivo in xenografted subcutaneous and metastatic animal models of cancer98,99. This group of compounds includes acetazolamide (AZ), brinzolamide, dorzolamide, methazolamide, and zonisamide with Ki's in the nanomolar range3,100. Aromatic sulfonamides were found to be effective in decreasing tumor cell proliferation and intracellular pH, which was associated with an increase in ceramide-mediated apoptosis. AZ and two newly synthesized aromatic sulfonamides (2-(4-sulfamoylphenyl-amino)-4,6-dichloro-1,3,5-triazine (TR1) and 4-[3-(N,N-dimethylaminopropyl)thioreidophenylsulfonylaminoethyl]benzenesulfonamide (GA15)) with high affinity for CAIX, were included in the study (Figure 3). All of the analyses revealed that tested aromatic sulfonamides had a more noticeable influence on tumor cells than AZ101.
Series of ureido-substituted benzenesulfonamides have been shown to inhibit carbonic anhydrase enzymes including CAIX. Depending on the substitution pattern of the urea moiety of the compounds excellent nanomolar inhibitory activity have been described. One of them, 4-[(3' nitrophenyl)carbamoyl]aminobenzenesulfonamide, significantly reduced the formation of metastases by the highly aggressive 4T1 mammary tumor cells, making it a promising candidate for the development of conceptually novel antimetastatic drugs102,103. Other studies have shown that indanesulfonamide exhibits acidification preventive activity during hypoxia and the effect is superior to non-selective CA inhibitor acetazolamide. The selective inhibition of CAIX by indanesulfonamide had no impact on lactic acid production under both normoxia and hypoxia conditions. The use of inhibitors reduced the tumor growth compared to controls55. More recently, a novel anticancer agent, the aromatic sulphonamide S-1, was tested on cervical cancer cells (HeLa) that were positive for CAIX expression as well as a normal prostate epithelial cell line (PNT1-A) that was negative for CAIX expression. S-1 caused a considerable reduction in cell viability, promoted apoptosis, and increased reactive oxygen species (ROS) generation in exposed cancer cells and may therefore be a promising candidate for future anticancer research104.
In another study, the apoptotic and cytotoxic activity of novel CAIX inhibitor H-4i was tested in CA-expressing HT-29 and HEK-293 cell line without expression of CAIX. When comparing HT-29 cells to human normal cells, it was discovered that the half inhibitory concentration (IC50 value) of the sulfonamide derivative was comparatively low in HT-29 cells. According to the findings of this study, H-4i induced a considerable increase in cytotoxicity and ROS production, as well as a significant increase in apoptosis levels of cancer cells. H-4i was more effective on HT-29 than HEK-293 and this may indicate that the new derivative H-4i has the potential to be exploited as an anticancer drug in colorectal cancer treatment105.
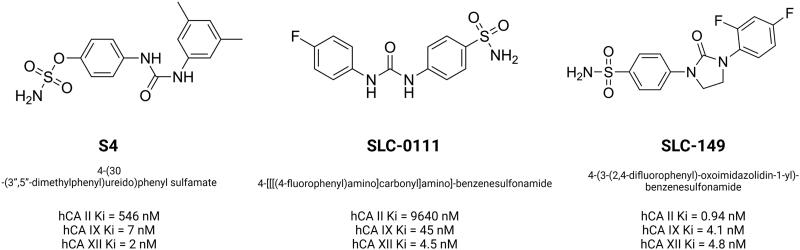
Furthermore, sulfamates, which work as inhibitors of steroid sulfatase involved in the production of active steroids have been found to inhibit CAIX activity as well106. The recent decade has seen a surge in interest in ureido-substituted sulfamates as tumor-associated small molecule inhibitors. A vast set of ureidosulfamates CAIX/XII derivatives were studied as part of the EU-funded METOXIA project107. The compounds demonstrated good selectivity at nanomolar concentrations for the tumor-associated CAIX/XII isoforms. A good response was observed in three of the particular CAIX/XII inhibitors tested in in vitro experiments for tumor cell migration and dissemination. It was decided to test one of them in a mouse orthotopic breast cancer model, and the results were promising. The 4-(3'-(3′′,5′′-dimethylphenyl)ureido)phenyl sulfate (S4) treatment with a 10 mg/kg maintenance dosage administered daily on a "5 days on, 2 days off" schedule reduced metastatic tumor burden in the mice lungs99. In HT29 cells, treatment with S4 resulted in a reduction in hypoxia-induced extracellular acidification as well as a reduction in clonogenic survival under hypoxic conditions and this can be the result of changes in CAIX ectodomain shedding108. 4-(3′-(4″-Chlorophenyl)ureido)phenyl sulfamate (FC9-398A), an S4 derivative with improved pharmacokinetic stability, reduced the primary growth of subcutaneous MDA-MB-231 xenograft109. Ureidosulfamates such as S4 and FC9-398A showed both antimetastatic potential and antitumorigenic activity in small cell lung cancer (SCLC) that is extremely sensitive to deregulation in CAIX signaling.
The aforementioned inhibitors can be used to improve chemotherapy efficacy, reduce adverse effects, and delay the onset of chemotherapy resistance/treatment failure. These are all clinically relevant obstacles, as chemotherapies are normally administered in a series of cycles. While cisplatin is commonly used in SCLC patients, acquired resistance is a known issue. After numerous treatment cycles, the cancer cells become cisplatin-resistant, resulting in relapse110. Mice with primary DMS 79 and COR-L24 SCLC tumors underwent up to four cycles of cisplatin alone or in conjunction with the sulfamate CAIX/XII inhibitor S4. The combination of cisplatin/S4 was more effective than either drug alone in reducing DMS 79 primary tumor development. Furthermore, the tumor response to cisplatin after S4 treatment mirrored the response to a single cisplatin dosage, showing that repeated cisplatin treatments did not result in resistance. It appears that the cisplatin/S4 combination reduced CAIX-positive cells in DMS 79 tumors111. Chemotherapeutic drug uptake is highly dependent on pH around tumor cells (pHe). The extracellular acidification caused by CAIX/XII reduces the uptake of weak base agents like doxorubicin by tumor cells. It has been recently shown that reducing CA activity with acetazolamide, a non-isoform selective CA inhibitor, improved doxorubicin uptake and cytotoxicity in CAIX-overexpressing MDA-MB-435 (melanoma) cells112.
Buller et al. performed a search of inhibitors from one million DNA-encoded compounds and a library selection against carbonic anhydrase IX discovered a novel class of submicromolar bis(sulfonamide) inhibitors. One of the inhibitors was created without the use of a DNA tag, and it was found to accumulate in hypoxic tumor tissue sections in vitro as well as to target tumors in vivo113. It has recently been demonstrated that the DNA-linked inhibitor antibody assay (DIANA) may be used for both the ultrasensitive identification of enzymes and the quantitative evaluation of enzyme inhibitor potency. DIANA's performance was evaluated by screening a unique compound collection consisting of 2816 compounds containing lead-like small molecules to determine its screening effectiveness. This subsequently led to the identification of three novel CAIX inhibitors as a result of the screening procedure. Through x-ray crystallography, Tykvart et al. were able to determine their mode of binding into the CAIX active site114. Computer-aided drug design approaches were also employed to identify new CA inhibitors. These include the use of pharmacophore modeling, 3D quantitative structure-activity relationship (QSAR), virtual screening, and molecular dynamics simulations115. Moreover, non-classical sulfonamide inhibitor design includes molecules of classical sulfonamides linked to a disaccharide (such as sucralose or sucrose) that have the potential to produce effective CAIX inhibitors. Furthermore, the chemical linker provides the spatial requirements for optimizing interactions with residues in the selective pocket of the active site, boosting selectivity for CAIX and minimizing off-target inhibition of CAII116–118. SLC-0111 (4-{[(4-Fluorophenyl)carbamoyl]amino}benzenesulfonamide), a ureidobenzenesulfonamide CAIX/XII inhibitor (Figure 4) increased the cytotoxicity of dacarbazine and temozolomide. SLC-0111 also increases the responsiveness of breast cancer cells to doxorubicin and the cytostatic activity of 5-fluorouracil in colon cancer cells119. Administration of SLC-0111 to mesenchymal stem cell (MSC) has been shown to inhibit proliferation, resistance to apoptosis, and anoikis in melanoma cells, but also the promotion of epithelial-to-mesenchymal transition (EMT) and the stemness phenotype. Furthermore, it was demonstrated for the first time that MSC can establish a vemurafenib-resistant phenotype in melanoma cells and that this impact was abolished when MSC cells were treated with the CAIX inhibitor120. A decrease in tumor cell growth and an increase in apoptotic cell death were observed after SLC-0111 was administered in the prostate cancer cell line. However, even though intracellular pH regulation was disrupted, targeting CAIX in combination with daunorubicin or cisplatin did not increase the apoptosis of cancer cells121. More recent investigations indicate that SLC-0111 can be used in the treatment of various malignancies including bladder, glioblastoma and pancreatic cancer based on in vitro cell culture studies122.
Figure 4.
Chemical structures of carbonic anhydrase IX and XII inhibitors from ureidosulfamates (S4), ureidobenzenesulfonamides (SLC-0111) and SLC-0111 derivative (SLC-149). The Ki values for CAII, CAIX and CAXII were indicated. Based on130,131. Created with BioRender.com
Since the development of SLC-0111 multiple modifications of the molecule has been proposed with different cellular response123. For advanced and metastatic solid cancers, SLC-0111 has recently completed a successful Phase I clinical trial in which it was found to be effective. This molecule is now being studied in Phase Ib/II clinical studies, where it is being evaluated as a monotherapy as well as in combination with other agents such as gemcitabine124,125. Nowadays, a multi-center open-label 1b clinical study of oral SLC-0111 in combination with IV gemcitabine in CAIX positive pancreatic ductal adenocarcinomas (PDAC) consisting of dose-escalation and dose-expansion phases is recruiting patients (status for 02.08.2021, ClinicalTrials.gov Identifier: NCT03450018). The studies involving SLC-0111 were recently reviewed by Supuran C.T.126.
SLC-149 (Figure 4) constitute another benezenesulfonamide developed based on the ureidosulfonamides scaffold. The structure of SLC-149 differs from SLC-0111 because of the ureido moiety was transformed into a five-membered cyclic oxoimidazolidin moiety. This resulted in profound increase in inhibitory potential towards CAII, IX, and XII, however diminished its selectivity from tumor-associated isoforms towards the cytosolic ones. Drug design of the compounds based on SLC-0111 was extensively described by the aforementioned author and his group123,126–129.
It has been suggested that targeting the CAIX may be effective in reducing triple-negative breast cancer (TNBC) cell phenotypic switching including vascular mimicry (VM) and the presence of cancer stem cells (CSCs). It was shown that the treatment of two TNBC cell lines (BT-549 and MDA-MB-231) with a particular CAIX siRNA or with a new benzoxaborole CAI (RC44) greatly affected their ability to produce a vascular-like network and mammospheres, as well as impeded their metastatic potential. Furthermore, the RC44 inhibitor was found to be effective in the inhibition of the signal pathways involved in the VM and CSC development. Based on these findings, it appears that targeting hypoxia-induced cell plasticity with CAIX inhibition may provide a novel option to selectively diminish VM and CSCs in TNBC, hence increasing the efficacy of currently available therapies132.
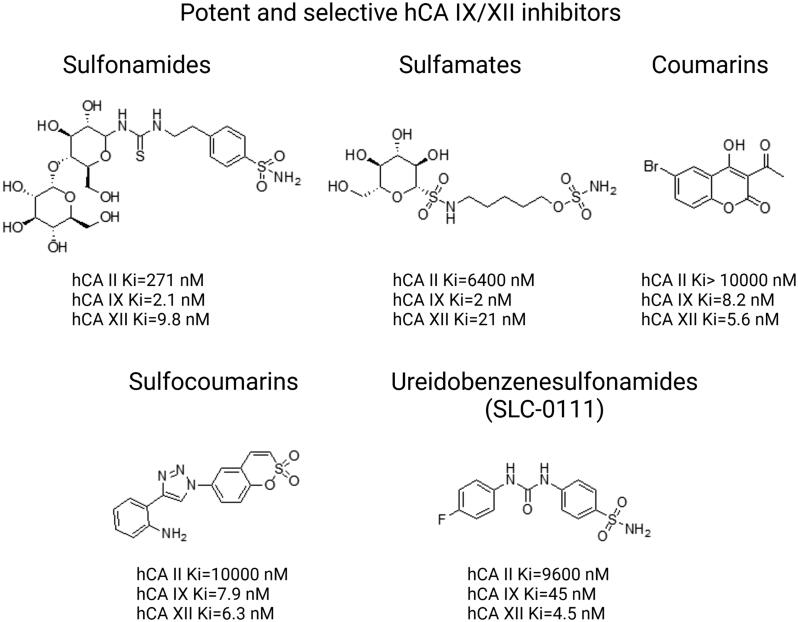
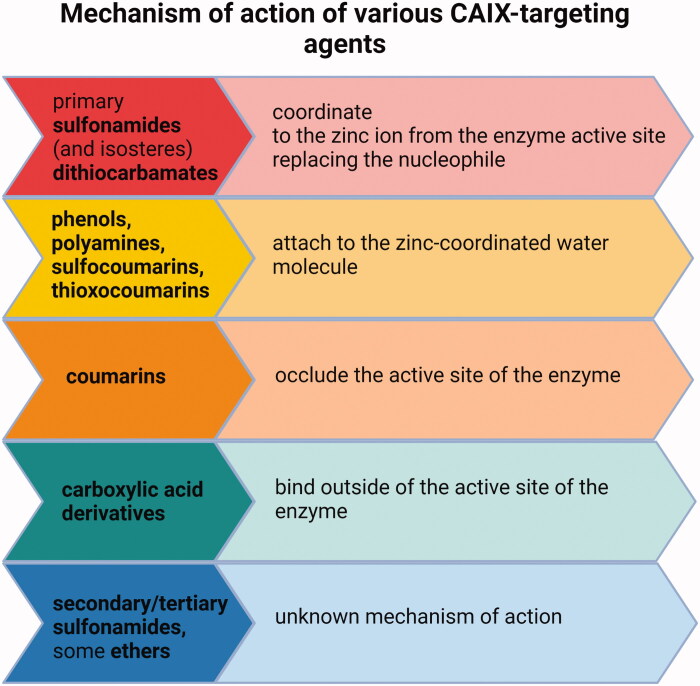
Various other chemical entities (Figure 5) with variable biological outcomes and mechanisms of action were tested (Figure 6).
Figure 5.
Structures of potent and selective hCA IX/XII inhibitors from distinct classes of compounds including sulfonamides, sulfamates, coumarins, sulfocoumarins, ureidobenzenesulfonamides. Based on133.
Figure 6.
Mechanisms of action of various types of CAIX inhibitors147,152–154.
These include sulfonamido carboranes134,135, benzenesulfonamides incorporating 1,3,4-oxadiazole hybrids136, benzimidazole derivatives137, 7-Acylamino-3H-1,2-benzoxathiepine 2,2-dioxides138, aryl derivatives of 3H-1,2-benzoxathiepine 2,2-dioxide139, S-substituted 2-mercaptoquinazolin-4(3H)-ones and 4-ethylbenzensulfonamides140, aryl selenols141, sulfocoumarins and coumarin derivatives142–145, coumarin-thiourea hybrids146, phenols, polyamines, carboxylates147, epacadostat148, pyridinium derivatives of 3-aminobenzenesulfonamide and sulfonamides containing various nitrogenous bases11,149,150 and short peptide-constructed nanofibers151.
Tyrosine kinase inhibitors such as imatinib and nilotinib exhibit inhibitory activity toward carbonic anhydrases, however without particular selectivity155. Among antiepileptic drugs, sulthiame has been demonstrated to inhibit carbonic anhydrases156. Sulfonamide inhibitors of cyclooxygenase 2 (COX-2) such as celecoxib157 and valdecoxib have also been found to effectively inhibit the activity of CAIX158. Research indicates that inhibition of CAIX may enhance the effectiveness of anti-VEGF therapy90, hexokinase II inhibition159, and traditional chemotherapy including cisplatin111, doxorubicin160 dacarbazine, and temozolomide119,161.
Moreover, the use of CAIX inhibitors may enhance the anticancer effects of DNA damage response (DDR) inhibitors including AP endonuclease-1/redox effector factor 1 (APE1/REF1) involved in single-strand DNA break repair and base excision repair162, cell cycle checkpoint proteins162, and histone deacetylases involved in the regulation of access of repair proteins to damage sites120,164.
Targeting CAIX with monoclonal antibodies
Two basic targeted approaches incorporating selective inhibition of CAIX have been described so far. The first one relies on the discovery of small-molecule inhibitors (depicted above and in Supuran165), while the second one focuses on the use of monoclonal antibodies that selectively target cancer cells without the induction of compensatory and resistance mechanisms. CAIX-specific monoclonal antibodies (mAbs) show therapeutic efficacy in a variety of ways. Direct mAb binding to CAIX can cause anti-tumor effects via antibody-mediated cell cytotoxicity (ADCC). Alternately, receptor-mediated internalization permits targeted delivery of cytotoxins and radionuclides to cancer cells. The development of mAbs targeting the CAIX active site may allow for selective CAIX inhibition15. This approach includes CAIX-targeting monoclonal antibodies such as G250 known under commercial names RENCAREX® or GIRENTUXIMAB®. Generic cG250 (girentuximab) is an IgG1 chimeric monoclonal antibody (mAb) that is now being investigated as immunotherapy for kidney cancer. The antibody recognizes soluble CAIX ectodomain (ECD), but not the splicing variant, and does not cross-react with CAI, II, and XII isoforms. The targeting of G250 antigen present on the surface of 95% of clear cell renal cell carcinomas (RCC) and restricted to the gastric epithelium, biliary ducts in the liver, astrocytes in the brain, and spinal cord offers limited toxicity due to favorable localization. G250 is internalized via clathrin-coated vesicles, escapes destruction in lysosomes, and is transported to and recycled by the perinuclear compartment of the cell. This results in long intracellular persistence and the ability to perform several internalization cycles in a single cell. Furthermore, the recycled antibody retains an intact Fc component that may be capable of continued stimulation of the ADCC response, which may account for its therapeutic efficacy. G250 therapy is effective against HT-29 colorectal cancer xenografts, which vary from RCC in that they express CAIX in a more diverse and hypoxia-related fashion166. In patients with metastatic RCC who participated in Phase I and II clinical studies, this antibody demonstrated good safety and tolerability, as well as a promising effectiveness profile167,168. A pilot open-label study to assess safety, tolerability, radiation dosimetry, and imaging properties of 89Zr-labeled girentuximab (89Zr-Girentuximab) will be performed in the clinical setting in patients with non-muscle-invasive bladder cancer (NMIBC) with the estimated study start date of July 2021 (NCT04897763).
There are two major techniques to combine chemotherapeutic agent toxicity and precise targeting of CAIX/XII overexpressing tumor tissues: antibody-drug conjugates (ADCs)169 and antibody-nanoparticle conjugates (ANCs)170–172. ADCs are antibodies covalently attached to a cytotoxic agent via a spacer. ADCs undergo chemical and enzymatic processing resulting in the release of the drug upon binding to CA, and endocytosis. ANCs are nanotechnology-based formulations and include lipid, polymeric and inorganic nanoparticles that preserve drugs against inactivation, allowing regulated release and passive accumulation in tumor tissues through increased permeability and retention. EGFR receptor-mediated endocytosis, formation of lysosomes results in the release of drug into the cytosol169,173.
Immunoglobulins are massive biomolecules that seem to have the problem reaching deeply into solid tumors because of their size and shape. Furthermore, they have the potential to be immunogenic83, and their normally long circulation durations can lead to early drug release and unwanted adverse effects. ADC synthesis is also expensive174. Despite the disadvantages, ADCs are a promising new class of pharmaceuticals that have the potential to revolutionize cancer treatment. To date, 11 ADCs are commercially available for the treatment of various malignancies. However, larger series of agents are currently undergoing Phase III investigative stages169. Testa et al.169 recently proposed a series of CAIX/XII-targeted ADCs that shown enhanced inhibitory activity compared with unconjugated monoclonal antibodies. This appears to be dependent on the conjugates small molecule CA inhibitor. Moreover, the benzenesulfonamide moiety was identified as crucial for the inhibition of the human CAII isoform169. Petrul et al. developed 3ee9 monoclonal antibody, which, when conjugated to monomethyl auristatin E through a self-immolative enzyme-cleavable linker, yielded the potent and selective CAIX antibody-drug conjugate CAIX-ADC (BAY 79-4620). BAY 79-4620 demonstrated significant anticancer effects in preclinical human xenograft models in mice reflecting a variety of tumor indications, with certain models demonstrating partial and total tumor reduction even after a single dose of the agent175(p9).
Recent investigations have focused on creating antibodies targeted against CAIX's catalytic domain. Rapid screening methods, such as phage display libraries [94-96], have been used to rapidly find CAIX antibodies. Some of the fragments feature catalytic site epitopes that impair CAIX activity in vitro15. The catalytic domain of CAIX can be targeted by the mouse monoclonal antibody VII/20. The antibody undergoes effective receptor-mediated internalization after binding to CAIX in mouse xenograft model of colorectal carcinoma (CRC), which is a mechanism that regulates the quantity and signaling of cell surface proteins and has a significant impact on immunotherapy176.
Targeting CAXII
Most if not all inhibitors described in the sections above target both CAIX and CAXII177–181 with exceptions182. More research focused on selective inhibition of CAXII by monoclonal antibodies. However, it is worth mentioning the efforts of Zeidler’s group who designed 6A10 that selectively binds catalytic domain of CAXII leading to inhibition of tumor cells in spheroid cultures, and a mouse xenograft model of cancer183,184. Moreover, the 6A10 reduced P-glycoprotein (P-GP) activity in CAXII/P‐GP double-positive chemoresistant cells. This resulted in cell death in vitro/in vivo and sensitization of cells to chemotherapeutic agents185. Uda et al. developed humanized monoclonal antibody blocking CAXII enzymatic activity that reduced growth of lung adenocarcinoma A549-cells in in vitro spheroid cultures186. On the other hand, Dekaminaviciute et al. developed monoclonal antibodies against the antigenic peptide of CAXII spanning 167–180 aa sequence187.
Structural differences between CAIX and CAXII
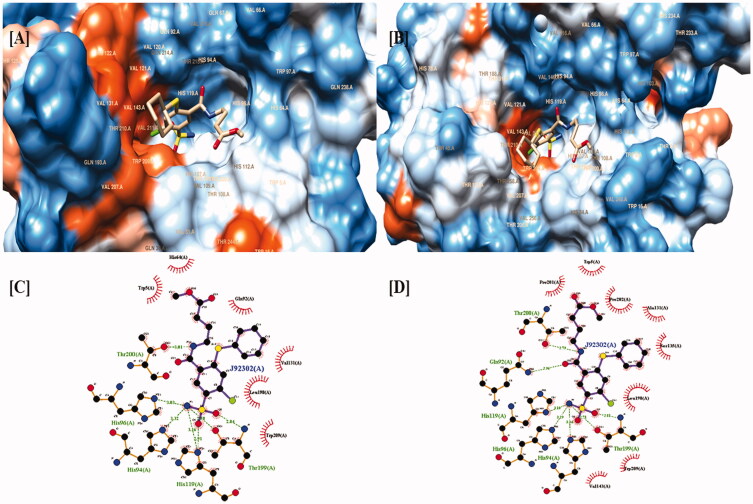
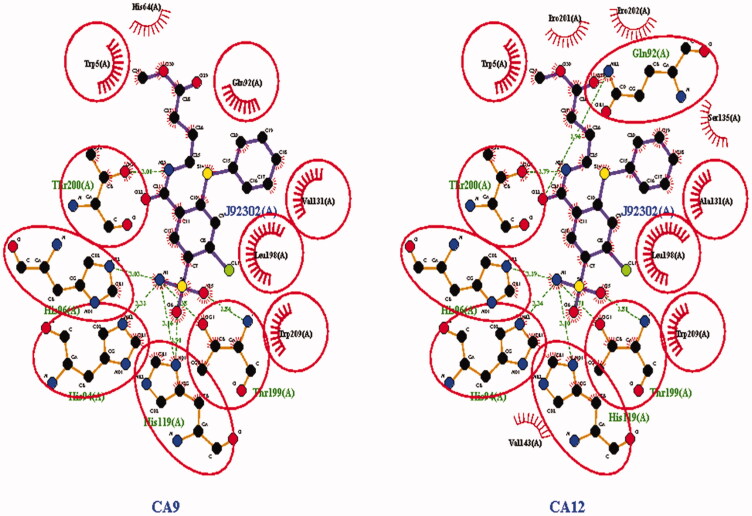
Mboge et. al compared the structural variations present in the different isoforms of CAs and concluded that the difference in the inhibitory effect of various CA inhibitors is because of the highly conserved interacting residues and the structural arrangement of the CA182. Based upon the comparative inhibitory activity of ureido-substituted benzene sulfonamides (USB) it has been revealed that the main structural variations between CAIX and CAXII are at positions 67, 91, 131, 132, and 135. CAIX is characterized by glutamine at 67 position, leucine at 91 position, valine at 131 position, alanine at 132 position, and leucine at 135 position while CAXII is having lysine at 67 position, threonine at 91 position, alanine at 131 position, serine at position 132 and 135. The similarity between valine and alanine present at the 131 position of CAIX and CAXII respectively favors the hydrophobic interactions with the ligand molecule. The presence of serine having a polar uncharged side chain in CAXII at 132 position placed nearer to the opening of the active binding site results in the steric hindrance of the inhibitor molecules within the CAXII. The presence of serine at positions 132 and 135 of CAXII develops a slender hydrophobic area within the active site which was absent in CAIX182. The active binding site of both CAIX and CAXII interacting with an inhibitor molecule is demonstrated in Figures 7–9.
Figure 7.
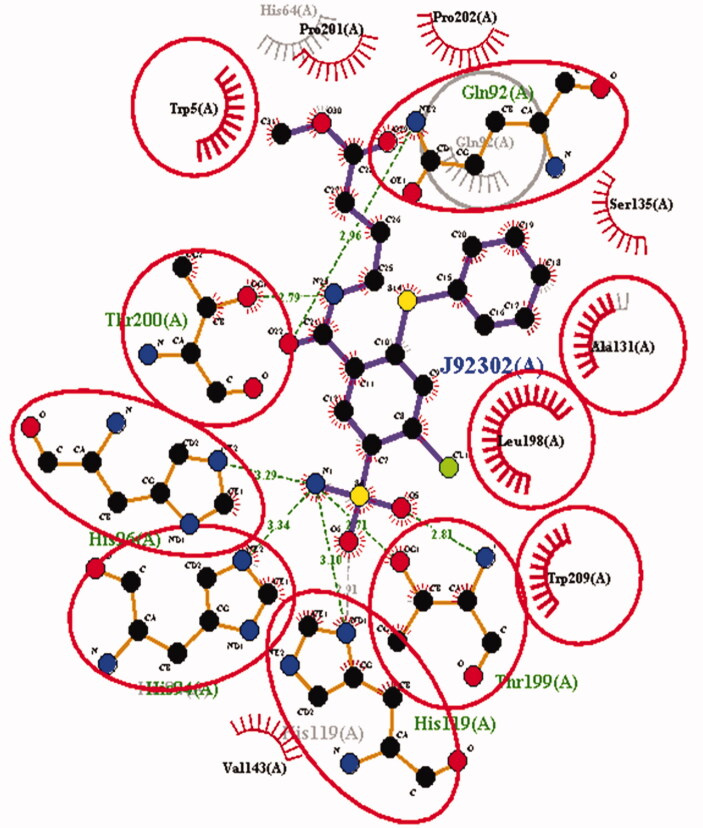
Two and three-dimensional structural representation of the binding cavity of CAIX and CAXII complexed with the same inhibitor molecule for comparing the binding pattern for both the isoforms. [A] Three-dimensional representation of the binding conformation of the ligand molecule within the binding cavity of the CAIX. [B] Three-dimensional representation of the binding conformation of the ligand molecule within the binding cavity of the CAXII. [C] Two-dimensional representation of binding interactions of the complexed inhibitor with CAIX. [D] Two-dimensional representation of binding interactions of the complexed inhibitor with CAXII.
Figure 8.
Two-dimensional interaction observed for the same ligand molecule observed against CAIX and CAXII isoforms. The binding residues highlighted by red circles were commonly involved in the interactions with the ligand molecule in both the isoforms. But non-highlighted residues were interacting in the single isoform out of the both.
Figure 9.
Comparative analysis of the binding interaction of the same ligand molecule against the CAIX as well as CAXII isoform.
The binding cavity of CAIX is highly lipophilic in nature compared with the same structural component in CAXII isoform. This lipophilic character of the active binding site of CAIX is because of the presence of large number of valine amino acids which possess non-polar character because of the presence of isopropyl side chain. The highly lipophilic character of the active binding site of CAIX is indicated as dark orange color in Figure 7(A). Thus, the presence of the non-polar group of valine results in the strong aromatic-aromatic interaction with the aromatic ring present.
On the other hand the active binding site of CAXII is characterized by the presence of serine amino acid which is hydrophilic in nature because of the presence of methanolic side chain. The low lipophilic character of the active binding site of CAXII is visible as light orange or white color in Figure 5(A). The polar methanolic group of serine interferes with the aromatic interactions of the aromatic ring of the ligand with the active residues present in the binding cavity of CAXII resulting in the weak binding.
CAIX/XII inhibitors development
In anticancer drug discovery, there is a growing interest in determining the effects treatments that disturb cancer cell pH homeostasis, and a critical element of this effort is the development of selective and effective inhibitors of CAIX/XII with profound anticancer activity. Mainly, primary sulfonamides (RSO2NH2) are well-known and good CA inhibitors. More than 200 protein X-ray crystal structures of this drug class-bound to CAII have been developed sharing common feature - a sulfonamide anion coordinated to the CA active site zinc cation. Thus scientific interest is focused mainly on this type of compounds. However, while targeting the CAIX/CAXII with traditional primary sulfonamide CA inhibitors, it is difficult to achieve selective binding to these cancer-relevant CAs. Secondary sulfonamides (RSO2NHR′) on the other hand, are typically considered to be poor CA inhibitors. Selectivity of the compounds is important for the reduction of both dose and side effects of the CA inhibition188. The inclusion of charged species, bulky entities such as FITC, albumin, or hydrophilic sugar moieties has been used to “dial in” the selectivity of small-molecule CAIX inhibitors. Other options include the use of sulfonamide or coumarin chemotypes that are naturally selective for extracellular CAs like CAIX15,189. It is however worth mentioning that CAIs have a spectrum of action from non-isoform-specific to isoform-specific, and hence whether to utilize pan-CAI treatment or isoform-specific CA inhibitor treatment depends on the type of therapy that is required190. Despite substantial progress, finding CA-targeted anticancer drugs is not an easy task. Various approaches have been applied in the development of new inhibitors165,191–194. However, many published structures have disappointingly low antiproliferative effects despite significant inhibition of isolated CAIX/XII. The contradictions between some CA inhibitors' capacity to disrupt recombinant enzyme catalytic function and their impact on cancer cells are an issue that has been less addressed than structure-activity relationship (SAR) and selectivity investigations. Despite the evidence supporting CAIX and XII as anticancer targets, including the efficacy of CA-inhibitory antibodies in vitro and in vivo, the success rate of small-molecule inhibitors in cell culture remains low. Figure 10 shows several potent hCA IX/XII inhibitors with good antiproliferative efficacy against cancer cells in hypoxia.
Figure 10.
Examples of potent hCA IX/XII inhibitors with good antiproliferative efficacy against cancer cells in hypoxia. Based on133.
Normoxic vs hypoxic conditions – cell line selection
The role of CAIX and XII inhibition in many situations is debatable because most papers on the subject provided cytotoxicity data obtained in normoxic settings. Although relevant in some cases, these variables are irrelevant when it comes to CA inhibitors' antiproliferative activity. This is owing to the low expression of target isoforms in many cancer cell lines. Some studies used cell lines with high CAIX activity in the cytosol, namely MDA-MB-231 and MCF-748 (discussed below). The non-default CAIX localization may lead to an overestimation of protein expression and activity and cause problems in data interpretation. Several murine and human cancer cell lines have been used to test the efficacy of CAIX/XII inhibitors. These cell lines are all obtained from solid tumors that exhibit upregulation of CAIX expression, which is associated with a poor prognosis due to a high rate of metastatic spread and/or the development of treatment resistance. One thing that the majority of these cancer cell lines have in common is that they exhibit no or extremely low expression of CAIX in normoxic conditions, with CAIX expression much higher under hypoxic conditions. There are a few exceptions to this rule. In contrast to HT29 colorectal cancer (CRC) cells with high expression of CAIX, HCT116 CRC cells and RT112 bladder carcinoma cells are both negative for CAIX in both normoxic and hypoxic environments. When CAIX/XII inhibitors are used in conjunction with other medicines, there is frequently an extra purpose for the inclusion of a specific cell line in the study (e.g., expression of V-ATPase on Me30966). For a variety of reasons, the human MDA-MB-231 and murine 4T1 triple-negative breast cancer cell lines have been widely used in research. Both cell lines are highly metastatic, which allows researchers to investigate the effectiveness of CAIX/XII inhibitors on both localized cancer growth and metastases, the latter of which can only be studied when cancer cells are injected into the mammary fat pad (orthotopic location) or intravenously injected into the organism (experimental metastasis). It was possible to identify and quantify cancer cell migration and colony formation in vitro and metastasis formation in vivo through the use of MDA-MB-231 cells that were tagged with enhanced green fluorescence protein (eGFP-MDA-MB-231 cells) or 4T1 cells that expressed luciferase (bioluminescence). In the case of the MDA-MB-231 and MCF-7 cells in CAI experiments, one word of caution is in order. In addition to the expression of CAIX activity on the plasma membrane (the normal expression pattern), MDA-MB-231 and MCF-7 cells have a significant amount of CAIX activity in their cytoplasm. Confocal microscopy investigation of CAIX expression (M75 antibody) in MDA-MB-231 and HT-1080 cells revealed that CAIX was partially localized in the cytoplasm of the MDA-MB-231 cells, whether it was completely localized on the plasma membrane of the HT-1080 cells. Speculations that at least a portion of the differences in sensitivity between cell lines to the selective inhibitors of CAIX and CAII are related to changes in the cellular localization of the respective isozymes are tempting130. Because experimental settings can be confusing, the results can be difficult to analyze. When tested against recombinant CAs, a significant proportion of the compounds had no detectable antiproliferative effect, although exhibiting different Ki values. The same is true for the situation, where antitumor effects are profound, but the Ki values are low. This may discourage further examination of such compound and their optimization for enhanced CA inhibition133.
Moreover, a growing body of evidence suggests that CAIX expression under hypoxic conditions may be dependent on the cancer cell line or the cancer type. In addition, it has been found that CAIX is overexpressed in some cancer cells, but not when the cells are exposed to hypoxia195. Adams et al. evaluated 30,216 immunohistochemical findings from 117 articles and discovered that CAIX expression rates in human invasive breast cancer were only 35%196, while Mayer et al. observed that elevated CAIX expression was present in hypoxic regions of some tumors, but it was absent in the others197. Moreover, it is of particular importance to distinguish between cancer cells that express CAIX as a result of an inactivating mutation in the pVHL tumor suppressor protein and tumors in which CAIX is present as a result of hypoxia and/or acidosis in the tumor microenvironment. The first category is dominated by clear cell renal cell carcinoma (ccRCC), which is characterized by the presence of an inactivating mutation or deletion of the VHL tumor suppressor gene and the presence of "constitutive" HIF stabilization and activation of HIF-regulated genes such as CAIX. This results in the expression of CAIX in more than 90% of these carcinomas, with CAIX being found in a significant proportion of tumor cells. The expression of CAIX, on the other hand, diminishes in more advanced ccRCC stages as a result of the transfer from the HIF-1 isoform to the HIF-2 isoform. This further complicates the use of CA inhibitors73.
Clinical translation
The dearth of adequate animal models and pharmacological agents that selectively inhibit tumor-related extracellular CAs without inhibiting intracellular CAs has hampered the preclinical and clinical evaluation of potential CAIX-selective treatments15. CA inhibitor design and effective CAIX targeting for anticancer purposes need further knowledge about their molecular characteristics, functional regulation mechanisms, and distribution, as well as different factors which may be unknown till now198. Furthermore, the perceived efficacy in a complex biological milieu can be influenced by many characteristics, including ligand residence time and metabolic stability. However, while screening for possible CAIX/XII inhibitors, the aforementioned criteria are not regularly taken into consideration133. Drug administration optimizations with phase I, II, and III clinical studies are required to determine the minimal effective inhibitory concentration in the brain and other human organ tissues suffering from cancer diseases to limit potential side effects. The topic of the relationship between in vitro CA IX/XII inhibition and in vivo anticancer activity of CAIs has been recently reviewed by Krasavin’s group133.
Despite the initial clinical success of anti-CAIX antibody therapy (girentuximab)167,168, further studies have not produced the expected results. ARISER (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Nonmetastatic RCC) was a phase 3 clinical trial that enrolled 864 high-risk patients with partial or complete nephrectomy associated with confirmed ccRCC (NCT00087022). The median age of the patients was 58 years. Participants were randomly assigned to 2 groups - the study group receiving an intravenous dose of 50 mg of girentximab in the first week, and 20 mg for the next 23 weeks199. In the same regimen, the placebo group was given as intravenous saline infusions. Disease recurrence was monitored by computed tomography. Immunohistological evaluation and exploratory biomarker analyzes were performed. The results showed no improvement in overall survival (OS) or disease-free survival (DFS). Despite the very good tolerance of the drug, no significant clinical benefit has been observed. It is suggested that the failure could have been related to the age of the patients. Changes in the immune system over time, reflecting impaired function and a deterioration in the immune response, seem to explain why girentuximab therapy has not been shown to be effective. However, confirmation of this theory requires detailed analysis199.
Resistance to CA inhibitors
In addition to CAs, there are other major molecules involved in pH regulation (described in section no. 2 of this review). Cancer cells targeted with CAIX/XII inhibitors will likely undergo a significant level of adaptation to maintain the intracellular pH (pHi). This hypothesis was supported by the finding that the combination of a proton pump inhibitor lansoprazole, which targets the V-ATPase ion/proton pump, and a CAIX antagonist, either S4 or FC9-399A, was more effective than either treatment alone in inhibiting the growth of Me30966 cells and triggering their apoptosis200. Chiche et al. found that silencing of CAIX causes overexpression of CAXII leaving extracellular acidification unaltered. CAIX also compensated for the absence of NHE1, another essential pH regulator201. Therefore, it is important to monitor the expression levels of other than CAIX crucial mediators of pH regulation in cells such as MCT1, MCT4, NHE1, or V-ATPase. It is worth mentioning that another compensatory mechanism include a decreased glucose metabolism and subsequent diminished production of acids202.
Combination therapies, synthetic lethality and multitargeting approach
Conventional monotherapies target actively proliferating cells, destroying both healthy and malignant cells. In contrast, combination therapy is nowadays a cornerstone of cancer treatment since it combines two or more therapeutic substances that target important pathways in a characteristically synergistic or additive manner190. Contrary to popular belief, patient-to-patient variability and independent pharmacological action is sufficient to explain the superiority of many FDA-approved medication combinations203. Nonetheless, if one of the drugs used in combination therapy is a chemotherapeutic agent, the toxicity is reduced since distinct pathways are addressed. Combination therapy may also be able to protect normal cells while killing cancer cells and be used as a way to combat drug resistance190. As a result of cancer cells' phenotypic plasticity, the therapeutic use of CAIX inhibitors is hampered by the possibility of undesired side effects as well as the development of compensatory mechanisms in cancer cells. Thus combination therapies involving various anticancer agents have been shown to exhibit profound antitumor efficiency when used with CAIX/CAXII inhibitors90,111,159–161. This topic has been recently reviewed in204.
Cancer cells can activate immune checkpoint pathways with immunosuppressive effects. Immune checkpoint monoclonal antibodies were a huge breakthrough in cancer therapy. Programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte protein 4 (CTLA-4) inhibitors have shown therapeutic promise, and some have been approved for use in cancer treatments, while others are still in clinical studies. However, the low response rate and immune-related side effects in some cancer patients prevent the widespread adoption of immune checkpoint therapy205. The tumor microenvironment is a barrier to immune function, as the acidic milieu of solid tumors caused by altered metabolism reduces immune activation. In metastatic melanoma and basal-like breast cancer TCGA cohorts, higher CAIX expression is associated with lower Th1 response. Combining SLC-0111 with immune checkpoint inhibitors increased Th1 response, lowered tumor growth, and reduced tumor metastasis. These findings imply that combined targeting CAIX with immune-checkpoint inhibitors may improve response and survival in hypoxic solid malignancies163.
Recently, the emphasis in cancer treatment has switched to the use of the synthetic lethality approach as a strategy to effectively tackle cancer without exerting a toxic effects in normal cells. Synthetic lethality occurs when two genes are connected in such a way that the absence of one maintains cell viability while the absence of both causes cell death. In its purest form, this would kill cancer cells while protecting healthy ones206. In response to hypoxia, pancreatic ductal adenocarcinomas (PDAC) cells that express activated GTPase KRAS upregulate the synthesis of CAIX, which regulates pH and glycolysis through the stabilization of HIF1A and HIF2A proteins. It is possible that disrupting this pathway may slow the formation of PDAC xenograft tumors in mice and may be used as the treatment modality in pancreatic cancer207. Unbalanced pH levels can also cause cell death. Intracellular acidification is associated with apoptosis, necroptosis, and autophagy. Intracellular alkalinization caused by the opioid painkiller JTC801 promotes alkaliptosis in cancer cells. Alkaliptosis appears to be a controlled kind of necrosis, as it releases endogenous damage-associated molecular patterns. For JTC801-induced alkaliptosis, oxidative stress and ion channel activation were not required. Rather, intracellular alkalinization is triggered by nuclear factor kappa B subunit p65 (NF-κB) activation. The NF-κB pathway is a prosurvival signaling system that acts in inflammatory conditions. Combining NF-κB activation with CAIX inhibition was shown to trigger alkaliptosis. CAIX knockdown restores alkalinization sensitivity in cells with low NF-κB activity. Furthermore, JCT801 was cytotoxic to cancer cells but not normal cells. One explanation is that the expression level of pH regulatory molecules like CAIX varies between cancer and normal cells208. Furthermore, it has been demonstrated that tumor hypoxia increases the radiation-sensitizing effects of olaparib through contextual synthetic lethality, and that tumor hypoxia may serve as a possible diagnostic for choosing patients who will benefit the most from the addition of olaparib to radiation treatment209. A similar approach was applied in the glioblastoma cells where E7070 sensitized cells to radio- and chemotherapy210. CAIX was used in a synthetic lethal CRISPR screen. Loss of CAIX affect multiple gene networks involved in cytoskeleton reorganization, DNA damage and cell cycle progression, redox regulation and mitochondria function, and others. Depletion of NFS1 cysteine desulfurase a critical factor in iron-sulfur cluster biogenesis or inhibition of xCT while targeting CAIX increased ferroptosis and decreased tumor development. Inhibition of CAIX decreased intracellular pH, increased reactive oxygen species buildup, and altered iron homeostasis. An alkaline intracellular pH may therefore suppress ferroptosis, a discovery that may lead to novel treatment approaches for solid tumors211. Recent years have seen the development of multitargeting approaches involving CA IX/XII inhibitors from a various different classes and different anticancer agents resulting in the generation of dual agents. These include the use of cytotoxins (monomethyl auristatin, tubulysin B, the maytansinoids, mertansine), antimalarial agents (artemisinin and dihydroartemisinin), EGFR antagonists, inhibitors of 15-lipoxygenase-cyclooxygenase 2, telomerase inhibitors, P-glycoprotein inhibitors, thioredoxin inhibitors, adenosine A2A receptor antagonists, pyrophosphatase/phosphodiesterase-3 inhibitors and others extensively reviewed by Supuran C.T.126,212
Conclusions
Cancer continues to be a major health burden worldwide. Despite continuous progress in anticancer drug development, most of the efforts fail to produce selective drug candidates for clinical use. This is attributed to many factors, including lack of specificity and concomitant side effects exhibited by non-selective agents. Nowadays, the approach to anti-cancer drug development has changed. Targeted therapies allow us to target cancerous cells without causing any effect on normal cells. This implies the need for the search for molecular targets specific to cancer cells and pivotal to their survival. Carbonic anhydrase IX and XII isoforms emerged as crucial anti-cancer molecular targets due to their expression being restricted to cancer cells and their pivotal function in cancer cell survival and metastasis. Many agents from distinct classes of chemical compounds were indicated as small-molecule CA-inhibitors. This field is dominated by sulfonamide group-containing compounds. Others include: sulfamates, coumarins, sulfocoumarins, and ureidobenzenesulfonamides. More recently, SLC-0111 ureidobenzenesulfonamide and its derivatives were identified as compounds with anti-cancer properties in solid tumors characterized by hypoxic and acidic conditions, which are likely to be non-responsive to chemotherapy and radiotherapy. SLC-0111 has also recently completed a Phase I clinical trial as an anti-cancer agent and is now being studied in Phase Ib/II clinical studies, where it is being evaluated as a monotherapy as well as in combination with other agents such as gemcitabine. Moreover, the aforementioned agents were also found to potentiate the anti-cancer effects of the existing conventional chemotherapeutic agents against colon, melanoma, and breast cancers by elevating the local pH to facilitate the permeability of the weakly basic anticancer agents like doxorubicin and 5-fluorouracil119. Various combinations of CA-inhibitors have been described in the literature. The majority of them exhibited synergic activity in cancer cells as described in204. Moreover, antibodies targeting CAIX and CAXII have been developed. Girentuximab (trade name Rencarex) was identified as an anti-tumor and anti-metastatic agent in patients with metastatic RCC who participated in Phase I and II clinical studies. Moreover, this antibody demonstrated good safety and tolerability, as well as a promising effectiveness profile167(p2),168.
In this review, we have presented the main approaches in targeting CAIX and CAXII with small molecules and monoclonal antibodies as well as indicated the main advantages and problems with their development and clinical implementation.
Author contributions
Conceptualization, M.K, A.G, S.M; writing—original draft preparation, M.K, A.G, S.M; writing—review and editing, M.K.; supervision, B.M, M.M, R.D and R.K. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. [DOI] [PubMed] [Google Scholar]
- 2.Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 3.Pinard MA, Mahon B, McKenna R.. Probing the surface of human carbonic anhydrase for clues towards the design of isoform specific inhibitors. Biomed Res Int 2015;2015:453543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward C, Meehan J, Gray M, Kunkler IH, Langdon SP, Argyle DJ.. Carbonic anhydrase IX (CAIX), cancer, and radiation responsiveness. Metabolites. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa Y, Uemura H, Hirao Y, Yoshida K, Saga S, Yoshikawa K.. Radiation hybrid mapping of the human MN/CA9 locus to chromosome band 9p12-p13. Genomics. 1998;53:118–19. [DOI] [PubMed] [Google Scholar]
- 6.Opavský R, Pastoreková S, Zelník V, et al. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics 1996;33:480–87. [DOI] [PubMed] [Google Scholar]
- 7.Waheed A, Sly WS, Doisy EA.. Carbonic anhydrase XII functions in health and disease. Gene 2017;623:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastorek J, Pastorekova S.. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64. [DOI] [PubMed] [Google Scholar]
- 9.Mboge MY, McKenna R, Frost SC.. Advances in anti-cancer drug development targeting carbonic anhydrase IX and XII. Top Anticancer Res 2015;5:3–42 [PMC free article] [PubMed] [Google Scholar]
- 10.Mahon BP, Pinard MA, McKenna R.. Targeting carbonic anhydrase IX activity and expression. Molecules 2015;20:2323–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Lomelino CL, Mboge MY, Frost SC, McKenna R.. Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules 2018;23:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS.. Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 2013;21:1570–82. [DOI] [PubMed] [Google Scholar]
- 13.De Simone G, Supuran CT.. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim Biophys Acta 2010;1804:404–9. [DOI] [PubMed] [Google Scholar]
- 14.Hulikova A, Zatovicova M, Svastova E, et al. Intact intracellular tail is critical for proper functioning of the tumor-associated, hypoxia-regulated carbonic anhydrase IX. FEBS Lett 2009;583:3563–8. [DOI] [PubMed] [Google Scholar]
- 15.McDonald PC, Winum JY, Supuran CT, Dedhar S.. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012;3:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch DR, Hurst DR.. Defining the hallmarks of metastasis. Cancer Res 2019;79:3011–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SA, Vanharanta S.. Epigenetic determinants of metastasis. Mol Oncol 2017;11:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García SA, Weitz J, Schölch S.. Circulating tumor cells. Methods Mol Biol 2018;1692:213–9. [DOI] [PubMed] [Google Scholar]
- 19.Balzer EM, Konstantopoulos K.. Intercellular adhesion: mechanisms for growth and metastasis of epithelial cancers. Wiley Interdiscip Rev Syst Biol Med 2012;4:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol 2013;591:2027–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muz B, de la Puente P, Azab F, Azab AK.. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015;3:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paredes F, Williams HC, San Martin A.. Metabolic adaptation in hypoxia and cancer. Cancer Lett 2021;502:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damaghi M, Wojtkowiak JW, Gillies RJ.. pH sensing and regulation in cancer. Front Physiol 2013;4:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre A, Harris AL.. The role of pH regulation in cancer progression. Recent Results Cancer Res 2016;207:93–134. [DOI] [PubMed] [Google Scholar]
- 25.Koltai T. Cancer: fundamentals behind pH targeting and the double-edged approach. Onco Targets Ther 2016;9:6343–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks SK, Pouysségur J.. Targeting pH regulating proteins for cancer therapy-progress and limitations. Semin Cancer Biol 2017;43:66–73. [DOI] [PubMed] [Google Scholar]
- 27.Asgharzadeh MR, Barar J, Pourseif MM, et al. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts 2017;7:115–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW.. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 2017;3:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Le A.. Glutamine metabolism in cancer. Adv Exp Med Biol 2018;1063:13–32. [DOI] [PubMed] [Google Scholar]
- 30.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y.. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 2020;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda-Gonçalves V, Granja S, Martinho O, et al. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget 2016;7:46335–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine AJ, Puzio-Kuter AM.. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010;330:1340–4. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen DX, Massagué J.. Genetic determinants of cancer metastasis. Nat Rev Genet 2007;8:341–52. [DOI] [PubMed] [Google Scholar]
- 34.Chiang AC, Massagué J.. Molecular basis of metastasis. N Engl J Med 2008;359:2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaelin WG, Ratcliffe PJ.. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393–402. [DOI] [PubMed] [Google Scholar]
- 36.Lendahl U, Lee KL, Yang H, Poellinger L.. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet 2009;10:821–32. [DOI] [PubMed] [Google Scholar]
- 37.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L.. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1α signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem 2006;281:25903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S.. Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL–/– renal cancer. Cancer Res 2005;65:5221–30. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Koo KH, Park JW, et al. HIF-1 is induced via EGFR activation and mediates resistance to anoikis-like cell death under lipid rafts/caveolae-disrupting stress. Carcinogenesis 2009;30:1997–2004. [DOI] [PubMed] [Google Scholar]
- 40.El Guerrab A, Zegrour R, Nemlin CC, et al. Differential impact of EGFR-targeted therapies on hypoxia responses: implications for treatment sensitivity in triple-negative metastatic breast cancer. PLOS One 2011;6:e25080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q li, Cui B ri, Li H yan, et al. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem Biophys Res Commun 2013;438:507–12. [DOI] [PubMed] [Google Scholar]
- 42.Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J.. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem 2003;278:14013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khandrika L, Lieberman R, Koul S, et al. Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer. Oncogene 2009;28:1248–60. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Zhang Z, Yao L, Yang J, Wang Z, Du G.. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol Med Rep 2018;18:3547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK.. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ 2001;12:363–9. [PubMed] [Google Scholar]
- 46.Ditte P, Dequiedt F, Svastova E, et al. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 2011;71:7558–67. [DOI] [PubMed] [Google Scholar]
- 47.Corbet C, Feron O.. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 2017;17:577–93. [DOI] [PubMed] [Google Scholar]
- 48.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 2000;60:7075–83. [PubMed] [Google Scholar]
- 49.Chia SK, Wykoff CC, Watson PH, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol 2001;19:3660–8. [DOI] [PubMed] [Google Scholar]
- 50.Hynninen P, Vaskivuo L, Saarnio J, et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology 2006;49:594–602. [DOI] [PubMed] [Google Scholar]
- 51.Panisova E, Kery M, Sedlakova O, et al. Lactate stimulates CA IX expression in normoxic cancer cells. Oncotarget 2017;8:77819–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiaschi T, Giannoni E, Taddei ML, et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial-mesenchymal transition in prostate carcinoma cells. Cell Cycle 2013;12:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaluz S, Kaluzová M, Liao SY, Lerman M, Stanbridge EJ.. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim Biophys Acta 2009;1795:162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stillebroer AB, Mulders PFA, Boerman OC, Oyen WJG, Oosterwijk E.. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol 2010;58:75–83. [DOI] [PubMed] [Google Scholar]
- 55.Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 2011;99:424–31. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Jiang L, Chew SH, Hirayama T, Sekido Y, Toyokuni S.. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol 2019;26:101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb BA, Chimenti M, Jacobson MP, Barber DL.. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011;11:671–7. [DOI] [PubMed] [Google Scholar]
- 58.Persi E, Duran-Frigola M, Damaghi M, et al. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat Commun 2018;9:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sansone P, Piazzi G, Paterini P, et al. Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J Cell Mol Med 2009;13:3876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson N, Potter C, Harris AL.. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 2004;64():6160–5. [DOI] [PubMed] [Google Scholar]
- 61.Swayampakula M, McDonald PC, Vallejo M, et al. The interactome of metabolic enzyme carbonic anhydrase IX reveals novel roles in tumor cell migration and invadopodia/MMP14-mediated invasion. Oncogene 2017;36:6244–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radvak P, Repic M, Svastova E, et al. Suppression of carbonic anhydrase IX leads to aberrant focal adhesion and decreased invasion of tumor cells. Oncol Rep 2013;29:1147–53. [DOI] [PubMed] [Google Scholar]
- 63.Fujiwara D, Kato K, Nohara S, Iwanuma Y, Kajiyama Y.. The usefulness of three-dimensional cell culture in induction of cancer stem cells from esophageal squamous cell carcinoma cell lines. Biochem Biophys Res Commun 2013;434:773–8. [DOI] [PubMed] [Google Scholar]
- 64.Axelson H, Fredlund E, Ovenberger M, Landberg G, Påhlman S.. Hypoxia-induced dedifferentiation of tumor cells–a mechanism behind heterogeneity and aggressiveness of solid tumors. Semin Cell Dev Biol 2005;16:554–63. [DOI] [PubMed] [Google Scholar]
- 65.Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013;32:5210–9. [DOI] [PubMed] [Google Scholar]
- 66.Currie MJ, Beardsley BE, Harris GC, et al. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: relationships with markers of tumor hypoxia and microvascularity. Hum Pathol 2013;44:402–11. [DOI] [PubMed] [Google Scholar]
- 67.Svastová E, Zilka N, Zat’ovicová M, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res 2003;290:332–45. [DOI] [PubMed] [Google Scholar]
- 68.Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY.. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci 2011;124:1077–87. [DOI] [PubMed] [Google Scholar]
- 69.Bourguignon LYW, Singleton PA, Diedrich F, Stern R, Gilad E.. CD44 interaction with Na+-H + exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem 2004;279:26991–7007. [DOI] [PubMed] [Google Scholar]
- 70.Bourguignon LY, Zhu H, Shao L, Chen YW.. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem 2001;276:7327–36. [DOI] [PubMed] [Google Scholar]
- 71.Csaderova L, Debreova M, Radvak P, et al. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Front Physiol 2013;4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riemann A, Rauschner M, Gießelmann M, Reime S, Haupt V, Thews O.. Extracellular acidosis modulates the expression of epithelial-mesenchymal transition (EMT) markers and adhesion of epithelial and tumor cells. Neoplasia. 2019;21:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pastorekova S, Gillies RJ.. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev 2019;38:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamali S, Klier M, Ames S, et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci Rep 2015;5:13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deitmer JW, Theparambil SM, Ruminot I, Becker HM.. The role of membrane acid/base transporters and carbonic anhydrases for cellular pH and metabolic processes. Front Neurosci 2015;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald PC, Swayampakula M, Dedhar S.. Coordinated regulation of metabolic transporters and migration/invasion by carbonic anhydrase IX. Metabolites 2018;8:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pore N, Jalla S, Liu Z, et al. In vivo loss of function screening reveals carbonic anhydrase IX as a key modulator of tumor initiating potential in primary pancreatic tumors. Neoplasia 2015;17:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zandberga E, Zayakin P, Ābols A, Pūpola D, Trapencieris P, Linē A.. Depletion of carbonic anhydrase IX abrogates hypoxia-induced overexpression of stanniocalcin-1 in triple negative breast cancer cells. Cancer Biol Ther 2017;18:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doyen J, Parks SK, Marcié S, Pouysségur J, Chiche J.. Knock-down of hypoxia-induced carbonic anhydrases IX and XII radiosensitizes tumor cells by increasing intracellular acidosis. Front Oncol 2013;2:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang L, Xu G, Li Z, et al. RNAi-mediated knockdown of CAIX enhances the radiosensitivity of nasopharyngeal carcinoma cell line, CNE-2. Onco Targets Ther 2017;10:4701–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gut MO, Parkkila S, Vernerová Z, et al. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology 2002;123:1889–1903. [DOI] [PubMed] [Google Scholar]
- 82.Pan P wen, Parkkila AK, Autio S, et al. Brain phenotype of carbonic anhydrase IX-deficient mice. Transgenic Res 2012;21:163–76. [DOI] [PubMed] [Google Scholar]
- 83.Li T, Liu X, Riederer B, et al. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol 2018;222:e12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pastoreková S, Parkkila S, Parkkila AK, et al. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997;112:398–408. [DOI] [PubMed] [Google Scholar]
- 85.Nordfors K, Haapasalo J, Korja M, et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer 2010;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ilie MI, Hofman V, Ortholan C, et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer 2011;128:1614–23. [DOI] [PubMed] [Google Scholar]
- 87.Tafreshi NK, Lloyd MC, Proemsey JB, et al. Evaluation of CAIX and CAXII expression in breast cancer at varied O2 levels: CAIX is the superior surrogate imaging biomarker of tumor hypoxia. Mol Imaging Biol 2016;18:219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Proescholdt MA, Merrill MJ, Stoerr EM, Lohmeier A, Pohl F, Brawanski A.. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol 2012;14:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cetin B, Gonul II, Gumusay O, et al. Carbonic anhydrase IX is a prognostic biomarker in glioblastoma multiforme. Neuropathology 2018;38:457–62. [DOI] [PubMed] [Google Scholar]
- 90.McIntyre A, Patiar S, Wigfield S, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res 2012;18:3100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ.. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm 2011;8:2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vullo D, Franchi M, Gallori E, et al. Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2003;13:1005–9. [DOI] [PubMed] [Google Scholar]
- 93.Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G.. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 94.McKenna R, Supuran CT.. Carbonic anhydrase inhibitors drug design. Subcell Biochem 2014;75:291–323. [DOI] [PubMed] [Google Scholar]
- 95.Anduran E, Aspatwar A, Parvathaneni NK, et al. Hypoxia-activated prodrug derivatives of carbonic anhydrase inhibitors in benzenesulfonamide series: synthesis and biological evaluation. Molecules 2020;25:E2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong BCK, Zhang H, Qin L, et al. Carbonic anhydrase IX-directed immunoliposomes for targeted drug delivery to human lung cancer cells in vitro. Drug Des Devel Ther 2014;8:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ng HLH, Lu A, Lin G, Qin L, Yang Z.. The potential of liposomes with carbonic anhydrase IX to deliver anticancer ingredients to cancer cells in vivo. Int J Mol Sci 2014;16:230–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 2011;71:3364–76. [DOI] [PubMed] [Google Scholar]
- 99.Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem 2012;55:5591–600. [DOI] [PubMed] [Google Scholar]
- 100.Ahlskog JKJ, Dumelin CE, Trüssel S, Mårlind J, Neri D.. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg Med Chem Lett. 2009;19:4851–56. [DOI] [PubMed] [Google Scholar]
- 101.Cianchi F, Vinci MC, Supuran CT, et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther 2010;334:710–9. [DOI] [PubMed] [Google Scholar]
- 102.Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 2011;54:1896–902. [DOI] [PubMed] [Google Scholar]
- 103.Vannozzi G, Vullo D, Angeli A, et al. One-pot procedure for the synthesis of asymmetric substituted ureido benzene sulfonamides as effective inhibitors of carbonic anhydrase enzymes. J Med Chem. 2022;65:824–37. [DOI] [PubMed] [Google Scholar]
- 104.Koyuncu I, Tülüce Y, Slahaddin Qadir H, Durgun M, Supuran CT.. Evaluation of the anticancer potential of a sulphonamide carbonic anhydrase IX inhibitor on cervical cancer cells. J Enzyme Inhib Med Chem 2019;34:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tülüce Y, Ahmed BA, Koyuncu İ, Durgun M.. The cytotoxic, apoptotic and oxidative effects of carbonic anhydrase IX inhibitor on colorectal cancer cells. J Bioenerg Biomembr 2018;50:107–16. [DOI] [PubMed] [Google Scholar]
- 106.Winum JY, Vullo D, Casini A, Montero JL, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Inhibition of cytosolic isozymes I and II and transmembrane, tumor-associated isozyme IX with sulfamates including EMATE also acting as steroid sulfatase inhibitors. J Med Chem 2003;46:2197–204. [DOI] [PubMed] [Google Scholar]
- 107.Pettersen EO, Ebbesen P, Gieling RG, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem 2015;30:689–721. [DOI] [PubMed] [Google Scholar]
- 108.Hektoen HH, Ree AH, Redalen KR, Flatmark K.. Sulfamate inhibitor S4 influences carbonic anhydrase IX ectodomain shedding in colorectal carcinoma cells. J Enzyme Inhib Med Chem 2016;31:779–86. [DOI] [PubMed] [Google Scholar]
- 109.Ward C, Meehan J, Mullen P, et al. Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget 2015;6:24856–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raez L, Samuels M, Lilenbaum R.. Combined modality therapy for limited-disease small cell lung cancer. Curr Treat Options Oncol 2005;6:69–74. [DOI] [PubMed] [Google Scholar]
- 111.Bryant JL, Gieling RG, Meredith SL, et al. Novel carbonic anhydrase IX-targeted therapy enhances the anti-tumour effects of cisplatin in small cell lung cancer. Int J Cancer 2018;142:191–201. [DOI] [PubMed] [Google Scholar]
- 112.Gieling RG, Parker CA, De Costa LA, et al. Inhibition of carbonic anhydrase activity modifies the toxicity of doxorubicin and melphalan in tumour cells in vitro. J Enzyme Inhib Med Chem 2013;28:360–369. [DOI] [PubMed] [Google Scholar]
- 113.Buller F, Steiner M, Frey K, et al. Selection of carbonic anhydrase IX inhibitors from one million DNA-encoded compounds. ACS Chem Biol. 2011;6:336–44. [DOI] [PubMed] [Google Scholar]
- 114.Tykvart J, Navrátil V, Kugler M, et al. Identification of novel carbonic anhydrase IX inhibitors using high-throughput screening of pooled compound libraries by DNA-linked inhibitor antibody assay (DIANA). SLAS Discov 2020;25:1026–37. [DOI] [PubMed] [Google Scholar]
- 115.Chahal V, Nirwan S, Pathak M, Kakkar R.. Identification of potent human carbonic anhydrase IX inhibitors: a combination of pharmacophore modeling, 3D-QSAR, virtual screening and molecular dynamics simulations. J Biomol Struct Dyn 2020;15:1–16. [DOI] [PubMed] [Google Scholar]
- 116.Lomelino CL, Murray AB, Supuran CT, McKenna R.. Sweet binders: carbonic anhydrase IX in complex with sucralose. ACS Med Chem Lett 2018;9:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cuffaro D, Nuti E, Rossello A.. An overview of carbohydrate-based carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2020;35:1906–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winum JY, Colinas PA, Supuran CT.. Glycosidic carbonic anhydrase IX inhibitors: a sweet approach against cancer. Bioorg Med Chem 2013;21:1419–26. [DOI] [PubMed] [Google Scholar]
- 119.Andreucci E, Ruzzolini J, Peppicelli S, et al. The carbonic anhydrase IX inhibitor SLC-0111 sensitises cancer cells to conventional chemotherapy. J Enzyme Inhib Med Chem. 2019;34:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peppicelli S, Andreucci E, Ruzzolini J, et al. The Carbonic Anhydrase IX inhibitor SLC-0111 as emerging agent against the mesenchymal stem cell-derived pro-survival effects on melanoma cells. J Enzyme Inhib Med Chem 2020;35:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Riemann A, Güttler A, Haupt V, et al. Inhibition of carbonic anhydrase IX by ureidosulfonamide inhibitor U104 reduces prostate cancer cell growth, but does not modulate daunorubicin or cisplatin cytotoxicity. Oncol Res 2018;26:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mussi S, Rezzola S, Chiodelli P, Nocentini A, Supuran CT, Ronca R.. Antiproliferative effects of sulphonamide carbonic anhydrase inhibitors C18, SLC-0111 and acetazolamide on bladder, glioblastoma and pancreatic cancer cell lines. J Enzyme Inhib Med Chem 2022;37:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Angeli A, Carta F, Nocentini A, et al. Carbonic anhydrase inhibitors targeting metabolism and tumor microenvironment. Metabolites. 2020;10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs. 2018;27:963–70. [DOI] [PubMed] [Google Scholar]
- 125.McDonald PC, Chia S, Bedard PL, et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol 2020;43:484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Supuran CT. Carbonic anhydrase inhibitors: an update on experimental agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2021;30:1197–208. [DOI] [PubMed] [Google Scholar]
- 127.Elimam DM, Eldehna WM, Salem R, et al. Natural inspired ligustrazine-based SLC-0111 analogues as novel carbonic anhydrase inhibitors. Eur J Med Chem 2022;228:114008. [DOI] [PubMed] [Google Scholar]
- 128.Elbadawi MM, Eldehna WM, Nocentini A, et al. Identification of N-phenyl-2-(phenylsulfonyl)acetamides/propanamides as new SLC-0111 analogues: synthesis and evaluation of the carbonic anhydrase inhibitory activities. Eur J Med Chem 2021;218:113360. [DOI] [PubMed] [Google Scholar]
- 129.Shaldam M, Eldehna WM, Nocentini A, et al. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur J Med Chem 2021;216:113283. [DOI] [PubMed] [Google Scholar]
- 130.Williams KJ, Gieling RG.. Preclinical evaluation of ureidosulfamate carbonic anhydrase IX/XII inhibitors in the treatment of cancers. Int J Mol Sci 2019;20:E6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mboge MY, Combs J, Singh S, et al. Inhibition of carbonic anhydrase using SLC-149: support for a noncatalytic function of CAIX in breast cancer. J Med Chem 2021;64:1713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarnella A, D’Avino G, Hill BS, et al. A novel inhibitor of carbonic anhydrases prevents hypoxia-induced TNBC cell plasticity. Int J Mol Sci 2020;21:8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krasavin M, Kalinin S, Sharonova T, Supuran CT.. Inhibitory activity against carbonic anhydrase IX and XII as a candidate selection criterion in the development of new anticancer agents. J Enzyme Inhib Med Chem 2020;35:1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dvořanová J, Kugler M, Holub J, et al. Sulfonamido carboranes as highly selective inhibitors of cancer-specific carbonic anhydrase IX. Eur J Med Chem 2020;200:112460. [DOI] [PubMed] [Google Scholar]
- 135.Kugler M, Holub J, Brynda J, et al. The structural basis for the selectivity of sulfonamido dicarbaboranes toward cancer-associated carbonic anhydrase IX. J Enzyme Inhib Med Chem 2020;35:1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma V, Kumar R, Angeli A, Supuran CT, Sharma PK.. Tail approach synthesis of novel benzenesulfonamides incorporating 1,3,4-oxadiazole hybrids as potent inhibitor of carbonic anhydrase I, II, IX, and XII isoenzymes. Eur J Med Chem 2020;193:112219. [DOI] [PubMed] [Google Scholar]
- 137.Uslu AG, Gür Maz T, Nocentini A, Banoglu E, Supuran CT, Çalışkan B.. Benzimidazole derivatives as potent and isoform selective tumor-associated carbonic anhydrase IX/XII inhibitors. Bioorg Chem 2020;95:103544. [DOI] [PubMed] [Google Scholar]
- 138.Pustenko A, Nocentini A, Balašova A, Krasavin M, Žalubovskis R, Supuran CT.. 7-Acylamino-3H-1,2-benzoxathiepine 2,2-dioxides as new isoform-selective carbonic anhydrase IX and XII inhibitors. J Enzyme Inhib Med Chem 2020;35:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pustenko A, Nocentini A, Balašova A, et al. Aryl derivatives of 3H-1,2-benzoxathiepine 2,2-dioxide as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2020;35:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.El-Azab AS, Abdel-Aziz AAM, Bua S, et al. S-substituted 2-mercaptoquinazolin-4(3H)-one and 4-ethylbenzensulfonamides act as potent and selective human carbonic anhydrase IX and XII inhibitors. J Enzyme Inhib Med Chem 2020;35:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Angeli A, Tanini D, Nocentini A, et al. Selenols: a new class of carbonic anhydrase inhibitors. Chem Commun. 2019;55:648–51. [DOI] [PubMed] [Google Scholar]
- 142.Krasavin M, Žalubovskis R, Grandāne A, Domračeva I, Zhmurov P, Supuran CT.. Sulfocoumarins as dual inhibitors of human carbonic anhydrase isoforms IX/XII and of human thioredoxin reductase. J Enzyme Inhib Med Chem 2020;35:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Buran K, Bua S, Poli G, Önen Bayram FE, Tuccinardi T, Supuran CT.. Novel 8-substituted coumarins that selectively inhibit human carbonic anhydrase IX and XII. Int J Mol Sci 2019;20:E1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chandra KM, Goud NS, Arifuddin M, et al. Synthesis and biological evaluation of novel 4,7-disubstituted coumarins as selective tumor-associated carbonic anhydrase IX and XII inhibitors. Bioorg Med Chem Lett 2021;39:127877. [DOI] [PubMed] [Google Scholar]
- 145.Meleddu R, Deplano S, Maccioni E, et al. Selective inhibition of carbonic anhydrase IX and XII by coumarin and psoralen derivatives. J Enzyme Inhib Med Chem 2021;36:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thacker PS, Srikanth D, Angeli A, et al. Coumarin-thiourea hybrids show potent carbonic anhydrase IX and XIII inhibitory action. ChemMedChem 2021;16:1252–6. [DOI] [PubMed] [Google Scholar]
- 147.Supuran CT, Alterio V, Di Fiore A, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836. [DOI] [PubMed] [Google Scholar]
- 148.Angeli A, Peat TS, Selleri S, Saleh Alfawaz Altamimi A, Supuran CT, Carta F.. X-ray crystallography of epacadostat in adduct with carbonic anhydrase IX. Bioorg Chem 2020;97:103669. [DOI] [PubMed] [Google Scholar]
- 149.Akocak S, Güzel-Akdemir Ö, Kishore Kumar Sanku R, et al. Pyridinium derivatives of 3-aminobenzenesulfonamide are nanomolar-potent inhibitors of tumor-expressed carbonic anhydrase isozymes CA IX and CA XII. Bioorg Chem 2020;103:104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nocentini A, Bua S, Lomelino CL, et al. Discovery of new sulfonamide carbonic anhydrase IX inhibitors incorporating nitrogenous bases. ACS Med Chem Lett 2017;8:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J, Shi K, Sabet ZF, et al. New power of self-assembling carbonic anhydrase inhibitor: short peptide-constructed nanofibers inspire hypoxic cancer therapy. Sci Adv 2019;5:eaax0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mishra CB, Tiwari M, Supuran CT.. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med Res Rev 2020;40:2485–565. [DOI] [PubMed] [Google Scholar]
- 154.Supuran CT. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin Drug Discov 2020;15:671–86. [DOI] [PubMed] [Google Scholar]
- 155.Parkkila S, Innocenti A, Kallio H, Hilvo M, Scozzafava A, Supuran CT.. The protein tyrosine kinase inhibitors imatinib and nilotinib strongly inhibit several mammalian alpha-carbonic anhydrase isoforms. Bioorg Med Chem Lett 2009;19:4102–6. [DOI] [PubMed] [Google Scholar]
- 156.Temperini C, Innocenti A, Mastrolorenzo A, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Interaction of the antiepileptic drug sulthiame with twelve mammalian isoforms: kinetic and X-ray crystallographic studies. Bioorg Med Chem Lett 2007;17:4866–72. [DOI] [PubMed] [Google Scholar]
- 157.Weber A, Casini A, Heine A, et al. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. J Med Chem 2004;47:550–7. [DOI] [PubMed] [Google Scholar]
- 158.Mahboubi-Rabbani M, Zarghi A.. Dual human carbonic anhydrase/cyclooxygenase-2 inhibitors: a promising approach for cancer treatment. Anticancer Agents Med Chem 2021. [DOI] [PubMed] [Google Scholar]
- 159.Cho EJ, Yu SJ, Kim K, et al. Carbonic anhydrase-IX inhibition enhances the efficacy of hexokinase II inhibitor for hepatocellular carcinoma in a murine model. J Bioenerg Biomembr 2019;51:121–9. [DOI] [PubMed] [Google Scholar]
- 160.Shabana AM, Mondal UK, Alam MR, et al. pH-sensitive multiligand gold nanoplatform targeting carbonic anhydrase IX enhances the delivery of doxorubicin to Hypoxic tumor spheroids and overcomes the hypoxia-induced chemoresistance. ACS Appl Mater Interfaces 2018;10:17792–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boyd NH, Walker K, Fried J, et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2017;2:92928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Logsdon DP, Grimard M, Luo M, et al. Regulation of HIF1α under hypoxia by APE1/Ref-1 impacts CA9 expression: dual targeting in patient-derived 3D pancreatic cancer models. Mol Cancer Ther 2016;15:2722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chafe SC, McDonald PC, Saberi S, et al. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol Res 2019;7:1064–78. [DOI] [PubMed] [Google Scholar]
- 164.Ruzzolini J, Laurenzana A, Andreucci E, et al. A potentiated cooperation of carbonic anhydrase IX and histone deacetylase inhibitors against cancer. J Enzyme Inhib Med Chem 2020;35:391–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Supuran CT. Experimental carbonic anhydrase inhibitors for the treatment of hypoxic tumors. J Exp Pharmacol 2020;12:603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zatovicova M, Jelenska L, Hulikova A, et al. Monoclonal antibody G250 targeting CA IX: binding specificity, internalization and therapeutic effects in a non-renal cancer model. Int J Oncol 2014;45:2455–67. [DOI] [PubMed] [Google Scholar]
- 167.Siebels M, Rohrmann K, Oberneder R, et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol 2011;29:121–6. [DOI] [PubMed] [Google Scholar]
- 168.Bleumer I, Knuth A, Oosterwijk E, et al. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer 2004;90:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Testa C, Papini AM, Zeidler R, et al. First studies on tumor associated carbonic anhydrases IX and XII monoclonal antibodies conjugated to small molecule inhibitors. J Enzyme Inhib Med Chem 2022;37:592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Antal I, Koneracka M, Kubovcikova M, et al. Targeting of carbonic anhydrase IX-positive cancer cells by glycine-coated superparamagnetic nanoparticles. Colloids Surf B Biointerfaces 2021;205:111893. [DOI] [PubMed] [Google Scholar]
- 171.Cazzamalli S, Dal Corso A, Widmayer F, Neri D.. Chemically defined antibody- and small molecule-drug conjugates for in vivo tumor targeting applications: a comparative analysis. J Am Chem Soc. 2018;140:1617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tatiparti K, Rauf MA, Sau S, Iyer AK.. Carbonic anhydrase-IX guided albumin nanoparticles for hypoxia-mediated triple-negative breast cancer cell killing and imaging of patient-derived tumor. Molecules 2020;25:2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Santos E da S, Nogueira KAB, Fernandes LCC, et al. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int J Pharm 2021;592:120082. [DOI] [PubMed] [Google Scholar]
- 174.Krall N, Pretto F, Decurtins W, Bernardes GJL, Supuran CT, Neri D.. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew Chem Int Ed Engl 2014;53:4231–5. [DOI] [PubMed] [Google Scholar]
- 175.Petrul HM, Schatz CA, Kopitz CC, et al. Therapeutic mechanism and efficacy of the antibody-drug conjugate BAY 79-4620 targeting human carbonic anhydrase 9. Mol Cancer Ther 2012;11:340–9. [DOI] [PubMed] [Google Scholar]
- 176.Zatovicova M, Jelenska L, Hulikova A, et al. Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des 2010;16:3255–63. [DOI] [PubMed] [Google Scholar]
- 177.Thacker PS, Alvala M, Arifuddin M, Angeli A, Supuran CT.. Design, synthesis and biological evaluation of coumarin-3-carboxamides as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem 2019;86:386–92. [DOI] [PubMed] [Google Scholar]
- 178.Thacker PS, Angeli A, Argulwar OS, Tiwari PL, Arifuddin M, Supuran CT.. Design, synthesis and biological evaluation of coumarin linked 1,2,4-oxadiazoles as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem 2020;98:103739. [DOI] [PubMed] [Google Scholar]
- 179.Thacker PS, Sridhar Goud N, Argulwar OS, et al. Synthesis and biological evaluation of some coumarin hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg Chem 2020;104:104272. [DOI] [PubMed] [Google Scholar]
- 180.Abdelrahman MA, Eldehna WM, Nocentini A, et al. Novel benzofuran-based sulphonamides as selective carbonic anhydrases IX and XII inhibitors: synthesis and in vitro biological evaluation. J Enzyme Inhib Med Chem 2020;35:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abdelrahman MA, Ibrahim HS, Nocentini A, et al. Novel 3-substituted coumarins as selective human carbonic anhydrase IX and XII inhibitors: synthesis, biological and molecular dynamics analysis. Eur J Med Chem 2021;209:112897. [DOI] [PubMed] [Google Scholar]
- 182.Mboge MY, Chen Z, Wolff A, et al. Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: disconnect between activity and growth inhibition. PLOS One 2018;13:e0207417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 183.Battke C, Kremmer E, Mysliwietz J, et al. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol Immunother 2011;60:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gondi G, Mysliwietz J, Hulikova A, et al. Antitumor efficacy of a monoclonal antibody that inhibits the activity of cancer-associated carbonic anhydrase XII. Cancer Res 2013;73:6494–503. [DOI] [PubMed] [Google Scholar]
- 185.von Neubeck B, Gondi G, Riganti C, et al. An inhibitory antibody targeting carbonic anhydrase XII abrogates chemoresistance and significantly reduces lung metastases in an orthotopic breast cancer model in vivo. Int J Cancer 2018;143:2065–75. [DOI] [PubMed] [Google Scholar]
- 186.Uda NR, Stenner F, Seibert V, et al. Humanized monoclonal antibody blocking carbonic anhydrase 12 enzymatic activity leads to reduced tumor growth in vitro. Anticancer Res 2019;39:4117–28. [DOI] [PubMed] [Google Scholar]
- 187.Dekaminaviciute D, Kairys V, Zilnyte M, et al. Monoclonal antibodies raised against 167-180 aa sequence of human carbonic anhydrase XII inhibit its enzymatic activity. J Enzyme Inhib Med Chem 2014;29:804–10. [DOI] [PubMed] [Google Scholar]
- 188.Moeker J, Peat TS, Bornaghi LF, Vullo D, Supuran CT, Poulsen SA.. Cyclic secondary sulfonamides: unusually good inhibitors of cancer-related carbonic anhydrase enzymes. J Med Chem 2014;57:3522–31. [DOI] [PubMed] [Google Scholar]
- 189.Eldehna WM, Taghour MS, Al-Warhi T, et al. Discovery of 2,4-thiazolidinedione-tethered coumarins as novel selective inhibitors for carbonic anhydrase IX and XII isoforms. J Enzyme Inhib Med Chem 2022;37:531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mokhtari RB, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget 2017;8:38022–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Havránková E, Garaj V, Mascaretti Š, et al. Novel 1,3,5-triazinyl aminobenzenesulfonamides incorporating aminoalcohol, aminochalcone and aminostilbene structural motifs as potent anti-VRE agents, and carbonic anhydrases I, II, VII, IX, and XII inhibitors. Int J Mol Sci 2021;23:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giovannuzzi S, D’Ambrosio M, Luceri C, et al. Aromatic sulfonamides including a sulfonic acid tail: new membrane impermeant carbonic anhydrase inhibitors for targeting selectively the cancer-associated isoforms. Int J Mol Sci 2021;23:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Abdel-Mohsen HT, El Kerdawy AM, Omar MA, et al. Application of the dual-tail approach for the design and synthesis of novel thiopyrimidine-benzenesulfonamide hybrids as selective carbonic anhydrase inhibitors. Eur J Med Chem 2022;228:114004. [DOI] [PubMed] [Google Scholar]
- 194.Eldeeb AH, Abo-Ashour MF, Angeli A, et al. Novel benzenesulfonamides aryl and arylsulfone conjugates adopting tail/dual tail approaches: synthesis, carbonic anhydrase inhibitory activity and molecular modeling studies. Eur J Med Chem 2021;221:113486. [DOI] [PubMed] [Google Scholar]
- 195.Li J, Zhang G, Wang X, Li XF.. Is carbonic anhydrase IX a validated target for molecular imaging of cancer and hypoxia? Future Oncol 2015;11:1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Adams A, van Brussel ASA, Vermeulen JF, et al. The potential of hypoxia markers as target for breast molecular imaging–a systematic review and meta-analysis of human marker expression. BMC Cancer 2013;13:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mayer A, Höckel M, Vaupel P.. Carbonic anhydrase IX expression and tumor oxygenation status do not correlate at the microregional level in locally advanced cancers of the uterine cervix. Clin Cancer Res 2005;11:7220–5. [DOI] [PubMed] [Google Scholar]
- 198.Said HM, Supuran CT, Hageman C, et al. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des 2010;16:3288–99. [DOI] [PubMed] [Google Scholar]
- 199.Chamie K, Donin NM, Klöpfer P, et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 2017;3:913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Federici C, Lugini L, Marino ML, et al. Lansoprazole and carbonic anhydrase IX inhibitors sinergize against human melanoma cells. J Enzyme Inhib Med Chem 2016;31:119–125. [DOI] [PubMed] [Google Scholar]
- 201.Chiche J, Ilc K, Laferrière J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009;69:358–68. [DOI] [PubMed] [Google Scholar]
- 202.Meijer TWH, Bussink J, Zatovicova M, et al. Tumor microenvironmental changes induced by the sulfamate carbonic anhydrase IX inhibitor S4 in a laryngeal tumor model. PLOS One. 2014;9:e108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Palmer AC, Sorger PK.. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell 2017;171:1678–1691.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kalinin S, Malkova A, Sharonova T, et al. Carbonic anhydrase IX inhibitors as candidates for combination therapy of solid tumors. Int J Mol Sci 2021;22:13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Darvin P, Toor SM, Sasidharan Nair V, Elkord E.. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Topatana W, Juengpanich S, Li S, et al. Advances in synthetic lethality for cancer therapy: cellular mechanism and clinical translation. J Hematol Oncol 2020;13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McDonald PC, Chafe SC, Brown WS, et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology 2019;157:823–37. [DOI] [PubMed] [Google Scholar]
- 208.Song X, Zhu S, Xie Y, et al. JTC801 induces pH-dependent death specifically in cancer cells and slows growth of tumors in mice. Gastroenterology 2018;154:1480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jiang Y, Verbiest T, Devery AM, et al. Hypoxia potentiates the radiation-sensitizing effect of olaparib in human non-small cell lung cancer xenografts by contextual synthetic lethality. Int J Radiat Oncol Biol Phys 2016;95:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Teixeira SA, Viapiano MS, Andrade AF, et al. The carbonic anhydrase inhibitor E7070 sensitizes glioblastoma cells to radio- and chemotherapy and reduces tumor growth. Mol Neurobiol 2021;58:4520–34. [DOI] [PubMed] [Google Scholar]
- 211.Chafe SC, Vizeacoumar FS, Venkateswaran G, et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv 2021;7:eabj0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Supuran CT. Multitargeting approaches involving carbonic anhydrase inhibitors: hybrid drugs against a variety of disorders. J Enzyme Inhib Med Chem. 2021;36:1702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]