Abstract
Ixodes scapularis, the tick vector of Lyme disease and human granulocytic ehrlichiosis (HGE), is prevalent in much of southern New York state. The distribution of this species has increased, as have reported cases of both Lyme disease and HGE. The unreliability of case reports, however, demonstrates the need for tick and pathogen surveillance in order to accurately define areas of high risk. In this study, a total of 89,550 m2 at 34 study sites was drag sampled in 1995 and a total of 51,540 m2 at 40 sites was sampled in 1996 to determine tick and pathogen distribution in southern New York state. I. scapularis was collected from 90% of the sites sampled, and regionally, a 2.5-fold increase in nymphal abundance occurred from 1995 to 1996. I. scapularis individuals from all sites were infected with Borrelia burgdorferi in 1995, while an examination of ticks for both B. burgdorferi and the agent of HGE in 1996 confirmed that these organisms were present in all counties; the average coinfection rate was 1.9%. No correlation was found between estimated risk and reported cases of Lyme disease. The geographic disparity of risk observed among sites in this study underscores the need for vector and pathogen surveillance on a regional level. An entomologic risk index can help identify sites for targeted tick control efforts.
Lyme disease, the most common vector-borne disease in the United States (more than 16,000 cases were reported from 45 states in 1996 [5]), is most prevalent in the northeastern United States. The primary vector of Lyme disease, the black-legged tick (Ixodes scapularis), has experienced significant population growth in recent years (12, 14). In New York, which accounts for approximately 30% of all Lyme disease cases nationally (5), the I. scapularis population has grown both north and west (1, 28) of Westchester County, where the disease is endemic (29). In fact, in the six lower Hudson Valley counties (Westchester, Putnam, Dutchess, Rockland, Orange, and Ulster), which straddle the Hudson River in southeastern New York, there have been significant increases in the number of Lyme disease cases since 1990, when 643 cases were reported; in 1996 3,227 cases were reported in these counties (19a), a fourfold increase.
Despite the increase in the number of Lyme disease cases reported, little information about the spatial distribution of ticks and Lyme disease risk in these counties has been available previously. The recent emergence in the region of a second I. scapularis-transmitted pathogen, an Ehrlichia equi-like agent that causes human granulocytic ehrlichiosis (HGE) (4), places added emphasis on tick and pathogen surveillance, particularly in the lower Hudson Valley. Evidence indicates that the agent of HGE has infected I. scapularis since at least 1984 (22), and of the 62 cases of HGE reported in New York in 1995, 53 (85%) occurred in five of the six lower Hudson Valley counties (19a). However, the reliability of human case reports can be questioned (22), and thus, the need for tick and pathogen surveillance to better define environmental risk is obvious.
The purpose of this study was to define risk of infection by the agents of Lyme disease and HGE in the lower Hudson Valley of New York, independent of human case reports, based on field surveillance of the distribution and density of I. scapularis, as well as on infection rates in ticks. Since the two agents may be transmitted simultaneously by a single tick (20) and subsequently have a more severe impact on patients than if a single agent had been transmitted, rates of coinfection also were determined.
MATERIALS AND METHODS
Study sites.
I. scapularis is primarily a woodland tick species (6, 11). Thus, while most risk of Lyme disease in suburban residential areas of Westchester County is periodomestic (10), it is due largely to the proximity of homes to woodland habitat (8), where the I. scapularis abundance is highest (18). Thus, the sites chosen for this study were wooded parks or recreation areas throughout the lower Hudson Valley (Fig. 1). We attempted to select sites that were approximately uniformly spaced and which provided a representative sample of woodland tick habitat throughout each county. A minimum of five sites were chosen per county, although additional sites were sampled under two conditions. The first of these conditions was that the relatively large sizes of some counties suggested that a greater number of sites might provide a more representative sample of woodland habitat with respect to tick abundance. The second condition was that too few ticks were collected from an individual site. While no formal recommendations on the sample size needed to reliably determine infection rates have been published previously, we attempted to obtain at least 50 nymphs per site; a threshold of 35 nymphs was deemed necessary before a site could be included in risk determination analyses.
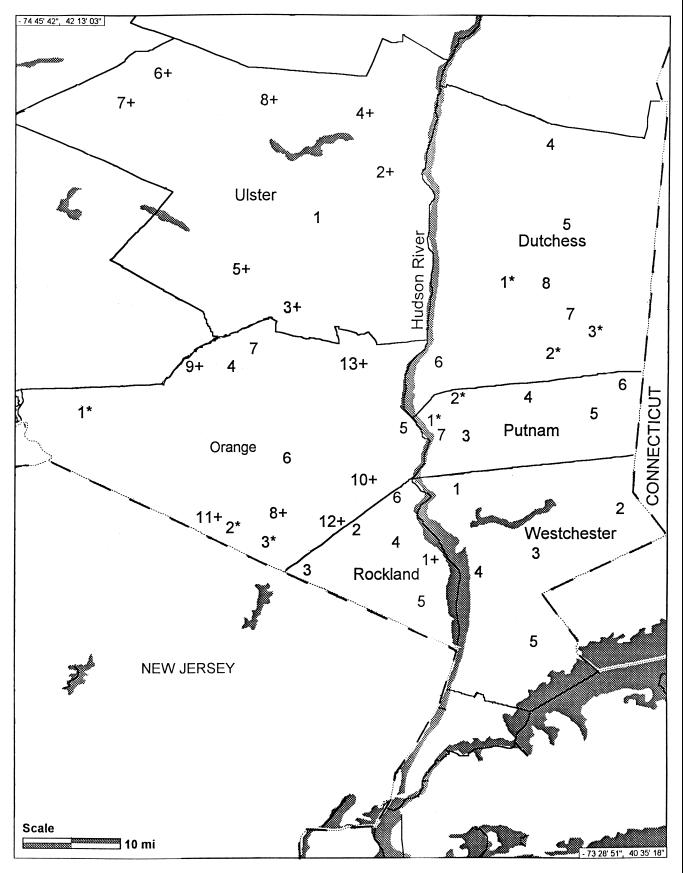
FIG. 1.
Map of study sites sampled in the lower Hudson Valley, New York, in 1995 and 1996. Asterisks indicate sites that were sampled only in 1995, and plus signs indicate sites that were sampled only in 1996.
Tick surveillance.
I. scapularis abundance was monitored by drag sampling, in which a 1-m2 panel of white corduroy cloth was pulled over the ground and vegetation along randomly selected transects at each study site. A minimum area of 1,000 m2 was sampled to provide an adequate index of tick density at each site. Additional sampling was conducted, if necessary, until at least 50 nymphs had been collected at each site. All ticks clinging to the drag cloth were removed with forceps, placed in vials containing 70% ethanol, and preserved for later identification. Specimens collected in 1995 subsequently were tested only for the presence of Borrelia burgdorferi, the etiologic agent of Lyme disease, while specimens collected in 1996 were tested for the presence of both B. burgdorferi and the Ehrlichia sp. that causes HGE.
Sampling was conducted through the late spring and summer months, when I. scapularis nymphs are active (13). Because nymphal abundance characteristically increases in late spring to a period of peak abundance before exhibiting a steady decline, comparisons among sites first required that drag data be standardized to a particular point in time. Otherwise, the observed differences among sites might have been the result of the time of sampling and not of actual differences in tick abundance. The data reported in this paper are limited to data obtained by woodland drag sampling; although I. scapularis nymphs were collected from stone walls, edge habitats, old fields, and ornamental shrubbery, only woodland habitat was present at all sites. Additional drag sampling data collected at the Louis Calder Center at Armonk in Westchester County as part of an ongoing long-term study of tick abundance were plotted weekly to provide an index of tick activity over time.
B. burgdorferi infection rates.
Because virtually all Lyme disease cases are the result of nymphal tick bites (9a), we focused on nymphs for infection rate determinations. Ticks from a representative sample of five sites per county were tested.
(i) Antigen capture ELISA.
Two methods for determining spirochete infection in I. scapularis were employed in this study. The first method, which was used to test ticks obtained in 1995, was an antigen capture enzyme-linked immunosorbent assay (ELISA) technique (3, 15) in which capture monoclonal antibodies (mAb) to OspA, the predominant outer surface protein of B. burgdorferi, were used in combination with rabbit antibodies to OspA and OspB to detect the presence of less than 1 ng of OspA or OspB (15).
Flat-bottom microtiter ELISA plates (Immulon I; Dynatech Laboratories, Inc., Chantilly, Va.) were coated for 3 days at 40°C with 100 μl of a solution containing mAb to OspA (L12-9B4-D8) at a concentration of 20 μg/ml in 0.15 M Na2CO3 buffer (pH 9.6). The plates were washed six times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-Tween) and then coated overnight at 40°C with 300 μl of PBS-Tween containing 0.5% bovine serum albumin (BSA).
Individual field-collected, preserved ticks were soaked in 25 μl of 1% Nonidet P-40 (NP-40) in 10 mM Tris-HCl (pH 10) for 15 min and then homogenized in glass microhomogenizer tubes (Gri-Tube; Fisher Scientific) with 5 μl of 2.5% NP-40 in PBS for 5 min. During homogenization, 10 μl of 2% BSA in PBS was added to each tube after the tick had disintegrated. PBS containing 0.25% NP-40 and 0.1% BSA was then added to bring the final volume to 200 μl. The tick homogenates were transferred to microtiter wells coated with the capture mAb. As a positive control, 200 μl of 0.5% BSA in PBS-Tween containing 40 ng of pooled OspA and OspB was added to duplicate wells, and twofold serial dilutions were prepared directly on the same plate. The plates were incubated at room temperature for 1 h and then washed again as described above. Rabbit antibodies (2 mg/ml) to OspA and OspB were diluted 1:1,000 in PBS-Tween containing 0.5% BSA, and 200 μl of this solution was added to each well. After 1 h of incubation, the plates were washed again. Two hundred microliters of a 1:1,000 dilution of goat anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Boehringer Manheim) was added to each well, and the preparations were incubated for 1 h at room temperature. After washing, 200 μl of p-nitrophenylphosphate substrate was added to each well. After incubation for 1 h at room temperature, the color reaction was stopped with 100 μl of 1 N NaOH. The absorbance was determined with a Bio-Tek Instruments microplate autoreader at a wavelength of 405 nm. Titration end points were calculated by determining the dilution at which the absorbance was equal to three standard deviations above the average absorbance of negative control wells.
(ii) PCR.
In 1996 infection rates were determined by PCR amplification. The PCR techniques allowed us to test individual ticks for both B. burgdorferi and the agent of HGE; thus, specimens could be used most efficiently, and levels of coinfection could be determined. This analysis involved amplification of a portion of the duplicated 23S rRNA genes with primers IS1 and IS2, as previously described (21). Each tick was broken apart with a sterile needle and placed in a tube with 100 μl of sterile lysis buffer (10 mM Tris HCl [pH 7.4], 0.5% NP-40, 0.5% Tween 20, 100 μg of proteinase K per ml). DNA was extracted from each tick with an Isoquick DNA extraction kit (ORCA Research, Bothell, Wash.) by using the manufacturer’s protocol, as previously described (23). Extracted DNA was collected by centrifugation, and the pellet was washed in 70% ethanol, dried, and resuspended in 50 μl of sterile H2O; 10 μl was used as a template in the PCR.
Strict procedures to prevent cross-contamination or carryover of amplified products were used throughout. These procedures included physical separation of the areas used for template preparation, PCR amplification, and post-PCR processing and analysis, as well as the use of barrier tips to prevent aerosol carryover during sample preparation and PCR setup. These procedures have been successfully used previously to detect B. burgdorferi in I. scapularis (23).
Ehrlichia infection rates.
The HGE agent was detected by PCR by using 16S ribosomal DNA primers specific for this organism. PCR amplification was performed in 50-μl mixtures containing 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, 50 μM dATP, 50 μM dGTP, 50 μM dCTP, 50 μM dTTP, 1.25 U of Taq polymerase (Fisher Scientific), and 45 pmol of each primer (GER 3 and GER 4) originally described by Munderloh et al. (19). A cycling program consisting of 45 s of denaturation at 94°C, 45 s of annealing at 60°C, and 45 s of extension at 72°C was carried out for 40 cycles. PCR products were separated by electrophoresis on 1.5 or 2% agarose gels and were visualized by staining with ethidium bromide. As noted above, strict procedures were used to prevent cross-contamination and carryover of amplified product.
Risk indices.
To provide a means of combining risk factors into a single quantity that realistically indicated the relative risk of encountering infected ticks at each site, an entomologic risk index (ERI) was calculated (16, 17). This index was the product of a measure of nymphal abundance, in this case an estimate of density (number of nymphs per square meter), and the infection rate in ticks (16) for each of the two disease agents investigated. Similarly, a combined risk index was calculated by multiplying the nymphal density by the total proportion of ticks infected with either or both disease agents. The resulting value was multiplied by 100 to bring the range of numbers into more common parlance and to reduce the number of decimal places. In essence, the index was a measure of environmental risk independent of the behavior of humans.
Data analysis and interpretation.
The number of ticks collected per square meter sampled was used to determine the relative tick density at each site. Because sampling in this study was conducted concurrently with routine sampling of a permanent, long-term grid site in Westchester County two to three times weekly, the data were used to determine (i) the period of peak abundance and (ii) temporal correction factors with which drag data could be standardized for the region. Standardization involved determining the percentage of ticks that were “absent” in a particular nonpeak week as nymphal numbers increased (prior to the peak) or declined (after the peak) over time. The actual counts for a particular week then were increased by the percentage absent to provide an estimate of the number of nymphs expected if sampling had been conducted at the time of peak abundance. Adjusted mean densities then were plotted to facilitate comparisons among counties. Previous comparisons of the temporal distributions of nymphs in Westchester and Dutchess counties (5a) supported the validity of standardizing regional abundance data based on data obtained at our permanent site in Westchester County. Mean fold increases were determined by averaging the changes in density determined for each of five study sites per county.
Infection rates were calculated by determining the percentage of ticks infected with B. burgdorferi or Ehrlichia sp. at each site. Mean risk indices were calculated as described above for each county to identify spatial patterns of risk in the lower Hudson Valley.
RESULTS
Study sites.
To determine the spatial distribution and abundance of I. scapularis in the lower Hudson Valley, at least five sites per county were used. However, because several sites yielded few or no ticks, additional sites were chosen during the study to provide tick specimens for infection rate determinations. Altogether, 34 and 40 sites were sampled in 1995 and 1996, respectively, and 27 of those sites were sampled in both years (Fig. 1).
Tick surveillance.
Nymphal sampling was conducted from 22 May to 27 June 1995 and from 6 June to 29 July 1996. In 1995 a total of 89,550 m2 at 34 study sites was drag sampled, and the mean area sampled per site was 2,634 m2. I. scapularis nymphs were collected from 31 (91%) of the sites (Table 1). A total of 51,540 m2 at 40 sites was drag sampled in 1996, and the average area sampled per site was 1,290 m2. A total of 36 (90%) of these sites yielded I. scapularis nymphs (Table 1). Once the data were standardized to the estimated peak abundance for each site, the mean nymphal density was calculated for each county.
TABLE 1.
Drag sampling results for 1995 and 1996
| County | 1995
|
1996
|
||||
|---|---|---|---|---|---|---|
| No. of sites sampled | No. of sites with I. scapularis | Area dragged (m2) | No. of sites sampled | No. of sites with I. scapularis | Area dragged (m2 | |
| Westchester | 5 | 5 | 14,480 | 5 | 5 | 5,100 |
| Putnam | 7 | 6 | 12,770 | 5 | 5 | 5,000 |
| Dutchess | 8 | 8 | 8,880 | 5 | 5 | 5,040 |
| Rockland | 5 | 5 | 22,100 | 6 | 6 | 9,040 |
| Orange | 8 | 7 | 28,200 | 10 | 8 | 13,460 |
| Ulster | 1 | 0 | 3,120 | 9 | 7 | 13,900 |
| Total | 34 | 31 | 89,550 | 40 | 36 | 51,540 |
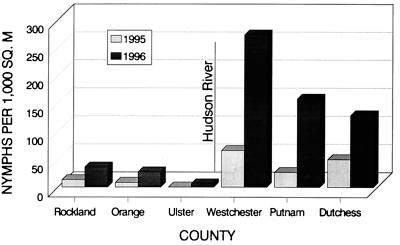
When the data obtained for 1995 and 1996 were compared, it was apparent that there was a marked increase in nymphal density from one year to the next (Fig. 2). On average, a 2.5-fold increase was found for five of the six counties sampled, with the individual county increases ranging from 1.0- to 3.9-fold (Table 2). Because of the low number of ticks collected from sites in Ulster County in 1995, this county was excluded from the analysis.
FIG. 2.
Comparison of woodland nymphal I. scapularis densities at sites in the lower Hudson Valley in 1995 and 1996. The means were adjusted for seasonal variation. The location of the Hudson River relative to the counties is shown. Rockland and Westchester counties are the southernmost counties; Ulster and Dutchess counties are the northernmost counties.
TABLE 2.
Mean nymphal I. scapularis densities at sites in the lower Hudson valley in 1995 and 1996 for the five sites per county sampled in both years
| County | Mean no. of ticks/1,000 m2 (SD)
|
Mean fold increase (SD) | |
|---|---|---|---|
| 1995 | 1996 | ||
| Westchester | 65.5 (43.4) | 271.8 (262.3) | 3.7 (3.2) |
| Putnam | 36.9 (18.8) | 157.4 (76.5) | 3.9 (2.6) |
| Dutchess | 75.2 (58.5) | 128.0 (108.1) | 2.1 (2.7) |
| Rockland | 13.0 (7.0) | 30.4 (20.7) | 1.0 (3.3) |
| Orange | 11.8 (14.2) | 46.0 (55.0) | 2.0 (2.8) |
In both years, the nymphal I. scapularis densities were markedly higher in the counties east of the Hudson River than in the counties across the river (Fig. 2). Likewise, there was a wide range of tick densities among study sites, even within counties. For instance, although Westchester County had the highest nymphal densities in both years, the densities at the five sites sampled in the county ranged from 18.4 to 113.1 nymphs per 1,000 m2 in 1995 and from 71.8 to 722.6 nymphs per 1,000 m2 in 1996.
B. burgdorferi and Ehrlichia sp. infection rates.
The infection rates in 1995, as determined by the antigen capture ELISA, ranged from 7 to 57% for the 22 sites from which at least 35 tick specimens were obtained and tested; the remaining 3 sites at which I. scapularis was collected were omitted from the analysis. Altogether, 1,091 nymphs were examined, and the mean infection rates in the counties ranged from 18.6 to 38.6% (Table 3). Ticks from all sites were found to be infected with B. burgdorferi.
TABLE 3.
B. burgdorferi infection rates as determined by antigen capture ELISA for I. scapularis nymphs in the lower Hudson Valley in 1995
| County | No. of sites tested | No. of ticks tested | Mean % positive (SD) |
|---|---|---|---|
| Westchester | 5 | 267 | 38.6 (18.6) |
| Putnam | 5 | 249 | 18.6 (8.6) |
| Dutchess | 4 | 200 | 31.0 (4.8) |
| Rockland | 4 | 195 | 27.3 (21.1) |
| Orange | 4 | 180 | 27.0 (4.8) |
| Total | 22 | 1,091 |
In 1996, the need to determine the prevalence of the HGE agent dictated that PCR be used to determine B. burgdorferi infection rates as well. Thus, samples consisting of approximately 50 nymphal ticks per site were examined for the presence of B. burgdorferi sensu stricto and the HGE Ehrlichia sp. concurrently. The B. burgdorferi infection rates ranged from zero at several sites in Ulster and Westchester counties to 43% at a site in Rockland County (Table 4). Regionally, the infection rate was 12.8% ± 9.1% (mean ± standard deviation).
TABLE 4.
Infection rates as determined by PCR for I. scapularis nymphs in the lower Hudson Valley in 1996
| County | No. of sites tested | No. of nymphs tested | Mean % positive (SD)
|
||
|---|---|---|---|---|---|
| B. burgdorferi | Ehrlichia sp. | Coinfection | |||
| Westchester | 5 | 250 | 16.0 (16.0) | 16.4 (10.8) | 4.4 (5.0) |
| Putnam | 5 | 252 | 9.2 (7.7) | 10.8 (5.4) | 0 |
| Dutchess | 5 | 253 | 15.0 (10.6) | 16.2 (11.1) | 1.6 (3.6) |
| Rockland | 5 | 252 | 28.2 (10.4) | 11.6 (7.7) | 4.8 (2.3) |
| Orange | 4a | 177 | 5.8 (3.9) | 14.3 (3.1) | 0.5 (1.0) |
| Ulster | 2a | 84 | 10.0 (5.7) | 8.5 (9.2) | 0 |
At other sites in the county not enough ticks were collected for testing.
Pairwise comparisons by county indicated that in four of the five counties examined (all but Ulster County) the B. burgdorferi infection rates were significantly lower in 1996 than in 1995 (arcsine transformation and test of two percentages; P = 0.05 significance level) (24). To determine if the apparent decrease was due to the change in the methods used to determine infection rates in the two years, six sites were selected arbitrarily as indicator sites, and groups of 50 nymphs collected at these sites in 1996 were tested by the antigen capture ELISA. Two of the sites had equivalent infection rates as determined by both methods (arcsine transformation and test of two percentages; P > 0.05), while for the remaining four sites significantly higher infection rates were obtained for the ticks tested by PCR than for the ticks tested by the antigen capture technique.
The Ehrlichia infection rates were comparable to the B. burgdorferi infection rates; the Ehrlichia infection rates were 13.0% ± 3.2% regionally and ranged from zero at several sites throughout the lower Hudson Valley to 28% at a site in Westchester County. The mean infection rates in the different counties are shown in Table 4.
When the 1996 B. burgdorferi infection rates were grouped spatially into data for southern (Westchester and Rockland), central (Putnam and Orange), and northern (Dutchess and Ulster) sites, the southern infection rate was 22.1%, which was markedly higher than the infection rates for the central counties (7.5%) and the northern counties (12.5%). However, when the Ehrlichia sp. infection rates were grouped spatially as described above, the equivalent mean infection rates were 14, 12.6, and 12.4% for the southern, central, and northern sites, respectively.
The proportions of ticks that were infected with both agents ranged from zero at most sites to 12% at a site in Westchester County. Ticks from 26 sites were tested, and individual ticks from 10 (38.5%) of these sites were infected with both pathogens. There was evidence of coinfection at 10 (45%) of the 22 sites at which both pathogens were present in the I. scapularis population. The coinfection rate was 1.9% ± 2.2% (average ± standard deviation) for all sites, and ticks containing both agents were found in four of the six counties (Table 4).
Risk index.
The risk indices in 1996 ranged from 0 to 11.6 for B. burgdorferi and from 0 to 14.5 for the agent of HGE (Table 5). The mean risk indices for individual counties ranged from 0.2 ± 0.2 for Orange and Ulster counties to 5.1 ± 5.2 for Westchester County for B. burgdorferi, and from 0.1 ± 0.1 for Ulster County to 4.8 ± 5.2 for Westchester County for the agent of HGE. The results revealed that the environmental risk decreased as one moved northward from Westchester and Rockland counties and as one moved westward across the Hudson River.
TABLE 5.
Risk indices for B. burgdorferi and the agent of HGE in the lower Hudson Valley region in 1996
| County | Sitea | Risk index
|
||
|---|---|---|---|---|
| B. burgdorferi | Ehrlichia sp. | Combined | ||
| Westchester | 1 | 0 | 0 | 0 |
| 2 | 11.6 | 14.5 | 30.4 | |
| 3 | 8.7 | 3.3 | 13.0 | |
| 4 | 5.3 | 4.6 | 11.9 | |
| 5 | 0 | 1.6 | 1.6 | |
| Mean | 5.1 (5.18)b | 4.8 (5.69) | 11.4 (12.14) | |
| Putnam | 3 | 0.4 | 0.4 | 0.9 |
| 4 | 0.8 | 0.8 | 1.6 | |
| 5 | 0 | 2.2 | 2.1 | |
| 6 | 2.5 | 2.2 | 4.7 | |
| 7 | 4.6 | 2.6 | 7.2 | |
| Mean | 1.7 (1.9) | 1.6 (0.97) | 3.3 (2.61) | |
| Dutchess | 4 | 0.7 | 1.3 | 2.5 |
| 5 | 6.4 | 0 | 6.4 | |
| 6 | 2.9 | 2.6 | 5.5 | |
| 7 | 0.5 | 1.8 | 2.3 | |
| 8 | 0.5 | 0.8 | 1.3 | |
| Mean | 2.2 (2.56) | 1.3 (0.98) | 3.6 (2.22) | |
| Rockland | 1 | 2.1 | 0.9 | 3.5 |
| 2 | 1.2 | 0.2 | 1.6 | |
| 3 | 1.1 | 0.2 | 1.4 | |
| 4 | 0.4 | 0.2 | 0.6 | |
| 5 | 1.3 | 1.4 | 2.9 | |
| Mean | 1.2 (0.61) | 0.6 (0.55) | 2.0 (1.18) | |
| Orange | 5 | 0.1 | 0.3 | 0.4 |
| 11 | 0.04 | 0.3 | 0.3 | |
| 12 | 0.5 | 0.9 | 1.5 | |
| 13 | 0.2 | 0.4 | 0.6 | |
| Mean | 0.2 (0.2) | 0.5 (0.29) | 0.7 (0.55) | |
| Ulster | 2 | 0.3 | 0.04 | 0.3 |
| 3 | 0.1 | 0.2 | 0.2 | |
| Mean | 0.2 (0.16) | 0.1 (0.11) | 0.3 (0.07) | |
The locations of sites are shown in Fig. 1.
The values in parentheses are standard deviations.
To determine the relationship between the risk indices calculated in this study and the 1996 Lyme disease cases reported to the New York State Department of Health (19a), a correlation analysis was performed. Likewise, risk indices were compared to Lyme disease incidence rates (numbers of cases per 100,000 people). Neither analysis revealed a significant relationship between entomologic risk, based on tick and pathogen surveillance, and number of cases reported (Spearman rank correlation; r = 0.75; P > 0.05) or incidence rate (r = 0.38, P > 0.05).
DISCUSSION
In both years of this study, I. scapularis was collected at approximately 90% of the sites sampled. In addition, almost all of the sites with black-legged ticks were found to harbor either the agent that causes Lyme disease or the agent that causes HGE or both; the only exception was Westchester County site 1 in 1996. At present, we have no explanation for the lack of infection of ticks that was noted that year. However, it is clear that populations of I. scapularis and their accompanying human pathogens are firmly established in counties throughout the lower Hudson Valley.
With respect to the nymphal abundance patterns, two points are noteworthy. First, intersite variation was evident within a county. This finding supports previous findings which showed that the risk of contracting tick-borne diseases apparently varied widely from site to site, even within an endemic area (7, 12). However, superimposed on this variation is a regional pattern in which the populations of I. scapularis nymphs may increase or decrease annually. For instance, long-term surveillance of two permanent grid sites in Westchester County has shown that nymphal populations may oscillate between relatively high and low densities on a yearly basis (5a); such oscillations have been noted at other sites as well (25). Although nymphal I. scapularis population size does not remain stable from year to year, the factors that influence the variation are not clear.
The geographic variation in tick abundance observed in this study supports the hypothesis that I. scapularis populations have spread northward and westward through the lower Hudson Valley from Westchester County (28). In both years of this study the nymphal populations were significantly lower in other counties than they were in Westchester County, where Lyme disease cases and I. scapularis have occurred since the early 1980s (29). The data also suggest that the northward spread of the tick population has occurred at a faster rate than the westward spread has. This is probably due to the Hudson River, which bisects the study region, effectively limiting mammal dispersal, which may play an important role in the emergence of new I. scapularis populations and disease foci (6). However, now that I. scapularis populations have become established in the counties west of the Hudson River, we anticipate that in the future differences in mean tick density on the two sides of the river will diminish, given the similarities in habitat types and wildlife populations that serve as hosts to ticks on the two sides.
Despite the variation in tick density among counties, the mean infection rates for the two etiologic agents were remarkably similar throughout the region in 1996. In fact, for counties east of the Hudson River, the infection rates were essentially identical. For both Rockland and Orange counties, which are west of the Hudson River, the infection rates for Ehrlichia sp. were higher than the infection rates for B. burgdorferi, although the differences were not statistically significant (P > 0.05).
This finding has several implications, most notably that I. scapularis feeds on a number of host species that are competent reservoirs of the Ehrlichia sp. In fact, it may be that the suite of reservoir species is the same for both B. burgdorferi and the agent of HGE or, at the very least, that there is significant overlap in reservoir competence of hosts of the two pathogens. For example, Peromyscus leucopus, the primary reservoir of B. burgdorferi in the northeastern United States (9), also is an important reservoir of the agent of HGE (22, 27). However, the low rate of coinfection with B. burgdorferi and the Ehrlichia sp. that causes HGE suggests that the relative importance of particular host species as reservoirs may vary for each agent.
In addition, the widespread distribution of the agent of HGE in ticks throughout the lower Hudson Valley suggests that infection with Ehrlichia sp. is not a new development despite the recent discovery of this agent in southern New York (4). This is consistent with the report of Schwartz et al. (22) which confirmed the presence of this agent in I. scapularis specimens collected in 1984.
The apparent regional decrease in B. burgdorferi infection rates from 1995 to 1996 seems to reflect a true change in the infection status of ticks in four of the five counties studied; the mean infection rate in Rockland County remained unchanged from 1995 to 1996. Site-by-site comparisons revealed that significant decreases (P < 0.05) occurred at 13 (65%) of 20 sites from which ticks were obtained and tested in both years; either no change (n = 5) or a significant increase (n = 2) was observed for the remaining sites. Thus, the trend toward reduced infection rates in 1996 reflected a regional phenomenon that had an impact at most sites.
The possibility that the reduced B. burgdorferi infection rate found in 1996 was an artifact of switching from the antigen capture ELISA to PCR testing was considered and discarded. The results of an antigen capture ELISA analysis of ticks from a subsample of six study sites in 1996 indicated that the infection rates were significantly lower in 1996 than in 1995 for four of the six sites examined in both years (arcsine transformation and test of two percentages; P < 0.05). Likewise, the infection rates determined by PCR in 1996 were significantly higher than the 1996 antigen capture ELISA rates for four of the sites. Together, these data suggest that PCR may be a more sensitive indicator of B. burgdorferi infection in ticks than antigen capture ELISA is, and, therefore, the lower infection rate observed in 1996 probably was not due to undetected infection.
At present, we have no explanation for the decrease in infection rates, although it is likely that individual sites can be influenced significantly by specific prevailing local environmental conditions that attenuate regional factors. For example, an ongoing long-term study of tick abundance and B. burgdorferi infection at a site in Westchester County has revealed that for the past 5 years the nymphal infection rates have been stable, averaging 22.1% ± 5.1% (5a). Long-term study of multiple sites is needed before adequate information on changes in infection rates from one tick cohort to the next at a particular site is available.
The coinfection rates obtained in this study were expected given the infection rates of the two pathogens and given the assumption that each pathogen is transmitted to I. scapularis independent of the other. In such cases, coinfection rates should be the product of the individual infection rates. Although the coinfection rates for a single site may vary from the values expected, regionally the observed and expected mean coinfection rates were not different. Since host-seeking I. scapularis nymphs can become coinfected only by feeding on a single reservoir-competent host (transovarial transmission is inconsequential for both pathogens), the low degree of coinfection observed in this study suggests that transmission of either pathogen to an uninfected tick is not facilitated by the presence of the other pathogen.
The environmental risk of infection with tick-borne diseases depends primarily on the following three factors: the density of ticks in an area, the distribution of those ticks in the environment, and the rates of infection (i.e., the percentages of ticks infected) with the pathogens. In general, the risk of infection with B. burgdorferi is seasonal (13) and spatially disjunct owing to the nonrandom, aggregated distribution of I. scapularis in nature (6, 13).
The use of an ERI provided a way to determine the relative risk from one site to another by considering two important factors that influence that risk: tick density and infection rate (17). Additional sampling and examination of ticks, insofar as they reduce the variance in infection rates within a county by minimizing the likelihood of obtaining an unusually high rate by chance, may place added dependence of the ERI on nymphal density. Thus, if tick population densities on both sides of the Hudson River become more homogeneous over time, the incidence of both Lyme disease and HGE may become more uniform throughout the region.
Regarding the utility of the ERI to predict the risk of tick-borne diseases, Mather et al. (17) noted that the ERI and the reported numbers of Lyme disease cases in six Rhode Island towns were highly correlated. In the present study, however, we found no correlation between environmental (i.e., entomologic) risk and either the number of reported cases or the incidence rates of the two diseases in each county. This finding was not unexpected in light of previously published concerns about the reliability of reported numbers of cases (2, 26). In fact, we suggest that the ERI should accurately depict the current risk of infection irrespective of the numbers of cases ultimately reported, and thus, using the ERI can help determine ways to minimize risk. For example, if high disease incidence rates are noted relative to the ERI, then changes in human behavior may be in order (perhaps people should spend less time in tick-infested areas or perform more thorough self-exams for the presence of ticks). Conversely, this situation may reflect the fact that disease reporting is more efficient or the fact that physicians are better able to diagnose the illnesses. Alternatively, a low incidence of Lyme disease relative to the ERI suggests that either preventive measures are having a significant impact and reducing exposure or there are severe shortcomings in the diagnosis and/or reporting of these diseases in the area. Areas with high ERIs could be targeted for rigorous tick control efforts and programs to prevent Lyme disease and HGE. Ultimately, differences in human exposure to infected ticks (i.e., differences in how residents of each county spend their time and use space relative to the location of host-seeking ticks) will dictate whether and to what extent disease incidence rates increase.
The differences in risk among the sites sampled in this study underscore the need for vector and pathogen surveillance on a regional level and highlight how such data can help clarify ecological issues that have an impact on risk, such as annual variations in infection rates and transmission of pathogens from vertebrate hosts to tick vectors. Understanding these factors should enhance our ability to devise and enact effective tick control strategies.
ACKNOWLEDGMENTS
We are grateful to B. Byrnes, B. Golson, M. Hoffmann, P. McCrosson, and E. Silano for help with the field work.
This study was funded by grant C-011999 from the New York State Department of Health Tick-Borne Disease Institute (to T.J.D.), by a grant from the American Lyme Disease Foundation (to R.C.F.), and by National Institutes of Health grant AR41511 (to I.S.).
Footnotes
Contribution no. 158 to the Louis Calder Center, Fordham University.
REFERENCES
- 1.Anderson J F, Magnarelli L A, McAninch J. Ixodes dammini and Borrelia burgdorferi in northern New England and upstate New York. J Parasitol. 1998;73:419–421. [PubMed] [Google Scholar]
- 2.Barbour A G, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 3.Burkot T R, Wirtz R A, Luft B, Piesman J. An OspA antigen-capture enzyme-linked immunosorbent assay for detecting North American isolates of Borrelia burgdorferi in larval and nymphal Ixodes dammini. J Clin Microbiol. 1993;31:272–278. doi: 10.1128/jcm.31.2.272-278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Human granulocytic ehrlichiosis—New York, 1995. Morbid Mortal Weekly Rep. 1995;44:593–595. [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 5a.Daniels, T. J., and R. C. Falco. Unpublished data.
- 6.Daniels T J, Fish D. Spatial distribution and dispersal of unfed larval Ixodes dammini (Acari: Ixodidae) in southern New York. Environ Entomol. 1990;19:1029–1033. [Google Scholar]
- 7.Daniels T J, Fish D, Levine J F, Greco M, Eaton A T, Padgett P J, LaPointe D A. Canine exposure to Borrelia burgdorferi and prevalence of Ixodes dammini (Acari: Ixodidae) on deer as a measure of Lyme disease risk in the northeastern United States. J Med Entomol. 1993;30:171–178. doi: 10.1093/jmedent/30.1.171. [DOI] [PubMed] [Google Scholar]
- 8.Dister S, Beck L, Wood B, Falco R C, Fish D. Proceedings, GIS’93: Geographic Information Systems in Forestry, Environmental, and Natural Resource Management. Vancouver, Canada. 1993. The use of GIS and remote sensing technologies in a landscape approach to the study of Lyme disease transmission risk. [Google Scholar]
- 9.Donahue J G, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 9a.Falco, R. C., et al. Unpublished data.
- 10.Falco R C, Fish D. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am J Epidemiol. 1988;127:826–830. doi: 10.1093/oxfordjournals.aje.a114865. [DOI] [PubMed] [Google Scholar]
- 11.Falco R C, Fish D. A comparison of methods for sampling the deer tick, Ixodes dammini. Exp Appl Acar. 1992;14:165–173. doi: 10.1007/BF01219108. [DOI] [PubMed] [Google Scholar]
- 12.Falco R C, Daniels T J, Fish D. Increase in abundance of immature Ixodes scapularis (Acari: Ixodidae) in an emergent Lyme disease endemic area. J Med Entomol. 1995;32:522–526. doi: 10.1093/jmedent/32.4.522. [DOI] [PubMed] [Google Scholar]
- 13.Fish D. Population ecology of Ixodes dammini. In: Ginsberg H S, editor. Ecology and environmental management of Lyme disease. New Brunswick, N.J: Rutgers University Press; 1993. pp. 25–42. [Google Scholar]
- 14.Lastavica G C, Wilson M L, Berardi V P, Spielman A, Deblinger R D. Rapid emergence of a focal epidemic of Lyme disease in coastal Massachusetts. N Engl J Med. 1989;320:133–137. doi: 10.1056/NEJM198901193200301. [DOI] [PubMed] [Google Scholar]
- 15.Mannelli A, Fish D, Daniels T J, Kharitonenkov I, Tun H, Cozzolino A C, Bucher D J. Detection of Borrelia burgdorferi OspA in Ixodes scapularis larvae by an antigen-capture enzyme-linked immunosorbent assay. Microbiologica (Bologna) 1997;20:355–359. [PubMed] [Google Scholar]
- 16.Mather T N. The dynamics of spirochete transmission between ticks and vertebrates. In: Ginsberg H S, editor. Ecology and environmental management of Lyme disease. New Brunswick, N.J: Rutgers University Press; 1993. pp. 43–60. [Google Scholar]
- 17.Mather T N, Nicholson M C, Donnelly E F, Matyas B T. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- 18.Maupin G O, Fish D, Zultowsky J, Campos E G, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, NY. Am J Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]
- 19.Munderloh U G, Madigan J E, Dumler J S, Goodman J L, Hayes S F, Barlough J E, Nelson C M, Kurtti T J. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J Clin Microbiol. 1996;34:664–670. doi: 10.1128/jcm.34.3.664-670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.New York State Department of Health. Unpublished data.
- 20.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz I, Wormser G P, Pavia C S. Diagnosis of early Lyme disease by polymerase chain reaction amplification or culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082–3088. doi: 10.1128/jcm.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz I, Fish D, Daniels T J. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz I S, Varde S, Nadelman R B, Wormser G P, Fish D. Inhibition of efficient polymerase chain reaction amplification of Borrelia burgdorferi DNA in blood-fed ticks. Am J Trop Med Hyg. 1997;56:339–342. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- 24.Sokal R R, Rohlf F J. Biometry. W. H. San Francisco, Calif: Freeman; 1969. [Google Scholar]
- 25.Stafford K C, III, Cartter M L, Magnarelli L A, Ertel S-H, Mshar P A. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–1244. doi: 10.1128/jcm.36.5.1240-1244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steere A E, Taylor E, McHugh G L, Logigian E L. Overdiagnosis of Lyme disease. JAMA. 1993;269:1812–1816. [PubMed] [Google Scholar]
- 27.Telford S R, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White D J, Chang H G, Benach J L, Bosler E M, Meldrum S C, Means R G, Debbie J G, Birkhead G S, Morse D L. The geographic spread and temporal increase of the Lyme disease epidemic. JAMA. 1991;266:1230–1236. [PubMed] [Google Scholar]
- 29.Williams C L, Curran A S, Lee A C, Sousa V O. Lyme disease: epidemiologic characteristics of an outbreak in Westchester County, NY. Am J Public Health. 1986;76:62–65. doi: 10.2105/ajph.76.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]