Abstract
Purpose
This case–control study assesses the independent roles of reproductive history, postmenopausal hormonal therapy (HT), socioeconomic status (SES), and occupational physical activity on the risk of breast cancer (BC).
Methods
Odds ratios (OR) were estimated from conditional logistic multivariate regression model in a data set of 19,253 Finnish women diagnosed with BC between 1994 and 2013 and 96,265 age-matched population controls.
Results
Both pre- and postmenopausal white-collar workers had significantly increased risk of ductal and lobular BC as compared to manual workers. Moderate occupational physical activity reduced risk of lobular BC by 14%. There was a transient increase in the risk of BC observed after each birth followed by a protective effect starting some years after the delivery. As the number of children increased, the short-term excess risk was lower and protective effect was observed earlier. Continuous estrogen-progestin therapy (EPT) significantly increased the risk of both ductal and lobular BC and the magnitude of risk was directly proportional to duration of use (OR for 5+ years of use 2.26, 95% confidence interval 2.12–2.42). Monthly EPT for 5+ years increased the risk (OR 1.32, 95% CI 1.20–1.45). Users of estradiol plus levonorgestrel intrauterine system devices showed ORs of 1.56 (95% CI 1.45–1.69) and 2.18 (95% CI 1.81–2.64) for ductal and lobular BC, respectively.
Conclusion
This study concludes that pregnancy has a dual effect on BC risk, with a transient increase in risk followed by a long-term protective effect. The SES and HT have a large effect on BC risk while occupational physical activity has only a small independent effect.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-022-06571-x.
Keywords: Breast cancer, Etiology, Risk factors, Hormonal replacement therapy, Socio-economic status, Physical activity, Parity
Introduction
Breast cancer is the most common malignancy worldwide, with around 2.26 million new diagnosis among female estimated in the year 2020 [1] In Finland, breast cancer is the leading cancer diagnosis among women with the age-standardized incidence rate of 92 per 100,000 person-years in 2018, standardized to the World Standard Population [2].
Reproductive factors such as low parity and high age at first birth, lack of breastfeeding, early menarche and late menopause are well established risk factors for breast cancer in epidemiological and clinical studies [3–5]. The hormonal mechanisms involved in reproductive processes influence the breast cancer development, either by stimulating or inhibiting the factors that are responsible for initiation of breast cancer or its early growth [6–8]. Especially, endogenous estrogens play several roles in neoplastic transformation of breast tissue, either as carcinogenic agents or as permissive, promotional and tumor growth-inducing agents [9, 10]. In addition to indigenous hormones, exogeneous hormone therapy to manage menopausal symptoms are also implicated as risk factors in the development of breast cancer [11–13].
Recently there has been increasing interest to associations of work environment and breast cancer risk. Physical inactivity has shown to be associated with breast cancer, but the results concerning the occupational physical activity are inconsistent [14–18]. In addition, some characteristics of the modern work life such as career planning and demands of work may lead to postponement of childbirths and thus indirectly influence breast cancer risk [19–23].
The risk of breast cancer is determined by complex mechanisms involving individual’s genetic, physiological, reproductive, lifestyle and environmental factors. Numerous epidemiologic studies have examined the relation between breast cancer and single risk factors individually but there are not many publications on the independent roles of multiple factors in same study. Although it has been acknowledged that the breast cancer is not a single disease with a uniform etiology, the etiological differences in risk according to age at diagnosis and other characteristics of tumor such as histology are not adequately studied [5, 24]. The present population-based study was designed to explore the risk of breast cancer in a multifactorial setting that allows assessment of independent roles of numerous components of reproductive history, postmenopausal hormonal therapy (HT), socio-economic status (SES) and occupational history stratified according to characteristics of breast cancer such as histology and age at diagnosis.
Materials and methods
Study subjects
This is a retrospective case–control study, which includes all Finnish women who were diagnosed with their first breast cancer (ICD-10 code C50) between 1st January 1994 and 31st December 2013. Altogether 19,253 cases were identified in the national population-based Finnish Cancer Registry. The cancers recorded by the Cancer Registry have been notified by hospitals, pathological and hematological laboratories, physicians, and dentists, and from death certificates [25].
For each case of breast cancer, five female controls were randomly selected from the Finnish National Population Registry matched by the year of birth. The controls had to live in Finland at the time of cancer diagnosis of the case (index date). The Population Registry also provided information on the dates of birth of biological children of the cases and controls.
Socioeconomic and occupational variables
Information about SES, education, and occupational history of all the study subjects was obtained from Statistics Finland. SES in our study is classified according to Statistics Finland’s classification of socioeconomic groups 1989, which is based on the classification of the United Nations Economic Commission for Europe (Appendix 1). The classification is formed by several criteria considering persons stage in life (family member, student, economically active, pensioner, etc.), occupational status, and nature of occupation [26]. For the purpose of our study, we categorized SES into upper-level white-collar employees, lower-level white-collar employees, manual workers and others as explained in Appendix 1. Educational levels were based national levels of education corresponding to the International Standard Classification of Education (ISCED 2011) of the United Nations Educational, Scientific and Cultural Organization.
Occupational physical activity
For all study participants, information on occupational history was obtained from Statistics Finland. Finland's national classification of occupations is based on the international classification of occupations 2010 (ISCO-08) of the International Labour Organization (ILO). The national classification of occupation has been revised in 1987, 2001 and 2010. We used a conversion key for converting the occupational codes to the 311 categories of the longitudinal occupational classification used in the NOCCA Job Exposure Matrix (NOCCA-JEM; [27]). In the NOCCA-JEM, physical activity at work is expressed as perceived physical workload (PPWL), which is a quantitative amount given to each occupation based on perceived workload reported in quality of the Finnish Work Life Survey in 1990 [27]. The PPWL is characterized by probability of exposure (P) and average exposure level among those exposed (L) in each occupation. L is expressed in scale 0–1 where values near 1 are categorized as very heavy workload and value approaching 0 as sedentary work. Cumulative exposure to PPWL for each case and control was calculated by multiplying P*L values with the time (T) worked in that occupation from the age of 20 (typical starting age in most occupations) to age of 65 years or age at index date, whichever was lower. If an individual changed occupation between the censuses, she/he was assumed to have changed occupations in the middle of the period between the known census years. The cumulative PPWL exposure non-zero values were divided into mild (lowest 50%; < 0.26 PPWL years), moderate (values between the 50 and 90 percentile; 0.26–3.89 PPWL years) and high level of physical activity (highest 10%; 3.90–28.35 PPWL years). The number of women in mild physical activity was small and cumulative PPWL was very low, we therefore combined this category to sedentary occupations with P*L*T value as 0. We classified economically inactive women as a separate category. This category includes mostly housewives and farmers’ wives, majority of which are physically active. Therefore, the final categories for occupational physical activity were sedentary, moderate, high and economically inactive.
Hormone therapy
Information on postmenopausal HT was obtained from the nationwide Prescription Registry of the Social Insurance Institution of Finland. The registry includes data on systemic HT purchases in Finland since 1994. Systemic HTs are available in Finland only with doctor’s prescription and automatically registered. In our study, purchases of HT at the age of ≥ 50 years for a minimum duration of 6 months was considered as postmenopausal HT.
Only estradiol (E) was used as the estrogen component during the study period. Systemic HT in our study was categories as E only, E combined with progestin therapy (EPT) and E (oral or transdermal) plus levonorgestrel-releasing intrauterine system (E+LNG-IUS). EPT was defined as continuous when oral or transdermal E was combined with daily progestin, as monthly when progestin was given for 10–14 days every month and once-in-3-months progestin when progestin was given for 10–14 days every 3 month. Information on the removal date of intrauterine devices is not available and therefore, the duration of LNG-IUS exposure was defined assuming that a woman who purchased one device used it for five years, which is the average duration of LNG-IUS use in Finland [28].
Statistical analyses
A conditional logistic regression model was used for matched cases and controls for both univariate and multivariate analyses. Odds ratio (OR) with 95% confidence intervals (95% CI) was used to evaluate the association between study variables and breast cancer. We tested for the correlation between SES, education and occupational physical activity as well as fitted several alternate models with the combination of these variables. We dropped education variable from our final analysis because educational information was correlated with SES and was unknown for a large proportion of study participants.
Reproductive variables in our study are parity (categorized as nulliparous, parous); number of children (1, 2, 3, 4, 5+); age at first birth (< 20, 20–24, 25–29, 30+ years); age at last birth (< 30, 30–34, 35–39, 40+ years). Duration of use of each type of HT was categorized into < 1 year, 1–< 5 years, 5+ years; except for E+LNG-IUS which is categorized as number of devices purchased (1 and 2+ purchase). The analyses were stratified according to age at breast cancer diagnosis (< 50 years, called “premenopausal”; ≥ 50 years, “postmenopausal”), and by histology (ductal, lobular).
To estimate the dual effect of pregnancy on breast cancer risk, a conditional logistic regression was run with parity, time since delivery and interaction between these two variables included in the model. The model was adjusted for SES. The fitted results of this model were plotted for the breast cancer risk by parity and time since the delivery. All analyses were performed using R statistical software, version 1.2.1335.
Results
Out of the 19,253 breast cancer cases, 82% were diagnosed at the age 50+ years, and 78% were of ductal and 16% of lobular subtype (Table 1).
Table 1.
Characteristics of cases and controls
| Cases | Controls | |||
|---|---|---|---|---|
| N | % | N | % | |
| All | 19,253 | 100 | 96,265 | 100 |
| Histologya | ||||
| Ductal | 15,031 | 78 | 75,155 | 78 |
| Lobular | 3095 | 16 | 15,475 | 16 |
| Other | 1127 | 6 | 5635 | 6 |
| Age at diagnosisa | ||||
| < 50 years | 3388 | 18 | 16,940 | 18 |
| 50+ years | 15,865 | 82 | 79,325 | 82 |
| Socio-economic status | ||||
| Manual workers | 3215 | 17 | 18,451 | 19 |
| Lower-level employees | 7489 | 39 | 35,917 | 37 |
| Upper-level employees | 3481 | 18 | 14,058 | 15 |
| Others | 5000 | 26 | 27,382 | 28 |
| Occupational physical activity | ||||
| Sedentary | 9460 | 49 | 44,687 | 46 |
| Moderately active | 6923 | 36 | 36,347 | 38 |
| Highly active | 1848 | 10 | 8977 | 9 |
| Economically inactive | 1022 | 5 | 6254 | 6 |
| Parity before index date | ||||
| 0 | 3463 | 18 | 15,406 | 16 |
| 1 | 3940 | 20 | 17,695 | 18 |
| 2 | 7317 | 38 | 36,378 | 38 |
| 3 | 3292 | 17 | 18,343 | 19 |
| 4 | 885 | 5 | 5739 | 6 |
| 5 + | 356 | 2 | 2704 | 3 |
| Age at first birth | ||||
| < 20 years | 1928 | 12 | 10,972 | 13 |
| 20–24 years | 6045 | 38 | 33,714 | 42 |
| 25–29 years | 4819 | 31 | 23,498 | 29 |
| 30+ years | 2998 | 19 | 12,672 | 16 |
| Age at last birth before index date | ||||
| < 30 years | 7568 | 48 | 40,278 | 50 |
| 30-34 years | 4815 | 30 | 24,172 | 30 |
| 55–39 years | 2670 | 17 | 12,872 | 16 |
| 40+ years | 737 | 5 | 3537 | 4 |
| Hormonal replacement Therapy (Used at least for 6 months) | ||||
| Estrogen only (50+ years) | 2353 | 22 | 12,957 | 31 |
| Continuous progestin (50+ years) | 3888 | 36 | 12,912 | 30 |
| Sequential progestin (50+ years) | 3092 | 28 | 11,566 | 27 |
| Once in 3 months progestin (50+ years) | 350 | 3 | 1453 | 3 |
| E+LNG-IUS (45+ years) | 1154 | 11 | 3820 | 9 |
aControls are classified according to the characteristics of the respective case
Tables 2 and 3 present the association of SES and occupational physical activity among pre- and postmenopausal women, respectively. Significantly increased risk of ductal and lobular breast cancer was observed among both pre- and postmenopausal women for white-collar employees as compared to manual workers. As compared to sedentary occupations, moderate physical activity at work was statistically protective for lobular subtype (OR 0.86, 95% CI 0.78–0.94) among postmenopausal women, while the same OR for premenopausal women remained insignificant (OR 0.86, 95% CI 0.65–1.14). Both pre- and postmenopausal women classified as economically inactive had significant protection against breast cancer risk as compared to sedentary workers.
Table 2.
Multivariate conditional logistics regression analysis for socio-economic status and occupational physical activity as predictor of breast cancer by histology among women diagnosed in age < 50 years
| Variables | Total | Ductal | Lobular | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| Socio-economic status | |||||||||
| Manual workers | 488 | 1 | Ref | 416 | 1 | Ref | 46 | 1 | Ref |
| Lower-level employees | 1548 | 1.20 | 1.07–1.34 | 1312 | 1.17 | 1.04–1.32 | 163 | 1.38 | 0.96–1.97 |
| Upper-level employees | 799 | 1.35 | 1.19–1.53 | 671 | 1.32 | 1.15–1.51 | 91 | 1.65 | 1.12–2.44 |
| Others | 539 | 1.00 | 0.87–1.14 | 439 | 0.93 | 0.81–1.08 | 63 | 1.27 | 0.84–1.92 |
| Occupational physical activity | |||||||||
| Sedentary | 2128 | 1 | Ref | 1790 | 1 | Ref | 237 | 1 | Ref |
| Moderate | 674 | 0.95 | 0.86–1.05 | 560 | 0.96 | 0.86–1.07 | 81 | 0.86 | 0.65–1.14 |
| High | 93 | 0.99 | 0.79–1.25 | 75 | 0.97 | 0.75–1.26 | 13 | 0.99 | 0.53–1.82 |
| Economically inactive | 493 | 0.77 | 0.67–0.88 | 423 | 0.77 | 0.67–0.89 | 34 | 0.76 | 0.48–1.18 |
Adjusted for parity
N number of cancer cases, OR odds ratio, 95%CI 95% confidence interval
Table 3.
Multivariate conditional logistics regression analysis for socio-economic status and occupational physical activity as predictor of breast cancer by histology among women diagnosed in age 50+ years
| Variables | Total | Ductal | Lobular | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| Socio-economic status | |||||||||
| Manual workers | 2727 | 1 | Ref | 2134 | 1 | Ref | 448 | 1 | Ref |
| Lower-level employees | 5941 | 1.12 | 1.06–1.17 | 4536 | 1.10 | 1.04–1.16 | 1060 | 1.15 | 1.01–1.30 |
| Upper-level employees | 2682 | 1.31 | 1.23–1.39 | 2040 | 1.27 | 1.19–1.36 | 490 | 1.40 | 1.21–1.62 |
| Others | 4461 | 1.04 | 0.98–1.10 | 3435 | 1.04 | 0.98–1.11 | 724 | 1.03 | 0.90–1.17 |
| Occupational physical activity | |||||||||
| Sedentary | 7332 | 1 | Ref | 5585 | 1 | Ref | 1321 | 1 | Ref |
| Moderate | 6249 | 0.95 | 0.91–0.98 | 4848 | 0.97 | 0.93–1.01 | 1037 | 0.86 | 0.78–0.94 |
| High | 1755 | 0.98 | 0.93–1.05 | 1347 | 1.00 | 0.94–1.07 | 296 | 0.93 | 0.80–1.08 |
| Economically inactive | 529 | 0.76 | 0.69–0.85 | 403 | 0.77 | 0.69–0.87 | 76 | 0.65 | 0.49–0.85 |
Adjusted for parity and hormonal replacement therapy use
N number of cancer cases, OR odds ratio, 95%CI 95% confidence interval
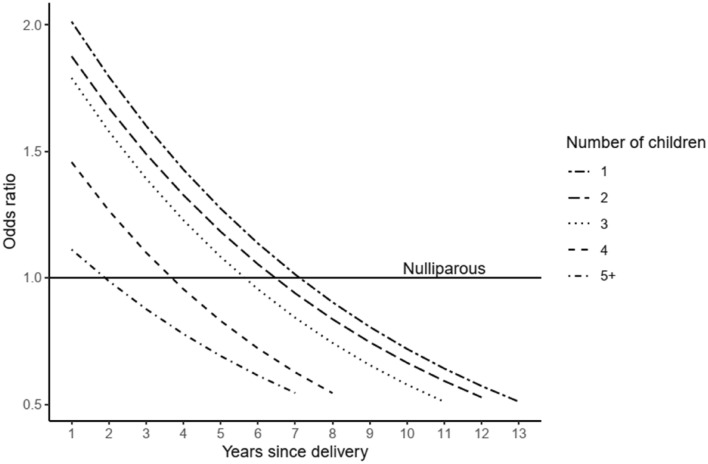
Parous women had 7% reduced risk of premenopausal and 17% reduced risk of postmenopausal breast cancer as compared to nulliparous women (Tables 4 and 5). Among parous women, the increasing number of children was strongly associated with decreasing breast cancer risk (Tables 4 and 5). However, there was a transient increase in the risk for several years after each birth before the incidence decreased below the level of nulliparous women (the model for premenopausal women illustrated in Fig. 1). The peak of transiently increased risk became lower and its duration shorter along with increasing number of children. The model for postmenopausal women is similar as Fig. 1 but with less elevated ORs for the first year after the birth. Increasing age at first and last birth was associated with increased risk of lobular breast cancer among both pre- and postmenopausal women (Tables 4 and 5). For ductal breast cancer, the ages at first and last birth had weaker associations with risk.
Table 4.
Multivariate conditional logistics regression analysis for reproductive factors as predictor of breast cancer by histology among women diagnosed < 50 years
| Variables | Total | Ductal | Lobular | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| Paritya | |||||||||
| Nulliparous | 753 | 1 | Ref | 628 | 1 | Ref | 77 | 1 | Ref |
| Parous | 2635 | 0.93 | 0.85–1.02 | 2220 | 0.96 | 0.87–1.06 | 288 | 0.89 | 0.66–1.19 |
| Number of childrenb | |||||||||
| 1 | 620 | 1 | Ref | 523 | 1 | Ref | 52 | 1 | Ref |
| 2 | 1223 | 0.92 | 0.82–1.04 | 1022 | 0.92 | 0.81–1.05 | 147 | 1.05 | 0.72–1.52 |
| 3 | 588 | 0.81 | 0.70–0.95 | 503 | 0.84 | 0.71–0.99 | 64 | 0.75 | 0.46–1.21 |
| 4 | 135 | 0.64 | 0.50–0.80 | 110 | 0.61 | 0.47–0.79 | 19 | 0.79 | 0.39–1.59 |
| 5 + | 69 | 0.63 | 0.46–0.86 | 62 | 0.69 | 0.49–0.96 | 6 | 0.39 | 0.14–1.06 |
| Age at first birthb | |||||||||
| < 20 years | 176 | 1 | Ref | 157 | 1 | Ref | 12 | 1 | Ref |
| 20–24 years | 661 | 1.06 | 0.88–1.28 | 573 | 1.05 | 0.86–1.28 | 65 | 1.37 | 0.71–2.66 |
| 25–29 years | 977 | 1.15 | 0.95–1.39 | 806 | 1.06 | 0.86–1.30 | 119 | 2.02 | 1.04–3.90 |
| 30+ years | 821 | 1.25 | 0.99–1.57 | 684 | 1.20 | 0.93–1.53 | 92 | 1.57 | 0.74–3.31 |
| Age at last birthb | |||||||||
| < 30 years | 907 | 1 | Ref | 780 | 1 | Ref | 80 | 1 | Ref |
| 30–34 years | 990 | 1.10 | 0.97–1.25 | 835 | 1.08 | 0.94–1.23 | 102 | 1.33 | 0.89–1.98 |
| 35–39 years | 608 | 1.21 | 1.03–1.42 | 498 | 1.16 | 0.97–1.38 | 85 | 1.92 | 1.19–3.12 |
| 40+ years | 130 | 1.18 | 0.93–1.50 | 107 | 1.12 | 0.86–1.46 | 21 | 2.64 | 1.33–5.24 |
Adjusted for socio-economic status, occupational physical activity
N number of cancer cases, OR odds ratio, 95%CI 95% confidence interval
aThe model only included parity
bThe model included number of children, and age at first and last birth
Table 5.
Multivariate conditional logistics regression analysis for reproductive factors as predictor of breast cancer by histology among women diagnosed 50+ years
| Variables | Total | Ductal | Lobular | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| Paritya | |||||||||
| Nulliparous | 2710 | 1 | Ref | 2084 | 1 | Ref | 436 | 1 | Ref |
| Parous | 13,155 | 0.83 | 0.79–0.87 | 10,099 | 0.81 | 0.77–0.86 | 2294 | 0.92 | 0.82–1.04 |
| Number of childrenb | |||||||||
| 1 | 3320 | 1 | Ref | 2539 | 1 | Ref | 568 | 1 | Ref |
| 2 | 6094 | 0.89 | 0.85–0.94 | 4669 | 0.88 | 0.83–0.93 | 1100 | 0.97 | 0.85–1.10 |
| 3 | 2704 | 0.79 | 0.74–0.85 | 2107 | 0.79 | 0.73–0.86 | 441 | 0.79 | 0.67–0.94 |
| 4 | 750 | 0.71 | 0.64–0.78 | 568 | 0.68 | 0.61–0.77 | 134 | 0.81 | 0.63–1.03 |
| 5 + | 287 | 0.60 | 0.52–0.70 | 216 | 0.59 | 0.50–0.70 | 51 | 0.59 | 0.41–0.84 |
| Age at first birthb | |||||||||
| < 20 years | 1752 | 1 | Ref | 1385 | 1 | Ref | 251 | 1 | Ref |
| 20–24 years | 5384 | 0.93 | 0.88–0.99 | 4225 | 0.92 | 0.86–0.99 | 843 | 1.02 | 0.87–1.20 |
| 25–29 years | 3842 | 0.97 | 0.91–1.04 | 2899 | 0.92 | 0.85–1.00 | 744 | 1.29 | 1.08–1.54 |
| 30+ years | 2177 | 0.98 | 0.89–1.08 | 1590 | 0.91 | 0.81–1.01 | 456 | 1.34 | 1.07–1.70 |
| Age at last birthb | |||||||||
| < 30 years | 6661 | 1 | Ref | 5209 | 1 | Ref | 1049 | 1 | Ref |
| 30–34 years | 3825 | 1.08 | 1.02–1.14 | 2920 | 1.07 | 1.01–1.14 | 689 | 1.12 | 0.97–1.28 |
| 35–39 years | 2062 | 1.13 | 1.05–1.22 | 1521 | 1.09 | 1.00–1.19 | 420 | 1.35 | 1.13–1.60 |
| 40+ years | 607 | 1.24 | 1.11–1.38 | 449 | 1.19 | 1.05–1.35 | 136 | 1.59 | 1.24–2.04 |
Adjusted for socio-economic status, occupational physical activity and hormonal replacement therapy use
N number of cancer cases, OR odds ratio, 95% CI 95% confidence interval
aThe model only included parity
bThe model included number of children, and age at first and last birth
Fig. 1.
Odds ratios of breast cancer among women diagnosed < 50 years for parous women in comparison to same-aged nulliparous women, by parity and time since the delivery. Multivariate conditional logistics regression analysis Adjusted for socio-economic status
Table 6 provides ORs for different types of postmenopausal HT therapy to breast cancer risk subtypes. Use of E only therapy for 5+ years significantly increased the risk of ductal (OR 1.17, 95% CI 1.08–1.27) and lobular (OR 1.18, 95% CI 1.00–1.40) breast cancer as compared to non-users. Continuous EPT increased the risk of both breast cancer subtypes, more strongly for lobular subtype and the strength of the association was directly proportional to duration of use. The use of EPT with monthly progestin for 1–< 5 years and 5+ years significantly increased the risk of breast cancer by 1.18 and 1.32-fold, respectively. EPT with progestin once in 3 months was not significantly associated with breast cancer risk. Use of one E+LNG-IUS device (typical use for 5 years) was associated with 1.56-fold increase in the risk of breast cancer (95% CI 1.45–1.69) as compared to never users, while use of more than one device was associated with 2.18-fold increase in risk (95% CI 1.81–2.64). The effect of E+LNG-IUS was similar for both ductal and lobular breast cancer.
Table 6.
Multivariate conditional logistics regression analysis for postmenopausal hormonal replacement therapy as predictor of breast cancer by histology among women diagnosed 50+ years
| Variables | Total | Ductal | Lobular | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | OR | 95% CI | N | OR | 95% CI | N | OR | 95% CI | |
| Estrogen only | |||||||||
| < 1 year | 212 | 0.95 | 0.82–1.10 | 165 | 0.98 | 0.83–1.15 | 34 | 0.91 | 0.63–1.31 |
| 1–< 5 years | 995 | 1.01 | 0.94–1.09 | 801 | 1.05 | 0.97–1.14 | 137 | 0.86 | 0.71–1.04 |
| 5+ years | 1146 | 1.18 | 1.10–1.27 | 875 | 1.17 | 1.08–1.27 | 185 | 1.18 | 1.00–1.40 |
| Continuous progestin | |||||||||
| < 1 year | 347 | 1.15 | 1.02–1.30 | 272 | 1.18 | 1.03–1.35 | 66 | 1.32 | 1.00–1.74 |
| 1–< 5 years | 1947 | 1.37 | 1.29–1.45 | 1476 | 1.34 | 1.25–1.43 | 363 | 1.57 | 1.37–1.81 |
| 5+ years | 1594 | 2.26 | 2.12–2.42 | 1151 | 2.12 | 1.97–2.30 | 374 | 3.34 | 2.87–3.90 |
| Monthly progestin | |||||||||
| < 1 year | 398 | 1.08 | 0.96–1.21 | 280 | 0.98 | 0.86–1.11 | 92 | 1.46 | 1.14–1.88 |
| 1–< 5 years | 2047 | 1.18 | 1.12–1.25 | 1568 | 1.20 | 1.13–1.29 | 383 | 1.11 | 0.97–1.28 |
| 5+ years | 647 | 1.32 | 1.20–1.45 | 485 | 1.35 | 1.21–1.51 | 121 | 1.14 | 0.91–1.42 |
| Once in 3-months progestin | |||||||||
| < 1 year | 65 | 0.84 | 0.64–1.10 | 45 | 0.78 | 0.57–1.08 | 16 | 0.94 | 0.53–1.66 |
| 1–< 5 years | 209 | 1.00 | 0.85–1.17 | 164 | 1.08 | 0.91–1.29 | 32 | 0.69 | 0.47–1.03 |
| 5+ years | 76 | 0.91 | 0.71–1.17 | 50 | 0.84 | 0.61–1.14 | 21 | 1.19 | 0.71–1.99 |
| E+LNG-IUS | |||||||||
| 1 purchase | 974 | 1.56 | 1.45–1.69 | 762 | 1.56 | 1.43–1.70 | 189 | 1.59 | 1.33–1.90 |
| 2+ purchases | 159 | 2.18 | 1.81–2.64 | 114 | 2.12 | 1.70–2.64 | 33 | 2.35 | 1.55–3.58 |
Adjusted for occupational physical activity, socioeconomic status and parity
N number of cancer cases, OR odds ratio, 95% CI 95% confidence interval
Discussion
Our findings showed that increasing age at first and last birth was associated with increased risk of breast cancer. Higher parity had a protective effect on the breast cancer but there was a transient increase in the risk after each pregnancy lasting for several years. Women in white-collar work and lower level of occupational physical activity had increased risk of breast cancer. Long-term use of EPT and E+LNG-IUS hormone therapy contributed strongly to the excess risk of breast cancer incidence.
Socioeconomic status
White-collar employees had higher risk of both pre- and postmenopausal breast cancer than blue-collar workers despite that our results were adjusted for parity and occupational physical activity and also for HT among the postmenopausal women. Most of the previous studies have shown similar association between different measures of increasing SES and breast cancer risk [20–22, 29, 30]. These studies however normally were not able to separate the effect of longer education and career planning leading to postponing of childbirth and resulting in lower parity and, the effects of other lifestyle factor in women with higher SES such as alcohol consumption and dietary habits nor take into account the greater access and use of exogenous hormones which may have been more is common among women in higher SES and increase their risk of postmenopausal breast cancer.
A previous study in Finland showed that age at first birth and average number of children did not vary by SES [31]. However, the non-modifiable reproductive risk factors such as early age at menarche and late age at menopause were observed among women with higher SES in Finland [32]. Therefore, these physiological differences and other aspects of SES such as alcohol consumption, which is more common among women with higher SES group in Finland might have been important risk contributor [33]. This might also explain that the greater strength of associations observed in our study for lobular breast cancer as compared to ductal subtypes in the upper-level employees, as lobular breast cancer is more sensitive to factors, such as alcohol consumption, that cause alterations in hormonal status.
Occupational physical activity
In our study, increased occupational physical activity was associated with lower breast cancer risk with the modest protective effect observed for lobular subtype and even smaller effect for ductal subtype. The smaller effect of physical activity in our study as compared to some earlier studies could be because our findings are adjusted for reproductive history and use of HT. We observed that the risk of breast cancer in both pre- and postmenopausal group was significantly reduced among the women who were economically inactive. Large proportion of this category comprised of spouses of farmers who are physically very active. Studies on association of breast cancer and physical activity have produced inconsistent results. While most studies show that occupational sedentariness is associated with increased risk of breast cancer [16, 34–38], few studies have shown borderline to no association [39–41]. Physical activity reduces the levels of circulating sex steroids that increases the risk of breast cancer among pre- and postmenopausal women [42–44]. Other mechanisms of breast cancer risk reduction through physical activity are via reduction in fat, boosting of immune system, and decreased insulin levels in body [42].
Reproductive factors
Increasing age at first birth was in our study associated with increased risk of lobular but not ductal breast cancer, similarly among pre- and postmenopausal women. Stronger association of the higher age at first birth with lobular breast cancer than with ductal subtypes was also observed in a large previous meta-analysis [45] and suggested in other studies [46–50]. First pregnancy exhibits maximum cellular differentiation and maturation of breast cells making them more resistant to carcinogenic effects [51, 52]. Increasing age at first birth and thereby longer duration between menarche and first birth, during which the undifferentiated breast tissue is subjected to tumor promoting effects of ovarian hormones in each menstrual cycle [50, 53]. Since lobular cancer is almost always estrogen receptor-positive and consequently more hormone sensitive than ductal cancer [54], an increase in the risk of lobular breast cancer with increasing age at first birth was expected.
Like previous studies [55–59], the present results showed that increasing parity is associated with lowered breast cancer risk. Furthermore, our results are well in line with earlier findings stating that pregnancy has dual effect on breast cancer risk, i.e., a long-term protective effect of a pregnancy is preceded by a transient increase of the risk after birth that can last up to 3–15 years [52, 60, 61]. Our findings among premenopausal women (Fig. 1) showed that women who had one child as compared to nulliparous women showed two-fold increased risk of breast cancer immediately after birth and the risk gradually decreased with protective effect observed about 7–8 years after first child. With each additional birth, the transient increase in risk was observed, however, the peak was smaller than in the previous birth. After fifth birth, there was only less than 20% transient excess risk as compared to nulliparous women, and protective effect started much earlier, about two years after the childbirth. Similar pattern of dual effect was seen for postmenopausal women but with a smaller transient increase in risk of breast cancer immediately after birth. We chose to present the findings for premenopausal breast cancer only because great majority of the postmenopausal breast cancers are diagnosed more than 10 years after childbirth and the modeling of the risk immediately after the birth is less strong. In a model combining both pre- and postmenopausal women, the peak of transient increase in the first-year peak was lower (OR about 1.5 after the first and second birth and below 1.0 for women after 4+ birth).
There is continuous increase of estrogens and other steroids during pregnancy, which are important for mammary gland development [9, 62, 63]. Consequently, they may play a role in proliferation and neoplastic transformation of breast cells [10, 63, 64] and cause transient increase in the risk after birth lasting for several years before protective effect is observed [51, 52].
Postmenopausal hormone therapy
Estrogen-only therapy was associated with elevated risk for both ductal and lobular cancers in long-term use of more than 5 years, but the magnitude of the effect was small compared to combined therapy. The findings from a recent meta-analysis of worldwide data showed that the effect of estrogen in breast cancer risk was doubled when the use was increased from 1 to 4 years to 5+ years, with 33% excess risk among estrogen-only users as compared with non-users [65]. Other previous studies suggest that the effect of estrogen-only HT is small and cannot be detected in short-term follow-up [66–68]. In Finland, previous studies have consistently shown that estradiol is associated with a moderate increase in breast cancer risk [11, 66].
Estrogen combined with progestin was more strongly associated with breast cancer than the use of estrogen-only therapy in our study, which has been shown also in previous studies [65, 69–71]. Addition of a progestin to estrogen therapy enhances the breast cells proliferation and number of cells present in terminal ductal lobular units increasing the risk of malignant transformation of breast tissues [72, 73]. Furthermore, the continuous use of progestin combined with estrogen was associated with higher relative risk than cyclic progestin used once a month or once in three months. This observation is also in line with previous studies [66, 71, 74]. Additionally, among continuous progestin therapy users, the risk of breast cancer increased linearly with duration of use and the relative risk was higher for lobular as compared to ductal cancer. Lobular breast cancer is hormonally more sensitive and continuous use of the combined EPT even for a short duration may cause significant proliferation of lobular cells resulting in cancer [69, 75].
We observed a strongly increased risk of both ductal and lobular breast cancer among E+LNG-IUS users. The magnitude of risk was higher for women who used more than one LNG-IUS device. A recent meta-analysis concluded an increased risk of breast cancer among LNG-IUS users regardless of age, and larger increase in risk was observed among older women [76]. The greater incidence of breast cancer among LNG-IUS users was observed also in some previous Finnish studies [77, 78].
Strengths and limitations
Our study has several strengths. The study is based on large number of breast cancer cases registered by the Finnish Cancer Registry, which is virtually complete as regard to cancer incidence since 1953 [79, 80], meaning that our results are strictly population-representative without selection bias. The Finnish Population Registry includes accurate information on childbirths of women born after the mid-1930s. Information on study variables had been registered in high-quality population-based registries similarly for cases and controls and we therefore do not have recall bias. We were able to include in the same model several important components of breast cancer etiology including reproductive history, SES, and occupational physical activity, and HT use, and hence to assess their independent roles in breast cancer etiology. However, the study has some limitations as well. A major limitation of our study is that we did not have information on estrogen receptor (ER) status of the breast cancer and were unable to distinguish the risk between ER positive and ER negative breast cancer. We did not have information on the family history of the breast cancer of cases and controls. Similarly, information such as age at menarche, age at menopause, body mass index (BMI), and history of breastfeeding among parous women were not available. Among Finnish female population, BMI is lower for higher SES and vice versa [81]. Therefore, we would expect to get even higher risk estimate for high SES if we would have been able to adjust the OR for the BMI.
Conclusion
Our study confirms that increasing age at first and last birth increases risk of breast cancer while increasing parity has a protective effect, but the effect is not straightforward: the long-term protective effect of parity is preceded by a transient increase in the risk after each pregnancy, which lasts for several years. We found that the selection of HT type affects the breast cancer incidence among postmenopausal women, and this effect remains after adjustment for parity and SES. Long-term estrogen treatment with continuous progestin or LNG-IUS was strongly associated with breast cancer risk irrespective of the histology of the cancer. Women with higher SES had elevated risk of breast cancer while occupational sedentariness only carried a minor excess after adjustment for parity and HT. Sedentary work is an important risk factor of BC from public health point of view because of large and increasing fraction of women in sedentary occupations. Multivariate setting with several factors of BC potentially associated with women’s reproductive, socio-economic and lifestyle selection is necessary to understand the true influences of these factors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the funds from Tampere University Foundation sr. The funding bodies had no role in the design of the study, collection, analysis, and interpretation of the data or in writing the manuscript.
Abbreviations
- CI
Confidence interval
- E
Estradiol
- EPT
Estradiol Progestin Therapy
- E+LNG-IUS
Estradiol (oral or transdermal) plus levonorgestrel-releasing intrauterine system
- HT
Hormonal therapy
- ICD
International Classification of Diseases
- ISCED
International Standard Classification of Education
- ISCO
International Standard Classification of Occupations
- NOCCA
Nordic Occupational Cancer Study
- NOCCA-JEM
Job Exposure Matrix of the NOCCA study
- OR
Odds ratio
- PPWL
Perceived physical workload
- SES
Socio-economic status
Data availability
Enquiries about data availability should be directed to the authors.
Declarations
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Pitkäniemi J, Malila N, Virtanen A, Degerlund H, Heikkinen S, Seppä K (2020) Cancer in Finland 2018. Cancer Society of Finland Publication No. 94, Helsinki
- 3.Zhang J, Wang M, Wang X, Li Y, Liang Z, Lin Y, et al. Associations of reproductive factors with breast cancer prognosis and the modifying effects of menopausal status. Cancer Med. 2020;9(1):385–393. doi: 10.1002/cam4.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. Reproductive risk factors in relation to molecular subtypes of breast cancer: results from the nurses' health studies. Int J Cancer. 2016;138(10):2346–2356. doi: 10.1002/ijc.29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troisi R, Bjørge T, Gissler M, Grotmol T, Kitahara CM, Myrtveit Saether SM, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med. 2018;283(5):430–445. doi: 10.1111/joim.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez SV, Russo J. Estrogen and xenoestrogens in breast cancer. Toxicol Pathol. 2010;38(1):110–122. doi: 10.1177/0192623309354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102(1):89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 10.Dickson RB, Lippman ME. Control of human breast cancer by estrogen, growth factors, and oncogenes. Cancer Treat Res. 1988;40:119–165. doi: 10.1007/978-1-4613-1733-3_6. [DOI] [PubMed] [Google Scholar]
- 11.Brusselaers N, Tamimi RM, Konings P, Rosner B, Adami HO, Lagergren J. Different menopausal hormone regimens and risk of breast cancer. Ann Oncol. 2018;29(8):1771–1776. doi: 10.1093/annonc/mdy212. [DOI] [PubMed] [Google Scholar]
- 12.Lyytinen K, Dyba T, Ylikorkala O, Pukkala E. A case-control study on hormone therapy as a risk factor for breast cancer in Finland: intrauterine system carries a risk as well. Int J Cancer. 2010;126(2):483–489. doi: 10.1002/ijc.24738. [DOI] [PubMed] [Google Scholar]
- 13.Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet 394(10204):1159–1168 (2019) [DOI] [PMC free article] [PubMed]
- 14.Moradi T, Adami H, Bergström R, Gridley G, Wolk A, Gerhardsson M, et al. Occupational physical activity and risk for breast cancer in a nationwide cohort study in Sweden. Cancer Causes Control. 1999;10(5):423–430. doi: 10.1023/A:1008922205665. [DOI] [PubMed] [Google Scholar]
- 15.Ekenga CC, Parks CG, Sandler DP. A prospective study of occupational physical activity and breast cancer risk. Cancer Causes Control. 2015;26(12):1779–1789. doi: 10.1007/s10552-015-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Wang Q, Zhang Y, Xie Q, Tan X. Physical Activity and Risk of Breast Cancer: a meta-analysis of 38 cohort studies in 45 study reports. Value in Health. 2019;22(1):104–128. doi: 10.1016/j.jval.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Luoto R, Latikka P, Pukkala E, Hakulinen T, Vihko V. The effect of physical activity on breast cancer risk: a cohort study of 30,548 women. Eur J Epidemiol. 2000;16(10):973–980. doi: 10.1023/A:1010847311422. [DOI] [PubMed] [Google Scholar]
- 18.Guo W, Fensom GK, Reeves GK, Key TJ. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br J Cancer. 2020;122(5):726–732. doi: 10.1038/s41416-019-0700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyle G, Hendrie GA, Hendrie D. Understanding the effects of socioeconomic status along the breast cancer continuum in Australian women: a systematic review of evidence. Int J Equity Health. 2017;16(1):182. doi: 10.1186/s12939-017-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundqvist A, Andersson E, Ahlberg I, Nilbert M, Gerdtham U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur J Public Health. 2016;26(5):804–813. doi: 10.1093/eurpub/ckw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen SB, Olsen A, Lynch J, Christensen J, Overvad K, Tjønneland A, et al. Socioeconomic position and lifestyle in relation to breast cancer incidence among postmenopausal women: a prospective cohort study, Denmark, 1993–2006. Cancer Epidemiol. 2011;35(5):438–441. doi: 10.1016/j.canep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Pukkala E, Weiderpass E. Time trends in socio-economic differences in incidence rates of cancers of the breast and female genital organs (Finland, 1971–1995) Int J Cancer. 1999;81(1):56–61. doi: 10.1002/(SICI)1097-0215(19990331)81:1<56::AID-IJC11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Katuwal S, Martinsen JI, Kjaerheim K, Sparen P, Tryggvadottir L, Lynge E, et al. Occupational variation in the risk of female breast cancer in the Nordic countries. Cancer Causes Control. 2018;29(11):1027–1038. doi: 10.1007/s10552-018-1076-2. [DOI] [PubMed] [Google Scholar]
- 24.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303(5920):767–770. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 25.Pukkala E, Engholm G, HøjsgaardSchmidt LK, Storm H, Khan S, Lambe M, et al. Nordic Cancer Registries—an overview of their procedures and data comparability. Acta Oncol. 2018;57:440–455. doi: 10.1080/0284186X.2017.1407039. [DOI] [PubMed] [Google Scholar]
- 26.Statistics Finland. Classification of Socio-economic Groups. https://www.stat.fi/en/luokitukset/sosioekon_asema/. Accessed 16 Dec 2020
- 27.Kauppinen T, Heikkilä P, Plato N, Woldbaek T, Lenvik K, Hansen J, et al. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA) Acta Oncol. 2009;48(5):791–800. doi: 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- 28.Backman T, Huhtala S, Tuominen J, Luoto R, Erkkola R, Blom T, et al. Sixty thousand woman-years of experience on the levonorgestrel intrauterine system: an epidemiological survey in Finland. Eur J Contracept Reprod Health Care. 2001;6(suppl 1):23–26. doi: 10.3109/ejc.6.s1.23.26. [DOI] [PubMed] [Google Scholar]
- 29.Danø H, Hansen KD, Jensen P, Petersen JH, Jacobsen R, Ewertz M, et al. Fertility pattern does not explain social gradient in breast cancer in Denmark. Int J Cancer. 2004;111(3):451–456. doi: 10.1002/ijc.20203. [DOI] [PubMed] [Google Scholar]
- 30.Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105(5):692–700. doi: 10.1002/ijc.11150. [DOI] [PubMed] [Google Scholar]
- 31.Rimpelä AH, Pukkala EI. Cancers of affluence: positive social class gradient and rising incidence trend in some cancer forms. Soc Sci Med. 1987;24(7):601–606. doi: 10.1016/0277-9536(87)90064-5. [DOI] [PubMed] [Google Scholar]
- 32.Rimpelä A, Rimpelä M (1983) Biological growth and maturation, in health habits among Finnish youth. Health Education. Series Original Reports 4/1984
- 33.Helakorpi S, Patja K, Prättälä R, Uutela A. Health behaviour and health among the Finnish adult population, publications of the National Public Health Institute 18/2005. Helsinki (in Finnish with English abstract): National Public Health Institute; 2005. [Google Scholar]
- 34.Johnsson A, Broberg P, Johnsson A, Tornberg ÅB, Olsson H. Occupational sedentariness and breast cancer risk. Acta Oncol. 2017;56(1):75–80. doi: 10.1080/0284186X.2016.1262547. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhao H, Peng C. Association of sedentary behavior with the risk of breast cancer in women: update meta-analysis of observational studies. Ann Epidemiol. 2015;25(9):687–697. doi: 10.1016/j.annepidem.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42(8):636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 37.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 38.Rintala P, Pukkala E, Läärä E, Vihko V. Physical activity and breast cancer risk among female physical education and language teachers: a 34-year follow-up. Int J Cancer. 2003;107(2):268–270. doi: 10.1002/ijc.11390. [DOI] [PubMed] [Google Scholar]
- 39.Lynch BM, Courneya KS, Friedenreich CM. A case-control study of lifetime occupational sitting and likelihood of breast cancer. Cancer Causes Control. 2013;24(6):1257–1262. doi: 10.1007/s10552-013-0194-0. [DOI] [PubMed] [Google Scholar]
- 40.Colditz GA, Feskanich D, Chen WY, Hunter DJ, Willett WC. Physical activity and risk of breast cancer in premenopausal women. Br J Cancer. 2003;89(5):847–851. doi: 10.1038/sj.bjc.6601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee IM, Cook NR, Rexrode KM, Buring JE. Lifetime physical activity and risk of breast cancer. Br J Cancer. 2001;85(7):962–965. doi: 10.1054/bjoc.2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin ML. Randomized controlled trials of physical activity and breast cancer prevention. Exerc Sport Sci Rev. 2006;34(4):182–193. doi: 10.1249/01.jes.0000240026.15126.ca. [DOI] [PubMed] [Google Scholar]
- 43.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 44.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132(11 Suppl):3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 45.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phipps AI, Li CI, Kerlikowske K, Barlow WE, Buist DSM. Risk factors for ductal, lobular, and mixed ductal-lobular breast cancer in a screening population. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1643–1654. doi: 10.1158/1055-9965.EPI-10-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ursin G, Bernstein L, Lord SJ, Karim R, Deapen D, Press MF, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93(3):364–371. doi: 10.1038/sj.bjc.6602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves GK, Pirie K, Green J, Bull D, Beral V, Million Women SC. Reproductive factors and specific histological types of breast cancer: prospective study and meta-analysis. Br J Cancer. 2009;100(3):538–544. doi: 10.1038/sj.bjc.6604853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newcomb PA, Trentham-Dietz A, Hampton JM, Egan KM, Titus-Ernstoff L, Warren Andersen S, et al. Late age at first full term birth is strongly associated with lobular breast cancer. Cancer. 2011;117(9):1946–1956. doi: 10.1002/cncr.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, et al. Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol. 2008;167(2):230–239. doi: 10.1093/aje/kwm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo J, Wilgus G, Russo IH. Susceptibility of the mammary gland to carcinogenesis: I differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am J Pathol. 1979;96(3):721–736. [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh C, Pavia M, Lambe M, Lan SJ, Colditz GA, Ekbom A, et al. Dual effect of parity on breast cancer risk. Eur J Cancer. 1994;30A(7):969–973. doi: 10.1016/0959-8049(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 53.Clavel-Chapelon F, E3N Group Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control. 2002;13(9):831–838. doi: 10.1023/A:1020684821837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li CI, Daling JR, Haugen KL, Tang MTC, Porter PL, Malone KE. Use of menopausal hormone therapy and risk of ductal and lobular breast cancer among women 55–74 years of age. Breast Cancer Res Treat. 2014;145(2):481–489. doi: 10.1007/s10549-014-2960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat. 1996;38(3):305–311. doi: 10.1007/BF01806150. [DOI] [PubMed] [Google Scholar]
- 56.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat. 2002;72(2):107–115. doi: 10.1023/A:1014891216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Högnäs E, Kauppila A, Pukkala E, Tapanainen JS. Cancer risk in women with 10 or more delveries. Obstet Gynecol. 2014;123(4):811–816. doi: 10.1097/AOG.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 58.Katuwal S, Tapanainen JS, Pukkala E, Kauppila A. The effect of length of birth interval on the risk of breast cancer by subtype in grand multiparous women. BMC Cancer. 2019;19(1):199. doi: 10.1186/s12885-019-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nyante SJ, Dallal CM, Gierach GL, Park Y, Hollenbeck AR, Brinton LA. Risk factors for specific histopathological types of postmenopausal breast cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;178(3):359–371. doi: 10.1093/aje/kws471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods KL, Smith SR, Morrison JM. Parity and breast cancer: evidence of a dual effect. Br Med J. 1980;281(6237):419–421. doi: 10.1136/bmj.281.6237.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q, Wuu J, Lambe M, Hsieh S, Ekbom A, Hsieh C. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13(4):299–305. doi: 10.1023/A:1015287208222. [DOI] [PubMed] [Google Scholar]
- 62.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(4):361. [PubMed] [Google Scholar]
- 64.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102(1–5):89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collaborative Group on Hormonal Factors in Breast Cancer Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159–1168. doi: 10.1016/S0140-6736(19)31709-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estrogen-only therapy. Obstet Gynecol. 2006;108(6):1354–1360. doi: 10.1097/01.AOG.0000241091.86268.6e. [DOI] [PubMed] [Google Scholar]
- 67.Zhang SM, Manson JE, Rexrode KM, Cook NR, Buring JE, Lee I. Use of oral conjugated estrogen alone and risk of breast cancer. Am J Epidemiol. 2007;165(5):524–529. doi: 10.1093/aje/kwk038. [DOI] [PubMed] [Google Scholar]
- 68.Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128(1):144–156. doi: 10.1002/ijc.25314. [DOI] [PubMed] [Google Scholar]
- 69.Li CI, Malone KE, Porter PL, Weiss NS, Tang MC, Cushing-Haugen KL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289(24):3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 70.Román M, Sakshaug S, Graff-Iversen S, Vangen S, Weiderpass E, Ursin G, et al. Postmenopausal hormone therapy and the risk of breast cancer in Norway. Int J Cancer. 2016;138(3):584–593. doi: 10.1002/ijc.29810. [DOI] [PubMed] [Google Scholar]
- 71.Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362(9382):419–427 (2003) [DOI] [PubMed]
- 72.Wood CE, Branstetter D, Jacob AP, Cline JM, Register TC, Rohrbach K, et al. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013;15(4):R62. doi: 10.1186/bcr3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santen RJ, Pinkerton J, McCartney C, Petroni GR. Risk of breast cancer with progestins in combination with estrogen as hormone replacement therapy. J Clin Endocrinol Metab. 2001;86(1):16–23. doi: 10.1210/jcem.86.1.7269. [DOI] [PubMed] [Google Scholar]
- 74.Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97(6):1387–1392. doi: 10.1002/cncr.11205. [DOI] [PubMed] [Google Scholar]
- 75.Newcomb PA, Titus-Ernstoff L, Egan KM, Trentham-Dietz A, Baron JA, Storer BE, et al. Postmenopausal estrogen and progestin use in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(7):593–600. [PubMed] [Google Scholar]
- 76.Conz L, Mota BS, Bahamondes L, TeixeiraDória M, Françoise MauricetteDerchain S, Rieira R, et al. Levonorgestrel-releasing intrauterine system and breast cancer risk: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99(8):970–982. doi: 10.1111/aogs.13817. [DOI] [PubMed] [Google Scholar]
- 77.Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Joensuu H, et al. Levonorgestrel-releasing intrauterine system and the risk of breast cancer: a nationwide cohort study. Acta Oncol. 2016;55(2):188–192. doi: 10.3109/0284186X.2015.1062538. [DOI] [PubMed] [Google Scholar]
- 78.Soini T, Hurskainen R, Grénman S, Mäenpää J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 Pt 1):292–299. doi: 10.1097/AOG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 79.Pukkala E. Biobanks and registers in epidemiologic research on cancer. Methods Mol Biol. 2011;11(675):127–164. doi: 10.1007/978-1-59745-423-0_5. [DOI] [PubMed] [Google Scholar]
- 80.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33(4):365–369. doi: 10.3109/02841869409098430. [DOI] [PubMed] [Google Scholar]
- 81.Prättälä R, Sippola R, Lahti-Koski M, Laaksonen MT, Mäkinen T, Roos E. Twenty-five-year trends in body mass index by education and income in Finland. BMC Public Health. 2012;12(1):936. doi: 10.1186/1471-2458-12-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Enquiries about data availability should be directed to the authors.