Abstract
Background:
Patients with metastatic renal cell carcinoma (mRCC) treated with systemic therapy sometimes progress at limited sites. The best treatment approach for patients with oligoprogression remains unclear.
Objective:
To determine the ability of stereotactic ablative radiation (SAbR) to extend ongoing systemic therapy in mRCC patients with oligoprogression.
Design, setting, and participants:
A single-arm phase II clinical trial was conducted at a university medical center and county hospital, including 20 patients with mRCC on first- to fourth-line systemic therapy with three or fewer sites of progression (including new sites) involving ≤30% of all sites.
Intervention:
SAbR to oligoprogressing metastases at outset and longitudinally, while radiated sites remain controlled and overall disease oligoprogressive.
Outcome measurements and statistical analysis:
The primary objective was to extend ongoing systemic therapy by >6 mo in >40% of patients. Secondary endpoints included overall survival, toxicity, and patient-reported quality of life.
Results and limitations:
Twenty patients were enrolled. Upfront and sequential SAbR was administered to a total of 37 sites. The local control rate was 100%. At a median follow-up of 10.4 mo (interquartile range: 5.8–16.4), SAbR extended the duration of the ongoing systemic therapy by >6 mo in 14 patients (70%, 95% confidence interval [CI]: 49.9–90.1). The median time from SAbR to the onset of new systemic therapy or death was 11.1 mo (95% CI: 4.5–19.3). The median duration of SAbR-aided systemic therapy was 24.4 mo (95% CI: 15.3–42.2). Median overall survival was not reached. One patient developed grade 3 gastrointestinal toxicity possibly related to treatment. There was no significant decline in quality of life. Limitations include nonrandomized design and a small patient cohort.
Conclusions:
SAbR extended the duration of the ongoing systemic therapy for patients with oligoprogressive mRCC without undermining quality of life. These data support the evaluation of SAbR for oligoprogressive mRCC in a prospective randomized clinical trial.
Patient summary:
Patients with metastatic kidney cancer on systemic therapy but progressing at limited sites may benefit from focused radiation to progressive sites. Focused radiation was safe and effective, and extended the duration of the ongoing systemic therapy.
Keywords: Oligometastasis, Oligoprogression, Renal cell carcinoma, Stereotactic ablative radiation, Stereotactic body radiation therapy, Stereotactic radiation
1. Introduction
Approximately 25% of patients with renal cell carcinoma (RCC) present with regional or distant metastasis, and another 20% of the patients with localized disease eventually develop metastasis [1]. Systemic therapy with immune checkpoint inhibitors (ICIs), tyrosine kinase inhibitors (TKIs), or a combination of the two is the standard of care for metastatic RCC (mRCC) [2–6]. However, the disease progresses in most patients.
To date, there has been limited research into patterns of progression. For example, the conventionally used criteria for response assessment in clinical trials, Response Evaluation Criteria in Solid Tumors (RECIST) criteria, do not distinguish patterns of progression. By RECIST, a 20% increase in the sum of the longest diameters of target lesions, even if involving a few sites, or the development of a single new site of disease, is regarded as a progression event. Similarly, in clinical practice, the current approach is to switch systemic therapy upon progression. This applies also to patients who tolerate the ongoing systemic therapy well and have limited progression. However, subsequent lines of systemic therapy are typically associated with shorter progression-free survival (PFS) intervals and are often associated with increased toxicity [7]. Hence, when progression occurs at a few sites, application of local therapy is a reasonable strategy—this may extend the duration of the ongoing systemic therapy and potentially prolong survival. The rationale for local therapy in oligoprogressive metastatic disease is supported by studies showing mutational heterogeneity and branched evolution that fosters tumor adaptation and therapeutic failure through Darwinian selection [8–10].
Stereotactic ablative radiation (SAbR) is safe and effective for treating RCC metastases [11–13]. Local control (LC) rates are typically >90%, although follow-up is usually limited [14]. A potential added benefit of radiotherapy is its ability to induce antigen presentation, which can synergize with ICIs [15]. In addition, SAbR may induce an abscopal effect that has been reported in up to 6% of patients [16]. In 2013, the first report of SAbR for oligoprogression was published in the medical literature [17]. We showed that SAbR extended the duration of systemic therapy from 14 to 22 mo. We subsequently reported our experience of SAbR for mRCC, which included 36 patients who had oligoprogressive disease, where SAbR extended the duration of systemic therapy by a median of 9.2 mo [18]. Others have also reported similar extensions in PFS of ongoing systemic therapy after SAbR [19]. These results set the foundation for a prospective phase II trial to evaluate SAbR to extend ongoing systemic therapy in patients with oligoprogressive mRCC.
2. Patients and methods
2.1. Study design and participants
This study is a single-arm, phase II trial conducted at a university medical center and affiliated county hospital (Parkland Health and Hospital System) that enrolled patients with mRCC and oligoprogression (Fig. 1). The essential criteria included at least one set of radiographic images showing overall disease control while on first- to fourth line of systemic therapy with no more than one to three sites of progression in ≤30% of all metastases. All sites of progression needed to be suitable for SAbR. Eligible patients were at least 18 yr old with pathology-proven RCC and an Eastern Cooperative Oncology Group performance status of 0–2 (full eligibility criteria can be found in the study protocol in the Supplementary material). Patients with high-risk disease by International Metastatic RCC Database Consortium (IMDC) criteria were excluded given the concerns that oligoprogression in the context of particularly aggressive disease may not be representative.
Fig. 1 –
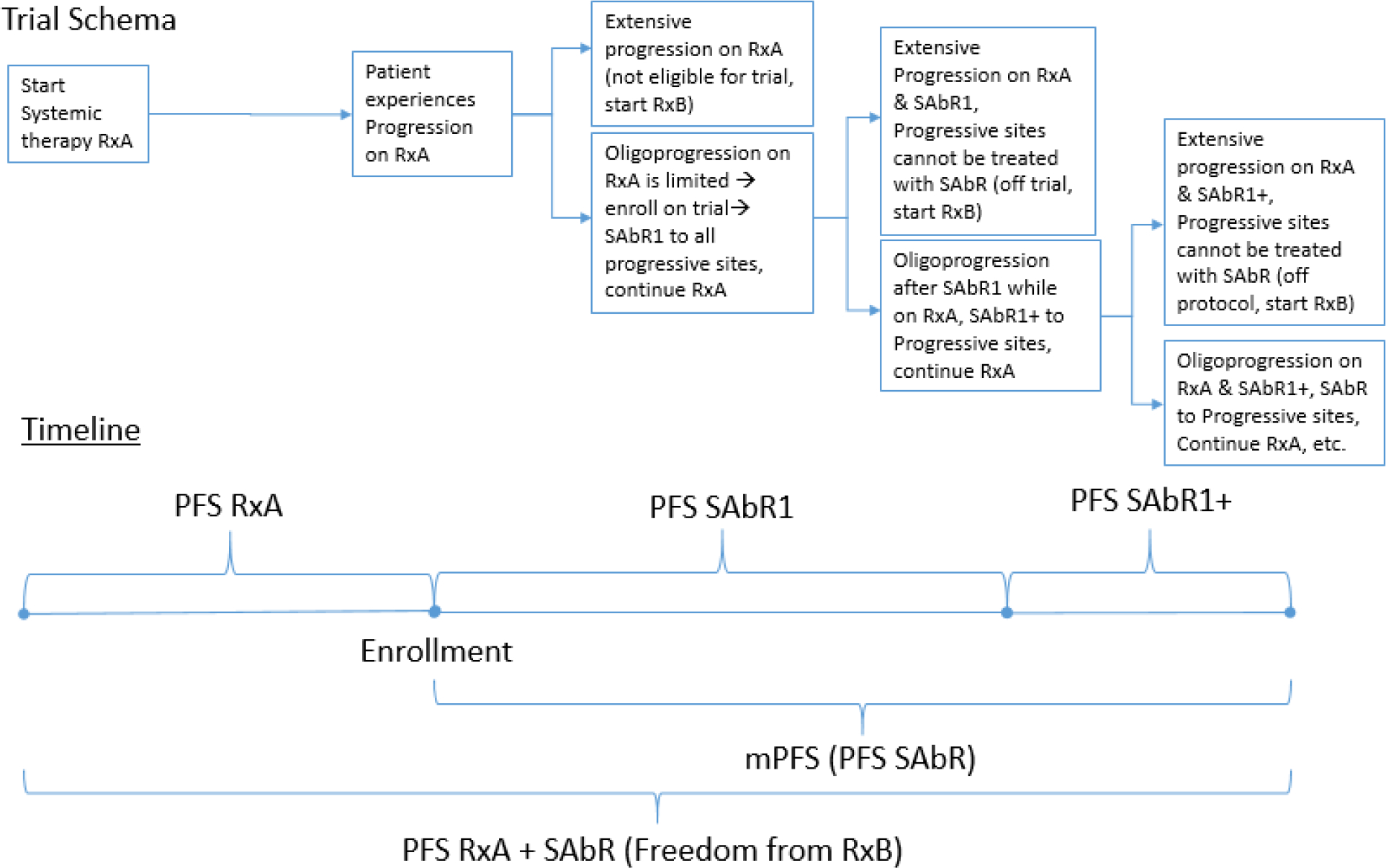
Trial schema. Oligoprogressive mRCC patients on systemic therapy treated with sequential SAbR until progression. mPFS = modified progression-free survival; mRCC = metastatic renal cell carcinoma; PFS = progression-free survival; RxA = initial systemic therapy; RxB = subsequent systemic therapy; SAbR = stereotactic ablative radiation; SAbR1 = initial SAbR; SAbR1+ = subsequent SAbR.
2.2. Treatment technique
Patients underwent computed tomography (CT)-based simulation with customized immobilization with tumor motion assessment and management, as needed. SAbR treatment planning and delivery were performed using standard techniques with on-board cone beam CT, as described previously [20]. Treatment regimens included ≥25 Gy × one fraction, ≥12 Gy × three fractions, or ≥8 Gy × five fractions to the periphery of the target to cover >95% of the target volume. Systemic therapy was optionally held from 3 d before to 3 d after SAbR at the discretion of the treating physicians.
2.3. Outcomes
The primary objective was to evaluate the probability of postponing next-line systemic therapy for >6 mo in >40% of patients, which was considered clinically meaningful. The benchmarks (6 mo and 40% of patients) were chosen based on the institutional experience of sequential SAbR for oligoprogressive mRCC and the literature [18,21]. For the purpose of the primary endpoint, progression mandating new systemic therapy was defined as follows: (1) progression at any irradiated site, (2) progression at a site unamenable to SAbR, (3) progression at more than three lesions (including both new and existing), (4) progression at >30% of all lesions, or (5) progression as determined by the medical or radiation oncologist.
Other endpoints included the following. PFS of SAbR-aided ongoing systemic therapy was defined as the time from the start of ongoing systemic therapy until the onset of new systemic therapy or death. Thus, for the purpose of this study, PFS was determined by progression not amenable to SAbR longitudinal control and largely coincided with the duration of the particular line of systemic therapy when SAbR was administered. We defined modified progression-free survival (mPFS) as the time from SAbR to the onset of new systemic therapy or death. Secondary endpoints included PFS of subsequent systemic therapy (PFS-SST), overall survival (OS), safety, and health-related quality of life (QOL). Toxicities were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 scale. Health-related QOL was assessed using the EuroQol Group’s five-level (EQ-5D-5L), Functional Assessment of Cancer Therapy—General (FACT-G), and Functional Assessment of Cancer Therapy—Kidney Symptom Index (FKSI) questionnaires. Follow-up included radiographic imaging at 3-mo intervals, which was evaluated for progression using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [22].
2.4. Statistical analysis
The primary objective was to extend ongoing systemic therapy by >6 mo in >40% of patients. Let P be the probability of postponing systemic therapy by >6 mo. The required sample size of using P as the primary endpoint for the one-arm design is based on excluding 40% from the lower limit of the 95% confidence interval (CI). The lower limit of the 95% CI is >40% when P = 70% and the sample size is 20 patients. The calculation is equivalent to estimating the sample size that yields P significantly different from 40% at a significance level of 0.05 when the actual P is 70%. An analysis from PASS (version 14) shows that a sample size of 20 patients will achieve 82% power to detect a difference of 30% (70% vs 40%) in the probability of postponing systemic therapy by >6 mo using a two-sided exact test with a significance level of 0.05, assuming that the population proportion of P under the null hypothesis is 70%. The probability of postponing subsequent systemic therapy by >6 mo and its 95% exact CI were determined by the Clopper and Pearson [23] methodology. Swimmer plots were drawn to show the timing of ongoing systemic therapy, SAbR treatments, and follow-up. The Kaplan-Meier method was used to estimate PFS and OS. Log-rank tests were used to investigate differences in PFS among study groups. Cox regression analyses were conducted to examine associations between PFS and prognostic factors. Paired t tests were used to examine changes in QOL scores from baseline to month 3.
3. Results
Twenty patients were enrolled between February 2019 and October 2020. Patient and treatment characteristics are summarized in Table 1. Most patients presented with synchronous metastases (60%) and had one lesion treated at initial SAbR (55%). At the time of initial SAbR, 12 patients had six or more metastases and eight patients had five or fewer metastases. Twenty patients received SAbR to 29 total lesions upfront, and six patients underwent SAbR to subsequent sites of disease progression for a total of 37 treated lesions. The median size of initial lesions treated with SAbR was 3.4 cm (interquartile range [IQR]: 2.4–4.9 cm). Lung (27%), liver (16.2%), and nonspine bone (16.2%) were the most common sites for SAbR. At enrollment, four patients were on first-line, ten on second-line, four on third-line, and two on fourth-line systemic therapy. Eight patients were on an ICI, eight were on a TKI, and four were on combination therapy (ICI + TKI, three patients; TKI + mTOR-I, one patient).
Table 1 –
Patient and treatment characteristics (n = 20 patients)
| Frequency | Percentage | |
|---|---|---|
| Median age (interquartile range) | 60.5 (55.5–64.75) | |
| Gender | ||
| Female | 4 | 20 |
| Male | 16 | 80 |
| Ethnicity | ||
| Hispanic | 3 | 15 |
| Non-Hispanic | 17 | 85 |
| Race | ||
| White | 15 | 75 |
| Asian | 1 | 5 |
| Black | 2 | 10 |
| Missing | 2 | 10 |
| IMDC risk score at enrollment | ||
| 0, favorable | 5 | 25 |
| 1–2, intermediate | 15 | 75 |
| Mean primary tumor diameter at initial diagnosis ± std. dev. | 7.7 ± 2.4 | |
| Histology | ||
| ccRCC | 18 | 90 |
| Papillary | 1 | 5 |
| RCC, NOS | 1 | 5 |
| Fuhrman grade | ||
| 2 | 7 | 35 |
| 3 | 7 | 35 |
| 4 | 6 | 30 |
| Nephrectomy | ||
| Cytoreductive | 12 | 60 |
| Localized/locoregional RCC | 7 | 35 |
| None | 1 | 5 |
| Initial staging (AJCC) | ||
| pT1 | 2 | 10 |
| pT2 | 1 | 5 |
| pT3 | 14 | 70 |
| pT4 | 2 | 10 |
| pTx | 1 | 5 |
| cN | ||
| cN1 | 2 | 10 |
| cNx | 8 | 40 |
| M | ||
| cM1 | 11 | 55 |
| Number of lines of systemic therapy prior to SAbR | ||
| 1 | 4 | 20 |
| 2 | 10 | 50 |
| 3 | 4 | 20 |
| 4 | 2 | 10 |
| Type of systemic therapy at SAbR | ||
| TKI | 8 | 40 |
| ICI | 8 | 40 |
| ICI + TKI | 3 | 15 |
| mTOR-I + TKI | 1 | 5 |
| Number of mets at SAbR | ||
| ≤5 | 8 | 40 |
| ≥6 | 12 | 60 |
| Synchronous metastasis | ||
| No | 8 | 40 |
| Yes | 12 | 60 |
| Number of SAbR sites treated initially | ||
| 1 | 11 | 55 |
| 2 | 9 | 45 |
| Size of SAbR lesions treated initially (cm), median (interquartile range) | 3.4 (2.4–4.9) | |
| All sites treated with SAbR (n = 37) | ||
| Kidney | 3 | 8.1 |
| Bone, nonspine | 6 | 16.2 |
| Spine | 5 | 13.5 |
| Lung | 10 | 27 |
| Liver | 6 | 16.2 |
| Adrenal | 3 | 8.1 |
| Abdomen | 2 | 5.4 |
| Soft tissue | 1 | 2.7 |
| Brain | 1 | 2.7 |
| SAbR fractionation (n = 37) | ||
| 1 fraction | 6 | 16.2 |
| 3 fractions | 21 | 56.8 |
| 5 fractions | 10 | 27 |
| Median/mode dose (range) | ||
| 1 fraction | 22/25 | (15–25) |
| 3 fractions | 36/36 | (36–45) |
| 5 fractions | 40/40 | (35–40) |
AJCC = American Joint Committee on Cancer Staging, eighth edition; ccRCC = clear cell renal cell carcinoma; ICI = immune checkpoint inhibitor; IMDC = International Metastatic RCC Database Consortium; mets = metastases; NOS = not otherwise specified; RCC = renal cell carcinoma; SAbR = stereotactic ablative radiotherapy; std. dev. = standard deviation; TKI = tyrosine kinase inhibitor.
The median follow-up was 10.4 mo (IQR: 5.8–16.4). At the time of this analysis, seven patients continue on systemic therapy without progressing to next line; nine patients switched systemic therapy, three of whom died later; and four patients died without changing systemic therapy (Fig. 2A and Supplementary Fig. 1). The duration of the ongoing systemic therapy was extended for >6 mo in 14/20 patients (70.0%, 95% CI: 49.9–90.1; Fig. 2B). Of these 14 patients, seven received ICIs, five received TKIs, and two received combination therapy at the time of SAbR. Among these 14 patients, four were on first-line, eight on second-line, and two were on third-line therapy. The median mPFS was 11.1 mo (95% CI: 4.5–19.3; Fig. 2B). At 6 mo after SAbR, the percent of patients remaining on the ongoing systemic therapy was 69.6% (95% CI: 44.5–85.1). At 1 yr, 44.2% of patients receiving SAbR remained on the same systemic therapy (95% CI: 20.5–65.7).
Fig. 2 –
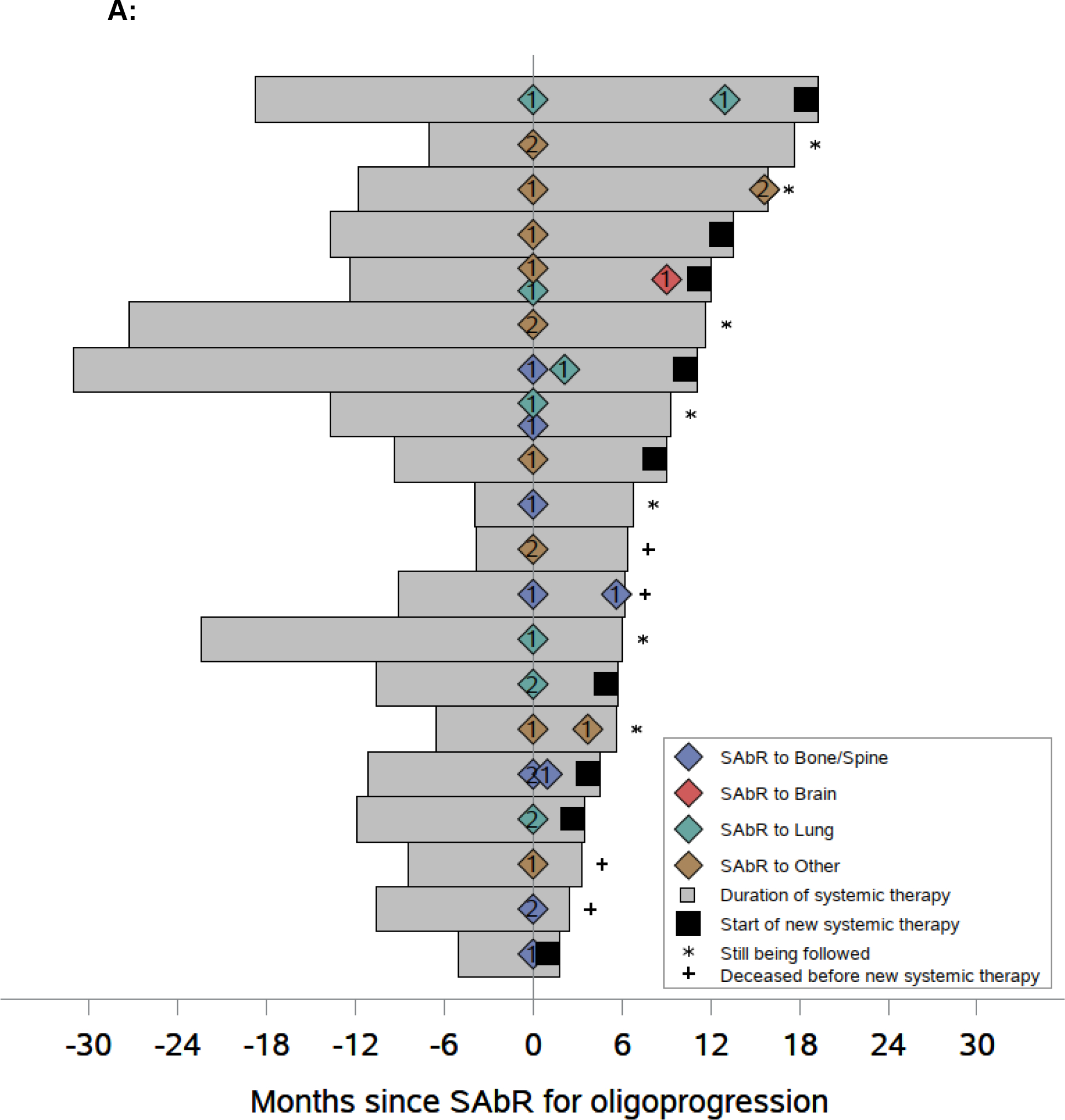
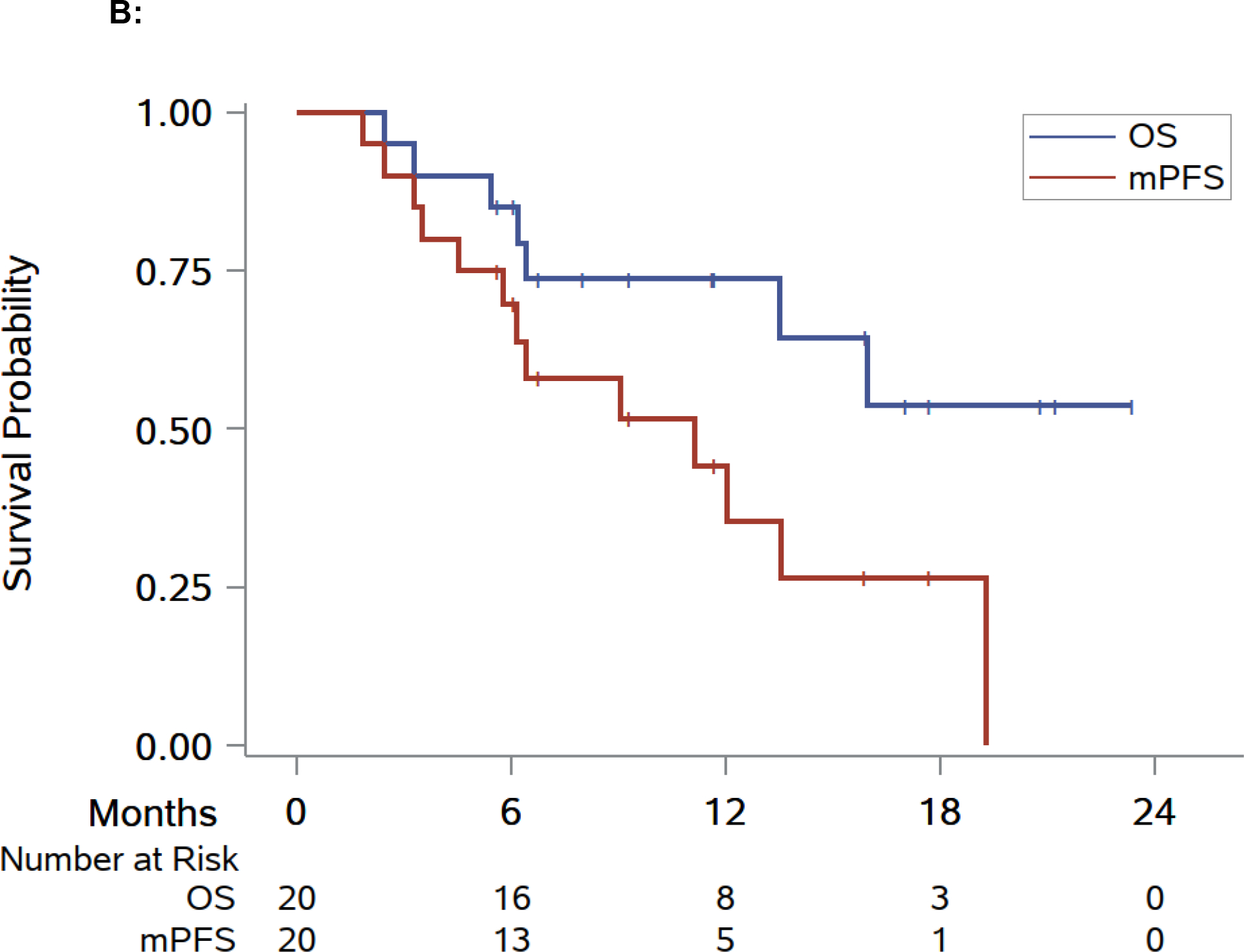
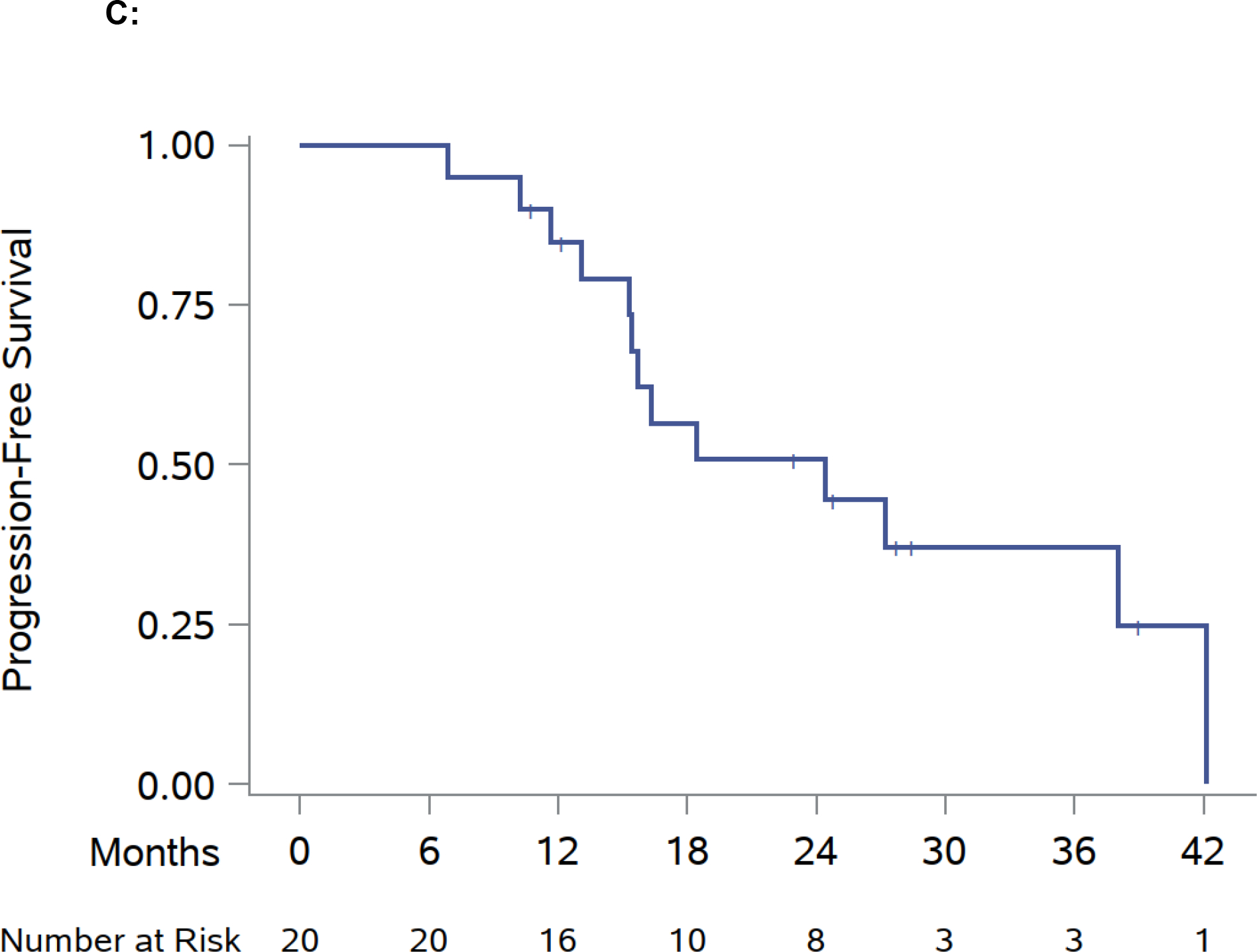
Swimmer plot and Kaplan-Meier analyses of modified progression-free survival (mPFS), overall survival (OS), and progression-free survival (PFS). (A) Swimmer plot centered on SAbR administration starting at the onset of systemic therapy at the time of SAbR. (B) Kaplan-Meier estimate of mPFS and OS. (C) Kaplan-Meier estimate of PFS. Diamonds indicate SAbR-treated lesions with numbers representing treatment sites and colors referring to location; multiple diamonds separated by time represent subsequent courses of SAbR. Black squares indicate the start of new systemic therapy. Asterisks indicate ongoing follow-up. Plus sign (+) indicates patients who died before a new line of systemic therapy. RT refers to SAbR. RT = radiation therapy; SAbR = stereotactic ablative radiation.
The median PFS of the SAbR-aided systemic therapy was 24.4 mo (95% CI: 15.3–42.2; Fig. 2C). A swimmer plot (Fig. 2A and Supplementary Fig. 1) shows the timing of the ongoing systemic therapy, SAbR treatments, and follow-up. The 1-yr PFS (from the start of systemic therapy until switch or death) was 84.7% (95% CI: 59.7–94.8), 2-yr PFS was 50.8% (95% CI: 26.6–70.8).
For the nine patients who switched to a new systemic therapy (three switched to ICIs, four to TKIs, and two to combination therapy), the median PFS on that subsequent therapy (PFS-SST) was 6.7 mo (95% CI: 0.9–20.8; Fig. 3). No local failures at the lesions that received SAbR were observed. Median OS was not reached. The 6-mo OS after initial SAbR was 85.0% (95% CI: 60.4–94.9), and 1-yr OS of 73.7% (95% CI: 47.7–88.2; Fig. 2B).
Fig. 3 –
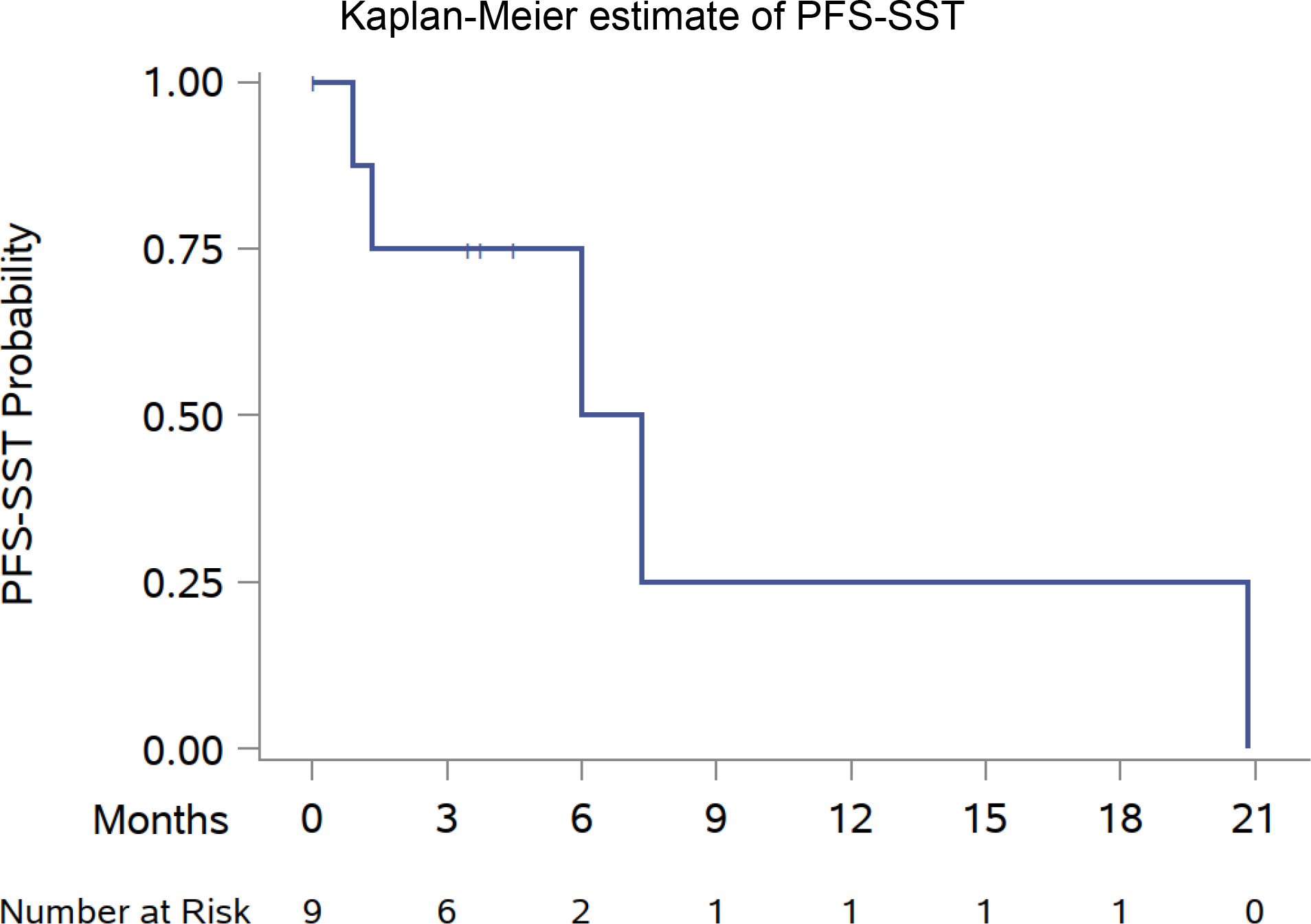
Kaplan-Meier estimate of PFS of subsequent systemic therapy. PFS = progression-free survival; PFS-SST = PFS of subsequent systemic therapy.
Treatment-related toxicities are summarized in Table 2. One patient had grade 3 colitis with small bowel perforation near the radiation field 5 mo after radiation in the setting of concurrent mTOR-I (everolimus) and TKI (lenvatinib) therapy. This patient was treated with SAbR to two abdominal/peritoneal metastases. No grade 4 or 5 treatment-related toxicities were observed.
Table 2 –
Summary of treatment-related toxicities
| AE terminology | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total | |
|---|---|---|---|---|---|---|---|
| 1 | Abdominal pain | 1 | 1 | 2 | |||
| 2 | Alkaline phosphatase increased | 2 | 2 | ||||
| 3 | ALT increased | 1 | 1 | ||||
| 4 | AST increased | 1 | 1 | ||||
| 5 | Epigastric pain | 1 | 1 | ||||
| 6 | Fatigue | 1 | 1 | 2 | |||
| 7 | Lymphocyte count decreased | 1 | 1 | ||||
| 8 | Nausea | 2 | 1 | 3 | |||
| 9 | Small intestinal perforation | 1 | 1 | ||||
| 10 | Vomiting | 1 | 1 | ||||
| Total: grade 1 | 11 | ||||||
| Total: grade 2 | 3 | ||||||
| Total: grade 3 | 1 | ||||||
| Total: grade:4 | 0 | ||||||
| Total: grade:5 | 0 | ||||||
| Sum of grades | 15 |
AE = adverse event; ALT = alanine aminotransferase; AST = aspartate transaminase.
Scores from health-related QOL outcomes (FACT-G, FKSI, and EQ-5D-5L questionnaires) for all patients with available follow-up (n = 16) were indistinguishable 3 mo after SAbR from those baseline. This analysis involved areas such as physical, emotional, social, and functional well-being (Table 3).
Table 3 –
Questionnaire responses at baseline and 3 mo
| Mean baseline ± Std. Dev. (N = 16) | Mean 3 mo ± Std. Dev. (N = 16) | Mean difference (95% CI) |
P value | |
|---|---|---|---|---|
| FACT-G | 82.2 ± 17.8 | 82.8 ± 17.5 | 0.6 (−5.2, 6.3) | 0.84 |
| PWB | 21.0 ± 6.8 | 21.6 ± 6.2 | 0.6 (−1.6, 2.7) | 0.59 |
| SWB | 22.9 ± 4.5 | 23.9 ± 5.3 | 1.1 (−0.5, 2.7) | 0.18 |
| EWB | 18.1 ± 4.0 | 17.8 ± 5.2 | −0.4 (−2.2, 1.5) | 0.67 |
| FWB | 20.1 ± 7.1 | 19.4 ± 7.0 | −0.7 (−3.1, 1.7) | 0.56 |
| FKSI | 44.4 ± 10.6 | 46.2 ± 9.1 | 1.8 (−1.6, 5.1) | 0.28 |
| EQ-5D-5L Health scale | 68.9 ± 22.2 | 73.9 ± 11.9 | 5.0 (−5.9, 15.9) | 0.34 |
EQ-5D-5L = EuroQol Group’s five-level; EWB = emotional well-being; FACT-G = Functional Assessment of Cancer Therapy—General; FKSI = Functional Assessment of Cancer Therapy Kidney Cancer Symptom Index; FWB = functional well-being; PWB = physical well-being; sWb = social well-being.
In exploratory, unspecified, univariate analyses, a statistically significant difference was observed in 6-mo mPFS for patients with five or fewer metastases at the time of SAbR compared with that for patients with six or more metastases (100% vs 50%; hazard ratio 12.10; log-rank p = 0.0037; Supplementary Table 1 and Supplementary Fig. 2).
Seven patients died, with none of the deaths attributed to SAbR. Four patients died prior to changing next line of systemic therapy. Of these four patients, one with an IMDC risk score of zero died because of complications surrounding an intestinal perforation outside of the radiation field while receiving fourth-line TKI therapy. One patient treated with SAbR to two liver lesions, with an IMDC risk score of 1, died on second-line TKI therapy after complications of disease progression including malignant ascites with an associated peritoneal fungal infection. One patient treated with SAbR to bone and spine, with an IMDC risk score of 1, died on second-line ICI + TKI therapy due to respiratory failure associated with disease progression. One patient treated with SAbR to bone and spine, with an IMDC risk score of 1, died on hospice after developing a colonic-cutaneous fistula and associated septic shock while on a third-line TKI. Three patients died of complications of disease progression after meeting the primary endpoint and initiating subsequent lines of therapy. These three patients died at approximately 1, 7, and 8 mo after initiating a subsequent line of systemic therapy.
4. Discussion
Metastatic RCC remains largely incurable. About 85–90% of patients fail to achieve a complete response with most active combinations of ICI and ICI/TKI. Even with these regimens, survival at 5 yr remains poor (≤40%) [2–4,6]. Thus, patients move from one systemic therapy to the next receiving on average three to four lines of therapy [24–26]. Each line of therapy is typically associated with reduced benefits: shorter disease control intervals and potentially cumulative toxicity. Today, little distinction is made about the mode of progression, whether generalized or more limited, and this is reflected in RECIST guidelines. However, different modes of progression likely reflect differential disease responsiveness to therapy and biology. Limited progression may indicate overall responsiveness to therapy, and may be explained by mutation heterogeneity and branched evolution. More indolent disease may also favor oligoprogression. Different modes of progression may be managed optimally with different approaches, and whereas a change of systemic therapy may be in order for patients with overt progression, the introduction of focal therapies for oligoprogression control could be advantageous. This would enable controlling sites of disease that could lead to in situ complications or seed other metastases while allowing the patient to remain on the ongoing systemic therapy, particularly if well tolerated. By extending the duration of the current systemic therapy and altering the course of the disease through elimination of resistant metastasis, this approach could also improve survival outcomes. Further, local therapy seems unlikely to undermine future systemic therapy, and such an approach may extend patient survival.
Multiple retrospective studies have evaluated SAbR for mRCC, but most have focused on oligometastasis and only a few on oligoprogression [11,18,19,27–31]. A retrospective analysis by Santini et al [19] evaluated 55 mRCC patients on first-line systemic therapy and oligoprogression managed with focal approaches including SAbR, and reported mPFS of 14 mo. SAbR was used in approximately 46% of patients and appeared to be effective. Another single-institution retrospective review of 72 patients with mRCC on systemic therapy treated with SAbR to oligoprogressive sites showed similar PFS regardless of systemic therapy [31]. In a multi-institutional study, Meyer et al [27] reported 180 patients with mRCC who had been treated with SAbR; of them, 101 patients were treated for oligoprogressive disease. The median local recurrence-free survival, PFS, time to systemic therapy, and OS were 19.3, 8.6, 10.5, and 23.2 mo, respectively. Our institution’s retrospective review of SAbR for oligoprogression in mRCC showed median mPFS of 9.2 mo [18].
This study met its primary endpoint showing that sequential SAbR to oligometastasis prolonged the duration of ongoing systemic therapy by >6 mo in at least 40% of patients. In fact, SAbR extended the duration of ongoing systemic by >6 mo in 70% of patients, and the median extension was 11.1 mo.
The median duration of SAbR-aided systemic therapy was 24.4 mo, including 11.1 mo after SAbR initiation. While this reflects a select patient population with oligoprogression and is subject to a selection (as well as immortal) bias, it compares favorably with median PFS for commonly used front-line combinations (8.2 mo for ipilimumab/nivolumab [2], 15.1 mo for pembrolizumab/axitinib [3], 13.8 mo for avelumab/axitinib [4], 24.0 mo for lenvatinib/pembrolizumab [5], and 16.6 mo for nivolumab/cabozantinib [6]). Furthermore, 80% of patients in our study were past first-line therapy, where duration of systemic therapy is typically shorter. The median extension of ongoing systemic therapy after SAbR of 11.1 mo compares favorably with the duration of second-line systemic therapy for mRCC patients [21,32]. In patients who went onto a subsequent line of systemic therapy due to progression after SAbR, the PFS-SST was 6.7 mo, which is comparable with PFS for second-line systemic therapy for mRCC [21,32]. These data suggest that the benefit accrued to SAbR may not undermine the benefit from subsequent lines of therapy and may ultimately translate into improvements in survival. This remains to be formally tested. In our study, median OS was not reached, but follow-up remains short.
Patient-reported QOL measures (as ascertained with multiple tools, FACT-G, FKSI, and EQ-5D-5L) at 3 mo after SAbR were undistinguishable from those at baseline. The ability of SAbR to prolong treatment duration without impacting QOL negatively is an important strength of this treatment approach.
SAbR for oligoprogressive mRCC was generally well tolerated, although caution should be exercised, especially when the SAbR sites are close to the hollow visceral organs of the gastrointestinal tract. Toxicity may also be exacerbated by both ICIs and TKIs, which can cause colitis and perforation. In this trial, systemic therapy was optionally held at the discretion of the treating physician for 3 d flanking SAbR treatment, which may make a difference particularly for patients on TKIs given their shorter half-life than ICIs. Although most of the treatment-related toxicities were either grade 1 or grade 2, and no grade 4 or 5 toxicities were observed, one patient experienced a grade 3 small intestine perforation possibly related to treatment. This patient was treated with SAbR to two abdominal/peritoneal metastases with concurrent combination of a TKI and an mTOR inhibitor, and subsequently developed colitis 5 mo after SAbR. The safety of SAbR in conjunction with systemic therapy continues to be evaluated. In our present study, one patient on a TKI and five on an ICI (for a total of six of 20, 30%) received SAbR concurrently without interruptions. SAbR with concurrent ICI/TKI was started with caution due to concerns for potential increased toxicity, but no enhanced toxicity was observed, and more prospective studies are needed [33–35]. Mohamad et al [36] evaluated the safety of concurrent ICI and hypofractionated radiotherapy in 59 patients with mRCC, and concluded that any-grade or grade ≥3 adverse events did not differ significantly from historical rates of ICI therapy alone. In a phase I trial, Tang et al treated 55 patients with ipilimumab and either concurrent or sequential SAbR. They reported 34% grade 3 toxicity comparable with ipilimumab alone. A recent meta-analysis of 13 prospective randomized trials with concurrent TKI and radiation therapy showed increased grade 3+ toxicity [37].
This single-arm phase II trial is limited by a small cohort and relatively short follow-up. Additional limitations include the lack of a control arm. While the study met its primary endpoint, a lack of a control arm makes it difficult to ascertain the overall benefit of SAbR. Specifically, four patients died before switching to next-line systemic therapy, including one where the death was directly attributed to disease progression. Whether an earlier switch of systemic therapy could have postponed the demise is unknown. However, it is important to note that x/4 of patients were on third-line systemic therapy or beyond. The patient population is also highly selected seeking to target those with overall responsive disease and true oligoprogression (as opposed to early evidence of more generalized progression). Accordingly, patients with high-risk disease were excluded.
These results expand upon those from a concurrent phase 2 study of patients with oligoprogressive mRCC similarly treated with SAbR [38]. Both studies sought to identify patients with oligoprogression by ensuring evidence of overall disease responsiveness to ongoing systemic therapy and excluding patients with aggressive disease. Both also leveraged longitudinal SAbR as a therapeutic strategy. The results were also quite similar, showing high local control rates and an extension of ongoing systemic therapy by approximately 1 yr. Some differences, however, are the inclusion of patients with oligoprogression while on ICI in our study, as well as the inclusion of patients with non–clear cell RCC. In addition, this study showed that patient QOL was not undermined by SAbR. Overall, both studies, despite being small, showed that SAbR effectively controlled progressive lesions, enabling meaningful extensions of the ongoing systemic therapy. Together, these studies support the evaluation of SAbR for oligoprogression in a randomized phase 3 trial.
5. Conclusions
This phase II single-arm study highlights the effectiveness of SAbR in extending ongoing systemic therapy for oligoprogressive mRCC without compromising QOL. These data support longitudinal cancer control with SAbR for disease that remains oligometastatic and should be validated further in prospective randomized trials.
Supplementary Material
Acknowledgments:
The authors acknowledge Dr. Damiana Chiavolini for the scientific editing of the manuscript.
Funding/Support and role of the sponsor:
Alana Christie, Kevin Courtney, and James Brugarolas are funded by P50CA196516. Raquibul Hannan is funded by American Cancer Society RSG-16-004-01-CCE. The clinical trial is funded by the Department of Radiation Oncology, UT Southwestern Medical Center.
Footnotes
Financial disclosures: Raquibul Hannan certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Stereotactic ablative radiation (SAbR) extended the duration of ongoing systemic therapy for patients with oligoprogressive metastatic renal cell carcinoma (mRCC) without undermining quality of life. These data support the evaluation of SAbR as the therapeutic modality of choice for oligoprogressive mRCC in a prospective randomized clinical trial.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol 1994;12:206–12. [DOI] [PubMed] [Google Scholar]
- [2].Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- [4].Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289–300. [DOI] [PubMed] [Google Scholar]
- [6].Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cao G, Wu X, Wang Z, et al. What is the optimum systemic treatment for advanced/metastatic renal cell carcinoma of favourable, intermediate and poor risk, respectively? A systematic review and network meta-analysis. BMJ Open 2020;10:e034626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326–32. [DOI] [PubMed] [Google Scholar]
- [9].Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Turajlic S, Xu H, Litchfield K, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 2018;173:581–94 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wersall PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005;77:88–95. [DOI] [PubMed] [Google Scholar]
- [12].Svedman C, Sandstrom P, Pisa P, et al. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006;45:870–5. [DOI] [PubMed] [Google Scholar]
- [13].Zaorsky NG, Lehrer EJ, Kothari G, Louie AV, Siva S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): a meta-analysis of 28 studies. Eur Urol Oncol 2019;2:515–23. [DOI] [PubMed] [Google Scholar]
- [14].Wang CJ, Christie A, Lin MH, et al. Safety and efficacy of stereotactic ablative radiation therapy for renal cell carcinoma extracranial metastases. Int J Radiat Oncol Biol Phys 2017;98:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol 2014;15:e170–7. [DOI] [PubMed] [Google Scholar]
- [16].Dengina N, Mitin T, Gamayunov S, Safina S, Kreinina Y, Tsimafeyeu I. Stereotactic body radiation therapy in combination with systemic therapy for metastatic renal cell carcinoma: a prospective multicentre study. ESMO Open 2019;4:e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Straka C, Kim DW, Timmerman RD, Pedrosa I, Jacobs C, Brugarolas J. Ablation of a site of progression with stereotactic body radiation therapy extends sunitinib treatment from 14 to 22 months. J Clin Oncol 2013;31:e401–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schoenhals J, Mohamad O, Christie A, et al. Stereotactic ablative radiation therapy for oligoprogressive renal cell carcinoma. Adv Radiat Oncol 2021;6:100692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Santini D, Ratta R, Pantano F, et al. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: a multicenter retrospective analysis. Oncotarget 2017;8:100708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824–30. [DOI] [PubMed] [Google Scholar]
- [21].Jain RK, Gandhi S, George S. Second-line systemic therapy in metastatic renal-cell carcinoma: a review. Urol Oncol 2017;35:640–6. [DOI] [PubMed] [Google Scholar]
- [22].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [23].Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. [Google Scholar]
- [24].Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293–300. [DOI] [PubMed] [Google Scholar]
- [25].Chen VJ, Hernandez-Meza G, Agrawal P, et al. Time on therapy for at least three months correlates with overall survival in metastatic renal cell carcinoma. Cancers (Basel) 2019;11:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Logan JE, Rampersaud EN, Sonn GA, et al. Systemic therapy for metastatic renal cell carcinoma: a review and update. Rev Urol 2012;14:65–78. [PMC free article] [PubMed] [Google Scholar]
- [27].Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: a study of the GETUG Group. Eur J Cancer 2018;98:38–47. [DOI] [PubMed] [Google Scholar]
- [28].Franzese C, Franceschini D, Di Brina L, et al. Role of stereotactic body radiation therapy for the management of oligometastatic renal cell carcinoma. J Urol 2019;201:70–5. [DOI] [PubMed] [Google Scholar]
- [29].Stenman M, Sinclair G, Paavola P, Wersall P, Harmenberg U, Lindskog M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005–2014. Radiother Oncol 2018;127:501–6. [DOI] [PubMed] [Google Scholar]
- [30].Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: a systematic review. Acta Oncol 2015;54:148–57. [DOI] [PubMed] [Google Scholar]
- [31].De B, Venkatesan AM, Msaouel P, et al. Definitive radiotherapy for extracranial oligoprogressive metastatic renal cell carcinoma as a strategy to defer systemic therapy escalation. BJU Int. In press. 10.1111/bju.15541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–62. [DOI] [PubMed] [Google Scholar]
- [33].Sha CM, Lehrer EJ, Hwang C, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol 2020;151:141–8. [DOI] [PubMed] [Google Scholar]
- [34].Verma V, Cushman TR, Tang C, Welsh JW. Toxicity of radiation and immunotherapy combinations. Adv Radiat Oncol 2018;3:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meng X, Feng R, Yang L, Xing L, Yu J. The role of radiation oncology in immuno-oncology. Oncologist 2019;24:S42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mohamad O, Diaz de Leon A, Schroeder S, et al. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology 2018;7:e1440168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tchelebi LT, Batchelder E, Wang M, et al. Radiotherapy and receptor tyrosine kinase inhibition for solid cancers (ROCKIT): a meta-analysis of 13 studies. JNCI Cancer Spectr 2021;5:pkab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheung P, Patel S, North SA, et al. Stereotactic radiotherapy for oligoprogression in metastatic renal cell cancer patients receiving tyrosine kinase inhibitor therapy: a phase 2 prospective multicenter study. Eur Urol 2021;80:693–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


