Abstract
Introduction:
Survivors of acute kidney injury (AKI) are at high risk of progression to chronic kidney disease (CKD), for which drugs may be a modifiable risk factor.
Methods:
We conducted a population-based cohort study of Olmsted County, MN residents who developed AKI while hospitalized between 1/1/2006 and 12/31/2014 using Rochester Epidemiology Project data. Adults with a hospitalization complicated by AKI who survived at least 90 days after AKI development were included. Medical records were queried for prescription of potentially nephrotoxic medications over the 3 years after discharge. The primary outcome was de novo or progressive CKD defined by either a new diagnosis code for CKD or ≥30% decline in estimated glomerular filtration rate (eGFR) from baseline. The composite of CKD, AKI readmission, or death was also evaluated.
Results:
Among 2,461 AKI survivors, 2,140 (87%) received a potentially nephrotoxic medication during the three years following discharge. When nephrotoxic medication use was analyzed in a time-dependent fashion, those actively prescribed at least one of these drugs experienced a significantly higher risk of de novo or progressive CKD (HR 1.38: 95% CI 1.24, 1.54). Similarly, active potentially nephrotoxic medication use predicted a greater risk of the composite endpoint of CKD, AKI readmission, or death within 3 years of discharge (HR 1.41: 95% CI 1.28, 1.56).
Discussion/Conclusion:
In this population-based cohort study, AKI survivors actively prescribed one or more potentially nephrotoxic medications were at significantly greater risk for de novo or progressive CKD. An opportunity exists to reassess nephrotoxin appropriateness following an AKI episode to improve patient outcomes.
Keywords: Acute kidney injury, adverse drug event, long-term outcomes, epidemiology
Introduction
Acute kidney injury (AKI) affects up to 40% of hospitalized patients and carries a 3.5-fold increased risk of mortality[1–3]. Beyond short-term consequences of AKI, including volume overload and electrolyte abnormalities, a single episode of AKI independently predicts recurrence of AKI, progression to chronic kidney disease (CKD), dialysis dependence and death[4–7]. Increasing evidence also indicates that AKI heightens the risk of cardiovascular events[8]. Given the myriad of potential AKI sequelae, it is imperative that adequate post-AKI care is provided[9].
Despite the presence of strong evidence supporting the association between AKI and worsened outcomes, little is known about the appropriate short- and long-term management of post-AKI patients[10]. During the course of hospitalization, AKI is often viewed as a transient event with even the most robust of decision support sequences ending abruptly upon resolution[11,12]. With such circumstances, suspension of directed care may allow for additional avoidable kidney insults to occur, as there is a potential lack of perceived risk when kidney function parameters return to baseline. In many cases, AKI resolves before discharge and is no longer considered an active problem requiring additional intervention[13,14]. Yet it is established that even when kidney function markers, such as serum creatinine, return to baseline before discharge, patients still experience a heightened risk for adverse kidney outcomes[15]. In the hospital setting, clinical and laboratory data are often evaluated on a daily basis, but upon discharge, a marked gap occurs in the care continuum for AKI patients who may receive no kidney-specific follow up.
One aspect of AKI follow-up care is nephrotoxin management[16,17]. Nephrotoxic medication use is ubiquitous among patients hospitalized with AKI. Nearly two-thirds of all medications are excreted extensively by the kidneys, and 23% are potentially nephrotoxic[18–20]. The Acute Disease Quality Initiative group recommended that patients with at least stage 1 AKI that persists for more than 7-days should receive comprehensive medication reconciliation and follow-up at discharge, yet available data suggest that medication optimization in the post-AKI period remains an unmet need[10,21]. Therefore, the purpose of this study was to assess the impact of nephrotoxin exposure post-hospitalization for AKI to determine if an opportunity for care optimization exists.
Methods
Study Design and Setting
Using data from the Rochester Epidemiology Project (REP), we performed a population-based cohort study of AKI survivors in Olmsted County, Minnesota, to determine the long-term risk of adverse kidney outcomes after exposure to nephrotoxins. IRB approval was obtained from both Mayo Clinic and Olmsted Medical Center. Included individuals were adult residents of Olmsted County, MN (≥ 18 years) with an episode of AKI during a hospitalization detected by electronic surveillance (see below) between January 1, 2006, and December 31, 2014. Longitudinal follow-up data from the REP was used to comprehensively capture outcomes similar to our previously described approach26.
The REP is a medical record linkage system established in the 1960s that amasses data from all of the main healthcare providers in Olmsted County, allowing for virtually complete ascertainment of medical data for local residents. The REP includes demographic data and comprehensive information about medical diagnoses, hospital admissions, surgical procedures, outpatient follow-up care, and prescription data from all participating institutions regardless of patient characteristics, including insurance status.
Patient Selection
AKI cases were identified and staged with an electronic surveillance tool developed and validated against a manually collected reference standard that uses both serum creatinine and urine output criteria using the Acute Kidney Injury Network criteria[22–24]. Baseline creatinine was calculated as the median of all creatinine values in the 6 months to 7 days before the index admission or if unavailable, estimated from back-calculation using the modification of diet in renal disease (MDRD) formula and an assumed eGFR of 60 mL/min/1.73m2 [25]. We excluded any individual who died in the 90 days after AKI development because the focus of the study was de novo or progressive CKD, which requires sustained evidence of kidney decline benchmarked at 3-months [26]. Patients with persistent dialysis dependence in the 3-month post-discharge interval or those who underwent kidney transplantation before, during, or in the 3-months after AKI admission were excluded. We also excluded patients who declined the use of their medical record for research, lacked any medical follow-up in the 3 years after their hospitalization, or did not remain an Olmsted County resident during the study period[27].
Data Collection and Definitions
The included cohort was followed for three years after discharge to characterize time-dependent use of potentially nephrotoxic medications. Potentially nephrotoxic systemic medications included proton pump inhibitors (PPIs), select antibiotics, antifungals, antivirals, non-steroidal anti-inflammatory drugs (NSAIDs), chemotherapeutic agents and immunosuppressants (Table S1). The medication list was validated and reconciled by study investigators, including pharmacists with expertise in evaluating drug-associated kidney injury, as well as with primary and tertiary references[28,29]. Angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs), were omitted from this medication list due to recent literature which suggests no relationship between use and worse kidney outcomes in the post-AKI period[30–32]. Further, use of ACEI/ARB is confounded by indication as the conditions they are associated with also affect kidney outcomes (i.e., heart failure, hypertension). For similar reasons, diuretics were omitted from this list [33]. Renally-eliminated medications that are not known to be nephrotoxic, were not included in the analyses.
Nephrotoxin use was identified through an electronic data pull from the REP. In cases of discrepancy, such as two prescriptions for the same medication class during a time period, discharge medication lists, prescription records, and clinical notes were manually reviewed to assess use. The total fill period of a prescription was used to calculate days of therapy. In cases where an order was placed to discontinue a prescription medication before completion of the total fill days, the duration was truncated to the medication discontinuation date. Medication use was analyzed in a time-dependent manner. Patients were classified in the “use” group only during the time periods that they were prescribed one or more potentially nephrotoxic mediations. If this period did not last the entirety of the three year follow up, patients were dynamically switched into the “no use” group as medications were stopped and started (Figure 1). These formed the two comparators, ‘use’ and ‘no-use’ of nephrotoxic agents. Patients without any potentially nephrotoxic medication use throughout the duration of follow up were kept in the ‘no use’ group throughout. By using the same patients but allowing their exposure status to change over time, we reduced the risk that differences found among groups were due to factors outside of nephrotoxin use (i.e., disease state or severity).
Figure 1.
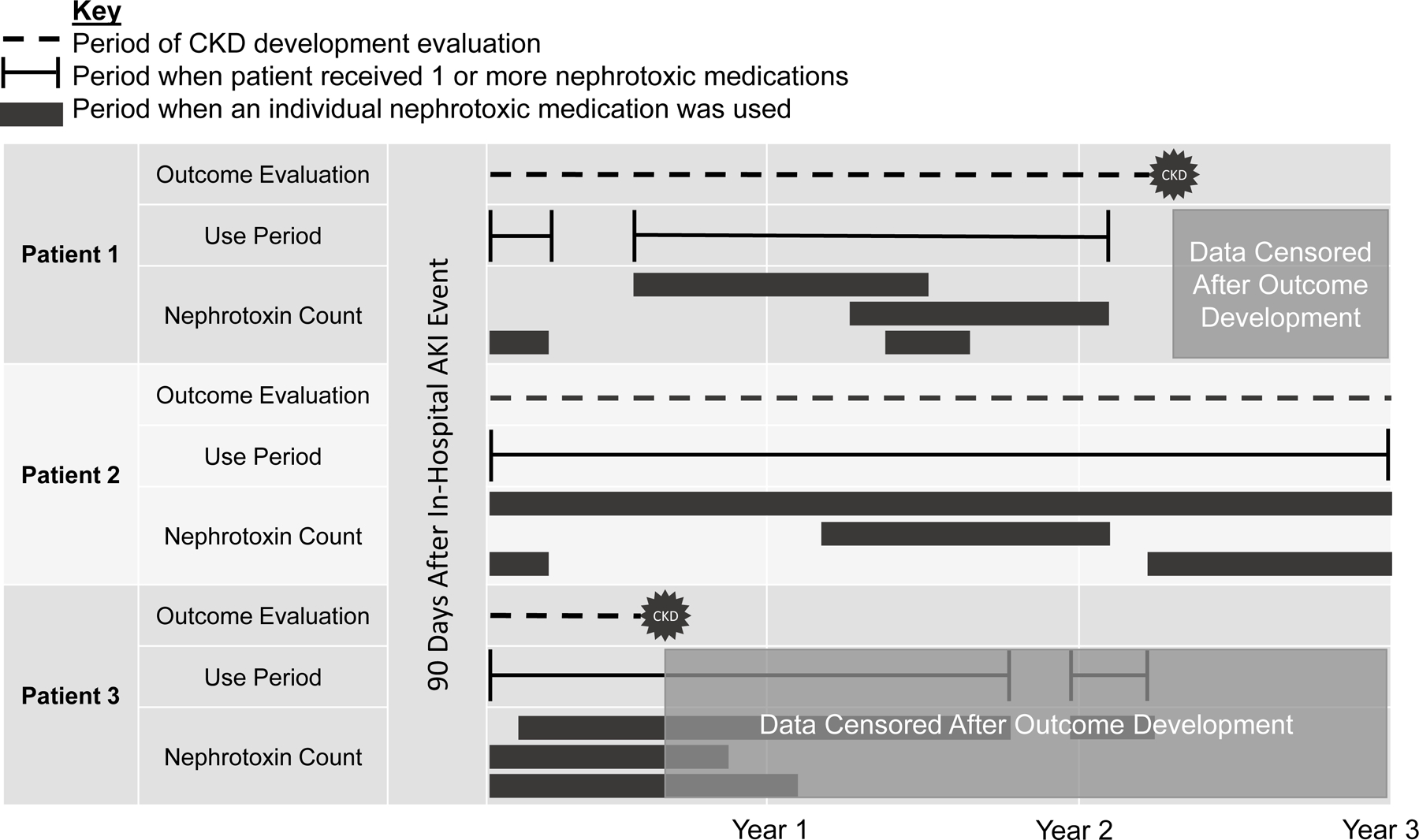
Time-dependent analysis methodology
Patients were followed for 3 years following acute kidney injury (AKI) development. Periods of nephrotoxin use were compared to periods without nephrotoxin use. Example patient scenarios are depicted. Patient 1 was categorized in and out of the use group during the first 2 follow up years, but eventually developed progressive or de novo chronic kidney disease (CKD) during a time of no nephrotoxin use and their CKD event was classified as a CKD event occurring without nephrotoxin use. Patient 2 was prescribed nephrotoxic medications throughout the follow-up period and was therefore categorized in the nephrotoxin use group throughout. Patient 2 did not develop CKD during the duration of the follow up period. Patient 3 received nephrotoxins off and on during the 3-year period, but their data was censored before the end of year 1 because they developed CKD early on during a period of nephrotoxin use. The CKD event for patient 3 was classified as a CKD event occurring with nephrotoxin use, and was further classified as a CKD event that occurred during a period when the patient was prescribed a count of 3 nephrotoxic medications. CKD: de novo or progressive chronic kidney disease; AKI: acute kidney injury
We captured patient demographics at baseline and comorbid conditions (e.g., cardiovascular disease, heart failure, peripheral vascular disease) using ICD 9/10 codes (Table S2). Hospital length of stay, maximum AKI severity (Stage 1, 2 or 3), duration of AKI, urine protein, kidney function based on serum creatinine, estimated glomerular filtration rate (eGFR), rehospitalization, and death were assessed during the study period. We also determined whether a patient developed transient or persistent AKI (< 48 hours or ≥ 48 hours, respectively)[10].
End Points
The primary outcome of de novo or progressive CKD was identified using multiple approaches to account for baseline pre-existing kidney dysfunction and potential detection bias from variable follow-up in the outpatient setting[34]. Given that the diagnostic criteria for CKD requires persistent kidney dysfunction for at least 90 days, we reviewed medical records for the study endpoint from 90 days to 3 years following the AKI event. Individuals that did not survive to 90 days after the AKI event were excluded, as they would not be able to fulfill the criteria for the diagnosis of CKD. An additional motivation for this strategy is dismissal medication lists for individuals with an expected lifespan of <90 days are often designed to focus on comfort and quality of life. Therefore, they may not be reflective of the study aims.
Patients met the endpoint of de novo or progressive CKD on the first date that one or both of the following criteria were true:
First, incident CKD, end-stage renal disease (ESRD), or kidney transplantation was identified using an a priori defined list of diagnosis codes (Table S3). As these diagnosis codes rarely accurately stage the severity of kidney dysfunction, the focus with this approach was to capture incident CKD as a binary event. For this aspect of the analysis, any patient with pre-existing kidney dysfunction based on these codes or eGFR < 60 mL/min/1.73 m2 prior to hospital admission was excluded.
Second, calculated eGFR was used to determine new or worsening kidney dysfunction from baseline. All patients with follow-up creatinine concentrations could be considered in this analysis. Pre-admission eGFR was calculated using the median baseline creatinine in the 6-months to −7 days prior to hospital admission and the MDRD equation. Upon dismissal, serum creatinine values were serially evaluated and eGFR was calculated. Patients with an eGFR < 60 mL/min/1.73m2 were staged based on standardized criteria (Stage 3a: 45–59 mL/min/1.73m2; Stage 3b: 30–44 mL/min/1.73m2; Stage 4: 15–29 mL/min/1.73m2; Stage 5: <15 mL/min/1.73m2). Serum creatinine values were only included if drawn in the outpatient setting, >7 days before or after an inpatient encounter to avoid classifying transient changes in kidney function as CKD. Medical records were reviewed for the 3-year follow-up interval or until the last available clinic visit or serum creatinine concentration in that timeframe, whichever was sooner. New or worsening kidney dysfunction was defined as at least one measurement with a 30% decline in eGFR from baseline; which was necessary even for those with a new estimated glomerular filtration rate (eGFR) <60mL/min/1.73m2. This criterion was established to avoid classification of individuals with very small changes in eGFR as positive for the outcome of interest (i.e. 61 mL/min/1.73m2 to 59 mL/min/1.73m2).
Data analysis
Univariable Cox proportional hazards regression models were fit to evaluate the time dependent incidence of de novo or progressive CKD during the three years of follow-up. A composite model was also developed to assess the incidence of de novo or progressive CKD, readmission with AKI, or death. Multivariable models were fit to evaluate the association of nephrotoxin use with long-term kidney outcomes after adjusting for baseline risk.[8,15,35,36]. Included risk factors were nephrotoxin use as a time-dependent variable, age, sex, body mass index, baseline eGFR, history of cardiovascular disease, heart failure, or peripheral vascular disease, maximum AKI stage, presence of persistent AKI, and change in eGFR during index hospitalization.
Results
Patient characteristics
Of the 4,551 patients screened for eligibility, 2,461 were included (Figure 2). The primary reasons for exclusion were death within 90 days of AKI development (n = 1,184) or a lack of Olmsted County, MN residence for the duration of the study period (n = 815). All patients included had clinical follow up at some point during the 3-year follow up period. Over the course of this follow up, 2,140 (87%) patients were prescribed at least one potentially nephrotoxic medication.
Figure 2.
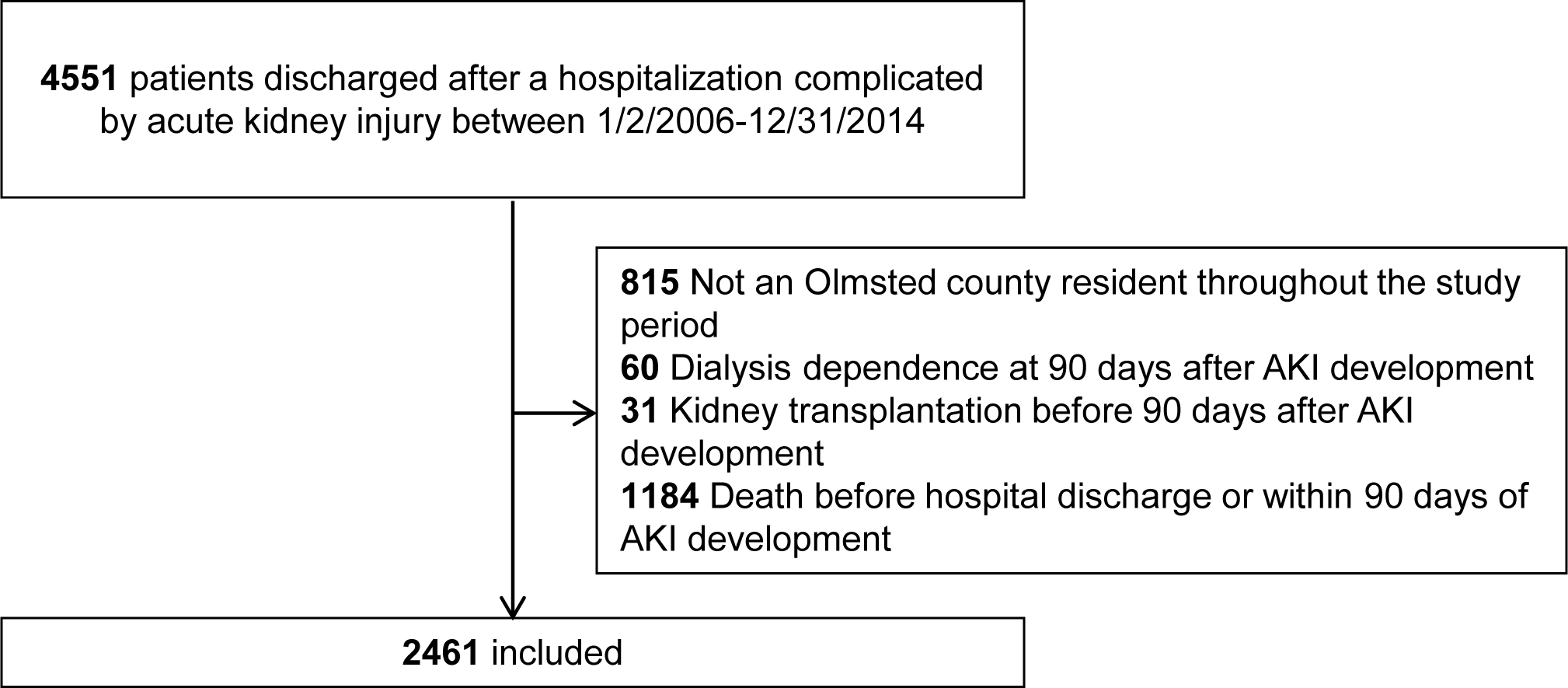
Study design and patient inclusion
Baseline SCr and eGFR at the time of the index hospital admission were 1.0 (IQR 0.9, 1.3) mg/dL and 59 (IQR 53, 74) mL/min/1.73 m2, respectively. Patients had a median of 1 (0, 2) outpatient SCr values in the 6 months to −7 days from admission (Table S4). Cardiovascular disease, congestive heart failure, and peripheral vascular disease were present in 61%, 47%, and 57% of patients, respectively (Table 1). During the index hospitalization, maximum AKI severity was most often stage 1 (77%) and the median duration was approximately 1 day (IQR 0.5, 5.5). Discharge SCr values increased by approximately 0.4 mg/dL from admission SCr values, and the corresponding mean eGFR fell by approximately 10 mL/min/1.73 m2 by discharge.
Table 1.
Patient demographics and characteristics
| Included patientsa (n = 2,461) | |
|---|---|
|
| |
| Patient information | |
| Age, y | 70 ± 17 |
| Male Sex | 1215 (49) |
| White race | 2242 (91) |
| BMI, kg/m2 | 29 ± 9 |
| Kidney function | |
| Baseline SCr, mg/dL | 1.0 (0.9, 1.3) |
| Baseline eGFR, mL/min/1.73 m2 | |
| eGFR category at baseline | 59 (53, 74) |
| G1 (≥ 90 mL/min/1.73 m2) | 316 (13) |
| G2 (60—89 mL/min/1.73 m2) | 804 (33) |
| G3A (45—59 mL/min/1.73 m2) | 951 (39) |
| G3B (30—44 mL/min/1.73 m2) | 236 (10) |
| G4 (15—29 mL/min/1.73 m2) | 145 (6) |
| G5 (< 15 mL/min/1.73 m2) | 9 (0.4) |
| Comorbid conditions | |
| Cardiovascular disease | 1496 (61) |
| Heart failure | 1153 (47) |
| Peripheral vascular disease | 1393 (57) |
| Diabetes mellitus | 848 (34) |
| Hypertension | 1794 (73) |
| Pulmonary hypertension | 52 (2) |
| Cirrhosis | 73 (3) |
| Index hospitalization details | |
| ICU during hospitalization | 855 (35) |
| Maximum AKI Severity | |
| Stage 1 | 1891 (77) |
| Stage 2 | 363 (15) |
| Stage 3 | 207 (8.4) |
| Time to AKI recovery, daysb | 1.0 (0.5, 5.5) |
| Number with persistent AKIc | 1040 (42) |
| Number with AKI ≥ 7 days | 704 (29) |
| Discharge urine protein, mg/dL | 24 (10, 55) |
| Discharge SCr, mg/dLd | 1.3 (1.0, 1.7) |
| Discharge eGFR, mL/min/1.73 m2 | 49 (35, 68) |
| CKD risk prediction score at DCe | 11.9 ± 5.0 |
| Hospital LOS, days | 6.4 ± 8.0 |
| Nephrotoxin details | |
| Nephrotoxin count at DC | 1.5 ± 1.2 |
| 3 year nephrotoxin count | 3.1 ± 2.0 |
| No nephrotoxin use during follow-up | 321 (13) |
| Number of patients prescribed nephrotoxins by class f | |
| Proton pump inhibitors | 1,929 (78) |
| Antibiotics | 1,160 (47) |
| NSAIDs | 600 (24) |
| Antivirals | 234 (10) |
| Chemotherapy | 106 (4) |
| Immunosuppressants | 45 (2) |
| Number of patients prescribed other relevant medications by class g | |
| ACEI/ARB | 1620 (66) |
| Diuretics | 1066 (43) |
DC: discharge; LOS: length of stay; ICU: intensive care unit; BMI: body mass index; eGFR: estimated glomerular filtration rate; SCr: serum creatinine; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; NSAID: nonsteroidal anti-inflammatory drug; AKI: acute kidney injury; SCr: CKD: chronic kidney disease
Reported as number (percent) for categorical variables and mean ± standard deviation or median (interquartile range) for continuous variables
Capped at 90 days, defined as creatinine returning to <1.5 × baseline[10]
Defined as AKI persisting for greater than 48 hours
Measured during the index hospitalization using the value closest to the discharge date.
This score assigns points for increasing risk related to age, sex, baseline serum creatinine, albuminuria, acute kidney injury stage and discharge serum creatinine. Scores range from 0 to 28 with higher scores indicating increased risk[37].
The number of patients that received a nephrotoxin in a therapeutic class at any point during the 3 year follow-up period
The number of patients that received a medication in a relevant therapeutic class at any point during the 3 year follow-up period. These medications were not considered nephrotoxic.
Among the 2,140 patients prescribed potentially nephrotoxic medications, PPIs, antibiotics and NSAIDs were the most commonly prescribed classes of medications (Table 1; Table S5). 24% of all patients (n = 600) had an NSAID on their prescribed medication list at some point over the three year follow up period.
Endpoints
For the primary endpoint, periods when patients were prescribed potentially nephrotoxic medications were associated with a significantly higher risk of de novo or progressive CKD during 3 years of follow-up (HR 1.38: 95% CI 1.24–1.54, p <0.001; Table 2). This association was preserved in analyses adjusted for baseline CKD risk (Adjusted HR 1.39: 95% CI 1.24–1.56, p <0.001; Table 3).
Table 2.
Association between nephrotoxin use and the development of study endpoints
| De novo or progressive CKD by either diagnosis code or eGFR decline | Composite of de novo or progressive CKD, readmission with AKI or death | |||
|---|---|---|---|---|
| Time Dependent covariates | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value |
|
| ||||
| Nephrotoxin (Y vs N) | 1.38 (1.24–1.54) | <0.001 | 1.41 (1.28–1.56) | <0.001 |
| Nephrotoxin count (continuous)a | 1.23 (1.16–1.31) | <0.001 | 1.25 (1.18–1.32) | <0.001 |
| Nephrotoxin count (categorical) | ||||
| 0 | Reference | Reference | ||
| 1 | 1.34 (1.18–1.51) | <0.001 | 1.39 (1.24–1.55) | <0.001 |
| 2 | 1.63 (1.35–1.96) | <0.001 | 1.59 (1.34–1.88) | <0.001 |
| 3 | 1.60 (1.11–2.33) | 0.013 | 1.84 (1.35–2.52) | 0.001 |
| 4+ | 2.11 (1.25–3.59) | 0.006 | 2.13 (1.32–3.45) | 0.002 |
CKD: chronic kidney disease, eGFR: estimated glomerular filtration rate, AKI: acute kidney injury
Per 1 nephrotoxin prescribed
Table 3:
Risk for outcome development. In all analyses, prescription of potentially nephrotoxic medications was associated with an increased incidence of new or worsening kidney dysfunction.
| Analysis Group | Sample | CKDa | Composite of CKD, Readmit with AKI or Death |
|---|---|---|---|
| All patients 3 year follow up (n=2461) | Hazard Ratiob | 1.38 (1.24–1.54) |
1.41 (1.28–1.56) |
| P Value | <0.001 |
<0.001 |
|
| Adjusted Hazard Ratioc | 1.39 (1.24–1.56) |
1.44 (1.30–1.59) |
|
| P Value | <0.001 | <0.001 | |
|
| |||
| All patients 1 year follow up (n=2461) | Hazard Ratioc | 1.36 (1.17–1.58) |
1.44 (1.26–1.63) |
| P Value | <0.001 | <0.001 | |
|
| |||
| Analysis of only patients with measured baseline eGFR (n=1599) | Hazard Ratioc | 1.35 (1.17–1.55) |
1.40 (1.23–1.58) |
| P Value | <0.001 | <0.001 | |
|
| |||
| Analysis of only patients with heart failure (n=1153) | Hazard Ratioc | 1.32 (1.14–1.54) |
1.37 (1.19–1.57) |
| P Value | <0.001 | <0.001 | |
|
| |||
| Analysis of only patients without heart failure (n=1308) | Hazard Ratioc | 1.48 (1.25–1.76) |
1.53 (1.31–1.78) |
| P Value | <0.001 | <0.001 | |
|
| |||
| Analysis of only patients with AKI recovery by discharged (n=1736) | Hazard Ratioc | 1.24 (1.08–1.43) |
1.32 (1.17–1.49) |
| P Value | <0.001 | <0.001 | |
|
| |||
| Analysis of only patients with baseline eGFR < 60 (n=1341) | Hazard Ratioc | 1.41 (1.21–1.65) |
1.47 (1.29–1.68) |
| P Value | <0.001 | <0.001 | |
CKD: chronic kidney disease; Dx: diagnosis; eGFR: estimated glomerular filtration rate; AKI: acute kidney injury; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; NTXN: potentially nephrotoxic medication
Outcome defined as de novo or progressive CKD.
Reported as hazard ratio (95% confidence interval)
Adjusted for gender, age, hospital length of stay. ICU during hospitalization, BMI, baseline eGFR, cardiovascular disease, CHF (in models not separating heart failure patients), PVD, maximum AKI severity, persistent AKI, and change in eGFR during hospitalization.
Recovery from AKI was defined as serum creatinine < 1.5 times baseline[10]
In multivariable analyses, we observed similar findings that indicated a risk with use of potential nephrotoxins (Table S6). When we used only the diagnosis code aspect of the CKD definition, we saw a significant increase in the incidence of CKD during periods of nephrotoxin exposure compared to periods of no use (unadjusted HR 1.50: 95% CI 1.17–1.91, p <0.001). When we used only the eGFR aspect of the definition (at minimum, a 30% decline in eGFR from baseline) there was a significantly higher risk of de novo or progressive CKD during potentially nephrotoxic medication use (unadjusted HR 1.35: 95% CI 1.20–1.51). 18 patients (0.7%) were excluded from this analysis due to absent SCr measurement in the 90 day to 3 year follow up period. Seven of these excluded individuals were prescribed a potentially nephrotoxic medication at some point during follow-up. Active use of potentially nephrotoxic medications also increased the risk of the composite secondary endpoint of de novo or progressive CKD, AKI readmission, or death during the 3- year follow up (HR 1.41; 95% CI 1.28–1.56; Table 2). This outcome was primarily driven by CKD development (n= 1,223, 73.9%) followed by death (n=360, 21.8%), AKI readmission (n=60, 3.6%) and combined CKD and AKI readmission on the same day (n=11, 0.7%).
Sensitivity and subgroup analyses
Several a priori specified sensitivity analyses and sub-group evaluations were performed to evaluate the robustness of the study findings (Table 3). Findings were general consistent including with truncated follow-up at 1-year, in the subgroup with renal recovery by discharge, in patients with and without heart failure, and in patients with a baseline eGFR < 60 mL/min/1.73 m2. The relative contribution to CKD development of each queried medication class was similar when ranked by c-statistic (Table S7).
Discussion
In AKI survivors, nephrotoxic medications are one of the few modifiable risk factors that may impact the development or progression of CKD. In this large population-based cohort study, we observed that nephrotoxic medication use following a hospitalization complicated by AKI increases the risk for de novo or progressive CKD in the three years after discharge. Increasing nephrotoxin burden[37] was associated with a stronger risk for long-term kidney dysfunction.
The implications of our findings are significant. Nearly two-thirds of medications are eliminated extensively by the kidney, and 20–40% are directly or indirectly nephrotoxic [18–20]. Our study has confirmed that AKI survivors are frequently prescribed potentially nephrotoxic medications at hospital discharge, and those medications are a modifiable risk factor for progression to CKD. We observed a step-wise increase in risk of de novo or progressive CKD during time periods when patients were prescribed increasing counts of nephrotoxic medications. This association remained significant even in patients that recovered from their AKI episode prior to discharge. These data demonstrate that a clear opportunity exists for clinician-guided nephrotoxin stewardship programs both at and after hospital discharge in AKI survivors. Our results could be used to support de-prescription of potentially nephrotoxic medications that are either unnecessary or can be interchanged for therapeutically similar alternative medications that carry less risk. Omeprazole and pantoprazole, PPIs that may be able to be exchanged for other acid-suppressive therapies during the at-risk period (e.g., histamine 2-receptor antagonists), were among the most commonly used potentially nephrotoxic medications in this cohort. In patients where nephrotoxic medications must be utilized for compelling indications such as transplantation or cancer care, stewardship initiatives could focus on an evaluation of the cumulative nephrotoxin burden and use of alternative non-nephrotoxic treatments for pain, heartburn, and some infections [37]. Further investigation is critical to test real-world feasibility and impact of nephrotoxin stewardship interventions.
In addition to nephrotoxin stewardship interventions, an opportunity exists to support patient education initiatives that explain the role that nephrotoxic medications can have on long-term kidney outcomes. Unfortunately, most adults are unaware of the function of their kidneys and their role in processing medications. In a large survey of community-dwelling adults, only 12% of individuals were aware that the kidneys processed medications[38]. As a consequence, patients may inadvertently take over the counter, potentially nephrotoxic medications after an episode of AKI because they are not aware of the risk. In the Southern Community Cohort Study, NSAID use was common among AKI survivors with as many as 19% of patients regularly using prescription or non-prescription NSAIDs[39]. These findings suggest that in addition to de-prescribing potentially nephrotoxic medications at discharge, educational programs are also imperative to prevent additional renal insults from over the counter medications such as NSAIDs[40]. We found that 24% of included individuals had prescriptions for NSAIDs during the follow-up period, likely only a subset of total use given the non-prescription status of such drugs like ibuprofen and naproxen sodium.
In our heart failure sub-analysis, we found a consistent risk of CKD development with nephrotoxin exposure. Previous studies have demonstrated that patients with heart failure are at greater risk for adverse kidney outcomes including repeat AKI episodes and CKD development[33,41]. Our study suggests that despite the overall increased risk of CKD development, nephrotoxins remain a potentially modifiable factor for CKD development in this subset of patients. Importantly, ACEI/ARBs and diuretics were not considered nephrotoxic in this study, a pertinent difference from previous research[42].
The current study is not without limitations. Due to the observational design, there is a potential that residual confounding from unmeasured or unknown factors that influence long-term kidney function could have played a role in the findings. To the extent possible this risk was minimized. We conducted multivariable analyses including numerous known risk factors for long-term kidney dysfunction in AKI survivors and the results were consistent. Several additional analyses were performed to evaluate the findings in different subgroups of AKI survivors (e.g., those with renal recovery by discharge, those with heart failure, those with baseline CKD). As previously acknowledged, some nephrotoxic medications may be unavoidable. Further investigation within this subset of patients is necessary to determine the real-world opportunity for modification of these therapies and the relative impact of disease processes on renal outcomes. We did observe that cumulative nephrotoxin burden was an important risk factor. In these cases where select nephrotoxic drugs are mandatory, the cumulative burden should be evaluated for other potential modifiable agents. It must also be acknowledged that nephrotoxins exhibit unique patterns and onset of injury which may be obscured with our aggregated approach[43]. For example, NSAIDs have multiple mechanisms by which they can contribute to nephrotoxicity. One such presentation is acute (< 7-days) due to altered intraglomerular hemodynamics. In contrast PPIs may present with acute interstitial nephritis which may occur over several months. Duration of exposure as it relates to the potential mechanism of injury was not included in this analysis. Given the available outpatient data in this historical study, we are also unable to ascertain whether patients actually utilized the medications as prescribed, or account for non-prescription medication use, herbals, and supplements.
To minimize the impact of this limitation, prescription data were reconciled from multiple sources to approximate true medication administration as closely as possible. Finally, although all of the patients included in our study had some form of medical follow up in the three years after their AKI event, not all patients had creatinine values obtained in the follow up period, potentially leading to underestimation of the true incidence of CKD in this cohort.
In conclusion, data from this cohort of patients discharged after a hospitalization complicated by AKI suggests that prescription of potentially nephrotoxic medications at or after dismissal increases the risk for new or worsening CKD, suggesting that an opportunity for care optimization may exist. Further research is needed to fully elucidate the risk associated with individual medication classes and the extent to which an intervention to adjust medication regimens would improve outcomes.
Supplementary Material
Conflict of Interest and Funding:
This project was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI143882 (PI; EFB), the American Society of Health System Pharmacy Resident Research Grant (PI; DJS), and the Agency for Healthcare Research and Quality HS028060–01 (PI; EFB). Additional funding was provided by the Mayo Clinic Department of Pharmacy. The funding sources had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the American Society of Health System Pharmacy. The authors have no other conflicts of interest to disclose.
Footnotes
Statement of Ethics: This study protocol was reviewed and approved by Institutional Review Boards from both Mayo Clinic (#18–007889) and Olmsted Medical Center (#032-OMC-18). All included individuals consented to the use of their medical records for research.
Consent to Participate: In accordance with IRB recommendations, informed consent was waived due to the retrospective nature of this study.
Data Availability:
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available from the corresponding author EFB upon reasonable request.
References
- 1.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–7. [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The Prognostic Importance of a Small Acute Decrement in Kidney Function in Hospitalized Patients: A Systematic Review and Meta-Analysis. Am J Kidney Dis. 2007;50(5):712–20. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2016;67(5):742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60(3):402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, et al. Predictors of Recurrent AKI. J Am Soc Nephrol. 2016. Apr;27(4):1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg R, Dennen P. Long-Term Outcomes of Acute Kidney Injury. Adv Chronic Kidney Dis. 2008;15(3):297–307. [DOI] [PubMed] [Google Scholar]
- 8.Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28(1):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashani K, Rosner MH, Haase M, Lewington AJP, O’Donoghue DJ, Wilson FP, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14(6). DOI: 10.2215/CJN.01250119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–57. [DOI] [PubMed] [Google Scholar]
- 11.Kolhe N V, Reilly T, Leung J, Fluck RJ, Swinscoe KE, Selby NM, et al. A simple care bundle for use in acute kidney injury: A propensity score-matched cohort study. Nephrol Dial Transplant. 2016;31(11):1846–54. [DOI] [PubMed] [Google Scholar]
- 12.Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA. Clinical Decision Support for In-Hospital AKI. J Am Soc Nephrol. 2018;29(2):654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: A cross-sectional study. BMC Health Serv Res. 2016;16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellum JA, Sileanu FE, Bihorac A, Hoste EAJ, Chawla LS. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6). DOI: 10.1164/rccm.201604-0799OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver SA, Adhikari NK, Bell CM, Chan CT, Harel Z, Kitchlu A, et al. Nephrologist Follow-Up versus Usual Care after an Acute Kidney Injury Hospitalization (FUSION): A Randomized Controlled Trial. Clin J Am Soc Nephrol. 2021. Jul;16(7):1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh G, Hu Y, Jacobs S, Brown J, George J, Bermudez M, et al. Post-Discharge Mortality and Rehospitalization among Participants in a Comprehensive Acute Kidney Injury Rehabilitation Program. Kidney360. 2021. Sep;2(9):1424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elinder CG, Bárány P, Heimbürger O. The use of estimated glomerular filtration rate for dose adjustment of medications in the elderly. Drugs and Aging. 2014;31(7):493–9. [DOI] [PubMed] [Google Scholar]
- 19.Seyffart G Seyffart’s Directory of Drug Dosage in Kidney Disease. 1st ed. Oberhaching, Germany: Dustri-Verlag Dr. Karl Feistle; 2011. [Google Scholar]
- 20.Taber SS, Mueller BA. Drug-associated renal dysfunction. Crit Care Clin. 2006. Apr;22(2):357–74, viii. [DOI] [PubMed] [Google Scholar]
- 21.Ostermann M, Chawla LS, Forni LG, Kane-Gill SL, Kellum JA, Koyner J, et al. Drug management in acute kidney disease - Report of the ADQI XVI meeting. Br J Clin Pharmacol. 2017;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A, Vairavan S, Akhoundi A, Wilson G, Chiofolo C, Chbat N, et al. Development and validation of electronic surveillance tool for acute kidney injury: A retrospective analysis. J Crit Care. 2015;30(5):988–93. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV., Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashani K, Shao M, Li G, Williams AW, Rule AD, Kremers WK, et al. No increase in the incidence of acute kidney injury in a population-based annual temporal trends epidemiology study. Kidney Int. 2017;92(3):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med. 1999. Mar;130(6):461. [DOI] [PubMed] [Google Scholar]
- 26.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013. Jan;3(1):1–150. [DOI] [PubMed] [Google Scholar]
- 27.Melton LJ. The threat to medical-records research. N Engl J Med. 1997. Nov;337(20):1466–70. [DOI] [PubMed] [Google Scholar]
- 28.Perazella MA. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;7(10):1713–21. [DOI] [PubMed] [Google Scholar]
- 29.Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayat E, Hollinger A, Cariou A, Deye N, Vieillard-Baron A, Jaber S, et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018;44(5). DOI: 10.1007/s00134-018-5160-6 [DOI] [PubMed] [Google Scholar]
- 31.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with Outcomes after Acute Kidney Injury. JAMA Intern Med. 2018;178(12). DOI: 10.1001/jamainternmed.2018.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CY, Liu KD, Yang J, Glidden DV., Tan TC, Pravoverov L, et al. Renin-angiotensin system blockade after acute kidney injury (AKI) and risk of recurrent AKI. Clin J Am Soc Nephrol. 2020;15(1). DOI: 10.2215/CJN.05800519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(6):1304–17. [DOI] [PubMed] [Google Scholar]
- 34.Rule AD, Bergstralh EJ, Melton LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009. Apr;4(4):804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu KD, Yang J, Tan TC, Glidden DV, Zheng S, Pravoverov L, et al. Risk Factors for Recurrent Acute Kidney Injury in a Large Population-Based Cohort. Am J Kidney Dis. 2013;(Fig 1). DOI: 10.1053/j.ajkd.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James MT, Pannu N, Hemmelgarn BR, Austin PC, Tan Z, McArthur E, et al. Derivation and External Validation of Prediction Models for Advanced Chronic Kidney Disease Following Acute Kidney Injury. JAMA. 2017;318(18):1787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane-Gill SL. Nephrotoxin Stewardship. Crit Care Clin. 2021;37(2). DOI: 10.1016/j.ccc.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 38.Slevin J, Programme TK, Officer D, Taylor A, Consultant C, Programme TK. Understanding what the public know about their kidneys and what they do. 2015;(July 2014).
- 39.Lipworth L, Abdel-Kader K, Morse J, Stewart TG, Kabagambe EK, Parr SK, et al. High prevalence of non-steroidal anti-inflammatory drug use among acute kidney injury survivors in the southern community cohort study. BMC Nephrol. 2016;17(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, et al. Chronic Kidney Disease Associated Mortality in Diastolic Versus Systolic Heart Failure: A Propensity Matched Study. Am J Cardiol. 2009;99(3):393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goswami E, Ogden RK, Bennett WE, Goldstein SL, Hackbarth R, Somers MJG, et al. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Heal Pharm. 2019. Oct;76(22):1869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, et al. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88(2):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available from the corresponding author EFB upon reasonable request.


