Abstract
Susceptibility to stress has long been considered important for the development of substance use disorders. Nonetheless, behavioral and physiological responses to stress are highly variable, making it difficult to identify the individuals who are most likely to abuse drugs. In the present study, we employed a comprehensive battery of tests for negative valence behaviors and nociception to identify individuals predisposed to opioid seeking following oral opioid self-administration. Furthermore, we examined how this profile was affected by a history of stress. We observed that mice receiving foot shock stress failed to exhibit a preference for sucrose, showed increased immobility in the forced swim task, and exhibited mechanical hypersensitivity when compared to controls. When considering these behaviors in light of future fentanyl-seeking responses, we observed that heightened mechanical sensitivity corresponded to higher opioid preference in mice with a history of stress, but not controls. Moreover, we were surprised to discover that paradoxically high sucrose preferences predicted fentanyl preference in shock mice, while signs of anhedonia predicted fentanyl preference in controls. Taken together, these results indicate that stress can act as a physiological modulator, shifting profiles of opioid abuse susceptibility depending on an individual’s history.
Keywords: stress, substance use disorder, anxiety, depression, pain
Stress can lead to several maladaptive responses including anxiety, depression, and substance use disorders (Koob, 2008; Krishnan & Nestler, 2008). Moreover, these disorders often co-occur, with high rates of comorbidity between depression or anxiety and substance use disorders (Feingold et al., 2018; Han et al., 2017). Unfortunately, less than half of the individuals experiencing both disorders receive any kind of mental health care, with only 9% seeking the appropriate treatment for both disorders (Han et al., 2017). In general, it is thought that this comorbidity involves the effects of stress on brain reward circuitry. For example, stress is known to impair positive valence behaviors, such as reward-seeking (Dieterich et al., 2019), much of which has been linked to key limbic system structures such as the amygdala (Dieterich et al., 2021) and nucleus accumbens (Bruchas et al., 2010).
Stress causes the release of corticotrophin releasing factor (CRF) in the brain, and a growing body of research has shown that this comes to affect brain reward circuits through interactions of CRF with the endogenous opioid system. Over time, these interactions are thought to produce maladaptive plasticity (Kreek & Koob, 1998). In mice, it has been shown that the aversive effects following inescapable footshock occur due to the activation of kappa opioid receptors and can be blocked by kappa antagonists or by knocking out the endogenous kappa ligand, dynorphin (Land et al., 2008). Moreover, immobility in the forced swim task, thought to model the clinical features of despondency or despair in depression, can be reduced by dynorphin knockouts or kappa antagonism (McLaughlin et al., 2003).
In parallel, it has also been shown that stress is an important indicator for vulnerability to drug seeking and the development of opioid addiction (Sofuoglu et al., 2014; Stafford et al., 2019). Stress is thought to modulate monoaminergic activity and drive negative affect through the kappa opioid receptor, leading to changes in drug reward and drug reinstatement behavior (Al-Hasani & Bruchas, 2011). For example, stress has been shown to increase oral fentanyl intake (Shaham et al., 1992; Shaham et al., 1993) and has also been shown to increase the efficacy of heroin reward in a progressive ratio task (Shaham & Stewart, 1994). Additionally, stress is a well-known cause of drug reinstatement (Shaham et al., 1996) and repeated drug use may even act as a source of stress, as opioid withdrawal has been shown to activate the HPA axis (Li et al., 2008).
Taken together, the findings above suggest an intimate interaction between stress and the endogenous opioid system. Nonetheless, viable predictors for opioid abuse susceptibility remain elusive (Swain et al., 2021). This problem may be due, at least in part, to individual differences in the presentation of disease states (Nestler, 2015), some aspects of which are intimately linked to an individual’s history. The goal of the present manuscript was therefore to generate a comprehensive behavioral assessment of mice with and without a history of stress. This assessment was explicitly designed to include tasks that can span the many roles of the endogenous opioid system, including emotionality (Zubieta et al., 2003), pain (Emery & Akil, 2020), and consummatory behaviors (Colantuoni et al., 2002). We have grouped the behaviors based on if they primarily measure negative valence or pain. Our negative valence behavior tests include the elevated plus maze, open field test, sucrose preference test, and forced swim test. The elevated plus-maze and open field test were used as measures of behavioral avoidance, while the sucrose preference test and forced swim were used to measure anhedonia and behavioral despair, respectively. Historically, avoidance behaviors towards the center of the open field and the open arms of the elevated plus-maze have been susceptible to the effects of anxiolytics, especially when examining naïve mice, suggesting that they represent a defensive, avoidant, or anxiety-like phenotype (Carobrez & Bertoglio, 2005; Simon et al., 1994). A failure to demonstrate a preference for a 1% sucrose solution over water is often caused by repeated stress (Pothion et al., 2004) and is a model of anhedonia, or inability to sense pleasure, which is a key clinical feature of depression. On the other hand, the time spent immobile in the forced swim task is used to represent amotivation or behavioral despair, another clinical feature of depression. Both tasks are responsive to the effects of common antidepressants used as treatment in humans (Liu et al., 2018; Porsolt, Bertin, & Jalfre, 1977; Porsolt, Le Pichon, & Jalfre, 1977; Willner et al., 1987). Pain testing consisted of mechanical von Frey stimulation, heat stimulation via the Hargreaves, and cold allodynia. These three paradigms test differing types of nociceptive stimuli with modalities that are anatomically distinct, and which can be activated independently of each other (Basbaum et al., 2009). These assays were then used as a method for predicting future opioid preference susceptibility following oral fentanyl self-administration.
Method
All Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23) and were approved by the Institutional Animal Care and Use Committee, Rutgers University.
Subjects
Adult C57BL/6 male and female mice (Jackson Labs) were used for behavioral testing starting at around 3–4 months of age. All mice were kept in a vivarium with a 12 hr:12 hr light:dark cycle with dawn at 7 AM. Mice were group housed and had ad libitum access to food and water throughout testing, unless otherwise noted. Experimental mice were randomly assigned to either shock (n = 15) or control (n = 14) groups, with all mice in a cage exposed to the same experimental condition.
Overview of Behavioral Testing
All mice were tested on a series of behaviors as described below. These behaviors included an initial stress protocol, followed by the examination of negative valence behaviors in the elevated plus maze, open field, sucrose preference test, and forced swim task (Fig. 1A), followed by tests of pain sensitivity in the von Frey, Hargreaves, and cold allodynia test (Fig. 2A). A subset of mice was then randomly selected to undergo fentanyl self-administration (Fig. 3A). The order of behavioral procedures was fixed for all mice. The fixed order was designed to prevent tasks that might have an inherent stress component (e.g., the Hargreaves test) from influencing more sensitive tasks such as the elevated plus maze. No more than one behavioral task was run per day.
Figure 1.
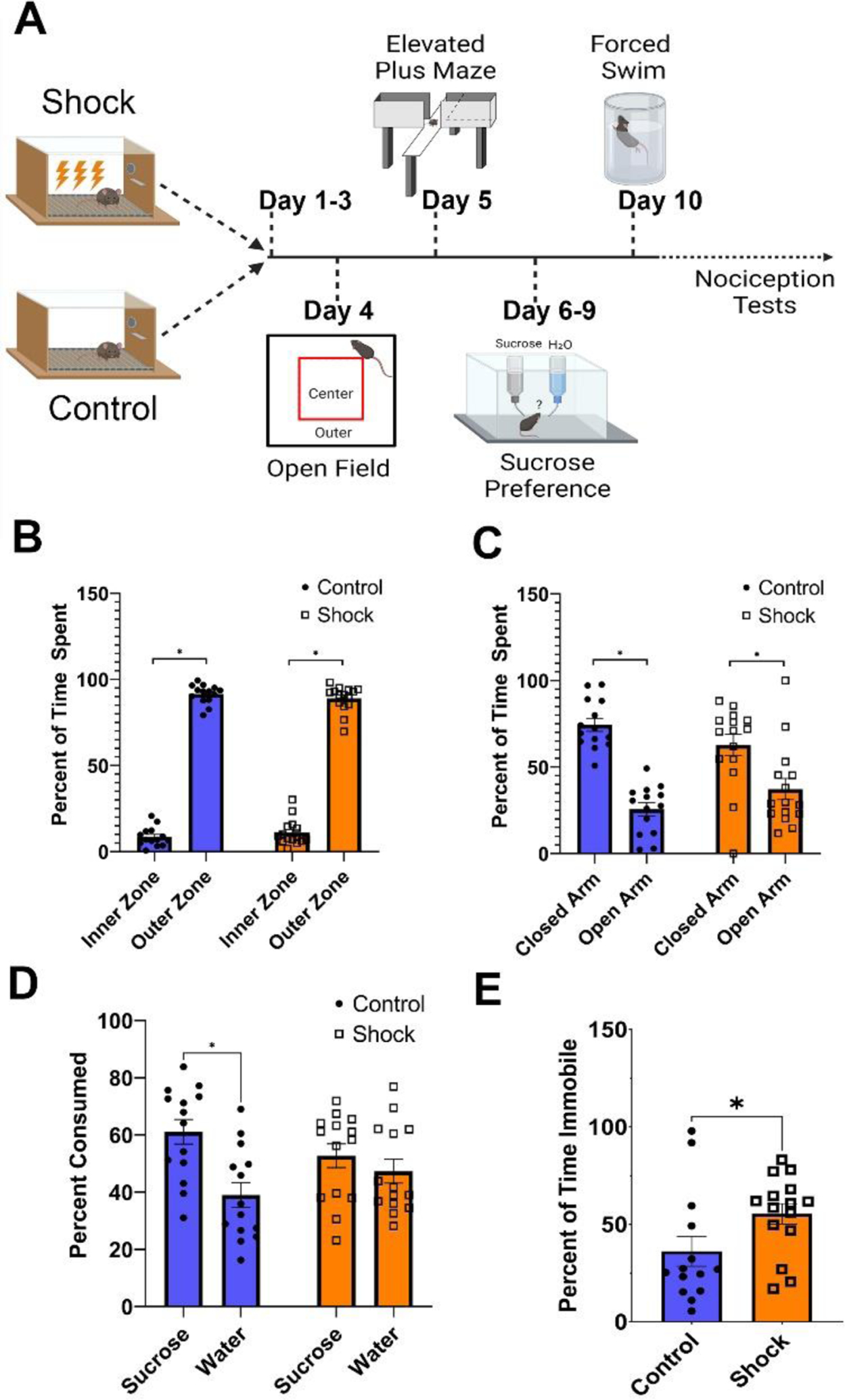
Footshock Stress Produces Behavioral Features of Anhedonia and Despair
Note. (A) Timeline of behavioral testing for negative valence behaviors including the open field test, elevated plus maze, sucrose preference test, and forced swim test. (B) Both shock and control mice spend more time in the outer zone of the open field than the inner zone (F [1,27] = 25.79, p < .0001), but no differences between groups were observed. (C) Shock and control mice also spent more time in the closed arms of the elevated plus maze than the open arms (F [1,27] = 1059, p < .001). (D) Control mice, but not shock mice, show a preference for 1% sucrose over water in the sucrose preference test (Control: t [13] = 2.567, p = .0234; Shock: [t [13] = 0.6518, p = .526). (E) Shock mice also spent more time immobile in the forced swim task than control mice (t [27] = 2.109, p = .044).
Figure 2.
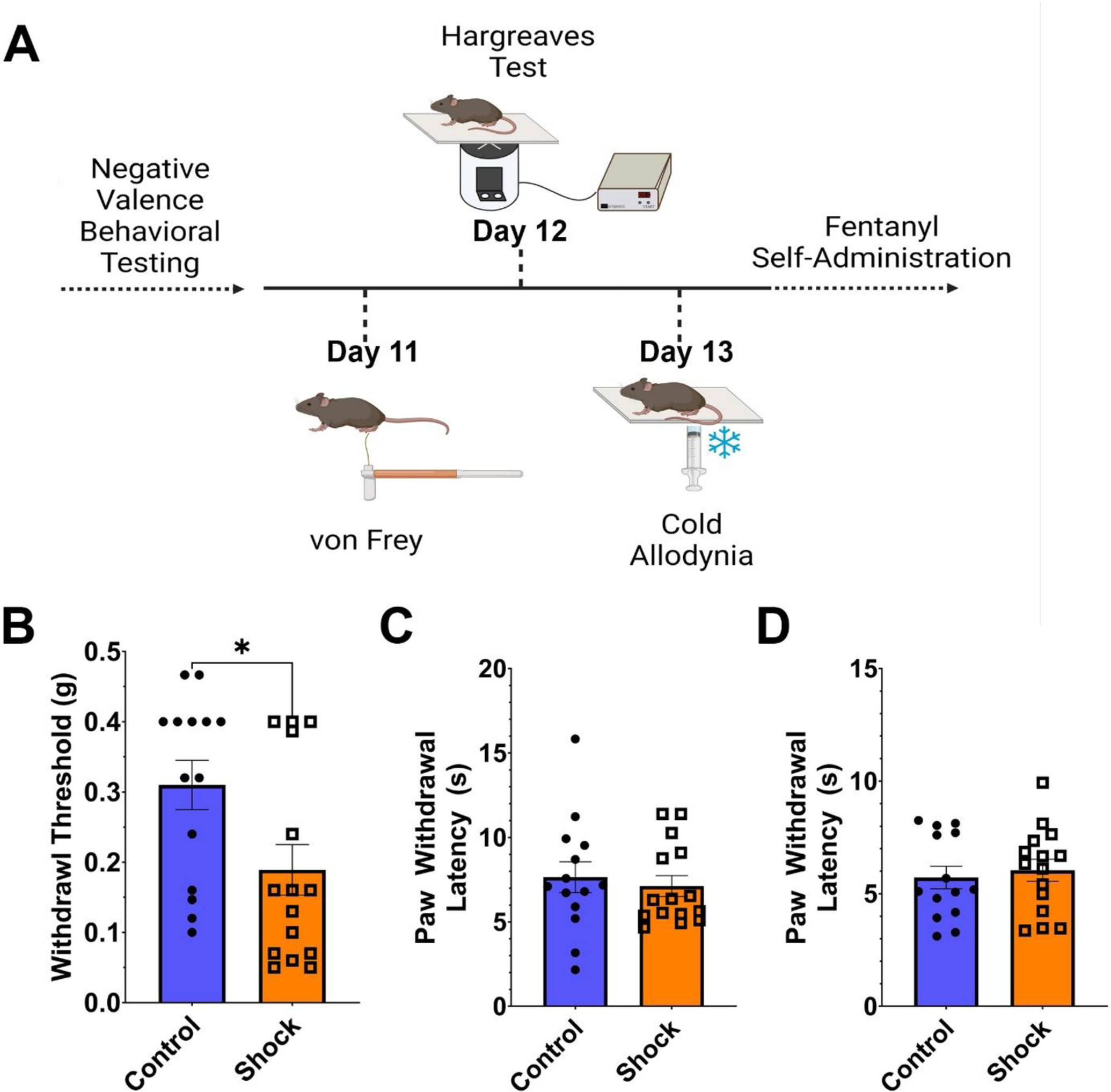
Footshock Stress Increases Mechanical Hypersensitivity but Not Thermal Sensitivity
Note. (A) Timeline for nociceptive assays including the von Frey task of mechanical sensitivity, the Hargreaves test for sensitivity to radiant heat, and the cold allodynia test for sensitivity to cold. (B) Shock mice were hypersensitive to mechanical stimulation of the paw, showing a lower withdrawal threshold than control mice (t [27] = 2.109, p = .044). On the other hand, no differences were observed between shock and control mice in the latency for paw withdrawal in the Hargreaves test of heat sensitivity (C) nor the cold allodynia test (D).
Figure 3.
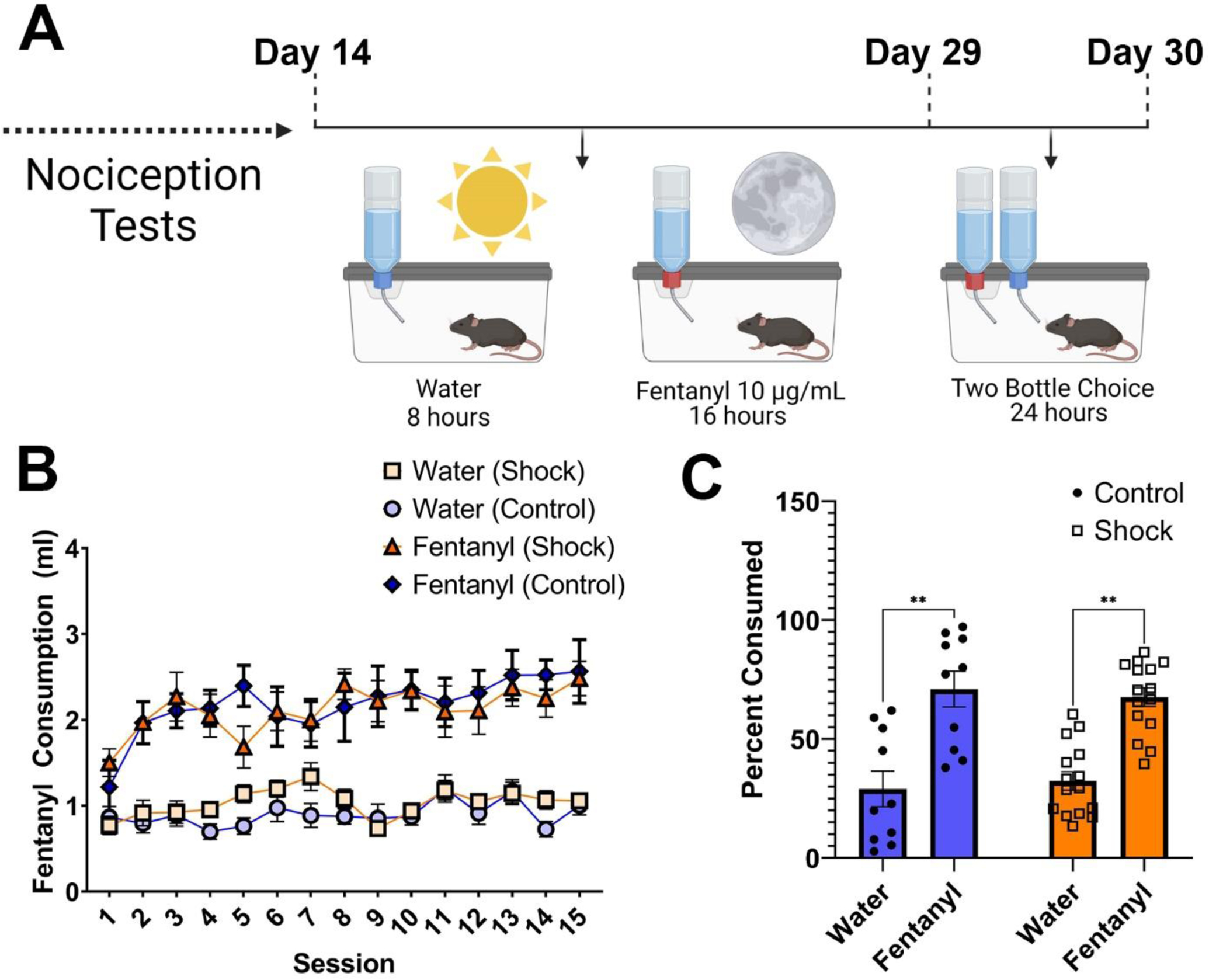
Oral Fentanyl Self-Administration over Two Weeks
Note. (A) Timeline of oral fentanyl self-administration. Mice had access to oral fentanyl (10μg/ml) during their active cycle (night) and access to water during the day for a period of 15 days. On day 16 mice were then given a choice between fentanyl and water in a 24-hr test. (B) Shock and control mice consumed similar total amounts of fentanyl (t [23] = 0.084, p = .9336), and both increased their intake of fentanyl over time (Control: R2 = .59; β = 0.013, F [1,13] = 19, p < .001; Shock: R2 = .45; β = 0.012, F [1,13] = 11, p = .006). No corresponding increase in water intake was observed (Control: R2 = .19; β = 0.008, F [1,13] = 19, p = .106 Shock: R2 = .11; β = 0.009, F [1,13] = 11, p = .223). (C) During the preference test, both groups showed a preference for fentanyl. (Bottle, F [1,23] = 24.95, p < .001), although responses did not differ across groups.
Stress Protocol
Footshock or tail-shocks have been used to model the effects of stress on various psychological disorders in rodent models (Bali & Jaggi, 2015; Chourbaji et al., 2005). There are known effects of footshock in animal models of opioid addiction (Barsy et al., 2011), mood disorders (Baratta et al., 2018; Greenwood et al., 2012; Landgraf et al., 2015), and pain (Wu et al., 2020). For the present study, we chose a model of inescapable shock known to promote negative valence behaviors (Qi et al., 2016), increase inflammatory markers (Frank et al., 2020), and to drive synaptic plasticity (Barrata et al., 2019). For shock stress, mice were placed in a Med-Associates chamber for three daily sessions. During each session, mice received 100 inescapable, 0.35 mA foot shocks. Shocks were delivered every 60 s, on average, and lasted 1 s. Control mice were placed in the chamber for an equivalent amount of time but received no footshocks.
Elevated Plus Maze
The elevated plus maze consisted of a plus-shaped, Plexiglas apparatus (Stoelting Co.) with two closed arms flanked by large sidewalls, and two open arms that lacked sidewalls. For testing in the elevated plus maze, mice were habituated to the experimental room in their home cage for at least 30 min. Mice were then allowed to freely explore the elevated plus maze for 5 min. During the test, the time spent in the open arms and closed arms were monitored by video tracking (AnyMaze). For analysis purposes, the time spent in the center square of the maze was considered part of the open-arm area. The apparatus was cleaned with 70% ethanol between subjects.
Open Field
Mice were habituated in the testing room at least 30 min prior to testing before being placed in the center of custom-built Plexiglas open field chambers measuring 40 cm × 40 cm. Video tracking software (AnyMaze) was used to measure the distance traveled, as well as the time spent along the outer edges and center of the maze over a period of a 30-min open-field test. The outer edge was defined as 10 cm from each wall, which partitioned the chamber such that 50% of the surface area belonged to the outer zone and the remaining 50% belonged to the inner zone. The apparatus was cleaned with 70% ethanol between subjects.
Sucrose Preference Test
The sucrose preference test is used as a measure of anhedonia-like behavior in mice. Mice were individually housed in a home cage and acclimated to two 15 ml sipper bottles of water (Drinko Bottles; Amuza) overnight. One bottle was then replaced with a 1% sucrose solution. Bottles of water and sucrose were weighed twice a day (morning and late afternoon) for 2 days and refilled as necessary. The position of bottles was switched each morning during weighing to combat any bias for bottle location. The total sucrose or water consumed was calculated by using the amount (g) of sucrose or water consumed over the total amount (g) of consumption for both sucrose and water to normalize for differences in consumption.
Forced Swim Test
Mice were habituated in the testing room at least 30 min prior to testing. Mice were placed in a forced swim chamber for a 6-min test. Chambers were 1 foot tall with a 4-inch inner diameter. Each apparatus was filled with ~10 inches of room-temperature water. Immobility was tracked during the final 4 min of the 6-min test via video tracking software (AnyMaze) using settings that were validated against human scorers. Following testing, mice were dried and transferred to a cage on a heating pad for recovery before returning to their home cage.
Von Frey Test of Mechanosensitivity
Mice were placed in 10 cm × 10 cm × 12 cm opaque plexiglas boxes and allowed to habituate on a wire-mesh von Frey table for 2 hr prior to testing. Mechanical sensitivity was measured by an experimenter using von Frey filaments (Stoelting), similar to methods described by others (Carrasquillo & Gereau, 2007). Briefly, von Frey filaments were applied using the ascending method (i.e., starting with the lowest level of force and incrementing to the largest). For each filament used, the right hindpaw was manually stimulated on the lateral mid plantar surface until the filament bent for ~ 2 s. A positive response was considered when a mouse exhibited a nocifensive response, such as a foot flick, licking, or stomping. Filaments were applied 5 times and if a filament evoked a response for 3 out of 5 (60%) of the stimulations, then that filament was considered the threshold for that trial. Each mouse was given three trials of ascending stimulation and the average of these trials was calculated and considered the mouse’s mechanical threshold.
Hargreaves Test
The Hargreaves test is a method for quantifying thermal sensitivity of the hindpaw following the application of an infrared heat stimulus. Prior to testing, mice were placed in 10 cm × 10 cm × 12 cm opaque plexiglas boxes and allowed to habituate on an elevated clear-glass platform for 2 hr prior to testing. The right hindpaw was stimulated on the lateral mid plantar surface by delivering radiant heat using an infrared stimulator (Ugo Basile). For all mice, the latency to paw withdrawal was defined as the time from laser onset until the time of an observed nocifensive response (i.e., a foot flick, licking, or stomping), with a maximum cutoff time of 20 s. The average of three response latencies was used for final data analysis.
Cold Allodynia
Prior to testing, mice were placed in 10 cm × 10 cm × 12 cm opaque plexiglas boxes and allowed to habituate on an elevated clear-glass platform for 2 hr prior to testing. For testing, dry ice was crushed into a fine powder and then packed into the body of a 1 ml syringe with the tip cut off to create a solid pellet, similar to previously described methods (Brenner et al., 2015). The pellet was then applied to the right hindpaw of the mouse and the latency to observe a nocifensive response was recorded. The average of three trials was taken for final data analysis.
Oral Fentanyl Self-Administration
A subset of mice was selected randomly to undergo oral fentanyl self-administration. Mice were housed individually in standard home cages with ad libitum access to food. Each evening at 5 PM, 2 hr before the onset of the night cycle, mice were given 15 ml bottles (Drinko Bottles; Amuza) containing fentanyl hydrochloride dissolved in water (10 μg/ml). Fentanyl bottles were then removed and replaced with bottles of water each morning at 9 AM. Bottles for both water and fentanyl were weighed daily and refilled as needed. The cycle continued for 15 days. On day 16, mice were given bottles containing both fentanyl and water overnight for a two-bottle choice paradigm.
Statistics
All data are plotted at Mean ± the standard error of the mean (SEM). Paired samples t-tests, two-way mixed ANOVAs, or two-way between-subjects ANOVAs were used, when appropriate. Nonnormal data were analyzed with either a Mann-Whitney U test or a two-way generalized linear mixed model. Statistics were run using a combination of GraphPad Prism or SPSS. Alpha was always set to.05. Post-hoc corrections for the familywise error rate were accomplished using either Sidak or Sidak-Holm corrections.
Results
Negative Valence Behaviors
To determine how footshock stress affected negative valence behaviors, we first examined responses for mice that had received footshocks and control mice that had not in the elevated plus maze, open field, sucrose preference test, and forced swim test (Fig. 1A).
We first examined the elevated plus maze and open field to assess baseline levels of anxiety-like behavior between shock and control mice. Both the shock and control groups spent significantly more time in the closed arms than the open arms (Arm: F [1,27] = 25.79, p < .0001; Fig. 1B). However, no group differences in the time spent in either the open or closed arms were observed (Group: F [1,27] = 0.02, p = .657; Group × Arm interaction: F [1,27] = 2.52, p = .124]. Similarly, all mice exhibited a similar preference for the outer zone of the open field over the inner zone (Zone: F [1,27] = 1059, p < .001), but responses did not vary by group (Group: F [1,27] = 2.103, p = .159; Group × Zone: F [1,27] = 1.026, p = .320; Fig. 1C).
We next examined responses in the sucrose preference test and forced swim task to test for anhedonia and behavioral despair. When testing for the presence of a sucrose preference, we observed that the presence of a sucrose preference differed across experimental groups (Group × Bottle: χ2 [1] = 4.196, p = .04, generalized linear model). Post-hoc tests revealed that control mice consumed more 1% sucrose than water (t [13] = 3.84, p < .001), while shock mice did not show a preference (t [13] = 0.939, p = .348; Fig. 1D). Moreover, shock (Mdn = 60.96; n =14) mice spent more time immobile in the forced swim task than control mice (Mdn = 26.15; n = 15) (Mann-Whitney U = 55, p = .029; Fig. 1E). Overall, these results show that shocked mice exhibited depression-like behavioral responses consistent with anhedonia and behavioral despair, without exhibiting differences in behavioral avoidance.
Responses to Nociceptive Stimuli
In addition to tests of negative valence behaviors, we also examined the responses of control and shock mice in three tasks associated with nociceptive sensitivity: the von Frey test for mechanosensitivity, the Hargreaves test for heat sensitivity, and the cold allodynia test for sensitivity to cold (Fig. 2A). When comparing shock mice to controls, we observed that shock mice were hypersensitive to mechanical stimulation in the von Frey task as compared to controls (t [27] = 2.109, p = .044; Fig. 2B), requiring a lower level of force on the hindpaw to elicit a nocifensive response. On the other hand, we did not observe any differences in the latency to paw withdrawal in response to radiant heat in the Hargreaves plantar test (t [27] = 0.482, p = .6339; Fig. 2C), nor did we observe differences in the latency to paw withdrawal in response to cold stimulation in the cold-allodynia task (t [27] = 0.459, p = .649; Fig. 2D). Thus, mice exposed to repeated stress exhibited heightened mechanical, but not thermal sensitivity.
Fentanyl Self-Administration Behaviors
Following our phenotypic characterization of negative valence behaviors and nociceptive sensitivity, a randomly selected subset of mice (n = 24) underwent oral fentanyl self-administration. During fentanyl self-administration, mice were given 16 hr of access to fentanyl (10μg/ml) during their active (night) cycle, and water during the remaining 8 hr of each day for a period of 15 days (Fig. 3A).
Over the course of self-administration, both control and shock mice showed an escalation in their intake of fentanyl, as evidenced by significant positive linear increase over time (Control: R2 = .59; β = 0.013, F [1,13] = 19, p < .001; Shock: R2 = .45; β = 0.012, F [1,13] = 11, p = .006; Fig. 3B). However, no corresponding increase in water intake was observed over the same time period (Control: R2 = .19; β =0.008, F [1,13] = 19, p = .106 Shock: R2 = .11; β = 0.009, F [1,13] = 11, p = .223; Fig. 3B). Moreover, the total consumption of fentanyl was similar in the forced choice task between the fentanyl group and control group (t [23] = 0.084, p = .9336]. Following chronic fentanyl intake, mice were given a two-bottle choice task to determine whether they exhibited a fentanyl preference. Both shock and control mice exhibited a preference for fentanyl over water (Bottle, F [1,23] = 24.95, p < .001; Fig. 3C), with no differences across groups (Group: F [1,23] = 0.145, p = .707; Bottle × Group: F [1,23] = 0.192, p = .665).
Individual Differences in Fentanyl Preference
While fentanyl was preferred over water in both groups, we also noted considerable behavioral variability in fentanyl-seeking behaviors, with fentanyl preference ranging from 37% to 97% (Fig. 3C; Fig. 4A). We therefore sought to further dissect the richness of these behavioral responses to determine whether an increased preference for opioids corresponded to differences in any of the other pre-drug behavioral measures. For these analyses, mice were grouped into fentanyl-preferring versus nonpreferring groups using a median-split approach, which corresponded well to the division point between two observed modes in the data (Fig. 4A).
Figure 4.
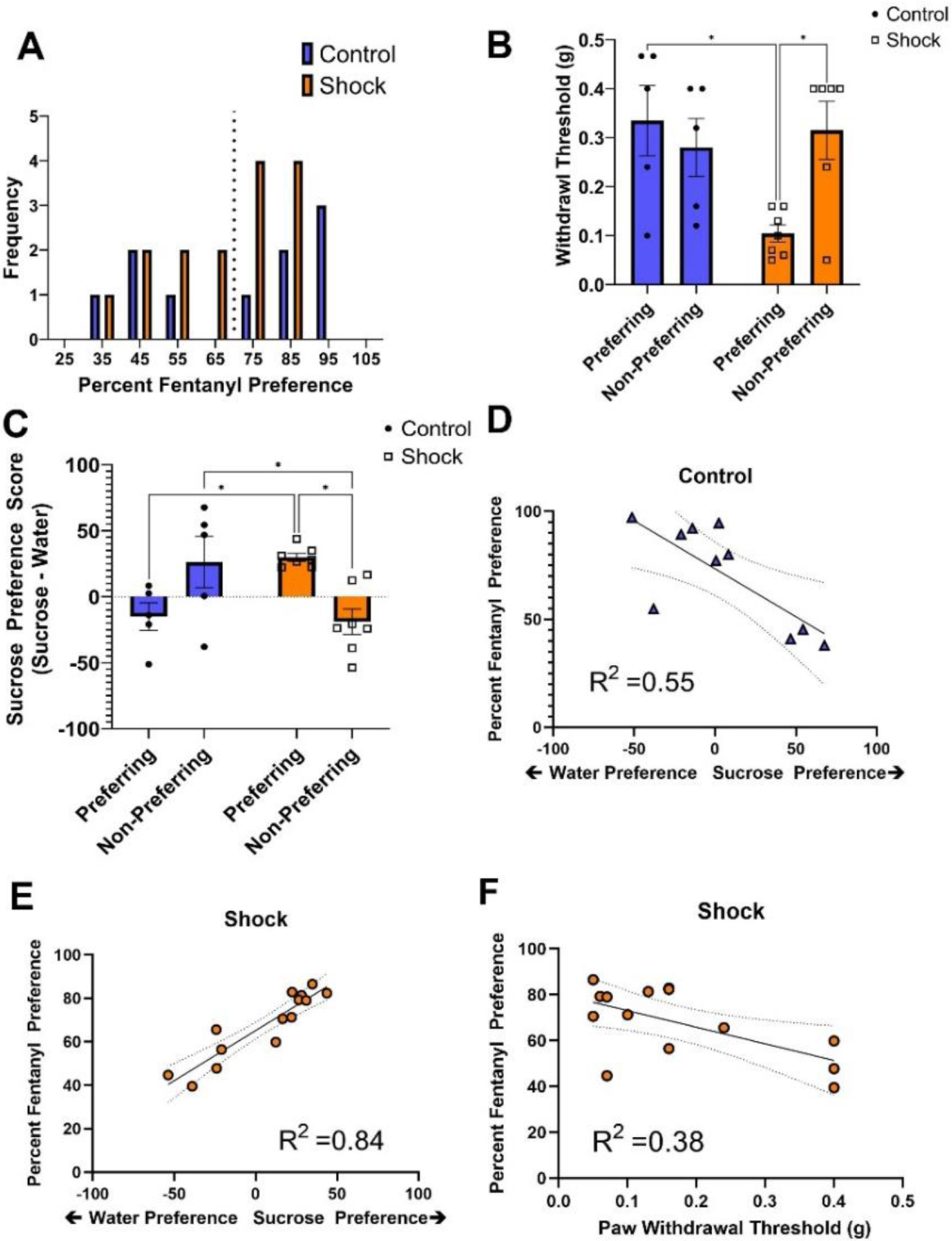
Individual Differences in Behavioral Responses and Stress History Differentially Predict Future Fentanyl Preference
Note. (A) Preferences for fentanyl were spread over a wide range from 37% to 97% in both shock and control mice. (B) A subset of shock mice exhibiting heightened mechanical sensitivity were also those most likely to exhibit heightened fentanyl preference (F [1,20] =7.494, p < .05) (C) Sucrose preference differed depending on both stress history and fentanyl preference (F [1,20] =16.73, p < .001). (C-D) In the control group, lower sucrose preference was associated with higher fentanyl preference (R2 = .55; F [1,9] = 9.919, p = .14). (C,E) In the shock group, higher sucrose preference predicted heightened fentanyl preference. (F) In addition to sucrose preference, mechanical sensitivity to pain also served as a reliable predictor of fentanyl preference. The combination of sucrose preference and mechanical sensitivity predicted 89.9% of the variability in sucrose preference (R2= .899; F [1,13] =48.901, p < .001). * p < .05.
When examining fentanyl susceptibility, we first considered individual differences in pre-drug mechanical sensitivity to pain. The endogenous opioid system plays an important role in pain regulation; however, tests of pain sensitivity are rarely incorporated into behavioral assays related to stress or mood disorders. Here we observed that the heightened mechanosensitivity observed in the shock group could be linked to fentanyl-preferring mice. (Intake × Group: F [1,20] =7.494, p < .05; Fig. 4B). Fentanyl-preferring mice in the shock group exhibited increased mechanical sensitivity as compared to both Fentanyl-preferring mice in the control group (t [20] = 3.24, p < .05), and nonpreferring mice in the shock group (t [20] = 3.40, p < .05). Additionally, reduced Von Frey thresholds were significant predictors of fentanyl preference in shock mice (R2 = .33, p = .025). In contrast, heightened mechanical sensitivity was not observed in nonpreferring shock mice when compared to fentanyl-preferring and nonpreferring controls (all p > .05, n.s.; Fig. 4B). From these findings, we infer that heightened mechanical pain sensitivity may be a key hallmark of opioid use susceptibility following stress exposure.
We also considered that differences in fentanyl susceptibility might coincide with the group differences observed in the forced swim and sucrose preference tasks. When examining the forced swim task, we observed no differences in the percent of time animals spent immobile between fentanyl-preferring and nonpreferring mice in either group (Intake: F [1,20[ =1.23, p = .280; Group: F [1,20] =1.58, p = .223; Group × Intake: F [1,20] = 0.02, p = .0889; data not shown). On the other hand, we discovered that individual differences in sucrose preference differentially predicted levels of future fentanyl preference in a manner that was dependent on prior stress history (Group × Intake interaction: F [1,20[ =16.73, p < .001; Fig. 4C). Surprisingly, fentanyl-preferring mice in the shock group exhibited greater sucrose preferences than their nonpreferring shock counterparts (t [20] = 3.43, p < .01). Additionally, lower sucrose preferences were observed in preferring control mice as compared to preferring mice with a history of stress (t [20] = 2.88, p < .05). In contrast, sucrose preference was greater in nonpreferring control mice when compared to nonpreferring shock mice (t [20] =2.91, p < .05).
To take a more unbiased approach and determine how well each of the pre-drug behavioral tests could predict the observed variability in fentanyl preference, we conducted a stepwise, linear regression which included measures from each behavioral test: 1) open arm time in the elevated plus maze, 2) inner zone time for the open field, 3) percent sucrose preference, 4) immobility time in the forced swim, 5) paw withdrawal latency in the Hargreaves task, 6) paw withdrawal latency in the cold allodynia task, and 7) the von Frey withdrawal threshold. For control mice, the percent of sucrose consumption was the only variable significantly related to fentanyl preference (F [1,9] = 9.919, p = .14), accounting for 55.4% of the variance in fentanyl preference (R2 =.554; Fentanyl Preference = −0.445 * Sucrose Preference +73.48). Specifically, we observed that heightened water preference corresponded with greater fentanyl preference in control mice (Fig. 4D). For shock mice, we observed that sucrose preference and von Frey thresholds were significantly related to fentanyl preference (F [1,13] = 48.901, p < .001). Sucrose preference accounted for 84.2% of the variance in fentanyl preference, while the addition of von Frey threshold in the second stage of the model accounted for an additional 5.7% of the variance in fentanyl preference (R2 = .899; Fentanyl Preference = 0.403* Sucrose Preference −31.18 * von Frey Threshold +70.944; Figs. 4E–F). Together, these data demonstrate that mice with high mechanical sensitivity and high sucrose-seeking behavior are those with the strongest fentanyl preferences. No other variables were retained in the stepwise regression for either control or shock mice.
Discussion
Opioids remain one of the most highly prescribed drugs, with more than 200 million opioid prescriptions issued each year (Volkow & McLellan, 2016). As 20%−30% of the individuals prescribed opioids misuse them and approximately half of those individuals develop an opioid use disorder (Vowles et al., 2015). While opioids are effective for the treatment of acute pain, their use as a chronic treatment remains questionable and represents a clear risk factor for developing an opioid use disorder. In clinical settings fentanyl is administered orally, transmucosally, intranasally, intravenously, epidurally, and transdermally; with oral administration as a frequent route of abuse (Gasior et al., 2015; Grape et al., 2010; Mystakidou et al., 2007). Despite their widespread use, predicting individual susceptibility to opioids has proven difficult in both clinical and preclinical settings. Here we demonstrate that comprehensive behavioral testing, using an expanded repertoire that includes assays for nociceptive processing and negative valence behaviors, can provide insights into opioid susceptibility in mice. Moreover, our results also reveal an important role for an individual’s history, suggesting that physiological modulators, such as stress, may act to differentiate predictors of opioid seeking.
The Role of Negative Valence Behaviors
Previous literature has suggested that susceptibility to stress may influence opioid-seeking behavior (Rubinstein et al., 1996, Swain et al 2021). In the present study, we observed behavioral differences between shock and control mice in the sucrose preference test and the forced swim task. These measures are typically associated with behavioral despair and anhedonia, two features often associated with learned helplessness and depression (Liu et al., 2018; Porsolt, Bertin, & Jalfre, 1977; Porsolt, Le Pichon, & Jalfre, 1977; Pothion et al., 2004; Willner et al., 1987). Indeed, the footshock protocol used here is consistent in both time course and intensity with those used to induce learned helplessness behaviors in other protocols (Chourbaji et al., 2005; Qi et al., 2016) and, while not directly measured, we consistently observed an attenuation or cessation of escape attempts (e.g., jumping) by the second or third session.
When examining forced swim immobility and sucrose consumption in relation to future susceptibility towards fentanyl preference, we were surprised to find no relationship between forced swim immobility and fentanyl preference. However, we did find a relationship between sucrose consumption and future fentanyl preference. What was most notable about the relationship between sucrose consumption and opioid use susceptibility was the juxtaposition between control mice and shock mice. In the shock group, higher sucrose preference predicted fentanyl preference, while in the control group lower sucrose preference was associated with fentanyl preference. Stress-coping models of addiction propose that reward seeking becomes a maladaptive coping strategy to reduce negative affect and increase positive affect (Wills & Hirky, 1996). In trying to reconcile these seemingly opposite results, we hypothesize that mice predisposed to anhedonia are those most sensitive to the negative effects of fentanyl. Moreover, we predict that exposure to stress in these same mice produces a shift towards persistent or maladaptive reward seeking, consistent with what we observe in shock mice. Indeed, previous studies using intercranial self-stimulation have shown that anhedonia in response to withdrawal was a predictor of multiple measures of morphine self-administration, indicating that an individual’s initial sensitivity to the negative effects of opioids may be an important predictor of drug use (Swain et al., 2020)
We were also surprised to observe that shock mice did not exhibit differences in the open field or elevated plus maze when compared to controls, nor did we observe any relation between responses in these tasks and opioid-seeking behavior. While surprising, the role of anxiety is unclear in clinical settings (Swain et al., 2021). Therefore, future studies might focus on expanding the predictive value of the behavioral battery used here to other aspects of opioid-use behavior. These tasks may provide novel insights into opioid withdrawal or relapse.
Nociception and Opioid Use Susceptibility
An individual’s inherent sensitivity to pain is rarely incorporated into models of opioid abuse susceptibility, stress, or mood disorders. This is particularly surprising as physical pain is often associated with clinical depression (Li, 2015). We discovered here that mechanical sensitivity to pain was a key predictor of opioid susceptibility in mice with a history of footshock stress but not for control mice. This same mechanical hypersensitivity was observed in another recent study (Wu et al., 2020), which similarly demonstrated that stress-induced hyperalgesia persisted for at least 28 days. Thus, tests of mechanical hypersensitivity may provide valuable evidence for opioid use susceptibility. In contrast, we failed to find any predictive value of tests of thermal sensitivity. While this finding may appear to contradict results from von Frey testing, multiple studies have shown that stress can cause vasoconstriction in the tail and paws, as well as an associated reduction in skin temperature (Blessing, 2003; Vianna & Carrive, 2005). With this in mind, tests of thermal sensitivity may provide less information than tests of mechanical sensitivity in the context of stress but may prove valuable in other models of opioid-seeking behavior.
Clinical Relevance
Opioids are both prescribed and abused through multiple routes of administration (Gasior et al., 2015; Grape et al., 2010; Mystakidou et al., 2007). Each of these administration routes is designed to provide long-lasting delivery for the continued relief of pain (Menahem & Shvartzman, 2004). In the present model, we employed a 10μg/ml dose of fentanyl over a long-access oral self-administration session. While it is difficult to directly compare dosing across rodents and humans, preclinical studies have shown that doses as small as 5 μg/ml can produce analgesic effects in mice (Alves et al., 2007). Moreover, 10μg/ml has previously been shown as an optimal concentration for the reinforcing properties of oral fentanyl (Monroe & Radke, 2021; Wade et al., 2008). Thus, the parameters chosen here appear to model key features of human opioid use and abuse.
Our results here also emphasize the notion that mental illness truly comprises multiple distinct disease states, and that expanded behavioral testing with attention to individual differences can help improve predictive and diagnostic criteria. As an example, the absence of a sucrose preference is typically associated with animal models of depression. Nonetheless, we demonstrate here that a subset of mice with paradoxically high sucrose-seeking are those most susceptible to fentanyl-seeking. For translating these findings into human patients, our results suggest that increased attention should be given to behaviors associated with the endogenous opioid system. This might include placing increased weight on clinical measures of sensation or reward-seeking behaviors as effective criteria when trying to determine the risk for comorbid opioid abuse. Additionally, it seems both simple and prudent to begin incorporating objective measures of pain sensitivity into the clinical repertoire before prescribing opioids. The von Frey is a highly translational task that could easily be employed in the clinic. Lastly, an individual’s history of stress, depression, or anxiety should be considered more carefully. Together, these factors might help identify patients best suited for nonopioid alternatives when considering pain management or other treatment approaches.
Acknowledgments
This study was supported by the National Institute on Drug Abuse Grants DA043572 (DJB) and fentanyl was generously supplied through the NIDA drug supply program. The funders had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation. We thank Dr. Yarimar Carrasquillo and Torri Wilson for their guidance on assays for nociception. CO and DJB conceptualized the project. CO performed the surgeries. CO and RV ran the behavioral experiments. CO and DJB Analyzed the data. CO and DJB wrote the manuscript with contributions from all coauthors. All authors have seen the manuscript and approved it for publication. The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- Al-Hasani R, & Bruchas MR (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology, 115(6), 1363–1381. 10.1097/ALN.0b013e318238bba6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves HC, Valentim AM, Olsson IA, & Antunes LM (2007). Intraperitoneal propofol and propofol fentanyl, sufentanil and remifentanil combinations for mouse anaesthesia. Laboratory Animals, 41(3), 329–336. 10.1258/002367707781282767 [DOI] [PubMed] [Google Scholar]
- Bali A, & Jaggi AS (2015). Electric foot shock stress: A useful tool in neuropsychiatric studies. Reviews in the Neurosciences, 26(6), 655–677). 10.1515/revneuro-2015-0015 [DOI] [PubMed] [Google Scholar]
- Baratta MV, Gruene TM, Dolzani SD, Chun LE, Maier SF, & Shansky RM (2019). Controllable stress elicits circuit-specific patterns of prefrontal plasticity in males, but not females. Brain Structure and Function, 224(5), 1831–1843. 10.1007/s00429-019-01875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, Watkins LR, & Maier SF (2018). Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. European Journal of Neuroscience, 47(8), 959–967. 10.1111/ejn.13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsy B, Mikics É, Barsvári B, & Haller J (2011). The long-term impact of footshock stress on addiction-related behaviors in rats. Neuropharmacology, 60(2–3), 267–273. 10.1016/j.neuropharm.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, & Julius D (2009). Cellular and molecular mechanisms of pain. Cell, 139(2), 267–284. 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW (2003). Lower brainstem pathways regulating sympathetically mediated changes in cutaneous blood flow. Cellular Molecular Neurobiology, 23(4–5), 527–538. 10.1023/a:1025020029037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DS, Golden JP, Vogt SK, & Gereau R (2015). A simple and inexpensive method for determining cold sensitivity and adaptation in mice. Journal of Visualized Experiments, 97, e52640. 10.3791/52640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, & Chavkin C (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Research, 1314, 44–55. 10.1016/j.brainres.2009.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, & Bertoglio LJ (2005). Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neuroscience & Biobehavioral Reviews, 29(8), 1193–1205. 10.1016/j.neubiorev.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Carrasquillo Y, & Gereau R (2007). Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. Journal of Neuroscience, 27(7), 1543–1551. 10.1523/JNEUROSCI.3536-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, & Gass P (2005). Learned helplessness: Validity and reliability of depressive-like states in mice. Brain Research Protocols, 16(1–3), 70–78. 10.1016/j.brainresprot.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, & Hoebel BG (2002). Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity Research, 10(6), 478–488. 10.1038/oby.2002.66 [DOI] [PubMed] [Google Scholar]
- Dieterich A, Floeder J, Stech K, Lee J, Srivastava P, Barker DJ, & Samuels BA (2021). Activation of basolateral amygdala to nucleus accumbens projection neurons attenuates chronic corticosterone-induced behavioral deficits in male mice. Frontiers in Behavioral Neuroscience,15, 643272. 10.3389/fnbeh.2021.643272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich A, Srivastava P, Sharif A, Stech K, Floeder J, Yohn SE, & Samuels BA (2019). Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Translational Psychiatry, 9(1), 337. 10.1038/s41398-019-0674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery MA, & Akil HJ (2020). Endogenous opioids at the intersection of opioid addiction, pain, and depression: The search for a precision medicine approach. Annual Review of Neuroscience, 43, 355–374. 10.1146/annurev-neuro-110719-095912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D, Brill S, Goor-Aryeh I, Delayahu Y, & Lev-Ran S (2018). The association between severity of depression and prescription opioid misuse among chronic pain patients with and without anxiety: A cross-sectional study. Journal of Affective Disorders, 235, 293–302. 10.1016/j.jad.2018.04.058 [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Zhang K, Fallon IP, Pearson MA, Liu G, Hutchinson MR, Watkins LR, Goldys EM, & Maier SF (2020). Acute stress induces the rapid and transient induction of caspase-1, gasdermin D and release of constitutive IL-1β protein in dorsal hippocampus. Brain, Behavior, and Immunity, 90, 70–80. 10.1016/j.bbi.2020.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Bond M, & Malamut R. (2016). Routes of abuse of prescription opioid analgesics: A review and assessment of the potential impact of abuse-deterrent formulations. Postgraduate Medicine, 128(1), 85–96. 10.1080/00325481.2016.1120642 [DOI] [PubMed] [Google Scholar]
- Grape S, Schug SA, Lauer S, & Schug BS (2010). Formulations of fentanyl for the management of pain. Drugs, 70(1), 57–72. 10.2165/11531740-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, & Fleshner M (2012). The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behavioural Brain Research, 233(2), 314–321. 10.1016/j.bbr.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, & Colpe L. J. J. H. a. (2017). Prevalence, treatment, and unmet treatment needs of US adults with mental health and substance use disorders. Health Affairs (Project Hope), 36(10), 1739–1747. 10.1377/hlthaff.2017.0584 [DOI] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron, 59(1), 11–34. doi: 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, & Koob GF (1998). Drug dependence: Stress and dysregulation of brain reward pathways. Drug & Alcohol Dependence, 51(1–2), 23–47. 10.1016/s0376-8716(98)00064-7 [DOI] [PubMed] [Google Scholar]
- Krishnan V, & Nestler EJ (2008). The molecular neurobiology of depression. Nature, 455(7215), 894–902. 10.1038/nature07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, & Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. Journal of Neuroscience, 28(2), 407–414. 10.1523/JNEUROSCI.4458-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long J, Der-Avakian A, Streets M, & Welsh DK (2015). Dissociation of learned helplessness and fear conditioning in mice: A mouse model of depression. PLoS ONE, 10(4). 10.1371/journal.pone.0125892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX (2015). Pain and depression comorbidity: A preclinical perspective. Behavioural Brain Research, 276, 92–98. 10.1016/j.bbr.2014.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Li J, Epstein DH, Zhang XY, Kosten TR, & Lu L (2008). Serum cortisol secretion during heroin abstinence is elevated only nocturnally. American Journal of Drug and Alcohol Abuse, 34(3), 321–328. 10.1080/00952990802013664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, & Zhou QG (2018). Sucrose preference test for measurement of stress-induced anhedonia in mice. Nature Protocols, 13(7), 1686–1698. 10.1038/s41596-018-0011-z [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, & Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. Journal of Neuroscience, 23(13), 5674–5683. 10.1523/jneurosci.23-13-05674.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahem S, & Shvartzman P (2004). High-dose fentanyl patch for cancer pain. The Journal of the American Board of Family Practice, 17(5), 388–390. 10.3122/jabfm.17.5.388 [DOI] [PubMed] [Google Scholar]
- Monroe SC, & Radke AK (2021). Aversion-resistant fentanyl self-administration in mice. Psychopharmacology, 238, 699–710. 10.1007/s00213-020-05722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, Tsiatas M, & Vlahos L (2007). Oral transmucosal fentanyl citrate in cancer pain management: A practical application of nanotechnology. International Journal of Nanomedicine, 2(1), 49–54. 10.2147/nano.2007.2.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2015). Role of the brain’s reward circuitry in depression: Transcriptional mechanisms. International Review of Neurobiology, 124, 151–170. 10.1016/bs.irn.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, & Jalfre M (1977). Behavioral despair in mice: A primary screening test for antidepressants. Archives Internationales de Pharmacodynamie et de Therapie, 229(2), 327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, & Jalfre M (1977). Depression: A new animal model sensitive to antidepressant treatments. Nature, 266(5604), 730–732. 10.1038/266730a0 [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, & Belzung C (2004). Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural Brain Research, 155(1), 135–146. 10.1016/j.bbr.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Barker DJ, Miranda-Barrientos J, & Morales M (2016). VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nature Neuroscience, 19(5), 725–733. 10.1038/nn.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Alvares K, Nespor SM, & Grunberg NE (1992). Effect of stress on oral morphine and fentanyl self-administration in rats. Pharmacology Biochemistry and Behavior, 41(3), 615–619. 10.1016/0091-3057(92)90382-p [DOI] [PubMed] [Google Scholar]
- Shaham Y, Klein LC, Alvares K, & Grunberg NE (1993). Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacology Biochemistry and Behavior, 46(2), 315–322. 10.1016/0091-3057(93)90359-2 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, & Stewart J (1996). Relapse to heroin-seeking in rats under opioid maintenance: The effects of stress, heroin priming, and withdrawal. Journal of Neuroscience, 16(5), 1957–1963. 10.1523/jneurosci.16-05-01957.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, & Stewart J (1994). Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berlin), 114(3), 523–527. 10.1007/BF02249346 [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, & Costentin JJ (1994). Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behavioural Brain Research, 61(1), 59–64. 10.1016/0166-4328(94)90008-6 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Rosenheck R, & Petrakis I (2014). Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addictive Behaviors, 39(2), 428–433. 10.1016/j.addbeh.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford NP, Kazan TN, Donovan CM, Hart EE, Drugan RC, & Charntikov S (2019). Individual vulnerability to stress is associated with increased demand for intravenous heroin self-administration in rats. Frontiers in Behavioral Neuroscience,13, 134. 10.3389/fnbeh.2019.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain Y, Gewirtz JC, & Harris A (2021). Behavioral predictors of individual differences in opioid addiction vulnerability as measured using iv self-administration in rats. Drug & Alcohol Dependence, 221,108561. 10.1016/j.drugalcdep.2021.108561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain Y, Muelken P, Skansberg A, Lanzdorf D, Haave Z, LeSage MG, Gewirtz JC, & Harris AC (2020). Higher anhedonia during withdrawal from initial opioid exposure is protective against subsequent opioid self-administration in rats. Psychopharmacology, 237(8), 2279–2291. 10.1007/s00213-020-05532-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DM, & Carrive P (2005). Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci, 21(9), 2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x [DOI] [PubMed] [Google Scholar]
- Volkow ND, & McLellan AT(2016). Opioid abuse in chronic pain—misconceptions and mitigation strategies. New England Journal of Medicine, 374(13), 1253–1263. 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, & van der Goes DN (2015). Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain, 156(4), 569–576. 10.1097/01.j.pain.0000460357.01998.f1 [DOI] [PubMed] [Google Scholar]
- Wade CL, Schuster DJ, Domingo KM, Kitto KF, & Fairbanks CA (2008). Supraspinally-administered agmatine attenuates the development of oral fentanyl self-administration. European Journal of Pharmacology, 587(1–3), 135–140. 10.1016/j.ejphar.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, & Muscat R (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl), 93(3), 358–364. 10.1007/BF00187257 [DOI] [PubMed] [Google Scholar]
- Wills TA, & Hirky AE (1996). Coping and substance abuse: A theoretical model and review of the evidence. In Zeidner M & Endler S (Eds.), Handbook of coping: Theory, research and application (pp. 279–302). John Wiley & Sons. [Google Scholar]
- Wu PY, Yang X, Wright DE, & Christianson JA (2020). Foot shock stress generates persistent widespread hypersensitivity and anhedonic behavior in an anxiety-prone strain of mice. Pain, 161(1), 211–219. 10.1097/j.pain.0000000000001703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, & Koeppe R (2003). Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Archives of General Psychiatry, 60(11), 1145–1153. 10.1001/archpsyc.60.11.1145 [DOI] [PubMed] [Google Scholar]


