Abstract
Per- and polyfluoroalkyl substances (PFAS) have become a target of rigorous scientific research due to their ubiquitous nature and adverse health effects. However, there are still gaps in knowledge about their environmental fate and health implications. More attention is needed for remote locations with source exposures. This study focuses on assessing PFAS exposure in Gustavus, a small Alaska community, located near a significant PFAS source from airport operations and fire training sites. Residential water (n = 25) and serum (n = 40) samples were collected from Gustavus residents and analyzed for 39 PFAS compounds. In addition, two water samples were collected from the previously identified PFAS source near the community. Fourteen distinct PFAS were detected in Gustavus water samples, including 6 perfluorinated carboxylic acids (PFCAs), 7 perfluorosulfonic acids (PFSAs), and 1 fluorotelomer sulfonate (FTS). ΣPFAS concentrations in residential drinking water ranged from not detected to 120 ng/L. High ΣPFAS levels were detected in two source samples collected from the Gustavus Department of Transportation (14,600 ng/L) and the Gustavus Airport (228 ng/L), confirming these two locations as a nearby major source of PFAS contamination. Seventeen PFAS were detected in serum and ΣPFAS concentrations ranged from 0.0170 to 13.1 ng/mL (median 0.0823 ng/mL). Perfluorooctanesulfonic acid (PFOS) and perfluorohexanesulfonic acid (PFHxS) were the most abundant PFAS in both water and serum samples and comprised up to 70% of ΣPFAS concentrations in these samples. Spearman’s correlation analysis revealed PFAS concentrations in water and sera were moderately and positively correlated (r = 0.495; p = 0.0192). Our results confirm a presence of a significant PFAS source near Gustavus, Alaska and suggest that contaminated drinking water from private wells contributes to the overall PFAS body burden in Gustavus residents.
Keywords: Aqueous film-forming foams (AFFFs), Arctic Health, Drinking water, Per- and polyfluoroalkyl substances (PFAS), Perfluorohexanesulfonic acid (PFHxS), Perfluorooctane sulfonate (PFOS)
Graphical Abstract
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of synthetic organic compounds that have been used in industrial and commercial applications since the 1940s and includes more than 9000 substances (EPA, 2020). For many PFAS, fluorinated alkyl chains give them high thermostability and water/grease-repellent properties. The ability of PFAS to repel both water and grease comes from their unique structure that includes both hydrophobic and hydrophilic functionalities (Kissa, 1994). Due to these properties, PFAS have been widely used as surfactants, adhesives, and emulsifiers in a variety of industrial applications and consumer products (Buck et al., 2011). In addition, their ability to lower aqueous surface-tension makes them a useful component in fluoropolymer manufacture and aqueous film-forming foams (AFFFs) that are used to extinguish fires from highly flammable liquids (Kissa, 2001).
As a result of their widespread use, PFAS have become ubiquitous in the environment and in humans. Although the presence of synthetic fluorinated substances in humans was first detected in the late 1960s, the interest in the environmental fate of PFAS significantly increased in the 2000s and has risen to a national priority in the United States and globally in the last few years (EPA, 2021a; Taves, 1968). Many PFAS are extremely persistent in the environment and resist biodegradation, direct photolysis, hydrolysis, and photooxidation (3M, 2000a; Schultz et al., 2003; Wang et al., 2017). The two most well-known PFAS, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), were extensively manufactured between 1940s and 2000s (Giesy and Kannan, 2001; Prevedouros et al., 2006), used in many industrial and consumer applications, and identified among the most ubiquitous PFAS in various environmental matrices and humans (Hansen et al., 2001). These PFAS have been detected in remote locations, such as the Arctic and Antarctic, as they can be transported through the atmosphere and by oceanic currents over long-distances (AMAP, 2017; Armitage et al., 2009; Butt et al., 2010; Nash et al., 2010; Yamashita et al., 2008).
PFAS production sites are major point sources of groundwater contamination in the United States and in other countries. For example, high levels of PFAS have been documented in the Cape Fear River in North Carolina due to wastewater discharges from a former fluorochemical production plant (EPA, 2006; Nakayama et al., 2007; Sun et al., 2016). PFOS and other associated compounds can still be detected in biota and humans as a consequence of releases from 3M’s PFAS production plants in Minnesota, despite being phased out almost two decades ago (Oliaei et al., 2013). In addition, PFAS-containing AFFFs used at commercial airports and military bases have been identified as the major sources of PFAS contamination of drinking water in the United States and other developed countries (Andrews and Naidenko, 2020; Banzhaf et al., 2017; Sunderland et al., 2019).
The Centers for Disease Control and Prevention has reported detectable serum PFAS levels in 97% of the U.S. population (CDC, 2019). Consumption of contaminated food and drinking water is a significant PFAS exposure pathway (Begley et al., 2005; Sunderland et al., 2019; Yuan et al., 2016). Drinking contaminated water in Uppsala, Sweden, has led to a significant increase in serum levels of perfluorohexanesulfonic acid (PFHxS). Concentrations in serum decreased by 20% in the next few years when the contaminated water source was substituted with uncontaminated sources (Stubleski et al., 2016). Hu et al. (2016) have shown that there was a significant link between PFAS detection in drinking water and the proximity of industrial sites, military fire training facilities, commercial airports, and wastewater treatment plants to contaminated water sources. In fact, in populations living near sites contaminated by point sources, drinking water can contribute up to 75% to total PFAS exposure (Hoffman et al., 2011; Vestergren and Cousins, 2009; Wang et al., 2017).
Epidemiological and toxicological studies suggest that PFAS exposure is associated with hepatic, cardiovascular, endocrine, immune, reproductive, and developmental adverse effects in animal models and humans (ATSDR, 2021; Fenton et al., 2021). Growing concerns about PFAS persistence and toxicity have led to the listing of PFOS and PFOA for global elimination under legally binding provisions of the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009 and 2019, respectively. The expert committee of the Stockholm Convention, the POPs Review Committee, has also recommended the global elimination of PFHxS with no exemptions (UNEP, 2019b). Canada nominated long-chain perfluorocarboxylic acids (PFCAs) in 2021 for inclusion under provisions of the Convention (Canada.ca, 2021) and in January 2022, the Committee decided that long-chain PFCAs met the criteria for inclusion (UNEP, 2022). The global fluoro-manufacturer, 3M, phased out PFOS, PFOA, and related compounds in 2000 to 2002 (3M, 1999, 2000b). In the United States, the Environmental Protection Agency (U.S. EPA) initiated a PFOA Stewardship Program, under which eight major fluoropolymer producers phased out PFOA and its precursors (EPA, 2021c).
Gustavus, a small community in southeast Alaska, serves as a gateway to the Glacier Bay National Park and Preserve and has a year-round population of 442 people (NPS, 2021). The majority of people in Gustavus obtain their drinking water from private wells that are generally 15–25 feet deep (McDowell, 2021). The Alaska Department of Environmental Conservation (DEC) and Department of Transportation & Public Facilities (DOT & PF) have prioritized Gustavus for PFAS investigation due to the known historical use of AFFFs at the Gustavus Airport and its potential impacts on drinking water (ACAT, 2019). DOT & PF began testing water for PFAS in Gustavus in August 2018 and initial tests showed that 19 Gustavus wells had PFAS concentrations above state action levels of 70 ng/L (the sum of PFOS and PFOA) (McDowell, 2021). As a result of further investigation, water samples from 101 wells, including the Airport Terminal well and the Firehouse well, were collected and analyzed for five PFAS (ACAT, 2019). For about 20% of the analyzed wells, PFAS concentrations were very close to or exceeded the limit of 70 ng/L recommended by the U.S. EPA for PFAS in drinking water (EPA, 2016a). The highest reported concentration of 6,729 ng/L exceeded the U.S. EPA critical level by almost two orders of magnitude (ACAT, 2019).
Here, we have analyzed drinking water samples collected from private homes (and public places) in Gustavus and blood serum samples from their residents for a range of PFAS. The goals of this pilot study were threefold: (1) to understand the overall occurrence of an expanded suite of 39 PFAS in drinking water and serum of Gustavus residents; (2) to estimate total daily intake through consumption of drinking water; and (3) to explore correlations between PFAS levels in water and serum of residents who provided water samples.
2. Materials and Methods
Water Collection.
Twenty-seven well water samples were collected from residences and public spaces in Gustavus, Alaska, during November 2019. Water samples were collected in polypropylene bottles precleaned with water, isopropyl alcohol, and methanol. The water was purged for 15 minutes prior to sample collection. Polypropylene bottles were rinsed twice with sample water, filled, sealed, and shipped to the laboratory on dry ice where they were stored at −20 °C until analysis.
Serum Collection.
Forty serum samples were collected from those Gustavus residents, who provided water from their residences. Participants were recruited via flyers posted in the Gustavus community. Serum samples were drawn by health care providers in the local community clinic. Serum samples were collected into 10 mL BD Vacutainer serum tubes by venipuncture, allowed to clot by leaving undisturbed at room temperature for 30 minutes, and then centrifuged at 2000 rpm for ten minutes to separate serum. The samples were shipped to the laboratory at Indiana University and were stored at −20 °C until analysis.
Information on demographics and drinking water sources was collected from all participants (Table 1). This study was approved by the Indiana University Institutional Review Board and each participant signed an informed consent (assent in case of children) before participation.
Table 1.
Summary of Participants’ Demographic Characteristics.
| parameters | N | percentage, % | |
|---|---|---|---|
| age (years) | <33 | 12 | 30 |
| >33 | 28 | 70 | |
| gender | Male | 17 | 40 |
| Female | 23 | 60 | |
| residence time (years) | <10 | 9 | 23 |
| ≥10 | 31 | 73 | |
| missing | 1 | 4 | |
| Water source | filtered | 18 | 45 |
| unfiltered | 9 | 23 | |
| missing | 13 | 32 | |
Water Analysis.
Water samples (250 mL, thawed at room temperature) were transferred into a new polypropylene bottle, precleaned with water, isopropyl alcohol, and methanol. The samples were fortified with surrogate standards (i.e., mass recovery standards; Table S4) and adjusted to pH = 4 with adding 25 μL of acetic acid. Oasis weak anion-exchange (WAX) cartridges (6 mL, 150 mg, 30 μm) were pre-conditioned with 3 mL of methanol with 0.5% ammonium acetate, 3 mL of methanol, and 3 mL of water with 2% formic acid. The samples were filtered using 0.45 μm glass fiber filters and loaded into 60 mL reservoirs connected to WAX cartridges. The cartridges were allowed to dry completely under vacuum for 10 minutes. Samples were then eluted using 6 mL of 0.5% methanolic ammonium hydroxide. The extracts were concentrated to 200 μL under a gentle stream of N2. Samples were then filtered through 0.2 μm nylon syringe filters (3000 rpm, 5 min) and the final extracts were spiked with isotopically labelled internal standards for instrumental quantitation (Table S1).
Serum Analysis.
Human serum samples (1 mL, thawed at room temperature) were fortified with surrogate standards and ultrasonicated in 4 mL of acetonitrile for 30 minutes. The samples were then centrifuged (3000 rpm, 5 minutes) and the supernatant was transferred into a new tube. These extraction steps were repeated twice, and all the supernatants were combined. The resulting extract was concentrated to ~1 mL using a gentle stream of N2 and diluted with 4 mL of water. WAX cartridges were preconditioned (3 mL, 60 mg, 30 μm) by passing 3 mL of methanol with 0.5% ammonium acetate, 3 mL of methanol, and 3 mL of water with 2% formic acid. Samples were then loaded onto the cartridges, and the cartridges were allowed to dry completely under vacuum for 10 minutes. After washing with 3 mL of water and 2% formic acid, the target compounds were eluted from the cartridge with 3 mL of 0.5% methanolic ammonium hydroxide. Eluted samples were concentrated using N2 and solvent exchanged to 0.5 mL of methanol. The samples were then passed through 0.2 μm nylon filters and the final samples were spiked with isotopically labeled internal standards for instrumental quantitation. The details on standards and reagents used in this study are provided in the Supporting Information.
Instrumental Analysis.
PFAS were analyzed using an ultra-performance liquid chromatograph coupled with a triple-quadrupole mass spectrometer (Agilent 1290 Infinity II UPLC – 6470 QQQ-MS) in the negative electrospray ionization (ESI-) mode. Chromatographic separation was performed on an Acquity UPLC BEH C18 column (50 mm, 2.1 mm i.d., 1.7 μm thickness, Waters, Milford, MA) at 40 °C. Mobile phases consisted of 2 mM ammonium acetate in water (A) and 2 mM ammonium acetate in methanol (B). The gradient was 10% B for 0.5 min initially, ramped to 40% B for 1 min, and then increased to 100% B for 17.5 min. The chromatograph was equilibrated for 3.5 min after every run and the sample injection volume was 5 μL. The nebulizer, gas flow, gas temperature, capillary voltage, sheath gas temperature, and sheath gas flow were set to be 25 psi, 10 L/min, 300 °C, 2800 V, 330 °C, and 11 L/min, respectively. Data acquisition was operated under dynamic multiple reaction monitoring mode. Optimized transition ions are listed in Table S1.
Quality Assurance and Control.
Procedural blanks and matrix spike samples were included in each batch of water and serum samples. Average blank levels were low and constituted only 6% and 7% of average PFAS levels in water and serum samples, respectively. All data were blank corrected by subtracting average blank concentrations from sample concentrations. Method detection limits (MDLs) were set as three times the standard deviation of the target analyte levels detected in blanks. For compounds not detected in blanks, MDLs were based on a signal-to-noise ratio of three. MDLs and average blank concentrations for all analytes are included in Table S2. The absolute matrix spike recoveries ranged from 50 to 144% for target analytes in water and from 30 to 123% for target analytes in serum (Table S3). Surrogate standards were spiked to each sample, and their recoveries ranged from 77 ± 4 to 153 ± 8% (mean ± standard error) in water and from 50 ± 3 to 100 ± 2% in serum samples (Table S4).
Quantification of target compounds was performed by isotope dilution using eight-point calibration curves with concentration ranges of 0.1 – 100 ng/mL. The regression coefficients of linearity tests were all > 0.99.
Data Analysis.
Estimated daily intake (EDI) rates for PFAS via drinking water were calculated as shown in Equation 1:
| (1) |
where EDIDW (ng/kg body weight [bw] /day) is the estimated daily intake via consumption of drinking water, the CDW (ng/L) is the concentration of a chemical in drinking water, DIDW (L/kg bw /day) is the daily average water volume intake per kg of body weight. Values used for DIDW were as follows: 0.011 L/kg bw /day for ages of 9 – 18 years old; 0.012 L/kg bw /day for ages of 19 – 59 years old; and 0.014 L/kg bw /day for ages over 59 years old based on the EPA Exposure Factors Handbook (EPA, 2011).
Plots were generated using Sigma Plot 13 (Systat Software Inc.). Statistical analysis, including Shapiro–Wilk, Mann-Whitney Rank Sum, and Spearman Rank Order Correlation tests, were performed using Sigma Plot 13. Descriptive statistics were computed using Microsoft Excel 2021 (Version 16.56). Concentrations below MDLs were replaced with MDL/2 values for the descriptive statistics and correlation analyses. The significance level was set at p < 0.05.
3. Results and Discussion
Population Characteristics.
A summary of demographic characteristics of the participants is presented in Table 1. Participants ranged in age from 8 to 97 years old (mean 45 ± 4 years) with 78% adults and 22% children. Sixty percent of the participants were female. Seventy three percent of the participants lived in Gustavus for ≥ 10 years. Forty five percent of the participants indicated that they use some type of water filters, 23% stated that they do not use any water filter, while 32% of participants did not provide a response.
PFAS Concentrations in Well Water.
The detection frequencies and median, mean (and their standard errors), minimum, and maximum concentrations for PFAS detected in water samples are provided in Table 2. Twelve PFAS were detected in Gustavus private well water samples and 7 of them were detected in ≥ 40% of the samples. The rest of the PFAS analytes were not detected in any of the samples and are not included in the further discussion.
Table 2.
Detection frequency (DF, %), minimum (min), median, mean (with their standard errors, SE), and maximum (max) concentrations of PFAS in water (ng/L) and serum (ng/mL) samples and the contribution of each individual PFAS compound to ΣPFAS concentrations.
| Residential water (ng/L, n = 25) | Source Zone Water (ng/L, n = 2) | Serum (ng/mL, n = 40) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOT | Airport | |||||||||||||
| compound (carbon chain) | DF | min. | median | mean +/− SE | max. | contr. | concentration | concentration | DF | min. | median | mean ± SE | max. | contr. |
| Short-Chain | Short-Chain | |||||||||||||
| PFPrS (C3) | 48 | <MDL | <MDL | 0.223 ± 0.0667 | 1.19 | 1 | 22.5 | 2.08 | 0 | <MDL | <MDL | <MDL | <MDL | 0 |
| PFBA (C4) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | 597 | 7.27 | 0 | <MDL | <MDL | <MDL | <MDL | 0 |
| PFBS (C4) | 80 | <MDL | 0.394 | 0.767 ± 0.174 | 3.16 | 3 | 91.8 | 4.2 | 90 | <MDL | 0.167 | 0.0557 ± 0.0074 | 0.192 | 1 |
| PFPeA (C5) | 20 | <MDL | <MDL | 0.674 ± 0.339 | 6.50 | 2 | 2,940 | 14.3 | 83 | <MDL | 0.0353 | 0.317 ± 0.0447 | 1.40 | 3 |
| PFPeS (C5) | 52 | <MDL | 0.059 | 0.743 ± 0.234 | 4.18 | 3 | 117 | 3.05 | 88 | <MDL | 0.238 | 0.0555 ± 0.0138 | 0.502 | 1 |
| PFHxA (C6) | 20 | <MDL | <MDL | 9.84 ± 1.09 | 28.4 | 12 | 3,240 | 28.9 | 15 | <MDL | <MDL | 0.00984 ± 0.00109 | 0.0325 | 0 |
| PFHxS (C6) | 40 | <MDL | <MDL | 4.38 ± 1.52 | 28.6 | 16 | 671 | 17.4 | 95 | <MDL | 1.17 | 2.46 ± 0.433 | 13.1 | 26 |
| 4:2 FTS (C6) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | 4.63 | <MDL | 5 | <MDL | <MDL | <MDL | 0.0393 | 3 |
| PFHpA (C7) | 24 | <MDL | <MDL | 0.949 ± 0.293 | 6.38 | 4 | 332 | <MDL | 93 | <MDL | 0.0267 | 0.0472 ± 0.00907 | 0.258 | 0 |
| PFHpS (C7) | 40 | <MDL | <MDL | 0.294 ± 0.102 | 1.76 | 1 | 98 | 1.56 | 100 | 0.018 | 0.150 | 0.256 ± 0.0366 | 1.07 | 3 |
| PFOS (C8) | 40 | <MDL | <MDL | 14.9 ± 5.87 | 120 | 55 | 6,300 | 146 | 100 | 0.278 | 3.38 | 3.78 ± 0.348 | 9.04 | 40 |
| PFOA (C8) | 48 | <MDL | <MDL | 0.636 ± 0.203 | 3.17 | 2 | 89.4 | <MDL | 95 | <MDL | 0.975 | 1.11 ± 0.107 | 3.69 | 12 |
| PFECHS (C8) | 20 | <MDL | <MDL | 0.0326 ± 0.0154 | 0.358 | 0 | 0.372 | <MDL | 43 | <MDL | <MDL | 0.0105 ± 0.00159 | 0.0452 | 0 |
| FOSA (C8) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | <MDL | <MDL | 58 | <MDL | 0.0163 | 0.0244 ± 0.00479 | 0.159 | 0 |
| PFNA (C9) | 8 | <MDL | <MDL | 0.0189 ± 0.00964 | 0.188 | 0 | 21.1 | <MDL | 100 | 0.026 | 0.521 | 0.553 ± 0.0446 | 1.32 | 6 |
| PFDA (C10) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | <MDL | <MDL | 100 | 0.017 | 0.240 | 0.258 ± 0.0201 | 0.548 | 3 |
| PFUdA (C11) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | <MDL | <MDL | 90 | <MDL | 0.143 | 0.185 ± 0.0189 | 0.472 | 2 |
| PFDoA (C12) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | <MDL | <MDL | 58 | <MDL | 0.034 | 0.0362 ± 0.00346 | 0.0824 | 0 |
| PFTrDA (C13) | 0 | <MDL | <MDL | <MDL | <MDL | 0 | <MDL | <MDL | 28 | <MDL | <MDL | 0.0290 ± 0.00260 | 0.0876 | 0 |
| ΣPFAS | <MDL | <MDL | 2.16 ± 1.08 | 120 | 100 | 14,600 | 228 | 0.017 | 0.0823 | 0.520 ± 0.204 | 13.1 | 100 | ||
MDL: method detection limit.
Total PFAS concentrations in residential water samples (ΣPFAS, the sum of 12 detected PFAS concentrations) ranged from not detected (n.d.) to 120 ng/L. Perfluoropropane sulfonic acid (PFPrS), perfluoro-1-butanesulfonic acid (PFBS), perfluoropentanesulfonic acid (PFPeS), and PFOA were frequently detected (48–80% of the samples) but measured at relatively low concentrations and only contributed ≤ 3% to the ΣPFAS concentrations. PFOS, PFHxS, and perfluorohexanoic acid (PFHxA) were less frequently detected although at higher concentrations. PFOS, PFHxS, and PFHxA were among the top contributors and constituted 55%, 16%, and 12% of the ΣPFAS concentrations, respectively. The remaining compounds were either detected less frequently or were less abundant.
PFAS Concentrations in Public Water.
Two water samples had elevated ΣPFAS concentrations of 14,600 and 228 ng/L (Table 2). These samples were collected from the Department of Transportation (DOT) and the Alaska Seaplanes terminal at the airport in Gustavus, Alaska, respectively. Overall, PFOS was the predominant compound (6,300 ng/L) in the DOT sample, followed by PFHxA (3,240 ng/L), perfluoropentanoic acid (PFPeA, 2,940 ng/L), and PFHxS (671 ng/L). The same PFAS compounds (PFOS at 146 ng/L, PFHxS at 28.9 ng/L, PFHxS at 17.4 ng/L, and PFPeA at 14.3 ng/L) were detected in the sample from the Gustavus airport (Table 2). PFOS and PFHxS were used as additives in legacy first generation AFFFs (D’Agostino and Mabury, 2014; Lin et al., 2021), and may also form from precursors from other foam components (Buck et al., 2011; Houtz et al., 2013; Rotander et al., 2015). Detection of elevated PFAS levels in water samples from these public facilities can be explained by the historical use of AFFFs, which resulted in nearby groundwater contamination (ACAT, 2019; McDowell, 2021; Rotander et al., 2015).
Our study confirms PFAS levels and congener patterns reported from previous investigations (DOT&PF, 2021; S&W, 2019). DOT & PF evaluated the potential for human exposure to PFAS contamination in Gustavus water supply wells during 2019 (S&W, 2020). Water samples were collected from private wells (unidentified) as well as the Gustavus airport and the results are generally consistent with our analysis. For example, the results from one sample collected at the airport in March 2019 revealed concentrations comparable to those found for airport sample in this study: PFOS at 270 ng/L, PFHxS at 30 ng/L, and PFBS at 4.3 ng/L (DOT&PF, 2021; S&W, 2019). However, in contract with our study, PFHxA and PFOA were not detected in this sample, and PFPeA was not analyzed.
Comparison of Drinking Water Concentrations.
The ΣPFAS concentrations detected in residential drinking water samples in this pilot study were similar to those measured across 5,000 public waterworks in the U.S. from 2013 to 2015 (25 to 180 ng/L) (Guelfo and Adamson, 2018). The levels of PFAS detected in public drinking water samples collected near the source zone (n = 2) were comparable to source zone levels reported in similar studies (McDonough et al., 2021; Pitter et al., 2020; Xu et al., 2021). Xu et al. (2021) assessed PFAS levels in contaminated drinking water in Sweden due to nearby fire training zones. Concentrations of PFOS ranged from n.d. to 8,000 ng/L in background and source zone waterworks, respectively (Xu et al., 2021). Similarly, Pitter et al. (2020) reported a maximum PFOS concentration of 1,480 ng/L in private wells impacted by a PFAS manufacturing plant.
PFAS at levels above the U.S. EPA lifetime health advisory have been correlated with fire training areas, industrial manufacturing, and wastewater treatment plants (Andrews and Naidenko, 2020). Overall, our findings show that, while PFAS concentrations in most of Gustavus residential wells are within the EPA’s non-regulatory lifetime health advisory of 70 ng/L, ~12% of the water samples exceed this level. Protective measures should be taken to prevent additional risks associated with elevated PFAS exposures (EPA, 2021d). Several states have established more stringent and enforceable drinking water standards based on scientific conclusions that the U.S. EPA health advisory levels are insufficiently protective (Hu et al., 2016; Post, 2021).
Estimated Daily Intake (EDI) from Drinking Water.
The PFAS EDIs for residents through the intake of drinking water are presented in Table 3. The EDIs increased with age because of the increased water consumption per body weight. The highest ΣPFAS EDI was found for residents between the ages of 60 – 97 years (0.310 ng/kg bw/day), followed by 19 – 59 years old (0.266 ng/kg bw/day), and 8 – 18 years old (0.244 ng/kg bw/day). The EDIs from this pilot study were lower than the U.S. EPA Reference Dose for PFOS (20 ng/kg bw/day) and the Agency for Toxic Substances and Disease Registry’s (ATSDR) intermediate oral Minimal Risk Level (MRL) for PFOS (2 ng/kg bw/day) (ATSDR, 2021; EPA, 2016b; Post, 2021). However, the EDI values for drinking water determined here were more comparable to the European Food Safety Authority’s (EFSA) Tolerable Daily Intake for sum of PFOA, PFNA, PFHxS, and PFOS (0.63 ng/kg bw/day) (EFSA, 2020). The U.S. EPA recently released draft health-based levels for PFAS in drinking water which are significantly more stringent than current standards (EPA, 2021b).
Table 3.
Estimated daily intakes (EDIs, ng/ kg body weight [bw]/day) for PFAS through consumption of drinking water (ng/kg bw/day). Only individual PFAS with detection frequency ≥40% were included in the EDI calculation.
| age, years | |||
|---|---|---|---|
| 8 – 18 | 19 – 59 | 60 – 97 | |
| PFPrS | 0.002 | 0.003 | 0.003 |
| PFBS | 0.008 | 0.009 | 0.011 |
| PFPeS | 0.008 | 0.009 | 0.010 |
| PFHxS | 0.048 | 0.053 | 0.061 |
| PFHpS | 0.003 | 0.003 | 0.004 |
| PFOA | 0.007 | 0.008 | 0.009 |
| PFOS | 0.164 | 0.179 | 0.209 |
| ΣPFAS | 0.244 | 0.266 | 0.310 |
PFAS Concentrations in Serum.
Table 2 includes the results of the descriptive statistics for the 17 PFAS detected in Gustavus serum samples. Of these 17, a total of 14 PFAS were detected in at least 40% of the samples. ΣPFAS concentrations (the sum of 17 detected PFAS concentrations) ranged from 0.017– 13.1 ng/mL (median 0.0823 ng/mL). Overall, PFOS was the most abundant PFAS detected in all the samples with a median of 3.38 ng/mL and contributed ~40% to ΣPFAS concentrations, similarly to the water samples. PFHxS and PFOA were also abundant and detected in 95 and 100% of the samples at median concentrations of 1.17 and 0.975 ng/mL, respectively. These two compounds contributed 26% and 12% to ΣPFAS concentrations, respectively. Strong positive Spearman correlation between PFOS and PFHxS serum concentrations (n = 40; r = 0.646; p < 0.0001) suggests that these compounds have a common exposure source.
Rotander et al. (2015) also reported PFOS and PFHxS as the most abundant PFAS measured in serum samples collected from firefighters with past AFFF exposure in Australia. Similar results were reported demonstrating PFOS and PFHxS as the most abundant targeted PFAS detected in serum samples of participants exposed to AFFF-contaminated drinking water (Barton et al., 2020; McDonough et al., 2021; Xu et al., 2021). Xu et al. (2021) found elevated PFAS serum levels in residents of Ronneby, Sweden, with long-term exposure to AFFF-contaminated drinking water. Xu et al. (2021) reported population geometric means for PFOS, PFOA, and PFHxS in serum were 135, 6.8, and 114 ng/mL, respectively. A neighboring city with uncontaminated drinking water served as a reference group and the population geometric means for PFOS, PFOA, and PFHxS in serum were 3.9, 1.5, and 0.84 ng/mL, respectively (Xu et al., 2021). The concentrations found in these two locations were higher than those found in our study. In addition, serum PFAS data collected in the U.S. National Health and Nutrition Examination Survey (NHANES) between 2015 and 2016 was compared to Gustavus results (https://wwwn.cdc.gov/nchs/nhanes/ accessed on December 21, 2021). The median serum levels of PFOS, PFOA, and PFHxS in NHANES (n = 1993) were 4.80, 1.57, and 1.20 ng/mL, respectively (CDC, 2019) and were generally comparable to the levels found in Gustavus residents (CDC, 2019; Graber et al., 2021; Moon, 2021).
PFAS Patterns in Serum and Water.
Figure 1 compares the individual contributions of 11 PFAS compounds to the ΣPFAS concentrations measured in ≥ 20% of both water and serum samples. Similarities in the PFAS profiles between the public and residential water and serum suggest a common source: PFOS and PFHxS were the two most abundant PFAS found in all three sample groups. PFHxA had comparable contributions to the ΣPFAS concentrations in well water (12%) and source water (22% for DOT and 13% for Airport). In contrast, PFPeA (C5) contributed 20% and 6% to the ΣPFAS concentrations in the DOT and Airport samples, respectively, but only 2% to the residential well water. The contributions of PFOS were similar in water and serum samples; however, serum samples showed greater contributions of PFHxS and PFOA. Serum had lower contributions from the short-chain PFAS, which is likely due to their shorter half-lives in human body (Jian et al., 2018; Zhang et al., 2013). Xu et al. (2020) found similar results in serum and drinking water samples from participants exposed to AFFF-contaminated water in Sweden. Spearman’s correlation analysis shows a significant positive correlation (r = 0.495; p = 0.0192) between the sum of the three most abundant PFAS compounds in paired water and serum samples (PFOS, PFOA, and PFHxS). These results suggest that drinking water is an important contributor to the PFAS body burden in Gustavus residents.
Figure 1.
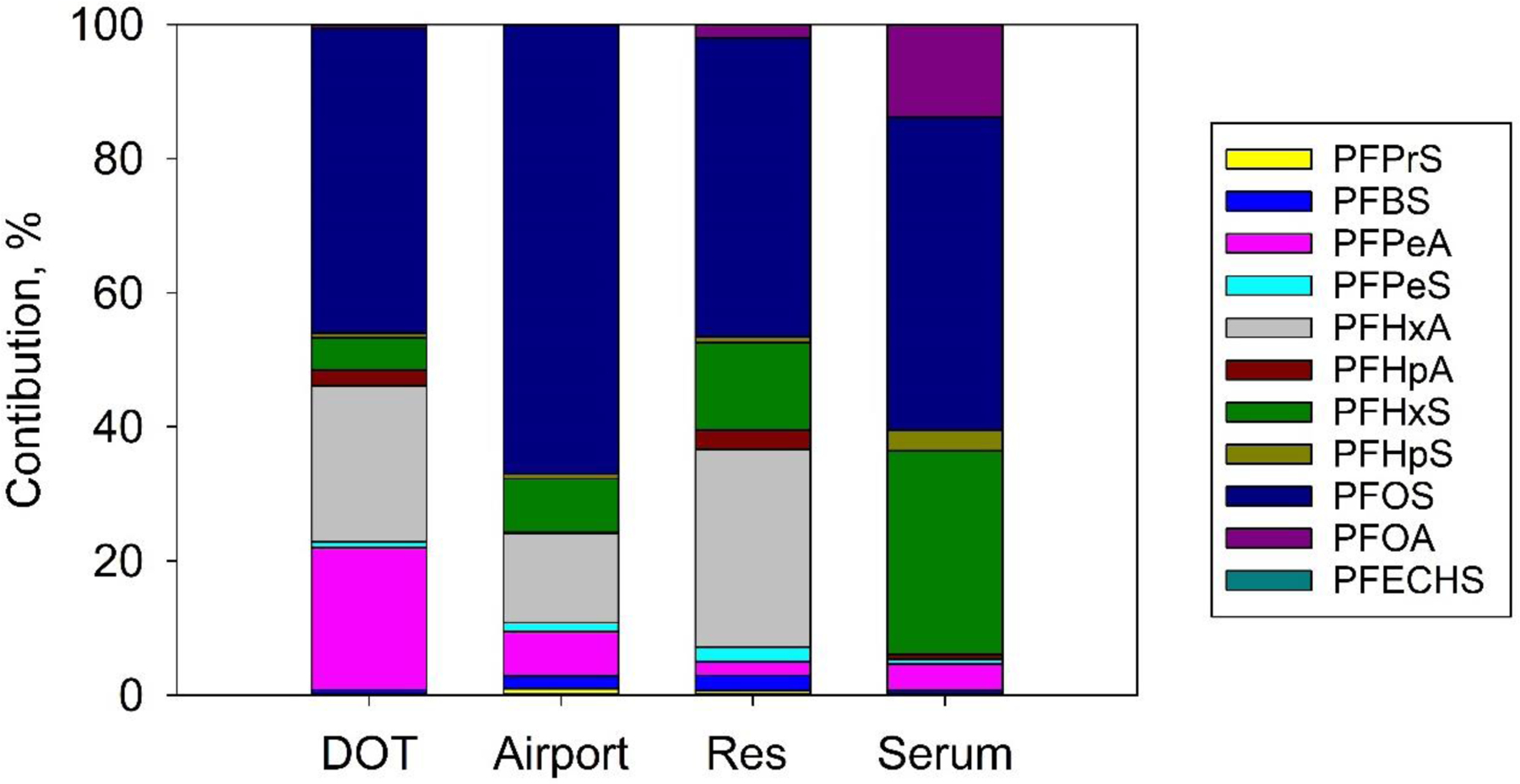
Contributions (%) of individual PFAS to the ΣPFAS concentrations in residential and public water (DOT and Airport) and serum samples collected from Gustavus, Alaska. Only compounds with detection frequency of ≥ 20% in both water and sera were included.
To further investigate the effect of the drinking water source on the PFAS body burden, serum samples were divided into two groups based on the source of drinking water indicated in the survey. The first group included those drinking from private wells and the second group included residents with alternate drinking sources, including bottled water, which started in 2018 when the source was initially identified. While there were no statistical differences among these groups based on the Mann-Whitney results (p = 0.659), the median level for the group drinking well water was higher than residents with alternate drinking water sources (7.89 ng/mL vs 5.46 ng/mL) (Figure 2). The lack of statistical difference may also be explained by the slow decline of PFAS levels in residents who have historically used well water but have switched to alternate water sources due to water contamination.
Figure 2.
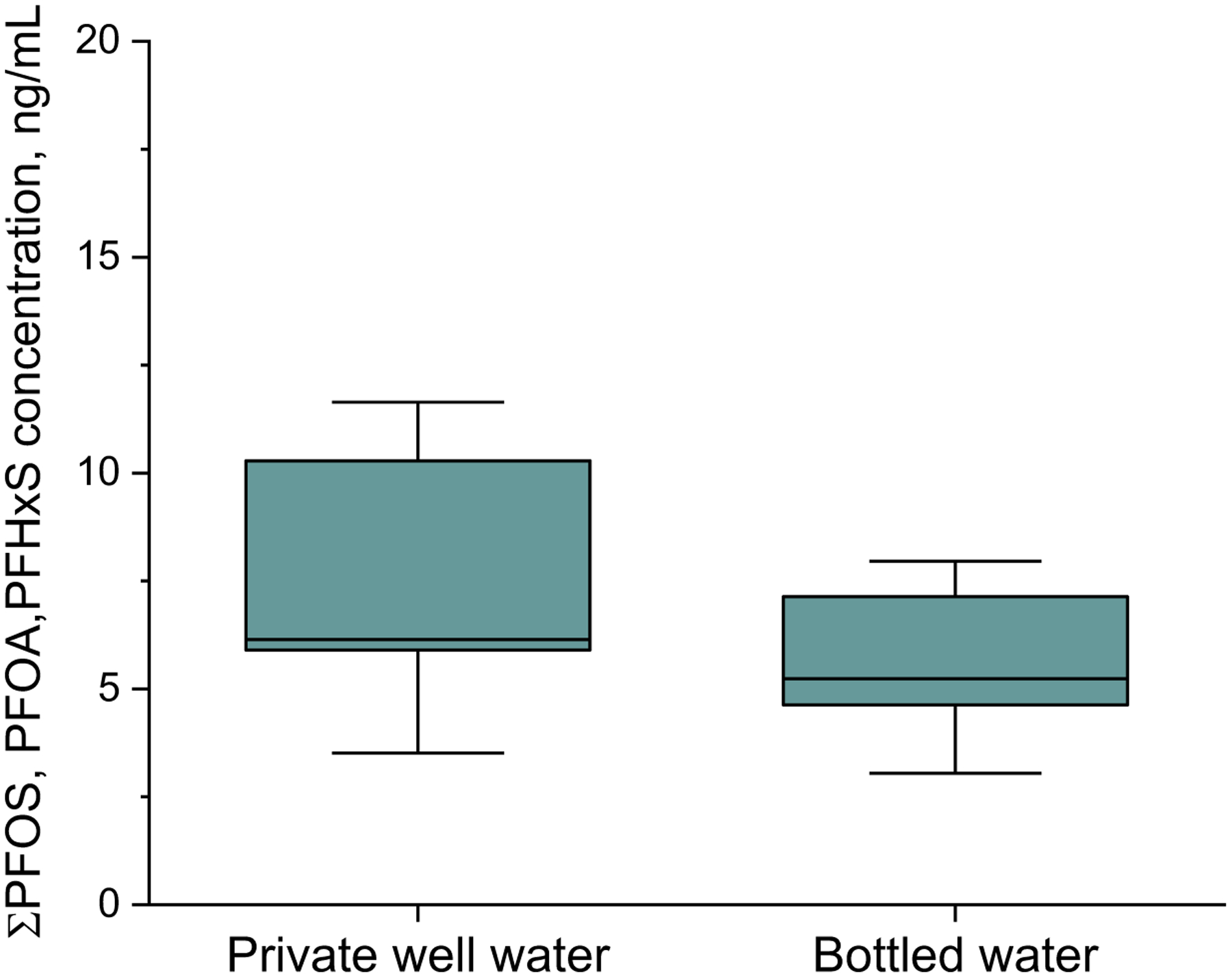
Total PFAS concentrations in human sera separated into two groups based on drinking water source: private well water and bottled water. The boxes represent the means with their standard errors, and the whiskers represent the 25th and 75th percentiles. The line inside each box represents the median.
4. Conclusions
Overall, this pilot study found extremely elevated levels of several PFAS in water samples collected near the airport in Gustavus, Alaska, and confirms this location as a significant source of PFAS. In total, up to seventeen PFAS were detected in paired residential water and serum samples collected from the Gustavus households. PFOS, PFOA, PFHxS, and PFHxA were the most abundant compounds in these samples and comprised up to ~80% of the ΣPFAS concentrations. A similarity of the PFAS distribution profile between the samples collected by the source and residential water suggests that contamination in private wells sampled in this study has likely originated from the airport. In addition, a significant correlation between the levels of select PFAS in paired drinking water and serum samples suggests drinking water as an important source contributing to body burden of PFAS in Gustavus residents. We cannot assess whether Gustavus residents’ exposure to PFAS has or will result in adverse health effects, however it is critical to take precautionary measures to prevent further exposures. In addition, it is also important to conduct regular water and serum testing, and to make medical screening available to affected individuals. Medical monitoring can discern any early signs of disease that might be associated with PFAS exposure, lead to earlier protective interventions, and reduce the effects of exposure.
Supplementary Material
Highlights.
The presence of a significant PFAS source near Gustavus, Alaska, was confirmed.
PFOS and PFHxS were most abundant in Gustavus resident serum and well water.
PFAS concentrations in serum and well water were positively associated.
Acknowledgments
The authors thank the community of Gustavus and participants for their generosity in sharing their time, water samples, and serum for this study. A.S. was supported by National Institute of Environmental Health Science R01 2R01ES019620-06A1. Sample collection was supported by funding from the Cedar Tree Foundation and the Groundswell Catalyst Fund. We would also like to thank the Gustavus Clinic for graciously providing space and assistance with blood draws.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Chemicals and reagents, details of analytical methods, and quality control measures.
References
- 3M, 1999. Fluorochemical use, Distribution and Release Overview. USEPA Administrative Record AR 226–0550. [Google Scholar]
- 3M, 2000a. Sulfonated Perfluorochemicals in the Environment: Sources; Dispersion, Fate and Effects. [Google Scholar]
- 3M, 2000b. Voluntary Use and Exposure Information Profile for Perfluorooctanoic Acid and Salts. USEPA Administrative Record AR226–0595. [Google Scholar]
- ACAT, 2019. Alaska Community Action on Toxics (ACAT): Threats to Drinking Water nad Public Health in Alaska. [Google Scholar]
- AMAP, 2017. Assessment 2016: Chemicals of Emerging Arctic Concern. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. [Google Scholar]
- Andrews DQ, Naidenko OV, 2020. Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environmental Science & Technology Letters 7, 931–936. [Google Scholar]
- Armitage JM, Macleod M, Cousins IT, 2009. Comparative assessment of the global fate and transport pathways of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environmental Science & Technology 43, 5830–5836. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2021. Toxicological profile for perfluoroalkyls, Atlanta, GA, USA. [PubMed] [Google Scholar]
- Banzhaf S, Filipovic M, Lewis J, Sparrenbom CJ, Barthel R, 2017. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). Ambio 46, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM, Adgate JL, 2020. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. International Journal of Hygiene and Environmental Health 223, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA, 2005. Perfluorochemicals: Potential sources of and migration from food packaging. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 22, 1023–1031. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT, 2010. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 408, 2936–2965. [DOI] [PubMed] [Google Scholar]
- Canada.ca, 2021. Government of Canada Health Science Summary: Long-chain perfluorocarboxylic acids (PFCAs), their salts and related compounds. [Google Scholar]
- CDC, 2019. U.S. Department of Health and Human Services and Centers for Disease Control and Prevention: Fourth National Report on Human Exposure to Environmental Chemicals. [Google Scholar]
- D’Agostino LA, Mabury SA, 2014. Identification of novel fluorinated surfactants in aqueous film forming foams and commercial surfactant concentrates. Environmental Science & Technology 48, 121–129. [DOI] [PubMed] [Google Scholar]
- DOT&PF, 2021. Alaska Department of Transportation and Public Facilities (DOT & PF): Gustavus Airport Firefighting Testing Area Contamination, https://dot.alaska.gov/airportwater/gustavus/.
- EFSA, 2020. European Food Safety Authority (EFSA): Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 18, e06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 2006. DuPont 2006 order on consent. http://www.epa.gov/region03/enforcement/dupont_order.pdf.
- EPA, 2011. Exposure Factors Handbook 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- EPA, 2016a. Drinking Water Health Advisories for PFOA and PFOS; https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos. [DOI] [PubMed]
- EPA, 2016b. Drinking water health advisory for perfluorooctane sulfonate (PFOS). EPA 822‐R‐16‐004, Washington, DC. [Google Scholar]
- EPA, 2020. CompTox Chemicals Dashboard PFASMASTER Chemicals (epa.gov). [Google Scholar]
- EPA, 2021a. Actions to Address PFAS (epa.gov). [Google Scholar]
- EPA, 2021b. Agenda EPA Science Advisory Board PFAS Review Panel Public Meeting, https://sab.epa.gov/ords/sab/f?p=100:19:31941373910133:::19:P19_ID:963.
- EPA, 2021c. Launches Stewardship Program to Reduce PFAS in the Marketplace (epa.gov). [Google Scholar]
- EPA, 2021d. PFAS Drinking Water Health Advisories (epa.gov). [Google Scholar]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, Smith JS, Roberts SM, 2021. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ Toxicol Chem 40, 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K, 2001. Global distribution of perfluorooctane sulfonate in wildlife. Environmental Science & Technology 35, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Graber JM, Black TM, Shah NN, Caban-Martinez AJ, Lu S. e., Brancard T, Yu CH, Turyk ME, Black K, Steinberg MB, Fan Z, Burgess JL, 2021. Prevalence and Predictors of Per- and Polyfluoroalkyl Substances (PFAS) Serum Levels among Members of a Suburban US Volunteer Fire Department. International Journal of Environmental Research and Public Health 18, 3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelfo JL, Adamson DT, 2018. Evaluation of a national data set for insights into sources, composition, and concentrations of per-and polyfluoroalkyl substances (PFASs) in US drinking water. Environmental Pollution 236, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KJ, Clemen LA, Ellefson ME, Johnson HO, 2001. Compound-specific, quantitative characterization of organic: Fluorochemicals in biological matrices. Environmental Science & Technology 35, 766–770. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM, 2011. Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid (PFOA) in Communities Surrounding a Fluoropolymer Production Facility. Environmental Health Perspectives 119, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz EF, Higgins CP, Field JA, Sedlak DL, 2013. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environmental science & technology 47, 8187–8195. [DOI] [PubMed] [Google Scholar]
- Hu XDC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM, 2016. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in US Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environmental Science & Technology Letters 3, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J-M, Chen D, Han F-J, Guo Y, Zeng L, Lu X, Wang F, 2018. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Science of The Total Environment 636, 1058–1069. [DOI] [PubMed] [Google Scholar]
- Kissa E, 1994. Fluorinated Surfactants in Blood. Journal of Fluorine Chemistry 66, 5–6. [Google Scholar]
- Kissa E, 2001. Fluorinated Surfactants and Repellents, Second ed. Marcel Dekker, Inc., New York. [Google Scholar]
- Lin Y, Capozzi SL, Lin L, Rodenburg LA, 2021. Source apportionment of perfluoroalkyl substances in Great Lakes fish. Environmental Pollution 290, 118047. [DOI] [PubMed] [Google Scholar]
- McDonough CA, Choyke S, Barton KE, Mass S, Starling AP, Adgate JL, Higgins CP, 2021. Unsaturated PFOS and Other PFASs in Human Serum and Drinking Water from an AFFF-Impacted Community. Environmental Science & Technology 55, 8139–8148. [DOI] [PubMed] [Google Scholar]
- McDowell, 2021. Gustavus PFAS Action Coalition: History, Accomplishments, and Next Steps [Google Scholar]
- Moon J, 2021. Perfluoroalkyl substances (PFASs) exposure and kidney damage: Causal interpretation using the US 2003–2018 National Health and Nutrition Examination Survey (NHANES) datasets. Environmental Pollution 288, 117707. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, Lindstrom AB, 2007. Perfluorinated compounds in the Cape Fear Drainage Basin in North Carolina. Environmental Science & Technology 41, 5271–5276. [DOI] [PubMed] [Google Scholar]
- Nash SB, Rintoul SR, Kawaguchi S, Staniland I, van den Hoff J, Tierney M, Bossi R, 2010. Perfluorinated compounds in the Antarctic region: Ocean circulation provides prolonged protection from distant sources. Environmental Pollution 158, 2985–2991. [DOI] [PubMed] [Google Scholar]
- NPS, 2021. National Park Service (NPS): Gustavus, https://www.nps.gov/glba/planyourvisit/gustavus.htm.
- Oliaei F, Kriens D, Weber R, Watson A, 2013. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res Int 20, 1977–1992. [DOI] [PubMed] [Google Scholar]
- Pitter G, Da Re F, Canova C, Barbieri G, Zare Jeddi M, Daprà F, Manea F, Zolin R, Bettega AM, Stopazzolo G, 2020. Serum levels of Perfluoroalkyl substances (PFAS) in adolescents and young adults exposed to contaminated drinking water in the Veneto region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environmental Health Perspectives 128, 027007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, 2021. Recent US State and Federal Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances. Environmental Toxicology and Chemistry 40, 550–563. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH, 2006. Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology 40, 32–44. [DOI] [PubMed] [Google Scholar]
- Rotander A, Toms L-ML, Aylward L, Kay M, Mueller JF, 2015. Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environment International 82, 28–34. [DOI] [PubMed] [Google Scholar]
- S&W, 2019. Shannon & Wilson, Inc., 2019, Summary report, August 2018 to November 2018 private well sampling, Gustavus, Alaska: Report prepared by Shannon & Wilson, Inc., Fairbanks, Alaska, 101543–001, for Alaska Department of Administration’s Division of Risk Management, Juneau, Alaska, March. [Google Scholar]
- S&W, 2020. Shannon & Wilson, Inc., 2020, Final Summary report, December 2018 to November 2019 Water Supply Sampling. Gustavus, Alaska: Report prepared by Shannon & Wilson, Inc., Fairbanks, Alaska, for Alaska Department of Transportation and Public Facilities, Fair-banks, Alaska, August. [Google Scholar]
- Schultz MM, Barofsky DF, Field JA, 2003. Fluorinated alkyl surfactants. Environmental Engineering Science 20, 487–501. [Google Scholar]
- Stubleski J, Salihovic S, Lind L, Lind PM, van Bavel B, Karrman A, 2016. Changes in serum levels of perfluoroalkyl substances during a 10-year follow-up period in a large population-based cohort. Environment International 95, 86–92. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, Pickett A, Smith C, Knappe DRU, 2016. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environmental Science & Technology Letters 3, 415–419. [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG, 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves DR, 1968. Evidence That There Are 2 Forms of Fluoride in Human Serum. Nature 217, 1050-&. [DOI] [PubMed] [Google Scholar]
- UNEP, 2022. United Nations Environment Programme (UNEP): Persistent Organic Pollutants Review Committee: Call for information and follow-up to the seventeenth meeting of the Persistent Organic Pollutants Review Committee. [Google Scholar]
- Vestergren R, Cousins IT, 2009. Tracking the Pathways of Human Exposure to Perfluorocarboxylates. Environmental Science & Technology 43, 5565–5575. [DOI] [PubMed] [Google Scholar]
- Wang ZY, DeWitt JC, Higgins CP, Cousins IT, 2017. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental Science & Technology 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- Xu Y, Fletcher T, Pineda D, Lindh CH, Nilsson C, Glynn A, Vogs C, Norström K, Lilja K, Jakobsson K, Li Y, 2020. Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ Health Perspect 128, 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nielsen C, Li Y, Hammarstrand S, Andersson EM, Li H, Olsson DS, Engström K, Pineda D, Lindh CH, Fletcher T, Jakobsson K, 2021. Serum perfluoroalkyl substances in residents following long-term drinking water contamination from firefighting foam in Ronneby, Sweden. Environment International 147, 106333. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Taniyasu S, Petrick G, Wei S, Gamo T, Lam PK, Kannan K, 2008. Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 70, 1247–1255. [DOI] [PubMed] [Google Scholar]
- Yuan GX, Peng H, Huang C, Hu JY, 2016. Ubiquitous Occurrence of Fluorotelomer Alcohols in Eco-Friendly Paper-Made Food-Contact Materials and Their Implication for Human Exposure. Environmental Science & Technology 50, 942–950. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW, 2013. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life. Environmental Science & Technology 47, 10619–10627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


