Abstract
Mannitol, a six-carbon sugar alcohol, is the main storage carbon in the button mushroom, Agaricus bisporus. Given the physiological importance of mannitol metabolism in growth, fruit body development, and salt tolerance of A. bisporus, the enzyme responsible for mannitol biosynthesis, NADP-dependent mannitol dehydrogenase (MtDH) (EC 1.1.1.138), was purified to homogeneity, and MtDH cDNA was cloned, sequenced, and characterized. To our knowledge, this represents the first report on the isolation of a cDNA encoding an NADP-dependent mannitol dehydrogenase. The MtDH cDNA contains an open reading frame of 789 bp encoding a protein of approximately 28 kDa. The N-terminal and internal amino acid sequences of the deduced protein exactly matched the ones determined from the purified MtDH subunit, whereas the amino acid composition of the deduced protein was nearly identical to that of the purified MtDH. The MtDH cDNA showed high homology with a plant-induced short-chain dehydrogenase from Uromyces fabae. Phylogenetic analysis based on amino acid sequences from mannitol(-1-phosphate) dehydrogenases indicated a close relationship between the substrate specificity of the enzymes and phylogenetic differentiation. Salt-stressed fruit bodies showed an overall increase in mannitol biosynthesis, as was evident from the increase in MtDH activity, MtDH abundance, and MtDH RNA accumulation. Furthermore, the MtDH transcript level seems to be under developmental control, as MtDH RNA accumulated during maturation of the fruit body.
The button mushroom, Agaricus bisporus, is the most important cultivated edible mushroom. Its cultivation, trade, and processing represent important economic activities, especially in the European Community and the United States. Due to the low degree of genetic variation in commercial strains (5), it is increasingly difficult to obtain strains that meet specific demands for the market of fresh or processed products. The production of improved strains by conventional breeding methods has been limited due to the aberrant life cycle of A. bisporus, which usually generates heterokaryotic spores with two parental nuclei that show almost no genetic exchange (46). Recently, a transformation procedure was developed for A. bisporus which allows for the production of transgenic mushrooms with altered characteristics (28, 47, 48). This biotechnological tool will certainly contribute to improvements in A. bisporus breeding programs, provided that information on genes involved in central metabolic processes becomes available.
In A. bisporus, mannitol is the main storage carbon, where it can contribute up to 20% of the mycelium dry weight and up to 50% of the fruit body dry weight (34). Mannitol is the most abundant sugar alcohol in nature, occurring in bacteria, algae, lichens, fungi, and many vascular plants (20, 43). Mannitol has been reported to accumulate in response to environmental stresses such as salt stress (20, 41). Recent evidence with plants and bacteria has shown that mannitol can act as a compatible solute, a compound that can accumulate in the cytosol and prevent inactivation of metabolic processes (23, 41). In fungi, mannitol’s function as an osmoregulatory compound might also be critical in providing an influx of water from the environment to support turgor and fruit body development (18, 20). Other physiological roles have been postulated for mannitol in fungi, including service as the main and most efficient respiratory source during postharvest development and fruit body senescence (13). Similarly, for celery suspension cultures, the conversion of mannitol to cell dry weight was 27% more efficient than the conversion of sucrose (33). The advantage of mannitol catabolism in plants and fungi may be explained by the fact that NAD(P)H is produced during mannitol oxidation and can then be indirectly shuttled into the mitochondrion and converted to ATP, resulting in a more efficient system than that in organisms that do not metabolize mannitol. Mannitol metabolism may also play an important role in the recycling of reductants (NADPH and NADP). NADP produced during mannitol synthesis becomes available for the oxidative reactions of the pentose phosphate shunt, which are controlled by the NADP/NADPH ratio, suggesting that mannitol synthesis has a growth-regulating function (9).
In A. bisporus, mannitol is synthesized from fructose by a reaction catalyzed by NADP-dependent mannitol dehydrogenase (MtDH) (EC 1.1.1.138). Although the enzyme has been purified previously (10, 29, 36), the MtDH cDNA has never been identified. Little is known about the regulation of MtDH in vivo. It has been postulated that MtDH activity, and thus mannitol synthesis, results in the reoxidation of NADPH generated in the pentose phosphate pathway, thereby allowing a vast conversion of glucose-6-phosphate into building blocks for DNA and proteins (36). A coordinated regulation of mannitol synthesis and the pentose phosphate pathway might also be involved in fruit body initiation, as both MtDH and glucose-6-phosphate dehydrogenase activities increase at the onset of fruit body formation (15, 16).
This report describes the isolation, sequencing, and characterization of the MtDH cDNA of A. bisporus. The results show that MtDH plays an important role in salt tolerance and fruit body development. The identification of MtDH cDNA and the recently developed transformation system will allow, for the first time, production of transgenic mushrooms with altered mannitol metabolism.
MATERIALS AND METHODS
Chemicals and mushroom cultivation.
Biochemicals were purchased from Sigma. Acrylamide, bisacrylamide, protein assay reagent, and DEAE–Bio-Gel-A agarose were obtained from Bio-Rad. Prestained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) molecular mass standards were from New England Biolabs. MiniS PC3.2/3, Superdex 200 PC3.2/3.0, and gel filtration molecular mass standards were obtained from Pharmacia. Alkaline phosphatase-linked anti-immunoglobulin G was purchased from Promega, as were chemicals for visualization. All buffers used for fast protein liquid chromatography were degassed with helium, filtered through 0.22-μm-pore-size MF-Millipore type GS membranes, and adjusted to their respective pHs at 25°C. A. bisporus U1 was purchased from Amycel (Ittervoort, The Netherlands), and mycelia were plated and maintained on MMP agar medium (1% malt extract, 0.5% mycological peptone, 1.5% agar). A. bisporus fruit bodies (Horst U1) were grown in plastic containers by using a commercial compost and casing layer (Mushroom Experimental Station, Horst, The Netherlands). For the salt stress study, A. bisporus U1 was grown in commercial compost topped with a casing layer saturated with water (0 mM NaCl control) or 150 mM NaCl.
MtDH purification.
All purification procedures were carried out at 4°C. Mushroom fruit bodies were harvested at stage 2 (14), sliced, frozen in liquid nitrogen, and stored at −80°C until used for extraction. Tissue was ground with a mortar and pestle, using a 1:4 tissue-to-buffer ratio. The extraction buffer contained 50 mM MOPS (morpholine propanesulfonic acid) (pH 7.5), 5 mM dithiothreitol (DTT), and 1 mM EDTA. Homogenates were centrifuged at 20,000 × g for 20 min at 4°C. Supernatant fractions were pooled and designated the crude extract. The crude extract was brought to 45% saturation with (NH4)2SO4, gently stirred for 1 h, and centrifuged at 20,000 × g for 20 min. The supernatant was retained, brought to 80% saturation by further addition of (NH4)2SO4, and stirred for an additional 1 h. After centrifugation as described above, the supernatant was discarded and the pellet was suspended in a minimum volume of 50 mM MOPS (pH 7.5) and 1 mM DTT. The dissolved pellet was applied at 1 ml/min to a DEAE–Bio-Gel-A agarose ion-exchange column (2.5 by 40 cm; 80-ml bed volume) equilibrated with 50 mM MOPS (pH 7.5)–5 mM DTT. The column was connected to a fast protein liquid chromatography system (Pharmacia) and washed with 50 mM MOPS (pH 7.5) and 5 mM DTT. The flow rate was 1 ml/min and 1.5-ml fractions were collected. Fractions containing MtDH activity were voided from the column and pooled. The pooled DEAE fraction was concentrated and desalted by buffer exchange in 50 mM MOPS (pH 7.5)–1 mM DTT by using Centricon-10 centrifugal concentrators (Amicon). The concentrated, desalted DEAE fraction was loaded on a 0.24-ml MiniS PC3.2/3 column (Pharmacia) equilibrated with 50 mM MOPS (pH 7.5) containing 1 mM DTT. The column was connected to a SMART system (Pharmacia), and proteins were eluted with a 4.8-ml linear gradient of 0 to 0.5 M NaCl in 50 mM MOPS (pH 7.5) and 1 mM DTT. The flow rate was 0.4 ml/min, and 80-μl fractions were collected. Fractions containing MtDH activity eluted at approximately 50 mM NaCl. MtDH activity remained constant for at least several weeks when the final preparation was stored at −80°C.
Enzyme activity, protein assays, and mannitol analysis.
Crude extracts and purification fractions were desalted by centrifugal filtration on a Sephadex G-25-50 column equilibrated with 50 mM MOPS-NaOH (pH 7.5) containing 1 mM DTT prior to assay for MtDH activity. MtDH activity was measured by monitoring the oxidation of NADPH (or reduction of NADP) spectrophotometrically at 340 nm. The reduction of fructose was assayed in 100 mM MOPS (pH 7.5) containing 0.2 mM NADPH, enzyme extract, and 800 mM d-fructose in a total volume of 1 ml. The reactions were initiated with d-fructose. The oxidation of mannitol was assayed in 100 mM Bis-Tris propane (pH 9.5) containing 2 mM NADP, enzyme extract, and 200 mM d-mannitol in a total volume of 1 ml. All assays were conducted in the direction of fructose reduction unless otherwise stated. One unit of activity was defined as the amount of enzyme which catalyzed the oxidation of 1 μmol of NADP(H) per min. Protein concentrations were determined spectrophotometrically by the Bradford method (1) with bovine serum albumin as a standard. Mannitol was extracted in 80% (vol/vol) ethanol and analyzed on a high-pressure liquid chromatography system as described by Stoop and Pharr (41).
PAGE.
Denaturing SDS-PAGE was carried out as described by Laemmli (24). The final acrylamide concentration was 12% (wt/vol) for the separating gel and 4.5% (wt/vol) for the stacking gel. All samples were preincubated in the presence of SDS sample buffer for 5 min at 100°C prior to being loaded on the gels. Electrophoresis was at a constant 150 V for approximately 60 min at room temperature on a Mini-Protean II gel apparatus (Bio-Rad). Gels were subsequently stained for proteins with Coomassie blue stain.
N-terminal and internal protein sequence analysis.
The N-terminal amino acid sequence was obtained from a purified MtDH sample (MiniS fraction) that was electrophoresed (SDS-PAGE) and blotted onto an Immobilon-P membrane (Millipore) by using a Bio-Rad wet-transfer apparatus. The blotting buffer consisted of 48 mM Tris, 39 mM glycine, 20% methanol, and 0.01% SDS. The internal amino acid sequence was obtained from purified MtDH cleaved with CNBr. One hundred picomoles (± 3 μg) of protein was incubated in 35 μl of 70% formic acid containing 50 mg of CNBr per ml at room temperature for 24 h in the dark. After digestion, 200 μl of MilliQ water (Millipore) was added, and the mixture was freeze-dried. The pellet was resuspended in a minimum volume of buffer. The cleavage products were separated on a 0.75-mm-thick 15% (wt/vol) SDS gel (Bio-Rad) and blotted onto an Immobilon-P membrane as described above. The protein sequences of the CNBr cleavage products together with the N-terminal amino acid sequence of the purified MtDH were determined by Sequentiecentrum, Utrecht, The Netherlands.
Characterization of polyclonal antibodies and immunoblotting.
Polyclonal antibodies were raised in rats against the purified MtDH fraction that eluted from the MiniS column. A total of 200 μg of protein (100 μg/rat) was subjected to electrophoresis on an SDS–12% polyacrylamide gel and stained with an aqueous solution of Coomassie blue. A band representing a single major 29-kDa species was sliced out of the gel and used for immunizations. Immunizations and bleedings were performed by Eurogentec BE SA (Seraing, Belgium). Protein samples were subjected to SDS-PAGE and blotted onto Immobilon-P as described above. Immunodetection of the antigen was carried out with the Protoblot Western Blot AP Systems kit (Promega).
Poly(A)+ RNA isolation and RT-PCR.
RNA was isolated from vegetative mycelium grown on MMP agar medium covered with cellophane and from stage 2 fruit bodies grown on commercial compost. Mycelium and fruit body tissue were immediately frozen in liquid nitrogen upon harvest. Total RNA was isolated as described by Lucas et al. (26). Poly(A)+ RNA was isolated from total RNA of stage 2 fruit bodies by using immobilized oligo(dT) residues on magnetic beads [Oligo(dT)25 Dynabeads; Dynal]. Reverse transcription (RT) was performed for 1 h at 37°C in 20 μl containing 100 ng of poly(A)+ RNA, 0.5 μl of oligo(dT)12–18, 20 nmol of deoxynucleoside triphosphates, and 35.7 U of RNA-guard (all from Pharmacia) and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco-BRL) in 1× TAQ buffer (Pharmacia). PCR was performed with 4 μl of the RT reaction mixture with forward primer 5′-CGCGGATCCTTCGTYAAYAARACIATYATCG-3′ and reverse primer 5′-GCGCCATGGTGRTCYCTDATYTTYTTRTC-3′ (primers were synthesized by Eurogentec). The oligonucleotide sequences of the primers were based on the N-terminal and internal amino acid sequences of the purified MtDH subunit, respectively. The RT-PCR resulted in a 650-bp fragment on a 1.5% agarose gel. This cDNA fragment was retrieved from the gel by using the Qiaquick gel extraction kit from Qiagen and cloned into pCR2.1 (Invitrogen) by using the manufacturer’s TA cloning protocol. The DNA sequences of clones containing the 650-bp fragment were determined by using the automatic sequencer ALFexpress (Pharmacia). Escherichia coli TOP10F′ One Shot cells (Invitrogen) were used for cloning and amplification of plasmids.
Cloning of the full length MtDH cDNA.
Gene-specific synthetic oligonucleotides (5′-GTGCCATGGAATCGCGGTATTGGTCTCG-3′ and 5′-GCGCCATGGTCGGTGTTGACATATCCTGG-3′) and nested synthetic oligonucleotides (5′-GCGCCATGGCTCCAGGATATGTCAACACC-3′ and 5′-GTGCCATGGGACCAATACCGCGATTTCC-3′) were constructed based on the nucleotide sequence of the 650-bp RT-PCR fragment described above. These synthetic oligonucleotides were used in a 3′ and 5′ RACE (rapid amplification of cDNA ends) protocol with Advantage Klentaq polymerase (Clontech), as described for the Marathon cDNA amplification kit (Clontech). DNA fragments resulting from this RACE protocol were cloned and sequenced as described above. Analysis of the obtained sequences revealed the translation initiation and termination codons and an open reading frame of 789 bp. New oligonucleotides were synthesized based on the 5′ and 3′ ends of this open reading frame (5′-ATGGCCCCAGGATTCACTATCAGC-3′ and 5′-CTACCAAATGAGTTGACCACCATC-3′, respectively) and used in an RT-PCR with the polymerase Pwo (Boehringer Mannheim). A 789-bp fragment corresponding to the full-length open reading frame was obtained and cloned in Pichia pastoris. The vector pPIC9 was chosen to enable large-scale MtDH production in the future. A clone encoding the full-length fragment was identified and characterized.
Southern and Northern blot analyses.
DNA was isolated as described by Reader and Broda (35). Southern blot analysis was performed with 1 μg of DNA per sample by standard procedures (37) with the 32P-random-primer-labeled 650-bp partial MtDH sequence. Northern blotting was performed with 10 μg of total RNA per sample by standard procedures (37) with the 32P-random-primer-labeled MtDH sequence and with a 32P-random-primer-labeled 470-bp rDNA fragment of the gene for the A. bisporus 28S rRNA (GenBank accession no. X91812).
Nucleotide sequence accession number.
The GenBank nucleotide sequence accession number for the A. bisporus MtDH cDNA is AFO53764.
RESULTS
N-terminal and internal amino acid sequence determination.
The MtDH protein was purified to apparent homogeneity by using ammonium sulfate fractionation followed by anion-exchange chromatography on a DEAE–Bio-Gel-A agarose column and cation-exchange chromatography on a MiniS PC3.2/3 column. Crude extracts of MtDH were obtained from A. bisporus U1 fruit bodies of stage 2 (see Materials and Methods). At each step of the purification, a single peak of MtDH activity was observed, indicating that, under the experimental conditions used, only one MtDH could be discerned. The purified MtDH was specific for NADP(H), confirming previous findings (29). Crude extracts did show a slight activity (<10% of the NADP-specific activity) with NAD when assayed in the mannitol oxidation direction; however, no evidence for the presence of an NAD-dependent mannitol dehydrogenase was found (data not shown). Throughout the purification, a protein with an apparent molecular mass of 29 kDa became more predominant on SDS-PAGE (data not shown), which coincides with previous findings (10, 29, 36).
The SDS-PAGE-purified MtDH subunit was blotted onto Immobilon-P and subjected to N-terminal amino acid sequencing, which resulted in the N-terminal amino acid sequence APGFTISFVNKTIIVTGGNRGIG. The amino acid sequence DKKIRDHQASNIPLNRFAQP was obtained from a peptide generated by a CNBr digest of the 29-kDa purified subunit of MtDH. Amino acid sequences were determined by Edman degradation and manually terminated (Sequentiecentrum).
Isolation and sequence analysis of MtDH cDNA.
Degenerate oligonucleotides derived from the N-terminal amino acid sequence and an internal peptide sequence from the purified MtDH subunit were used for RT-PCR experiments. Poly(A)+ RNA isolated from mushroom fruit bodies was used as the template in an RT-PCR with the above-described degenerate oligonucleotides, generating a 650-bp cDNA fragment. This fragment was cloned into E. coli, sequenced, and used for Northern analysis. The 32P-labeled 650-bp fragment hybridized to a 1,000-nucleotide (nt) RNA (data not shown). To obtain a full-length coding sequence, 3′ and 5′ RACE were performed with gene-specific and nested primers (see Materials and Methods). The 3′ RACE resulted in two cDNA fragments of approximately 950 and 180 bp, while the 5′ RACE resulted in 700- and 350-bp cDNA fragments. By combining the 5′ and 3′ RACE data with those from the 650-bp cDNA fragment, a complete sequence was obtained and deposited in GenBank. Analysis of that sequence revealed the translation initiation and termination codons together with 5′ and 3′ untranslated regions. Downstream from the first in-frame translation initiation codon (ATG), no transit peptide was found. The full-length coding sequence consisted of 789 bp. To enable cloning of the 789-bp open reading frame, RT-PCR was performed with the proofreading polymerase Pwo and gene specific primers based on coding sequences adjacent to and including the translation initiation and termination codon sequences. The resulting 789-bp fragment was cloned into P. pastoris to enable large-scale MtDH production in the future.
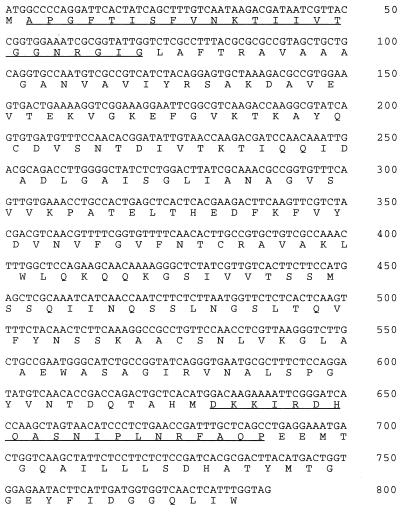
The clone thus obtained (pPIC9MTDH2.1) consisted of the entire open reading frame, which could encode a 262-amino-acid polypeptide with a molecular mass of 28 kDa (Fig. 1). This deduced molecular mass is similar to the apparent molecular mass (29 kDa) of the MtDH subunit purified from A. bisporus fruit bodies. Analysis of the deduced amino acid sequence of this cDNA revealed that it contained sequences identical to the N-terminal and internal amino acid sequences from the purified MtDH subunit (Fig. 1). The isoelectric point deduced from the MtDH amino acid sequence by using DNAsis2.1 software was 8.4. A high isoelectric point of 9.1 was previously reported for MtDH (36). A protein motif search with the Prosite database yielded the presence of a short-chain dehydrogenase family signature (accession no. PDOC00060), as is expected for a mannitol dehydrogenase cDNA (Fig. 1, bp 466 to 552). Furthermore, the deduced amino acid composition was nearly identical to the amino acid composition of the purified MtDH subunit (Table 1). These data provide several lines of evidence that clearly indicate that clone pPIC9MTDH2.1 contains the MtDH cDNA.
FIG. 1.
Nucleotide sequence and deduced amino acid sequence of the open reading frame of MtDH cDNA. Peptide sequences confirmed by direct amino acid sequencing of purified MtDH are underlined.
TABLE 1.
Comparison of the deduced amino acid composition of cloned MtDH with the analytically determined amino acid composition of purified MtDH from stage 2 fruit bodies
| Amino acid(s) | Deduced MtDH (mol%) | Purified MtDH (mol%) |
|---|---|---|
| Ala | 11.45 | 11.68 |
| Arg | 2.67 | 2.82 |
| Asn + Asp | 10.31 | 10.66 |
| Gln + Glu | 8.78 | 8.78 |
| Cys | 1.15 | 0.00 |
| Gly | 8.40 | 9.76 |
| His | 1.53 | 1.55 |
| Ile | 7.25 | 6.66 |
| Leu | 6.11 | 6.33 |
| Lys | 6.11 | 6.34 |
| Met | 1.91 | 0.39 |
| Phe | 4.20 | 4.37 |
| Pro | 1.91 | 2.61 |
| Ser | 7.63 | 8.58 |
| Thr | 7.25 | 7.46 |
| Trp | 1.15 | 0.00 |
| Tyr | 2.67 | 2.73 |
| Val | 9.54 | 9.01 |
The MtDH cDNA and its deduced amino acid sequence were compared with DNA and protein sequences on file in the databases by using a FASTA search. The deduced amino acid sequence showed the highest homology (76% similarity and 60% identity) to a plant-induced short-chain dehydrogenase of Uromyces fabae (GenBank accession no. U81790 [12]) with an unknown function (Fig. 2). Comparison at the DNA level by using the SWALL database showed highest homology with U. fabae and relatively low similarity to other oxidoreductases. We also compared the amino acid sequence of MtDH with amino acid sequences of different types of mannitol(-1-phosphate) dehydrogenases available in the databases. Similarity and identity percentages were obtained with the DNAsis software by comparing amino acid sequences by using (percent similarity) or by not using (percent identity) the amino acid substitution table. MtDH showed a similarity of 41 to 44% (23 to 25% identity) with NAD-dependent mannitol dehydrogenases of Rhodobacter sphaeroides (accession no. L13697 [39]) and Pseudomonas fluorescens (U39468 [2]) and 45 to 48% similarity (26 to 28% identity) with NAD-dependent mannitol-1-phosphate dehydrogenases of Streptococcus mutans (M94225 [19]), Streptococcus faecalis (M38386 [11]), Bacillus stearothermophilus (U18943 [17]), Bacillus subtilis (D38161 [31]), E. coli X51359 [21]), and Mycoplasma mycoides (U61140 [8]). Similar scores (48% similarity and 26% identity) were obtained when MtDH was compared to plant mannitol 1-oxidoreductases from Apium graveolens (U24561 [49]) and Lycopersicon esculentum (X92855 [25]).
FIG. 2.
Multiple sequence alignment of the amino acid sequences of the MtDH protein from A. bisporus and a short-chain dehydrogenase with an unknown function from U. fabae. Identical residues in the two sequences are marked with asterisks, while conserved substitutions are marked with dots.
Southern analysis revealed only one or two band patterns, indicating that the MtDH gene may be a single-copy gene or a low-copy-number gene (data not shown).
Increased mannitol biosynthesis in salt-stressed fruit bodies.
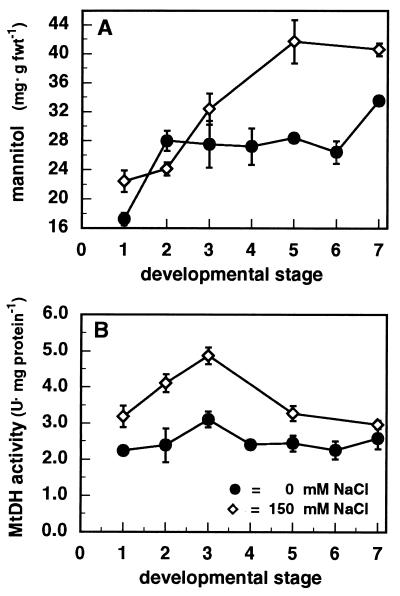
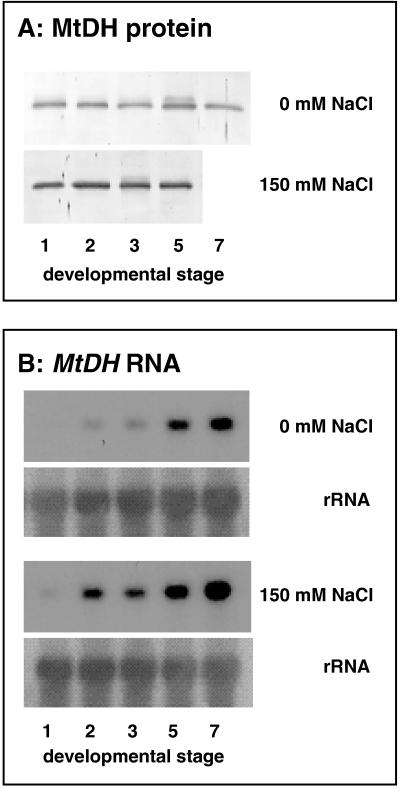
A typical pattern of mannitol concentration during fruit body development is shown in Fig. 3A. The mannitol concentration in nonstressed fruit bodies increased rapidly early in development, whereafter it remained relatively constant during fruit body maturation. Mushrooms grown under salt stress, by addition of 150 mM NaCl to the casing layer, accumulated larger amounts of mannitol than the nonstressed mushrooms (those with additions of water [0 mM NaCl] to the casing layer). Mannitol was accumulated up to 40 mg per g (fresh weight), which is equivalent to 60% of the total dry weight (Fig. 3A). Throughout the fruit body development, MtDH specific activity was highest in salt-stressed mushrooms (Fig. 3B). Salt-stressed mushrooms also showed increased MtDH protein abundance, as was evident from immunoblot analysis with polyclonal antibodies raised against the purified MtDH subunit (Fig. 4A). Immunoblot analysis of crude extracts from A. bisporus and from purified MtDH probed with anti-MtDH serum showed a single major immunoreactive band corresponding to an apparent molecular mass of 29 kDa (data not shown). Blots probed with preimmune serum had no major immunoreactive bands (data not shown). Furthermore, at each developmental stage the MtDH transcript level was higher in salt-stressed fruit bodies than in nonstressed fruit bodies (Fig. 4B). The MtDH transcript level also seemed to be under developmental control, as the MtDH RNA accumulation increased during fruit body development in both nonstressed and salt-stressed fruit bodies (Fig. 4B).
FIG. 3.
Mannitol concentration (A) and MtDH specific activity (B) during fruit body development of A. bisporus U1 grown on commercial compost with addition of 0 or 150 mM NaCl to the casing layer. Bars indicate the standard errors of the means.
FIG. 4.
Effect of NaCl on MtDH protein abundance (A) and MtDH RNA accumulation (B) in different developmental stages of A. bisporus. Mushrooms were grown on commercial compost with addition of 0 or 150 mM NaCl to the casing layer. The immunoblot of crude extracts (10 μg per lane) showed one major band of 29 kDa (A). Total RNA was extracted from each developmental stage, and relative amounts of MtDH transcript (1,000 nt) were determined by RNA blot analysis (B). The blot was hybridized to the 32P-labeled MtDH fragment. Equal RNA loading of lanes was confirmed by hybridization to a ribosomal DNA probe (rRNA).
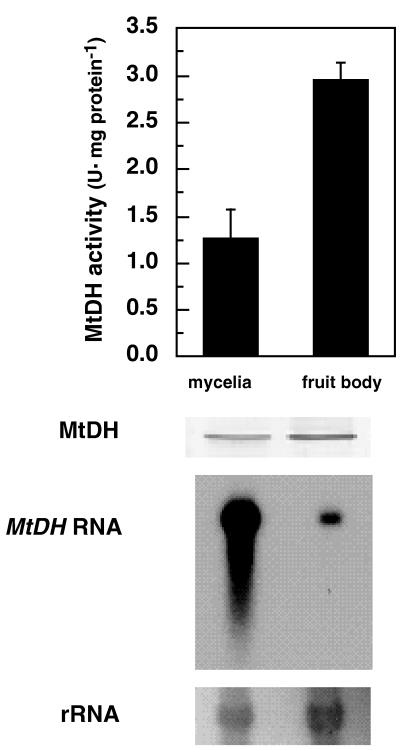
Differential MtDH activities, MtDH protein levels, and MtDH transcript levels in mycelia and stage 2 fruit bodies.
Mycelia grown on MMP medium showed a lower MtDH specific activity (units per milligram of protein) than commercial stage 2 fruit bodies (Fig. 5). MtDH specific activity was related to the MtDH protein abundance, as was evident from the lower MtDH protein abundance observed in mycelia. Interestingly, mycelia contained a higher MtDH transcript level than stage 2 fruit bodies (Fig. 5, MtDH RNA). The amount of RNA and the development time for the blot were chosen so that a clear signal for the MtDH transcript from fruit bodies was visible, resulting in a very strong signal for the transcript from mycelia. When the development time for the blot was decreased, no streaking of the MtDH transcript from mycelia was observed, indicating that the RNA was not degraded (not shown).
FIG. 5.
Differential MtDH activities and MtDH protein and MtDH transcript accumulations in A. biporus U1 mycelia grown on defined medium and in stage 2 fruit bodies of A. biporus U1 grown on commercial compost. The blot was hybridized to the 32P-labeled MtDH fragment. Equal RNA loading of lanes was confirmed by hybridization to a ribosomal DNA probe (rRNA). Bars indicate the standard errors of the means.
DISCUSSION
Our understanding of the role of MtDH and mannitol metabolism in fruit body development and the salt stress response of A. bisporus can be enhanced substantially by increased knowledge at the molecular and gene levels. Towards this end, we isolated and characterized the MtDH cDNA and studied its expression in response to NaCl stress. In order to obtain more information about the MtDH protein of A. bisporus, MtDH was purified to apparent electrophoretic homogeneity. The SDS-PAGE-determined molecular mass of the MtDH subunit was approximately 29 kDa, which confirms earlier findings (29, 32). N-terminal and internal peptide sequences of the purified MtDH subunit were obtained, and degenerate oligonucleotides based on these peptide sequences were used in an RT-PCR resulting in a 650-bp cDNA. Based on 5′ and 3′ RACE experiments, a 950-bp cDNA that included translational initiation and termination codons and an open reading frame of 789 bp was deduced. The length of this cDNA fragment was comparable to the transcript size revealed by RNA blot analysis (1,000 nt), further indicating that the cDNA is nearly full length. A clone (pPIC9MTDH2.1) containing the 789-bp open reading frame, encoding a protein with a deduced molecular mass of 28 kDa, was identified. The size of the deduced protein, the identity of the deduced amino acid sequence with the N-terminal and internal amino acid sequence of the purified MtDH subunit (Fig. 1), the similarity in amino acid composition (Table 1), and the presence of a conserved short-chain dehydrogenase family motif (Fig. 1) clearly identify this open reading frame as MtDH cDNA.
A FASTA search of the DNA and protein databases revealed that the deduced MtDH protein sequence was very homologous to that of a plant-induced short-chain dehydrogenase of U. fabae (8) with an unknown function (Fig. 2). The high similarity (76%) between the A. bisporus and U. fabae protein sequences suggests that U. fabae contains an NADP-dependent mannitol dehydrogenase, which may be involved in the plant-induced infection process. Mannitol biosynthesis has been reported to be required for wild-type virulence of Cryptococcus neoformans, a fungus that can cause serious human and animal infections (6, 7). The involvement of mannitol metabolism in pathogenesis has previously been suggested by Williamson et al. (49), who identified a plant mannitol dehydrogenase as a pathogenesis-related protein.
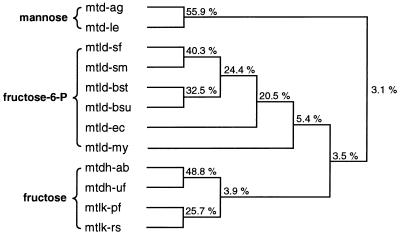
Pairwise comparisons of the amino acid sequence of A. bisporus and those of different types of mannitol(-1-phosphate) dehydrogenases available in the FASTA database revealed a relatively low similarity (41 to 48%) between MtDH and the dehydrogenases analyzed. However, a phylogenetic tree analysis with the protein sequences of the above-mentioned dehydrogenases resulted in a clear relationship among the dehydrogenases (Fig. 6). The dendrogram reveals that all mannitol-1-phosphate dehydrogenases (fructose-6-phosphate oxidoreductases) are clustered together and are distinct from all mannitol dehydrogenases. Interestingly, the cluster of the plant mannitol dehydrogenases known to date (Fig. 6, mtd-ag and mtd-le) was clearly distinct from all other dehydrogenases. This is not surprising, as these plant mannitol dehydrogenases are 1-oxidoreductases, interconverting mannitol and mannose but not fructose (42, 44, 45). Thus, the phylogenetic tree consists of three major clusters that correspond to the different types of mannitol dehydrogenases based on their substrate specificities, namely, the mannose oxidoreductases, the fructose-6-phosphate oxidoreductases, and the fructose oxidoreductases. The last cluster, which includes the mushroom MtDH cDNA, contains two distinct clusters which correspond to the cofactor requirements (NADPH or NADH) of the fructose oxidoreductase. The divergence of these oxidoreductases based on substrate specificity predates the divergence based on cofactor requirement or species, suggesting that these dehydrogenases have evolved based on substrate requirements followed by cofactor and species requirements.
FIG. 6.
Phylogenetic tree based on the amino acid sequences of different mannitol(-1-phosphate) dehydrogenases available in FASTA databases. The tree was composed of NAD-dependent mannitol dehydrogenases of A. graveolens (mtd-ag) and L. esculentum (mtd-le); the NAD-dependent mannitol-1-phosphate dehydrogenases of S. faecalis (mtld-sf), Streptococcus mutans (mtld-sm), Bacillus stearothermophilus (mtld-bst), B. subtilis (mtld-bsu), E. coli (mtld-ec), and M. mycoides (mtld-mm); the MtDH of A. bisporus (mtdh-ab); the short-chain dehydrogenase of U. fabae (mtdh-uf); and the NAD-dependent mannitol dehydrogenases of P. fluorescens (mtlk-pf) and R. sphaeroides (mtlk-rs). Calculated matching percentages are indicated at each branch point of the dendrogram and were generated by using DNAsis v. 2.0 (Hitachi) software. The bracket encompasses dehydrogenases with either mannose, fructose, or fructose-6-phosphate as the substrate.
Salt-stressed mushrooms responded to an increased NaCl concentration in their growth environment by accumulating a large amount of mannitol, up to 40 mg per gram (fresh weight) or 60% of their dry weight, confirming earlier observations that mannitol can act as an osmolite in growing fruit bodies (20, 22). Throughout the fruit body development, salt-stressed mushrooms contained a higher MtDH specific activity, MtDH protein abundance, and MtDH RNA accumulation than nonstressed mushrooms, indicating that mannitol metabolism plays an important role in salt tolerance of A. bisporus.
Besides being affected by NaCl, mannitol metabolism seems to be under developmental control. Throughout the fruit body development, the MtDH specific activity and MtDH protein level remained relatively constant. One can postulate that an equal abundance of MtDH protein throughout development might be necessary to ensure a constant production of mannitol, which in turn is important for regulation of the internal osmotic potential and possibly water uptake (18). Interestingly, MtDH RNA accumulation increased during fruit body development in both nonstressed and salt-stressed fruit bodies. Young fruit bodies (stages 1 and 2) accumulated less MtDH RNA than older fruit bodies (stages 5 and 7), despite the relatively equal amounts of MtDH protein present in these tissues. This might indicate that maturing fruit bodies have a higher turnover of MtDH protein and/or a lower protein stability. Increased protease activity has been observed in maturing fruit bodies (3, 4), which may, in part, explain these observations.
Comparison of the MtDH specific activity (units per milligram of protein) of mycelium and stage 2 fruit body tissue indicates that young fruit bodies contain a higher MtDH specific activity. The MtDH specific activity was related to the amount of MtDH protein present; e.g., stage 2 fruit bodies accumulated more MtDH protein than mycelia grown on defined medium. However, the MtDH transcript level was higher in mycelia than in fruit bodies. This apparent contradiction may be explained, in part, by differences in the composition of the growth media (carbohydrate and salt concentrations in particular) and/or differences inherent to the developmental stage or maturation of the mushroom. The mycelia used for this study were grown for approximately 20 days on defined medium and were initiated with a 5-mm inoculum placed in the center of the plate. A gradient in maturation of the mycelium, from the center towards the outer layer of the plate, is therefore likely to occur, with aging mycelia representing the majority of the harvested mycelia. Aging mycelia, as described above for aging fruit bodies, may have a high protease activity and thus high MtDH turnover. Furthermore, mycelia and fruit bodies may have different mechanisms for regulating MtDH expression, which are currently not understood.
Besides its physiological importance in the cultivated mushroom, mannitol also plays an important role in the pharmaceutical and food industry, where it is increasingly used as a nutritive sweetener and antioxidant. Commercial production of mannitol occurs mainly through chemical reduction of fructose, a process that yields small amounts of both mannitol and its by-product sorbitol (27). More efficient and selective mannitol production methods, using organisms such as E. coli (38) and Leuconostoc mesenteroides (40) or enzyme reactors (30), are being explored. The availability of different types of mannitol dehydrogenases may prove to be useful in optimizing industrial mannitol production processes.
This report presents the first isolation of a cDNA for MtDH of A. bisporus. Evidence that mannitol biosynthesis, and MtDH RNA accumulation in particular, is affected by the developmental stage of the mushroom as well as by salt stress is shown. The identification of the MtDH cDNA allows for the production of transgenic mushrooms with altered mannitol metabolism in order to further elucidate the role of mannitol in A. bisporus. Furthermore, the MtDH cDNA can be expressed in fermentative organisms such as P. pastoris or L. mesenteroides to ultimately produce large amounts of this enzyme, which in turn can be used for commercial mannitol production.
ACKNOWLEDGMENTS
This research was supported in part by European Commission BIO4-CT965002 grant to J. M. H. Stoop.
We thank Marc W. T. Werten and John B. Hammond for helpful discussions.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Brünker P, Altenbuchner J, Kulbe K D, Mattes R. Cloning, nucleotide sequence and expression of a mannitol dehydrogenase from Pseudomonas fluorescens DSM 50106 in Escherichia coli. Biochim Biophys Acta. 1997;1351:157–167. doi: 10.1016/s0167-4781(96)00189-3. [DOI] [PubMed] [Google Scholar]
- 3.Burton K S, Hammond J B W, Minamide T. Protease activity in Agaricus bisporus during periodic fruiting (flushing) and sporophore development. Curr Microbiol. 1994;28:275–278. [Google Scholar]
- 4.Burton K S, Partis M D, Wood D A, Thurston C F. Accumulation of serine proteinase in senescent sporophores of the cultivated mushroom Agaricus bisporus. Mycol Res. 1997;101:146–152. [Google Scholar]
- 5.Castle A J, Horgen P A, Anderson J B. Restriction fragment length polymorphisms in the mushrooms Agaricus brunnescens and Agaricus bitorquis. Appl Environ Microbiol. 1987;53:816–822. doi: 10.1128/aem.53.4.816-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi V, Flynn T, Niehaus W G, Wong B. Stress tolerance and pathogenic potential of a mannitol mutant of Cryptococcus neoformans. Microbiol. 1996;142:937–943. doi: 10.1099/00221287-142-4-937. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi V, Bartiss A, Wong B. Expression of bacterial mtlD in Saccharomyces cerevisiae results in mannitol synthesis and protects a glycerol-deficient mutant from high-salt and oxidative stress. J Bacteriol. 1997;179:157–162. doi: 10.1128/jb.179.1.157-162.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Nicolet J, Miserez R, Kuhnert P, Krampe M, Pilourd T, Abdo E M, Griot C, Frey J. Characterization of the gene for an immunodominant 73 kDa lipoprotein of Mycoplasma mycoides subsp. mycoides small colony type. Microbiology. 1996;142:3515–3524. doi: 10.1099/13500872-142-12-3515. [DOI] [PubMed] [Google Scholar]
- 9.Dütsch G A, Rast D. Biochemische beziehung zwischen Mannitbildung und Hexosemonophosphatzyklus in Agaricus bisporus. Phytochemistry. 1972;11:2677–2681. [Google Scholar]
- 10.Edmundowicz J M, Wriston J C. Mannitol dehydrogenase from Agaricus campestris. J Biol Chem. 1963;238:3539–3541. [PubMed] [Google Scholar]
- 11.Fischer R, von Strandmann R P, Hengstenberg W. Mannitol-specific phosphoenolpyruvate-dependent phosphotransferase system of Enterococcus faecalis: molecular cloning and nucleotide sequences of the enzyme IIIMtl gene and the mannitol-1-phosphate dehydrogenase gene, expression in Escherichia coli, and comparison of the gene products with similar enzymes. J Bacteriol. 1991;173:3709–3715. doi: 10.1128/jb.173.12.3709-3715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn M, Mendgen K. Characterization of in planta-induced rust genes isolated from a haustorium-specific cDNA library. Mol Plant-Microbe Interact. 1997;10:427–437. doi: 10.1094/MPMI.1997.10.4.427. [DOI] [PubMed] [Google Scholar]
- 13.Hammond J B W, Nichols R. Changes in respiration and soluble carbohydrates during the post-harvest storage of mushrooms (Agaricus bisporus) J Sci Food Agric. 1975;26:835–842. [Google Scholar]
- 14.Hammond J B W, Nichols R. Carbohydrate metabolism in Agaricus bisporus (Lange) Sing.: changes in soluble carbohydrates during growth of mycelium and sporophore. J Gen Microbiol. 1976;93:309–320. doi: 10.1099/00221287-93-2-309. [DOI] [PubMed] [Google Scholar]
- 15.Hammond J B W. Variations in enzyme activity during periodic fruiting of Agaricus bisporus. New Phytol. 1981;89:419–428. [Google Scholar]
- 16.Hammond J B W. Sugar, sugar phosphate and NADP(H) levels in Agaricus bisporus fruit bodies. J Gen Microbiol. 1985;131:329–333. [Google Scholar]
- 17.Henstra S A, Tolner B, ten hoeve Duurkens R H, Konings W N, Robillard G T. Cloning, expression, and isolation of the mannitol transport protein from the thermophilic bacterium Bacillus stearothermophilus. J Bacteriol. 1996;178:5586–5591. doi: 10.1128/jb.178.19.5586-5591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtz R B. Qualitative and quantitative analyses of free neutral carbohydrates in mushroom tissue by gas-liquid chromatography and mass spectrometry. J Agric Food Chem. 1971;19:172–1273. [Google Scholar]
- 19.Honeyman A L, Curtiss R., III Isolation, characterization, and nucleotide sequence of the Streptococcus mutans mannitol-phosphate dehydrogenase gene and the mannitol-specific factor III gene of the phosphoenolpyruvate phosphotransferase system. Infect Immun. 1992;60:3369–3375. doi: 10.1128/iai.60.8.3369-3375.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings D H. Polyol metabolism in fungi. Adv Microb Physiol. 1984;25:149–193. doi: 10.1016/s0065-2911(08)60292-1. [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Wu L F, Tomich J, Saier M H, Jr, Niehaus W G. Corrected sequence of the mannitol (mtl) operon in Escherichia coli. Mol Microbiol. 1990;4:2003–2006. doi: 10.1111/j.1365-2958.1990.tb02050.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalberer P P. Influence of water potential of the casing soil on crop yield and on dry-matter content, osmotic potential and mannitol content of the fruit bodies of Agaricus bisporus. J Hort Sci. 1990;65:573–581. [Google Scholar]
- 23.Kets E P W, Galinski E A, de Wit M, de Bont J A M, Heipieper H J. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J Bacteriol. 1996;178:6665–6670. doi: 10.1128/jb.178.23.6665-6670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lauter F R. Root-specific expression of the LeRse-1 gene in tomato is induced by exposure of the shoot to light. Mol Gen Genet. 1996;252:751–754. doi: 10.1007/BF02173983. [DOI] [PubMed] [Google Scholar]
- 26.Lucas M C, Jacobsen J W, Giles N H. Characterization and in vitro translation of polyadenylated messenger ribonucleic acid from Neurospora crassa [Fungi] J Bacteriol. 1977;130:1192–1198. doi: 10.1128/jb.130.3.1192-1198.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makkee M, Kieboom A P G, van Bekkum H. Production of d-mannitol. Starch/Starke. 1985;37:136–141. [Google Scholar]
- 28.Mooibroek H, van de Rhee M, Soler-Rivas C, Mendes O, Werten M, Huizing H, Wichers H. Progress in transformation of the common mushroom Agaricus bisporus. In: Royse D J, editor. Mushroom biology and mushroom products. Proceedings of the second international conference. University Park, Pa: The Pennsylvania State University; 1996. pp. 37–46. [Google Scholar]
- 29.Morton N, Dickerson A G, Hammond J B W. Mannitol metabolism in Agaricus bisporus: purification and properties of mannitol dehydrogenase. J Gen Microbiol. 1985;131:2885–2890. [Google Scholar]
- 30.Nidetzky B, Haltrich D, Schmidt K, Schmidt H, Weber A, Kulbe K D. Simultaneous enzymatic synthesis of mannitol and gluconic acid. II. Development of a continuous process for a coupled NAD(H)-dependent enzyme system. Biocatal Biotransform. 1996;14:47–65. [Google Scholar]
- 31.Ogasawara N, Fujita Y, Kobayashi Y, Sadaie Y, Tanaka T, Takahashi H, Yamane K, Yoshikawa H. Systematic sequencing of the Bacillus subtilis genome: progress report of the Japanese group. Microbiology. 1995;141:257–259. doi: 10.1099/13500872-141-2-257. [DOI] [PubMed] [Google Scholar]
- 32.Pfyffer G E, Hübscher U, Rast D M. Antibodies against the fungal enzyme mannitol dehydrogenase. Exp Mycol. 1989;13:321–331. [Google Scholar]
- 33.Pharr D M, Stoop J M H, Williamson J D, Studer-Feusi M E, Massel M O, Conkling M A. The dual role of mannitol as osmoprotectant and photoassimilate in celery. Hort Sci. 1995;30:1182–1188. [Google Scholar]
- 34.Rast D. Zur stoffwechselphysiologischen bedeutung van Mannit und Trehalose in Agaricus bisporus. Planta. 1965;64:81–93. [Google Scholar]
- 35.Reader U, Broda P. Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol. 1985;1:17–20. [Google Scholar]
- 36.Ruffner H P, Rast D, Tobler H, Karesch H. Purification and properties of mannitol dehydrogenase from Agaricus bisporus sporocarps. Phytochemistry. 1978;17:865–868. [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schafer A, Stein M A, Schneider K H, Giffhorn F. Mannitol dehydrogenase from Rhodobacter sphaeroides Si4: subcloning, overexpression in Escherichia coli and characterization of the recombinant enzyme. Appl Microbiol Biotechnol. 1997;48:47–52. doi: 10.1007/s002530051013. [DOI] [PubMed] [Google Scholar]
- 39.Schneider K H, Giffhorn F, Kaplan S. Cloning, nucleotide sequence and characterization of the mannitol dehydrogenase gene from Rhodobacter sphaeroides. J Gen Microbiol. 1993;139:2475–2484. doi: 10.1099/00221287-139-10-2475. [DOI] [PubMed] [Google Scholar]
- 40.Soetaert W, Buchholz K, Vandamme E J. Production of d-mannitol and d-lactic acid by fermentation with Leuconostoc mesenteroides. Agro Food Ind Hi tech. 1995;6:41–44. [Google Scholar]
- 41.Stoop J M H, Pharr D M. Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiol. 1994;106:503–511. doi: 10.1104/pp.106.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoop J M H, Williamson J D, Conkling M A, Pharr D M. Purification of NAD-dependent mannitol dehydrogenase from celery suspension cultures. Plant Physiol. 1995;108:1219–1225. doi: 10.1104/pp.108.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoop J M H, Williamson J D, Pharr D M. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1996;5:139–144. [Google Scholar]
- 44.Stoop J M H, Chilton W S, Pharr D M. Substrate specificity of the NAD-dependent mannitol dehydrogenase from celery. Phytochemistry. 1996;43:1145–1150. [Google Scholar]
- 45.Stoop J M H, Williamson J D, Conkling M A, MacKay J J, Pharr D M. Characterization of NAD-dependent mannitol dehydrogenase from celery as affected by ions, chelators, reducing agents and metabolites. Plant Sci. 1998;131:43–51. [Google Scholar]
- 46.Summerbell R C, Castle A J, Horgen P A, Anderson J B. Inheritance of restriction fragment length polymorphisms in Agaricus brunnescens. Genetics. 1989;123:293–300. doi: 10.1093/genetics/123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van de Rhee M D, Graça P M A, Huizing H J, Mooibroek H. Transformation of the cultivated mushroom, Agaricus bisporus, to hygromycin B resistance. Mol Gen Genet. 1996;250:252–258. doi: 10.1007/BF02174382. [DOI] [PubMed] [Google Scholar]
- 48.Van de Rhee M D, Mendes O, Werten M W T, Huizing H J, Mooibroek H. Highly efficient homologous integration via tandem exo-β-1,3-glucanase genes in the common mushroom, Agaricus bisporus. Curr Genet. 1996;30:166–173. doi: 10.1007/s002940050116. [DOI] [PubMed] [Google Scholar]
- 49.Williamson J D, Stoop J M H, Massel M O, Conkling M A, Pharr D M. Sequence analysis of a mannitol dehydrogenase cDNA from plants reveals a function for the pathogenesis related protein ELI3. Proc Natl Acad Sci USA. 1995;92:7148–7152. doi: 10.1073/pnas.92.16.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]