Abstract
Purpose
Radiation dose intensification improves outcome in men with high-risk prostate cancer (HR-PCa). A prospective trial was conducted to determine safety, feasibility, and maximal tolerated dose (MTD) of multi-level MRI-based 5-fraction stereotactic radiation (SAbR) in patients with HR-PCa.
Methods and Materials
This phase I clinical trial enrolled HR-PCa patients with grade group ≥4, PSA ≥ 20ng/ml, or radiographic ≥T3, and well-defined prostatic lesions on multi-parametric MRI (mpMRI) into 4 dose-escalation cohorts. The initial cohort received 47.5Gy to the prostate, 50Gy to mpMRI-defined intra-prostatic lesion(s), and 22.5Gy to pelvic lymph nodes in 5 fractions. Radiation doses were escalated for pelvic nodes to 25Gy and mpMRI lesion(s) to 52.5Gy and then 55Gy. Escalation was performed sequentially according to rule-based trial design with 7–15 patients per cohort and a 90-day observation period. All men received peri-rectal hydrogel spacer, intra-prostatic fiducial placement, and 2 years of androgen deprivation. The primary endpoint was MTD according to a 90-day acute dose-limiting toxicity (DLT) rate <33%. DLT was defined as NCI Common Toxicity Criteria for Adverse Events (CTCAE) ≥ grade 3 treatment-related toxicity. Secondary outcomes include acute and delayed gastrointestinal (GI)/genitourinary (GU) toxicity graded with CTCAE.
Results
Fifty-five of the 62 enrolled patients were included in the analysis. Dose was escalated through all 4 cohorts without observing any DLTs. Median overall follow-up was 18 months, with a median follow-up of 42, 24, 12, and 7.5 months for cohorts 1–4 respectively. Acute and late grade 2 GU toxicities were 25% and 20%, while GI were 13% and 7%, respectively. Late grade 3 GU & GI toxicities were 2% and 0%, respectively.
Conclusions
SAbR dose for HR-PCa was safely escalated with multi-level dose painting of 47.5Gy to prostate, 55Gy to mpMRI-defined intra-prostatic lesions, and 25Gy to pelvic nodal region in 5 fractions. Longer and ongoing follow-up will be required to assess late toxicity.
Introduction
Approximately 30–60% of men with high-risk prostate cancer (HR-PCa) develop biochemical recurrence within 10 years, and many develop metastatic cancer leading to mortality.(1) Multiple studies have reported improved biochemical control (BC) with higher conventionally fractionated radiation therapy (CFRT) dose for prostate cancer (PCa).(2–4) Yet, BC for CFRT remains poor for HR-PCa. CFRT has reached its limitations for further dose escalation for multiple reasons, including organs at-risk (OAR) dosimetry, patient inconvenience, and cost. The recently published randomized phase III trial, FLAME, attempted to overcome this limitation by incorporating a simultaneous integrated boost (SIB) to intraprostatic lesions. Biochemical disease-free survival (bDFS) benefits were reported, but boost doses were limited by normal tissue toxicity constraints.(5)
Stereotactic ablative radiotherapy (SAbR) delivers an ablative dose of radiation therapy (RT) typically in 4–7 fractions.(6,7) The low alpha/beta ratio of PCa relative to nearby OARs suggests that SAbR may offer higher therapeutic advantage in treating PCa than CFRT and allow for further dose escalation.(8) Multiple studies have successfully treated low and intermediate risk PCa patients with SAbR.(3,4,9,10) Zelefsky et. al. reported increased pathologic local control for low-intermediate risk PCa when the SAbR dose was escalated from 32.5Gy to 40Gy in 5 fractions.(3) Interestingly, a 7.7% 2-year local failure was observed at the highest 5-fraction dose level of 40Gy. This is expected to be even higher in HR-PCa, justifying further dose escalation requirements in this patient population. Magnetic resonance imaging (MRI), particularly with multiparametric approach (mpMRI), can now detect intra-prostatic lesions with high accuracy.(11)
A few studies have evaluated SAbR for treating HR-PCa, which has the highest mortality, due to the high risk of pelvic lymph node metastasis and challenges in simultaneously treating elective nodal regions. Several approaches have been considered for SAbR in HR-PCa, including treating the prostate but not pelvic lymph nodes (12–14), CFRT to pelvic lymph nodes and SAbR boost to the prostate(12), or treating both pelvic lymph nodes and prostate with SAbR and SIB(15,16). While electively treating pelvic lymph nodes remains controversial given lack of randomized trials showing overall survival (OS) benefits, multiple studies have reported improved BC.(17,18) Furthermore, retrospective as well as prospective database studies suggest that SAbR to prostate and pelvic lymph node is well tolerated.(19,20)
Therefore, a phase I dose escalation trial was designed to determine feasibility, safety, and maximum tolerated dose (MTD) for SAbR treatment of the prostate, proximal seminal vesicles, intra-prostatic lesions, and pelvic lymph nodes regions with varying doses.
Methods
Trial design and participants
Patients were enrolled in an IRB-approved single institutional phase I dose escalation study from November 2015 to October 2019 (ClinicalTrials.gov:NCT02353819). Eligible patients required at least one of the following HR-PCa features: PSA ≥ 20ng/ml, Grade Group ≥ 4, or AJCC clinical/radiographic ≥T3. For radiographic staging and for delineating target lesion(s), mpMRI was required with at least one identifiable lesion.
Exclusion criteria included prior pelvic radiotherapy, chemotherapy, surgery for PCa, history of inflammatory bowel disease, and regional or distant metastasis. Our protocol also excluded patients with prior TURP due to increased risk of toxicity.(21)
Study interventions
All patients received a peri-rectal hydrogel spacer (SpaceOAR, Boston Scientific, Marlborough MA) and intraprostatic metallic fiducial markers. A two-year course of androgen deprivation therapy (ADT) was planned, consisting of gonadotropin releasing hormone agonist/antagonist and anti-androgen bicalutamide starting 10–20 weeks before SAbR. Bicalutamide was stopped at the end of SAbR. Patients performed two enemas and filled their bladder in preparation for CT simulation scan and each treatment. Patients were prescribed prophylactic alpha-blockers before SAbR and received dexamethasone (4 mg) before each treatment.
Patients received 5 fractions with a minimum of 36 hours between treatments and a maximum of 3 fractions per week. Building on the safety experience of 5-fraction whole pelvis RT from rectal cancer, a dose of 22.5Gy was initially selected for the pelvic lymph nodes and distal seminal vesicles.(22) The RTOG contouring atlas (23) was used to delineate the pelvic lymph nodes CTV followed by a 5mm expansion to generate PTV1. The prostate and proximal 1 cm of seminal vesicles were delineated and a 3mm expansion was used to generated PTV2. In cases of seminal vesicle invasion, coverage of a larger length of seminal vesicles up its full extent was to be included at the discretion of the investigator in PTV2. PTV2 was included in PTV1. A PTV2 dose of 47.5Gy was kept constant for all cohorts based on our previous dose escalation trial.(9,10) PTV3 consisted of the mpMRI-based intra-prostatic lesion(s) with 0–3mm expansion (at investigator discretion) and the dose was started at 50Gy. The dose was sequentially escalated to 25Gy to the pelvic nodal regions and to 52.5Gy followed by 55Gy to the intraprostatic lesion(s) (Table 1).
Table 1.
Characteristics by dose cohort with statistical analysis comparing cohorts. PSA and baseline IPSS were analyzed as both categorical (Fisher’s exact test) and continuous variable (one-way ANOVA).
| Total dose (Gy) | 47.5/50/22.5 | 47.5/50/25 | 47.5/52.5/25 | 47.5/55/25 | ||||||||
| Dose per fraction (Gy) (prostate/lesion/nodes) | 9.5/10/4.5 | 9.5/10/5 | 9.5/10.5/5 | 9.5/11/5 | ||||||||
| Characteristic | No. | % | No. | % | No. | % | No. | % | No. | % | Fischer's exact test | ANOVA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Number of patients | 12 | 22% | 15 | 27% | 13 | 24% | 15 | 27% | 55 | |||
| Median follow-up, month (range) | 42 | (30–48) | 24 | (12–36) | 12 | (12–18) | 7.5 | (3–9) | 18 | |||
| Average age, years (IQR) | 70 | (65–76) | 76 | (73–81) | 70 | (64–76) | 71 | (67–77) | 72 | (67–77) | p=0.039 | |
| Grade group | p=0.73 | |||||||||||
| 1 | 1 | 8% | 0 | 0% | 0 | 0% | 1 | 7% | 2 | 4% | ||
| 2 | 3 | 25% | 2 | 13% | 2 | 15% | 5 | 33% | 12 | 22% | ||
| 3 | 2 | 17% | 3 | 20% | 2 | 15% | 0 | 0% | 7 | 13% | ||
| 4 | 3 | 25% | 7 | 47% | 4 | 31% | 5 | 33% | 19 | 35% | ||
| 5 | 3 | 25% | 3 | 20% | 5 | 38% | 4 | 27% | 15 | 27% | ||
| Baseline PSA (ng/mL) | p=0.60 | |||||||||||
| <10 | 8 | 67% | 7 | 47% | 7 | 54% | 7 | 47% | 29 | 53% | ||
| 10–20 | 1 | 8% | 6 | 40% | 3 | 23% | 3 | 20% | 13 | 24% | ||
| >20 | 3 | 25% | 2 | 13% | 3 | 23% | 5 | 33% | 13 | 24% | ||
| Clinical stage | p=0.002 | |||||||||||
| T1c | 4 | 33% | 8 | 53% | 10 | 77% | 12 | 80% | 34 | 62% | ||
| T2 | 7 | 58% | 6 | 40% | 0 | 0% | 1 | 7% | 14 | 25% | ||
| T2a | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | ||
| T2b | 6 | 50% | 2 | 13% | 0 | 0% | 1 | 7% | 9 | 16% | ||
| T2c | 1 | 8% | 4 | 27% | 0 | 0% | 0 | 0% | 5 | 9% | ||
| T3 | 1 | 8% | 1 | 7% | 0 | 0% | 0 | 0% | 2 | 4% | ||
| T3a | 1 | 8% | 1 | 7% | 0 | 0% | 0 | 0% | 2 | 4% | ||
| T3b | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | ||
| Unspecified | 0 | 0% | 0 | 0% | 3 | 23% | 2 | 13% | 5 | 9% | ||
| MRI EPE Present | p=0.52 | |||||||||||
| Yes | 5 | 42% | 9 | 60% | 6 | 46% | 5 | 33% | 25 | 45% | ||
| No | 7 | 58% | 6 | 40% | 7 | 54% | 10 | 67% | 30 | 55% | ||
| MRI SVI Present | p=0.40 | |||||||||||
| Yes | 3 | 25% | 1 | 7% | 3 | 23% | 1 | 7% | 8 | 15% | ||
| No | 9 | 75% | 14 | 93% | 10 | 77% | 14 | 93% | 47 | 85% | ||
| Number of MRI lesions | p=0.97 | |||||||||||
| 1 | 7 | 58% | 6 | 40% | 5 | 38% | 8 | 53% | 26 | 47% | ||
| 2 | 3 | 25% | 6 | 40% | 6 | 46% | 4 | 27% | 19 | 35% | ||
| 3 | l | 8% | 2 | l3% | 2 | 15% | 2 | l3% | 7 | l3% | ||
| >4 | l | 8% | l | 7% | 0 | 0% | l | 7% | 3 | 5% | ||
| Baseline IPSS | P=0.76 | |||||||||||
| 0-4 | l | 8% | 3 | 2l% | 2 | l7% | 4 | 29% | 10 | 19% | ||
| 4–8 | 3 | 25% | 4 | 29% | 4 | 33% | 5 | 36% | l6 | 31% | ||
| 8–12 | 3 | 25% | 5 | 36% | 2 | l7% | l | 7% | 11 | 2l% | ||
| 12–l6 | 4 | 33% | l | 7% | 4 | 33% | 3 | 2l% | 12 | 23% | ||
| 16–20 | l | 8% | l | 7% | 0 | 0% | l | 7% | 3 | 6% | ||
Toxicity was graded using the NCI Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0. Dose-limiting toxicity (DLT) was defined as any treatment-related grade 3, 4, or 5 toxicity in the following categories: gastrointestinal (GI), renal, genitourinary (GU), sexual-reproductive, or neurological. DLT also included any treatment-related grade 4 or 5 toxicity. Seven to 15 patients were planned per dose cohort (Table 1). Toxicity data were continuously monitored with a 90-day window period. The dose will be escalated if zero out of the first seven patients, 2 or fewer out of the first nine, 3 or fewer out of the first twelve, or 4 or fewer out of the first fifteen patients experience a DLT within 90-days.
Prostate lesion(s) were annotated by a radiologist with 15 years of experience in prostate cancer imaging using a free-hand region-of-interest tool in a commercially available post-processing workstation (iCAD, VersaVue, Nashua, NH). The diagnostic mpMRI protocol was previously published (24) and complies with recommendations from the Prostate Imaging Reporting & Data System (PI-RADS, version 2.1)(25). A separate treatment planning MRI was obtained in the treatment position, without an endorectal coil to prevent prostate deformation. The treatment planning MRI included high spatial-resolution spin-echo axial T2-weighted images to delineate the lesion and prostatic boundaries, and axial gradient-echo images to locate the fiducial markers. To determine the location of the intraprostatic targets on the treatment planning MRI, the radiologist integrated findings from the diagnostic mpMRI and treatment planning MRI using anatomical landmarks such as location, texture, and morphology of BPH nodule. The lesion(s) of interest were delineated on the treatment planning MRI and exported from iCAD to Eclipse treatment planning software (Varian, Palo Alto, CA) and used as lesion GTV without further modifications. Subsequently, the treatment planning MRIs were registered to the treatment planning CT simulation using metallic fiducial markers.
The details of patient immobilization, target delineation, beam optimization, OAR constraints, and image-guided radiation delivery have been previously described.(26) Priority was given to meeting OAR constraints over coverage of PTV targets. The goal for target coverage was at least 95% of each PTV volume receiving prescription dose (%VPrescription) and 99% of PTV being covered by at least 90% of prescription dose (D99%). Dose to the MRI-defined prostatic urethra was maintained <105% of the prostate dose (Figure 1). Dose constraints are included in Table S1. After immobilization in a stereotactic body frame (Elekta, Stockholm, Sweden) with a vaclock bag, a cone-beam CT was obtained, prior to each treatment, for image guidance. The metallic fiducial markers in the prostate were used for daily alignment and pelvic bony anatomy was used as surrogate for pelvic nodal field alignment. Bladder and rectal volume was also checked with cone-beam CT as this may impact both toxicity as well as pelvic lymph node coverage(27). Dynamic Arc-IMRT was used to deliver SAbR with 6 degree of freedom on the treatment couch.
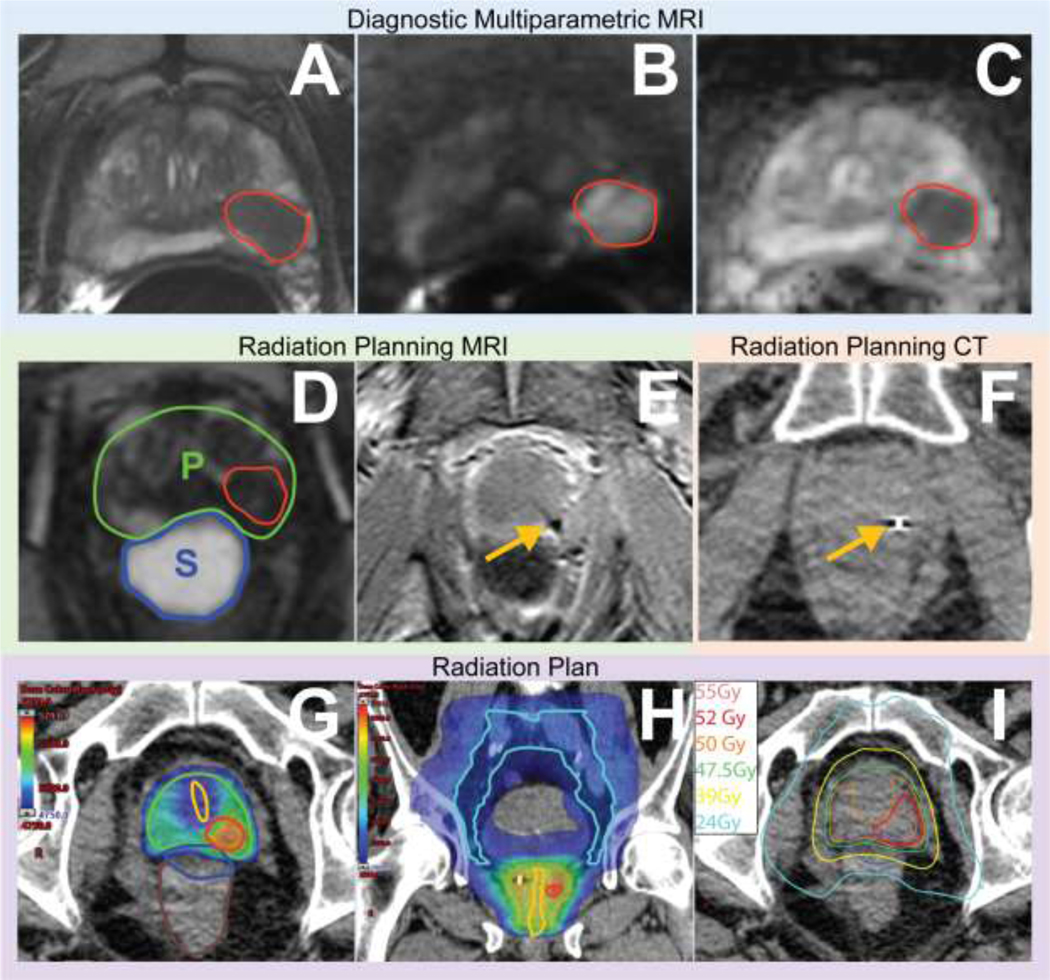
Figure 1.
Integrating diagnostic mpMRI, radiation planning MRI, and CT to delineate lesion(s). Images of a 65-year-old patient with elevated PSA (5.25 ng/mL) and grade group 4 cancer. Diagnostic mpMRI revealed a PI-RADS 5 lesion in the left mid gland peripheral zone shown as a homogeneous, moderately hypointense lesion (red contour) on axial T2-weighted images (A); hyperintense on high b-value diffusion weighted images (B) and hypointense on apparent diffusion coefficient map (C) images. After androgen deprivation and hydrogel spacer placement, and before radiation therapy initiation, radiation planning MRI axial T2-weighted images (D) indicate adequate spacer (S) distribution in the rectoprostatic space and decrease in prostate (P) and lesion size. Although less conspicuous than on diagnostic MRI, the correlation between studies allows us to delineate lesions on treatment planning MR images. Given its vulnerability to artifacts caused by metal, axial gradient-echo MR imaging (E) can easily identify the fiducial markers (yellow arrow) and facilitate fusion of the MRI and radiation planning CT (F) datasets. Radiation plans showing intralesional boost to contoured tumors (red contour G and H), urethral dose sparing (yellow contour G and H), and treatment of pelvic lymph nodes (cyan contour I). Isodose lines show location of 24Gy and 39Gy rectal constraints (I) of the same axial slice as G.
Endpoints
The primary endpoint was the maximum tolerated dose (MTD) as determined by <33% of patients per dose cohort having DLT within 90 days of starting RT. Secondary endpoints included acute (within 90 days of starting RT) and late (>90 days after starting RT) GI/GU toxicity, BC, OS, disease-specific survival (DSS), and health-related quality of life (HRQOL) via the Expanded Prostate Cancer Index Composite for Clinical Practice questionnaire (EPIC). International Prostate Symptoms Score (IPSS) were also collected. Patients were evaluated at 1–1.5, 3, 6, 9, 12, 18, 24, 30, 36, 42, and 48 months.
Data Analysis
All DLTs were continuously monitored and verified by the principal investigator and independently reviewed by the cancer centers’ and departments’ data and safety monitoring committees. Data were analyzed using Microsoft Excel 2017 (Microsoft, Redmond, Washington), Matlab2020b (Mathworks, Natick, Massachusetts), and SAS 9.4 (SAS institute, Cary, North Carolina). Differences in categorical variable were analyzed with chi-squared or Fisher’s exact test while continuous variables were compared with one-way ANOVA. OS, BC, and DSS were estimated with the Kaplan-Meier method.
Differences in baseline characteristics were analyzed with chi-square or Fisher’s exact test for categorical and grouped variables while continuous variables were compared with one-way ANOVA. Differences between cohorts were analyzed with pair-wise comparison with Bonferroni correction.
Differences in physician reported toxicity between cohorts were assessed with chi-square or Fisher’s exact test and pair-wise comparison with Bonferroni correction. Differences in IPSS symptom score were assessed, relative to baseline values, using one-way ANOVA comparing all 4 cohorts.
Minimal clinically important change (MCIC) in an EPIC domain was determined by the standard deviation (SD) method at each time point as well as the anchor-based method.(28) A difference of 5 points in the bowel domain and 6 points in the urinary domain was considered significant with the anchor-based method.(28) A SD of 0.5 was considered a small MCIC whereas a SD of 1 was considered a large MCIC.
Results
Patient Demographics
Sixty-two men were enrolled in the trial from 11/2015 to 10/2019 with 55 patients included in the analysis (Table 1). Seven patients were excluded due to the following reasons in accordance with the protocol: no follow up (1), withdrawal of consent before (4) or after treatment (1), and ineligibility (1). At baseline, the median age was 73 (IQR, 67–77) with PSA 9.7 (IQR, 5.8–19.9) and IPSS 8 (IQR, 4.25–12). There were more patients with clinical T3 disease in cohort 1 and 2 than in cohort 3 and 4 (p=0.002 Fisher’s exact test; p=0.004 and p=0.067 for pair-wise comparison between cohort 3 and cohort 1 as well as 2, respectively; p=0.008 and p=0.072 for pair-wise comparison between cohort 4 and cohort 1 as well as 2, respectively); however, no difference was observed in MRI-defined extra-prostatic or seminal vesicle invasion (Table 1 & Table S2). Patients in cohort 2 were marginally older than patients in cohort 1 and cohort 3 (p=0.039 for one-way ANOVA; p=0.07 and 0.09 for pair-wise comparison, respectively). Additional comparisons are in Table S2.
Target dosimetry
The average prescription dose coverage for all patients was 93%, 94%, and 88% for PTV1 (pelvic lymph nodes and distal seminal vesicle), PTV2 (prostate and proximal seminal vesicle), and PTV3 (MRI-defined lesion boost), respectively (Table 2). The average dose to 99% of PTV1, PTV2, PTV3 was 90%, 96%, and 97% of the prescription dose. Cohort 2 had a statistically higher D99% PTV1 coverage than cohort 3 (p=0.02 and p=0.03 by absolute and relative dose, respectively) but not cohort 4 (p=0.054 and p=0.06 by absolute and relative dose, respectively). There were no statistically significant differences in coverage of PTV2 between cohorts (Table 2). Cohort 4 had a lower PTV3 coverage when normalized to the prescribed dose than cohort 3 (p<0.01 for %Vprescription and %D99%) but the absolute D99% was similar in the two cohorts. On review of cohort 3 and 4 radiation plans, there was difficulty achieving the higher PTV3 dose in some cohort 4 patients when the tumor was located near the urethra, due to the urethral dose constraint in our protocol.
Table 2.
Radiation planning dose achieved in PTV for all patients and by cohort. ANOVA analysis between cohorts.
| PTV1 | PTV2 | PTV3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vprescription | D99% | V47.5Gy | D99% | Vprescription | D99% | ||||
| % | Gy | % | % | Gy | % | % | Gy | % | |
|
|
|||||||||
| All Patients | |||||||||
| Avg | 93% | 22 | 90% | 94% | 46 | 96% | 88% | 50 | 97% |
| IQR1 | 90% | 21 | 84% | 93% | 45 | 95% | 88% | 50 | 97% |
| Median | 94% | 23 | 93% | 95% | 46 | 97% | 95% | 50 | 98% |
| IQR3 | 97% | 24 | 96% | 97% | 47 | 99% | 95% | 52 | 99% |
| Cohort 1 | 22.5Gy | 47.5Gy | 50 Gy | ||||||
| Avg | 93% | 22 | 91% | 92% | 45 | 95% | 93% | 50 | 99% |
| IQR1 | 91% | 19 | 83% | 89% | 44 | 93% | 93% | 49 | 99% |
| Median | 94% | 21 | 95% | 95% | 46 | 97% | 95% | 50 | 99% |
| IQR3 | 96% | 22 | 96% | 96% | 47 | 98% | 98% | 50 | 100% |
| Cohort 2 | 25Gy | 47.5Gy | 50 Gy | ||||||
| Avg | 93% | 23 | 93% | 95% | 46 | 97% | 96% | 50 | 99% |
| Median | 93% | 24 | 94% | 95% | 46 | 98% | 95% | 50 | 100% |
| IQR1 | 90% | 23 | 90% | 94% | 46 | 96% | 95% | 49 | 98% |
| IQR3 | 100% | 25 | 101% | 98% | 47 | 99% | 95% | 50 | 100% |
| Cohort 3 | 25Gy | 47.5Gy | 52.5Gy | ||||||
| Avg | 91% | 21 | 84% | 95% | 46 | 97% | 93% | 51 | 98% |
| IQR1 | 87% | 19 | 76% | 93% | 45 | 95% | 95% | 51 | 97% |
| Median | 91% | 21 | 83% | 95% | 46 | 97% | 95% | 52 | 98% |
| IQR3 | 97% | 23 | 94% | 97% | 47 | 99% | 95% | 52 | 99% |
| Cohort 4 | 25Gy | 47.5Gy | 55 Gy | ||||||
| Avg | 92% | 23 | 92% | 94% | 46 | 97% | 74% | 51 | 93% |
| IQR1 | 89% | 22 | 89% | 94% | 45 | 95% | 64% | 49 | 88% |
| Median | 95% | 23 | 93% | 96% | 46 | 97% | 73% | 51 | 93% |
| IQR3 | 97% | 24 | 97% | 97% | 47 | 98% | 95% | 54 | 97% |
| ANOVA | 0.71 | <0.01 | 0.02 | 0.19 | 0.19 | 0.19 | <0.01 | <0.01 | <0.01 |
Acute toxicity
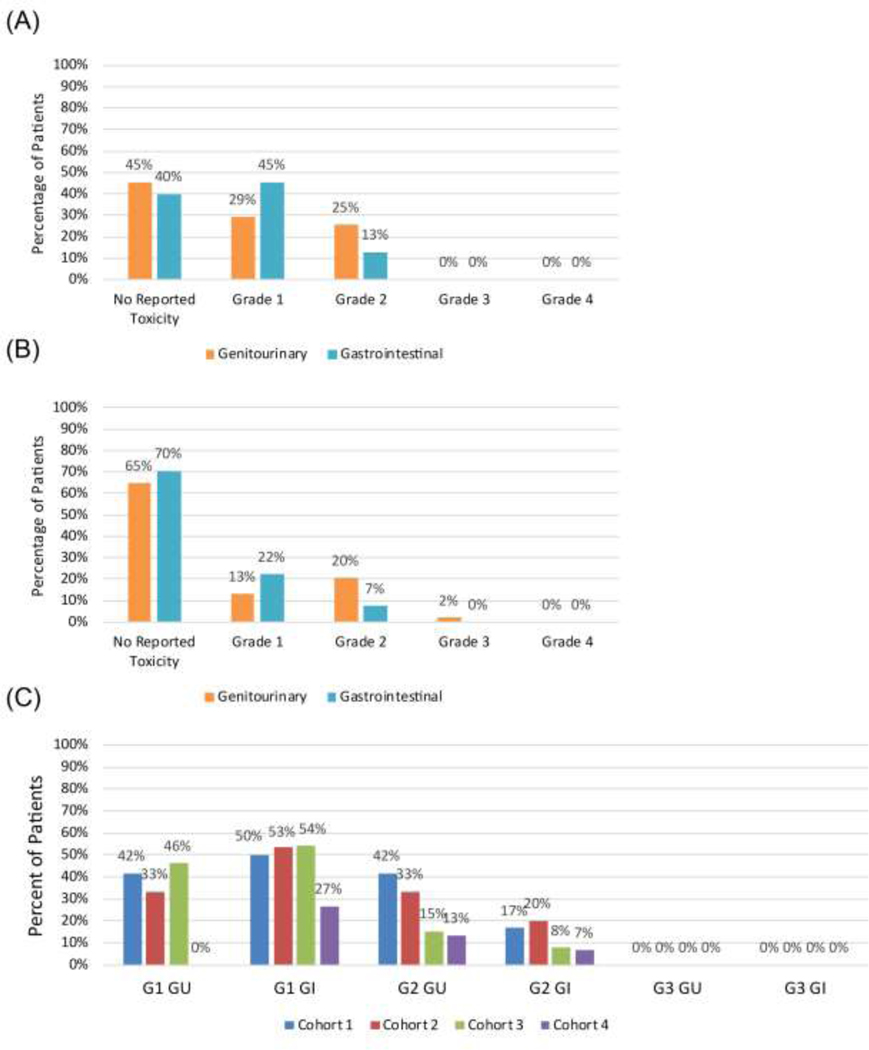
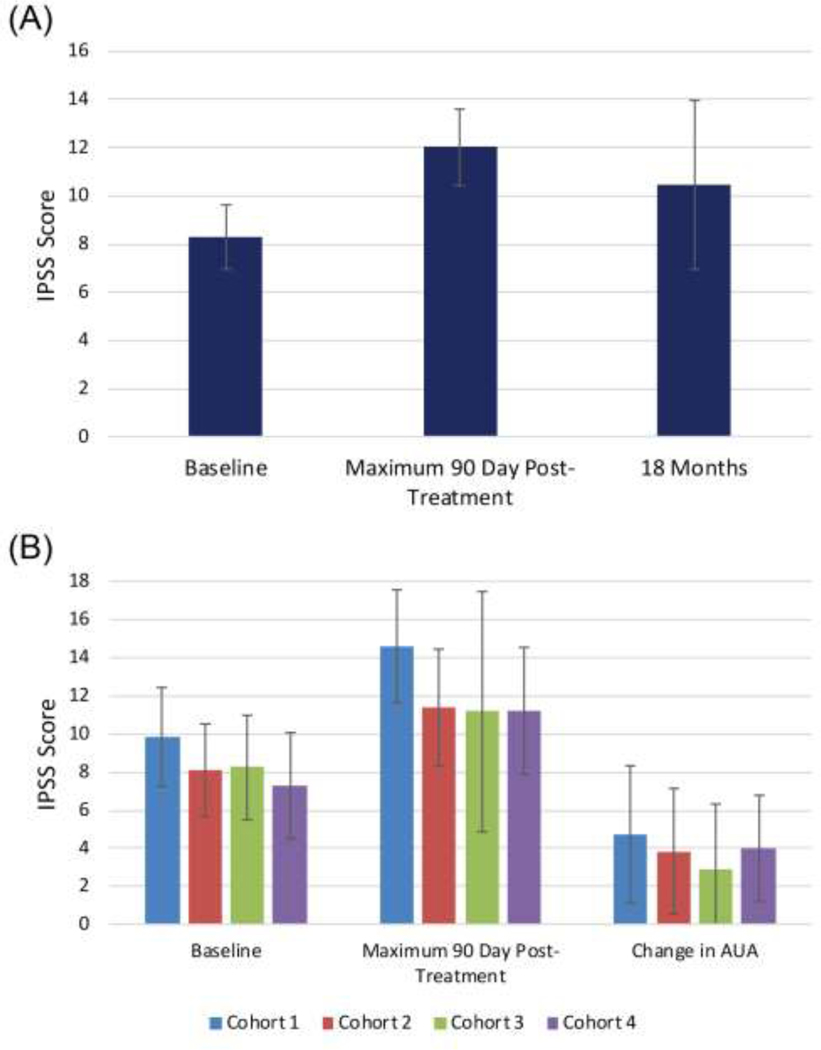
Median follow-up was 18 months, and all patients, except for 1 who unexpectedly died after his 3-month follow-up (unrelated to treatment or cancer), achieved a minimum follow-up of 6 months (range 3–48 months). Median follow-up for cohorts 1–4 were 42, 24, 12, and 7.5 months, respectively (Table 1). Patients completed treatment in all 4 cohorts with no dose limiting toxicities within 90 days of treatment. Twenty-five percent of men experienced grade 2 GU toxicity and 13% experienced grade 2 GI toxicity during this time (Figure 2A). No acute grade 3 or 4 toxicity was seen. There was no statistical increase in acute toxicity with dose-escalation; indeed, acute GU toxicity was the lowest in the highest dose cohort compared to the other 3 cohorts (p=0.002, Figure 2C), driven by fewer G1 GU toxicities (p=0.01). Grade 1 and 2 GI and grade 2 GU acute toxicities were less common in later cohorts but did not reach statistical significance (Table S3). Baseline IPSS score increased from a mean of 8.3 to 12.0 during the 90-day window (95% CI, [7.0–9.6] to [10.4–13.6], p=0.001) (Figure 3A). No significant difference was observed at baseline, maximum score within 90 days, or change in IPSS score between cohorts (Figure 3B).
Figure 2.
Maximum physician-reported CTCAE acute (A) and late (B) GU and GI toxicities. Acute toxicity by cohort (C).
Figure 3.
Patient-reported outcomes on IPSS symptom score questionnaire with 95% CI at baseline, maximum 90 days post-treatment, and 18 months post-treatment (A). Average IPSS, maximum 90-day IPSS, and change in IPSS from baseline to maximum 90-day with 95% CI within each cohort(B).
Late toxicity
Twenty percent of participants developed late grade 2 GU toxicity and 7% had late grade 2 GI toxicity (Figure 2B). One participant (2%) treated in the lowest dose cohort developed late grade 3 GU toxicity. He developed urinary retention 15 months after treatment and required TURP with resection of lateral and median prostate lobes. No ≥ grade 4 late GU or ≥ grade 3 late GI toxicity was seen. Mean IPSS score at 18 months was 10.5 (95% CI, 7.0–14.0) and was not statistically different from baseline (p=0.17) (Figure 3A).
Patient-reported outcomes
A small decrease in EPIC score was observed in the GI domain at 1.5 months relative to baseline (difference [95% CI], −4.6 [−0.7 to −1.8]). At 3 months, a minimal change was observed in EPIC score in the GI domain relative to baseline (difference [95% CI], 1.3 [−1.2 to 3.8]). Similarly, a trend toward lower GU EPIC scores was seen at 1.5 months (difference [95% CI], −1.9 [−5.4 to 1.7]) and a trend toward improved scores at 3 months relative to baseline (difference [95% CI], 2.2 [−1.2 to 5.6]) (Figure S1). Fewer patients completed EPIC questionnaires at 12 and 18 months; however, a lower EPIC score was seen in the GU domain at 12 months (difference [95% CI], −10.8 [−17.6 to −4]) and GI domain at 18 months (difference [95% CI], −9.6[−16.6 to −2.6]) relative to both baseline and 3 months (Figure S1).
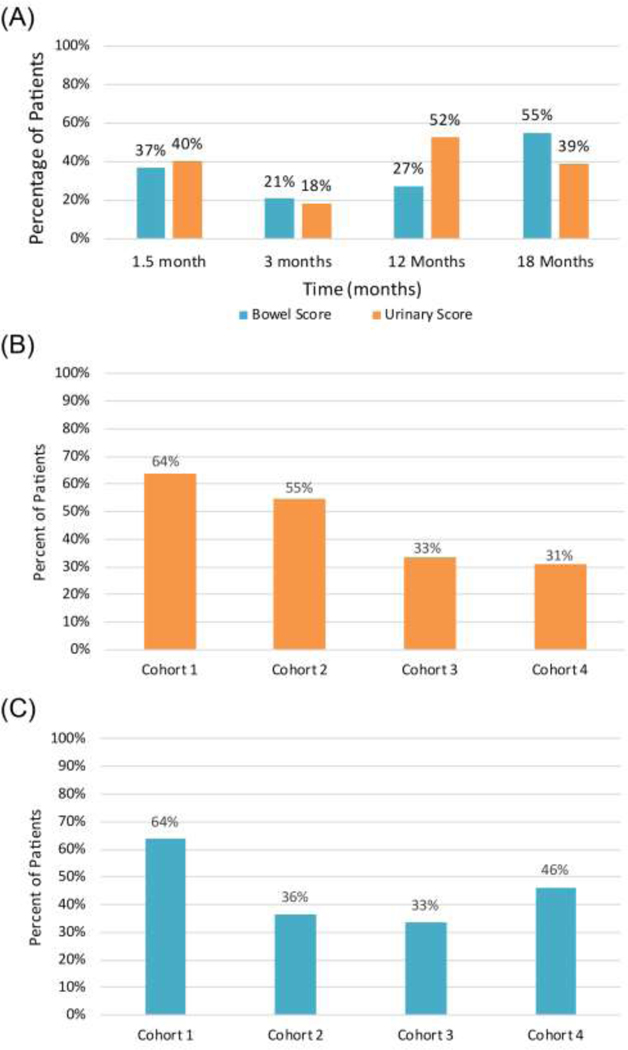
Using anchor-based MCIC, at 1.5 months, 40% and 37% of patients showed MCIC in urinary and bowel domains, respectively (Figure 4A). MCIC decreased to 18% and 21% at 3 months for urinary and bowel domains, respectively. At 18 months, 39% and 55% of patients had MCIC in urinary and bowel domains, respectively. Using the lowest score within the first 3 months for each patient, 45% of patients developed GI and 45% developed GU MCIC. Numerically, more patients reached GU MCIC within 90 days in cohort 1–2 than in cohort 3–4 but this was not statistically different (p=0.36, Figure 4B). No differences were observed in patients who reached MCIC in the GI domain (p=0.49, Figure 4C). The number of patients who have small MCIC with the SD method is similar to that observed with the anchor-based method (Figure S2).
Figure 4.
Patient-reported EPIC score reaching MCIC using the anchor-based method. A threshold change for MCIC of 5 for the bowel domain and 6 for the urinary domain was used (A). Percent of patients who reached MCIC within the first 90 days stratified by cohort in the GU domain (B) and the GI domain (C).
Androgen deprivation therapy compliance
At the time of data analysis, 26 patients received ADT for 24 months (47%), 10 patients (18%) stopped ADT prematurely due to side effects/insurance/non-compliance, and the remaining 19 patients (35%) continued to receive ADT. Fourteen patients (25%) recovered their testosterone levels.
Oncologic outcomes
The 2-year actuarial BC is 96.6%, bPFS 94.8%, DSS is 100%, and OS is 98.2%. A single patient developed biochemical recurrence 18 months after treatment. The patient’s PSA increased from 0.06 to 1.2 ng/mL 12 months after radiation treatment, while he was receiving ADT. PET Axumin did not reveal abnormality at that time. The patient did not tolerate ADT and elected to stop therapy, having received 18 months of ADT. At the 18-month follow-up, PSA rose to 5.1, testosterone increased to 217ng/dL, and PET Axumin scan revealed solitary uptake in the right iliac bone. Upon retrospective review, the area was sclerotic on CT simulation scan. The patient received focal SBRT to the area and, shortly thereafter, developed widely metastatic disease and was re-started on ADT.
Discussion
With a median follow-up of 18 months, we successfully escalated doses of 5-fraction SAbR treatment in patients with HR-PCa to 55Gy for mpMRI delineated target lesions, 47.5Gy to the entire prostate gland along with proximal seminal vesicles, and 25Gy to pelvic lymph nodes without reaching dose-limiting acute toxicity. This is the highest reported dose of SAbR treatment for HR-PCa and represents significant treatment intensification compared to previously reported studies. This is also one of the first SAbR trials of HR-PCa to incorporate elective nodal RT and a peri-rectal hydrogel spacer.
Varying dose regimens have been used for prostate SAbR but the optimal dose remains controversial. We previously escalated SAbR dose for patients with intermediate risk PCa to 47.5Gy in 5 fractions, which was used as the prostate dose for all patients in the current trial. (9) Recognizing that failures probably predominate at initial disease sites, we sought to leverage the accuracy of mpMRI in detecting intraprostatic disease sites for focal dose escalation. The FLAME randomized phase III trial treated patients with intermediate-risk and HR-PCa to either standard 77Gy in 2.2Gy/fraction to the entire prostate or 77Gy in 2.2Gy/fraction to the prostate with an SIB boost to mpMRI-delineated intraprostatic lesions up to 95Gy (2.7Gy/fraction). They recently reported improved biochemical DFS (HR 0.45) with intraprostatic boost.(5)
The intraprostatic lesion dose in our study is a significant dose escalation compared to previous reports and rivals the expected biologic effective doses in interstitial brachytherapy. The alpha-beta ratio of PCa is approximately 1.5–4.(8) Assuming an alpha-beta ratio of 3 and using the linear-quadratic model, the target lesions in our highest dose cohort received an EQD2 of 154Gy, while the boost dose in the FLAME trial has an EQD2 of 108.3Gy. A common monotherapy HDR treatment regimen is 27Gy in 2 fractions(29), which corresponds to a nominal EQD2 of 89 Gy, 145 Gy with a 30% hotspot, and 188Gy with a 50% hotspot in the lesion.
In our cohort, acute 90-day grade 2 GU toxicity was 25% and GI toxicity was 13%, which are comparable to other CFRT/SAbR reports. PACE-B treated low and intermediate risk patients with SAbR using 36.25Gy in 5, 62Gy in 20, or 78Gy in 39 fractions and reported similar toxicity (21% grade 2 GU and 10% grade 2 GI toxicity in the SAbR arm; 27% grade 2 GU and 12% grade 2 GI toxicity in other arms).(30) HYPO-RT-PC compared 78Gy in 39 fractions to 42.7Gy in 7 fractions for intermediate and HR-PCa.(31) Grade ≥2 GU toxicity was 28% in the SAbR arm and 23% in the CFRT arm; about 6–8% grade ≥2 GI toxicity was observed at the end of treatment.(31) FLAME reported about 40% acute grade ≥2 GU toxicity for both CFRT and SAbR, and about 10–12% acute grade ≥2 GI toxicity by the end of treatment.(32) In hypoFLAME, the entire prostate received 35Gy in 5 fractions with an mpMRI-defined intraprostatic boost to 50Gy; 34% grade ≥2 acute GU and 5% acute grade ≥2 GI toxicity were reported.(14) The studies described similar rates of grade ≥2 GU acute toxicity and similar or lower rates of GI toxicity; however, pelvic lymph nodes were not treated.
Elective treatment of pelvic lymph nodes in HR-PCa remains controversial because some trials (i.e. RTOG 9413, GETUG-01) showed no benefit and others (i.e. POP-RT) showed biochemical failure-free survival and distant metastasis-free survival benefit.(33–35) We included elective pelvic nodal coverage in all cohorts and escalated from 22.5Gy to 25Gy. Treating pelvic nodes is expected to increase GI toxicity, as seen in CFRT trials that compared whole pelvis treatment with prostate-only treatment.(18) FASTR attempted to treat both prostate with 50Gy and pelvic lymph nodes with 25Gy in 5 fractions.(15) The trial was terminated early after accruing 16 patients because 3 patients developed grade 3 toxicity and 1 patient had grade 4 toxicity. The group’s subsequent trial, FASTR-2, only treated the prostate with 35Gy in 5 fractions and reported good toxicity outcomes (14% acute grade 2 GU and 3.7% acute grade 2 GI toxicity).(13) In SATURN, HR-PCa was treated with 40Gy and pelvic lymph nodes with 25Gy all in 5 fractions.(16) They reported 46% acute grade 2 GU toxicity and 3% acute grade 2 GI toxicity.(16) GU toxicity was significantly higher than that observed in our trial and may be attributed to differences in reporting and high level of baseline grade 2 GU symptom (30%) in SATURN. Also in SATURN, the 3 month EPIC large MCIC was 5.6% for GU and 24% for GI domains, which is similar to 8% in the GU domain and significantly higher than 10% in the GI domain in this trial.(13) Patients in our study had similar or better GI toxicity than other reported SAbR prospective trials that targeted pelvic lymph nodes.
The favorable toxicity profile in this trial is likely attributed to a combination of systematically using a peri-rectal hydrogel spacer(36), prophylactic steroids as well as alpha blocker, evidence-derived constraints, stereotactic setup, small PTV margins, and image guidance. We previously escalated the SAbR dose for treating intermediate PCa in a phase 1 study based on acute toxicity; however, unacceptable long-term rectal toxicity was observed.(9,10,26) This was addressed by using a rectal hydrogel spacer and defining new rectal constraints, which were used in our protocol. While randomized data on the benefit of hydrogel spacer in the setting of SAbR have not been published, a phase II study reported decreased rectal ulceration than previous phase I/II trials(37). Interestingly, acute grade 2 toxicity was higher in that study (46.5% for GU and 24% for GI) than in the current study. This may be due to differences in defining “acute” period (270 days compared with 90 days), lack of prophylactic steroids/alpha blocker, lack of urethral constraint, and possibly more limited experience with spacer placement/planning/treatment delivery. Increased experience with the protocol may also explain why fewer GU toxicities and trend toward lower GI toxicity were reported in later cohorts of this study, despite receiving higher doses than earlier cohorts. Further improvements in toxicity may be achieved with adaptive treatment and MR-guided radiotherapy.
The significance of urethral dose in developing urinary toxicity in interstitial brachytherapy is well established, and guidelines recommend minimizing hotspots around the urethra.(38) The role of the urethral dose in CFRT/SAbR is more controversial. A randomized phase II clinical trial comparing CFRT with 75.85Gy in 41 fractions to CFRT with urethral sparing (mean ≤65Gy to proximal prostatic urethra and ≤74Gy to distal prostatic urethra) in men with low-risk prostate cancer was performed.(39) The trial was accruing slowly and closed after enrolling 16 patients. They failed to show a benefit in toxicity but there was a higher PSA nadir with urethra sparing and strong trend toward increased PSA failure (p=0.06). Yet voxel-based dosimetry suggests that prostatic urethra and portions of the bladder wall are predictors of urinary toxicity.(40) Rather than deescalating the urethra dose, we elected to minimize hotspots with a maximum point dose <49.87 Gy.
Limitations
Limited follow-up to assess late toxicity, particularly for cohorts 3 and 4, and oncologic outcomes are the main limitations of the study. While the overall median follow-up of the study is 18 months, cohort 1 and cohort 2 had 42 and 24 months of follow-up, respectively. Although one patient developed late grade 3 GU toxicity at 15 months, this toxicity is likely uncommon given that 28 other participants had longer than 18 months of follow-up without experiencing ≥grade 3 toxicities. Nevertheless, additional toxicity events, especially for cohort 3 and 4, are expected with longer follow-up.
Conclusions
SAbR for HR-PCa using multi-level dose painting is feasible and can be safely delivered with 47.5Gy to prostate, 55Gy to mpMRI-defined intra-prostatic lesion(s), and 25Gy to pelvic lymph nodes in 5 fractions. The treatment was well tolerated with acceptable acute 90-day GU/GI toxicity. This is the highest reported SAbR dose for treating HR-PCa in 5 fractions. Long-term follow-up is required to assess late toxicity and outcomes.
Supplementary Material
Acknowledgements
We thank Dr. Damiana Chiavolini for the scientific editing of this manuscript.
Funding Statement
The trial is funded by the Department of Radiation Oncology, UT Southwestern Medical Center. Boston Scientific provided the peri-rectal hydrogel spacers. Dr. Hannan and Dr. Wang are supported by NIH R01 EB027898.
Dr. Timmerman reported research funding from Varian Medical Systems, Elekta Oncology, and Accuray, outside the submitted work. Dr. Hannan reported non-financial funding from Boston Scientific who provided hydrogel spacer for the trial. Dr. Desai reported consultation and research funding from Boston Scientific, outside the submitted work. Dr. Folkert reports research funding and travel reimbursement from Boston Scientific and travel reimbursement from Varian Medical Systems, unrelated to submitted work. Dr. Lotan reported grants from GENOMEDX and mdxhealth, unrelated to submitted work. Dr. Costa reported institutional grant from Bayer, consultation fees from Profound, scientific review Honoria from NIH, unrelated to submitted work.
Footnotes
Conflict of Interest Statement for All Authors
The remaining authors do not have any conflicts of interest to report.
Data Availability Statement for this Work
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zaorsky NG, Trabulsi EJ, Lin J, et al. Multimodality therapy for patients with high-risk prostate cancer: Current status and future directions. Semin Oncol 2013;40:308–321. [DOI] [PubMed] [Google Scholar]
- 2.Zaorsky NG, Palmer JD, Hurwitz MD, et al. What is the ideal radio-therapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol 2015;115:295–300. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Kollmeier M, McBride S, et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol 2019;104:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson WC, Silva J, Hartman HE, et al. Stereotactic body radiation therapy for localized prostate cancer: A systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol 2019;104:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intrapro-static tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the flame randomized phase III trial. J Clin Oncol 2021. JCO2002873. [DOI] [PubMed] [Google Scholar]
- 6.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: An ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol 2018. JCO1801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogelius IR, Bentzen SM. Dose response and fractionation sensitivity of prostate cancer after external beam radiation therapy: A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 2018;100:858–865. [DOI] [PubMed] [Google Scholar]
- 8.Vogelius IR, Bentzen SM. Diminishing returns from ultrahypofractionated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2020;107:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannan R, Tumati V, Xie XJ, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-results from a multi-institutional clinical trial. Eur J Cancer 2016;59:142–151. [DOI] [PubMed] [Google Scholar]
- 10.Boike TP, Lotan Y, Cho LC, et al. Phase i dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rooij M, Hamoen EH, Futterer JJ, et al. Accuracy of multiparametric mri for prostate cancer detection: A meta-analysis. AJR Am J Roentgenol 2014;202:343–351. [DOI] [PubMed] [Google Scholar]
- 12.Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: A six year study. Radiat Oncol 2014;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callan L, Bauman G, Chen J, et al. A phase I/II trial of fairly brief androgen suppression and stereotactic radiation therapy for high-risk prostate cancer (fastr-2): Preliminary results and toxicity analysis. Adv Radiat Oncol 2019;4:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draulans C, van der Heide UA, Haustermans K, et al. Primary endpoint analysis of the multicentre phase II hypo-flame trial for intermediate and high risk prostate cancer. Radiother Oncol 2020;147:92–98. [DOI] [PubMed] [Google Scholar]
- 15.Bauman G, Ferguson M, Lock M, et al. A phase 1/2 trial of brief androgen suppression and stereotactic radiation therapy (fastr) for high-risk prostate cancer. Int J Radiat Oncol 2015;92:856–862. [DOI] [PubMed] [Google Scholar]
- 16.Musunuru HB, D’Alimonte L, Davidson M, et al. Phase 1–2 study of stereotactic ablative radiotherapy including regional lymph node irradiation in patients with high-risk prostate cancer (saturn): Early toxicity and quality of life. Int J Radiat Oncol Biol Phys 2018;102:1438–1447. [DOI] [PubMed] [Google Scholar]
- 17.Sandler KA, Cook RR, Ciezki JP, et al. Prostate-only versus whole-pelvis radiation with or without a brachytherapy boost for gleason grade group 5 prostate cancer: A retrospective analysis. Eur Urol 2020;77:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol 2018;19:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy V, Gupta M, Mulye G, et al. Early results of extreme hypofractionation using stereotactic body radiation therapy for high-risk, very high-risk and node-positive prostate cancer. Clin Oncol (R Coll Radiol) 2018;30:442–447. [DOI] [PubMed] [Google Scholar]
- 20.Pinitpatcharalert A, Happersett L, Kollmeier M, et al. Early tolerance outcomes of stereotactic hypofractionated accelerated radiation therapy concomitant with pelvic node irradiation in high-risk prostate cancer. Adv Radiat Oncol 2019;4:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiyama H, Hirayama T, Jhaveri P, et al. Is there an increase in genitourinary toxicity in patients treated with transurethral resection of the prostate and radiotherapy? A systematic review. Am J Clin Oncol 2014;37:297–304. [DOI] [PubMed] [Google Scholar]
- 22.Cisel B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 × 5 gy and consolidation chemotherapy for clinical t4 and fixed clinical t3 rectal cancer: Long-term results of the randomized Polish II study. Ann Oncol 2019;30:1298–1303. [DOI] [PubMed] [Google Scholar]
- 23.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012;83:e353–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa DN, Pedrosa I, Roehrborn C, et al. Multiparametric magnetic resonance imaging of the prostate: Technical aspects and role in clinical management. Top Magn Reson Imaging 2014;23:243–257. [DOI] [PubMed] [Google Scholar]
- 25.Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019;76:340–351. [DOI] [PubMed] [Google Scholar]
- 26.Kim DWN, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1–2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol 2014;89:509–517. [DOI] [PubMed] [Google Scholar]
- 27.Kishan AU, Tyran M, Weng J, et al. Stereotactic body radiotherapy to the prostate and pelvic lymph nodes: A detailed dosimetric analysis of a phase ii prospective trial. Br J Radiol 2019;92 20181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology 2015;85:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton G, McGuffin M, Chung HT, et al. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Efficacy results from a randomized phase II clinical trial of one fraction of 19 Gy or two fractions of 13.5 Gy. Radiother Oncol 2020;146:90–96. [DOI] [PubMed] [Google Scholar]
- 30.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–395. [DOI] [PubMed] [Google Scholar]
- 32.Monninkhof EM, van Loon JWL, van Vulpen M, et al. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the flame randomized controlled trial. Radiother Oncol 2018;127:74–80. [DOI] [PubMed] [Google Scholar]
- 33.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the getug-01 randomized study. Int J Radiat Oncol Biol Phys 2016;96:759–769. [DOI] [PubMed] [Google Scholar]
- 34.Murthy V, Maitre P, Kannan S, et al. Prostate-only versus whole-pelvic radiation therapy in high-risk and very high-risk prostate cancer (POP-RT): Outcomes from phase III randomized controlled trial. J Clin Oncol 2021:JCO2003282. [DOI] [PubMed] [Google Scholar]
- 35.Miller LE, Efstathiou JA, Bhattacharyya SK, et al. Association of the placement of a peri-rectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: A systematic review and meta-analysis. JAMA Netw Open 2020;3:e208221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folkert MR, Zelefsky MJ, Hannan R, et al. A multi-institutional phase ii trial of high-dose SAbR for prostate cancer using rectal spacer. Int J Radiat Oncol Biol Phys 2021;111:101–109. [DOI] [PubMed] [Google Scholar]
- 37.Yamada Y, Rogers L, Demanes DJ, et al. American brachytherapy society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy 2012;11:20–32. [DOI] [PubMed] [Google Scholar]
- 38.Vainshtein J, Abu-Isa E, Olson KB, et al. Randomized phase II trial of urethral sparing intensity modulated radiation therapy in low-risk prostate cancer: Implications for focal therapy. Radiat Oncol 2012;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mylona E, Ebert M, Kennedy A, et al. Rectal and urethro-vesical subregions for toxicity prediction after prostate cancer radiation therapy: Validation of voxel-based models in an independent population. Int J Radiat Oncol Biol Phys 2020;108:1189–1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.