Abstract
Nasojejunal tubes (NJTs) are increasingly used in critically ill patients. NJT insertion with endoscopic- or x-ray-guidance can be achieved with success rates above 90%. This systematic review and meta-analysis of randomized controlled trials (RCTs) compares the efficiency and safety of these two methods in critically ill patients. We searched Chinese and English databases for RCTs comparing endoscopy- and x-ray-guided NJT placement published up to July 5, 2021. Meta-analyses were performed using RevMan5 software to compute mean differences (MDs) and odds ratios (ORs). Eleven RCTs (n=676) were included. The endoscopic group had a higher procedure success rate (OR=2.14, 95% CI [1.19, 3.85], Z=2.52, P=0.01) and shorter insertion time (MD=-3.70 min, 95% CI [-6.90, -0.50], Z=2.27, P=0.02) than the x-ray group. NJT indwelling time and post-insertion complications were similar between groups. The x-ray group had fewer complications during placement (OR=8.08, 95% CI [3.58, 18.22], Z=5.03, P<0.00001]; on subgroup analysis, only gastrointestinal non-bleeding adverse events differed significantly between groups (OR=2.78, 95% CI [1.43, 5.39], Z=3.03, P=0.002). Visual analog scale discomfort scores were better in the x-ray group (MD=4.10, 95% CI [3.57, 4.63], Z=15.07, P<0.00001). Compared with x-ray-guided NJT placement, endoscopy-guided placement was faster, had a higher success rate, and was associated with fewer gastrointestinal non-bleeding adverse events and less discomfort during insertion. Endoscopic guidance is recommended for NJT placement in critically ill patients to improve placement efficiency. X-ray guidance is a good alternative, depending on the hospital setting, as it is convenient, economical, and potentially safer.
Keywords: Nasojejunal tube, endoscope, x-ray, meta-analysis
Introduction
The latest guidelines issued by the American Critical Medical Association and the American Society of Enteral and Parenteral Nutrition in 2016 recommended the use of nasojejunal tubes (NJTs) for patients with a high gastric residual volume, high risk of aspiration, or gastric tube intolerance [1]. NJTs have a number of advantages over nasogastric tubes in critically ill patients, including reducing the risk of reflux and aspiration of gastric content and increasing the tolerance of enteral nutrition [2]. A 2013 systematic review showed that NJTs also reduce the risk of pneumonia and ventilator-associated pneumonia in critically ill patients, compared with nasogastric tubes [3].
NJT use for nutritional support is increasing among critically ill patients [4-6]. Establishing methods for improving the success rate and safety of NJT placement is an urgent clinical issue. A randomized controlled study showed that the overall success rate of NJT placement with gastric prokinetic agent use was approximately 40.4% [7]. Another study found that the success rate of placing spiral NJTs was only 53% in patients with normal gastrointestinal motility [8]. NJTs can be placed into the desired position by many methods, including “blind” insertion, use of gastric prokinetic agents, or guidance with endoscopy, x-ray, B-ultrasonography, or electromagnetic devices [9,10].
Many studies have reported NJT placement success rates of 90%-100% with endoscopic guidance [11,12] and 84% with x-ray guidance [13-15]. Endoscopy-guided NJT placement has the advantages of high efficiency, time saving, and accurate direct vision. Although x-ray-guided placement is noninvasive and inexpensive, it involves radiation exposure. A previous meta-analysis comparing endoscopic- and X-ray-guidance for NJT insertion found no differences between these techniques, only there was a slight difference in safety [16]. However, multiple randomized controlled trials (RCTs) [17-21] have reported significant differences in insertion time and rate of successful placement between the two methods.
Currently, both endoscopy and x-ray are considered to have advantages, as well as disadvantages, when used for NJT placement. There is no clear consensus regarding which is superior for critically ill patients. To this end, we conducted a systematic review with meta-analysis of RCTs comparing endoscopy- and x-ray-guided NJT placement, with the objectives of exploring differences in efficiency and safety between the two methods and establishing a basis for rapid and safe NJT placement in clinical practice.
Methods
Study reporting and registration
This systematic review with meta-analysis was reported and completed according to the Preferred Reporting Item Reporting Guidelines for Systematic Evaluation and Meta-analysis (PRISMA). It is registered with PROSPERO (CRD42021262267).
Eligibility criteria
We included all RCTs published in English or Chinese that directly compared endoscopy- and x-ray-guided NJT placement. The exclusion criteria were randomized crossover, cluster randomized, or quasi-experimental trials. We excluded studies that included subjects younger than 18 years of age but did not exclude studies with different sample sizes, follow-up times, publication years, or language of publication.
Search strategy and study selection
On July 5, 2021, two authors (GZ L, QX) conducted searches of the Cochrane Database of Systematic Reviews, PubMed, Web of Science database, EMBASE (Ovid), China Knowledge Network Infrastructure, Chinese Biomedicine Literature Database, VIP Database, and Wan Fang Data Knowledge Platform. The searches were conducted using a combination of terms: ‘nasointestinal tube’ OR ‘jejunal feeding’ OR ‘naso intestinal tube’ OR ‘nasoenteric tubes’ OR ‘small-bowel feeding tube’ OR ‘feeding tube’ OR ‘gastrojejunal tube’, AND ‘endoscopy’, ‘endoscopic’ OR ‘fluoroscopy’ OR ‘fluoroscopic’ OR ‘X ray’.
The first step was to download the articles into Endnote. The two authors then independently screened the titles/abstracts of the articles to identify potentially relevant articles. The full-text version of these articles was evaluated to determine whether they met the inclusion and exclusion criteria. The final selection of studies was made jointly by the two authors, and differences were resolved through symposium, with input from a third author (SW).
Data extraction
The authors (GZ L, QX) independently extracted the research material using a standard data collection form. The retained data included the name of the first author, the publication year, number of patients, patient characteristics (including mean age), study population, type of NJT, and study outcomes.
When data were missing from the initial study, the corresponding author was emailed two times to try to obtain the information. If there was no response after the second email, the data were considered unreported.
Study outcomes
The primary outcome measures of this systematic review with meta-analysis were the procedure success rate (defined as the percentage of successful placement of the NJT at the desired location), insertion time (defined as the time from onset of the insertion attempt until the tube was fixed at the nostril), and insertion-related complications (e.g., epistaxis, gastrointestinal (GI) bleeding, abdominal pain, aspiration, dyspnea). Secondary outcomes were post-insertion complications (e.g., dislodgement, sinusitis, lung infection) and NJT indwelling time (defined as the time from insertion until the tube was removed).
Validity assessment
Two independent researchers (MY P, XX) performed independent assessments of the risk of bias for the main outcome of the included studies using the risk of bias assessment tool in the Cochrane Reviewers Handbook 5.4.0. Differences between the two authors were resolved through discussion or after consultation with the third author (SW).
Statistical analysis
Odds ratios (ORs) and their 95% confidence intervals (CIs) were used to analyze classification variables. In the meta-analysis of ORs, studies without events were excluded [22]. Mean differences (MDs) and their CIs were calculated for continuous variables. P values of effect size estimates were two-sided, and P values <0.05 were considered statistically significant. Heterogeneity between studies was assessed by calculating the I2 statistic, with an I2>50% or a P value <0.10 indicating the presence of significant heterogeneity. Random effects models were used for meta-analysis of outcomes with higher between-study heterogeneity [17]. A funnel plot was constructed to assess publication bias [22]. Statistical analyses were performed using Review Manager 5.4.0.
Results
Study screening
The PRISMA flowchart is shown in Figure 1. We obtained 1,857 records using our database search strategy and identified 12 records from other sources. A total of 1,546 records underwent title and abstract screening after removing duplicates. The full text of the 39 studies remaining after the initial screening was reviewed to determine whether they met the inclusion/exclusion criteria. A total of 11 studies meeting these criteria were included in the final meta-analysis.
Figure 1.
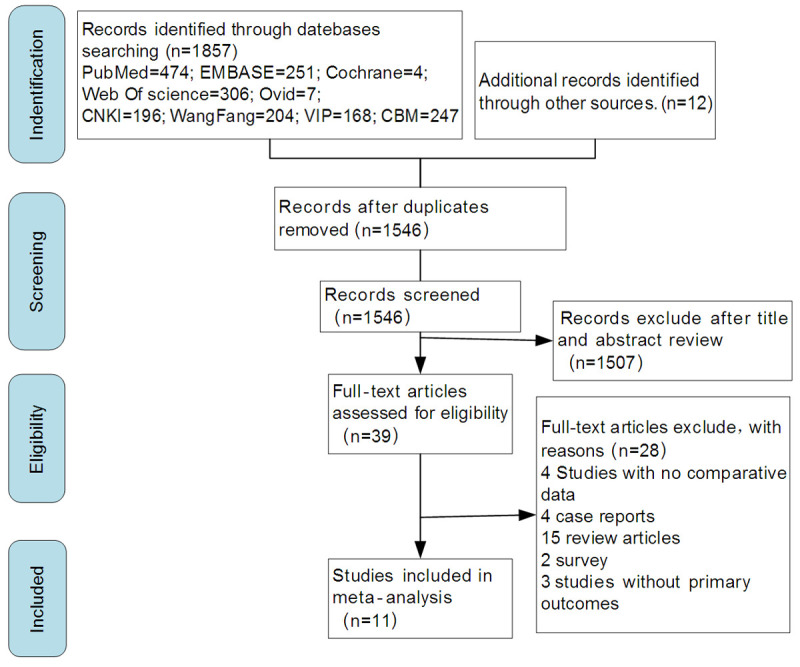
PRISMA flowchart for study selection. Abbreviations: CBM, Chinese Biomedicine Literature Database; CNKI, China Knowledge Network Infrastructure.
Characteristics of the included studies
The characteristics of the 11 included studies are displayed in Table 1. The studies included a total of 676 patients.
Table 1.
Characteristics of the included studies
| Author (year) | Placement method | Sample size | Age (y)a | Study population | Type of tube | Outcomes |
|---|---|---|---|---|---|---|
| Foote 2004 [15] | Endoscopy | 26 | 59.0±4.1 | Surgical ICU patients | 109 cm, 8 F (Corpak Medsystems, Wheeling, IL) | ①②③ |
| X-ray | 17 | 58.1±5.6 | Surgical ICU patients | 109 cm, 8 F (Corpak Medsystems) | ①②③ | |
| Fang 2005 [23] | Endoscopy | 50 | 52 (15-98) | Critically ill patients | 9 F (Sandoz Nutrition, Minneapolis, MN, USA) | ①②③⑤ |
| X-ray | 50 | 55 (13-90) | Critically ill patients | 8 F, 120 cm polyurethane Fredrick-Miller tube | ①②③⑤ | |
| Tong 2009 [14] | Endoscopy | 50 | 50 (20-64) | SAP | 3.33 F | ①②③ |
| X-ray | 50 | 48 (18-63) | SAP | 3.33 F | ①②③ | |
| Sun 2012 [17] | Endoscopy | 31 | 51.6±10.9 | Critically ill patients | 3.33 F | ①②③ |
| X-ray | 31 | 52.6±11.7 | Critically ill patients | 3.33 F | ①②③ | |
| Xie 2012 [24] | Endoscopy | 19 | 37.3 (27-69) | SAP | 3.33 F | ①②③ |
| X-ray | 20 | 39.2 (31-74) | SAP | 3.33 F | ①②③ | |
| Song 2013 [25] | Endoscopy | 24 | 55.6 (35-76) | SAP | 3.33 F | ①②③ |
| X-ray | 28 | 55.3 (32-73) | SAP | 3.33 F | ①②③ | |
| Yang 2015 [26] | Endoscopy | 15 | 49.3 (28-71) | SAP | 3.33 F | ①③④⑤ |
| X-ray | 15 | 50 (29-79) | SAP | 3.33 F | ①③④⑤ | |
| Ma 2016 [18] | Endoscopy | 30 | 57.7±9.1 | Critically ill patients | NA | ①② |
| X-ray | 30 | 58.2±8.7 | Critically ill patients | NA | ①② | |
| Guan 2018 [19] | Endoscopy | 38 | 58.1 (25-79) | SAP | NA | ①②③ |
| X-ray | 39 | 57.2 (27-84) | SAP | NA | ①②③ | |
| Shen 2012 [20] | Endoscopy | 21 | 49.1±12.2 | ICU patients | 3.33 F | ①②③④ |
| X-ray | 22 | 50.1±10.3 | ICU patients | 3.33 F | ①②③④ | |
| Li 2017 [21] | Endoscopy | 20 | 53.8±9.7 | Critically ill patients | 3.33 F | ①② |
| X-ray | 20 | 52.4±10.2 | Critically ill patients | 3.33 F | ①② |
Note: ① Procedure success rate; ② insertion time; ③ placement-related complications; ④ post-insertion tube-related complications; ⑤ nasojejunal tube indwelling time.
Mean ± standard deviation or median (range).
Abbreviations: ICU, Intensive care unit; 3.33 F, Flocare; 3.33 mm, 130 cm; NA, Not available (data not provided); SAP, Severe acute pancreatitis.
Study quality assessment
All of the included studies were RCTs, and each had clearly defined inclusion and exclusion criteria for the study population. However, 5 papers [17,19,21,25,26] did not describe in detail the method of random sequence generation, and 1 study [15] made the allocation concealment public, which was associated with a high risk of bias. As endoscopy- and x-ray-guided NJT placement are different methods, it is difficult to blind researchers and patients to the type of placement. However, because the outcomes of the current meta-analysis are objective, the lack of blinding would not have a substantial impact on our outcomes. Therefore, after discussion among our group, we determined the risk of bias associated with blinding was low in all studies, as shown in Figure 2.
Figure 2.
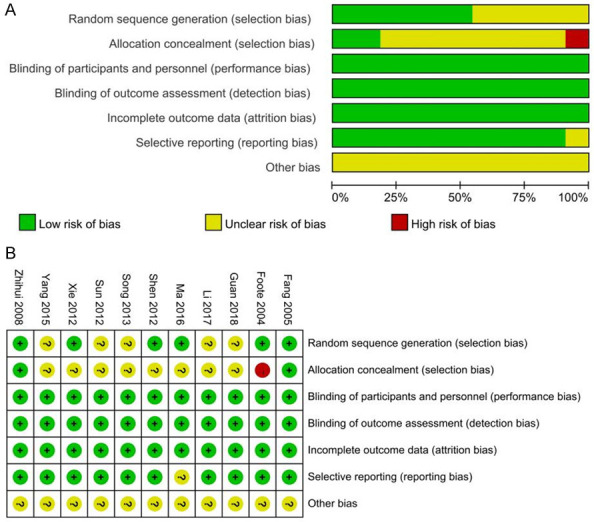
Risk of bias assessment. (A) shows the risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies, and (B) shows the risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Comparison of placement efficiency between groups
Comparison of placement success rate
All studies [14,15,17-21,23-26] reported the success rate of endoscopy- and x-ray-guided NJT placement. The heterogeneity of each study was small P=0.21, I2=26%). Fixed-effects meta-analysis showed that the success rate was significantly higher in the endoscopic group than in the x-ray group (OR=2.14, 95% CI [1.19, 3.85], Z=2.52, P=0.01) (Figure 3).
Figure 3.
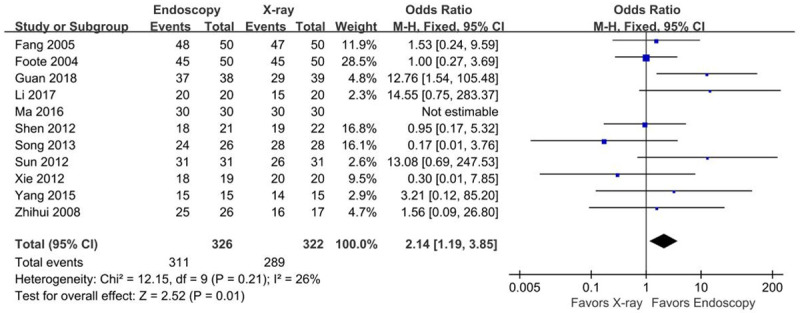
Forest plot for insertion success rate.
Comparison of tube insertion time
Ten studies [14,15,17-21,23-25] reported the insertion time of the endoscopic and x-ray groups, and the heterogeneity among these studies was large (P<0.00001, I2=95%). Sensitivity analysis revealed that deleting any study did not significantly reduce this heterogeneity. A random-effects model was therefore used for the meta-analysis, which showed that the insertion time was significantly shorter in the endoscopic group than in the x-ray group (MD=-3.70 min, 95% CI [-6.90, -0.50], Z=2.27, P=0.02) (Figure 4).
Figure 4.
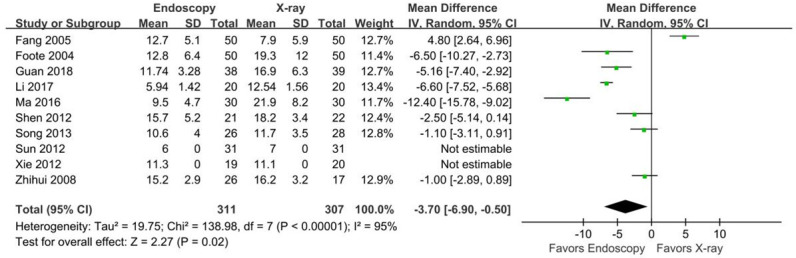
Forest plot for tube insertion time.
Comparison of tube indwelling time
Two studies [23,26] reported the tube indwelling time of NJTs inserted using endoscopy or x-ray guidance. There was no heterogeneity between these studies (P=0.92, I2=0%). Fixed-effects meta-analysis showed no significant difference in indwelling time between groups (MD=0.53 days, 95% CI [-2.49, 3.55], Z=0.34, P=0.73) (Figure 5).
Figure 5.
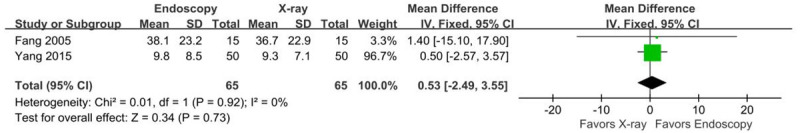
Forest plot for tube indwelling time.
Comparison of safety between insertion methods
Comparison of placement-related complications
Nine studies [14,15,17,19,20,23-26] reported the incidence of placement-related complications in the endoscopy and X-ray groups. Three studies reported 0 complications. Placement-related complications occurred in 61 cases (35%) in the endoscopy group, including 6 cases of epistaxis (9.6%), 37 cases of GI non-bleeding adverse events (25%; abdominal pain, abdominal distension, diarrhea), 5 cases of GI tract bleeding (6.7%), 9 cases of tachypnea (10.4%), and 4 causes of aspiration (11.1%). Placement-related complications occurred in 37 cases (21.6%) in the x-ray group, including 4 cases of epistaxis (7.4%), 20 cases of GI non-bleeding adverse events (12.9%; abdominal pain, abdominal distension, diarrhea), 6 cases of Gl tract bleeding (7.8%), 5 cases of tachypnea (5.7%), and 2 cases of aspiration (5.4%).
There was high heterogeneity among the 9 studies [14,15,17,19,20,23-26] (P<0.000001, I2=86%). Sensitivity analysis revealed no heterogeneity among studies (P=0.98, I2=0%) after excluding 1 heterogeneous source [19]. Fixed-effects meta-analysis including the 3 studies with no complications but excluding the study producing high heterogeneity, showed that the rate of complications was lower in the x-ray group than in the endoscopy group (OR=8.08, 95% CI [3.58, 18.22], Z=5.03, P<0.00001) (Figure 6). In subgroup analysis, only GI non-bleeding adverse events were significantly different between the two groups (OR=2.78, 95% CI [1.43, 5.39], I2=68%), as shown in Figure 7.
Figure 6.
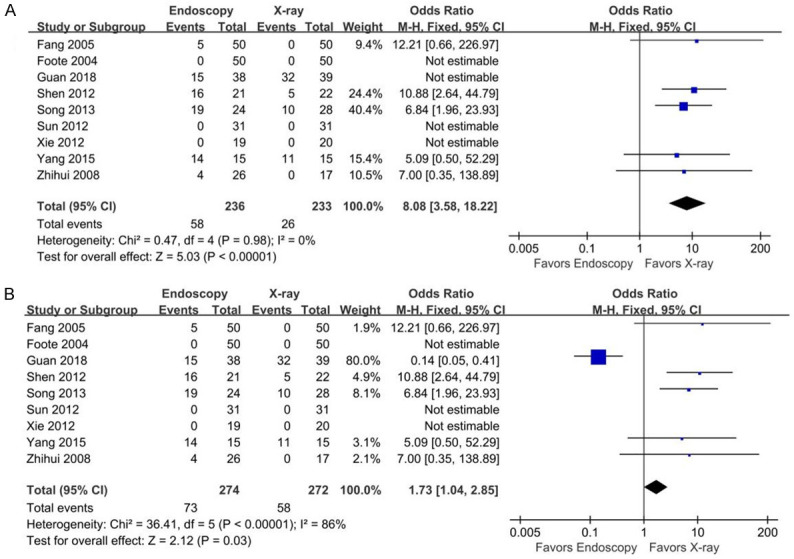
Forest plots for tube placement-related complications. (A) shows the plot with all studies included in the analysis, and (B) shows the plot after the study producing high heterogeneity (Guan 2018) was removed from the analysis.
Figure 7.
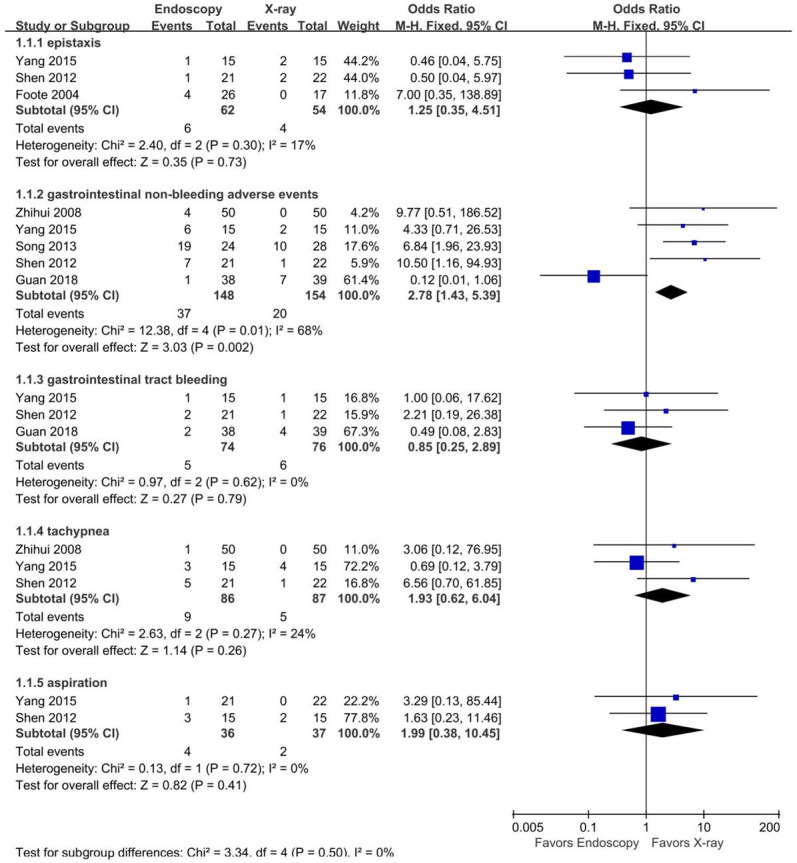
Forest plots of subgroup analysis for placement-related complications.
Comparison of post-insertion tube-related complications
Three studies [20,25,26] reported 125 NJT-related longer-term complications. Post-insertion complications occurred in 27 patients (45.0%) in the endoscopy group, including 3 cases of nasopharyngitis (8.3%), 11 lung infections (18.3%), and 13 tube dislodgements (21.6%). Post-catheter complications occurred in 21 patients (32.3%) in the x-ray group, including 3 cases of nasopharyngitis (8.1%), 9 lung infections (13.8%), and 9 tube dislodgements (13.8%). There were no significant differences in post-insertion complications between groups for the total complications or any of the three subgroups of complications (Figure 8).
Figure 8.
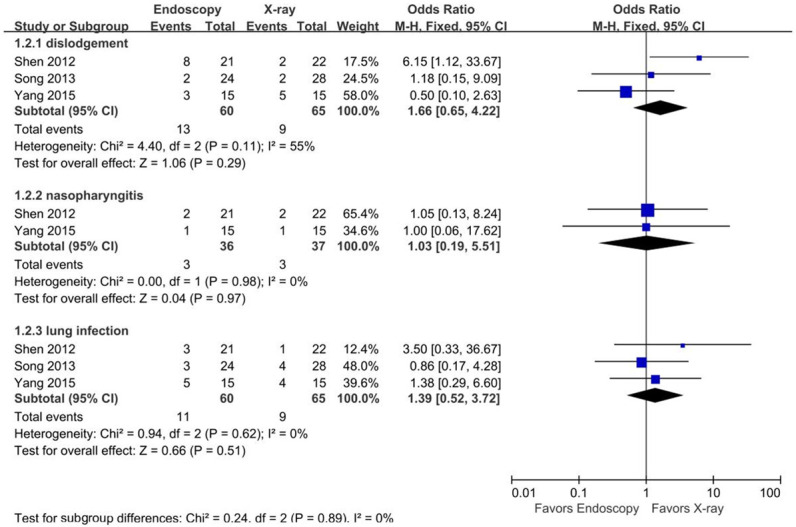
Forest plots for tube-related complications.
Comparison of VAS discomfort scores
Two studies [14,26] used visual analogue scale (VAS) discomfort scores to quantify patient comfort associated with NJT placement. VAS discomfort scores were used to objectively evaluate the degree of pain, with lower scores representing less subjective patient discomfort. The results showed that the mean VAS discomfort score during the procedure was significantly lower in the x-ray group than in the endoscopic group (MD=4.10, 95% CI [3.57, 4.63], Z=15.07, P<0.00001], as shown in Figure 9.
Figure 9.
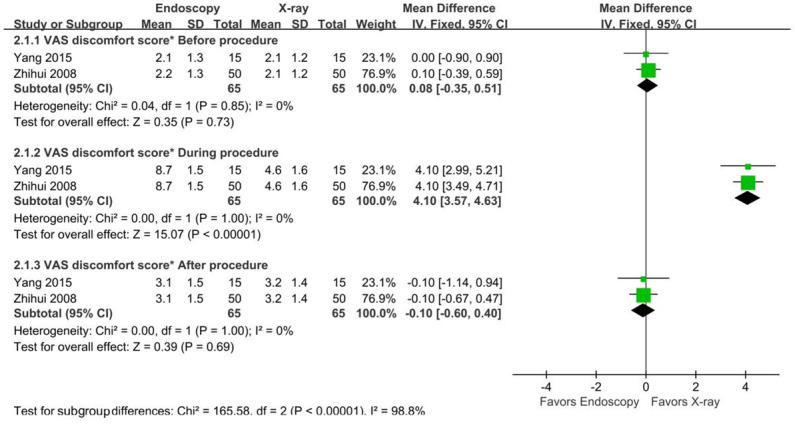
Forest plots for VAS discomfort score*.
Publication bias
We assessed publication bias using a funnel plot. The primary outcome of the included studies (insertion success rate) was used to create the plot. As shown in Figure 10, there was no significant funnel plot asymmetry.
Figure 10.
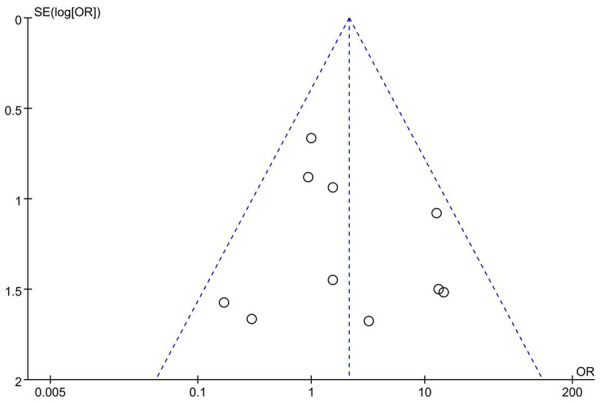
Funnel plot for assessment of publication bias.
Discussion
Endoscopy-guided placement of NJTs is typically performed by endoscopists at the bedside (usually in an ICU). After applying topical anesthetic spray to the nasopharyngeal area, the NJT is inserted into the stomach through one naris, while the endoscope is passed via the other naris into the stomach. The endoscope aids NJT insertion by allowing visualization of the anatomy of the upper GI tract. It also provides a means to help maneuver the tube into the appropriate position: if there is difficulty passing the tube through the pylorus, closed biopsy forceps can be placed through the endoscope’s working channel and be used to grasp the tip of the NJT, moving it through the pylorus and into the proper place. At the end of the procedure, an x-ray is obtained to confirm that the NJT is properly positioned [14,15,23].
X-ray-guided placement of NJTs is performed by radiologists in the radiology department, using a fixed-type C-arm x-ray fluoroscopy machine positioned at the abdomen. After applying local anesthetic (lidocaine) gel to the throat, the NJT is inserted 50 to 55 cm through one nostril. At this point, the tip of the NJT is located at the pylorus. The NJT guidewire is then removed and replaced with a radiation guidewire, which is inserted until it is 3 cm beyond the tip of the NJT. Intermittent or continuous x-ray visualization is used, depending on the individual patient. Next, the NJT is advanced until it is at the level of the ligament of Treitz. The radiation guidewire is then removed, and the NJT is fixed after confirming it is in proper position by fluoroscopy [14,15,23].
X-ray-guidance during NJT placement has the advantages of being non-invasive, requiring no special equipment, and providing direct visualization. The direction of the GI tract is seen by radiography and the position of the tip of the NJT can also be well visualized. However, domestic and foreign scholars generally believe that endoscopy-guided nasoenteral nutrition tube placement has a higher one-time success rate [11,12].
Our study showed that the efficiency of endoscopy-guided NJT placement was higher than that of x-ray-guided placement. Specifically, the endoscopic group had a significantly higher insertion success rate (OR=2.14) and shorter tube insertion time (MD=-3.70 min). In the 3 included studies [18,21,26], the success rate of endoscopic tube placement was 100%. The tube indwelling time was not significantly different between the two placement methods. Studies [27] have shown that experience and techniques of the operators affect the success rate and insertion time of NJT placement. Endoscopy technology is evolving and now includes deep enteroscopy, endoscopic ultrasonography, ultra-thin transnasal endoscopy, and laparoscopic-assisted surgery. Advances have improved the efficiency of NJT placement and enabled endoscopists to successfully place NJTs in patients who previously required open surgery [28]. Endoscopy-guided NJT placement is not only efficient, time-saving, and technologically advanced, but it is also especially suitable for critically ill patients [29]. A disadvantage is the need for endoscopy physicians and equipment to be moved to critically ill patients for bedside catheter insertion, which increases the workload of medical staff. Patients also have more discomfort during endoscopy-guided NJT placement (with higher VAS discomfort scores), but they can benefit from concurrent diagnostic upper gastrointestinal endoscopy for screening suspicious lesions [30]. Therefore, this method has been widely used in clinical settings.
Among the 8 studies included in the final complication analysis (excluding the 1 study leading to high heterogeneity), 58 patients (24.5%) in the endoscopic group developed epistaxis, abdominal pain, dyspnea, or other complications during placement. Only 26 of 233 patients (11.1%) developed complications in the x-ray group. Our meta-analysis results revealed no serious complications (perforation, hemodynamic instability, or death) in any patient, suggesting that endoscopy- and x-ray-guided NJT placement are both safe. However, x-ray-guided placement was associated with a lower risk of complications during placement and less patient discomfort during placement, suggesting that the safety and comfort of NJT placement were better using x-ray guidance. By comparing VAS discomfort scores before and after NJT insertion with the scores during insertion, it is obvious that patients experience substantial discomfort during the insertion process. Therefore, it is important to prioritize the catheterization method that will most likely reduce patient discomfort. The reduced complication risk and discomfort with x-ray guidance may be attributed to being able to directly determine the location of the tube at any time during insertion using this method, thereby allowing the position to be adjusted whenever necessary. The x-ray method avoids the possibility of direct stimulation (or even damage) of the GI tract lining by the endoscope [25].
The relative frequency of x-ray-guided NJT placement increased from 11.8% in 2010 to 19.4% in 2017, while there was a corresponding decrease in frequency of endoscopic placement from 87.3% to 78.8% [31]. X-ray guidance as a standard method of NJT placement has the advantages of not requiring preoperative medication or a skilled endoscopic operator and being able to be performed in a hospital of any size. However, it requires patients who are conscious, cooperative, and breathing on their own to complete the procedure. It also involves radiation exposure, which may lead to radiation-induced side effects and is thereby contraindicated in patients with leukopenia or aplastic anemia and in pregnant women.
While conducting this review, we also noted a difference in the cost of NJT placement between the two methods. The single study that reported cost results showed that in 2004, the cost of radiographic NJT placement was $250.99, while the cost of endoscopic NJT placement was $619.24 (including $51.66 for an abdominal x-ray to confirm successful placement) [23]. Unfortunately, because only 1 included study examined this issue, we could not perform a meta-analysis. By contrast, a survey study [31] reported that between 2010 and 2017, the cost of x-ray-guided placement decreased from $186 to $167, while the cost of endoscopy-guided placement decreased from $189 to $154. These differing results may be related to insufficient outcome indicators included in the survey study. Further investigations are required to evaluate differences in costs of the two methods of NJT placement.
Conclusions
Based on the results of this systematic review with meta-analysis, we suggest endoscopic technology as first-choice guidance when placing NJTs to increase the likelihood of success and shorten the insertion time. However, the choice of insertion method requires consideration of the specific conditions at the individual hospital. In smaller hospitals with more limited medical resources, x-ray-guided NJT placement is a good alternative for patients with no contraindications to radiation exposure. In the future, we plan to conduct a multi-center, large-sample, randomized controlled trial comparing endoscopy- and x-ray-guided NJT insertion to confirm our results. We also plan to evaluate whether other methods, such as ultrasound or electromagnetic guides, are more suitable for NJT placement in critically ill patients.
Disclosure of conflict of interest
None.
References
- 1.Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) (vol 40, pg 159, 2016) JPEN J Parenter Enteral Nutr. 2016;40:1200. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 2.Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: results of a randomized controlled trial. Crit Care Med. 2001;29:1495–1501. doi: 10.1097/00003246-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Alhazzani W, Almasoud A, Jaeschke R, Lo BW, Sindi A, Altayyar S, Fox-Robichaud AE. Small bowel feeding and risk of pneumonia in adult critically ill patients: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17:127. doi: 10.1186/cc12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn SR, Early BJ, Zenati MS, Ochoa JB. Use of a nasal bridle prevents accidental nasoenteral feeding tube removal. JPEN J Parenter Enteral Nutr. 2009;33:50–54. doi: 10.1177/0148607108321704. [DOI] [PubMed] [Google Scholar]
- 5.Krzak A, Pleva M, Napolitano LM. Nutrition therapy for ALI and ARDS. Crit Care Clin. 2011;27:647–659. doi: 10.1016/j.ccc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Wiggins TR, DeLegge MH. Evaluation of a new technique for endoscopic nasojejunal feeding-tube placement. Gastrointest Endosc. 2006;63:590–595. doi: 10.1016/j.gie.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Hu B, Ye H, Sun C, Zhang Y, Lao Z, Wu F, Liu Z, Huang L, Qu C, Xian L. Metoclopramide or domperidone improves post-pyloric placement of spiral nasojejunal tubes in critically ill patients: a prospective, multicenter, open-label, randomized, controlled clinical trial. Crit Care. 2015;19:784. doi: 10.1186/s13054-015-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch S, Witteman E, Kho Y, Tan A. Erythromycin to promote bedside placement of a self-propelled nasojejunal feeding tube in non-critically ill patients having pancreatitis: a randomized, double-blind, placebo-controlled study. Nutr Clin Prac. 2011;26:181–185. doi: 10.1177/0884533611399924. [DOI] [PubMed] [Google Scholar]
- 9.Kaffarnik MF, Lock JF, Wassilew G, Neuhaus P. The use of bedside electromagnetically guided nasointestinal tube for jejunal feeding of critical ill surgical patients. Technol Health Care. 2013;21:1–8. doi: 10.3233/THC-120704. [DOI] [PubMed] [Google Scholar]
- 10.Huang TC, Jing JY, Yan M. How to promote bedside placement of the postpyloric feeding tube: a network meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2015;39:521–530. doi: 10.1177/0148607114546166. [DOI] [PubMed] [Google Scholar]
- 11.Fan AC, Baron TH, Rumalla A, Harewood GC. Comparison of direct percutaneous endoscopic jejunostomy and PEG with jejunal extension. Gastrointest Endosc. 2002;56:890–894. doi: 10.1067/mge.2002.129607. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe SJ, Foody W, Gill S. Transnasal endoscopic placement of feeding tubes in the intensive care unit. JPEN J Parenter Enteral Nutr. 2003;27:349–354. doi: 10.1177/0148607103027005349. [DOI] [PubMed] [Google Scholar]
- 13.Stănescu D, Mihalache D, Nistor A, Buciu A, Irimescu O, Tiron C, Gorceag R. Importance of enteral nutrition support in necrotic hemorrhagic pancreatitis. Rev Med Chir Soc Med Nat Iasi. 2010;114:91–94. [PubMed] [Google Scholar]
- 14.Tong ZH, Yu WK, Li WQ, Wang ZM, Ye XH, Li N, Li JS. A randomised clinical trial of transnasal endoscopy versus fluoroscopy for the placement of nasojejunal feeding tubes in patients with severe acute pancreatitis. Postgrad Med J. 2009;85:59–63. doi: 10.1136/pgmj.2008.070326. [DOI] [PubMed] [Google Scholar]
- 15.Foote JA, Kemmeter PR, Prichard PA, Baker RS, Paauw JD, Gawel JC, Davis AT. A randomized trial of endoscopic and fluoroscopic placement of postpyloric feeding tubes in critically ill patients. JPEN J Parenter Enteral Nutr. 2004;28:154–157. doi: 10.1177/0148607104028003154. [DOI] [PubMed] [Google Scholar]
- 16.Zhu YF, Yin HY, Zhang R, Ye XL, Wei JR. Endoscopy versus fluoroscopy for the placement of postpyloric nasoenteric tubes in critically ill patients: a meta-analysis of randomized controlled trials. J Crit Care. 2016;33:207–212. doi: 10.1016/j.jcrc.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Xia BT, Su XH. Discussion on the method of placing spiral nasojejunal nutrition tube. Inner Mongolia Medical Journal. 2012;44:1356–1357. [Google Scholar]
- 18.Ma T. Comparison of three different nasojejunal tube placement methods and intraoperative cooperation. Journal of Nursing Education. 2016;31:1997–1999. [Google Scholar]
- 19.Guan FH, Zhong ZX. Analysis on the therapeutic effect and nutritional effect of modified oral-duodenoscopic catheterization in the treatment of severe acute pancreatitis. China Modern Doctor. 2018;56:8–11. [Google Scholar]
- 20.Shen GG, Jiang XG, Lu WH, Wu JY, Wang J, Jin XJ. Clinical effects of the placement of nose-jejunum nutrition tube guided by x-ray and endoscopy on critically ill patient. Chinese General Practice. 2012;15:3396–3398. [Google Scholar]
- 21.Li N, Huang L, Zhang YH, Song C, Wang WJ, Jiao JY. Clinical application of endoscopic nasojejunal nutrition tube. Modern Diagnosis and Treatment. 2017;28:748–750. [Google Scholar]
- 22.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang JC, Hilden K, Holubkov R, DiSario JA. Transnasal endoscopy vs. fluoroscopy for the placement of nasoenteric feeding tubes in critically ill patients. Gastrointest Endosc. 2005;62:661–666. doi: 10.1016/j.gie.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Xie M. Comparison of nasoduodenal nutrition tube placement under X-ray and gastroscope. Zhejiang Clinical Medicine. 2012;14:746–747. [Google Scholar]
- 25.Song YG, Wang CG, Zhu GX. Comparison of the efficacy of two guiding methods of nasojejunal tube placement for early enteral nutrition in the treatment of acute pancreatitis. Zhejiang Clinical Medicine. 2013;10:1484–1485. [Google Scholar]
- 26.Yang YS, Tang XJ, Liu D, Lu J, Li WH. Comparison of the nutritional effects for patients with severe acute pancreatitis by three different naso-enteric feed tube placement. Journal of Tropical Diseases and Parasitology. 2015;13:94–96. [Google Scholar]
- 27.Schwab D, Muhldorfer S, Nusko G, Radespiel-Troger M, Hahn EG, Strauss R. Endoscopic placement of nasojejunal tubes: a randomized, controlled, prospective trial comparing suitability and technical success for two different tubes. Gastrointest Endosc. 2002;56:858–863. doi: 10.1067/mge.2002.129870. [DOI] [PubMed] [Google Scholar]
- 28.Paski SC, Dominitz JA. Endoscopic solutions to challenging enteral feeding problems. Curr Opin Gastroenterol. 2012;28:427–431. doi: 10.1097/MOG.0b013e328355ecc9. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Liu L, Wang J, Zhang YZ, Wu ZY, Lu FL, Mao CH, Yu Q, Cao DZ. Efficacy and safety of placing nasoenteral feeding tube with transnasal ultrathin endoscope in critically ill patients. Chin Med J. 2009;122:2608–2611. [PubMed] [Google Scholar]
- 30.Long C, Yu Y, Cui B, Jagessar SAR, Zhang J, Ji G, Huang G, Zhang F. A novel quick transendoscopic enteral tubing in mid-gut: technique and training with video. BMC Gastroenterol. 2018;1:37. doi: 10.1186/s12876-018-0766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez Garcia RJ, Lindquester W, Dhangana R, Warhadpande S, Amesur N. An expanding role for interventional radiology: medicare trends in fluoroscopic, endoscopic, and surgical enteric tube placement and maintenance from 2010 to 2018. Clin Imaging. 2021;78:201–205. doi: 10.1016/j.clinimag.2021.05.008. [DOI] [PubMed] [Google Scholar]


