Abstract
Objective: This study aimed to explore the application values of modified radical mastectomy in female patients with mammary cancer of different molecular types and from this we conducted a prognosis study. Methods: A total of 204 Breast Cancer (BC) patients who were admitted to our hospital from March 2015 to March 2017 were included and divided into Group A (Luminal A type, n = 68), Group B (Luminal B type, n = 48), Group C (ERBB2: Erb-B2 Receptor Tyrosine Kinase 2 + type, n = 42), and Group D (Basal-like type, n = 46) according to their molecular cancer types. Patients in Groups A and B demonstrated superior treatment efficacy and lower incidence of adverse reactions than those in Groups C and D (P < 0.05), while no statistical difference was observed among the 4 groups in terms of the total operation time, intraoperative blood loss, and postoperative 48-h drainage volume (P > 0.05). Before treatment, the 4 groups exhibited similar results from the EORTC breast cancer-specific quality of life questionnaire (EORTCQLQ-BR23) (P > 0.05). Results: After treatment, Group A was superior to the other 3 groups in this regard (P < 0.05). Further, no significant difference was observed among the 4 groups in terms of the prognosis of 3-year survival (P > 0.05). Conclusion: The clinical application of modified radical mastectomy does not depend on the molecular typing of BC; however, the treatment was more effective in the treatment of Luminal A type BC.
Keywords: Breast cancer, ERBB2+ TYPE, luminal A type, modified radical mastectomy, prognosis
Introduction
Breast cancer (BC) is currently an extremely common malignant tumor seen in the clinical setting, accounting for 9%-12% of all malignant tumors [1]. In 2015, there were 231,840 female BC patients in the United States [2]. An increasing number of studies have demonstrated a continuous rise in the incidence of BC. The prevalence of BC is predicted to potentially exceed that of lung cancer in the coming 50 years, with BC becoming the 2nd most common malignant tumor worldwide [3,4]. Currently, the onset mechanism of BC is unclear. At the early stage, no specific symptoms are observed. Therefore, most patients, when diagnosed, may have entered the middle and advanced stages; treatment becomes more challenging in these stages. This is one of the major reasons for poor patient prognosis [3]. BC-related mortality is expected to reach 13.4/100,000 people by 2020 [5]. Due to the high incidence and risk, BC is always studied as a key disease in the clinic setting. Research is being conducted worldwide to identify more effective diagnosis and treatment methods for BC [6-8].
With further study on BC as well as transformation and upgrades in treatment approaches, the clinical treatment of BC has entered the comprehensive era, including operation, radiotherapy, chemotherapy, endocrine therapy, biological targeting treatment, and TCM-assisted treatment [9]. However, surgical treatment remains the most common method of treatment. In recent years, the application value of modified radical mastectomy in treating BC has been gradually verified through more in-depth studies [10,11]. Compared to traditional radical operation, modified radical mastectomy has more obvious clinical efficacy and little postoperative negative impact, making it the first choice in BC treatment [12]. However, few studies have been conducted regarding the difference of related modified radical mastectomy in the treatment of various types of BC. To further establish the application of modified radical mastectomy in the clinic, this study was performed to target the application of modified radical mastectomy in various subtypes of BC to provide reference and instruction for clinical practice.
Materials and methods
General materials
A total of 204 BC patients who were admitted to our hospital from March 2015 to March 2017 were included and divided into Group A (Luminal A type, n = 68), Group B (Luminal B type, n = 48), Group C (ERBB2+ type, n = 42), and Group D (Basal-like type, n = 46) according to their molecular cancer types. This study was approved by the ethics committee of Tongji University Affiliated Yangpu Hospital, Tongji University, and all subjects or their immediate family members signed the informed consent.
Inclusion and exclusion criteria
Inclusion criteria
Patients who exhibited the clinical manifestations of BC with their molecular typing criteria proposed on the St. Gallen in 2013 (Table 1 for details); those who were diagnosed with BC clinical stages I and II using biopsy at the Pathology Department; those who did not have fascia pectoralis involved by the tumor; those who provided complete medical records; patients who agreed to cooperation with the investigations performed by our medical staff; and those who not undergone other radiotherapy, chemotherapy, or antibiotic treatment within 3 months before the operation were included.
Table 1.
Molecular typing criteria
| Type | Morbid State |
|---|---|
| Luminal A type | ER/PR positive and high PR expression (≥ 20%); HER2 negative; low Ki-67 expression, and high CK18, CK8, and AR expression; Luminal A type, a.k.a., hormone-dependent BC, is more common among individuals aged > 50 years old. |
| Luminal B type | 2 types: type 1 is Luminal B type (HER-2 negative), with pathological IHC expressed as ER positive or PR positive, HER-2 negative and high Ki-67 expression; type 2 is Luminal B type (HER-2 positive) with pathological IHC expressed as ER positive or PR positive, and HER-2 positive, mostly found in senior BC patients. |
| ERBB2+ type | ER and PR negative, HER-2 positive, and high Ki-67 expression in most cases. The criterion for HER-2 positive is a p53 mutation rate between 40% and 86% in this subtype of BC except for HER-2 protein in high expression. It is characterized by poor tumor differentiation at Grade III in histology. |
| Basal-like type | Total 75% of the patients with this type of tumor may experience TP53 mutation and BRCAI (an inhibitory gene) mutation. In addition, Ki-67 will express at a high level. |
Exclusion criteria
Some patients were excluded because they were concurrently experiencing multiple tumors, other cardiovascular diseases, autoimmune disorders, mental disorders, organ failure, disability that required them to lie in the bed for a long time or if they showed low treatment compliance, patients who were pregnant or those with cancer that invaded the pectorals or experienced severe auxiliary lymph nodes, or demanded transfer to another hospital.
Methods
Modified radical mastectomy was performed for all the patients by senior surgeons of our hospital. Operation method: the patients were made to lie in the dorsal position with the upper extremities extending outward by 90°. After anaesthetization, the surgical incision was made based on the size of the diseased breast and the tumor site, while maintaining a distance of about 2-5 cm from the edge of the tumor. As the breast skin was cut open, an electrotome was used to isolate the flap and cut off the mammary tissue. The patients’ fossa axillaris was dissected to lift the ectopectoralis and the entopectoralis upward from the inside with a goiter retractor to expose the fossa axillaris thoroughly. Auxiliary nodes and the nodes between the ectopectoralis and the entopectoralis were removed. The surgical incision was rinsed and soaked in steamed water (45°C), into which, a drainage tube was placed as per the routine procedure; the tissue was then sutured.
Observation indicators
Major observation indicators
All the patients were investigated for clinical treatment efficacy 3 weeks after the operation. The healing efficacy was denoted as: CR if the tumor site and clinical syndromes disappear completely; PR if the product of the maximal tumor diameter and the maximal vertical diameter reduced by 50% while other lesions remain the same; SD if the product of the maximal tumor diameter and the maximal vertical diameter were reduced less than 50% or expanded no more than 25%; PD if the product of the maximal tumor diameter and the maximal vertical diameter expanded more than 25%. Effective treatment rate = (CR + PR)/total number of cases × 100%.
Secondary observation indicators
Operation-related indicators: the total operation time, intraoperative blood loss, and postoperative 48-h drainage volume. 2. QOL: patients’ QOL was evaluated using EORTCQLQ-BR23 before and 3 weeks after the operation. 3. Prognosis: patients were followed up for 3 years via return visits to the hospital to record their 3-year survival.
Statistical analyses
Statistical analyses were performed with SPSS 22.0 (Shanghai Yuchuang Network Technology Co., Ltd.). Data were illustrated using GraphPad Prism 7. Nominal data were expressed as %, and comparison studies were performed using chi-squared test for intergroup comparisons. Numerical data were expressed as mean ± standard deviation values, and comparison studies were performed using t test for intergroup comparisons and one-way ANOVA and LSD post-test for comparison among multiple groups; the survival was calculated with Kaplan-Meier analysis and compared using Log-rank test. For all statistical comparisons, significance was defined as P < 0.05.
Results
Comparison of general characteristics and clinical efficacy
The general characteristics of the 4 groups were compared; no significant difference was found in the age, disease duration, BMI, concurrent disease, smoking, exercise, living environment, LVD, and LAS (P > 0.05, Table 2).
Table 2.
Comparison of the general characteristics of the 4 groups [n (%)]
| Factor | A (n = 68) | B (n = 48) | C (n = 42) | D (n = 46) | F or X2 | P |
|---|---|---|---|---|---|---|
| Age | 59.2 ± 6.7 | 58.2 ± 7.1 | 58.9 ± 7.6 | 59.2 ± 6.7 | 0.231 | 0.875 |
| Disease duration (years) | 0.54 ± 0.24 | 0.53 ± 0.30 | 0.55 ± 0.32 | 0.54 ± 0.27 | 0.511 | 0.765 |
| BMI (KG) | 25.31 ± 3.94 | 25.43 ± 3.76 | 26.21 ± 4.03 | 25.54 ± 3.65 | 0.511 | 0.675 |
| Concurrent disease | 0.471 | 0.998 | ||||
| Hypertension | 15 (22.06) | 10 (20.83) | 10 (23.81) | 12 (26.09) | ||
| Diabetes | 20 (29.41) | 14 (29.17) | 11 (26.19) | 12 (26.09) | ||
| Nil | 35 (51.47) | 24 (50.00) | 21 (52.17) | 24 (52.17) | ||
| Smoking | 0.523 | 0.909 | ||||
| Y | 34 (50.00) | 25 (52.08) | 23 (54.76) | 26 (56.52) | ||
| N | 34 (50.00) | 23 (47.92) | 19 (45.24) | 20 (43.48) | ||
| Exercise | 0.070 | 0.995 | ||||
| Y | 12 (17.65) | 9 (18.75) | 7 (16.67) | 8 (17.39) | ||
| N | 56 (82.35) | 39 (81.23) | 35 (83.33) | 38 (82.61) | ||
| Living environment | 0.207 | 0.977 | ||||
| Downtown | 47 (69.12) | 32 (66.67) | 28 (66.67) | 30 (65.22) | ||
| Rural | ||||||
| LVD (mm) | 50.14 ± 5.26 | 50.56 ± 5.58 | 51.63 ± 6.04 | 50.17 ± 5.48 | 0.731 | 0.535 |
| LAS (mm) | 33.64 ± 4.08 | 33.16 ± 4.45 | 32.32 ± 4.56 | 32.45 ± 4.56 | 1.085 | 0.357 |
A comparison of the clinical efficacy in the 4 groups showed that Groups C, D were not significantly different from each other (P > 0.05); however, Groups C and D had significantly lower clinical efficacy than Groups A and B. Further, Group A demonstrated the best clinical efficacy (P < 0.001, Table 3).
Table 3.
Comparison among the 4 groups for clinical efficacy
| Factor | A (n = 68) | B (n = 48) | C (n = 42) | D (n = 46) | X2 | P |
|---|---|---|---|---|---|---|
| CR | 42 (70.59) | 24 (50.00) | 18 (42.86) | 16 (34.78) | ||
| PR | 15 (22.06) | 13 (27.08) | 8 (19.05) | 12 (26.09) | ||
| CD | 8 (11.76) | 9 (18.75) | 10 (23.81) | 10 (21.74) | ||
| PD | 3 (4.41) | 4 (8.33) | 6 (14.29) | 8 (17.39) | ||
| Cure rate (%) | 83.82 | 72.92 | 61.90*,# | 60.87*,# | 9.577 | 0.023 |
represents P < 0.05 as compared with the Group A;
P < 0.05 as compared with Group B.
Comparison of adverse reactions and Operation-related indicators
The 4 groups were compared for incidence of adverse reactions. No significant difference was observed between Groups A and B as well as Groups C and D (P > 0.05). However, the incidences of adverse reactions in Groups A and B were significantly lower than those in Groups C and D (P < 0.05, Table 4).
Table 4.
Comparison among the 4 groups for incidence of adverse reactions
| A (n = 68) | B (n = 48) | C (n = 42) | D (n = 46) | X2 | P | |
|---|---|---|---|---|---|---|
| Postoperative bleeding | 3 (4.41) | 2 (4.17) | 3 (7.14) | 2 (4.35) | ||
| Postoperative infection | 0 (0.00) | 1 (2.08) | 3 (7.14) | 2 (4.35) | ||
| Subcutaneous fluid accumulation | 1 (1.47) | 1 (2.08) | 2 (4.76) | 3 (6.52) | ||
| Skin flap necrosis | 1 (1.47) | 1 (2.08) | 2 (4.76) | 4 (8.70) | ||
| Edema of the upper extremity | 2 (2.94) | 1 (2.08) | 3 (7.14) | 3 (6.52) | ||
| Fever | 3 (4.41) | 4 (8.33) | 2 (4.76) | 6 (13.04) | ||
| Incidence (%) | 14.71 | 22.92 | 35.71*,# | 43.48*,# | 13.420 | 0.004 |
represents P < 0.05 as compared with the Group A;
P < 0.05 as compared with Group B.
There was no significant difference in the total operation time, intraoperative blood loss, and postoperative 48-h drainage volume of the 4 groups (P > 0.05, Figure 1).
Figure 1.
Comparison of operation-related indicators. A: Comparison of operation time; B: Comparison of intraoperative blood loss; C: Comparison of 48 h drainage volume.
Comparison of operation-related indicators, the QOL scores and prognostic survival
Before treatment, the 4 groups reported similar EORTCQLQ-BR23 scores (P > 0.05). After treatment, Group A had the highest score among all 4 groups (P < 0.05). The scores of Groups B and C were not significantly different from each other (P > 0.05) but were higher than that of Group D (P < 0.05). There was a significant increase in the EORTCQLQ-BR23 scores of all 4 groups after treatment (P < 0.05, Figure 2).
Figure 2.
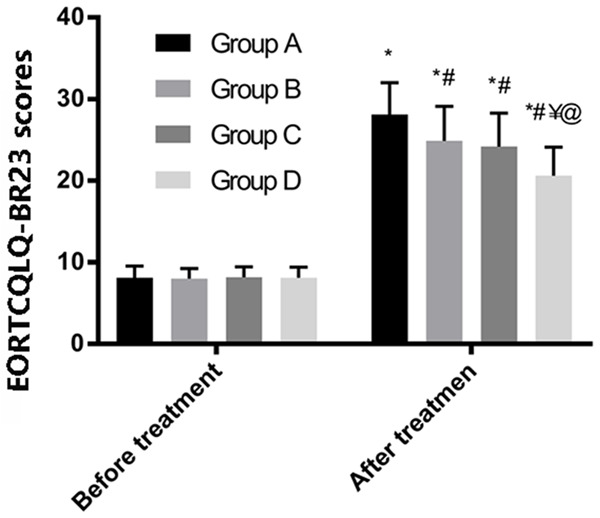
EORTCQLQ-BR23 scores. * represents the comparison with the conditions before treatment in the same group; # as compared with Group A after treatment; ¥ as compared with Group B after treatment; @ as compared with Group C after treatment (P < 0.05).
Of the 204 patients, 194 returned for a subsequent visit, contributing to a follow-up success rate of 95.59%. Three patients in Group A, 4 in Group B, 2 in Group C, and 0 patients in Group D failed to follow-up. A comparison of the prognosis of 3-year survival showed no significant difference among the 4 groups (P > 0.05, Figure 3).
Figure 3.
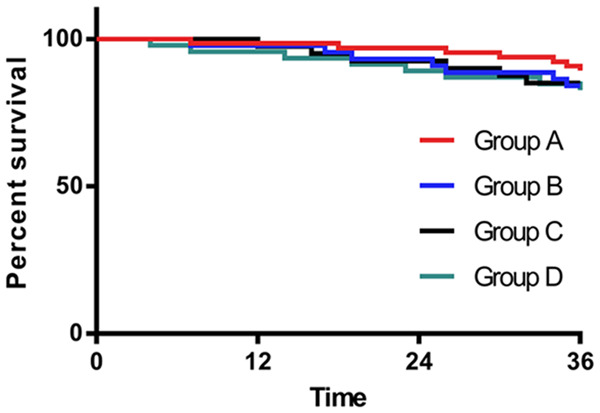
Three-year prognostic survival curves of the 4 groups.
Discussion
At present, BC is a very common malignant tumor with a high incidence all the year round in clinic, with an increasing trend being observed in recent years [13]. In order to improve the diagnosis and treatment efficacy of BC in the clinical setting, further understanding of the disease is crucial. Thus far, the 4 most common BC molecular types have been identified [14]; however, which type benefits the most from radical mastectomy is unclear. This study focused on exploring the efficacy of modified radical mastectomy in regard to different molecular types of BC to provide guidance for future clinical treatment.
According to the study results, the clinical treatment efficacy was highest in Group A than in the other 3 groups, indicating that modified radical mastectomy is most effective for Luminal A type BC. A possible reason may be that Luminal A type BC has been clinically testified as a hormone-dependent BC, for which, endocrine therapy is the best solution [15]. Zhang et al. [16] also observed the same results in their study on the treatment efficacy of paclitaxel in Luminal A type BC, supporting the conclusions from this study. The incidences of postoperative adverse reactions in Groups C and D were not significantly different from each other; however, these were obviously higher than those in Groups A and B, indicating that patients with RBB2+ type and Basal-like type BC have a higher chance of experiencing postoperative adverse reactions. We believe that the reasons for this could be that this type of BC is characterized by a high degree of deterioration and possibility of tumor metastasis or invasion [17]. Therefore, after the operation, patients may experience a higher possibility of stress response or abnormal immune function rehabilitation, leading to a sharp rise in the incidence of adverse reactions. In their study, D’ Alesio et al. [18] also observed poor prognosis in patients with ERBB2+ type or Basal-like type BC as compared with those in the other 2 groups, which was consistent with our hypothesis. However, to our knowledge, no study has verified the mechanism based on which the difference in molecular type impacts the surgical treatment effects. In this study, only conjectures were proposed after observing the patients’ clinical efficacy that may differ from the reality owing to study limitations. In the future, we shall explore this subject as a key research direction for more in-depth exploration and observation. Further comparison of the 4 groups for operation-related indicators indicated no significant difference in terms of the total operation time, intraoperative blood loss, and postoperative drainage volume. This also indicated that the invasion that patients experienced during modified radical mastectomy was not dependent on the molecular type of BC. Wang et al. [19] reported significant difference of chemotherapy in BC patients of different molecular types. Thus, we inferred that as an invasive procedure of resection, surgery is not affected by the internal effect of molecular cells [20], while chemotherapy and radiotherapy heavily depended on interference with cellular molecules to remove the cancer. Therefore, different molecular types may have comparatively obvious impact on the results of chemotherapy. However, most patients included in this study were in the early stage of BC. It is impossible to establish the prognosis of patients with different molecular typing after radiotherapy or chemotherapy, and this is a limitation of this study. Based on the investigation of the patients’ QOL scores, Groups A and B reported superior prognosis and QOL than the other 2 groups, further confirming our hypothesis. Takano et al. [21] also proposed that the key factor in deciding the prognosis of tumor lies in the conditions of the lymph and molecular type. Compared to patients with negative lymph, patients with positive lymph have worse prognosis, while the lymph examination results of Luminal A and B types were mostly negative. This may be one of the reasons accounting for the different prognosis of patients in those groups. To further clarify the difference in the prognosis of patients with BC of different molecular types, we performed a 3-year prognostic follow-up and found no significant difference in their 3-year survival. Previous studies have also verified that the best prognosis was found in patients with Luminal A type BC [22], contradictory to our conclusions possibly because of the statistical calculation owing to fewer patients in each group. In addition, we also observed the specific survival of each group; Group A had superior survival compared to the other 3 groups. The deviation in the study results may be attributed to the large proportion of BC patients in the early stage. Therefore, the sample size needs to be increased to improve the discussion over the results.
This study aimed to explore the treatment efficacy of modified radical mastectomy in patients with BC of different molecular types. However, this study was limited in that it failed to establish the biological changes with the administration of surgical treatment to patients with BC of different molecular types. The high re-striction in study subjects may also result in variability in the study results. There are many methods (such as retention of breast) to treat BC in the clinic, the impact of which for different BC molecular types is not established. The study shall be improved in terms of those shortages to carry out more comprehensive experiments and analysis for the best results.
In conclusion, the clinical application of modified radical mastectomy was not dependent on the molecular type of BC; however, best efficacy was observed in the treatment of Luminal A type BC.
Acknowledgements
This work was supported by funding from a grant entitled The Mechanism of cxcr2-Regulated Inhibition of Aging and Promotion of Chemotherapy Resistance in Three Negative Breast Cancer by TRAF6 [grant number 19YF1442300].
Disclosure of conflict of interest
None.
References
- 1.Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline update on ovarian suppression summary. J Oncol Pract. 2016;12:390–393. doi: 10.1200/JOP.2016.011239. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, Members P, André F. Tailoring therapies-improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Du S, Zhang J, Liang A, Liu Y. Exosomes and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Cancer Gene Ther. 2017;24:6. doi: 10.1038/cgt.2016.69. [DOI] [PubMed] [Google Scholar]
- 7.Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19:157–168. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinker K, Chin J, Melsaether AN, Morris EA, Moy L. Precision medicine and radiogenomics in breast cancer: new approaches toward diagnosis and treatment. Radiology. 2018;287:732–747. doi: 10.1148/radiol.2018172171. [DOI] [PubMed] [Google Scholar]
- 9.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer. 2016;8:93. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69:126. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Othman AH, El-Rahman A, El Sherif F. Efficacy and safety of ketamine added to local anesthetic in modified pectoral block for management of postoperative pain in patients undergoing modified radical mastectomy. Pain Physician. 2016;19:485–494. [PubMed] [Google Scholar]
- 12.Chang HT, Shi HY, Wang BW, Yeh SJ. Breast cancer incidence and predictors of surgical outcome: a nationwide longitudinal study in Taiwan. Clin Oncol. 2017;29:362–369. doi: 10.1016/j.clon.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 13.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67:439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 14.Edenfield J, Schammel C, Collins J, Schammel D, Edenfield WJ. Metaplastic breast cancer: molecular typing and identification of potential targeted therapies at a single institution. Clin Breast Cancer. 2017;17:e1–e10. doi: 10.1016/j.clbc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Jang SH, Lee JE, Oh MH, Lee JH, Cho HD, Kim KJ, Kim SY, Han SW, Kim HJ, Bae SB. High EZH2 protein expression is associated with poor overall survival in patients with luminal a breast cancer. J Breast Cancer. 2016;19:53–60. doi: 10.4048/jbc.2016.19.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Zhao R, He Y, Fu X, Fu L, Zhu Z, Fu L, Dong JT. Micro RNA 100 sensitizes luminal A breast cancer cells to paclitaxel treatment in part by targeting mTOR. Oncotarget. 2016;7:5702. doi: 10.18632/oncotarget.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Zhu L, Yu X, Fu Q, Xu W, Wang P. Quantitative assessment of metabolic tumor burden in molecular subtypes of primary breast cancer with FDG PET/CT. Diagn Interv Radiol. 2018;24:336. doi: 10.5152/dir.2018.17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alesio C, Bellese G, Gagliani MC, Aiello C, Grasselli E, Marcocci G, Bisio A, Tavella S, Daniele T, Cortese K. Cooperative antitumor activities of carnosic acid and Trastuzumab in ERBB2+ breast cancer cells. J Exp Clin Cancer Res. 2017;36:154. doi: 10.1186/s13046-017-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Sang D, Xu B, Yuan P, Ma F, Luo Y, Li Q, Zhang P, Cai R, Fan Y. Value of breast cancer molecular subtypes and Ki67 expression for the prediction of efficacy and prognosis of neoadjuvant chemotherapy in a Chinese population. Medicine. 2016;95:e3518. doi: 10.1097/MD.0000000000003518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Su X, Yang L, Qiao F, Fang Y, Yu L, Yang Q, Wang Y, Yin Y, Chen R. The influence of myeloid-derived suppressor cells on angiogenesis and tumor growth after cancer surgery. Int J Cancer. 2016;138:2688–2699. doi: 10.1002/ijc.29998. [DOI] [PubMed] [Google Scholar]
- 21.Takano S, Ishikawa E, Sakamoto N, Matsuda M, Akutsu H, Noguchi M, Kato Y, Yamamoto T, Matsumura A. Immunohistochemistry on IDH 1/2, ATRX, p53 and Ki-67 substitute molecular genetic testing and predict patient prognosis in grade III adult diffuse gliomas. Brain Tumor Pathol. 2016;33:107–116. doi: 10.1007/s10014-016-0260-x. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Tang H, Wang J, Xie X, Liu P, Kong Y, Ye F, Shuang Z, Xie Z, Xie X. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast. 2017;32:1–6. doi: 10.1016/j.breast.2016.11.024. [DOI] [PubMed] [Google Scholar]


