Abstract
Objective: To analyze the clinical efficacy and possible mechanism of butylphthalide in treatment of acute ischemic stroke. Methods: In this retrospective study, 127 patients with ischemic stroke, hospitalized during Jan. 2019 to Jan. 2021, were enrolled and as assigned to observation group (n=65) and control group (n=62) according to treatment methods. The control group received routine treatment, and the observation group was treated with butylphthalide injection in addition to conventional cure. The treatments lasted for 2 weeks in both groups. Subsequently, the recovery of neurological deficits (NIHSS) and Barthel index (BI) of the two groups of patients, cerebrovascular vascular reserve function (CVR) values and pulsation index (PI) before and after treatment, and the levels of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF) and recombinant basic fibroblast growth factor (bFGF) were detected. The expression of Keap1-Nrf2/ARE signaling pathway related molecules was detected by ELISA. Results: The overall response rate (ORR) of observation group was remarkably superior to that of control group (P<0.05). NIHSS score obviously decreased while BI remarkably increased in both groups after treatment (all P<0.05); and the observation group showed an significantly higher BI score but significantly lower NIHSS score compared with the control group (all P<0.05). The CVR of the two groups of patients after treatment was significantly higher than that before treatment (P<0.05), while PI was significantly lower than before treatment (P<0.05); The CVR of observation-group after treatment was substantially higher than that of control-group (P<0.05), while PI was lower than control-group (P<0.05). Serum Keap1 levels of the two groups of patients after treatment were significantly higher than that before treatment (P<0.05), while serum levels of NQO1, Nrf2, and ARE were significantly lower than that before treatment (P<0.05). The serum level of Keap1 in the observation group was remarkably higher than that of the control group (P<0.05), while the serum levels of NQO1, Nrf2 and ARE were evidently lower than those in the control group (P<0.05). The two groups had insignificant difference in incidence of adverse reactions (P>0.05). Conclusion: The butylphthalide can effectively improve the clinical efficacy of acute ischemic stroke, and promote patients’ neurological function and activities of daily living. The mechanism may be that butylphthalide improves the CVR of patients, enhances the establishment of collateral compensatory vessels, and changes the expression of the Keap1-Nrf2/ARE signaling pathway, thereby exerting the neuroprotective effect. Clinically, butylphthalide may have good safety in adjuvant therapy of acute ischemic stroke.
Keywords: Butylphthalide, acute ischemic stroke, clinical efficacy, mechanism
Introduction
Ischemic stroke accounts for about 60%-80% of all cerebrovascular accidents and is the most frequent in clinical practice. The occurrence of ischemic stroke severely impairs people’s health of life and is the leading cause of mortality and disability in middle-aged and elderly people [1]. Early diagnosis, early treatment and prevention should be emphasized in clinical practice. The guideline of diagnosis and treatment include thrombolysis, anticoagulation, antiplatelet, neuroprotective agents, fibrosis reduction, and blood pressure control [2]. It has been demonstrated that early thrombolysis (within 6 hours after onset of stroke) is the most effective way to treat ischemic stroke. However, most people had already missed the best timing when they seek for medical treatment [3,4]. Therefore, it is particularly important to protect neural function and actively intervene other pathological links of ischemic stroke so as to improve the prognosis of patients [5]. Researchers have studied the effects of various neuroprotective agents in numerous animal studies over the years, but most of the effects are still unsatisfactory.
Butylphthalide (racemic-3-n-butylphthalide) is a new anticerebral ischemia drug developed independently in China. It was first extracted from the volatile oil of rapeseed of southern watercress and can be synthesized via artificial chemistry [6]. According to animal experiments, butylphthalide is curative for ischemic stroke [7]. Studies have reported that butylphthalide can effectively improve cerebral vascular microcirculation and cellular energy metabolism of patients with ischemic stroke, thus protecting the cognitive function of patients, and has better effect and are safe in clinical applications [8]. However, the specific mechanism of butylphthalide on patients with ischemic stroke has yet been elucidated. Current studies suggest that the process of pathological injury in patients with acute ischemic stroke is very complex, which includes oxidative stress injury, disorder of energy metabolism in brain tissue, inflammatory response and excitatory aminoacidosis. The Keap1-Nrf2/ARE oxidative stress pathway with nuclear factor e2-related factor 2 (Nrf2) as its core is considered to exert an important role in maintaining the REDOX equilibrium state in brain cells. Many downstream factors regulated by Nrf2, such as quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), glutathione peroxidase (GSH-Px) etc., have multiple effects such as antioxidant stress, alleviation of calcium overload, anti-inflammatory injury and anti-apoptosis [9]. Studies have shown [10] that butylphthalide in the treatment of ischemic stroke may be related to the improvement of oxidative stress mechanism, but whether it is related to the KEAP1-NRF2/ARE pathway has not been reported in clinical studies. In order to further confirm its clinical efficacy and analyze its possible mechanism, this study explored the clinical curative effect of butylphthalide on acute ischemic stroke and its possible mechanism.
Materials and methods
Clinical data
In this retrospective study, 127 patients with ischemic stroke, who were hospitalized during Jan. 2019 to Jan. 2021, were enrolled and classified into observation group (n=65) and control group (n=62) according to the treatment methods. The study was approved by ethics committee of hospital.
Inclusion and exclusion criteria
Inclusion criteria
(1) The patients who met the diagnostic criteria of ischemic stroke in Chinese Guideline on Diagnosis and Treatment of Acute ischemic Stroke 2014 issued by Chinese Medical Association [10]; (2) The patients with age of 45-70 years old; (3) The patients with cerebral infarction of internal carotid artery system confirmed by cranial CT and MRI; (4) The patients with time window of stroke symptoms and signs from onset to treatment within 48 h, and without indications of thrombolytic therapy; (5) The patients developed ischemic stroke for the first time; (6) The patients who cooperated with the treatment and signed the informed consent form.
Exclusion criteria
(1) Patients with acute insufficiency of vital organs, e.g., heart, liver, and kidneys, and those with hemorrhagic, metabolic, immunological, neoplastic and systemic infectious diseases; (2) Patients with cerebral vascular diseases such as cerebral hemorrhage and cerebral vascular malformation; (3) Patients with a recent (within 4 weeks) history of trauma and surgery; (4) Patients with allergy to drugs used in this study; (5) Patients with severe mental disorders that cannot cooperate with this study; (6) Patients with incomplete clinical data.
Methods
The control group was executed with routine therapy, including anti-platelet aggregation (aspirin 150-300 mg/d) and neuroprotective agent (citicoline) according to the requirements of Chinese Guideline on Diagnosis and Treatment of Acute Ischemic Stroke [10], and those with cerebral edema were given 20% mannitol or glycerol fructose dehydration treatment to maintain water electrolyte balance. In addition, the high-risk factors of patients should be controlled, such as blood pressure control for patients with hypertension, blood lipid control for patients with hyperlipidemia, and blood glucose control for patients with diabetes, etc.
In addition to the above-mentioned therapy, the observation group took 100 ml of butylphthalide (Shijiazhuang Pharmaceutical Group Enbipu Pharmaceutical Co., Ltd, H20100041) by intravenous drip. The infusion time was controlled within 1-2 h, and the next instillation was performed at least 6 hours later. The patients received intravenous injection twice a day.
Observation of indicators
(1) Neurological deficits and recovery of daily activities: Neurological deficits and recovery of daily activities of the two groups were compared before and after treatment. The degree of neurological impairment was assessed by National Institutes of Health Stroke Scale (NIHSS) score [11], which includes 11 dimensions with a full score of 42 points. The higher score indicates a severer degree of the patient’s neurological deficit. The daily living activity of patients was assessed by Barthel index (BI) [12], which contains 10 aspects and scored 0-10 points in each aspect. The higher score indicates the superior ability of daily living activities of patients.
(2) Cerebral Vascular Reserve Function (CVR): The CVR and Pulsatility Index (PI) of the two groups of patients were measured by transcranial doppler ultrasound diagnostic apparatus (German ENE) before and after treatment. The patients were examined in supine position, and two probes with a frequency of 2.0 MHz were placed in bilateral temporal window of them, with a sampling depth of 50-65 mm. The probe was fixed until the blood flow signal was stable. The mean flow rate (MFV) of middle cerebral artery (MCA) in fine state and after calm breathing with mixed gas (95% O2 and 5% CO2) for 1 min was recorded as MFV1 and MFV2, respectively. At the same time, the systolic blood flow velocity (Vs), diastolic blood flow velocity (Vd), and average blood flow velocity (Vm) of the MCA were detected to calculate the CVR and PI. The calculation formula: CVR value = (MFV2-MFV1) ×100%; PI = (Vs-Vd) Vm.
(3) The levels of brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and recombinant basic fibroblast growth factor (bFGF): Early morning fasting venous blood (3 ml) was drawn from patients of the two groups before and after treatment, and the serum was separated after centrifugation. The expression of serum BDNF, VEGF and bFGF of the two groups of patients were detected by ELISA in strict accordance with kit instructions. Human BDNF ELISA Kit (Abcam American, ab212166); Human VEGF ELISA Kit (Abcam American, ab222510); Human bFGF ELISA Kit (Abcam American, ab246531).
(4) Expression of KEAP1-NRF2/ARE signaling pathway related molecules: The morning fasting venous blood of the two groups was extracted before and after treatment, and the serum was separated after centrifugation. The expression of KEAP1-NrF2/ARE signaling pathway related molecules was detected by ELISA. Human NQO1 ELISA kit, human Keap1 ELISA kit, human Nrf2 ELISA kit and human ARE kit were purchased from Shanghai Kexing Biotechnology Co., Ltd. and the operation was performed in strict accordance with kit instructions.
(5) Safety analysis: Incidence of adverse reactions of the two groups during treatment was observed and compared.
Clinical efficacy
The clinical efficacy was graded into 4 levels: recovery, markedly effective, effective and ineffective [13]. Recovery: the patient’s symptoms and signs disappeared completely after treatment, the ability of daily activities was restored, and NIHSS score was decreased by 91% to 100% comparing to pre-treatment level. Markedly effective: patient’s symptoms and signs improved significantly after treatment, the ability of daily activities was largely restored, and NIHSS score was decreased by 46% to 90% comparing to pre-treatment level. Effective: patient’s symptoms and signs improved after treatment, the ability of daily activities was partially restored, and NIHSS score decreased by 18% to 45% comparing to pre-treatment level. Ineffective: patient’s symptoms, signs and daily activities were not changed or even deteriorated after treatment, and the NIHSS scores decreased or increased by <18% comparing to pre-treatment level.
Statistical analysis
The statistical analysis was conducted by SPSS25.0. The measurement data were expressed by (x̅±s) and the enumeration data were expressed by percentage; The comparison of measurement data between groups was performed by t-test, the comparison before and after treatment in the same group was performed by paired t-test, and the comparison of count data was performed by χ2 test. P<0.05 was deemed as statistically significant difference.
Results
Clinical data
There was no significant difference in gender, BMI, TC, TG, LDL-C, basic diseases (hypertension, diabetes, hyperlipidemia), onset time, NIHSS score and BI between the two groups before treatment (P>0.05) (Table 1).
Table 1.
Comparison of clinical data between the two groups (x̅±s)
| index | Observation group (n=65) | Control group (n=62) | t/χ 2 | P |
|---|---|---|---|---|
| Gender (n) | ||||
| Male | 39 | 40 | 0.275 | 0.600 |
| Female | 26 | 22 | ||
| BMI (kg/m2) | 23.47±5.28 | 23.69±5.02 | 0.240 | 0.810 |
| TC (mmol/L) | 4.10±2.16 | 4.02±2.03 | 0.215 | 0.830 |
| TG (mmol/L) | 3.17±0.97 | 3.26±0.89 | 0.544 | 0.587 |
| LDL-C (mmol/L) | 2.93±0.69 | 2.87±0.77 | 0.463 | 0.644 |
| Hypertension (n, %) | 37 (56.92) | 39 (62.90) | 0.472 | 0.492 |
| Diabetes mellitus (n, %) | 19 (29.23) | 16 (25.81) | 0.186 | 0.666 |
| Hyperlipidemia (n, %) | 8 (12.31) | 10 (16.13) | 0.381 | 0.537 |
| Onset time (h) | 17.28±3.11 | 18.02±3.64 | 1.234 | 0.220 |
| NIHSS score (scores) | 12.37±2.15 | 12.45±2.03 | 0.215 | 0.830 |
| BI | 33.47±11.39 | 34.22±12.74 | 0.350 | 0.727 |
Note: BMI: body mass index; TC: total cholesterol; TG: Triglycerides; LDL-C: low-density lipoprotein cholesterol; NIHSS: National Institutes of Health Stroke Scale; BI: Barthel Index.
Clinical efficacy
In the observation group, 27 cases (41.54%) were cured after treatment, 19 cases were markedly effective (29.23%), 16 cases were effective (24.62%), and 3 cases were ineffective (4.62%), and the total effective rate was 95.38%; In the control group, 21 cases were cured (33.87%), 15 cases were markedly effective (24.19%), 16 cases were effective (25.81%), 10 cases were ineffective (16.13%), and the total effective rate was 83.87%. The total effective rate of observation group was remarkably higher than that of the control group (P<0.05) (Table 2).
Table 2.
Comparison of clinical efficacy between two groups [n (%)]
| Group | Number of cases | Cured | Markedly effective | Effective | Invalid | Total effective rate (%) |
|---|---|---|---|---|---|---|
| Observation group | 65 | 27 (41.54) | 19 (29.23) | 16 (24.62) | 3 (4.62) | 95.38 |
| Control group | 62 | 21 (33.87) | 15 (24.19) | 16 (25.81) | 10 (16.13) | 83.87 |
| χ 2 | - | - | - | - | - | 4.578 |
| P | - | - | - | - | - | 0.032 |
Comparison of NIHSS score and BI
There was no significant difference in NIHSS score or BI between the two groups before treatment (all P>0.05). The NIHSS score significantly decreased while the BI significantly increased after treatment (all P<0.05). The NIHSS score after treatment in observation group was obviously lower than that in control-group (P<0.05), while BI after treatment in observation-group was notably higher than that in control-group (P<0.05) (Table 3).
Table 3.
Comparison of NIHSS score and BI score between two groups before and after treatment (x̅±s)
| Group | Time | NIHSS (points) | BI |
|---|---|---|---|
| Observation group (n=65) | Before treatment | 12.37±2.15 | 33.47±11.39 |
| After treatment | 4.58±1.20* | 63.29±15.20* | |
| t | 25.508 | 12.658 | |
| P | 0.000 | 0.000 | |
| Control group (n=62) | Before treatment | 12.45±2.03 | 34.22±12.74 |
| After treatment | 6.32±1.74 | 52.39±16.42 | |
| t | 18.053 | 6.884 | |
| P | 0.000 | 0.000 |
Note: Compared with the control group in the same period, t-test;
P<0.05.
Comparison of CVR and PI
There was no significant difference in CVR or PI between the two groups before treatment (all P>0.05). After treatment, the CVR was substantially increased while PI was significantly decreased comparing with pre-treatment level (all P<0.05). The CVR in observation group after treatment was substantially higher than that the control group (P<0.05), while PI was significantly lower (all P<0.05) (as shown in Table 4).
Table 4.
Comparison of CVR and PI between the two groups before and after treatment
| Group | Time | CVR (%) | PI |
|---|---|---|---|
| Observation group (n=65) | Before treatment | 19.34±3.25 | 0.93±0.15 |
| After treatment | 39.80±7.53* | 0.70±0.09* | |
| t | 20.113 | 10.600 | |
| P | 0.000 | 0.000 | |
| Control group (n=62) | Before treatment | 19.85±3.11 | 0.92±0.17 |
| After treatment | 30.32±6.57 | 0.80±0.11 | |
| t | 11.342 | 4.666 | |
| P | 0.000 | 0.000 |
Note: Compared with the control group in the same period, t-test;
P<0.05.
Comparison of serum BDNF, VEGF and bFGF between the two groups before and after treatment
There were no statistically significant differences in serum BDNF, VEGF and bFGF between the two groups before treatment (all P>0.05). After treatment, the serum levels of BDNF, VEGF, and bFGF of the two groups were remarkably higher than those before treatment (P<0.05), and the indicators of the observation group after treatment were obviously higher than those of the control group (all P<0.05) (Figure 1).
Figure 1.
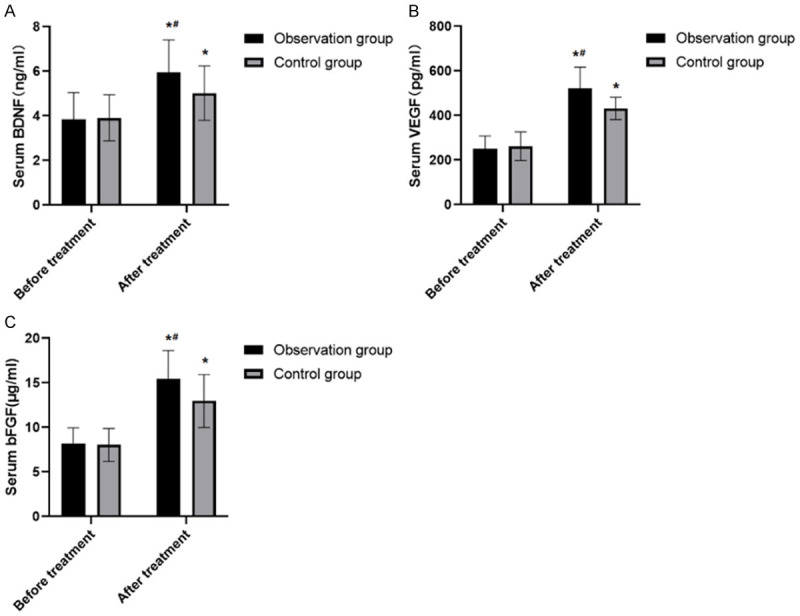
Comparison of serum BDNF, VEGF, bFGF levels before and after treatment between the two groups. A. There were no statistically significant differences in serum BDNF, between the two groups before treatment (P>0.05). After treatment, the serum levels of BDNF, of the two groups were remarkably higher than those before treatment (P<0.05), and the BDNF of the observation group after treatment were obviously higher than those of the control group (P<0.05). B. There were no statistically significant differences in serum VEGF, between the two groups before treatment (P>0.05). After treatment, the serum levels of VEGF, of the two groups were remarkably higher than those before treatment (P<0.05), and the VEGF of the observation group after treatment were obviously higher than those of the control group (P<0.05). C. There were no statistically significant differences in serum bFGF, between the two groups before treatment (P>0.05). After treatment, the serum levels of bFGF of the two groups were remarkably higher than those before treatment (P<0.05), and the bFGF of the observation group after treatment were obviously higher than those of the control group (P<0.05). Note: The comparison of the same group before and after treatment was performed by paired t test, *P<0.05; The comparison with the same group of treatment was by t-test, #P<0.05.
Comparison of Keap1-Nrf2/ARE signaling pathway related molecules
There were no significant differences in the levels of NQO1, Keap1, Nrf2 and ARE between the two groups before treatment (all P>0.05). The levels of Keap1 after treatment were significantly higher than those before treatment (P<0.05), while the levels of NQO1, Nrf2 and ARE were significantly lower than those before treatment (all P<0.05). The Keap1 of the observation group after treatment was significantly higher than that of the control group (P<0.05), while NQO1, Nrf2 and ARE were markedly lower than those of the control group (all P<0.05) (Figure 2).
Figure 2.
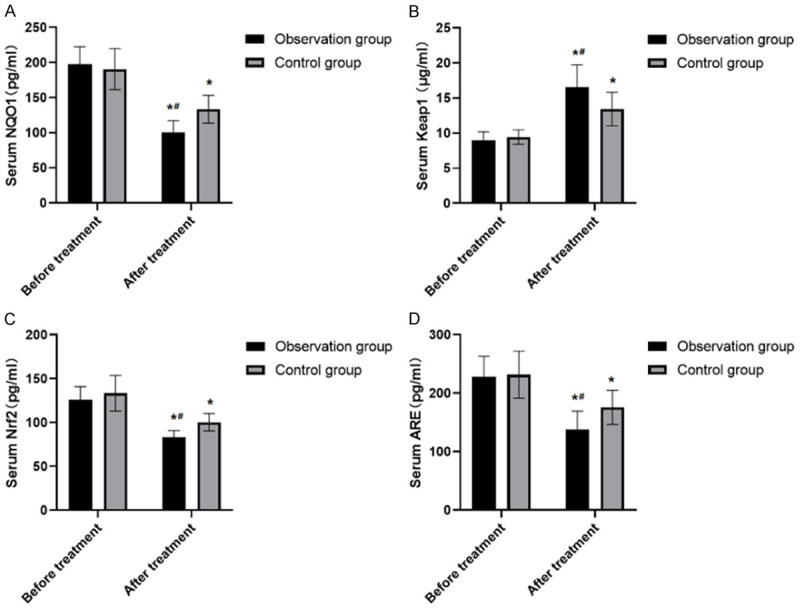
Expression of serum levels of Keap1-Nrf2/ARE signal pathway related molecules between two groups before and after treatment. A. There were no significant differences in the levels of NQO1 between the two groups before treatment (P>0.05). The the levels of NQO1 were significantly lower than those before treatment (P<0.05). The NQO1 were markedly lower than those of the control group (P<0.05). B. There were no significant differences in the levels of Keap1 between the two groups before treatment (P>0.05). The levels of Keap1 after treatment were significantly higher than those before treatment (P<0.05). The Keap1 of the observation group after treatment was significantly higher than that of the control group (P<0.05). C. There were no significant differences in the levels of Nrf2 between the two groups before treatment (P>0.05). The the levels of Nrf2 were significantly lower than those before treatment (P<0.05). The Nrf2 were markedly lower than those of the control group (P<0.05). D. There were no significant differences in the levels of ARE between the two groups before treatment (P>0.05). The the levels of ARE were significantly lower than those before treatment (P<0.05). The ARE were markedly lower than those of the control group (P<0.05). Note: The comparison of the same group before and after treatment was performed by paired t test, *P<0.05; The comparison with the same group of treatment was by t-test, #P<0.05.
Safety analysis
In the observation group, dizziness and headache occurred in 2 cases (3.07%), nausea and vomiting in 5 cases (7.69%), abdominal pain and diarrhea in 3 cases (4.62%), bleeding in 2 cases (3.07%), with an incidence of 18.46%. In control group, there were 4 cases of dizziness and headache (6.45%), 6 cases of nausea and vomiting (9.68%), 2 cases of abdominal pain and diarrhea (3.23%) and 1 case of bleeding (1.61%), with an incidence of 20.97%. There were no statistical differences in the incidence of adverse reactions between the two groups (P>0.05) (Table 5).
Table 5.
The incidence of adverse reactions in two groups during treatment [n (%)]
| Group | Number of cases | Dizziness/headache | Nausea and vomiting | Abdominal pain and diarrhea | Bleeding | Total incidence |
|---|---|---|---|---|---|---|
| Observation group | 65 | 2 (3.07) | 5 (7.69) | 3 (4.62) | 2 (3.07) | 12 (18.46) |
| Control group | 62 | 4 (6.45) | 6 (9.68) | 2 (3.23) | 1 (1.61) | 13 (20.97) |
| χ 2 | - | - | - | - | - | 0.126 |
| P | - | - | - | - | - | 0.723 |
Discussion
At present, the most effective method for treating acute ischemic stroke is thrombolysis. However, thrombolytic therapy has certain limitations, as most people have lost the optimal chance of thrombolytic therapy during treatment [13]. The symptoms of neuronal necrosis in acute ischemic stroke can appear in very short time after onset. Therefore, the key to treatment is to save the ischemic penumbra of cerebral infarction and improve or restore the blood perfusion of ischemic brain tissue [14]. The rapid establishment of collateral circulation is an effective measure to improve blood circulation in the ischemic penumbra. Since the establishment of collateral compensatory vessels can ensure the supply of blood and oxygen supply of brain tissue, the volume of infarcts and the damage of nerve function can be reduced, thus promoting the recovery of brain tissue function [15,16].
Butylphthalide is a new anti-cerebral ischemia drug extracted from celery seed. It has been confirmed that butylphthalide can intercept pathological links of irreversible cerebral damage caused by acute ischemic stroke, thereby exerting a strong protective effect on the brain [17]. Some of the scholars suggest the following mechanism of butylphthalide in acute ischemic stroke [18,19]: (1) The establishment of collateral circulation is accelerated by preserving the integrity of the patient’s blood vessels and restoring or increasing blood perfusion to the ischemic area. (2) By maintaining stability in mitochondrial membrane and improving the activity of mitochondrial enzymes, it protects the mitochondrial structure of patients, enhances the energy metabolism of their body tissues and reduces the death of nerve cells. (3) The airway can enhance the activity of antioxidant enzymes, inhibit the apoptosis of nerve cells and help the recovery of nerve function by inhibiting the inflammatory reaction and the release of arachidonic acid and glutamate.
In order to further understand the effect of butylphthalide injection, we explored and analyzed the clinical effect of butylphthalide injection in acute ischemic stroke and its possible mechanism. The results showed that NIHSS score decreased and BI index increased after treatment, and the amelioration of NIHSS and BI in observation-group was better than in control-group. It is consistent with the results of other researches [20,21], that the adjuvant therapy of butylphthalide injection can not only reduce the neurological impairment, exert its protective effect on nerves, but also effectively improve their quality of life. CVR refers to a compensatory effect that occurs in the brain to maintain the normal and stable blood flow under the pathological stimulation of ischemic stroke. The stable blood flow in the ischemic state is provided by the open collateral circulation of cerebral blood vessels, and the establishment of collateral circulation depends on the compensatory contraction or expansion of cerebral capillaries and arterioles in brain [22,23]. Scholars reported that [24] CVR is beneficial for assessing acute ischemic stroke, and is connected with neurological deficit. The results of our study revealed that CVR increased and PI decreased in the two groups after treatment, and the reduction of PI and the increase of CVR in observation group were substantially superior to those in control group. These suggest that the adjuvant therapy with butylphthalide injection could effectively increase patient’s CVR, which is related to the uplifting of cerebral perfusion and the improvement of microcirculation and vascular compliance.
BDNF, bFGF and VEGF are substantially expressed in the nervous system. They are involved in multiple pathological processes of ischemic stroke and promote the establishment of collateral circulation [25,26]. BDNF, bFGF and VEGF can reduce the further damage of nerve tissue, save the ischemic penumbra, and promote the repair and regeneration of neurons and axons. Our results demonstrated that BDNF, bFGF and VEGF in the two groups were obviously increased after treatment, and the increase in observation group after treatment was superior to that in control group. It is consistent with study of Rali P et al. [26], that butylphthalide promotes the expression of BDNF, bFGF and VEGF, thus improving angiogenesis and cerebral microcirculation, and this may be one of the mechanisms of butylphthalide in treating ischemic stroke.
As an oxidative stress signaling pathway, Keap1-Nrf2/ARE signaling pathway may exert the anti-inflammatory, anti-oxidative stress and anti-apoptosis function by activating the expression of its downstream factors after acute ischemic stroke [27,28]. Nrf2 and its cytoplasmic adaptor protein Keap1 are important central regulators of antioxidant stress response. Nrf2 can induce the expression of phase II detoxifying enzymes and encode antioxidant proteins by coordinating with antioxidant response elements (ARE), which plays an important protective role in cell defense. When the body is stimulated by electrophiles or oxidative stress, the cysteine residue of Keap1 is immediately modified, and the stability of Nrf2 is immediately reduced, causing the two to dissociate rapidly. Thus, nuclear metastasis is initiated and binds to the nuclear antioxidant response element ARE and the small Maf nuclear protein in vivo to form a dimer, which activates the expression of some downstream genes (such as HO-1, GSH, GCLC, etc.) and regulates the level of antioxidant enzymes. Keap1-nrf2/ARE pathway is involved in neuroprotection, anti-oxidative stress, anti-apoptosis and regulation of GSH synthesis, etc. Animal studies have also confirmed that activation of KEAP1-NRF2/ARE pathway can effectively play an endogenous neuroprotection function to certain extent. The results in this study showed that the adjuvant therapy of butylphthalide could effectively inhibit the expression of Keap1, promote the expression of Nrf2, ARE and NQO1, and then activate the KEAP1-NRF2/ARE signaling pathway. These results suggest that butylphthalide may play an anti-oxidative stress and neuroprotective role by activating the KEAP1-NrF2/ARE pathway. In addition, there were no statistically significant differences in the adverse reactions between the two groups during treatment, suggesting that the adjuvant treatment of butylphthalide does not increase the adverse reactions of patients, and the clinical treatment is safe.
However, due to the small sample size of this study, more animal and clinical studies are required to confirm the specific mechanism of action of butylphthalide in the treatment of ischemic stroke, thereby to provide a better basis for improving the clinical prognosis of patients. In future study, we will further expand the sample size, and at the same time, further clarify the mechanism of the effect of butylphthalide on acute ischemic stroke patients.
To conclude, butylphthalide can improve the clinical efficacy of patients with acute ischemic stroke, and promote neurological function and activities of daily living. Its mechanism is likely that butylphthalide improves the CVR of patients, enhances the establishment of collateral compensatory vessels, and changes the expression of Keap1-Nrf2/ARE signaling pathway, thereby exerting the neuroprotective effect. It also has a good safety that is worth of clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Amin OSM, Sheikhbzeni AS, Siddiq AN. Relationship of QTc interval prolongation with acute ischemic stroke. Med Arch. 2020;74:195–198. doi: 10.5455/medarh.2020.74.195-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyedsaadat SM, Neuhaus AA, Pederson JM, Brinjikji W, Rabinstein AA, Kallmes DF. Location-specific ASPECTS paradigm in acute ischemic stroke: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2020;41:2020–2026. doi: 10.3174/ajnr.A6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez JR, Hobbs KS, Johnson LL, Vu QD, Bennett J, Tegeler C, Wolfe SQ, Sarwal A. The clinical contribution of neurovascular ultrasonography in acute ischemic stroke. J Neuroimaging. 2020;30:867–874. doi: 10.1111/jon.12771. [DOI] [PubMed] [Google Scholar]
- 4.Tiedt S, Brandmaier S, Kollmeier H, Duering M, Artati A, Adamski J, Klein M, Liebig T, Holdt LM, Teupser D, Wang-Sattler R, Schwedhelm E, Gieger C, Dichgans M. Circulating metabolites differentiate acute ischemic stroke from stroke mimics. Ann Neurol. 2020;88:736–746. doi: 10.1002/ana.25859. [DOI] [PubMed] [Google Scholar]
- 5.Mengozzi L, Widimsky P. The potential value of histological analysis of thrombi extracted through mechanical thrombectomy during acute ischemic stroke treatment. Anatol J Cardiol. 2020;23:254–259. doi: 10.14744/AnatolJCardiol.2020.81342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karaszewski B, Jabłoński B, Żukowicz W. The salvageable brain in acute ischemic stroke. The concept of a reverse mismatch: a mini-review. Metab Brain Dis. 2020;35:237–240. doi: 10.1007/s11011-019-00517-x. [DOI] [PubMed] [Google Scholar]
- 7.Katyal A, Bhaskar S. CTP-guided reperfusion therapy in acute ischemic stroke: a meta-analysis. Acta Neurol Scand. 2021;143:355–366. doi: 10.1111/ane.13374. [DOI] [PubMed] [Google Scholar]
- 8.Qu JF, Chen YK, Luo GP, Qiu DH, Liu YL, Zhong HH, Wu ZQ. Does the Babinski sign predict functional outcome in acute ischemic stroke? Brain Behav. 2020;10:e01575. doi: 10.1002/brb3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Tian X, Pei LL, Niu PP, Guo Y, Hu R, Liu K, Tian M, Li Y, Wang C, Wang X, Xu Y, Song B. The association between serum apelin-13 and the prognosis of acute ischemic stroke. Transl Stroke Res. 2020;11:700–707. doi: 10.1007/s12975-019-00769-w. [DOI] [PubMed] [Google Scholar]
- 10.Chinese Medical Association Neurology Branch, Chinese Medical Association Neurology Branch Cerebrovascular Disease Group. Chinese acute ischemic stroke diagnosis and treatment guidelines 2014. Chinese Journal of Neurology. 2015;48:11–12. [Google Scholar]
- 11.Lee H, Yang Y, Liu B, Castro SA, Shi T. Patients with acute ischemic stroke who receive brain magnetic resonance imaging demonstrate favorable in-hospital outcomes. J Am Heart Assoc. 2020;9:e016987. doi: 10.1161/JAHA.120.016987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Park SY, Jang MU, Kim Y, Lee J, Kim C, Kim YJ, Sohn JH. Association between osteoporosis and cognitive impairment during the acute and recovery phases of ischemic stroke. Medicina (Kaunas) 2020;56:307. doi: 10.3390/medicina56060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi AI, Aslam H, Zafar W, Huang W, Lobanova I, Naqvi SH, Malhotra K, Arora N, Chandrasekaran PN, Siddiq F, French BR, Gomez CR. Acute kidney injury in acute ischemic stroke patients in clinical trials. Crit Care Med. 2020;48:1334–1339. doi: 10.1097/CCM.0000000000004464. [DOI] [PubMed] [Google Scholar]
- 14.Gopal M, Lakhani S, Lee VH. Intravenous thrombolysis in acute ischemic stroke patients with unsuspected infective endocarditis. J Stroke Cerebrovasc Dis. 2021;30:105502. doi: 10.1016/j.jstrokecerebrovasdis.2020.105502. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Pan C, Zhang P, Tang Y, Tang Z. Clinical characteristics of inpatients with coronavirus disease 2019 and acute ischemic stroke: from epidemiology to outcomes. Curr Neurovasc Res. 2020;17:760–764. doi: 10.2174/1567202617999201110200410. [DOI] [PubMed] [Google Scholar]
- 16.Young GH, Tang SC, Wu VC, Wang KC, Nong JY, Huang PY, Hu CJ, Chiou HY, Jeng JS, Hsu CY. The functional role of hemojuvelin in acute ischemic stroke. J Cereb Blood Flow Metab. 2020;40:1316–1327. doi: 10.1177/0271678X19861448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ontario Health (Quality) Automated CT perfusion imaging to aid in the selection of patients with acute ischemic stroke for mechanical thrombectomy: a health technology assessment. Ont Health Technol Assess Ser. 2020;20:1–87. [PMC free article] [PubMed] [Google Scholar]
- 18.Qian S, Li R, Zhang C, Zhang R, Guo D, Bu X, Wang A, Peng H, Chen J, Zhang Y, He J, Xu T, Zhong C. Plasma endostatin levels at acute phase of ischemic stroke are associated with post-stroke cognitive impairment. Neurotox Res. 2020;37:956–964. doi: 10.1007/s12640-020-00173-5. [DOI] [PubMed] [Google Scholar]
- 19.Puig J, Shankar J, Liebeskind D, Terceño M, Nael K, Demchuk AM, Menon B, Dowlatshahi D, Leiva-Salinas C, Wintermark M, Thomalla G, Silva Y, Serena J, Pedraza S, Essig M. From “time is brain” to “imaging is brain”: a paradigm shift in the management of acute ischemic stroke. J Neuroimaging. 2020;30:562–571. doi: 10.1111/jon.12693. [DOI] [PubMed] [Google Scholar]
- 20.Bruce SS, Merkler AE, Bassi M, Chen ML, Salehi Omran S, Navi BB, Kamel H. Differences in diagnostic evaluation in women and men after acute ischemic stroke. J Am Heart Assoc. 2020;9:e015625. doi: 10.1161/JAHA.119.015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugnara G, Neuberger U, Mahmutoglu MA, Foltyn M, Herweh C, Nagel S, Schönenberger S, Heiland S, Ulfert C, Ringleb PA, Bendszus M, Möhlenbruch MA, Pfaff JAR, Vollmuth P. Multimodal predictive modeling of endovascular treatment outcome for acute ischemic stroke using machine-learning. Stroke. 2020;51:3541–3551. doi: 10.1161/STROKEAHA.120.030287. [DOI] [PubMed] [Google Scholar]
- 22.Comertpay E, Vural S, Eroğlu O, Dindar Badem N, Karadeniz Bilgili Y, Coşkun F. The diagnostic value of sTWEAK in acute ischemic Stroke. Balkan Med J. 2020;37:336–340. doi: 10.4274/balkanmedj.galenos.2020.2020.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg K, Bykowski J. Modern neuroimaging techniques in diagnosing transient ischemic attack and acute ischemic stroke. Emerg Med Clin North Am. 2021;39:29–46. doi: 10.1016/j.emc.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Link TW, Santillan A, Patsalides A. Intra-arterial neuroprotective therapy as an adjunct to endovascular intervention in acute ischemic stroke: a review of the literature and future directions. Interv Neuroradiol. 2020;26:405–415. doi: 10.1177/1591019920925677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto K, Shiga H, Nakamura H, Matsui M, Miwa T. Relationship between olfactory disturbance after acute ischemic stroke and latent thalamic hypoperfusion. Chem Senses. 2020;45:111–118. doi: 10.1093/chemse/bjz077. [DOI] [PubMed] [Google Scholar]
- 26.Lio KU, Jiménez D, Moores L, Rali P. Clinical conundrum: concomitant high-risk pulmonary embolism and acute ischemic stroke. Emerg Radiol. 2020;27:433–439. doi: 10.1007/s10140-020-01772-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, Kim Y, Park SY, Kim C, Kim YJ, Sohn JH. Pre-stroke glycemic variability estimated by glycated albumin is associated with early neurological deterioration and poor functional outcome in prediabetic patients with acute ischemic stroke. Cerebrovasc Dis. 2021;50:26–33. doi: 10.1159/000511938. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X, Chen J, Sun Z, Zeng H, Xue J, Zhang Y, Liu G, Huang X. Nutrition program selection in acute ischemic stroke patients with GI hemorrhage. Asia Pac J Clin Nutr. 2020;29:55–60. doi: 10.6133/apjcn.202003_29(1).0008. [DOI] [PubMed] [Google Scholar]


