Abstract
Objective: To determine the efficacy and safety of 5-aminolevulinic acid-mediated photodynamic therapy (ALA-PDT) in cervical intraepithelial neoplasia (CIN) infected by HPV. Methods: The clinical data of 115 patients with HPV-Infected CIN admitted to our hospital were selected for this retrospective analysis. They were divided into a control group (n=53) and an experimental group (n=62) according to different treatment methods. Patients in the control group were treated by laser therapy, while those in the experimental group were treated by ALA-PDT. The two cohorts of patients were compared with respect to clinical efficacy, LSIL cure rate, adverse reactions, and complications. Patients were followed up for 3 months and 6 months, and their clinical outcomes were compared based on HPV persistence and residual/recurrent low-grade squamous intraepithelial lesion (LSIL). In the experimental group, the negative conversion rate of HPV and the degree of squamous epithelial lesions in patients with different HPV infection types and various visible degrees of transformation zones (TZs) were compared 6 months after surgery. Results: After 6 months of follow-up, the HPV clearance rate and LSIL reversal rate in the control group were 62.3% and 64.2%, respectively, while those in the experimental group were 79.0% and 80.6%, respectively, with significant differences between the two groups (P<0.05). There was no significant difference between the two groups in terms of the LSIL cure rate of lesions located in cervicovaginal region and cervicovaginal region + cervical canal (P>0.05). Neither were there any significant differences in HPV negative conversion rate and LSIL residual/recurrence rate in patients with HPV 16/18 and non-HPV 16/18 between the control group and the experimental group (P>0.05). However, the HPV negative conversion rate in partially visible TZs was lower than that in completely visible TZs, and the LSIL residual/recurrence rate in the completely visible TZs in the control group was higher than that in the experimental group (P<0.05). The incidence of adverse reactions was not significantly different between the two groups (P>0.05), but the incidence of complications in the control group was higher than that in the experimental group (P<0.05). Neither the experimental group nor the control group had any cases of pathologic escalation to high-grade squamous intraepithelial lesion (HSIL). Conclusion: ALA-PDT can effectively treat HPV-infected CIN and promote HPV clearance.
Keywords: Cervical intraepithelial neoplasia, HPV infection, laser surgery, photodynamic therapy
Introduction
Human papillomavirus (HPV) infection is a risk factor for invasive cervical cancer (CC) and precancerous lesions, especially the persistence of high-risk HPV infection, which is a high-risk factor for CC [1,2]. CC is the fourth most common malignancy in women worldwide [3]. Persistent HPV infection is closely associated with CC and its precancerous lesions. HPV, a circular double-stranded DNA virus, can be divided into high- and low-risk types according to the benign or malignant nature of the skin lesions [4]. In cervical squamous cell carcinoma, 95% of HPV genotypes are HPV-16, 18, 45, 31, 33, 35, 52 and 58 [5]. Although some cervical HPV infections can be spontaneously resolved by the autoimmune system, research has shown that high-risk HPV infection is a major trigger for cervical lesions [6]. Therefore, for high-risk patients with persistent infection for more than one year, timely intervention should be adopted to prevent malignant lesions. As to those with low-grade cervical lesions, prompt and active treatment should be given to promote the negative conversion of HPV and prevent the further progression of cervical intraepithelial neoplasia (CIN). The occurrence of CC can be defined as a complex mechanism of uncontrolled cell division that may involve cellular changes and epigenetic factors such as HPV gene integration. When HPV infection occurs, DNA mutates under cellular and other environmental conditions, resulting in the integration and operation of viral DNA and host DNA synthesis mechanisms. Therefore, viruses can escape cellular and immune defense mechanisms, promote cell proliferation, and inhibit apoptosis [7].
CIN, which refers to a series of pathomorphological changes such as abnormal cell proliferation, poor differentiation, nuclear abnormalities and increased mitosis in cervical epithelial cells under the stimulation of HPV, is closely related to CC and is a stage before the occurrence of CC [8,9]. Previously, pathologists divided CIN into three grades according to the degree of dysplasia of neoplastic cells, namely CIN I (mild), CIN II (moderate), and CIN III (severe with carcinoma in situ), reflecting a continuous pathologic process of the occurrence and development of CC [10]. The Lower Anogenital Squamous Terminology (LAST) Standardization project used squamous intraepithelial lesions (SIL) to rename HPV infection-associated squamous epithelial lesions of the lower genital tract, including the cervix, into low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) [11]. HSIL has the potential to be cancerous, and without proper treatment, it has a high risk of progressing to invasive carcinoma of the cervix. Clinically, loop electrosurgical excision procedure (LEEP) or cold knife conization (CKC) is often selected for intervention, depending on the range of lesions and the depth of invasion [12-14]. As for the clinical treatment of LSIL, some experts believe that LSIL is only an instantaneous expression after HPV infection, which has different biological properties from malignant tumors, with a high natural regression rate [15]. Rouzier et al. [16] found that 60%-90% of LSIL can be reversed naturally within 2 years. While 30% of LSILs persist, 10% progress to HSIL, and only 1% may progress to CC [17]. Therefore, early detection and intervention of high-risk HPV persistent infection and CIN can effectively prevent the occurrence of CC.
For virus-infected people, the long-term existence of HPV will increase the mental burden, and there is a possibility that the disease may be transmitted to their spouses through sexual contact [18]. Currently, there is no effective treatment for HPV infection. Traditional treatment methods are divided into physical methods and surgical resection, but with the common characteristics of easy recurrence, serious tissue damage, and the risk of fertility due to damage of cervical structure caused by improper treatment [19]. Photodynamic therapy (PDT) is a new minimally invasive treatment technique [20]. 5-aminolevulinic acid-mediated photodynamic therapy (ALA-PDT) is a relatively novel technology for the treatment of epithelial, superficial and non-melanoma skin tumors, infectious lesions, and skin inflammatory diseases [21]. Fan et al. [22] reported that after 1-4 courses of ALA-PDT treatment, lichen planus lesions were significantly improved, with a total effectiveness rate up to 71% and no obvious side effects except for tolerable pain in most patients. 5-aminolevulinic acid (ALA) is a precursor of hemoglobin synthesis in vivo, which does not produce photosensitivity due to its small content in normal cells. When ALA is applied to the damaged skin surface, it crosses the skin barrier, selectively aggregates in the target cells, and is metabolized into photoactive-free porphyrin in the target cells, known as protoporphyrin IX (PpIX, heme precursor) [23]. ALA-PDT can selectively act on rapidly proliferating cells and exert a specific killing effect, with no or less damage to normal tissues and cells [24]. Moreover, it generated no local scar compared to laser or LEEP, while effectively protecting cervical function and minimizing the impact on fertility. The purpose of this study was to investigate the ameliorative effect of ALA-PDT on HPV-infected CIN.
Materials and methods
Study population
The clinical data of 115 patients with HPV-infected CIN admitted to our hospital from October 2020 to June 2021 were selected for this retrospective analysis. Based on different treatment methods they received, the patients were divided into a control group (n=53) and an experimental group (n=62). The average age of patients was (35.71±2.51) years old in the control group and (36.23±2.69) years old in the experimental group. The general data were similar in two groups (all P>0.05), indicating the comparability between two groups (Table 1). All patients underwent gynecological examinations, including liquid-based cytology test (LCT), high-risk HPV (Hr-HPV) test (Roche Cobas 4800), colposcopy and biopsy, and HPV-infected low-grade CIN was confirmed. Patients with CC or other malignancies of the reproductive system, severe immune diseases and complicated gonorrhea, and acute pelvic inflammatory disease were excluded. The choice of treatment plan was initially based on the patient’s willingness and the specific condition of each patient. All patients signed the informed consent form before enrollment. The study was approved by the Medical Research Ethics Committee of the International Peace Maternity & Child Health Hospital of China Welfare Institute (ID: GKLW 2018-18).
Table 1.
General information
| Age | Course of disease (month) | Lesion location | ||
|---|---|---|---|---|
|
| ||||
| Cervicovaginal region | Cervicovaginal region + cervical canal | |||
| Control group (n=53) | 35.71±2.51 | 15.87±3.86 | 34 | 19 |
| Experimental group (n=62) | 36.23±2.69 | 16.24±4.39 | 39 | 23 |
| χ2/t | 1.0655 | 0.4761 | 0.0192 | |
| P | 0.2889 | 0.6349 | 0.8898 | |
Inclusion criteria: (1) Patients with an age of 25-45 years old and confirmed HPV cervical persistent infection by HPV genotyping tests; (2) Patients with LSIL on cervical cytology, which was confirmed by colposcopy and histopathological biopsy; (3) Patients without any other treatment for more than 1 year.
Exclusion criteria: (1) Patients with HSIL, malignant cells, or suspected cancerous lesions and cancerous infiltration indicated by cytological and histological examination; (2) Patients with CIN II or CIN III, CC or invasive cancer as indicated by Colposcopic biopsy; (3) Patients with severe pelvic inflammation and cervical inflammation or other serious gynecologic inflammation revealed by clinical examination; (4) Patients with with undiagnosed vaginal bleeding; (5) Patients with allergy to drugs used in this study or known to have porphyria; (6) Pregnant women; (7) Patients with severe organic disease, mental disorders, autoimmune diseases or immunosuppression.
Treatment methods
Patients in the control group received one-time laser therapy 3-7 days after menstruation. CO2 laser therapy was performed with the JC-25 CO2 laser therapeutic instrument (Shanghai Juehua Medical Instrument Co., Ltd) with an output power of 600VA for 2-5 min. Sexual activity was forbidden for 3 months after treatment.
ALA-PDT was performed on patients in the experimental group 3-7 days after menstruation. The bladder was emptied before the procedure. Three vials of ALA (Shanghai Fudan Zhangjiang Biomedical Co., Ltd., 118 mg/vial) were dissolved in 1.5 mL thermosensitive gel to make ALA pads by cotton pieces, and ALA pads were applied on the surface of the cervix and cervical canal for 3-4 h, then PDT was performed. LED-IB photodynamic therapy instrument (Wuhan Yage Optic and Electronic Technique Co., Ltd.) was used for cervical and vaginal lesions, LD600-C photodynamic therapy instrument (Wuhan Yage Optic and Electronic Technique Co., Ltd.) was applied to cervical canal lesions. The lesion area was irradiated with 635 nm red light wave at 80 mW/cm2 for 30 minutes, once every 7-14 days for 3 consecutive times. Also, sexual activity was prohibited for 3 months after treatment.
Outcome measures
(1) Comparison of clinical efficacy: LCT, Hr-HPV test and colposcopy biopsy were performed after three months of follow-up. LCT and HR-HPV tests were performed again after 6 months of follow-up, and colposcopy biopsy was performed for those with abnormal LCT results and HR-HPV positive. A cure was defined as HR-HPV negative, no cytological findings of malignant cells or intraepithelial lesions, and no cervical lesions by colposcopy biopsy. Residual was defined as continuous cervical lesions revealed by colposcopic biopsy after 3 and 6 months of follow-up. Recurrence was defined as no cervical lesions at 3 months follow-up and recurrence at 6 months follow-up. According to the site of cervical lesions in the cervix (cervicovaginal region/cervicovaginal region + cervical canal), we compared the curative effect and residual/recurrence of different treatments in the same site and different sites with the same treatment method.
(2) Adverse reactions of patients were counted, including colporrhagia in the month after operation, menstrual abdominal pain and colpitis.
(3) The complications, including bleeding, infection, menstrual flow obstruction, menostaxis and cervical tube adhesions, were recorded.
(4) Clinical outcomes of patients in the two groups were compared 6 months after surgery, with HPV persistence and LSIL residual/recurrence (or escalating to HSIL) as the outcome measures. HPV persistence: Genotyping of HPV was performed to identify persistent cervical infection. Recurrence was defined as abnormal uterine cervical fluid after surgery detected by cytology or colposcopy, and the presence of negative lesions confirmed by pathological examination. Follow-up and reexamination of abnormal cervical liquid-based cytology and pathological detection by multi-point biopsy under colposcopy confirmed the presence of CIN lesions.
Statistical processing
SPSS21.0 statistical software was used to process the data. Counted data were recorded as cases (%) and compared using the χ2 test. Measured data were presented as (mean ± SD), and the comparison between groups was analyzed by the independent samples t-test while the comparison within the group was performed by paired t test, with P<0.05 as the level of significance.
Results
Clinical efficacy
The experimental group showed significantly higher HPV negative conversion rates and LSIL reversal rates than the control group at 3 and 6 months after treatment (P<0.05). See Table 2.
Table 2.
Clinical efficacy in both groups of patients
| At 3 months after treatment | At 6 months after treatment | |||
|---|---|---|---|---|
|
|
|
|||
| HPV negative conversion | LSIL reversion | HPV negative conversion | LSIL reversion | |
| Control group (n=53) | 30 (56.6) | 31 (58.5) | 33 (62.3) | 34 (64.2) |
| Experimental group (n=62) | 47 (75.8) | 48 (77.4) | 49 (79.0) | 50 (80.6) |
| χ2/t | 4.7621 | 4.7611 | 3.9271 | 3.9481 |
| P | 0.0291 | 0.0291 | 0.0475 | 0.0469 |
Negative conversion rates of patients with different types of HPV and transformation zones (TZs)
In the experimental group, there was no significant difference in the HPV negative conversion rate between HPV 16/18 type and non-HPV 16/18 type patients (P>0.05), but the HPV negative conversion rate of patients with partially visible TZs was lower than those with completely visible TZs (P<0.05). Tables 3 and 4.
Table 3.
Negative conversion rates of patients with different types of HPV and transformation zones
| Negative conversion of different types of HPV | Negative conversion of different transformation zones | |||
|---|---|---|---|---|
|
|
|
|||
| HPV 16/18 type | Non-HPV 16/18 type | Completely visible | Partially visible | |
| Control group (n=53) | 22 (41.5) | 19 (35.8) | 28 (52.8) | 13 (24.5) |
| Experimental group (n=62) | 36 (58.1) | 26 (41.9) | 35 (56.5) | 22 (35.5)* |
| χ2/t | 1.3631 | 0.4930 | ||
| P | 0.2431 | 0.4826 | ||
Compared with the visible transformation zone of the same group, P<0.05.
Table 4.
LSIL residual/recurrence in patients with different types and transformation zones
| Types of HPV infection | Transformation zones | |||
|---|---|---|---|---|
|
|
|
|||
| HPV 16/18 type | Non-HPV 16/18 type | Completely visible | Partially visible | |
| Control group (n=53) | 7 (13.2) | 8 (15.1) | 11 (20.8) | 5 (9.4) |
| Experimental group (n=62) | 3 (4.8) | 3 (4.8) | 1 (1.6) | 5 (8.1) |
| χ2/t | 0.0191 | 4.1021 | ||
| P | 0.8901 | 0.0428 | ||
LSIL residual/recurrence in patients with different types and TZs
No significant difference was observed in the LSIL residual/recurrence rate in HPV 16/18 type and non-HPV 16/18 type between the experimental group and control group (P>0.05), but a significant difference was present in LSIL residual/recurrence rate in the different conditions of transformation zones between the experimental group and the control group (P<0.05). See Table 4.
Cure rate in patients with lesions at different sites
A significant difference was observed in the total cure rate between the control group and the experimental group 6 months after follow-up (P<0.05). However, there was no significant difference between the two groups in terms of the cure rate of lesions located in cervicovaginal region and cervicovaginal region + cervical canal (P>0.05). See Table 5.
Table 5.
LSIL cure rate in different sites
| Total cure rate | Cervicovaginal region | Cervicovaginal region + cervical canal | |
|---|---|---|---|
| Control group (n=53) | 33 (62.3) | 19 (57.6) | 14 (42.4) |
| Experimental group (n=62) | 49 (79.0) | 25 (51.0) | 24 (49.0) |
| χ2/t | 3.9271 | 0.3408 | |
| P | 0.0475 | 0.5594 | |
Incidence of adverse reactions in both groups
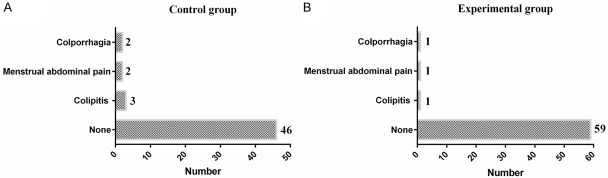
In the experimental group, there was 1 case of colporrhagia in the month after operation, 1 case of menstrual abdominal pain and 1 case of colpitis, with a total incidence of 4.8%. In the control group, there were 2 cases of colporrhagia in the month after operation, 2 cases of menstrual abdominal pain and 3 cases of colpitis, with a total incidence of 13.2%. The incidence of adverse reactions was significantly different between the two groups (P<0.05). See Figure 1.
Figure 1.
Incidence of adverse reactions in both groups.
Incidence of complications in two groups
The total incidence of complications in the experimental group was significantly lower than that of the control group (1.6% vs 11.3%, P<0.05). See Table 6.
Table 6.
Incidence of complications in both groups
| Hemorrhage | Infection | Menstrual flow obstruction | Menostaxis | Adhesion of cervical canal | Total incidence | |
|---|---|---|---|---|---|---|
| Control group (n=53) | 1 (1.9) | 1 (1.9) | 2 (3.8) | 1 (1.9) | 1 (1.9) | 6 (11.3) |
| Experimental group (n=62) | 0 | 0 | 0 | 1 (1.6) | 0 | 1 (1.6) |
| χ2/t | 4.7111 | |||||
| P | 0.0300 |
Clinical outcomes
There were 6 cases of HPV persistence and 8 cases of LSIL residual/recurrence in the experimental group, which were significantly less than those of the control group (13 cases of HPV persistence and 16 cases of LSIL residual/recurrence) (all P<0.05). Neither group had any case of pathological escalation to HSIL. See Table 7.
Table 7.
Clinical outcome
| HPV persistence | LSIL residual/recurrence | Escalation to high-grade cervical intraepithelial neoplasia | |
|---|---|---|---|
| Control group (n=53) | 13 (24.5) | 16 (30.2) | 0 (0) |
| Experimental group (n=62) | 6 (9.7) | 8 (12.9) | 0 (0) |
| χ2/t | 4.5691 | 5.1701 | - |
| P | 0.0326 | 0.0230 | - |
Discussion
The common treatment methods for cervical lesions include cervical LEEP and CKC, as well as electrocautery, freezing, microwave and laser therapy [24,25]. However, for young women with fertility requirements, surgery and invasive physical therapy can easily cause cervical insufficiency and affect female fertility. ALA-PDT, on the other hand, is a highly selective minimally invasive treatment method combining medicine and machinery, which can effectively prevent the further progression of CIN and preserve the cervical structure through non-invasive treatment by laser combined with photosensitizer [26].
This article mainly discussed the efficacy and safety of ALA-PDT in HPV-infected CIN. The cervical epithelium is composed of cervical/vaginal squamous epithelium and cervical columnar epithelium, while CIN is confined to the epithelial tissue above the basement membrane of the cervical epithelium, the thickness of which is about 2-3 mm [27,28]. The effective depth of interaction of PDT is 8 mm, so PDT can treat CIN. The results of this study showed that the HPV negative conversion rate and the LSIL reversal rate in the PDT group were significantly higher than those of the control group. In PDT group, the total cure rate was 79.0%, which were significantly better than those in the control group (62.3%); Moreover, the clinical outcome and the incidence of complications were better in the PDT group. All these findings are consistent with previous research conclusions [29-31]. HPV is an epitheliophilic virus. After infecting the cervix, it binds to basal cells, and the free viral DNA is often integrated into the host cell genome, which results in up-regulated protein expression levels of E6 and E7 with synergistic effects, thus enabling the cells to acquire greater proliferation ability and leading to the transformation of cancerous cells [32]. We believe that ALA-PDT can eliminate virus infection by killing proliferating active cells infected with HPV, and that by inhibiting the expression of E6 and E7, the host cells cannot provide the conditions required for the complete life cycle of HPV. Although the LSIL total cure rate in the experimental group was significantly higher than that in the control group, there was no significant difference in the cure rate of different parts, indicating that ALA-PDT can protect fertility function without leaving scars, and the curative effect of different sites was significantly better than that in the CO2 laser therapy group. ALA-PDT can target the HPV local infection. Recent evidence has shown that [33] ALA-PDT can induce apoptosis of the p13K/Akt pathway through autophagy, up-regulation of Ras/Raf/MEK/ERK expression and down-regulation of Ras/Raf/MEK expression. Exogenous ALA can be selectively absorbed by hyperproliferative cells to produce protoporphyrin IX, and generate reactive oxygen species after illumination, killing the hyperproliferative cells [34]. After application, the cells infected by HPV selectively absorb and aggregate; and under the irradiation of a laser with specific wavelength, singlet oxygen and other substances are generated under the action of photodynamic, which leads to damage to mitochondria, the failure of replication and transcription of nucleic acids, and the selective apoptosis of abnormally hyperplastic cells and potentially infected cells [35], thus reducing the recurrence rate.
There are some deficiencies in this study. For example, the sample size was small, the follow-up time for curative effect observation was short, and there is a lack of large sample research on the long-term negative conversion rate and cancelation rate. It is hoped that a well-designed, randomized, and controlled trial with prospective data collection and sample size calculation could be performed in the future to confirm the findings in our study. In conclusion, ALA-PDT can effectively treat HPV-infected CIN and promote HPV clearance, which is a better choice for the treatment of cervical HPV infection and CIN for women with fertility needs.
Currently, PDT is widely used not only in dermatology, but also in otolaryngology, ophthalmology, neurology, gastroenterology, urology, and other medical fields. This study also discussed the efficacy and safety of PDT in the treatment of HPV-infected CIN, and determined that ALA-PDT is safe and effective to treat cervical high-risk HPV infection, which can improve the negative conversion rate of high-risk HPV in cervical epithelial cells and reduce the incidence of complications.
Acknowledgements
Medicine and Engineering Interdisciplinary Research Fund of Shanghai Jiaotong University (Grant Number: ZH2018ZDA32).
Disclosure of conflict of interest
None.
References
- 1.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 2.Torres-Poveda K, Ruiz-Fraga I, Madrid-Marina V, Chavez M, Richardson V. High risk HPV infection prevalence and associated cofactors: a population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC Cancer. 2019;19:1205. doi: 10.1186/s12885-019-6388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olusola P, Banerjee HN, Philley JV, Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. 2019;8:622. doi: 10.3390/cells8060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattoscio D, Medda A, Chiocca S. Human papilloma virus and autophagy. Int J Mol Sci. 2018;19:1775. doi: 10.3390/ijms19061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onuki M, Matsumoto K, Iwata T, Yamamoto K, Aoki Y, Maenohara S, Tsuda N, Kamiura S, Takehara K, Horie K, Tasaka N, Yahata H, Takei Y, Aoki Y, Kato H, Motohara T, Nakamura K, Ishikawa M, Kato T, Yoshida H, Matsumura N, Nakai H, Shigeta S, Takahashi F, Noda K, Yaegashi N, Yoshikawa H. Human papillomavirus genotype contribution to cervical cancer and precancer: implications for screening and vaccination in Japan. Cancer Sci. 2020;111:2546–2557. doi: 10.1111/cas.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng JJ, Song JH, Yu CX, Wang F, Wang PC, Meng JW. Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health. 2019;19:109. doi: 10.1186/s12905-019-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives. J Oncol. 2019;2019:3257939. doi: 10.1155/2019/3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra A, Tzafetas M, Lyons D, Fotopoulou C, Paraskevaidis E, Kyrgiou M. Cervical intraepithelial neoplasia: screening and management. Br J Hosp Med (Lond) 2016;77:C118–C123. doi: 10.12968/hmed.2016.77.8.C118. [DOI] [PubMed] [Google Scholar]
- 9.Curty G, de Carvalho PS, Soares MA. The role of the Cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int J Mol Sci. 2019;21:222. doi: 10.3390/ijms21010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castle PE, Murokora D, Perez C, Alvarez M, Quek SC, Campbell C. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstet. 2017;138:20–25. doi: 10.1002/ijgo.12191. [DOI] [PubMed] [Google Scholar]
- 11.Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, Wilkinson EJ, Zaino RJ, Wilbur DC Members of LAST Project Work Groups. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266–1297. doi: 10.5858/arpa.LGT200570. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Shi X, Li Y, Luan H, Liu W. LEEP/Cone combined with photodynamic therapy for successful treatment of high-grade squamous intraepithelial lesion. Photodiagnosis Photodyn Ther. 2019;25:237–238. doi: 10.1016/j.pdpdt.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Liu L, Tao X, Guo L, Zhang H, Sui L. Risk factor analysis of persistent high-grade squamous intraepithelial lesion after loop electrosurgical excision procedure Conization. J Low Genit Tract Dis. 2019;23:24–27. doi: 10.1097/LGT.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Liu M, Ji Y, Qu P. The effectiveness of cold-knife conization (CKC) for post-menopausal women with cervical high-grade squamous intraepithelial lesion: a retrospective study. BMC Surg. 2021;21:241. doi: 10.1186/s12893-021-01238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segura SE, Ramos-Rivera G, Hakima L, Suhrland M, Khader S. Low-grade squamous intraepithelial lesion, cannot rule out high-grade lesion: diagnosis, histological outcomes and human papillomavirus results. Cytopathology. 2019;30:99–104. doi: 10.1111/cyt.12629. [DOI] [PubMed] [Google Scholar]
- 16.Rouzier R. Management of CIN1. J Gynecol Obstet Biol Reprod (Paris) 2008;37(Suppl 1):S114–120. doi: 10.1016/j.jgyn.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Kanthiya K, Khunnarong J, Tangjitgamol S, Puripat N, Tanvanich S. Expression of the p16 and Ki67 in cervical squamous intraepithelial lesions and cancer. Asian Pac J Cancer Prev. 2016;17:3201–3206. [PubMed] [Google Scholar]
- 18.Peasant C, Foster RH, Russell KM, Favaro BE, Klosky JL. Caregiver sexual and HPV communication among female survivors of childhood cancer. J Pediatr Oncol Nurs. 2016;33:199–208. doi: 10.1177/1043454215607339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittmaack A, Dudley D, Boyle A. Maternal history of cervical surgery and preterm delivery: a retrospective cohort study. J Womens Health (Larchmt) 2019;28:1538–1542. doi: 10.1089/jwh.2018.7457. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Wen X, Li Y, Hamblin MR. Photodynamic therapy in dermatology beyond non-melanoma cancer: an update. Photodiagnosis Photodyn Ther. 2017;19:140–152. doi: 10.1016/j.pdpdt.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan ZX, Zhang LL, Wang HW, Wang PR, Huang Z, Wang XL. Treatment of cutaneous lichen planus with ALA-mediated topical photodynamic therapy. J Innov Opt Heal Sci. 2015;8:1540004. [Google Scholar]
- 23.Lan T, Zou Y, Hamblin MR, Yin R. 5-Aminolevulinic acid photodynamic therapy in refractory vulvar lichen sclerosus et atrophicus: series of ten cases. Photodiagnosis Photodyn Ther. 2018;21:234–238. doi: 10.1016/j.pdpdt.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int J Urol. 2017;24:97–101. doi: 10.1111/iju.13291. [DOI] [PubMed] [Google Scholar]
- 25.Maza M, Figueroa R, Laskow B, Juárez A, Alfaro K, Alonzo TA, Felix JC, Gage JC, Cremer M. Effects of maintenance on quality of performance of cryotherapy devices for treatment of precancerous cervical lesions. J Low Genit Tract Dis. 2018;22:47–51. doi: 10.1097/LGT.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Lai Y, Wan T, Zhou B, Qiang X, Ge S, Zhang K, Mao Z. A randomized clinical study of the treatment of white lesions of the vulva with a fractional ultrapulsed CO2 laser. Ann Palliat Med. 2020;9:2229–2236. doi: 10.21037/apm-20-1085. [DOI] [PubMed] [Google Scholar]
- 27.Wang HW, Zhang LL, Miao F, Lv T, Wang XL, Huang Z. Treatment of HPV infection-associated cervical condylomata acuminata with 5-aminolevulinic acid-mediated photodynamic therapy. Photochem Photobiol. 2012;88:565–569. doi: 10.1111/j.1751-1097.2011.01060.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh I, Mittal S, Banerjee D, Chowdhury N, Basu P. Study of correlation of cervical epithelial thickness with the grade of colposcopic abnormality. Int J Gynecol Pathol. 2016;35:269–274. doi: 10.1097/PGP.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 29.Tao XH, Guan Y, Shao D, Xue W, Ye FS, Wang M, He MH. Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia: a systemic review. Photodiagnosis Photodyn Ther. 2014;11:104–112. doi: 10.1016/j.pdpdt.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Hillemanns P, Petry KU, Soergel P, Collinet P, Ardaens K, Gallwas J, Luyten A, Dannecker C. Efficacy and safety of hexaminolevulinate photodynamic therapy in patients with low-grade cervical intraepithelial neoplasia. Lasers Surg Med. 2014;46:456–461. doi: 10.1002/lsm.22255. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado Alvarado E, Osorio Peralta MO, Moreno Vázquez A, Martínez Guzmán LA, Melo Petrone ME, Enriquez Mar ZI, Jovel Galdamez DE, Carrión Solana B, Balderas Martínez G, Parra E, Castellanos Oliveros RI, Bello Leiva RL, Espinosa Montesinos A, Barrera Mendoza C, Medina García SE, Ramón Gallegos E. Effectiveness of photodynamic therapy in elimination of HPV-16 and HPV-18 associated with CIN I in Mexican women. Photochem Photobiol. 2017;93:1269–1275. doi: 10.1111/php.12769. [DOI] [PubMed] [Google Scholar]
- 32.Poreba E, Broniarczyk JK, Gozdzicka-Jozefiak A. Epigenetic mechanisms in virus-induced tumorigenesis. Clin Epigenetics. 2011;2:233–247. doi: 10.1007/s13148-011-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Wang S, Li Z, Ao C, Wang J, Wang L, Peng X, Zeng K. 5-aminolevulinic acid photodynamic therapy reduces HPV viral load via autophagy and apoptosis by modulating Ras/Raf/MEK/ERK and PI3K/AKT pathways in HeLa cells. J Photochem Photobiol B. 2019;194:46–55. doi: 10.1016/j.jphotobiol.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Valdes PA, Millesi M, Widhalm G, Roberts DW. 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J Neurooncol. 2019;141:555–565. doi: 10.1007/s11060-018-03079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu ES, Yow CM. Modulation of telomerase and signal transduction proteins by hexyl-ALA-photodynamic therapy (PDT) in human doxorubicin resistant cancer cell models. Photodiagnosis Photodyn Ther. 2012;9:243–255. doi: 10.1016/j.pdpdt.2011.12.005. [DOI] [PubMed] [Google Scholar]