Abstract
Objective: The aim of this study was to investigate whether curcumin has a therapeutic effect on endometriosis (EM) and to determine the specific mechanism. Methods: Network pharmacology was used to obtain the core targets of curcumin for the treatment of EM and the specific biologic processes involved. A mouse model of EM was constructed and divided into different groups, as follows: control, negative control, curcumin, and denogestrel. The number, volume, and degree of adhesions of the lesions in each group were measured. The levels of IL-1β, IL-6, and VEGFA in the peritoneal cavity were measured by enzyme-linked immunosorbent assay (ELISA). Western blot and Q-PCR were used to detect HIF-1α and VEGFA proteins and gene expression levels in the lesion tissues. Results: Network pharmacology suggested that curcumin treated EM through the HIF signaling pathway, of which IL-6, HIF-1α, and VEGFA are key targets. The number of lesions, volume, and degree of adhesions were significantly reduced in the curcumin group compared to the negative control group and the control group (P < 0.05). IL-6, IL-1β, and VEGFA levels were reduced in the peritoneal fluid (P < 0.05). HIF-1α and VEGFA protein and gene levels were significantly reduced in the lesions (P < 0.05). No modulation of HIF-1α was shown by denogestins. Conclusion: Curcumin played a role in the treatment of EM by modulating the HIF signaling pathway, improving the local hypoxia of the lesion, and reducing the inflammatory state of EM.
Keywords: Network pharmacology, curcumin, endometriosis, hypoxia-inducible factor
Introduction
Curcumin is a polyphenol extracted from turmeric, a member of the ginger family. It has the chemical formula 1,7-bis-(4-hydroxy-3-methoxyphenyl)-hepta-1,6-diene-3,5-dione and is one of the world’s largest selling natural food colors. Curcumin has become a hot topic in medical research because of its various pharmacological activities, such as anti-inflammatory and antioxidant [1]. Curcumin has therapeutic effects on a variety of human diseases, including cancer, cardiovascular disease, diabetes, arthritis, neurologic disorders, Crohn’s disease, and cardiovascular disease [2]. The modulation of curcumin for tumors has received the most attention from researchers; approximately 37% of curcumin studies are related to tumors. Curcumin can modulate multiple cellular signaling pathways simultaneously to alleviate or prevent different types of cancer, including multiple myeloma, colorectal, pancreatic, breast, prostate, and lung cancers, because it can modulate growth factors, enzymes, transcription factors, kinases, inflammatory cytokines, and apoptotic proteins [3,4].
Endometriosis (EM) is an estrogen-dependent disease, in which endometrial glands and mesenchyme with growth functions appear outside the mucosa and myometrium of the uterine cavity [5,6]. Although EM is a benign disease, it has the aggressiveness and metastatic characteristics of a malignancy; recent studies found that EM involves somatic mutations, a significant proportion of which involve known cancer-related mutations [7]. EM is also an inflammation-associated disease and is characterized by the presence of immune cell aggregates in the pelvis and ectopic lesions. In EM, the levels of inflammatory cytokines and oxygen free radicals are significantly elevated. Based on curcumin’s tumor-modulating effects and anti-inflammatory and antioxidant effects, we hypothesized that curcumin may have therapeutic effects on EM. However, current studies on EM and curcumin are few and insufficient [8].
Traditional research on new drug targets and pathways consumes much time, and emerging network pharmacologic technology provides us with a new way of thinking. Network pharmacology uses database information to analyze the connection among genes, proteins, diseases, and drugs. Biologic networks are used to determine the mechanism of drugs in a more systematic and complete way. Different effects of drugs on multiple targets and multiple pathways are shown. The method identifies the synergistic effects of drugs on multiple targets and pathways consistent with the holistic approach of drug development and has been widely used in the study of Chinese medicine extracts and Chinese medicine compounding [9,10]. Therefore, the present study was designed to investigate the therapeutic mechanism of curcumin in EM by using network pharmacology. Validation was conducted using animal experiments to provide a scientific basis for the further development of curcumin in EM treatment (Figure 1).
Figure 1.
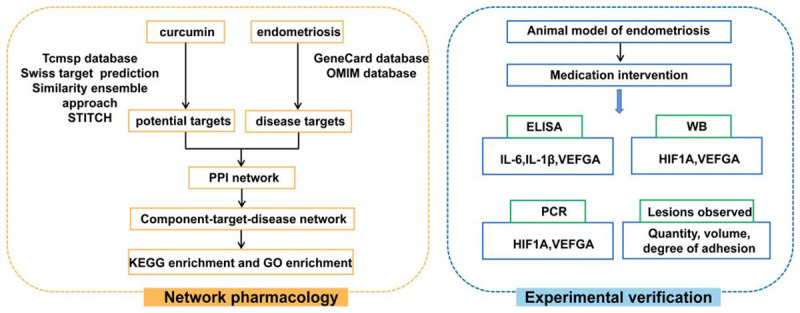
Technical procedure based on network pharmacology and experimental verification.
Materials and methods
Chemicals and reagents
The following chemicals and reagents were used: curcumin (Sigma, St. Louis, MO, USA, No. C1386); dienogest and estradiol benzoate (MedChemExpress, No. HY-B0084, HY-B1192); IL-6, IL-1β, and VEGFA ELISA kits (Cloud-Clone Crop, Wuhan, China, No. SEA079Mu, SEA563Mu, SEA143Mu); polyclonal antibodies HIF1A, VEGFA, and β-ACTIN (Boster Biological Technology Co., Ltd., No. A00013-1, A00045, BA2305); and PCR primer HIF1A, VEGFA, and GAPDH (Sangon Biotech Co., Ltd., Shanghai, China; Primer sequences are shown in supplementary materials). Strand cDNA Synthesis SuperMix for qPCR and qPCR SYBR Master Mix (Yeasen Biotechnology Co., Ltd., Shanghai, China, No. 11141ES10, 11184ES03) and electrogenerated chemiluminescence (ECL) color liquid (Biyuntian Biological Products Co., Ltd., Shanghai, China, No. 9966444330) were obtained.
Intersection analysis of curcumin and endometriosis-related targets
The 2D structure of curcumin was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) platform with the Canonical SMILES by using “curcumin (CAS: 458-37-7)” as a search keyword. The obtained structure was imported into SWISS TARGET PREDICTION (http://www.swisstargetprediction.ch/), and the setting possibility was > 0. Canonical SMILES was imported into the similarity ensemble approach (https://sea.bkslab.org/), and the species was set to Homo sapiens, P-Value ≤ 0.01. In the Traditional Chinese Medicine Database and Analysis Platform (TCMSP, https://tcmsp-e.com/) and STITCH (http://stitch.embl.de/), “curcumin” was the search keyword, and the species was H. sapiens. All potential targets for curcumin were obtained by pooling the targets from the four databases and removing duplicates [11,12]. The results were published in GeneCards (https://www.genecards.org/, Gene Cards Version 5.2), OMIM (http://www.omim.org/, Updated 17 September 2021) under the keyword “endometriosis”. “EM” was searched to obtain EM-related targets, which were intersected and matched with the corresponding gene targets of curcumin by using the online Venn diagram tool (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain potential targets of curcumin for EM treatment. The obtained potential targets were imported into Cytoscape 3.8.0 to obtain a disease-drug-target network map.
Screening of key targets in curcumin for endometriosis treatment
Studying the interactions between protein networks can help uncover core genes within them. Potential targets were obtained from the Venn diagram and fed into STRING (https://string-db.org/, version 11.0) for protein-protein interaction (PPI) analysis. The PPI network was obtained by removing free genes and using the highest value of the platform default settings with a confidence level of ≥ 0.900 to increase the confidence of the data. In the PPI network, a number of tightly connected regions were defined as clusters or topological modules, which were constructed from multiple nodes and edges. They reflect the relevant molecular biologic functions and core protein processes, in which the highly valued targets play a pivotal role [13]. The analysis and Hubba plugins of Cytoscape 3.8.0 were used to explore the core targets of curcumin for EM treatment.
KEGG enrichment analysis and GO enrichment analysis
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were performed on the core target clusters obtained from the PPI network by using the DAVID tool (https://david.ncifcrf.gov) to obtain the biological processes, cellular components, molecular functions, and key signaling pathways of the intersecting targets and to explore the core mechanisms of action and related biologic pathways of curcumin for EM treatment [14]. The functional annotations with P-values < 0.05 in the enrichment results were further analyzed, and bubble maps of the enrichment results were plotted using R language.
Molecular docking map
Curcumin was molecularly docked to the screened proteins with high degree values to further validate the regulatory effects of curcumin on EM-related key targets. The 3D crystal structures of the target proteins VEGFA, IL-6, and HIF1α were downloaded from the PDB database (https://www.rcsb.org/) (PDB DOI 10.2210/pdb1KAT/pdb, 10.2210/pdb2L3Y/pdb, 10.2210/pdb4NQ0/pdb). The 3D structure of curcumin (CAS No. 458-37-7) was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The AutoDock Tools 1.5.6 software was used to remove the water molecules from the target protein, separate the ligand and receptor, add non-polar hydrogen, and calculate the Gasteiger charge. The files were saved in pdbqt format. We imported the compound already saved in PDB format into AutoDock Tools 1.5.6, added the atomic charge, and assigned the atomic type. All flexible keys were rotatable by default and saved as a docking ligand in pdbqt format. Docking was performed by running the Auto Dock software, and the docking results were visualized using Pymol software.
Establishment of mouse endometriosis model and animal administration
Female BALB/c mice (18-22 g) were provided by Animal Room, Laboratory Center, Changhai Hospital. The animals were kept in a sterile laboratory room and given free access to food and water at 20°C-24°C, 45%-55% humidity, and 12 h/12 h light/dark cycle. All experiments were carried out in strict accordance with the guidelines of the Experimental Animal Center of Shanghai Changhai Hospital. The principles of animal protection, animal welfare, and ethics were applied.
To establish the EM mouse model, 48 BALB/c mice were grouped as donors (16) and recipients (32) according to randomized grouping and at 1:2 ratio. The model establishment steps were as follows. (1) For estrogen injection, all experimental mice were injected subcutaneously with estradiol benzoate solution at 150 μg/kg on the back of neck, once every 4 days, and twice in total. (2) Specimen preparation before implantation was as follows: the uterus of the donor mice was removed and washed with pre-cooled saline for blood and mucus, and then, it was divided into two. The two parts were placed in separate Petri dishes containing 0.5 ml of pre-cooled RPMI1640 medium. The uterus was dissected longitudinally with ophthalmic scissors. The inner layer of the uterus was peeled off and quickly cut into endometrial fragments ≤ 1 mm3 in volume and transformed into a suspension. (3) Intraperitoneal injection was performed as follows. After disinfection of the abdominal skin of the recipient mouse, the endometrial fragments were injected into the abdominal cavity of the recipient mouse with a 1 ml syringe needle connected to a 20 ml syringe needle at a point 0.5 cm above the urethral orifice in the lower abdomen of the mouse and at a ratio of 1:2. All operations from the removal of the donor mouse’s uterus to the injection of the endometrial fragments into the recipient mouse should be completed within 5 min of each other. Asepsis should be maintained throughout the operations. Post-implantation treatment was as follows. Estradiol benzoate solution was injected subcutaneously into the dorsal neck of the recipient mice at 150 μg/kg on the day of intraperitoneal implantation and at 4 days later, for a total of two injections. All recipient mice were randomly divided into four groups, namely, control, negative control, curcumin, and dinogestrel. Four mice were in each group. After 1 week of molding, curcumin was administered by gavage once daily for 21 days. Curcumin sesame oil solution (300 mg/kg) was administered to the curcumin group, denogestrel sesame oil solution (300 ug/kg) was administered to the denogestrel group, and an equal volume of sesame oil was administered to the negative control group. On the third day after gavage, the mice were sacrificed by spinal dislocation, and the abdominal fluid and ectopic lesions were extracted as follows. The skin of the lower abdomen of the mice was debrided and disinfected with 75% alcohol cotton balls, and 1.5 ml of saline was injected by gently stabbing vertically with a 5 ml syringe along the lower abdomen against the midline of the abdominal wall. The abdominal wall was gently squeezed by hand more than 20 times so that the saline could fully diffuse into the abdominal cavity. The saline was then aspirated from the abdominal cavity with a 2 ml syringe and centrifuged at 4°C for 15 min at 2000 rpm. The supernatant was stored in a refrigerator at -80°C. Finally, the peritoneal cavity was opened, and the ectopic lesion was stripped and extracted (Figure 2).
Figure 2.
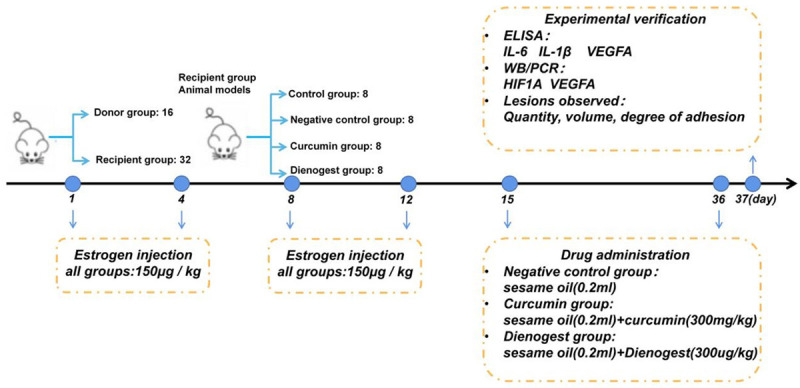
Experimental procedures of the in vivo EM mouse model.
Adhesion scoring and recording of the number and size of lesions
After opening the abdominal cavity, the mice were scored for the degree of abdominal adhesion by using the Blauer adhesion scoring system. Scoring criteria were as follows: 0, no adhesions; 1, slight membranous adhesions in the pelvis; 2, dense adhesions, usually with the uterine horns adhering to both the intestinal canal and the bladder; 3, more dense and extensive adhesions, with both uterine horns adhering to the intestinal canal and the bladder and with some uterine mobility; 4, severe adhesions, with both uterine horns adhering to both the intestinal canal and the bladder and with the uterus immobilized. Adhesion scoring was performed independently by two experimentalists, and the average of the scores was considered the final adhesion score [15]. The number of ectopic lesions was counted. The length, width, and height of the lesions were measured with Vernier calipers after removal. Implant volume was calculated according to the formula of V = Π/6 × length × width × height, and recorded [16].
ELISA assay
The sera of VEGFA, IL-6, and IL-1β, were detected by ELISA method. OD values were measured by Infite M200 microplate reader (Tecan Co., Ltd., Shanghai, China), and the corresponding concentrations were calculated.
Polymerase chain reaction assay
Ectopic lesions (60 mg) were ground into fragments of ≤ 1 mm3 volume. The fragments were combined with 1 ml of Trizol reagent, and the mixture was shaken and mixed before lysis for 10 min. The supernatant was removed by centrifugation at 12000 rpm for 10 min and added with 200 µl of chloroform to each tube. The tube was shaken for 15 s, left for 10 min at room temperature, and centrifuged at 12,000 rpm for 15 min. The sample tube was carefully removed from the centrifuge. The contents were divided into three layers. The tube was prechilled and centrifuged at 12,000 rpm for 15 min. We freshly prepared 75% ethanol with DEPC water. After centrifugation, a small amount of white precipitate appeared at the bottom of the tube, and this was the total RNA. The supernatant was aspirated and carefully added with 1 ml of 75% ethanol. The precipitate was washed by gently turning. When the RNA had just become clear, an appropriate amount of nuclease-free water was added to the water bath at 55°C for 5 min to completely dissolve the RNA. Then, the concentration of the extracted RNA was measured by UV analysis. The concentration of the extracted RNA was determined by UV analysis. cDNA was reverse transcribed according to the Strand cDNA Synthesis SuperMix for qPCR and qPCR SYBR Master Mix instructions. Real-time PCR was performed.
Western blot analysis
A tissue homogenate was prepared by grinding 60 mg of the ectopic lesions into fragments ≤ 1 mm3 in volume, mixing with 540 µl of tissue protein lysis solution, and lysing on ice for 1 h. The homogenate was centrifuged for 15 min (4°C, 12500 rpm), and the supernatant was retained. The protein concentration of the supernatant was determined using the BCA method. The protein was denatured by adding 5 × loading buffer and boiled at 99°C for 10 min. Protein samples were separated by 10% SDS polyacrylamide gel and then transferred onto PVDF membranes. The membranes were blocked with blocking buffer for 20 min and incubated overnight with the first antibodies of HIF-1α, VEGFA, and β-ACTIN at 4°C. The membranes were washed with TBST 3 times for 10 min each time and then incubated with the corresponding secondary antibodies at room temperature for 1 h. The protein was visualized by using an ECL developer and a ChampChemi professional + automatic multicolor fluorescence and chemometrics gel imaging system. The gray levels of the proteins were analyzed. Proteins were relatively expressed as target protein/β-actin.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 8 software. Experimental data were described by mean ± standard deviation, and two independent samples t-test was used to compare two groups. Differences were considered significant when P < 0.05.
Results
Screening of curcumin and endometriosis related-targets
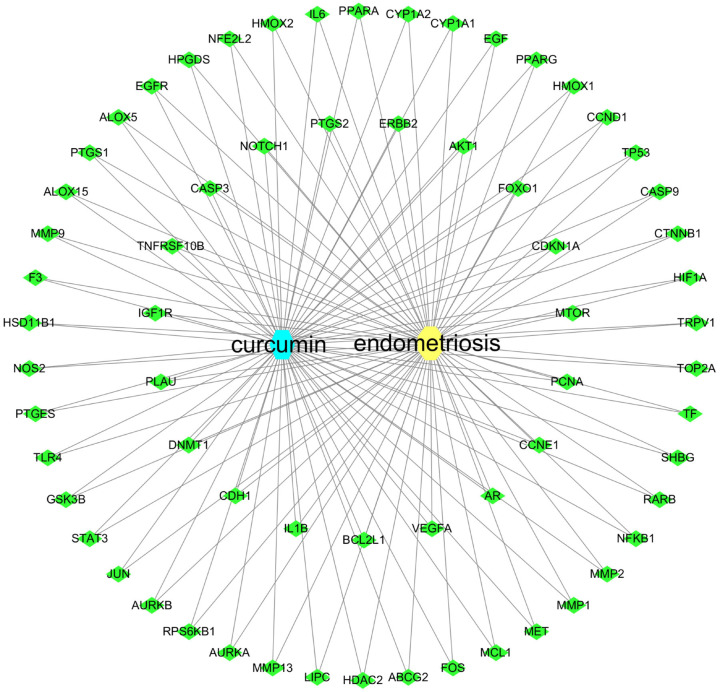
A total of 180 curcumin-related targets were returned from the four databases after filtering according to the conditions, and these were intersected with the 1618 EM-related targets returned by GeneCards, OMIM using the Venn plot tool. Sixty-five therapeutic EM targets for curcumin were obtained (Figure 3). The obtained potential targets were imported into Cytoscape 3.8.0 to obtain the EM-curcumin-target network map (Figure 4).
Figure 3.
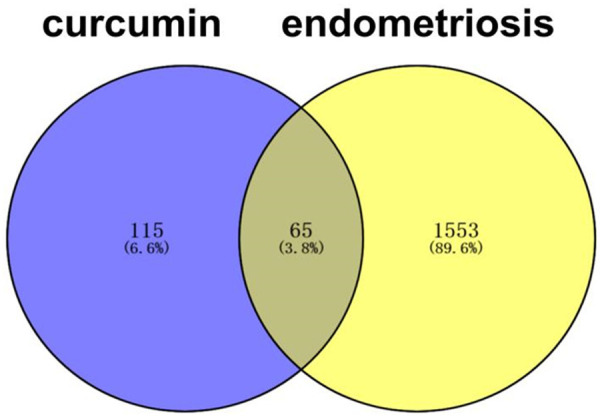
Venn Diagram of curcumin and EM related targets.
Figure 4.
Curcumin-target-disease network.
Screening of key targets by PPI network
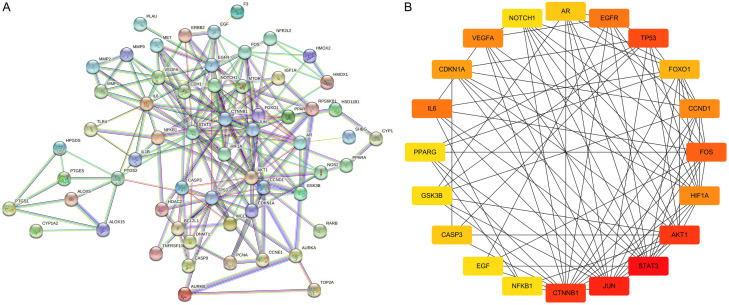
The curcumin-treated EM genes obtained from the intersection were imported into STRING, which is a PPI network containing 65 nodes and 209 edges (Figure 5). The PPI network was imported into Cytoscape 3.8.0. The top 20 targets, including STAT3, VEGFA, HIF-1α, IL-6, and others, were selected as core targets using the HUBBA plugin with degree as the calculation method (Figure 5).
Figure 5.
PPI network of intersect targets (A), Network diagram of the core target (B).
Enrichment analysis of related pathways and biological process
GO enrichment and KEGG analyses were performed to elucidate the biologic processes and pathways invoked by the potential gene targets of EM treatment with curcumin. The results showed 129 significant pathways corresponding to the EM targets of curcumin treatment (P ≤ 0.05). A total of 224 significantly enriched GO terms were obtained from the GO analysis (P ≤ 0.05). Among the 129 pathways, the HIF1 signaling pathway had the smallest P and FDR values and the highest reliability for several core targets, such as VEGFA, IL-6, and HIF-1α (Figure 6), in addition to some tumor-related pathways. In the GO analysis, the P values for hypoxia and vascular processes, such as positive regulation of angiogenesis, positive regulation of blood vessel endothelial cell migration, and cellular response to hypoxia, were also less than 0.001, which corresponded to the KEGG analysis (Figure 7). Thus, we verified this pathway of HIF1, which was closely related to hypoxia and angiogenesis, and applied it to our subsequent experiments.
Figure 6.
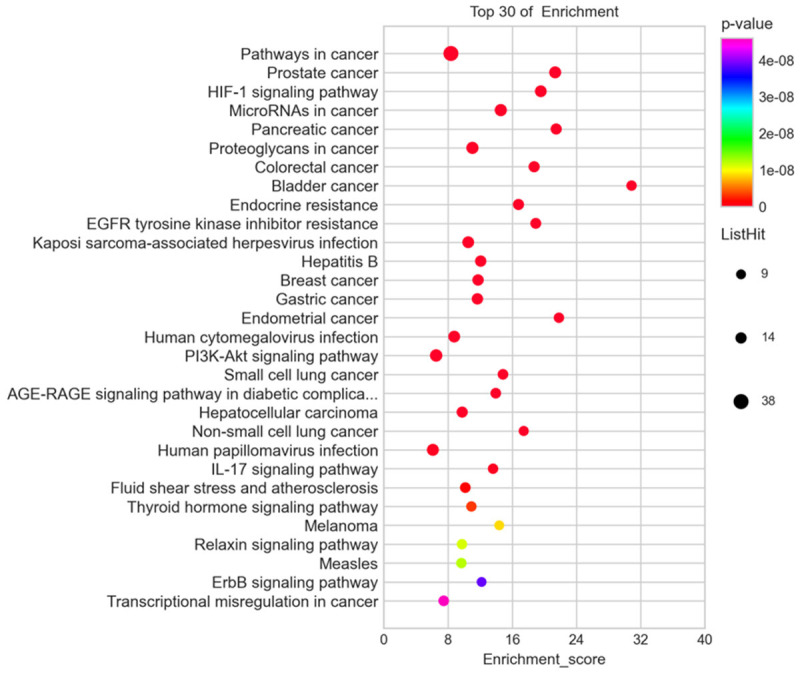
KEGG Pathways enrichment analysis of intersect targets.
Figure 7.
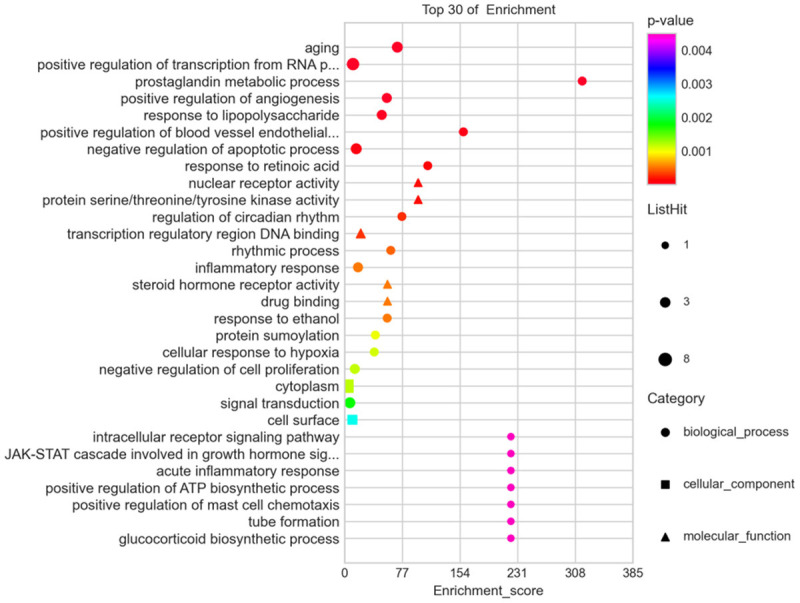
GO enrichment analysis of intersect targets.
Docking diagram of curcumin and key molecules
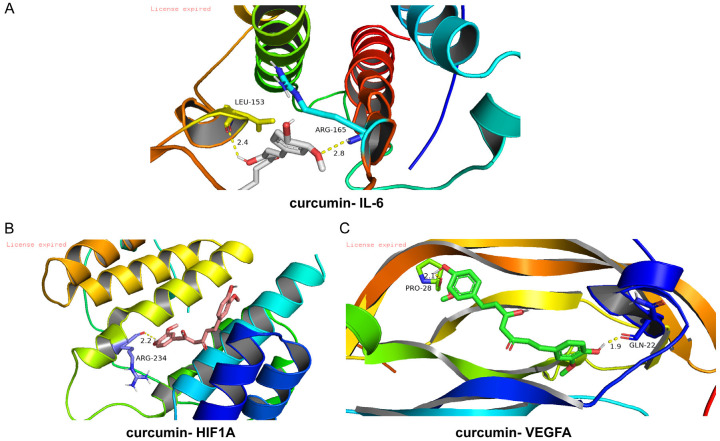
The lower the steady-state conformational energy of the ligand-receptor binding was, the stronger the effect was. We docked curcumin with IL-6, VEGFA, and HIF1-α and found that the binding energy of the three was less than -5, indicating that curcumin was able to bind well to the three proteins and had a strong effect (Figure 8).
Figure 8.
Molecular docking diagram of curcumin and IL-6 (A), curcumin and HIF-1α (B), curcumin and VEGFA (C).
Effects of curcumin on lesion number, lesion size, and adhesion score
No differences in adhesion score, lesion number, or lesion volume levels were found in the negative control group compared with the control group. In the curcumin and denogestrel groups, the adhesion score, lesion number, and volume significantly increased compared with the control group (P < 0.05) (Figure 9).
Figure 9.
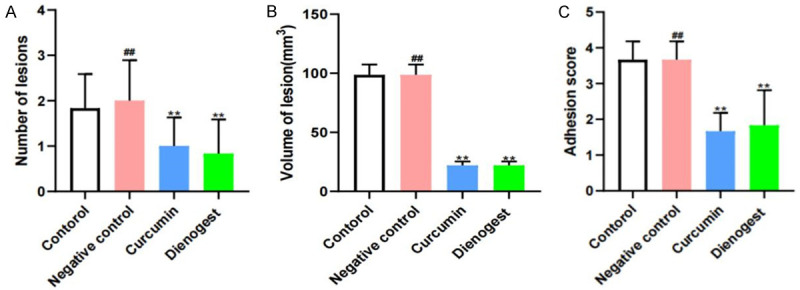
Effects of curcumin on the number of lesions (A), volume of lesion (B), adhesion score (C). Notes: **: P < 0.05, vs. Control; ##: P > 0.05, vs. Control. Statistical method: t test.
Effects of curcumin on inflammatory factors and VEGFA
No difference was found in the levels of IL-1β, IL-6, and VEGFA in the peritoneal fluid of the negative control group compared with those in the control group. The levels of IL-1β, IL-6, and VEGFA in the peritoneal fluid of the curcumin and denogestrel groups were significantly reduced compared to those in the control group (P < 0.05) (Figure 10).
Figure 10.
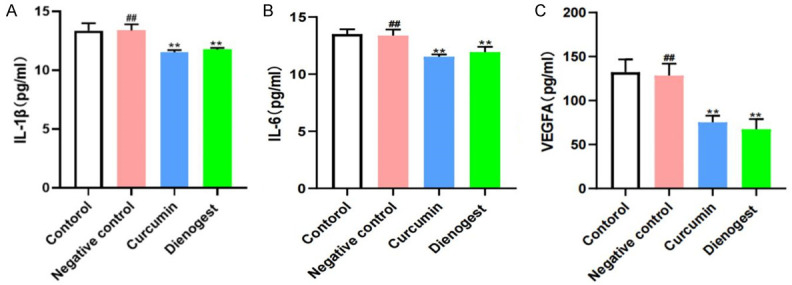
Effects of curcumin on IL-1β (A), IL-6 (B), VEGFA (C). Notes: **: P < 0.01, vs. Control; ##: P > 0.05, vs. Control. Statistical method: t test.
Effects of curcumin on protein and gene expression of HIF-1α and VEGFA
The PCR results were consistent with the WB results, and no difference was found in the HIF-1α and VEGFA levels in the negative-control lesions compared with those in the control group. The HIF-1α and VEGFA expression levels were significantly lower in the curcumin group than in the control group (P < 0.05). No effect on HIF-1α and VEGFA expression was found in the dinogestrel group. No significant difference in the expression of HIF-1α and VEGFA was detected in the denogestrel group compared with those in the control group (Figure 11).
Figure 11.
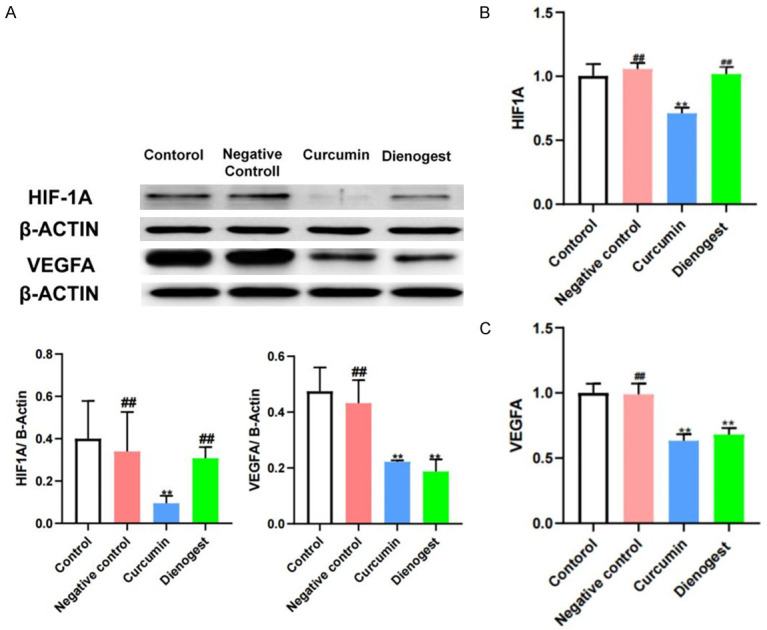
Effects of curcumin on the expression of VEGFA and HIF-1α proteins in the endometriosis lesion. WB results of HIF-1α, WB results of VEGFA (A), PCR results of HIF-1α (B) and VEGFA (C). Notes: **: P < 0.05, vs. Control; ##: P > 0.05, vs. Control. Statistical method: t test.
Discussion
As a new research method, network pharmacology and molecular docking technology can effectively and systematically explore the association between Chinese herbal medicines and their active ingredients in disease treatment. Their mechanisms of action can be elucidated by integrating data from multiple platforms and constructing models. By analyzing the protein structure of curcumin, we obtained the possible targets of curcumin, and 65 targets in curcumin were associated with EM through Venn diagrams. However, computer analysis techniques can reflect only the correlation between the two. They cannot reflect the biologic activity of the targets up- or down-regulated by curcumin. Therefore, the effect of target modulation was verified through certain experiments.
The results of the animal studies showed that the number and size of lesions and the degree of adhesions in the curcumin group were significantly improved after curcumin treatment compared to the control group, indicating that the disease progression in EM was significantly inhibited.
The KEGG enrichment results suggested that the HIF1 pathway may be the core pathway of curcumin treatment for EM, whereas several HIF1A-related targets, such as VEGFA, HIF-1α, and IL-6, were also suggested as core targets in the PPI core target analysis. Hence, the HIF1 pathway should be the focus of studies on curcumin for EM. HIF-1 is an adaptive system that regulates the transcription of multiple genes associated with growth, angiogenesis, proliferation, glucose transport, metabolism, pH regulation, and cell death under hypoxic conditions [17]. Increasing lines of evidence show that curcumin, a naturally occurring bioactive compound of turmeric root, had significant targeting effects on HIF-1 subunits and had a stronger targeting effect on HIF-1α [18]. The HIF1 pathway was studied in EM, but it was still unclear which drugs can act through the HIF-1α pathway in EM, because few clinical and basic studies were conducted on this topic. Filippi found in a clinical study that HIF-1/2α, PAR-1/4, and VEGF-A levels were significantly higher in ectopic lesions of ovarian endometriosis than those in the endometrium of healthy women. The expressions of HIF-1/2α and VEGF-A mRNA in OMA lesions were positively correlated [19]. A previous clinical study confirmed this finding, that is, VEGFR2, HIF-1α, HGF, PDGFB, NRP1, and EPHB4 were highly expressed in ectopic tissues and promoted further angiogenesis [20]. In terms of mechanism exploration, the hypoxic environment could induce epithelial-mesenchymal transition (EMT) in endometrial cells and increase the invasive capacity of endometrial cells. The overexpression of HIF-1α was closely correlated with EMT. The invasiveness of Ishikawa cells was decreased by knocking down HIF-1α under hypoxic conditions. The upregulation of HIF-1α induced characteristic changes in EMT, whereas the downregulation of HIF-1α had the opposite effect [21].
EM is accompanied by inflammatory manifestations, which cause endothelial dysfunction and may even lead to cancer [22]. The role of HIF-1α in regulating the executive function of immune cells may be an important factor in the progression of EM disease. The adaptation of macrophages to hypoxia is regulated by HIF-1α, and the overexpression of HIF-1α in macrophages can increase the proportion of pro-inflammatory M1-type macrophages, thereby increasing the inflammatory response [23]. LPS activation of macrophages leads to a shift in their metabolism toward the glycolytic and pentose phosphate pathways. Activation of this glycolytic pathway increases the hypoxic environment and leads to further accumulation of HIF-1, thereby exacerbating the inflammatory response, in which elevated IL-1β expression is most pronounced [24,25]. In our previous study, LPS levels were significantly higher in the peritoneal fluid of EM mice compared with normal mice, which may be an important reason for the increased proportion of M1-type macrophages in the EM peritoneal cavity [26]. Macrophages are the core cells in the EM state; they are essential for lesion growth, development, angiogenesis, innervation, and pain symptoms, and an increased proportion of M1 macrophages is one of the primary causes of the inflammatory state of EM [27].
Levels of IL-6 and VGEF-A expression in peritoneal fluid and VEGFA expression in lesions were downregulated after curcumin treatment. Abnormal IL-6 levels were closely related to EM disease development and progression. IL-6 and SIL-6R levels increased in the peritoneal fluid of EM patients [28]. SIL-6R directly binds IL-6/SIL-6R complex to gp130, thereby enhancing the biological activity of IL-6 [29]. IL-6 increases the secretion of the binding bead protein, which helps ectopic endometrial cells evade immune surveillance and elimination, promotes ectopic endometrial survival, and accelerates the conversion of macrophages to M1, whereas activated macrophages can further secrete IL-6, thereby creating a vicious cycle that exacerbates EM [30,31]. Sustained high levels of IL-6 can induce the epithelial-mesenchymal transition of the peritoneum, thereby forming adhesions and fibrosis, disrupting the peritoneal glycocalyx, and exposing the basement membrane. Low molecular weight hyaluronic acid is released, which triggers a series of pro-inflammatory mediators, including cytokines (TNF-α, IL-1, IL-6, and prostaglandins), growth factors (TGF-α, TGF-β, platelet-derived growth factor VEGF, and epidermal growth factor), and the fibrin/coagulation cascade (thrombin, tissue factor, and fibrinogen activator inhibitor [PAI]-1/2). Growth factors induce HIF-1α expression, which drives cell growth, extracellular matrix production, and cell migration, thereby creating a vicious cycle that further promotes EM progression [32]. Hu found that HIF-1α expression could be permanently amplified through Th1 and Th17 cells, inhibiting regulatory B10 and innate-like B cells and promoting the expressions of IL-6, IL-8, TNF-α, and IL-1β, as well as VCAM-1 and TSP-1 [33]. Ying Zhang found that HIF-1α knockdown significantly reduced the secretion of TNF-α, IL-6, and IL-8 by LPS-stimulated cells [34]. Previous studies have shown that curcumin treatment of endometriotic stromal cells inhibited the activation of the transcription factor NF-κB, which in turn significantly reduced the TNF-α-induced secretion of IL-6, IL-8, and MCP-1 [35]. Our study identified the HIF signaling pathway as a possible new pathway for curcumin to inhibit IL-6 expression.
VEGF is currently the most recognized pro-angiogenic factor. VEGFA and VEGFR-2 expressions are significantly higher in ectopic lesions of EM patients and are accompanied by higher levels of VEGFA in the serum and peritoneal fluid [36]. VEGFA and VEGFR-2 expressions are further increased in deep infiltrating EM [37]. In hypoxia, HIF-1α is transferred to the nucleus and acts on the hypoxia-responsive element binding sites in the regulatory regions of VEGFA-encoding and VEGF receptor-encoding genes, binding specifically to them and promoting the increased expression of VEGF mRNA. These result in increased local VEGF expression. Under hypoxic conditions, vascular smooth muscle cells continue to proliferate and secrete large amounts of HIF-1α, forming a positive cycle. Blocking the expression of HIF-1α is an important step in the inhibition of angiogenesis [38,39]. HIF-1α mRNA and protein syntheses can be efficiently inhibited by curcumin [40]. Survival of endometrial stromal cells was significantly reduced after high-dose curcumin treatment. At the same time, VEGFA expression was downregulated after curcumin treatment [41]. We found that VEGFA levels were down-regulated in the lesions and in the peritoneal fluid in the animal model, in line with previous studies.
Previous studies focused on the antioxidant stress capacity of curcumin, which reduced ROS directly by enhancing the intracellular levels of reduced glutathione and counteracted antioxidants by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway [42]. Our study demonstrated for the first time that curcumin can treat EM and improve disease hypoxia through the HIF-1α pathway. Consistent with previous studies, curcumin exhibited therapeutic effect on EM by inhibiting oxidative stress, thus alleviating inflammation [8,43,44]. The real situation may be that curcumin inhibits oxidative stress on the one hand and improves hypoxia on the other hand. Further studies are needed to determine how the balance between oxidative stress and hypoxia is achieved in the intervention of curcumin to treat EM, and this will be the direction of our future work.
Conclusion
The HIF-1 signaling pathway, which is the core pathway of curcumin in EM treatment, was obtained for the first time using a network pharmacology approach. In subsequent animal validation experiments, curcumin significantly reduced disease progression in EM mice, decreased the levels of IL-6 and IL-1β, and down-regulated the expressions of HIF-1α and VEGFA. In summary, the use of network pharmacology provided new ideas for the study of curcumin in the treatment of EM. In the future, we will study curcumin’s dual regulatory effect on oxidative stress and hypoxia.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82074206, 81774352, 81703874), Shanghai 3-Year Action Plan for Traditional Chinese Medicine [ZY (2018-2020)-FWTX-1107], Medical Innovation Research Special Project of “Science and Technology Innovation Action Plan” of Shanghai Science and Technology Commission (21Y21920500).
Disclosure of conflict of interest
None.
References
- 1.Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24:2930. doi: 10.3390/molecules24162930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 3.Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. 2015;73:155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- 4.Giordano A, Tommonaro G. Curcumin and cancer. Nutrients. 2019;11:2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget. 2017;8:41679–41689. doi: 10.18632/oncotarget.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 7.Guo SW. Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology. Hum Reprod Update. 2020;26:423–444. doi: 10.1093/humupd/dmz047. [DOI] [PubMed] [Google Scholar]
- 8.Vallée A, Lecarpentier Y. Curcumin and endometriosis. Int J Mol Sci. 2020;21:2440. doi: 10.3390/ijms21072440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W, Piao G. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017;22:1135. doi: 10.3390/molecules22071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Liu J, Yu Y, Chen Y, Wang Y. Modular pharmacology: the next paradigm in drug discovery. Expert Opin Drug Discov. 2012;7:667–677. doi: 10.1517/17460441.2012.692673. [DOI] [PubMed] [Google Scholar]
- 11.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 12.Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altaf-Ul-Amin M, Shinbo Y, Mihara K, Kurokawa K, Kanaya S. Development and implementation of an algorithm for detection of protein complexes in large interaction networks. BMC Bioinformatics. 2006;7:207. doi: 10.1186/1471-2105-7-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 15.Saltan G, Süntar I, Ozbilgin S, Ilhan M, Demirel MA, Oz BE, Keleş H, Akkol EK. Viburnum opulus L.: a remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J Ethnopharmacol. 2016;193:450–455. doi: 10.1016/j.jep.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Zhou A, Hong Y, Lv Y. Sulforaphane attenuates endometriosis in rat models through inhibiting PI3K/Akt signaling pathway. Dose Response. 2019;17:1559325819855538. doi: 10.1177/1559325819855538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 18.Bahrami A, Atkin SL, Majeed M, Sahebkar A. Effects of curcumin on hypoxia-inducible factor as a new therapeutic target. Pharmacol Res. 2018;137:159–169. doi: 10.1016/j.phrs.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Filippi I, Carrarelli P, Luisi S, Batteux F, Chapron C, Naldini A, Petraglia F. Different expression of hypoxic and angiogenic factors in human endometriotic lesions. Reprod Sci. 2016;23:492–497. doi: 10.1177/1933719115607978. [DOI] [PubMed] [Google Scholar]
- 20.Yerlikaya G, Balendran S, Pröstling K, Reischer T, Birner P, Wenzl R, Kuessel L, Streubel B, Husslein H. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;204:88–98. doi: 10.1016/j.ejogrb.2016.07.500. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y, Liu Y, Xiong W, Zhang L, Liu H, Du Y, Li N. Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum Reprod. 2016;31:1327–1338. doi: 10.1093/humrep/dew081. [DOI] [PubMed] [Google Scholar]
- 22.Jiang L, Yan Y, Liu Z, Wang Y. Inflammation and endometriosis. Front Biosci (Landmark Ed) 2016;21:941–948. doi: 10.2741/4431. [DOI] [PubMed] [Google Scholar]
- 23.Corcoran SE, O’Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm. 2017;2017:9029327. doi: 10.1155/2017/9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni Z, Ding J, Zhao Q, Cheng W, Yu J, Zhou L, Sun S, Yu C. Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. Am J Reprod Immunol. 2021;86:e13471. doi: 10.1111/aji.13471. [DOI] [PubMed] [Google Scholar]
- 27.Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol (Lausanne) 2020;11:7. doi: 10.3389/fendo.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Fu X, Wu T, Yang L, Hu C, Wu R. Role of interleukin-6 and its receptor in endometriosis. Med Sci Monit. 2017;23:3801–3807. doi: 10.12659/MSM.905226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida S, Harada T, Iwabe T, Taniguchi F, Mitsunari M, Yamauchi N, Deura I, Horie S, Terakawa N. A combination of interleukin-6 and its soluble receptor impairs sperm motility: implications in infertility associated with endometriosis. Hum Reprod. 2004;19:1821–1825. doi: 10.1093/humrep/deh324. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe-Timms KL, Zimmer RL, Ricke EA, Piva M, Horowitz GM. Endometriotic haptoglobin binds to peritoneal macrophages and alters their function in women with endometriosis. Fertil Steril. 2002;78:810–819. doi: 10.1016/s0015-0282(02)03317-4. [DOI] [PubMed] [Google Scholar]
- 31.Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, Marín-Sánchez P, Machado-Linde F, García-Peñarrubia P. The role of peritoneal macrophages in endometriosis. Int J Mol Sci. 2021;22:10792. doi: 10.3390/ijms221910792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RB. Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum. 2018;3:20180103. doi: 10.1515/pp-2018-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu F, Liu H, Xu L, Li Y, Liu X, Shi L, Su Y, Qiu X, Zhang X, Yang Y, Zhang J, Li Z. Hypoxia-inducible factor-1α perpetuates synovial fibroblast interactions with T cells and B cells in rheumatoid arthritis. Eur J Immunol. 2016;46:742–751. doi: 10.1002/eji.201545784. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Xu Y, Zhou K, Kao G, Yan M, Xiao J. Hypoxia-inducible transcription factor-1α inhibition by topotecan protects against lipopolysaccharide-induced inflammation and apoptosis of cardiomyocytes. Biomed Eng Online. 2021;20:88. doi: 10.1186/s12938-021-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KH, Lee EN, Park JK, Lee JR, Kim JH, Choi HJ, Kim BS, Lee HW, Lee KS, Yoon S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26:1037–1047. doi: 10.1002/ptr.3694. [DOI] [PubMed] [Google Scholar]
- 36.Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006;132:501–509. doi: 10.1530/rep.1.01110. [DOI] [PubMed] [Google Scholar]
- 37.Machado DE, Abrao MS, Berardo PT, Takiya CM, Nasciutti LE. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil Steril. 2008;90:148–155. doi: 10.1016/j.fertnstert.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 38.Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb. 2008;15:26–33. doi: 10.5551/jat.e533. [DOI] [PubMed] [Google Scholar]
- 39.Liu LX, Lu H, Luo Y, Date T, Belanger AJ, Vincent KA, Akita GY, Goldberg M, Cheng SH, Gregory RJ, Jiang C. Stabilization of vascular endothelial growth factor mRNA by hypoxia-inducible factor 1. Biochem Biophys Res Commun. 2002;291:908–914. doi: 10.1006/bbrc.2002.6551. [DOI] [PubMed] [Google Scholar]
- 40.Shan B, Schaaf C, Schmidt A, Lucia K, Buchfelder M, Losa M, Kuhlen D, Kreutzer J, Perone MJ, Arzt E, Stalla GK, Renner U. Curcumin suppresses HIF1A synthesis and VEGFA release in pituitary adenomas. J Endocrinol. 2012;214:389–398. doi: 10.1530/JOE-12-0207. [DOI] [PubMed] [Google Scholar]
- 41.Cao H, Wei YX, Zhou Q, Zhang Y, Guo XP, Zhang J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol Med Rep. 2017;16:5611–5617. doi: 10.3892/mmr.2017.7250. [DOI] [PubMed] [Google Scholar]
- 42.Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019;14:e0216711. doi: 10.1371/journal.pone.0216711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kizilay G, Uz YH, Seren G, Ulucam E, Yilmaz A, Cukur Z, Kayisli UA. In vivo effects of curcumin and deferoxamine in experimental endometriosis. Adv Clin Exp Med. 2017;26:207–213. doi: 10.17219/acem/31186. [DOI] [PubMed] [Google Scholar]
- 44.Arablou T, Kolahdouz-Mohammadi R. Curcumin and endometriosis: review on potential roles and molecular mechanisms. Biomed Pharmacother. 2018;97:91–97. doi: 10.1016/j.biopha.2017.10.119. [DOI] [PubMed] [Google Scholar]