Abstract
Objectives: To explore and analyze the correlation between lncRNA NEAT1 and serum hepcidin (HEPC) in the peripheral blood of non-alcoholic fatty liver disease patients. Methods: 119 patients, confirmed to have non-alcoholic fatty liver disease (NAFLD) and admitted to our hospital from January 2017 to June 2019, were enrolled in the NAFLD group, and 100 healthy subjects during the same period were enrolled in the control group. We recorded the two groups’ general information and routine laboratory examination results and performed correlation analyses on the lncRNA NEAT1 expressions in their peripheral blood mononuclear cells (PBMCs) and HEPC. Results: The BMI, the waist circumferences, and the ALT, GGT, TC, and TG levels in the NAFLD group were critically higher than they were in the control group (P<0.05). The relative expressions of lncRNA NEAT1 in the PBMCs of the NAFLD group were remarkably higher than they were in the control group (P<0.05). The HEPC levels in the NAFLD group were significantly higher than they were in the control group (P<0.05). The lncRNA NEAT1 expressions in the NAFLD patients presented a remarkable positive correlation with the ALT, GGT, TC, and TG levels (P<0.05). The HEPC levels were positively correlated with the ALT, GGT, TC, and TG levels in the NAFLD patients (P<0.05), and the lncRNA NEAT1 expressions in the peripheral blood had a positive correlation with HEPC (P<0.05). We used ROC curves to analyze the diagnostic value of lncRNA NEAT1 in the peripheral blood to NAFLD, and the area under the curve was 0.822 (95% confidence interval of overall probability: 0.612~0.921). The sensitivity was 86.47%, and the specificity was 82.03%. Conclusion: lncRNA NEAT1 is abnormally overexpressed in the PBMCs of patients with NAFLD. The regulatory effect of lncRNA NEAT1 on NAFLD may be related to the mechanism of HEPC, which is expected to be a potential biological indicator for the prevention and treatment of NAFLD.
Keywords: Non-alcoholic fatty liver (NAFLD), peripheral blood, lncRNA NEAT1, serum hepcidin (HEPC), correlation
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a metabolic stress hepatic injury represented by hepatocyte steatosis and viral inflammation of liver. NAFLD includes simple hepatic steatosis, non-alcoholic steatohepatitis, and eventually cirrhosis and even hepatocellular carcinoma [1]. Long non-coding RNA (lncRNA) is a type of non-coding RNA with a transcription length over 200 nt. Current studies suggest that lncRNA can target multiple genes such as transcription factors, activators and suppressors, regulate the transcription and expression of genes, and participate in the occurrence and progression of a variety of diseases [2,3]. In addition, lncRNA can participate in the regulation of genes and the synthesis of proteins in cells in a variety of ways, affecting the metabolism of intracellular substances, and then participating in the occurrence and progression of NAFLD [5]. lncRNA NEAT1, a member of the lncRNA family, is abnormally expressed in a variety of tumors. Studies suggest that lncRNA NEAT1 is involved in hepatocyte injury [6]. Also, iron metabolism has been a research focus in the pathogenesis of NAFLD, the mechanism of which is yet to be fully clarified. Some scholars have proposed that iron metabolism affects the disease progression of NAFLD, and it may be related to factors such as lipid peroxidation, oxidative stress, and insulin resistance [7]. Currently, the influence mechanism of lncRNA on NAFLD has not been clarified, and we speculate that lncRNA-NEAT1 affects the disease progression through the regulation of iron metabolism. In this study, the correlation between the peripheral blood lncRNA-NEAT1 levels in patients with NAFLD and the hepcidin (HEPC) levels and the TLR4/NF-κB pathway were investigated and analyzed. The report is as follows.
Materials and methods
Clinical data
In this retrospective study, 119 subjects that confirmed to have non-alcoholic fatty liver disease (NAFLD) and admitted to our hospital from January 2017 to June 2019 were enrolled in the NAFLD group, and 100 healthy subjects who visited our hospital during the same period were enrolled in the control group. The study was authorized by the hospital’s ethics committee.
Inclusion and exclusion criteria
Inclusion criteria: (1) The patient diagnoses were in line with the diagnostic criteria in the Guidelines for the Prevention and Treatment of Non-alcoholic Fatty Liver Disease (2018 update) [8]; (2) Patients ≥18 years old; and (3) Both groups of subjects voluntarily signed the informed consent forms.
Exclusion criteria: (1) Subjects with viral hepatitis, liver tumors or liver cirrhosis; (2) Patients with type 2 diabetes; (3) Patients with acute or chronic inflammation; (4) Patients with other diseases, such as tumors, immune system diseases, tuberculosis, hematological diseases, chronic atrophic gastritis, etc.; (5) Patient who had used immunomodulators within one month before their enrollment; (6) Patients with hepatolenticular degeneration; and (7) Patients who had consumed large quantities of alcohol.
Methods
General information
The two groups’ general data, including their genders, ages, body weights, waist circumferences, hip circumferences, blood pressure, etc., were recorded. At the same time, we performed routine laboratory tests on the patients, including determining their aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), total cholesterol (TC), triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels.
Reagents and equipment
Ficoll lymphocyte separation solution (Sigma, USA); Trizol reagent (Invitrogen, USA); Prime ScriptTM RT reagent Kit with gDNA Eraser and SYBR® Premix Ex TaqTM (Dalian TaKaRa, Japan); Human Hepcidin Quantikine ELISA Kit (American R&D, DHP250); Spectrophotometer (Bio-Rad Company, USA); and 7600 Fluorescence quantitative PCR (Applied Biosystems).
Measuring the lncRNA-NEAT1 levels in the peripheral blood
Early morning fasting venous blood (5 ml) from the two groups of subjects was drawn and mixed well in heparin sodium anticoagulation tubes. PBMC was extracted using a Ficoll lymphocyte separation solution. The total RNA of PBMC was extracted according to instructions of the Trizol reagent, and its concentration and purity were measured using a spectrophotometer. After passing the test, the total RNA was frozen at -80°C. The lncRNA NEAT1 expressions were analyzed by taking GAPDH as internal reference gene. Prime ScriptTM RT reagent Kits with a gDNA Eraser were used to reverse-transcript the RNA into cDNA. The RT-PCR quantification was conducted using the SYBR method, and the measurement routines and reaction requirements were carried out in accordance with the kit’s instructions. The primer sequences were synthesized by the Shanghai Shenggong Biological Engineering Co., Ltd. The forward primer of lncRNA NEAT1 was 5’-CTTCCTCCCTTTAACTTATCCATTCAC-3’ and the reverse primer was 5’-CTCTTCCTC CACCATTACCAACAATAC-3’. The reaction system: pre-denaturation at 95°C for 10 min, 95°C for 15 s, 60°C for 1 min and 72°C for 30 s, with a total of 40 cycles. All the samples were made with 3 holes, and the relative expressions of lncRNA NEAT1 were calculated using the 2-ΔΔCt method.
Measuring the HEPC
Early morning fasting venous blood was drawn from the two groups of subjects, centrifuged at 3000 r/min for 10 min to take the supernatant. ELISA was used to measure the HEPC levels in both groups strictly according to the instructions.
Statistical analysis
SPSS 22.0 was used to examine the collected data. The measurement data were presented as (x̅±s) and compared using t tests between the two groups. The enumeration data were recorded as percentages and compared using X 2 tests. Pearson correlation coefficients were used for the correlation analyses, with P<0.05 considered to be a statistically significant difference.
Results
Comparison of the two group’s clinical data
There were no statistically significant differences in terms of gender, age, hip circumference, or ALP, HDL-C and LDL-C levels between the two groups (P>0.05). The BMI, waist circumferences, and ALT, GGT, TC, and TG levels in the NAFLD group were remarkably greater than they were in the control group (P<0.05), as shown in Table 1.
Table 1.
Comparison of the two groups’ clinical data
| Clinical data | NAFLD Group (n=119) | Control Group (n=100) | t/χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 65 | 59 | 0.424 | 0.515 |
| Female | 54 | 41 | ||
| Age (years old, x̅±s) | 55.62±7.22 | 54.97±8.15 | 0.626 | 0.532 |
| BMI (kg/m2, x̅±s) | 27.85±4.15 | 25.64±3.29 | 4.308 | 0.000 |
| Waist circumference (cm, x̅±s) | 87.09±7.83 | 83.78±6.42 | 3.379 | 0.001 |
| Hip circumference (cm, x̅±s) | 96.44±9.36 | 94.58±7.03 | 1.637 | 0.103 |
| ALT (U/L, x̅±s) | 33.97±4.63 | 27.96±3.11 | 11.052 | 0.000 |
| GGT (U/L, x̅±s) | 29.86±7.28 | 24.52±8.30 | 5.071 | 0.000 |
| ALP (U/L, x̅±s) | 64.85±15.62 | 63.72±13.29 | 0.570 | 0.569 |
| TC (mmol/L, x̅±s) | 4.69±1.55 | 3.98±1.42 | 3.508 | 0.001 |
| TG (mmol/L, x̅±s) | 1.97±0.46 | 1.74±0.51 | 3.507 | 0.001 |
| HDL-C (mmol/L, x̅±s) | 1.27±0.35 | 1.34±0.47 | 1.261 | 0.209 |
| LDL-C (mmol/L, x̅±s) | 3.08±0.37 | 3.04±0.41 | 0.759 | 0.449 |
The lncRNA NEAT1 expressions in the two groups’ peripheral blood
The relative lncRNA NEAT1 expressions in the PBMC of the NAFLD group were notably higher than they were in the control group [(2.974±0.642) vs. (1.240±0.339), P<0.05], as shown in Figure 1.
Figure 1.
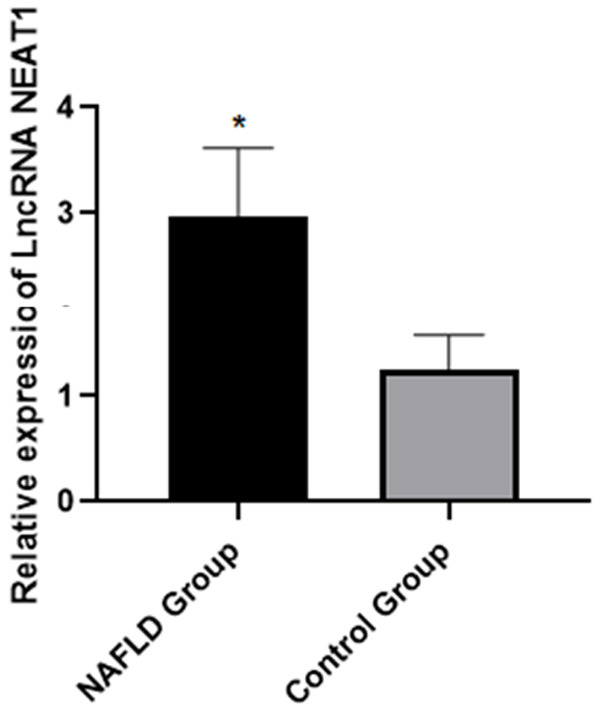
The relative lncRNA NEAT1 expressions in the peripheral blood of the patients in two groups. Note: Compared with the control group, *P<0.05.
Comparison of the two groups’ serum HEPC levels
The serum HEPC levels in the NAFLD group were critically higher than they were in the control group [(98.84±13.42) vs. (61.08±6.45), P<0.05], as elaborated in Figure 2.
Figure 2.
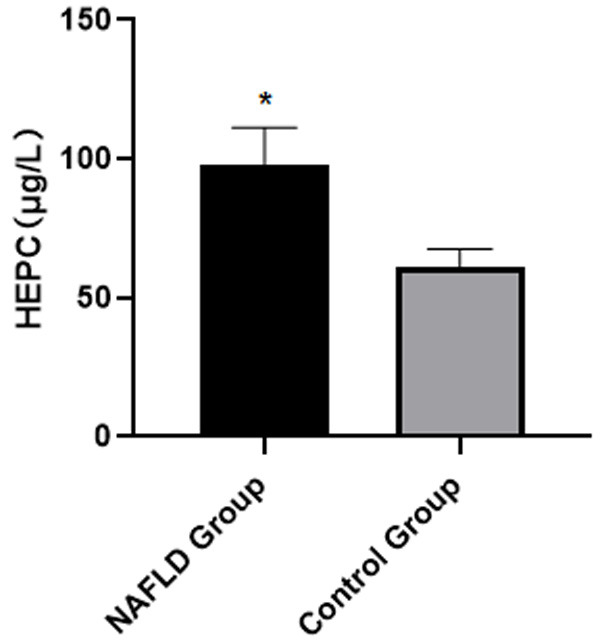
Comparison of the serum HEPC levels in the two groups of patients. Note: Compared with the control group, *P<0.05.
Analysis of the correlation between the serological indicators and the lncRNA NEAT1 expressions
The lncRNA NEAT1 expressions in the peripheral blood had a remarkably positive connection with the ALT, GGT, TC, and TG levels in the NAFLD group (r=0.467, 0.389, 0.399, 0.344, P<0.05), as shown in Figure 3.
Figure 3.
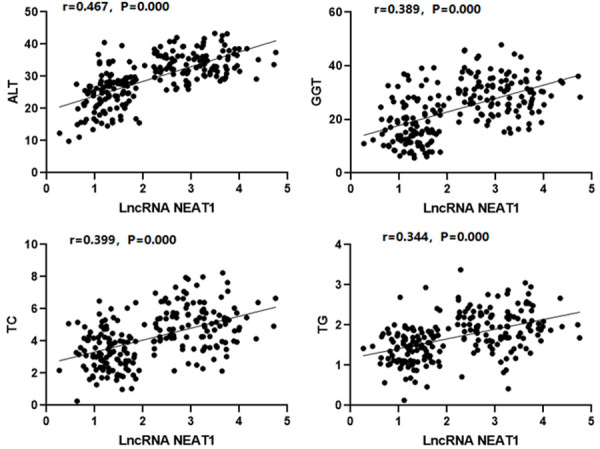
The correlation between the serological indicators and the lncRNA NEAT1 expressions.
Analysis of the correlation between the serological indicators and the HEPC levels
There was a significant positive correlation between serum HEPC levels and the ALT, GGT, TC, and TG levels in the patients with NAFLD (r=0.457, 0.512, 0.506, 0.391, P<0.05), as indicated in Figure 4.
Figure 4.
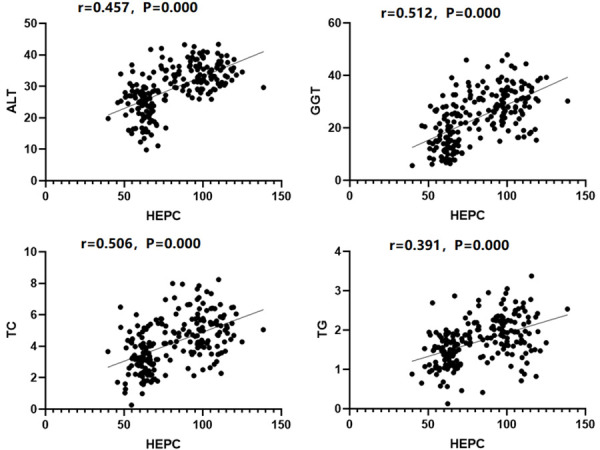
The correlation between the serological indicators and the HEPC levels.
Analysis of the correlation between the lncRNA NEAT1 expressions and HEPC
The lncRNA NEAT1 expressions in the peripheral blood of the NAFLD group were significantly positively related with the HEPC levels (r=0.443, P<0.05), as illustrated in Figure 5.
Figure 5.
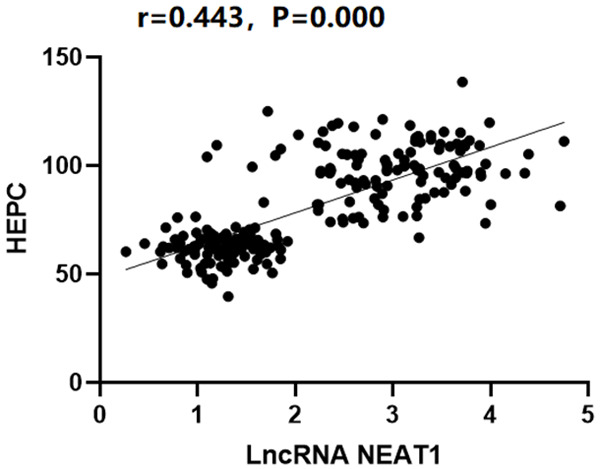
The correlation between the lncRNA NEAT1 expressions and the HEPC levels.
The diagnostic evaluation of lncRNA NEAT1 in the peripheral blood to NAFLD
The diagnostic evaluation of lncRNA NEAT1 in the peripheral blood to NAFLD was analyzed using an ROC curve, and the area under the curve was 0.822 (95% confidence interval of overall probability: 0.612~0.921), and the sensitivity and the specificity were 86.47% and 82.03% respectively, as shown in Figure 6. The peripheral blood lncRNA NEAT1 has a good diagnostic value for NAFLD.
Figure 6.
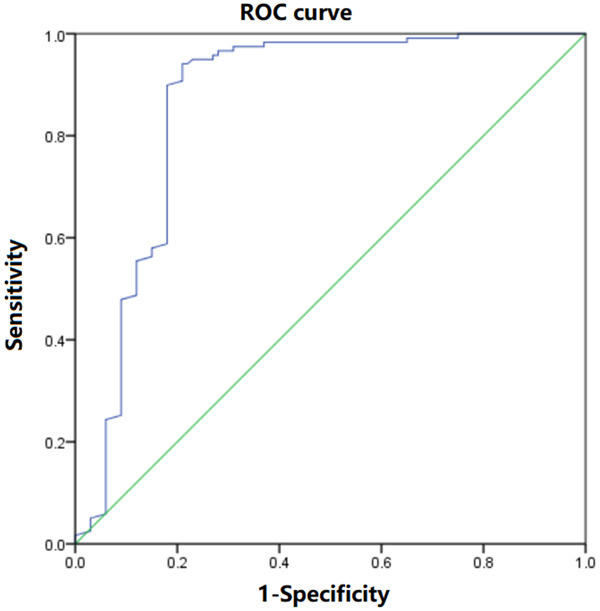
A diagnostic evaluation of the lncRNA NEAT1 expressions in the peripheral blood in NAFLD analyzed using ROC curves.
Discussion
NAFLD, a type of metabolic stress-caused liver injury, is frequently connected to obesity, diabetes, and other diseases. Among which, the influence of obesity is most closely related [9]. Investigations and studies in recent years have shown that [10] the incidence of NAFLD in China is on the rise yearly and gradually tends to occur among younger patients. NAFLD is one of the crucial causes of portal hypertension, liver transplantation, cirrhosis and liver cancer in patients. For obese patients, the presence of NAFLD may indicate “malignant obesity”, with a marked increase in the probability of diabetes, hypertension, dyslipidemia, coronary heart disease, and stroke [11,12]. However, the pathogenesis of NAFLD has not yet been completely clarified, and there is a lack of effective drug treatment in clinical practice. Therefore, it is urgent to clarify the pathogenesis of NAFLD and to seek effective precautions and treatments.
lncRNA is a group of RNA transcripts with a length of over 200 nt and no protein coding function. It has been found that lncRNA participates in many biological processes of liver cells and plays a crucial role in a variety of liver pathological processes. lncRNA is related to endoplasmic reticulum function, mitochondria, and energy metabolism [13,14]. lncRNA-H19 directly regulates the miR130a/PPARγ axis to promote the synthesis of NAFLD fat [15]. The liver fibrosis and hepatocellular apoptosis in NAFLD rats can be promoted by inhibiting the expression of lncRNA HULC in the MAPK signaling pathway. lncRNA MIRT2 sponges miR-34a-5p, thereby upregulating the expression of USP10 and inhibiting liver steatosis [17]. As an endogenous and competitive RNA, lncRNA MEG3 regulates hepatic adipogenesis by competitively binding miR-21 to low-density lipoprotein receptor-related protein-6 (LRP6) [18]. The above study results suggest that lncRNA plays an essential role in NAFLD. It shows that the relative expression of lncRNA NEAT1 in the PBMC of the NAFLD group was notably higher than it was in the control group. The results are similar to those reported by other researchers [19,20], suggesting that there is abnormal regulation of lncRNA NEAT1 expression in the process of NAFLD disease. In addition, the lncRNA NEAT1 expressions in the peripheral blood of NAFLD patients is remarkably positively correlated with the ALT, GGT, TC, and TG levels, suggesting that the regulation of lncRNA NEAT1 expression in NAFLD subjects is related to liver function and lipid metabolism.
The small intestine is the main organ for iron absorption, and the liver is the main site for iron storage. Since most people consume far more iron in their diet than the body needs, the liver, as the core organ for iron metabolism, is likely to cause iron deposition in the liver after being damaged [21,22]. There are a variety of iron regulative proteins in human body. In recent years, researchers have found a new iron regulative protein, HEPC. Researchers have found that HEPC plays a vital function in the process of iron metabolism, especially in some chronic anemia diseases [23]. HEPC is a small molecule antimicrobial peptide secreted by the liver, and its target cells include hepatocytes, intestinal epithelial cells, and macrophages. HEPC can be bonded to the iron transporter-1 on such cell membranes to inhibit the transport of iron by transporters, thereby promoting the deposition of iron in cells [24]. The results of recent animal experiments show that iron and fat accumulated in liver cells can synergistically induce the injury and the death of hepatocytes [25]. This study’s results show that the serum HEPC in the NAFLD group was critically higher than it was in the control group. The results, similar to those reported by other researchers [26], suggest that there is abnormal iron metabolism in the process of NAFLD disease. In addition, the serum HEPC level in the NAFLD patients was notably positively correlated with the ALT, GGT, TC, and TG levels, indicating that the HEPC levels in NAFLD patients are related to their liver function and lipid metabolism.
The lncRNA NEAT1 expression in the peripheral blood of NAFLD subjects was significantly positively correlated with the HEPC levels, suggesting that the regulative mechanism of lncRNA NEAT1 on NAFLD disease may be related to the regulation of iron metabolism in the human body. Using ROC curves to analyze the diagnostic value of lncRNA NEAT1 in the peripheral blood to NAFLD, the area under the curve was 0.822 (95% confidence interval of overall probability: 0.612~0.921), the sensitivity and the specificity were 86.47% and 82.03% respectively. This suggests that lncRNA NEAT1 may be a possible biological target for the prevention and treatment of NAFLD. The results of this study are similar to those reported by other researchers [27], namely that lncRNA NEAT1 plays a key role in the occurrence and progression of NAFLD disease. In addition, the regulation of this disease may be connected to the regulation of iron metabolism in the body. In other words, a patient’s lipid metabolism is further affected through the regulation of iron metabolism, so the further progression of the disease is promoted or blocked.
However, there are still deficiencies in this study, including the limited sample size as well as the lack of an in-depth analysis of the specific pathway between lncRNA NEAT1 and iron metabolism. Therefore, it is necessary to expand the sample size and analyze the specific mechanism of lncRNA NEAT1 regulation in subsequent studies in order to make better progress in clinical work.
Disclosure of conflict of interest
None.
References
- 1.Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol. 2019;25:1307–1326. doi: 10.3748/wjg.v25.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin SS, Lin XF, Zheng JZ, Wang Q, Guan HQ. lncRNA NEAT1 regulates fibrosis and inflammatory response induced by nonalcoholic fatty liver by regulating miR-506/GLI3. Eur Cytokine Netw. 2019;30:98–106. doi: 10.1684/ecn.2019.0432. [DOI] [PubMed] [Google Scholar]
- 3.Ma TT, Huang C, Ni Y, Yang Y, Li J. ATP citrate lyase and LncRNA NONMMUT010685 play crucial role in nonalcoholic fatty liver disease based on analysis of microarray data. Cell Physiol Biochem. 2018;51:871–885. doi: 10.1159/000495384. [DOI] [PubMed] [Google Scholar]
- 4.Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Li X, Hu W, Zhou Y, Din Y. Silencing of lncRNA SNHG20 delays the progression of nonalcoholic fatty liver disease to hepatocellular carcinoma via regulating liver Kupffer cells polarization. IUBMB Life. 2019;71:1952–1961. doi: 10.1002/iub.2137. [DOI] [PubMed] [Google Scholar]
- 6.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2016;65:1080–1086. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao XY, Xiong X, Liu T, Mi L, Peng X, Rui C, Guo L, Li S, Li X, Lin JD. Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 2018;9:2986. doi: 10.1038/s41467-018-05383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263–8276. doi: 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suksangrat T, Phannasil P, Jitrapakdee S. miRNA regulation of glucose and lipid metabolism in relation to diabetes and non-alcoholic fatty liver disease. Adv Exp Med Biol. 2019;1134:129–148. doi: 10.1007/978-3-030-12668-1_7. [DOI] [PubMed] [Google Scholar]
- 10.Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 11.Torres JL, Novo-Veleiro I, Manzanedo L, Alvela-Suárez L, Macías R, Laso FJ, Marcos M. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J Gastroenterol. 2018;24:4104–4118. doi: 10.3748/wjg.v24.i36.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin North Am. 2016;45:639–652. doi: 10.1016/j.gtc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CH, Ampuero J, Gil-Gómez A, Montero-Vallejo R, Rojas Á, Muñoz-Hernández R, Gallego-Durán R, Romero-Gómez M. miRNAs in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2018;69:1335–1348. doi: 10.1016/j.jhep.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9:387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Xu Y, Zhao D, Chen T, Gu C, Yu G, Chen K, Zhong Y, He J, Liu S, Nie Y, Yang H. LncRNA-AK012226 is involved in fat accumulation in db/db mice fatty liver and non-alcoholic fatty liver disease cell model. Front Pharmacol. 2018;9:888. doi: 10.3389/fphar.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X. Down-regulation of lncRNA-NEAT1 alleviated the non-alcoholic fatty liver disease via mTOR/S6K1 signaling pathway. J Cell Biochem. 2018;119:1567–1574. doi: 10.1002/jcb.26317. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Song Y, Liu C, Geng J. LncRNA NEAT1-MicroRNA-140 axis exacerbates nonalcoholic fatty liver through interrupting AMPK/SREBP-1 signaling. Biochem Biophys Res Commun. 2019;516:584–590. doi: 10.1016/j.bbrc.2019.06.104. [DOI] [PubMed] [Google Scholar]
- 18.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Stacchiotti A, Grossi I, García-Gómez R, Patel GA, Salvi A, Lavazza A, De Petro G, Monsalve M, Rezzani R. Melatonin effects on non-alcoholic fatty liver disease are related to MicroRNA-34a-5p/Sirt1 axis and autophagy. Cells. 2019;8:1053. doi: 10.3390/cells8091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jampoka K, Muangpaisarn P, Khongnomnan K, Treeprasertsuk S, Tangkijvanich P, Payungporn S. Serum miR-29a and miR-122 as Potential biomarkers for Non-Alcoholic Fatty Liver Disease (NAFLD) Microrna. 2018;7:215–222. doi: 10.2174/2211536607666180531093302. [DOI] [PubMed] [Google Scholar]
- 21.Shen X, Guo H, Xu J, Wang J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J Cell Physiol. 2019;234:18169–18179. doi: 10.1002/jcp.28450. [DOI] [PubMed] [Google Scholar]
- 22.Dongiovanni P, Meroni M, Longo M, Fargion S, Fracanzani AL. miRNA signature in NAFLD: a turning point for a non-invasive diagnosis. Int J Mol Sci. 2018;19:3966. doi: 10.3390/ijms19123966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long JK, Dai W, Zheng YW, Zhao SP. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol Med. 2019;25:26. doi: 10.1186/s10020-019-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76:99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrecht J, Verhulst S, Reynaert H, Grunsven LA. The miRFIB-score: a serological miRNA-based scoring algorithm for the diagnosis of significant liver fibrosis. Cells. 2019;8:1003. doi: 10.3390/cells8091003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Chen X, Gao J, Xu C, Xu P, Li Y, Zhu Y, Yu C. Long noncoding RNA FLRL2 alleviated nonalcoholic fatty liver disease through Arntl-Sirt1 pathway. FASEB J. 2019;33:11411–11419. doi: 10.1096/fj.201900643RRR. [DOI] [PubMed] [Google Scholar]
- 27.Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ, Sookoian S. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64:800–812. doi: 10.1136/gutjnl-2014-306996. [DOI] [PMC free article] [PubMed] [Google Scholar]


