Abstract
Objective: To reveal the cerebral hypoperperfusion characteristics of White matter lesions (WMLs), we monitored the blood pressure (BP) fluctuation in patients with orthostatic hypotension (OH) and WMLs. Methods: A total of 2265 syncope patients were enrolled in this retrospective study. Clinical outcomes of brain MRI or CT, tilt test and continuous electrocardiogram monitoring were reviewed. All patients were divided into two groups according to WMLs status, and the WMLs group was further classified into three subgroups according to Fazekas grade (1-3). BP fluctuation in these subgroups was compared. The risk factors of WMLs and OH were determined by a multivariate logistic regression test. Results: A total of 2265 syncope patients were enrolled, among which 56% patients were male. The average age of patients with WMLs was (61±12) years old. ΔTIME (Odds ratio [OR]: 1.014, 95% confidence interval [CI]: (1.005, 1.023), P=0.0015) and ΔSBP1 (OR: 0.990, 95% CI: (0.980, 1.000), P=0.0579) were demonstrated to be the risk factors of WMLs. The number of cases of repeated drops in blood pressure was twice as high as the cases with only drop in BP . The median and mean ΔSBP and ΔTIME of patients with WMLs were higher than those without WMLs. The incidence of diabetes, hypertension, age and Parkinson Plus Syndromes in patients with WMLs significantly decreased in comparison to those without WMLs (OR-diabetes: 2.558, OR-hypertension: 1.713, OR-age: 0.924 and OR-Parkinson Plus Syndromes: 0.476, P<0.05). Conclusion: WMLs occurs in patients with hypoperfusion of recurrent OH. Vascular WMLs is associated with diabetes, hypertension, and age is at higher risk than WMLs associated with Parkinson Plus Syndromes.
Keywords: White mater lesions, orthostatic hypotension
Introduction
White matter lesions (WMLs) are commonly observed with high-signal foci and blurred edges on T2-weighted and fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), or hypoattenuations or hypodensities on computed tomography (CT) of the brain, especially in periventricular and subcortical areas [1-3]. Previous studies have focused on the genetic characteristics of WMLs, and the Fazekas scale has been widely used as a grading standard for disease severity [4-12].Other research found that levels of systolic (SBP) and diastolic blood pressure (DBP) are associated with WML severity [13-16]. Reports on the relationship between BP change and WML progression are scarce [17]. Neither did these studies show relationship between the degree of blood pressure change with WMLs and the degree of hypoperfusion which is manifested as clinical symptoms, nor did they reflect the cerebral autoregulation that occurs when blood pressure changes. We usually use the increase of pulsatility index (PI) and the decrease of diastolic blood flow velocity by transcranial doppler (TCD) to estimate the change of intracranial pressure and reflect the fluctuation of cerebral perfusion pressure. However, PI value is affected by many factors (BP and cardiac output) which limit its clinical application. Diastolic blood flow velocity with TCD has not been popular either because of the degree of cerebrovascular disease and the failure ratio of detecting temple window.
We have found that many patients with syncope have WMLs as indicated by MRI. The various forms of syncope and transient loss of consciousness (TLOC) are due to cerebral hypoperfusion. The degree of hypoperfusion has not been quantitatively distinguished in the past literature. Orthostatic hypotension (OH) is confirmed when syncope occurs while standing [18-24]. The onset may occur within 3 minutes of a change in position (typical OH) or after long standing (delayed OH). Tilt testing may assist in finding such OH [18-22]. This method is practical and has rigorous standards, and parameters which can be used to quantify indicators.
Our aim was to determine whether blood pressure changes in OH patients with syncope are more representative to reflect the degree of hypoperfusion with WMLs.
Materials and methods
Patient profiles
This was a retrospective study. During June 2015 and March 2019, we recruited 2265 consecutive patients with syncope. All patients were informed and provided written consent before participating in the study, which was approved by the Ethics Committee of Beijing Tiantan Hospital Affiliated to Capital Medical University (approval No. KY2019-109-01). All the patients with syncope were referred to China National Clinical Research Center for Neurological Diseases by their neurologists or physicians, with confirmation of the referral diagnosis by clinical and laboratory evaluations.
Patient enrolment
Inclusion criteria
Patients with a history of syncope ranging from single to several times; patients with pressure alteration from lying to standing position (SBP decreased by ≥20 mmHg or DBP decreased by ≥10 mmHg or SBP was lower than 90 mmHg) in the upright tilt test, which meets the standard of upright hypotension.
Office hypertension, diabetes, Parkinson’s disease (PD), Parkinsonian Syndrome and Parkinson Plus Syndromes (e.g. multiple system atrophy (MSA), dementia with Lewy bodies (DLB), corticobasal degeneration (CBD), and progressive supranuclear palsy (PSP)) were involved [22-27].
Exclusion criteria
Patients with any of the following conditions were excluded: leukodystrophy, heart disease or heart failure; low blood volume (after menstruation, intracerebral hemorrhage, gastrointestinal bleeding and diarrhea); renal insufficiency; unable or refuse to accept brain MRI or CT examination due to metal implants in the body or psychological and emotional problems.
Imaging data collection
The following protocols were recommended for all patients: brain imaging, including brain MRI (T1 weighted, T2 weighted, Fluid-attenuated Inversion Recovery (FLAIR)) or CT (if contraindicated to MRI). Image data were collected in DICOM format on discs and analyzed by the Image Research Centre in Beijing Tiantan Hospital. All patients were divided into two groups: WMLs group and non-WMLs group. And the WMLs group was classified into three sub-groups according to Fazekas grade [11,12].
Blood pressure data collection and data management
Heart examination included 12-lead ECG, precordial echocardiography, cardiac monitoring for ≥24 hours with automated rhythm detection. Antihypertensive drugs were prohibited on the day of monitoring. Head-up tilt test and continuous electrocardiogram monitoring were completed. Blood pressure was measured noninvasively by using a to nometric device placed on the radial pulse when underwent the head-up tilt test. The position of the arm was at heart level for measurements of blood pressure in both supine and upright positions. And then blood pressure was recorded sustained from supine to upright position. OH was defined as a decrease in SBP≥20 mmHg and/or DBP≥10 mmHg, or SBP<90 mmHg from supine to standing >60 minutes (or until symptomatic hypotension after standing) [18-22].
Symptoms of OH to end the head-up tilt test included dizziness, transient loss of consciousness (TLOC), and syncope.
Blood pressure outcomes
The angle of head-up tilt test was 70° by upright position. Blood pressure was recorded sustained from supine to upright position as diagram A and B. The monitored values are listed below: SBP0/DBP0: supine BP; TIME1: the first time for falling down of upright BP; SBP1/DBP1: the first falling down of upright BP with or without symptoms; ΔSBP1/ΔDBP1: the first BP fluctuation; TIME2: the time for the maximum falling down of upright BP; SBP2/DBP2: the maximum falling down of upright BP with or without symptoms; ΔSBP2/ΔDBP2: the maximum BP fluctuation; ΔTIME: interval time of twice falling down of upright BP. All of ΔBPs are primary indicators, and ΔTIMEs are secondary one.
Statistical analysis
Quantitative data were expressed as median and mean ± standard deviation (x̅±sd). Categorical variables were expressed as numbers (percentages). The outcomes were tested by the Wilcoxon test, Fisher’s exact test, Kruskal-Wallis test, and Logistic regression analysis. All analyses were performed with SAS 9.4 Software, and values of P<0.05 were considered statistically significant.
Data availability
The clinical data involved in this paper were from the clinical databases of Beijing Tiantan Hospital and Beijing Chaoyang Hospital. Others who want to share information must first get approval from the two hospitals and the authors. This study was not involved in any industry-sponsored research and corporate activities.
Results
There were 3315 syncope patients who consented and were enrolled in this study during June 2015 and March 2019. We initially excluded 572 patients by exclusion criteria. After excluding 478 patients with missing data, there were a total of 2265 eligible patients with complete information. Overall, 56% patients were male, and the average age was 54 years old (7 to 92). 2010 OH patients were monitored by tilt test and divided into WMLs group and non-WMLs groups according to MRI.
Over half of the patients had symptoms such as dizziness, TLOC or syncope. There were 1342 patients with WMLs as presented in Table 1. Age, gender and diseases had significant difference between patients with and without WMLs.
Table 1.
Comparison of the distribution of characteristics between individuals with and without WMLs
| N=2265 | with WMLs n=1342 | without WMLs n=923 |
|---|---|---|
| Age (year), x̅±sd (median) | 61±12 (62) | 43±17 (45) |
| Male, n (%) | 790 (34.9%) | 468 (20.7%) |
| Symptom, n (%) | 884 (39.0%) | 576 (25.4%) |
| Hypertension, n (%) | 536 (23.7%) | 153 (6.8%) |
| Diabetes, n (%) | 293 (12.9%) | 59 (2.6%) |
| PD, n (%) | 150 (6.6%) | 78 (3.4%) |
| Parkinsonian syndrome, n (%) | 197 (8.7%) | 65 (2.9%) |
| Parkinson Plus Syndromes, n (%) | 45 (2.0%) | 34 (1.5%) |
Note: Fisher test and Kruskal-Wallis test: P<0.05. WMLs: white matter lesions; PD: Parkinson’s disease.
Latencies and durations of orthostatic hypotension
Blood pressure was recorded from supine (0°) to upright position (70°) as shown in Figures 1A and 2A. As shown in Figures 1B and 2B, ΔTIME had two types: the one was that the blood pressure decreased repeatedly, and the other was that blood pressure dropped one and only, then ΔTIME is zero.
Figure 1.
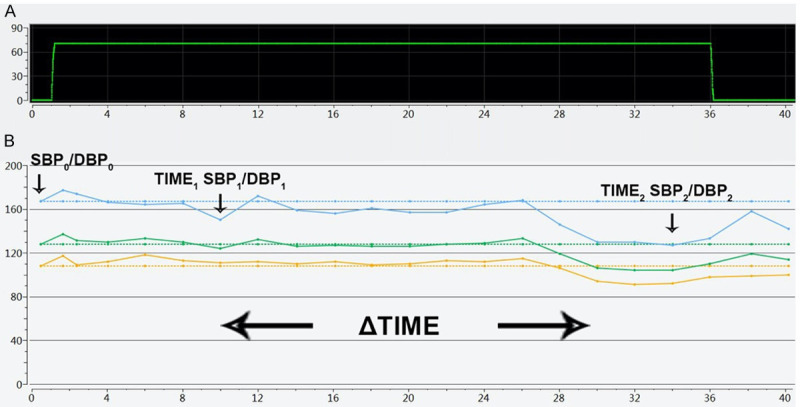
A (Time-angle): The X-axis was the time of tilt testing (minite), and the Y-axis was the angle of head-up tilt test; B (Time-BP mmHg): The X-axis was the time of tilt testing (minite), and the Y-axis was blood pressure. The three lines of the broken line diagram represented systolic blood pressure, mean blood pressure, and diastolic blood pressure. Blood pressure had decreased repeatedly and slowly.
Figure 2.
A (Time-angle): The X-axis was the time of tilt testing (minite), and the Y-axis was the angle of head-up tilt test; B (Time-BP mmHg): The X-axis is the time of tilt testing (minite), and the Y-axis is blood pressure. The three lines of the broken line diagram represent systolic blood pressure, mean blood pressure, and diastolic blood pressure. Blood pressure had dropped one and only, then ΔTIME is zero.
According to our calculations, the number of cases with BP decreased repeatedly was twice as much as the cases with BP dropped one and only (Table 2).
Table 2.
Type for ΔTIME oforthostatic hypotension (n)
| ΔTIME of OH | With WMLs | Without WMLs | Total |
|---|---|---|---|
| BP decreased repeatedly (non-zero) | 734 | 656 | 1390 |
| BP dropped one and only (zero) | 468 | 152 | 620 |
Note: OH: orthostatic hypotension; WMLs: white matter lesions.
There were significant differences in BP, ΔSBP and ΔTIME between patients with and without WMLs. The median and mean of BP parameters of patients with WMLs were slightly higher than those without WMLs. The time parameters of patients with WMLs were slightly higher than or equal to those without WMLs (Table 3).
Table 3.
BP fluctuations in patients with or without WMLs (BP: mmHg, time: min)
| n=2265 | with WMLs (n=1342) | without WMLs (n=923) | P | ||
|---|---|---|---|---|---|
|
|
|
||||
| median | x̅±sd | median | x̅±sd | ||
| SBP0 | 131 | 134±18 | 122 | 123±14 | <0.0001 |
| DBP0 | 82 | 82±11 | 77 | 78±11 | <0.0001 |
| SBP1 | 104 | 104±25 | 101 | 100±22 | <0.0001 |
| DBP1 | 72 | 70±17 | 70 | 68±17 | 0.0001 |
| ΔSBP1 | 25 | 30±21 | 21 | 23±19 | <0.0001 |
| ΔDBP1 | 10 | 12±15 | 9 | 10±15 | 0.0050 |
| SBP2 | 95 | 93±25 | 92 | 91±23 | 0.0375 |
| DBP2 | 65 | 64±18 | 62 | 61±17 | <0.0001 |
| ΔSBP2 | 36 | 40±25 | 28 | 32±23 | <0.0001 |
| ΔDBP2 | 15 | 18±18 | 14 | 16±18 | 0.0908 |
| TIME1 | 35 | 27±17 | 35 | 28±15 | 0.9262 |
| TIME2 | 40 | 37±10 | 37.5 | 37±10 | 0.5310 |
| ΔTIME | 2.5 | 10±14 | 2.5 | 9±12 | 0.0110 |
Note: Kruskal-Wallis test: P<0.05; WMLs: white matter lesions.
We compared differences in blood pressure fluctuations among patients with Fazekas scales (Table 4). Some parameters were of significant difference between patients with Fazekas 1 and Fazekas 2, and between patients with Fazekas 1 and Fazekas 3. There was faint difference between patients with Fazekas 2 and Fazekas 3.
Table 4.
BP fluctuations in patients with different Fazekas scales (BP: mmHg, time: min)
| n=2265 | Fazekas 1 n=758 | Fazekas 2 n=290 | Fazekas 3 n=294 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| median | x̅±sd | median | x̅±sd | median | x̅±sd | |
| SBP0 | 128 | 131±16 | 137 | 138±20 | 134 | 136±18 |
| DBP0 | 81 | 81±11 | 83 | 83±11 | 83 | 83±11 |
| SBP1 | 101 | 100±25 | 111 | 109±26 | 109 | 107±23 |
| DBP1 | 71 | 69±18 | 73 | 72±16 | 74 | 73±16 |
| ΔSBP1 | 24 | 31±22 | 25 | 30±20 | 25 | 29±18 |
| ΔDBP1 | 10 | 13±16 | 9 | 10±14 | 9 | 11±13 |
| SBP2 | 92 | 91±24 | 98 | 96±26 | 97 | 97±24 |
| DBP2 | 63 | 62±18 | 65 | 65±18 | 66 | 67±18 |
| ΔSBP2 | 35 | 40±25 | 38 | 43±26 | 33 | 39±24 |
| ΔDBP2 | 16 | 19±18 | 14 | 18±18 | 13 | 16±17 |
| TIME1 | 35 | 28±16 | 35 | 25±17 | 35 | 26±17 |
| TIME2 | 40 | 38±10 | 38 | 36±11 | 38 | 36±11 |
| ΔTIME | 2.5 | 10±14 | 2.5 | 11±14 | 2.5 | 10±14 |
Note: x̅±sd: Mean ± standard deviation. P<0.05: between Fazekas 1 and Fazekas 2: SBP0 (P<0.0001), DBP0 (P=0.0167), SBP1 (P<0.0001), DBP1 (P=0.0010), ΔDBP1 (P=0.0362), SBP2 (P=0.0037), DBP2 (P=0.0401), TIME1 (P=0.0046), and TIME2 (P=0.0020); between Fazekas 1 and Fazekas 3: SBP0 (P<0.0001), DBP0 (P=0.0108), SBP1 (P<0.0001), DBP1 (P=0.0005), SBP2 (P=0.0003), DBP2 (P=0.0008), and ΔDBP2 (P=0.0071). P≈0.05: between Fazekas 2 and Fazekas 3: ΔSBP2 (P=0.0558).
Risk factors analysis
The multivariate analysis showed 5 impact factors: age, ΔTIME, hypertension, diabetes, and Parkinson Plus Syndromes had significant difference (all P<0.05) between patients with and without WMLs (Table 5) after controlling for confounders. After we set sub-variables for BP fluctuations, there was faint difference in ΔSBP1 (P=0.0579) between those with and without WMLs (Table 5).
Table 5.
Risk factors for WMLs
| WMLs | ||
|---|---|---|
|
|
||
| OR*(95% CI) | P | |
| BP fluctuations | ||
| ΔSBP1 | 0.990 (0.980, 1.000) | 0.0579 |
| ΔDBP1 | 0.991 (0.977, 1.004) | 0.1749 |
| ΔSBP2 | 0.996 (0.987, 1.006) | 0.4457 |
| ΔDBP2 | 1.010 (0.997, 1.024) | 0.1450 |
| ΔTIME | 1.014 (1.005, 1.023) | 0.0015 |
| Age | 0.924 (0.916, 0.932) | <0.0001 |
| Sex | 0.959 (0.777, 1.184) | 0.6978 |
| Hypertension | 1.713 (1.342, 2.185) | <0.0001 |
| Diabetes | 2.558 (1.839, 3.558) | <0.0001 |
| PD | 0.577 (0.348, 0.957) | 0.1901 |
| Parkinsonian syndrome | 0.683 (0.386, 1.209) | 0.7859 |
| Parkinson Plus Syndromes | 0.476 (0.268, 0.846) | 0.0208 |
OR: odds ratio; 95% CI: 95% confidence interval of the difference;
WMLs: white matter lesions; PD: Parkinson’s disease.
Discussion
WMLs were commonly considered as a result of chronic cerebral ischemia [7-15]. We have shown that the median and mean of BP parameters in patients with WMLs were higher than those without WMLs (Table 3). Most of the BP parameters of patients with Fazekas 2 and 3 of WMLs had similar trends to patients with Fazekas 1 of WMLs. The higher the WMLs scale is, the more obvious change in blood pressure (Table 4). Because Fazekas scale is a semi-quantitative judgment of the weight and degree of WMLs, there will be fuzzy transition between the levels of the score. The difference between patients with Fazekas 2 and 3 in our study was not as significant as that between patients with Fazekas 1 and 3 or Fazekas 1 and 2. Semi-quantitative methods cannot accurately estimate the decrease in cerebral perfusion. Primary and final levels of WMLs can be distinguished from measured changes, however, the intermediate transition levels cannot be easily distinguished. The BP level could not reflect hypoperfusion. So, variables for BP fluctuations were set, in which ΔSBP1 (OR: 0.990, 95% CI: (0.980, 1.000), P=0.0579) did not significantly increase the risk of WMLs, and ΔDBP1, ΔSBP2 and ΔDBP2 were not the impact factors (Table 5). The standard we applied was a drop of 20 mmHg in SBP and/or 10 mmHg in DBP, or SBP<90 mmHg after standing position. The data of ΔSBP1 would be smaller when SBP dropped from 100 mmHg to 89 mmHg. So, there may be difference when p value close to 0.05.
In healthy people, upright positioning leads to decreased blood pressure (BP) in a transient period within 30 seconds, at the same time, intact cerebral auto-regulation maintained a constant cerebral perfusion through increased cardiac output and cerebral vasodilatation [28]. However, the responses to upright positioning may be disturbed due to diminished cerebral autoregulation or ischemic lesions that disturb autonomic nervous function [29-35]. So, we paid attention to the interval time (ΔTIME) of twice falling down of upright BP. TIME1 and TIME2, the time for falling down of upright BP, showed no specificity (P>0.05), but ΔTIME (P=0.0110) had significant difference between those with and without WMLs in our present study. The risk of WMLs increased by 1.4% with each additional second in ΔTIME (OR: 1.014, 95% CI: 1.005, 1.023, P=0.0015) of twice falling down of upright BP. We found that the median and mean ΔTIME of patients with WMLs were equal to or longer than those without WMLs (P=0.0110, Table 2), suggesting that abnormality of autonomic nerve in patients with WMLs is breaking out later than those without WMLs. And recurrent hypotension is characteristic. Among the patients with WMLs, the number of non-zero ΔTIME cases far exceeded the number of zero ΔTIME cases (Table 2).
Our study showed that the OR of diabetes, hypertension, age and Parkinson Plus Syndromes for WMLs were 2.558, 1.713, 0.924 and 0.476 respectively (Table 5). This means that diabetes is the most distinct risk factor, but such comparisons have never been made before. Previous studies focused on the incidence, pathogenesis and baroreflex sensitivity of OH in patients with different age, hypertension and diabetes conditions [36-46]. Rotterdam Scan Study displayed that the middle-aged people with subcortical and periventricular WMLs were higher than those of old-aged people [47]. The three factors are all related to atherosclerosis. So, the reasons for hypoperfusion with WMLs might be that: (1) Atherosclerotic narrowing of the arteriole leads to decreased blood flow and leads directly to chronic hypoperfusion. (2) Atherosclerosis leads to dysfunction of the arterial baroreceptor which causes dysregulation of autonomic nervous system. (3) While blood-brain barrier permeability increased with vascular endotheliocyte incompetence, plasma protein components may leak into the perivascular of brain white matter [46].
Comprehensive analysis shows that PD and PD syndrome have no significant influence, regardless of the number of enrolled cases. The minimum impact factor of WMLs is Parkinson Plus Syndromes (OR: 0.476, 95% CI: (0.268, 0.846)). OH is one of the non-motor symptoms of movement disorders [23-27,48,49]. Although OH occurs in several diseases, Parkinson Plus Syndromes that progresses to autonomic failure has a OR value of 0.476 (95% CI: 0.268, 0.846, P<0.05) for WMLs (Table 5). Gregor KW found that long latencies of OH with PD (161 months) was significantly different with MSA (24 months), DLB (34 months), or PSP (30 months) [49]. Our study, as a cross-sectional design, was likely to be in the early stages of PD Syndrome. In addition, MDS Clinical Diagnostic Criteria for Parkinson’s Disease defined an orthostatic decrease of at least 30 mmHg in systolic or 15 mmHg in diastolic to diagnose severe autonomic failure [23-27]. The standard we applied was a drop of 20 mmHg in SBP and/or 10 mmHg in DBP, or SBP<90 mmHg after standing position [18-21]. We think that autonomic dysfunction or autonomic disorder is equivalent to the latency period of PD or other diseases.
There are some limitations in this study. Firstly, it is uncertain whether the length of ischemia time is the only index to determine the degree of WMLs. In the future, we plan to solve it through animal experiments, such as making animal models of different Fazekas grades of WMLs to study the relationship between ischemia and blood pressure change time (ΔTIME). Secondly, autonomic dysfunction or autonomic disorder could be reversed in a period of time, while the change in WMLs is irreversible. Autonomic disorder cannot define the pathological stage of the change about WMLs. Especially for those patients with improved symptoms of dizziness and syncope after treatment, quantitative indicators such as perfusion volume will be more useful to evaluate WMLs.
In conclusion, we have found indirect indices, as ΔSBP and ΔTIME, can reflect the change of scale of WMLs.
Disclosure of conflict of interest
None.
References
- 1.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 2.Mäntylä R, Aronen HJ, Salonen O, Korpelainen M, Peltonen T, Standertskjöld-Nordenstam C, Erkinjuntti T. The prevalence and distribution of white-matter changes on different MRI pulse sequences in a post-stroke cohort. Neuroradiology. 1999;41:657–665. doi: 10.1007/s002340050820. [DOI] [PubMed] [Google Scholar]
- 3.Rudilosso S, San Román L, Blasco J, Hernández-Pérez M, Urra X, Chamorro Á. Evaluation of white matter hypodensities on computed tomography in stroke patients using the Fazekas score. Clin Imaging. 2017;46:24–27. doi: 10.1016/j.clinimag.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology. 2007;68:1020–1024. doi: 10.1212/01.wnl.0000257817.29883.48. [DOI] [PubMed] [Google Scholar]
- 5.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 6.Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis. 2012;33:525–531. doi: 10.1159/000337335. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, Wu J, Zeng J, Yao C, Huang Y. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 8.Cognat E, Cleophax S, Domenga-Denier V, Joutel A. Early white matter changes in CADASIL: evidence of segmental intramyelinic oedema in A pre-clinical mouse model. Acta Neuropathol Commun. 2014;2:49. doi: 10.1186/2051-5960-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim GM, Park KY, Avery R, Helenius J, Rost N, Rosand J, Rosen B, Ay H. Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke. 2014;45:479–485. doi: 10.1161/STROKEAHA.113.003004. [DOI] [PubMed] [Google Scholar]
- 10.Ren XM, Qiu SW, Liu RY, Wu WB, Xu Y, Zhou H. White matter lesions predict recurrent vascular events in patients with transient ischemic attacks. Chin Med J (Engl) 2018;131:130–136. doi: 10.4103/0366-6999.222341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 12.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 13.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 14.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian stroke prevention study. Neurology. 1999;53:132–139. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- 17.Godin O, Tzourio C, Maillard P, Mazoyer B, Dufouil C. Antihypertensive treatment and change in blood pressure are associated with the progression of white matter lesion volumes: the three-city (3C)-dijon magnetic resonance imaging study. Circulation. 2011;123:266–273. doi: 10.1161/CIRCULATIONAHA.110.961052. [DOI] [PubMed] [Google Scholar]
- 18.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (Version 2009) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheldon RS, Morillo CA, Krahn AD, O’Neill B, Thiruganasambandamoorthy V, Parkash R, Talajic M, Tu JV, Seifer C, Johnstone D, Leather R. Standardized approaches to the investigation of syncope: canadian cardiovascular society position paper. Can J Cardiol. 2011;27:246–253. doi: 10.1016/j.cjca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, Olshansky B, Raj SR, Sandhu RK, Sorajja D, Sun BC, Yancy CW. 2017 ACC/AHA/HRS Guideline for the evaluation and management of patients with syncope: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2017;136:e60–e122. doi: 10.1161/CIR.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 21.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 22.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESC/ESH guidelines for the management of arterial hypertension. the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) G Ital Cardiol (Rome) 2018;19(Suppl 1):3S–73S. doi: 10.1714/3026.30245. [DOI] [PubMed] [Google Scholar]
- 23.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 24.Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Höglinger GU. Accuracy of the national institute for neurological disorders and stroke/society for progressive supranuclear palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord. 2013;28:504–509. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- 25.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 26.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korczyn AD. Autonomic nervous system disturbances in Parkinson’s disease. Adv Neurol. 1990;53:463–468. [PubMed] [Google Scholar]
- 28.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. doi: 10.1002/j.1552-4604.1994.tb04977.x. [DOI] [PubMed] [Google Scholar]
- 29.Myers MG, Norris JW, Hachniski VC, Sole MJ. Plasma norepinephrine in stroke. Stroke. 1981;12:200–204. doi: 10.1161/01.str.12.2.200. [DOI] [PubMed] [Google Scholar]
- 30.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial doppler studies. Stroke. 2010;41:2697–2704. doi: 10.1161/STROKEAHA.110.594168. [DOI] [PubMed] [Google Scholar]
- 31.Korpelainen JT, Sotaniemi KA, Suominen K, Tolonen U, Myllylä VV. Cardiovascular autonomic reflexes in brain infarction. Stroke. 1994;25:787–792. doi: 10.1161/01.str.25.4.787. [DOI] [PubMed] [Google Scholar]
- 32.McLaren A, Kerr S, Allan L, Steen IN, Ballard C, Allen J, Murray A, Kenny RA. Autonomic function is impaired in elderly stroke survivors. Stroke. 2005;36:1026–1030. doi: 10.1161/01.STR.0000160748.88374.ce. [DOI] [PubMed] [Google Scholar]
- 33.Roumie CL, Zillich AJ, Bravata DM, Jaynes HA, Myers LJ, Yoder J, Cheng EM. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46:465–470. doi: 10.1161/STROKEAHA.114.007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, Mattheus M, Woerle HJ, Broedl UC, Johansen OE, Albers GW, Diener HC. Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017;48:1218–1225. doi: 10.1161/STROKEAHA.116.015756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42:136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- 36.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952–957. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 37.Mader SL. Aging and postural hypotension. An update. J Am Geriatr Soc. 1989;37:129–137. doi: 10.1111/j.1532-5415.1989.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 38.Kwon HS, Lim YH, Kim HY, Kim HT, Kwon HM, Lim JS, Lee YJ, Kim JY, Kim YS. Association of ambulatory blood pressure and heart rate with advanced white matter lesions in ischemic stroke patients. Am J Hypertens. 2014;27:177–183. doi: 10.1093/ajh/hpt199. [DOI] [PubMed] [Google Scholar]
- 39.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Frewen J, Finucane C, Savva GM, Boyle G, Kenny RA. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J Gerontol A Biol Sci Med Sci. 2014;69:878–885. doi: 10.1093/gerona/glt171. [DOI] [PubMed] [Google Scholar]
- 41.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspar L, Kruzliak P, Komornikova A, Celecova Z, Krahulec B, Balaz D, Sabaka P, Caprnda M, Kucera M, Rodrigo L, Uehara Y, Dukat A. Orthostatic hypotension in diabetic patients-10-year follow-up study. J Diabetes Complications. 2016;30:67–71. doi: 10.1016/j.jdiacomp.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Simó R. Neurodegeneration as an early event in diabetic retinopathy. Endocrinol Nutr. 2011;58:211–213. doi: 10.1016/j.endonu.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 44.James MA, Potter JF. Orthostatic blood pressure changes and arterial baroreflex sensitivity in elderly subjects. Age Ageing. 1999;28:522–530. doi: 10.1093/ageing/28.6.522. [DOI] [PubMed] [Google Scholar]
- 45.Harrington F, Murray A, Ford GA. Relationship of baroreflex sensitivity and blood pressure in an older population. J Hypertens. 2000;18:1629–1633. doi: 10.1097/00004872-200018110-00014. [DOI] [PubMed] [Google Scholar]
- 46.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: a model system for studying vascular inflammation in diabetes and atherosclerosis. Curr Diab Rep. 2011;11:193–202. doi: 10.1007/s11892-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 48.Uchiyama T, Sakakibara R, Asahina M, Yamanishi T, Hattori T. Post-micturitional hypotension in patients with multiple system atrophy. J Neurol Neurosurg Psychiatry. 2005;76:186–190. doi: 10.1136/jnnp.2004.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenning GK, Scherfler C, Granata R, Bösch S, Verny M, Chaudhuri KR, Jellinger K, Poewe W, Litvan I. Time course of symptomatic orthostatic hypotension and urinary incontinence in patients with postmortem confirmed Parkinsonian syndromes: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1999;67:620–623. doi: 10.1136/jnnp.67.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical data involved in this paper were from the clinical databases of Beijing Tiantan Hospital and Beijing Chaoyang Hospital. Others who want to share information must first get approval from the two hospitals and the authors. This study was not involved in any industry-sponsored research and corporate activities.



