Abstract
Mesenchymal stem cells (MSC) are promising candidates to combat the growing rates of chronic degenerative diseases. These cells provide regeneration and/or differentiation into other cell types, and secrete various trophic factors that participate in migration, proliferation, and immunomodulation. However, the novelty of MSC research has noticeably declined as common barriers and unresolved challenges prevent further progress. A common issue is the low survivability and migration of systemically infused MSC towards targeted regions. Nevertheless, successful clinical treatment of various chronic diseases suggests that the MSCs may have an alternative mechanism. Recent advancements have shown labelling and imaging techniques to be a reliable source of data. These data not only illustrate the biodistribution but can be referenced to either support and/or improve the specificities of the cellular therapy construct. In this review, we compile recent studies between 2017 and 2021 to determine the homing and migration of MSCs by specific and peripherally-targeted organs. We also compare the different cell-tracking assays with the safety and efficacy of their therapeutic construct. We found that the common route of MSCs occurred in the lungs, liver, kidney and spleen. Furthermore, MSCs were also able to home and migrate towards targeted or injured organs such as the heart and lymph nodes. Although the MSCs were not detectable by the end of the study, the tested animals had significantly improved in terms of the disease symptoms and their related comorbidities. Thus, we hypothesize that the secretion of exosomes had contributed to this phenomenon.
Keywords: Mesenchymal stem/stromal cells, biodistribution, imaging, cell tracking, pharmacokinetics, in vivo
Introduction
The emergence of cell-based therapies brought many novelties and opportunities into the field of regenerative medicine. Among them, the applications of mesenchymal stem cells (MSC) have been triumphant. Although stem cells remain controversial due to the deliberate manipulation of a living organism, they produce a highly regenerative effect [1]. Furthermore, these cells are known to be immune-privileged with the extended function of modulating the recipient’s immunity. The cells act as a direct replacement for dead or irrecoverable cells through their large differentiation capacities [2,3]. MSCs have an innate affinity towards adipogenic, chondrogenic, and osteogenic differentiation. However, these cells can transdifferentiate into other cell niches (myocyte, fibroblasts, neurons) depending on local cues from endogenously targeted cells [4]. MSCs also release a concentrated secretion of their functional metabolites. Previously thought of as waste products, these secretory vesicles are an enriched body of proteins, genes, and other useful materials. They partake in metabolic activities, recruitment of immune bodies, cell cycles, apoptosis, angiogenesis and more [5-7]. The MSCs have been applied to various chronic and degenerative diseases. Among them were cardiovascular complications (e.g., stroke), chronic kidney disease, liver abnormalities (e.g., NASH), osteo-degeneration, and cancers [8,9].
In recent years, there have been shortcomings in the novelty of research efforts. This indicated the closure of possible adaptations and evolutionary studies of MSC, and yet, challenges and barriers for clinical translation remained [10,11]. In retrospect, most data utilized for clinical trials rely on rudimentary tests such as toxicology or the safety and efficacy of their medical products [12]. As more complex experimental drugs and advanced configurations surface, future progress would demand laborious examinations and/or specific evidence. However, stem cell therapy remains an innovative and flexible technique and the continuous developments in the field have suggested exceptional therapeutic possibilities. The topic of methods to introduce drugs or cells has been strongly debated. While the most convenient route of administration is the systemic or indirect route, there are several challenges that suggest otherwise.
The basic search keywords were derived from medical subject headings (MESH) from the vocabulary thesaurus of PUBMED/MEDLINE. An advanced search and relevant modifications for common words and terms associated with each base keyword were added. SCOPUS, PUBMED and Web of Science (WOS) was selected from the available access provided by the Faculty of Medicine, National University of Malaysia. The search was filtered for “research articles” or “journal articles” published within 5 years (2017-2021). All results from each database were downloaded as bibliographies containing titles, keywords, and abstracts for each article. The bibliography files were labelled appropriately as the source, date of access and results (i.e., PUBMED_210721_196 results). Bibliographies were uploaded and viewed using citation software, Mendeley. Bibliographies were uploaded into individual folders and combined separately. The 100% matching duplicates were automatically merged by the software but a manual merging of duplicates was also performed. The first screen of articles was performed on title, abstract, and keywords that were appropriately matched to the topic of interest. The second screening involved the retrieval of full-text research articles. The selection and removal were performed following the inclusion and exclusion criteria prepared during the concept and design of the study. Inclusion criteria: (i) biodistribution, migration, or homing, (ii) MSC therapy, (iii) systemic delivery route and (iv) controlled experimental studies. Exclusion criteria: (i) no biodistribution, migration or homing, (ii) differentiated MSC, (iii) direct or topical delivery route and (iv) uncontrolled experimental study. Methods and results were screened to ensure no false representation or absent data occurred in the articles. In Figure 1, we report a total of 646 records compiled from the three databases; PUBMED (196), SCOPUS (145), and WOS (305). A total duplication of 247 was merged or removed which left 399 individual research articles. The first screening process generated 54 candidate reports by title, abstracts, and keywords and removed 345 reports. The retrieved 54 reports were further screened and yielded 12 suitable reports and 42 excluded reports. Both reviewers individually screened the articles, discussed and achieved consensus after the final screen. The contents of the final accepted articles were extracted and tabulated in Tables 1, 2 and 3.
Figure 1.
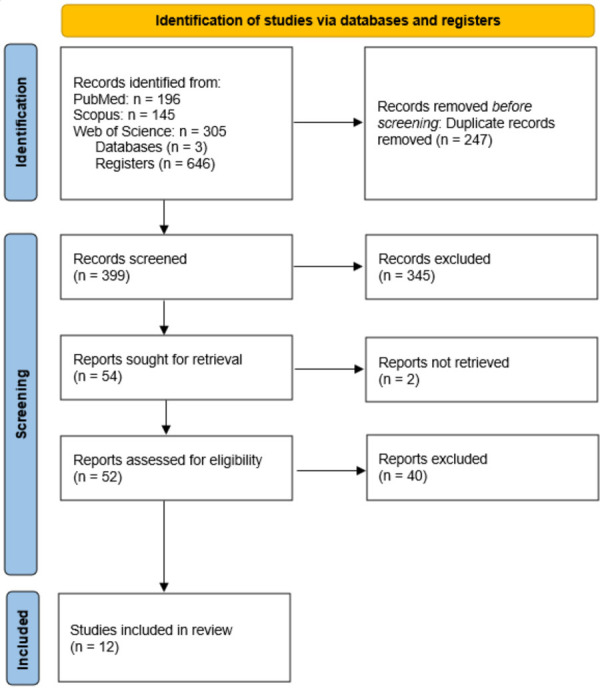
PRISMA flow diagram for systematic review.
Table 1.
Biodistribution pattern of MSCs in target and peripheral organs of reviewed studies
| Organ | Reference | Studies that detected MSCs |
|---|---|---|
| Targeted Organ | ||
| Heart | [19-23] | [19-23] |
| Lung | [24] | [24] |
| Central Nervous System | [22,23,26,28] | [26] |
| Brain | ||
| Spinal Cord | ||
| Kidney | [25] | [25] |
| Skin | [29] | - |
| Gingiva | [27] | - |
| Peripheral Organ | ||
| Lungs | [19-23,25,28,29] | [19-23,25,27-30] |
| Liver | [19,21-23,25,27,28] | [19,21-23,25,27,28] |
| Kidney | [19,20,22,23-25,27] | [19,20,22-24,27] |
| Spleen | [19-22,25,28,30] | [20,21,22,25,28,30] |
| Pancreas | [21,25] | [21] |
| Thymus | [24,25,28] | - |
| Lymph nodes | [19,22,25,28] | [19,22,25] |
| Mesenteric Lymph Nodes | ||
| Inguinal lymph nodes | ||
| Gut | [19,23,25] | [19] |
| Stomach | ||
| Small intestine | ||
| Colon | ||
| Bladder | [25] | - |
| Ovaries | [25] | - |
| Bone | [25] | - |
| Bone marrow | [23,27] | [23] |
| Blood | [19,22,25,29] | [19,22] |
(-) indicates no results.
Table 2.
Type and source of MSCs with cell tracing methods in animal models
| First Author and Year | Animal Model | Type and source of MSC | Cell tracking assays and organs involved | Biodistribution, homing and migration of MSC |
|---|---|---|---|---|
| Islamov et al. 2017 [26] | SOD-1 mice | Human UCBC | IF microscopy of Hoechst 33258-labeled UCBC and RT-PCR of transduced NCAM1, VEGF, and GDNF in lumbar spinal cord, liver, spleen | RT-PCR confirmed presence of UCBC after 5 days in the lumbar spinal cord. After 1 month, IF staining detected UCBC after euthanasia of animals. However, spleen and liver did not detect UCBC. |
| Liao et al. 2017 [19] | C57BL/6J mice and mountain goats | Goat and mouse BMSCs | IF microscopy of Hoechst 33342-stained BMSCs in lung, heart, liver, kidney, spleen, colon and mesenteric lymph nodes. FCM of counterstained Hoechst+ BMSC in blood | The BMSCs were successfully detected but also showed improved engraftment after anticoagulant co-treatment as seen in the blood, colon, mesenteric lymph nodes, liver, and heart but decreasedconcentrations in lungs of the latter group. |
| Gaafar et al. 2017 [20] | Wistar rats | Human WJ-MSC | IF microscopy of PKH-26 fluorescent-labeled MSCs in the lung, heart, kidney and spleen. | WJ-MSC were found primarily located in the ischemic myocardium while minor concentrations were detected in lungs, kidney and spleen. |
| Van Linthout et al. 2017 [21] | C57BL6/J mice | PMSC | RT-PCR of Alu specific primers with fluorescent probe in left ventricle, lung, liver, spleen and pancreas | Engraftment of PMSC was greater in the diabetic group compared to controls as detected in the left ventricle (4.5-fold, P<0.005), lung (19-fold, P<0.005), kidney (47-fold, P<0.05) and spleen (2.4-fold, P=0.1694) |
| Fabian et al. 2017 [22] | APP/PS1 mice model | Young and aged mice syngeneic BMSC | IF microscopy of GFP-labelled cells and RT-PCR of Y-chromosome specific primers in brain, peripheral organs (lung, heart, liver, kidney, lymph nodes, spleen, bone marrow) and blood. | Young MSCs were detected in the lung, axillary lymph nodes, blood, kidney, bone marrow, spleen, liver, heart, and brain of young, aged, and APP-PS1 mice. However, the aged MSC were not detected in all three mice models. |
| Tan et al. 2018 [28] | F344/NSIc rats | F344/NSIc rat BMSCs | PET/CT imaging and IHC of EdU-labelled BMSCs with Hoechst 33342 in rat brain, spleen, thymus, and lymph nodes | After 12 hr post-transplantation, much detection of BMSC was found in the lung and some were distributed in the spleen and liver. Conversely, cells were undetected in the brain parenchyma throughout the study. |
| In control and treatment groups, the PET image showed high SUV around the ischemic area after 3 days. SUV further increased after 10 days but was significantly inhibited in the BMSC group. | ||||
| Gallagher et al. 2019 [30] | NOD/SCID mice | Human first-trimester and term-UCMSC | IHC and FCM of brain, lungs and spleen. Whole-animal cryoimaging for Qtracker-625 nanocrystals-labelled UCMSC | Biodistribution analysis indicated the infused MSCs were distributed in lungs and spleen but not in the brain at 20 hours and 120 hours in both unstressed control and stressed mice. |
| Ueda et al. 2019 [27] | C57BL/6N mice | Green fluorescent protein (GFP)-transgenic C57BL/6N mice BMSC | IF microscopy of GFP-labelled BMSC in lung, kidney, liver, spleen and bone marrow. | After tail vein injection of MSC, they initially and mainly accumulated in the lungs and lesser amounts in kidney, liver, spleen and bone marrow. |
| Baer et al. 2020 [24] | ATM-deficient mice | Luciferase transgenic mice mASC/AMSC | Luc+ AMSC were tracked via BLI on days 1, 3, 6, and 9 and RT-PCR was conducted later as the signals were too low at the indicated endpoints (day 15 and 50) of the lung, kidney, and thymus. | BLI was able to detect increased retention of ASCs in the lungs. From day 15 to day 50, the ASCs decreased in the lungs by 50% but a minor increase was observed in the kidneys by 20%. No observable differences were apparent in the thymus throughout the experiment. |
| Kosaric et al. 2020 [29] | BALB/CJ mice | Human BMSC | BLI of Luc+GFP+ BMSC in mouse. FCM and RT-PCR in lung, spleen, blood, and wound area of mice | BLI images revealed localization of the BMSCs in the lungs which significantly decreased during 48 hours and undetected 3 days and thereafter. Similarly, FCM shows similar outcomes with BLI. RT-PCR confirmed presence of BMSC entrapped in the lungs but also expression of intrinsic therapeutic proteins. |
| Levy et al. 2021 [23] | C57BL/6 mice model | Human MSCs | FCM of CFSE-labelled MSC in brain, spinal cord, kidneys, lungs, spleen, gut, and heart) | MSCs were detected in the lung, liver, heart, and kidney, but not the brain. It was noted that the bio-distribution was not factored by the incorporation of Ro-31-8425 drug into MSCs since the same distribution was observed in controlled MSCs. |
| Yudintceva et al. 2021 [25] | Chinchilla rabbits | Rabbit BMSC | BLI, NLR-M2 and IHC of SPION-labelled MSC in kidney, lungs, liver, spleen, paratracheal lymph nodes, heart, brain, pancreas, stomach, small intestine, colon, bladder, femur, ovaries, inguinal lymph nodes, and blood | Following the IV administration of the MSCs, there was a large accumulation in the lungs during the first 72 hours and a shorter retention in the liver and spleen. The NLR-M2 measurements was able to confirm the homing properties of MSC to Mtb-affected kidneys and paratracheal lymph nodes. Subsequent histologic analysis also confirmed the presence of nanoparticle-labeled MSCs in the lung parenchyma, liver, spleen, and kidneys. |
*Abbreviations: Luc, Luciferase; GFP, Green Fluorescent Protein; CFSE, Carboxyfluorescein Succinimidyl Ester; SPION, Superparamagnetic Iron Oxide Nanoparticles; IF, Immunofluorescence; BLI, Bioluminescence Imaging; IHC, Immunohistochemistry; FCM, Flow Cytometry; RT-PCR, Reverse-Transcription Polymerase Chain Reaction; NLR-M2, Nonlinear Longitudinal Magnetic Response; PET, Positron Emission Tomography; ASC, Adipose-Derived Mesenchymal Stem Cells; BMSC, Bone Marrow Mesenchymal Stem Cells; PMSC, Placenta-Derived Mesenchymal Stem Cells; UCMSC, umbilical cord mesenchymal stem cells; UCBSC, Umbilical Cord Blood Stem Cells; WJ-MSC, Wharton’s Jelly Mesenchymal Stem Cell.
Table 3.
Disease and therapeutic outcomes of traced MSC in animal model
| First Author and Year | Disease | Therapeutic outcomes of MSC therapy |
|---|---|---|
| Islamov et al. 2017 [26] | ALS | Successful detection of UCBC in the lumbar spinal cord highlights the survival and homing ability of cells into the CNS. The animal model showed significant improvements in the physiologic and neurologic functions. |
| Liao et al. 2017 [19] | Experimental colitis | Heparin-induced BMSCs (400 U/kg) displayed lower coagulation rate and greater penetration through the lung capillary network with subsequent distribution to other organs. In the experimental colitis model, authors confirmed reduced weight loss, inflammation, tissue injury and mortality. |
| Gaafar et al. 2017 [20] | Myocardial infarction | WJ-MSC positively affected the cardiac markers and had differentiated into cardiomyocytes in vivo. This suggests that the cardioprotective function of the WJ-MSC may serve as therapeutic strategy. |
| Van Linthout et al. 2017 [21] | Cardiomyopathy related Diabetes Mellitus | The systemic infusion of PMSC had cardioprotective effects inferenced by the improved diastolic pressure, cardiomyocyte stiffness, and inflammation. These benefits support the use of PMSC as therapy. |
| Fabian et al. 2017 [22] | Alzheimer’s Disease | The ageing of BMSC and mice or neuronal status of mice affects the biodistribution and therapy. Transplantation of the young BMSC in young mice greatly displaces the neuronal defects and still maintain regenerative properties in aged or APP/PS1 mice model. The data also suggest that aged MSC will not work as therapy. |
| Tan et al. 2018 [28] | Transient MCAO | In both vehicle and BMSC groups, [18F]DPA-714 PET showed a high standardized uptake value (SUV) around the ischemic area 3 days after MCAO. Although SUV was increased further 10 days after MCAO in both groups, the increase was inhibited in the BMSC group, significantly. Histologic analysis showed that an inflammatory reaction occurred in the lymphoid organs and brain after MCAO, which was suppressed in the BMSC group. |
| Gallagher et al. 2019 [30] | MDD | Although the authors did not successfully confirm the presence of MSC in the CNS, they were able to show improved behavioral conditions of the mice. They inferenced a similar mechanism from previous studies where the infused MSC were able to resolve the stress-induced inflammation and improve the cognitive conditions in the mice model. |
| Ueda et al. 2019 [27] | Wound socket from tooth extraction | Authors found that MSC administered with scaffold and subcutaneous injection did not accumulate in the lungs compared to systemic administration. However, they found that MSC-infused scaffold had better homing and wound healing properties with low risk of adverse effects in tooth extraction sockets. |
| Baer et al. 2020 [24] | A-T | Author states the efficacy/effects will be explored following this paper. Therefore, no therapeutic results were available to determine safety and efficacy of the cell therapy. |
| Kosaric et al. 2020 [29] | Excisional wounds | Similar to other studies, authors demonstrated that the effect of MSC infusion on tissue repair is significant, observing acceleration of time to closure using an excisional wound model, and show that the therapeutic effect of intravenous infusion is comparable to direct injection of hMSCs in the context of excisional wound healing. |
| Levy et al. 2021 [23] | Multiple sclerosis | The systemic administration of Ro-31-8425-loaded MSCs was able to better alleviate symptoms of EAE compared to control MSCs. Additionally, the serum levels of EAE mice show sustained drug levels which had immunomodulatory properties in response to the EAE. |
| Yudintceva et al. 2021 [25] | Renal tuberculosis | This study demonstrates the recruitment of intravenously administered MSCs to the Mtb-affected sites in a preclinical model of renal tuberculosis in rabbits that can be further explored for the development of novel anti-TB treatment approaches. Furthermore, the study also demonstrates a highly sensitive method of non-linear magnetic response measurements for a sensitive biodistribution analysis of SPIONs-labeled stem cells and for the tracing of their state transformation over a period of time into different organs by the change of M2(H) dependencies. |
*Abbreviations: ALS, Amyotrophic Lateral Sclerosis; A-T, Ataxia-Telangiectasia; EAE, Experimental Autoimmune Encephalomyelitis; MCAO, Middle Cerebral Artery Occlusion; Mtb, Mycobacterium Tuberculosis; MDD, Major Depressive Disorder; NOD/SCID, Nonobese Diabetic/Severe Combined Immunodeficient; TB, Tuberculosis; GFP, Green Fluorescent Protein; CNS, Central Nervous System.
Challenges of systemic delivery vs. topical administration
Biodistribution is fundamentally used to identify the homing and migration properties of the therapeutic product. Usually, safety and efficacy studies are unable to directly associate their results due to unconfirmed status, position or engraftment of cells to the targeted site. The pharmacodynamic and pharmacokinetic aspects are mostly overlooked in cell therapies [13,14]. These are extremely vital concepts addressing the initial response from biodistribution (or circulation), migration, and homing abilities of cells. Furthermore, it analyzes the modalities of molecular and chemical interactions that determine the efficacy of the cell therapy model. The primary factor that defines the overall route of medical products is the method of administration. While a large majority of studies prefer the systemic route due to convenience, its limitations and challenges can contribute towards an inefficient delivery system.
Volatility of blood vessel system
The intravenous, intracardiac, or any vascular route carries the risk of ineffective delivery of drugs. Hence, drugs are often designed in larger doses to ensure ample particles reach the targeted site and exert an effect above the threshold. Furthermore, blood vessels may be positioned distally from organs or mechanistically selective to filter the diffusion of specific particles. Hence, it is not guaranteed that the therapeutic product may migrate in situ after successful circulation. The blood is a solvent for many proteins, chemicals, and genetic material but also hosts pathogens and toxins. In a diseased individual, the inflation of the latter could alter the physicochemical properties of the product. Additionally, the mechanical obstruction must also be considered since the blood vessels are a constantly mobile and pulsing entity [15,16]. Bloodstreams are created from the pumping of the heart and constriction of vascular walls. Together, they exert a force known as blood pressure. Thus, bloodstreams can easily exert physical forces or cause micro-collisions with circulating products onto the product. Otherwise, the surface contact could also disable a key protein’s recognition and function. Consequently, the theoretically-superior approach to minimize all mentioned factors is through the topical route. Direct administration limits the need for an expanded dose, providing only the accurate dose or volume as necessary. The risk of diffusing into non-target regions or misrouting, followed by unspecified changes can be avoided. The only downside of relying upon this method is the deliberate invasive procedure and the risk of induced cytotoxicity due to concentrated physiologic or chemical activity. These are common issues with most drug interventions. However, stem cell therapies seem to have a natural predisposition in overcoming the ordeals of systemic administration.
Despite that, the number of studies conducted on MSCs by 2018 registered at approximately 900 total studies [10,17]. Furthermore, 43% majority of these studies were administered systemically in vivo. This is because the MSCs were seen able to survive and migrate in harsh micro-environmental factors induced by chronic diseases. These may include hypoxia (insufficient oxygen supply), cytokine storms (inflammation, pH instability), hyper-immune activity (macrophagic and apoptotic activity), and more. While this is an uncommon approach, there were previous efforts to incorporate state-of-the-art live-cell imaging, but the unpredictability of MSCs have led to inconsistent results and poor simplification of its mechanisms [18].
Homing and migration properties of MSCS
In Table 1, we compiled and distinguished results between the targeted organs for the cell therapy from the peripheral organs included within each study. We categorized the results based on the organs observed and the presence of MSC after treatment. We found that the studies conducted on cardiac, renal, and pulmonary complications successfully detected the presence of MSCs in the heart [19-23], lungs [24] and kidneys [25]. Conversely, only one study was able to locate MSCs in the CNS while the rest did not report any significant outcomes [26]. Additionally, there were no MSCs detected in the site of the skin and gingiva wound model of mice [27]. In the peripheral organs observed, nine of the 12 selected studies observed the biodistribution of MSCs in the lungs [19-23,25,27-29] and one study did not conduct a specific analysis of the lungs but observed through the whole-animal cryo-imaging [22]. We confirmed that all ten individual studies found a significant volume of MSC in the lungs across multiple various periods, as early as hours into treatment to weeks after [19-23,25,27-30]. In several instances, the number of MSCs had decreased but still maintained sufficient levels for detection.
Common biodistribution of systemically infused MSC
Overall, the biodistribution of intravenously administered MSC has remained unchanged as previously reported [15,31]. We report that the course of migration for MSCs appears most frequent to the metabolically active organs of lungs, spleen and kidneys. We identified that the MSC may also occur in the extension of those organs such as the heart, liver and lymph nodes in response to inflammatory markers. For targeted studies such as the CNS, the MSCs were not successfully engrafted [22,23,28] as depicted in Figure 2. This has been a common theme and limitation of MSC since the brain is enclosed by the blood-brain barrier (BBB) which tightly prevents the influx of most blood-diffused materials [32]. In most designs, the endothelial barrier is mechanistically homogeneous, so that large matter such as cells are not permitted any passage. Therefore, the peripheral organs such as the pancreas [21], gut [19], and bone marrow [23,27] have less frequent detection from biodistribution analysis of MSC, whereas the gingiva [27], skin [29] bladder, ovaries and bone [25] did not show any significant outcomes.
Figure 2.
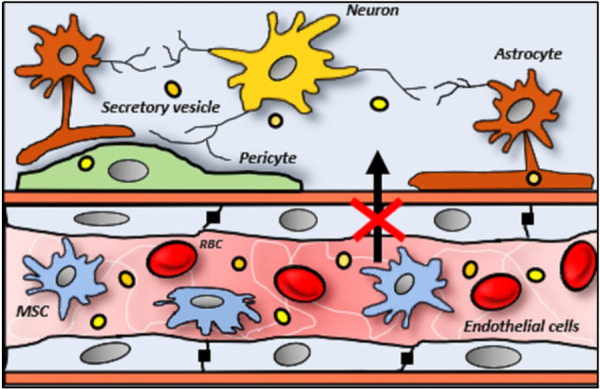
Inability of MSCs (irregular-shaped), like RBCs (red discs), to penetrate the selective endothelial (elongated-shaped) barriers of CNS. Conversely, the exosomes (yellow beads) are sufficiently small to be able to diffuse through and reach various nerve cells (dendritic-shaped).
MSC cannot efficiently penetrate lung capillaries
Following up on their 2012 exploration [33], Eggenhofer et al. (2014) [34] summarized that no method distinguishes between live or dead cells after redistribution of MSCs from the lungs towards various organs. In some cases, a small percentage of viable cells may leave the pulmonary capillaries. Saat et al. (2016) [35] later confirmed this hypothesis based on a similar murine model for ischemia-reperfusion injury of the liver. They further explained that the labelled luminescent particles could remain active despite the fragmentation of the MSCs, thus, inferencing a downstream detection of the signals in various organs or tissues of the body. However, that may not be the case from our observation in the targeted organs and further findings below. The positive signals in the heart also suggest that MSCs may operate through a gradient of migratory potential based on the proximity of organs to the lungs. Intravenous administration of MSCs has frequently led to embolized blood capillaries as seen in Figure 3 [36,37]. Cells en masse disrupt micro-circulation inciting ischemic injury, increased inflammation and downstream reperfusion injury. Furlani et al. [2009] traced the post-intravascular administered MSCs [38]. The authors also illustrated concentrated mass in the lungs similar to previous findings.
Figure 3.
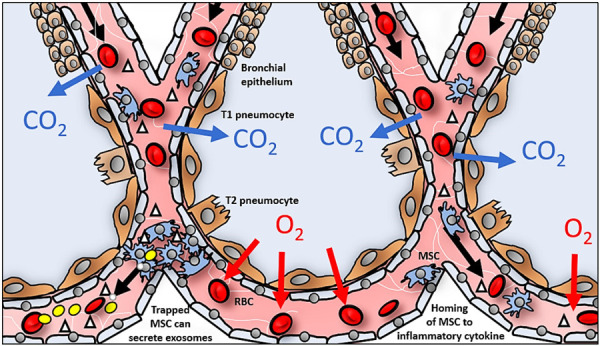
Migration and engraftment of MSCs is hindered by coagulation of MSCs in lung capillaries. Homing of MSCs through lung capillaries (right) through chemotaxis to source of inflammation or injury. Embolized MSCs in capillaries in the lungs (left) actively secrete exosomes (yellow) in response to accumulated cytokines.
Drainage of MSC into metabolic spaces such as liver and kidney
After the lungs, MSCs frequently present in the liver [19,21-23,25,27,28] and kidneys [19,20,22,23-25,27]. The liver requires a larger than average blood supply as the major metabolic organ of the body. Therefore, it often aligns with the larger circulatory pathway that may have led to the accumulation of circulated MSCs. As shown in Figure 4, MSCs have a natural affinity for chemotactic migration towards pro-inflammatory markers (i.e., IL-1β, IFN-γ and TNF-α) [39,40], that are secreted from damaged tissues, lymphocytes or macrophages. These cytokines are promptly sent to the liver for either functional purposes or neutralization and detoxification for removal.
Figure 4.
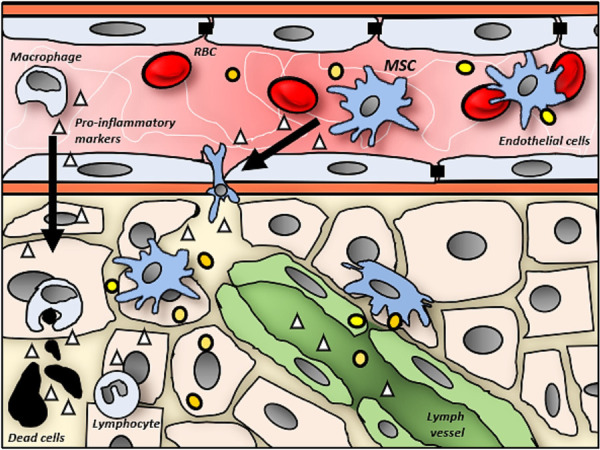
Upon death or injured of cells (black), local lymphocytes (irregular nucleus) secrete pro-inflammatory markers (triangle) to surrounding area. Diffusion of these cytokines into bloodstream with circulating RBC (red disc), attracts macrophages (irregular-shaped) and MSCs (spindle-shaped). The macrophages and MSCs attach and migrate across the endothelial cells (elongated-shaped) to reach the site of tissue damage. While the macrophages actively digest and remove the dead cells, MSCs secrete exosomes (yellow beads) to stimulate regeneration and reduce inflammation. Furthermore, the drainage of cytokines into the lymph vessels (green) could explain the detection of MSCs orderivatives in the lymphatic system.
The notion of MSCs in the renal space immediately suggests the end process or excretion from the body through the kidneys. However, the minimal-to-absent cellular concentration does not sufficiently justify its presence [19,20,22-24,27]. Likely, most degenerative diseases that resulted in the production of inflammatory cytokines would eventually drain into the kidneys with or without the liver’s intervention. In addition to their adhesive properties, the MSCs are substantially large and cannot be excreted through passive diffusion or filtration of the renal glomeruli. Otherwise, the cells and their derivatives would have been detected downstream in the bladder, which was not reported in the results of studies reported in this review [25].
Extravasation of MSC into spleen and lymph nodes
As the major medium of transportation, the blood carries the majority of MSC [19,22]. However, there are also MSCs found in the lymphatic system. Several studies had reported a significant volume of MSC detected in the spleen [20,21,22,25,28,30] and lymph [19,22,25]. The spleen is also known to have a large capacity for blood like the liver, as it qualitatively filters the healthy from old or damaged erythrocytes. These are removed by active immune cells concentrated in the lymph nodes or spleen. The lymph nodes act as sedimentary sites and a regulator of most lymphocytes. In this scenario, we hypothesize that the accumulation of MSC in the lymph nodes were more likely associated with increased inflammatory particles from tissue damage, as opposed to the spleen that serves as a common blood circulatory route. This was confirmed when the control group reported a comparable level of MSCs in the spleen [25] but the same was not determined in the lymph nodes [19]. Thejaswi et al. [2012] proved that allogeneic infusion of MSCs reduced immune activity in lymph nodes and spleen of immunocompetent BALB/c mice model. Moreover, their in-vitro analysis described that the post-differentiated MSCs still managed to suppress lymphocytic activities (downregulated TNF-α, IL-1α and IL-2) of co-cultured PBMCs. Conversely, the absence of MSC in the thymus is very apparent [25,28] and is perhaps due to its physiological niche. The thymus is a major organ mediating its role in the endocrinologic system and lymphatic system. This gland can function independently of the lymph circulation and only acts as a one-way supplier of hormones responsible for priming or regulating T-cells.
Methods of tracing MSC biodistribution in animal model
In Table 2, we briefly list the type and source of MSCs with the different methods of labelling and/or pre-treatment of MSC and the subsequent in-vivo live-cell imaging and/or cell-tracking assays employed in reviewed studies. There were three commonly applied methods in this review, namely the direct labelling of the MSC [19,20,23,28,30], transduction and/or detection of cell-specific DNA markers in donor cells [21,22,25,30] and isolation of cells from transgenic animal models (i.e., GFP) [22,25,27,29]. The whole animal observation conducted in reviewed studies were through bioluminescent imaging (BLI) [24,25,29] and nonlinear longitudinal magnetic response (NLR-M2) [24]. Conversely, the most common ex-vivo assays performed on organs/tissue/blood of euthanized animals are in order of, immunofluorescent (IF) staining [19,20,26,27], immunohistochemistry (IHC) [22,25,28,30], reverse-transcription polymerase chain reaction (RT-PCR) [21,22,24,26,29] and flow cytometry (FCM) [19,23,29,30]. Less common practices include positron emission tomography (PET/CT) scan [28] and whole-animal cryo-imaging [30] which were present in our review.
Sensitivity of biomarkers and/or tests for biodistribution
The act of migration calls forth complex interactions between the medical product and the host’s system through chemoattractant and adhesive molecules (ICAM-1, VCAM-1) [39,40]. The recipient body is responsible for resisting the invasion of foreign bodies, which makes it difficult for medicinal products to penetrate. Therefore, it is necessary for researchers to examine and identify a suitable micro-construct of their therapeutic product that is biocompatible in both healthy and distressed hosts. Additionally, the internal conditions of a live organism vary drastically from an in-vitro simulation and may subject endogenous MSC to stress which translates to either adaptation or death of cells. This is where visualization and identification of the medicinal products in-vivo become a crucial determinant.
The ligation of fluorescent molecules on cells is considered a traditional method for biodistribution studies. This can be achieved through direct labelling of the MSC [19,20,23,28,30] or isolating cells from transgenic animal models (i.e., GFP) [22,27]. We report that the former method is practiced most often but carries several limitations in today’s application. This route cannot be followed up with live-cell imaging, and more often, diluted signals and false positives can occur due to cell division or cell death [31]. Although radioactive labelling [27] and nanoparticles [25] offer a solution, the safety, design and stability of these molecules require greater consideration towards induced in-vivo cytotoxicity. In overcoming both limitations, transduction and/or detection of cell-specific DNA markers in donor cells [21,22,25,30] or cells from a transgenic model [25,29] are becoming more applicable due to the continuous propagation of internalized signals in new cells. Furthermore, the addition of the ‘proofreading’ function during DNA replication preserves the stability and accuracy of the genetic markers [29].
The practice of in-vivo imaging of the cells in a live model enables a better understanding of the interaction and mobility of MSC through various anatomical structures [24,40]. Bioluminescent imaging (BLI) [24,25,29] and with the nonlinear longitudinal magnetic response (NLR-M2) [24] are some examples for observing animals as a whole. This method allows continuous survival which enables chronic evaluation and preservation of minimally conducted sample size. Even though the availability to conduct these tests is strictly dependent on the resources and access to equipment, we encourage the integration of higher sensitivity analysis (i.e., RT-PCR), taking into account signals (i.e., BLI) that diminishes in a short amount of time [24].
Therapeutic effect of MSC therapy despite limitations
In Table 3, we list the diseases and therapeutic outcomes from the MSC therapy employed in reviewed studies. All studies had reported that the MSCs recovered lost functions, controlled inflammation, and prevent further degeneration in animal model. Thus, the MSC was shown to be versatile as it was able to enhance the survivability of animals with neurological complications [22,24,26,38,30], open wound [27,29], induced-colitis [19], renal damage [21,25], and cardiac impairment [20,21]. However, only one study did not observe the therapeutic outcome which was conducted in the ataxia-telangiectasia (A-T) animal model [24].
Mirror of MSC: exosomes to overcome niche barriers
Although we find that the infused MSC did not reach most organs or targeted organs, the animals in reviewed studies have exhibited significant recovery and return of function as tabulated in Table 3. Hence, we propose a second narrative for the mechanism of MSCs in this review through secretion of proliferative and anti-inflammatory cytokines, that were produced exogenously but function at the site of tissue damage. Arguably, the regenerative capacity of MSC is more often referenced to the extracellular secretions (hereafter referred to as exosomes) in lieu of the whole cell as the therapeutic unit [42]. Therefore, MSCs are better encapsulated as both a vehicle and generator of the medicinal products which can have intracellular (autocrine) or intercellular (proximal/paracrine or distal/exocrine) functions [43,44]. It is highly likely that the MSCs that successfully penetrate the lungs would have a greater reach and more direct effect on the wound or injury. A heparin pre-treatment of cultured MSC by Liao et al. in 2017 [19] confirmed greater penetration and biodistribution of MSC which eventually reduced mortality of their experimental colitis mice model. Consequently, we hypothesized the positive outcomes attributed by infused MSCs not found in situ, would have likely been an effect of exosomes [45].
Exosomes carry an abundance of material, as a care package of various functional proteins and genetic instructions (Figure 5). Often, they are an external manifestation of their parent or derived cell [46]. Interestingly, these nano-sized particles exist and are secreted by all types of cells. For example, the tumor-derived exosomes have been shown to have an abnormally high concentration of anti-apoptotic, immuno-suppression and growth factors [47,48]. Other isoforms such as natural killer (NK) cell-derived exosomes are able to elicit a greater immune response from cells that are under-responsive or impaired against infections. The NK-derived exosome boasts anti-tumor potential by delivering and further stimulating key apoptotic factors (i.e., PD-1, FasL, etc) and supplying cytotoxic proteins (i.e., perforin, granzyme, etc) [49,50]. Zhu et al. (2018) [51] was able to demonstrate the suppression of glioblastoma by NK-derived exosomes in their in-vitro and in-vivo mice model. In the case of MSCs, we find growth and immunomodulatory factors which can safely recapture the optimal homeostatic conditions and recover loss functions [52-54]. Therefore, we propose the simultaneous incorporation of the listed or novel imaging techniques, to confirm the role of endogenous exosomes materialized in the scenario of coagulated MSCs in lungs.
Figure 5.
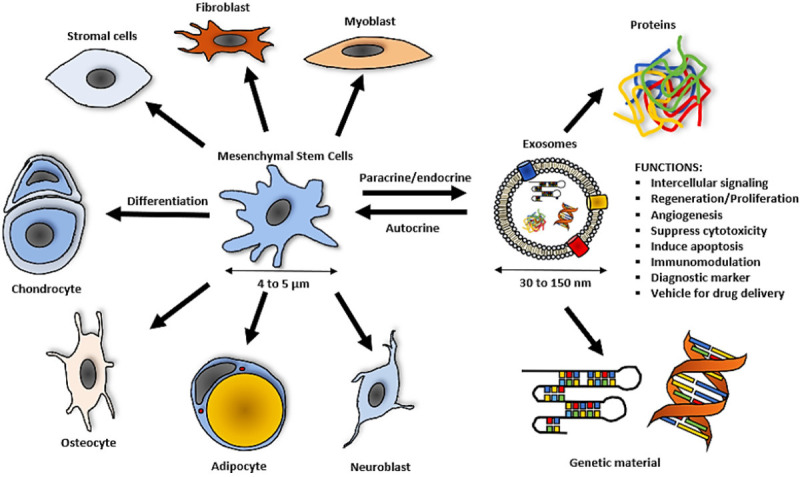
Functions of exosomes and size comparison to MSCs.
While most stem cells efforts are consistently applied in the clinical scene to accomplish medical board approvals for practical use, exosomes present an exciting opportunity for cell-free therapeutic agents. Owing to the compact size of exosomes (30-150 nm), this can open avenues for regenerative medicine in the BBB, CNS or other related barriers [55-57]. Albeit, exosomes closely resemble chemical drugs and the production or sourcing from live cells presents an even greater challenge. Incidentally, exosomes as a secondary product of cell/tissue cultures will demand further optimization of protocols and more stringent qualitative tests to ensure uniform therapeutic characteristics and to divert from any risks of adverse/side effects [58,59]. The achieved model must also be reasonably cost-effective and efficient in maximizing valuable tissue samples [60].
Challenge of defining exosomes and developing an optimized protocol
However, these ‘exosomes’ are not without consequences and challenges. Compared to the MSCs, these secreted factors are much more complex and heterogeneous in nature [58,61]. Not to mention, its size complicates quantitative and qualitative solutions. In 2020, a systematic review by Tieu et al. (2020) compiled the exploration of MSC-derived extracellular vesicles (EV) in various pre-clinical models [62]. At the end, the authors highlighted the prospects, challenges and limitations to be addressed. They urge to improve pre-clinical study designs and optimize manufacturing protocols for rapidly growing medical innovation. In the 206 studies, 60% obeyed the size-inclusion factor of exosomes. The remaining studies determine the identity of EVs isolation techniques like protein markers, morphology, and others. For isolation techniques, the majority (70%) performed ultracentrifugation or (23%) through isolation kits. Each was noted for differences in EV yield and purity [63]. Characterization by size-exclusion, antibody recognition and morphology are diverse, thus preventing accurate and systematic comparison. To further complicate matters, the range of its content is also not established since an unknown volume or concentration could exist, with the interference of other cell-free products. This single variability mixed with the unpredictability of the MSCs, complicates the process to establish a set of effective and translatable doses [64]. Ultimately, the identity, purity, quality, safety and functionality of exosomes are still unknown [65]. Similar to the beginnings of MSC exploration, the perception of exosome studies seems overwhelmingly positive although knowledge gaps and barriers remain unanswered. These will be critical challenges to be addressed because the challenges of exosomes are further added onto the pre-existing challenges of cell manufacturing.
Future considerations
In this review, we did not address the different MSC sources and their subsequent infusion into animals. Although, all MSCs do share a similar characterization profile as standardized by the International Society for Cellular Therapy (ISCT) [1] by expression of positive markers (CD73, CD90 and CD105), and negative markers (CD11b or CD14, CD19 or CD79a, CD34, CD45 and HLA-DR) [2]. Note that MSCs from different sources do not exhibit the same physiological properties. For example, WJ-MSCs have an age-independent proliferation when compared to BMSCs. The human MSCs are also physically larger than rodent MSCs which could be the reason for lung embolization in the mice model. Secondly, we did not discuss the effects of syngeneic, allogeneic, or xenogeneic infusion of MSC into animal models. However, MSCs have long-established their innate immunocompatibility to host recipients and have not illicit any harm or severe illnesses [66]. Fortunately, this review mainly consisted of syngeneic MSC [19,22,24,25,27,28] and the xenogeneic or pre-clinical models were based on human-derived MSC [20,21,23,26,29,30] for the purpose of clinical and translational medicine. Lastly, we did not compare our results to the topical/direct route of MSC. Perhaps this administration may have better efficacy in confined spaces with access to protected organs compared to the systemic route.
Conclusions
We report that the common biodistribution of systemically infused MSC occurs most frequently in the lungs, liver, spleen, and kidneys. The MSCs can also migrate towards the heart and lymph nodes in response to inflammation. Meanwhile, MSC are less frequent in the blood, gut, bone marrow, pancreas and lumbar spinal cord. In any case, the external detection and/or identification of MSC outside the pulmonary space were not necessarily live cells. The incorporation of greater sensitivity or specific imaging for viable cells will help to solve this conundrum. Lastly, we hypothesize that the therapeutic success of undetected MSC towards targeted regions may be an effect of released exosomes.
Acknowledgements
All authors have read and approved the final manuscript. This work was supported by grants provided by University Kebangsaan Malaysia (DPK-2021-006 & FF-2020-469/1). The granting agencies played no role in activities pertaining to the execution of the study and the submission of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Fekete N, Rojewski MT, Fürst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7:e43255. doi: 10.1371/journal.pone.0043255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14:145. doi: 10.1186/s12967-016-0892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan MNFB, Yazid MD, Yunus MHM, Chowdhury SR, Lokanathan Y, Idrus RBH, Ng AMH, Law JX. Large-scale expansion of human mesenchymal stem cells. Stem Cells Int. 2020;2020:9529465. doi: 10.1155/2020/9529465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liau LL, Al-Masawa ME, Koh B, Looi QH, Foo JB, Lee SH, Cheah FC, Law JX. The potential of mesenchymal stromal cell as therapy in neonatal diseases. Front Pediatr. 2020;8:591693. doi: 10.3389/fped.2020.591693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looi QH, Eng SP, Liau LL, Tor YS, Bajuri MY, Ng MH, Law JX. Mesenchymal stem cell therapy for sports injuries - from research to clinical practice. Sains Malaysiana. 2020;49:825–838. [Google Scholar]
- 8.Gu LH, Zhang TT, Li Y, Yan HJ, Qi H, Li FR. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell Mol Immunol. 2015;12:444–55. doi: 10.1038/cmi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocini D, Colivicchi F, Santini M. Stem cell therapy for cardiac arrhythmias. Ital Heart J. 2005;6:267–71. [PubMed] [Google Scholar]
- 10.Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9:17–27. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planat-Benard V, Varin A, Casteilla L. MSCs and inflammatory cells crosstalk in regenerative medicine: concerted actions for optimized resolution driven by energy metabolism. Front Immunol. 2021;12:626755. doi: 10.3389/fimmu.2021.626755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks A, Futrega K, Liang X, Hu X, Liu X, Crawford DHG, Doran MR, Roberts MS, Wang H. Concise review: quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl Med. 2018;7:78–86. doi: 10.1002/sctm.17-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvadori M, Cesari N, Murgia A, Puccini P, Riccardi B, Dominici M. Dissecting the pharmacodynamics and pharmacokinetics of MSCs to overcome limitations in their clinical translation. Mol Ther Methods Clin Dev. 2019;14:1–15. doi: 10.1016/j.omtm.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi D, Lee H, Kim H, Yang M, Heo J, Won Y, Jang S, Park JK, Son Y, Oh T, Lee E, Hong J. Cytoprotective self-assembled RGD peptide nanofilms for surface modification of viable mesenchymal stem cells. Chem Mater. 2017;29:5. [Google Scholar]
- 17.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:652034. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao L, Shi B, Chang H, Su X, Zhang L, Bi C, Shuai Y, Du X, Deng Z, Jin Y. Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7:106–116. doi: 10.7150/thno.16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaafar T, Attia W, Mahmoud S, Sabry D, Aziz OA, Rasheed D, Hamza H. Cardioprotective effects of wharton jelly derived mesenchymal stem cell transplantation in a rodent model of myocardial injury. Int J Stem Cells. 2017;10:48–59. doi: 10.15283/ijsc16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Linthout S, Hamdani N, Miteva K, Koschel A, Muller I, Pinzur L, Aberman Z, Pappritz K, Linke WA, Tschope C. Placenta-derived adherent stromal cells improve diabetes mellitus-associated left ventricular diastolic performance. Stem Cells Transl Med. 2017;6:2135–2145. doi: 10.1002/sctm.17-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabian C, Naaldijk Y, Leovsky C, Johnson AA, Rudolph L, Jaegar C, Arnold K, Stolzing A. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res Ther. 2017;8:85. doi: 10.1186/s13287-017-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy O, Rothhammer V, Mascanfroni I, Tong Z, Kuai R, De Biasio M, Wang Q, Majid T, Perrault C, Yeste A, Kenison JE, Safaee H, Musabeyezu J, Heinelt M, Milton Y, Kuang H, Lan H, Siders W, Multon MC, Rothblatt J, Massadeh S, Alaamery M, Alhasan AH, Quintana FJ, Karp JM. A cell-based drug delivery platform for treating central nervous system inflammation. J Mol Med (Berl) 2021;99:663–671. doi: 10.1007/s00109-020-02003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baer PC, Sann J, Duecker RP, Ullrich E, Geiger H, Bader P, Zielen S, Schubert R. Tracking of infused mesenchymal stem cells in injured pulmonary tissue in atm-deficient mice. Cells. 2020;9:1444. doi: 10.3390/cells9061444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yudintceva N, Mikhailova N, Bobkov D, Yakovleva L, Nikolaev B, Krasavina D, Muraviov A, Vinogradova T, Yablonskiy P, Samusenko I, Ryzhov V, Deriglazov V, Marchenko Y, Multhoff G, Klapproth AP, Li WB, Nayak B, Sonawane A, Shevtsov M. Evaluation of the biodistribution of mesenchymal stem cells in a pre-clinical renal tuberculosis model by non-linear magnetic response measurements. Front Phys. 2021:9. [Google Scholar]
- 26.Islamov RR, Rizvanov AA, Fedotova VY, Izmailov AA, Safiullov ZZ, Garanina EE, Salafutdinov II, Sokolov ME, Mukhamedyarov MA, Palotás A. Tandem delivery of multiple therapeutic genes using umbilical cord blood cells improves symptomatic outcomes in ALS. Mol Neurobiol. 2017;54:4756–4763. doi: 10.1007/s12035-016-0017-x. [DOI] [PubMed] [Google Scholar]
- 27.Ueda N, Atsuta I, Ayukawa Y, Yamaza T, Furuhashi A, Narimatsu I, Matsuura Y, Kondo R, Watanabe Y, Zhang X, Koyano K. Novel application method for mesenchymal stem cell therapy utilizing its attractant-responsive accumulation property. Applied Sciences. 2019;9:4908. [Google Scholar]
- 28.Tan C, Zhao S, Higashikawa K, Wang Z, Kawabori M, Abumiya T, Nakayama N, Kazumata K, Ukon N, Yasui H, Tamaki N, Kuge Y, Shichinohe H, Houkin K. [18F]DPA-714 PET imaging shows immunomodulatory effect of intravenous administration of bone marrow stromal cells after transient focal ischemia. EJNMMI Res. 2018;8:35. doi: 10.1186/s13550-018-0392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosaric N, Srifa W, Bonham CA, Kiwanuka H, Chen K, Kuehlmann BA, Maan ZN, Noishiki C, Porteus MH, Longaker MT, Gurtner GC. Macrophage subpopulation dynamics shift following intravenous infusion of mesenchymal stromal cells. Mol Ther. 2020;28:2007–2022. doi: 10.1016/j.ymthe.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher D, Siddiqui F, Fish J, Charlat M, Chaudry E, Moolla S, Gauthier-Fisher A, Librach C. Mesenchymal stromal cells modulate peripheral stress-induced innate immune activation indirectly limiting the emergence of neuroinflammation-driven depressive and anxiety-like behaviors. Biol Psychiatry. 2019;86:712–724. doi: 10.1016/j.biopsych.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Spaeth EL, Kidd S, Marini FC. Tracking inflammation-induced mobilization of mesenchymal stem cells. Methods Mol Biol. 2012;904:173–90. doi: 10.1007/978-1-61779-943-3_15. [DOI] [PubMed] [Google Scholar]
- 32.Sensebé L, Fleury-Cappellesso S. Biodistribution of mesenchymal stem/stromal cells in a preclinical setting. Stem Cells Int. 2013;2013:678063. doi: 10.1155/2013/678063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saat TC, van den Engel S, Bijman-Lachger W, Korevaar SS, Hoogduijn MJ, IJzermans JN, de Bruin RW. Fate and effect of intravenously infused mesenchymal stem cells in a mouse model of hepatic ischemia reperfusion injury and resection. Stem Cells Int. 2016;2016:5761487. doi: 10.1155/2016/5761487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, Janowski M, Walczak P, Boltze J, Lukomska B, Jolkkonen J. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–6. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Bębenek A, Ziuzia-Graczyk I. Fidelity of DNA replication - a matter of proofreading. Curr Genet. 2018;64:985–996. doi: 10.1007/s00294-018-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–8. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 41.Thejaswi K, Amarnath M, Gunda S, Jerald M, Raj TA, Singh S. Immune modulatory responses of mesenchymal stem cells from different sources in cultures and in vivo. Cell & Tissue Transplantation & Therapy. 2012;4:1–13. [Google Scholar]
- 42.Salari V, Mengoni F, Del Gallo F, Bertini G, Fabene PF. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: possible treatments and future perspectives. Int J Mol Sci. 2020;21:9683. doi: 10.3390/ijms21249683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12:71. doi: 10.1186/s13287-021-02138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araldi RP, D’Amelio F, Vigerelli H, de Melo TC, Kerkis I. Stem cell-derived exosomes as therapeutic approach for neurodegenerative disorders: from biology to biotechnology. Cells. 2020;9:2663. doi: 10.3390/cells9122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011;2011:842849. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Y, Luo X, Lv W, Hu W, Zhao C, Xiong M, Yi Y, Wang D, Wang Y, Wang H, Wu Y, Zhang Q. Tumor-derived exosomal components: the multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis. 2021;12:547. doi: 10.1038/s41419-021-03825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu L, Kalimuthu S, Gangadaran P, Oh JM, Lee HW, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7:2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Pace AL, Tumino N, Besi F, Alicata C, Conti LA, Munari E, Maggi E, Vacca P, Moretta L. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers (Basel) 2020;12:661. doi: 10.3390/cancers12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Novel alternatives to extracellular vesicle-based immunotherapy - exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 2018;46(Suppl 3):S166–S179. doi: 10.1080/21691401.2018.1489824. [DOI] [PubMed] [Google Scholar]
- 52.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 53.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, Tian X. mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2021;11:590470. doi: 10.3389/fphar.2020.590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elman JS, Murray RC, Wang F, Shen K, Gao S, Conway KE, Yarmush ML, Tannous BA, Weissleder R, Parekkadan B. Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells. PLoS One. 2014;9:e89882. doi: 10.1371/journal.pone.0089882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases. J Biol Chem. 2016;291:26589–26597. doi: 10.1074/jbc.R116.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan Q, Li XD, Zhang SM, Wang HW, Wang YL. Extracellular vesicles in neurodegenerative diseases: insights and new perspectives. Genes Dis. 2019;8:124–132. doi: 10.1016/j.gendis.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906. doi: 10.1016/j.jconrel.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J. Clinical applications for exosomes: are we there yet? Br J Pharmacol. 2021;178:2375–2392. doi: 10.1111/bph.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JH, Ha DH, Go HK, Youn J, Kim HK, Jin RC, Miller RB, Kim DH, Cho BS, Yi YW. Reproducible large-scale isolation of exosomes from adipose tissue-derived mesenchymal stem/stromal cells and their application in acute kidney injury. Int J Mol Sci. 2020;21:4774. doi: 10.3390/ijms21134774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.González-Cubero E, González-Fernández ML, Gutiérrez-Velasco L, Navarro-Ramírez E, Villar-Suárez V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J Anat. 2021;238:1203–1217. doi: 10.1111/joa.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tieu A, Lalu MM, Slobodian M, Gnyra C, Fergusson DA, Montroy J, Burger D, Stewart DJ, Allan DS. An analysis of mesenchymal stem cell-derived extracellular vesicles for preclinical use. ACS Nano. 2020;14:9728–9743. doi: 10.1021/acsnano.0c01363. [DOI] [PubMed] [Google Scholar]
- 63.Kim JY, Rhim WK, Yoo YI, Kim DS, Ko KW, Heo Y, Park CG, Han DK. Defined MSC exosome with high yield and purity to improve regenerative activity. J Tissue Eng. 2021;12:20417314211008626. doi: 10.1177/20417314211008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27:585–598. doi: 10.1080/10717544.2020.1748758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M, Wu SY. The advances and challenges in utilizing exosomes for delivering cancer therapeutics. Front Pharmacol. 2018;9:735. doi: 10.3389/fphar.2018.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28:801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]


