Abstract
Background: The literature data regarding colon cancer patients with liver-only metastases (CLM) show that NLR determined before metastasectomy is a prognostic marker of shorter relapse-free survival (RFS), but no results has been reported to date for rectal cancer patients with liver-only metastases (RLM). This study aimed to investigate the NLR and SII in CLM and RLM.
Methods: Relapse-free (RFS) and overall survival (OS) were evaluated in 67 CLM and 103 RLM patients with a median follow-up of 46.5 and 59.8 months, respectively. Pre- and/or postoperative chemotherapy ± targeted treatment was applied in 96% and 87% of CLM and RLM patients, respectively. The cut-off level for hematologic parameters were determined by receiver operating characteristic (ROC) analysis. Univariate analysis was performed by Kaplan-Meier method and log rank test. For multivariate analysis Cox regression was applied.
Results: In univariate analysis low NLR (cut-off 2) and SII (535) were predictors of longer RFS in case of CLM (p < 0.01). In contrast, for RLM high NLR (2.42) and SII (792) were predictors of longer RFS (p < 0.001). For RLM both NLR and SII proved to be independent markers of RFS (HR 0.66 (95% CI 0.52–0.84) and 0.73 (0.57–0.91), respectively) and OS (0.76 (0.58–0.99) and 0.66 (0.5–0.87), respectively). Only NLR (1.44 (1.04–1.99)) was independent marker of RFS for CLM. The preoperative treatment has not influenced the role of NLR or SII.
Conclusion: In contrast to CLM, in RLM the high NLR or SII determined before metastasectomy proved to be independent prognostic factors of longer RFS and OS.
Keywords: neutrophil-to-lymphocyte ratio, liver-only metastases of rectal cancer, metastasectomy, relapse-free survival, systemic immune-inflammation index, liver-only metastases of colon cancer, preoperative treatment
Background
In Hungary the incidence and mortality of colorectal cancer (CRC) is currently the second most common among malignancies [1]. Liver metastases develop in nearly half of CRC patients, and the best treatment for patients with colorectal liver-only metastases (CRLM) is the surgical resection, however, 60–80% of them experience recurrence after resection [2]. Knowledge of derived preoperative hematologic parameters, which are readily available data, may be important in assessing the risk of recurrence. The best known prognostic parameter is the neutrophil-to-lymphocyte ratio (NLR), which has also been studied in several studies evaluating patients with CRLM [3, 4, 5]. In these studies, patients were dichotomized based on a calculated or from the literature taken cut-off value, and based on this, significantly different relapse-free survival (RFS) curves were found. In general, the lower NLR has been identified as a marker of longer RFS, but at the same cut-off value (e.g., Ref. 5), strongly significant [6, 7] or non-significant [8, 9] differences of RFS curves were demonstrated. All these data together reflect the incoherent association. The systemic immune-inflammation index (SII = platelet count x NLR), which has been shown to be the best prognostic marker for occurrence of liver metastasis in CRC [10], was investigated in only two studies for the recurrence in CRLM [11, 12].
In previous studies, the presence of pre- and postoperative chemo- and targeted therapies was not an exclusion criterion, although in some studies all patients received pseudoneoadjuvant (hereafter preoperative) [13, 14, 15] or pseudoadjuvant (hereafter postoperative) treatment [16, 17]. The difference in rate of pre- and postoperative treatments reflects data from everyday practice. The localization of the primary tumor has not been detailed in many studies, and the calculation method of cut-off values is also not uniform; the most common is based on ROC analysis. Several studies exclusively investigated the overall survival (OS), but their results are also non convergent [18, 19, 20].
Based on our previous experience [21] and moreover on the histologic, genetic, behavioral, etc. differences between colon and rectum tumors detailed by Paschke et al. [22] and [23, 24], we hypothesized that the role of NLR may depend on the site of primary tumor of CRLM patients. For colon cancer Chang et al. [25] proved that low NLR is a significant marker of RFS, but no report was found for rectal cancer. The aim of the present study was to separately investigate the colon and rectal cancer patients with liver-only metastases wether NLR and SII determined before metastasectomy are possible markers of RFS and OS.
Materials and Methods
Patients
All patients underwent curative resection of primary cancer. Those patients whom liver metastases were surgically treated between 2001 and 2018 were reviewed (n = 205). The exclusion criteria were: 1) Radio-frequency thermal ablation (RFTA) or radiofrequency ablation (RFA) of liver metastases (n = 16); 2) unavailable laboratory parameters (n = 16); 3) presence of other synchronous malignancies (n = 3). A total of 170 patients were included in the study, 67 of whom had a primary tumor in the colon (CLM) and 103 in the rectum (RLM). Besides the clinicopathologic parameters the presence of chemotherapy (±targeted treatment) before and/or after metastasectomy was recorded. The 5-FU-based chemotherapy was administered alone or combined with oxaliplatin or irinotecan. Targeted therapy (cetuximab, bevacizumab or panitumumab) was also applied in several cases. All hematological parameters were determined from the blood samples taken before metastasectomy.
Metastasectomy was laparoscopic (16 and 18%, for CLM and RLM, respectively) or classic, including synchronous surgery of primary tumor and liver metastasis (10%, both CLM and RLM). In case of 10 RLM patients the “liver first” strategy was chosen. Hepatic magnetic resonance imaging (MRI) to assess the local disease extension and to evaluate chemotherapy response, thoraco-abdominal computed tomography (CT) and positron emission tomography (PET-CT) were systematically performed to evaluate the presence of disease. Follow-up of all patients was performed every 3 months (physical examination, abdominal ultrasonography, CT, MRI or PET-CT, and routine laboratory).
Statistics
The primary objective was the prognostic value of NLR and SII for RFS; secondary objectives included OS and the effect of preoperative treatment on the role of NLR and SII. RFS was calculated from date of metastasectomy to date of progression or end of follow-up. OS was calculated from date of metastasectomy to date of cancer-related death or end of follow-up. The cut-off values for dichotomization of continuous variables were determined by ROC analysis of relapse or death for RFS and OS, respectively. The ratio of relapse was not underestimated because of enough follow-up duration. Survival curves were constructed by Kaplan-Meier method and compared by log rank test. Multivariate Cox regression analysis was used to find independent markers of survival. To avoid multicollinearity only uncorrelated variables were used in the Cox regression analysis. The NCSS program (NCSS 2019 Statistical Software (2019). NCSS, LLC. Kaysville, Utah, United States, ncss.com/software/ncss.) was used for statistical analyses.
Results
The clinical and laboratory parameters of patients are presented in Table 1 and Table 2.
TABLE 1.
Clinicopathological characteristics and preoperative laboratory parameters of patients with colon cancer liver-only metastases.
| Parameters | N (%) | Median (range) | Cut-off value RFS/OS | |
|---|---|---|---|---|
| Age (yrs) | 65 (38–80) | 62/66 | ||
| <62 | 23 (34) | |||
| ≥62 | 44 (66) | |||
| <66 | 35 (52) | |||
| ≥66 | 32 (48) | |||
| Gender | ||||
| male | 36 (54) | |||
| female | 31 (46) | |||
| Type of surgery used for metastasectomy | ||||
| laparoscopy | 11 (16) | |||
| open | 56 (84) | |||
| Resection margin | ||||
| R0 | 46 (69) | |||
| R1 | 21 (31) | |||
| Synchronicity of primary surgery and metastasectomy | ||||
| synchronous | 7 (10) | |||
| metachronous | 60 (90) | |||
| Preoperative (metastasectomy) chemotherapy ± targeted therapy | ||||
| none | 16 (24) | |||
| yes | 51 (76) | |||
| targeted | 43 (84) | |||
| Postoperative (metastasectomy) chemotherapy ± targeted therapy | ||||
| none | 18 (27) | |||
| yes | 49 (73) | |||
| targeted | 27 (55) | |||
| Pre- or postoperative chemotherapy ± targeted therapy | ||||
| none | 3 (4) | |||
| yes | 64 (96) | |||
| targeted | 48 (75) | |||
| WBC (G/l) | 6.8 (3.5–12.5) | 6.8/7.1 | ||
| <6.8 | 32 (48) | |||
| ≥6.8 | 35 (52) | |||
| <7.1 | 35 (52) | |||
| ≥7.1 | 32 (48) | |||
| neutrophil (G/l) | 4.3 (1.2–9.2) | 4/4.8 | ||
| <4 | 27 (40) | |||
| ≥4 | 40 (60) | |||
| <4.8 | 41 (61) | |||
| ≥4.8 | 26 (39) | |||
| lymphocyte (G/l) | 2 (0.8–3.7) | 1.94/2 | ||
| <1.94 | 32 (48) | |||
| ≥1.94 | 35 (52) | |||
| <2 | 36 (54) | |||
| ≥2 | 31 (46) | |||
| platelet (G/l) | 244 (105–446) | 210/184 | ||
| <210 | 20 (30) | |||
| ≥210 | 47 (70) | |||
| <184 | 10 (15) | |||
| ≥184 | 57 (85) | |||
| NLR | 2 (0.7–8.8) | 2/1.7 | ||
| <2 | 30 (45) | |||
| ≥2 | 37 (55) | |||
| <1.7 | 18 (27) | |||
| >1.7 | 49 (73) | |||
| SII (G/l) | 502 (125–1952) | 535/290 | ||
| <535 | 39 (58) | |||
| ≥535 | 28 (42) | |||
| <290 | 9 (16) | |||
| ≥290 | 58 (84) | |||
| GOT (U/l) | 25 (13–343) | 24/24 | ||
| <24 | 22 (37) | |||
| ≥24 | 37 (63) | |||
| NA | 8 | |||
| GPT (U/l) | 22 (9–296) | 31/17 | ||
| <31 | 48 (80) | |||
| ≥31 | 12 (20) | |||
| <17 | 14 (23) | |||
| ≥17 | 46 (77) | |||
| NA | 7 | |||
| Site of progression | ||||
| liver | 33 (63) | |||
| lung | 5 (10) | |||
| liver+lung | 7 (13) | |||
| other | 7 (13) | |||
| Extent of progression | ||||
| single | 41 (61) | |||
| multiple | 11 (16) | |||
| none | 15 (22) | |||
TABLE 2.
Clinicopathological characteristics and preoperative laboratory parameters of patients with rectal cancer liver-only metastases.
| Parameters | N (%) | Median (range) | Cut-off value RFS/OS | |
|---|---|---|---|---|
| Age (yrs) | 62 (31–81) | 68/64 | ||
| <68 | 68 (66) | |||
| ≥68 | 35 (34) | |||
| <64 | 58 (56) | |||
| ≥64 | 45 (44) | |||
| Gender | ||||
| male | 69 (67) | |||
| female | 34 (33) | |||
| Type of surgery used for metastasectomy | ||||
| laparoscopy | 19 (18) | |||
| open | 84 (82) | |||
| Resection margin | ||||
| R0 | 65 (63) | |||
| R1 | 38 (37) | |||
| Synchronicity of primary surgery and metastasectomy | ||||
| synchronous | 10 (10) | |||
| metachronous | 93 (90) | |||
| “liver first” | 10 (11) | |||
| Preoperative (metastasectomy) chemotherapy ± targeted therapy | ||||
| none | 37 (36) | |||
| yes | 66 (64) | |||
| targeted | 41 (62) | |||
| Postoperative (metastasectomy) chemotherapy ± targeted therapy | ||||
| none | 29 (28) | |||
| yes | 74 (72) | |||
| targeted | 23 (31) | |||
| Pre- or postoperative chemotherapy ± targeted therapy | ||||
| none | 13 (13) | |||
| yes | 90 (87) | |||
| targeted | 49 (54) | |||
| WBC (G/l) | 5.9 (3.4–18.6) | 7.3/4.2 | ||
| <7.3 | 79 (77) | |||
| ≥7.3 | 24 (23) | |||
| <4.2 | 14 (14) | |||
| ≥4.2 | 89 (86) | |||
| neutrophil (G/l) | 3.8 (2–13.5) | 5.5/3.5 | ||
| <5.5 | 85 (83) | |||
| ≥5.5 | 18 (17) | |||
| <3.5 | 37 (36) | |||
| ≥3.5 | 66 (64) | |||
| lymphocyte (G/l) | 1.4 (0.4–3.6) | 0.97/1.7 | ||
| <0.97 | 32 (31) | |||
| ≥0.97 | 71 (69) | |||
| <1.7 | 77 (75) | |||
| ≥1.7 | 26 (25) | |||
| platelet (G/l) | 210 (111–396) | 161/314 | ||
| <161 | 18 (17) | |||
| ≥161 | 85 (83) | |||
| <314 | 90 (87) | |||
| ≥314 | 13 (13) | |||
| NLR | 2.9 (0.9–11.3) | 2.42/2.56 | ||
| <2.42 | 31 (30) | |||
| ≥2.42 | 72 (70) | |||
| <2.56 | 39 (38) | |||
| >2.56 | 64 (62) | |||
| SII (G/l) | 616 (189–3,500) | 792/742 | ||
| <792 | 65 (63) | |||
| ≥792 | 38 (37) | |||
| <742 | 63 (61) | |||
| ≥742 | 40 (39) | |||
| GOT (U/l) | 23 (9–72) | 25/20 | ||
| <25 | 50 (58) | |||
| ≥25 | 36 (42) | |||
| <20 | 20 (23) | |||
| ≥20 | 66 (77) | |||
| NA | 17 | |||
| GPT (U/l) | 19 (5–77) | 22/13 | ||
| <22 | 52 (60) | |||
| ≥22 | 34 (40) | |||
| <13 | 13 (15) | |||
| ≥13 | 73 (85) | |||
| NA | 17 | |||
| Site of progression | ||||
| liver | 43 (42) | |||
| lung | 21 (20) | |||
| liver+lung | 8 (8) | |||
| other | 13 (13) | |||
| Extent of progression | ||||
| single | 69 (67) | |||
| multiple | 16 (16) | |||
| none | 18 (17) | |||
GOT, aspartate aminotransferase; GPT, alanine aminotransferase; NA, not available; NLR, neutrophil-to lymphocyte ratio; OS, overall survival; RFS, relapse-free survival; SII, systemic immune-inflammation index; WBC, white blood cells.
The median follow-up was 46.5 (95% CI 43.5–50.1) and 59.8 (48.8–73.9) months for CLM and RLM patients, respectively. For CLM the median RFS and OS was 10.2 (95% CI 5.8–14.4) and 34.4 (30.1–42.1) months, respectively. In case of RLM the median RFS and OS were 8.6 (6.6–12.4) and 41.1 (35.2–48.6) months, respectively.
Primarily, the prognostic role of different parameters for RFS was tested. The parameters with significant effect (p < 0.05) or close to the significance level (p < 0.1) in univariate analysis or significant (p < 0.05) in multivariate analysis were included in Tables 3 and 4. To avoid multicollinearity some parameters had to be omitted, therefore NLR and SII were tested in two different multivariate model (Cox1 and Cox2). Both, NLR and SII proved to be independent markers of RFS in case of RLM, while only NLR was independent marker of RFS of CLM (Tables 3, 4).
TABLE 3.
Uni- and multivariate analysis of RFS and OS for CLM.
| Parameters | mRFS (95%CI) | p | HRCox1 (95%CI) | pCox1 | HRCox2 (95%CI) | pCox2 |
|---|---|---|---|---|---|---|
| Age | ||||||
| <62 | 6.6 (4.4–8.9) | 7 × 10−4 | - | - | ||
| ≥62 | 14.6 (10.2–16.8) | |||||
| Resection margin | ||||||
| R0 | 13.8 (8.3–17.8) | 0.001 | 1 (ref) | 0.048 | 1 (ref) | 0.182 |
| R1 | 6.6 (5.2–10) | 1.42 (1.003–2.1) | 1.3 (0.89–1.9) | |||
| Synchronous | ||||||
| yes | 8.2 (2–8.2) | 0.012 | - | - | ||
| no | 12.9 (7–14.6) | |||||
| Postoperative chemotherapy ± targeted therapy | ||||||
| none | 5.5 (2–5.8) | 3 × 10−4 | - | 1 (ref) | 0.012 | |
| yes | 13.4 (9.9–16.3) | 0.61 (0.42–0.9) | ||||
| Pre- or postoperative chemotherapy ± targeted therapy | ||||||
| none | 2.5 (2–2.5) | 0.006 | - | - | ||
| yes | 10.8 (8.2–13.9) | |||||
| WBC | ||||||
| <6.8 | 14.6 (10.8–19.3) | 0.011 | - | - | ||
| ≥6.8 | 8.3 (5.5–10.2) | |||||
| Neutrophil | ||||||
| <4 | 19.3 (10.8–24.9) | 0.002 | - | - | ||
| ≥4 | 8.3 (5.8–10.5) | |||||
| NLR | ||||||
| <2 | 14.6 (6.8–24.2) | 0.004 | 1 (ref) | 0.03 | - | |
| ≥2 | 9.9 (6–12.7) | 1.44 (1.04–1.99) | ||||
| SII | ||||||
| <535 | 14.4 (10.5–19.3) | 0.005 | - | 1 (ref) | 0.229 | |
| ≥535 | 8.2 (5–9.9) | 1.24 (0.87–1.78) | ||||
| GOT | ||||||
| <24 | 12.9 (8.3–17.8) | 0.043 | 1 (ref) | 0.043 | 1 (ref) | 0.013 |
| ≥24 | 6.8 (5.5–10.5) | 1.43 (1.01–2.02) | 1.6 (1.1–2.33) | |||
| GPT | ||||||
| <31 | 12.7 (7–14.4) | 0.03 | - | - | ||
| ≥31 | 5 (3.2–8.9) | |||||
| mOS (95% CI) | ||||||
| WBC | ||||||
| <7.1 | NR (40.5–46.6) | 0.004 | - | - | ||
| ≥7.1 | 30.1 (23.2–37.5) | |||||
| Neutrophil | ||||||
| <4.8 | NR (34.4–46.6) | 0.004 | - | 1 (ref) | 0.114 | |
| ≥4.8 | 30.1 (23.2–37.5) | 1.45 (0.91–2.31) | ||||
| Lymphocyte | ||||||
| <2 | 68.3 (42.1–68.3) | 0.005 | - | 1 (ref) | 0.053 | |
| ≥2 | 31.3 (24.2–34.4) | 1.63 (0.99–2.67) | ||||
| Platelet | ||||||
| <184 | 24.2 (17.9–33.1) | 0.045 | 1 (ref) | 0.853 | - | |
| ≥184 | 42.1 (31.3–68.3) | 1.06 (0.59–1.9) | ||||
| GOT | ||||||
| <24 | NR (39.7–42.1) | 0.021 | 1 (ref) | 0.073 | 1 (ref) | 0.233 |
| ≥24 | 31.3 (24.6–40.8) | 1.64 (0.96–2.81) | 1.4 (0.81–2.41) | |||
| GPT | ||||||
| <17 | NR (−33.1) | 0.011 | - | - | ||
| ≥17 | 34.4 (25–40.8) | |||||
| Extent of progression | ||||||
| single | 37.5 (31.1–46.6) | 0.016 | 1 (ref) | 0.043 | 1 (ref) | 0.011 |
| multiple | 20.3 (16.3–24.2) | 1.71 (1.02–2.88) | 1.98 (1.17–3.34) | |||
CI, confidence interval; Cox(1 or 2), multivariate Cox regression analysis (model 1 or 2); GOT, aspartate aminotransferase; GPT, alanine aminotransferase; HR, hazard ratio; mOS, median overall survival; mRFS, median relapse-free survival; NLR, neutrophil-to lymphocyte ratio; NR, not reached; ns, not significant; ref, reference; SII, systemic immune-inflammation index; WBC, white blood cells.
TABLE 4.
Uni- and multivariate analysis of RFS and OS for RLM.
| Parameters | mRFS (95%CI) | p | HRCox1 (95%CI) | pCox1 | HRCox2 (95%CI) | pCox2 |
|---|---|---|---|---|---|---|
| Resection margin | ||||||
| R0 | 11.7 (7.1–17.3) | 0.005 | 1 (ref) | 0.001 | - | |
| R1 | 6.5 (4.8–11.1) | 1.58 (1.2–2.07) | ||||
| Postoperative chemo ± targeted therapy | ||||||
| none | 6.6 (4.1–8.6) | 0.084 | 1 (ref) | 0.014 | 1 (ref) | 0.114 |
| yes | 11.1 (7–15.4) | 0.69 (0.52–0.93) | 0.79 (0.6–1.06) | |||
| WBC | ||||||
| <7.3 | 10.9 (6.5–15.6) | 0.06 | 1 (ref) | 0.568 | 1 (ref) | 0.416 |
| ≥7.3 | 6.9 (4.8–11.5) | 1.09 (0.81–1.48) | 1.13 (0.84–1.52) | |||
| Neutrophil | ||||||
| <5.5 | 11.1 (7.1–15.6) | 0.014 | - | - | ||
| ≥5.5 | 6.6 (4.2–8.3) | |||||
| Lymphocyte | ||||||
| <0.97 | 13.6 (6.6–23.7) | 0.025 | - | - | ||
| ≥0.97 | 7.1 (6.2–11.2) | |||||
| NLR | ||||||
| <2.42 | 6.3 (4.8–7.6) | 1.6 × 10−4 | 1 (ref) | 0.021 | - | |
| ≥2.42 | 14.9 (7.8–19.2) | 0.71 (0.54–0.95) | ||||
| SII | ||||||
| <792 | 6.5 (5.7–7.6) | 1.8 × 10−4 | - | 1 (ref) | 0.002 | |
| ≥792 | 19.2 (11.9–23.7) | 0.65 (0.5–0.85) | ||||
| GPT | ||||||
| <22 | 8.6 (5.9–14.2) | 0.085 | 1 (ref) | 0.151 | 1 (ref) | 0.139 |
| ≥22 | 11.2 (6.2–24.2) | 0.82 (0.63–1.07) | 0.82 (0.63–1.07) | |||
| mOS (95% CI) | ||||||
| Age | ||||||
| <64 | 47.3 (40.2–62.4) | 0.054 | 1 (ref) | 0.004 | 1 (ref) | 0.012 |
| ≥64 | 33.1 (26.1–39.2) | 1.57 (1.15–2.14) | 1.47 (1.09–1.99) | |||
| Resection margin | ||||||
| R0 | 52.5 (37.8–71.4) | 0.002 | 1 (ref) | 0.025 | - | |
| R1 | 31 (20.9–41.1) | 1.4 (1.04–1.88) | ||||
| Postoperative targeted therapy | ||||||
| none | 46.5 (36.8–62) | 0.059 | - | - | ||
| yes | 31.7 (20.3–40.5) | |||||
| WBC | ||||||
| <4.2 | 31.2 (20.3–35.2) | 0.036 | 1 (ref) | 0.031 | 1 (ref) | 0.005 |
| ≥4.2 | 46 (39.2–62.4) | 0.67 (0.47–0.96) | 0.58 (0.40–0.85) | |||
| Lymphocyte | ||||||
| <1.7 | 39.3 (31.7–46) | 0.059 | - | - | ||
| ≥1.7 | 101 (39.2–101) | |||||
| Platelet | ||||||
| <314 | 39.2 (31.7–46) | 0.032 | 1 (ref) | 0.044 | - | |
| ≥314 | NR (52.5–62.4) | 0.47 (0.22–0.98) | ||||
| NLR | ||||||
| <2.56 | 39.2 (26.7–41.1) | 0.084 | 1 (ref) | 0.038 | - | |
| >2.56 | 47.3 (35.2–63.1) | 0.72 (0.53–0.98) | ||||
| SII | ||||||
| <742 | 36.8 (26.7–41.1) | 0.011 | - | 1 (ref) | 0.001 | |
| ≥742 | 62 (41.2–138) | 0.57 (0.41–0.79) | ||||
| GPT | ||||||
| <13 | 26.5 (16.6–41.2) | 0.237 | 1 (ref) | 0.002 | 1 (ref) | 0.006 |
| ≥13 | 40.2 (33.8–50.3) | 0.54 (0.34–0.8) | 0.58 (0.39–0.86) | |||
CI, confidence interval; Cox(1 or 2), multivariate Cox regression analysis (model 1 or 2); GPT, alanine aminotransferase; HR, hazard ratio; mOS, median overall survival; mRFS, median relapse-free survival; NLR, neutrophil-to lymphocyte ratio; NR, not reached; ns, not significant; ref, reference; SII, systemic immune-inflammation index; WBC, white blood cells.
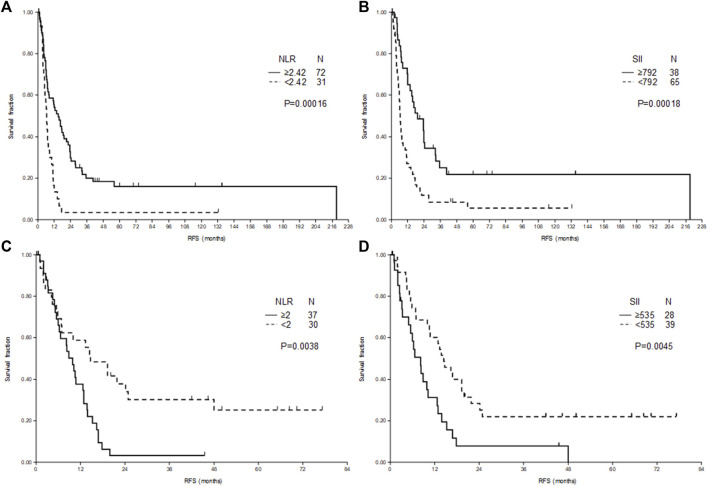
At 18 months only one patient (3%) remained free of relapse in the low NLR group of RLM, while in the high NLR group 28 patients (39%) were free of progression (Figure 1A). In contrast, at 18 months in low NLR group of CLM 50% of patients were free of relapse, while in the high NLR group all, but three patients progressed (Figure 1C). The number of patients free from relapse at 18 months for low and high SII of RLM was 10 (15%) and 19 (50%), respectively (Figure 1B) and for CLM was 15 (38%) and 3 (11%), respectively (Figure 1D).
FIGURE 1.
Relapse-free survival (RFS) after liver metastasectomy of patients with rectal cancer (A,B) and colon cancer (C,D) according to neutrophil-to-lymphocyte ratio (NLR) (A,C) and systemic immune-inflammation index (SII) (B,D).
Survival analysis of OS was conducted with the recalculated cut-off values for continuous variables. Besides, age, resection margin, GPT, platelet and WBC count, both NLR or SII proved to be significant predictors of OS in RLM (Table 4). In univariate analysis for CLM nor NLR neither SII was statistically significant predictor of OS (Table 3).
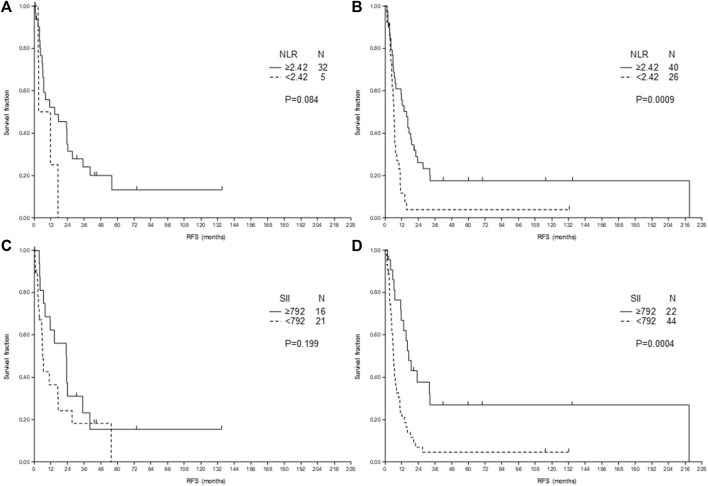
As Hand et al. [17] reported that preoperative chemotherapy influenced the role of NLR in CRLM, we analyzed the RFS stratified according to treatment before metastasectomy. In case of RLM the longer RFS of high NLR (Figures 2A,B) or SII (Figures 2C,D) was present both for treated and untreated patients, however, in case of untreated patients the significance level was not reached because of relatively low number of cases (Figure 2).
FIGURE 2.
Relapse-free survival (RFS) after liver metastasectomy of patients with rectal cancer according to neutrophil-to-lymphocyte ratio (NLR) (A,B) and systemic immune-inflammation index (SII) (C,D) and according to treatment: no treatment (A,C) or chemotherapy (±targeted treatment) before metastasectomy (B,D).
Preoperative treatment also did not influence the effect of NLR and SII in case of CLM (data not shown).
Moreover, if the preoperative treatment was included as covariate in multivariate tests of PFS and OS its effect was non-significant. A very similar result was found for SII, and the presence or not of targeted treatment prior metastasectomy also did not influence the results for NLR or SII (data not shown).
Discussion
There are some articles which analyzed the role of NLR in prediction of RFS after resection of liver metastases of CRC patients. In some of them the localization of primary tumor was not reported or rectal tumors were studied together with the left sided colon tumors (Table 5).
TABLE 5.
Literature data about RFS according to primary colorectal cancer (CRC) localization after metastasectomy of liver-only metastases.
| Primary tumor localization | Ref no | N | Colon | Longer RFS | p | p | Preop | Sync | Postop | Follow-up a months |
|---|---|---|---|---|---|---|---|---|---|---|
| % | NLR (SII) cut-off | Univ | Multiv | % | % | % | ||||
| CRC | ||||||||||
| 3, 26 | 586 | NA | >5 | 0.272 | - | 23 | 38 | 27 | >6 | |
| 6 | 440 | NA | <5 | <0.001 | <0.001 | 11 | 33 | >33 | 24 | |
| 8 | 247 | NA | <5 | 0.77 | - | - | - | - | 20 | |
| 13 | 169 | NA | <2.5 | 0.09 | 0.347 | 100 | 72 | 76 | 34.6 | |
| 14 | 140 | NA | <2.4 | 0.033 | 0.609 | 100 | 71 | 74 | 33 | |
| 16 | 92 | NA | <5 | 0.047 | 0.022 | 76 | 0 | 100 | 27.1 b | |
| COLON cancer | ||||||||||
| 25 | 98 | 100 | <2.5 | 0.044 | 0.029 | - | 0 | - | 35.2 b | |
| Our study | 67 | 100 | <2 | 0.004 | 0.03 | 76 | 10 | 73 | 46.5 | |
| (<535) | 0.005 | 0.229 | ||||||||
| >50% of patients with COLON cancer | ||||||||||
| 9 | 575 | 78 | ≤5 | 0.104 | - | 86 | 66 | 90 | 37 | |
| 11 | 452 | 56 | <2.6 | 0.163 | - | 63 | 53 | 74 | 28 | |
| (≤517) | 0.068 | - | ||||||||
| 4 | 343 | 58 | <2.5 | 0.017 | - | 58 | 49 | - | 49 | |
| 5 | 295 | 60 | <2 | <0.001? | 0.001 | 19 | 78 | 37 | 63.2 | |
| 12 | 283 | 66 | (≤0.0135) | 0.003 | 0.005 | 51 | 58 | 77 | 35.4 | |
| 31 | 231 | 67 | ≤3 | 0.049 | 0.06 | 76 | 35 | 72 | 73.2 | |
| 30 | 197 | 62 | <3 | 0.284 | 0.617 | 26 | 57 | - | - | |
| 15 | 183 | 57 | <2.3 | ns | - | 100 | 85 | 78 | 36.3 | |
| 29 | 150 | 58 | <4.63 | 0.017 | 0.452 | 39 | 63 | 73 | 36 | |
| 28 | 182 | 70 | >3 | 0.939 | - | 19 | 65 | 91 | 32.5 b | |
| 27 | 128 | 79 c | <1.71 | ns | - | 74 | 63 | - | 45 | |
| 32 | 130 | 54 | ≤5 | 0.044 | 0.03 | 16 | 38 | 26 | 44 b | |
| >50% of patients with RECTAL cancer | ||||||||||
| 17 | 83 | 24 | <1.94 | 0.026 | 0.006 | 29 | 100 | 100 | - | |
| 7, 33 | 174 | 46 | <5 | 0.008 | ns | 42 | 0 | 39 | 36 | |
| RECTAL cancer | ||||||||||
| Our study | 103 | 0 | ≥ 2.42 | <0.001 | <0.001 | 64 | 10 | 72 | 59.8 | |
| (≥ 792) | <0.001 | 0.002 | ||||||||
CRC, colorectal cancer; multiv, multivariate; NA, not available; ns, non-significant; Postop, postoperative treatment; Preop, preoperative treatment; Ref, reference; RFS, relapse-free survival; SII, systemic immune-inflammation index; Sync, synchronous; univ, univariate.
Median.
Average.
Personal communication.
In the few articles where the colon and rectum appears among patients’ characteristics, no stratified test was performed based on localization. There is only one article where exclusively colon tumors were analyzed. To the best of our knowledge, the present study is the first investigation, which performed a separate analysis of rectal tumors. All relevant studies have been summarized in Table 5.
Since no study has been found where the high NLR would be a significant marker of longer RFS, in case of CLM the association between high NLR and shorter RFS is obvious. For RLM there are very few articles where patients with primary rectal cancer were in the majority. [7, 33] examined the role of NLR in two articles (88% overlap with patient data), and found longer RFS and OS for low NLR, however their result was in correlation with the high frequency of postoperative infectious complications (77% of all postoperative complications) [33], which was lower in our study (29%, data not shown). Kim et al. [17] reported similar result, however, their study investigated only patients with synchronous surgery of primary and metastases and all patients received adjuvant chemotherapy. Moreover, there were far fewer preoperatively-treated patients in Kim’s article (29%) than in ours (64%) or in Neal’s reports (42%). Can the preoperative treatment alter the results? Interestingly, in Table 3 of the six reports where colon cancer patients were in the majority, in five where preoperative treatment was common (>60%), NLR was not significant for RFS. On the other hand, in four out of six reports with lower preoperative treatment frequency (<60%) the NLR was significant marker of RFS. Hand et al. [18] reported NLR as a non-significant or significant marker of OS (RFS was not studied) after liver metastasectomy of CRC patients (the location of primary tumor was not reported) depending whether the patients received or not preoperative chemotherapy, respectively. In our study, in contrast to the results of [18] in patients who received preoperative treatment the high NLR proved to be a very significant predictor of longer RFS. The discrepancy can be explained by different preoperative treatment (chemotherapy only in Hand’s study vs chemotherapy+targeted therapy in >50% of patients in our study) and different location of the primary tumor, as we have seen that in case of rectum and colon, the role of NLR is completely different. In spite that the influence of chemo- and targeted therapies on NLR of patients with advanced CRC [34] and on immunologic characteristics of liver metastases of CRC tumors [35] was already reported, further studies should clarify the effect of preoperative treatment on NLR in case of liver metastases of colon and separately rectal cancer.
A publication studying SII in CRLM patients was reported by [11], but neither NLR (Table 3), nor SII was significant for RFS after metastasectomy. Another recent study by [12] found significantly longer survival for low SII, which proved to be independent predictor of RFS. Their results may differ from that ours because they investigated colon (56–66%) and rectal cancer patients together (Table 3).
Our results can also be an explanation why [4] concluded that an integrated cut-off value can’t be determined for the preoperatively measured NLR in CRLM patients. The various colon/rectum ratio and different frequencies of preoperative treatment in reviewed studies made the results inconsistent. Therefore, it is important to primarily shed light on the difference between colon and rectum, and not to find a cut-off that could be used in the clinic.
The histologic, genetic, behavioral, etc. differences between colon and rectum tumors detailed by [22] and moreover the inflammation pattern (IL-6, CRP), which differs in colon and rectum tumors described by [23, 24] may explain our results.
McCoy et al. [36] studied the relation between the presence of stromal Foxp3 and RFS in rectal cancer patients after preoperative treatment and reported a significantly longer RFS (p = 0.025) for low Foxp3+ cell density. In another study, [37] demonstrated a strong negative correlation (p = 0.006) between stromal Foxp3+ infiltration and preoperative serum CRP levels in CRC patients. A study by [38] showed that high preoperative CRP levels were associated (p < 0.001) with high NLR in CRC patients. According to the above data it can be hypothesized that RLM patients with high NLR, which is associated with high CRP and subsequently with low Foxp3 levels may have longer RFS, as a consequence of a specific tumor immune microenvironment (TIME).
The longer RFS for colon in cases of low NLR may be explained by the presence of tumor-infiltrating lymphocytes (TILs). According to the results of [39] the low NLR in CRC patients (73% colon cancer) was significantly associated with higher TILs (p = 0.005) at the invasive margin of the tumor and a significantly longer DFS. CD8+ TILs may account for antitumoral effect, resulting in an unfavorable tumor immune microenvironment (TIME) and longer RFS. [40] studied 94 liver metastases of CRCs (65% colon cancer) and the level of CD8+ TILs. The distribution of high and low CD8+ density was significantly different for rectal and colon origin. A high CD8+ was more frequently observed for colon (54%) than for rectal origin (30%, p = 0.027). There was no difference between left and right sided colon. The RFS was significantly better (p = 0.018) for cases with high CD8+. Similarly, [41] reported a similar result, that high CD8/CD3 ratio was significantly more frequent in intra- and peritumoral tissue of liver metastases of colon tumors (60% and 54%) than that of rectal tumors (37% and 35%, p = 0.011 and 0.035, respectively). The RFS for high CD8/CD3 was significantly longer (p = 0.035). Other results of studies investigating NLR on recurrence of primary colon or rectal tumors can be used for comparison only with reservations, because the CRLM differ from primary lesions in terms of immune cell infiltration [42, 43, 44].
In accordance with [45] the location of primary tumor did not influence the RFS after hepatic resection. Instead, NLR, which reflects TIME, can influence the survival in different manner depending on primary localization.
The limitation of this study consist in its retrospective character. The treatments administered after relapse (surgery, chemotherapy, etc) were not considered for OS analysis. The prognostic factor (RAS mutation) [2, 8] and other tumor markers (e.g. fibrinogen/albumin index [11] or prognostic nutrition index [5], etc.) were also not available. In spite of limitations, this study has the power to clarify the controversies, which still exists between NLR and RFS after liver resection of patients with CRLM.
Conclusion
The NLR and SII determined before surgery of liver metastases of patients with RLM proved to be an independent prognostic factor of RFS and OS in an opposite manner as for CLM where low NLR predicts longer RFS, namely NLR and SII above the cut-off level predicts longer RFS. Further prospective studies stratified according to primary tumor location and TIME may strengthen our findings.
Data Availability Statement
The datasets presented in this article are not readily available due to patient privacy. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of the institute and by the Medical Research Council (21679-2/2016/EKU). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study concepts and design: NP, BB, EH, AP, TM. Data acquisition: NP, BB, EH, TM. Quality control of data and algorithms: NP, BB, EH, TM. Data analysis and interpretation: NP, BB, EH, AP, TM. Statistical analysis: BB. Manuscript preparation: NP, BB. Manuscript editing: NP, BB, EH, AP, TM. Manuscript revising critically for important intellectual content: AP, TM. Final approval of the manuscript: NP, BB, EH, AP, TM.
Funding
TM acknowledges financial support from the National Laboratories Excellence program (under the National Tumorbiology Laboratory project (NLP-17)) and the Hungarian Thematic Excellence Programme (TKP2020-NKA-26).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Liu W, Zhang W, Xu Y, Li Y-H, Xing B-C. A Prognostic Scoring System to Predict Survival Outcome of Resectable Colorectal Liver Metastases in This Modern Era. Ann Surg Oncol (2021) 28:7709–18. 10.1245/s10434-021-10143-6 [DOI] [PubMed] [Google Scholar]
- 3. Alabraba E, Ibrahim H, Olaru A, Cameron I, Gomez D, Group NHS. Retrospective Cohort Study of Statin Therapy Effect on Resected Colorectal Liver Metastases. Wjgs (2020) 12:34–44. 10.4240/wjgs.v12.i2.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dupré A, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ, Malik HZ. Preoperative Leucocyte-Based Inflammatory Scores in Patients with Colorectal Liver Metastases: Can We Count on Them? World J Surg (2019) 43:1351–9. 10.1007/s00268-019-04914-2 [DOI] [PubMed] [Google Scholar]
- 5. Kim W-J, Lim T-W, Kang S-H, Park P-J, Choi S-B, Lee S-i., et al. Development and Validation of Novel Scoring System for the Prediction of Disease Recurrence Following Resection of Colorectal Liver Metastasis. Asian J Surg (2020) 43(2):438–46. 10.1016/j.asjsur.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 6. Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated Preoperative Neutrophil to Lymphocyte Ratio Predicts Survival Following Hepatic Resection for Colorectal Liver Metastases. Eur J Surg Oncol (Ejso) (2008) 34:55–60. 10.1016/j.ejso.2007.02.014 [DOI] [PubMed] [Google Scholar]
- 7. Neal CP, Mann CD, Sutton CD, Garcea G, Ong SL, Steward WP, et al. Evaluation of the Prognostic Value of Systemic Inflammation and Socioeconomic Deprivation in Patients with Resectable Colorectal Liver Metastases. Eur J Cancer (2009) 45:56–64. 10.1016/j.ejca.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 8. Western CE, Wiggans M, Aroori S, Bowles M, Stell D. PMO-089 Preoperative Neutrophil: Lymphocyte Ratio Is Not a Predictor of Outcome Following Hepatic Resection for Colorectal Metastases. Gut (2012) 61:2–A109. 10.1136/gutjnl-2012-302514b.89 21997557 [DOI] [Google Scholar]
- 9. Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, et al. Comprehensive Complication index Predicts Cancer-specific Survival after Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg (2017) 266:1045–54. 10.1097/SLA.0000000000002018 [DOI] [PubMed] [Google Scholar]
- 10. Lu Y, Xin D, Wang F. Predictive Significance of Preoperative Systemic Immune-Inflammation index Determination in Postoperative Liver Metastasis of Colorectal Cancer. Ott (2019) Vol. 12:7791–9. 10.2147/OTT.S223419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y-Y, Liu Z-Z, Xu D, Liu M, Wang K, Xing B-C. Fibrinogen-albumin Ratio index (FARI): a More Promising Inflammation-Based Prognostic Marker for Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann Surg Oncol (2019) 26:3682–92. 10.1245/s10434-019-07586-3 [DOI] [PubMed] [Google Scholar]
- 12. Deng Y, Zhao Y, Qin J, Huang X, Wu R, Zhou C, et al. Prognostic Value of the C-Reactive Protein/Albumin Ratio and Systemic Immune-Inflammation Index for Patients with Colorectal Liver Metastasis Undergoing Curative Resection. Pathol Oncol Res (2021) 27:633480. 10.3389/pore.2021.633480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giakoustidis A, Neofytou K, Khan AZ, Mudan S. Neutrophil to Lymphocyte Ratio Predicts Pattern of Recurrence in Patients Undergoing Liver Resection for Colorectal Liver Metastasis and Thus the Overall Survival. J Surg Oncol (2015) 111:445–50. 10.1002/jso.23845 [DOI] [PubMed] [Google Scholar]
- 14. Neofytou K, Smyth EC, Giakoustidis A, Khan AZ, Cunningham D, Mudan S. Elevated Platelet to Lymphocyte Ratio Predicts Poor Prognosis after Hepatectomy for Liver-Only Colorectal Metastases, and it Is superior to Neutrophil to Lymphocyte Ratio as an Adverse Prognostic Factor. Med Oncol (2014) 31:239. 10.1007/s12032-014-0239-6 [DOI] [PubMed] [Google Scholar]
- 15. Mao R, Zhao J-J, Bi X-Y, Zhang Y-F, Li Z-Y, Huang Z, et al. A Low Neutrophil to Lymphocyte Ratio before Preoperative Chemotherapy Predicts Good Outcomes after the Resection of Colorectal Liver Metastases. J Gastrointest Surg (2019) 23:563–70. 10.1007/s11605-018-3796-8 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Peng Z, Chen M, Liu F, Huang J, Xu L, et al. Elevated Neutrophil to Lymphocyte Ratio Might Predict Poor Prognosis for Colorectal Liver Metastasis after Percutaneous Radiofrequency Ablation. Int J Hyperthermia (2012) 28:132–40. 10.3109/02656736.2011.654374 [DOI] [PubMed] [Google Scholar]
- 17. Kim H, Jung HI, Kwon SH, Bae SH, Kim HC, Baek M-J, et al. Preoperative Neutrophil-Lymphocyte Ratio and CEA Is Associated with Poor Prognosis in Patients with Synchronous Colorectal Cancer Liver Metastasis. Ann Surg Treat Res (2019) 96:191–200. 10.4174/astr.2019.96.4.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hand F, Ryan EJ, Harrington C, Durand M, Maguire D, O'Farrelly C, et al. Chemotherapy and Repeat Resection Abrogate the Prognostic Value of Neutrophil Lymphocyte Ratio in Colorectal Liver Metastases. Hpb (2020) 22:670–6. 10.1016/j.hpb.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 19. Hamada T, Ishizaki H, Haruyama Y, Hamada R, Yano K, Kondo K, et al. Neutrophil-to-lymphocyte Ratio and Intratumoral CD45RO-Positive T Cells as Predictive Factors for Longer Survival of Patients with Colorectal Liver Metastasis after Hepatectomy. Tohoku J Exp Med (2020) 251:303–11. 10.1620/tjem.251.303 [DOI] [PubMed] [Google Scholar]
- 20. Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey J-N. Blood Neutrophil-To-Lymphocyte Ratio Predicts Survival in Patients with Colorectal Liver Metastases Treated with Systemic Chemotherapy. Ann Surg Oncol (2009) 16:614–22. 10.1245/s10434-008-0267-6 [DOI] [PubMed] [Google Scholar]
- 21. Komlósi V, Hitre E, Pap É, Adleff V, Réti A, Székely É, et al. SHMT1 1420 and MTHFR 677 Variants Are Associated with Rectal but Not colon Cancer. BMC Cancer (2010) 10:525. 10.1186/1471-2407-10-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paschke S, Jafarov S, Staib L, Kreuser E-D, Maulbecker-Armstrong C, Roitman M, et al. Are colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Ijms (2018) 19:2577. 10.3390/ijms19092577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 Genotypes and colon and Rectal Cancer. Cancer Causes Control (2007) 18:1095–105. 10.1007/s10552-007-9049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slattery ML, Curtin K, Poole EM, Duggan DJ, Samowitz WS, Peters U, et al. Genetic Variation in C-Reactive Protein in Relation to colon and Rectal Cancer Risk and Survival. Int J Cancer (2011) 128:2726–34. 10.1002/ijc.25721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang Z, Zheng J, Ma Y, Zhao J, Wang C, Liu Z. The Neutrophil-To-Lymphocyte Ratio as a Predictor for Recurrence of Colorectal Liver Metastases Following Radiofrequency Ablation. Med Oncol (2014) 31:855. 10.1007/s12032-014-0855-1 [DOI] [PubMed] [Google Scholar]
- 26. Sanyal S, Alabraba E, Ibrahim H, Olaru A, Cameron I, Gomez D. ACE Inhibitor Therapy Does Not Influence the Survival Outcomes of Patients with Colorectal Liver Metastases Following Liver Resection. J Gastrointest Canc (2021) 52:106–12. 10.1007/s12029-019-00350-6 [DOI] [PubMed] [Google Scholar]
- 27. Cimino MM, Donadon M, Giudici S, Sacerdote C, Di Tommaso L, Roncalli M, et al. Peri-tumoural CD3+ Inflammation and Neutrophil-To-Lymphocyte Ratio Predict Overall Survival in Patients Affected by Colorectal Liver Metastases Treated with Surgery. J Gastrointest Surg (2020) 24:1061–70. 10.1007/s11605-019-04458-9 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y-W, Lu C-C, Chang C-D, Lee K-C, Chen HH, Yeh WS, et al. Prognostic Value of Sarcopenia in Patients with Colorectal Liver Metastases Undergoing Hepatic Resection. Sci Rep (2020) 10:6459. 10.1038/s41598-020-63644-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng J, Li H, Ou Q, Lin J, Wu X, Lu Z, et al. Preoperative Lymphocyte-To-Monocyte Ratio Represents a superior Predictor Compared with Neutrophil-To-Lymphocyte and Platelet-To-Lymphocyte Ratios for Colorectal Liver-Only Metastases Survival. Ott (2017) Vol. 10:3789–99. 10.2147/OTT.S140872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taniai T, Haruki K, Hamura R, Fujiwara Y, Furukawa K, Gocho T, et al. The Prognostic Significance of C-Reactive Protein-To-Lymphocyte Ratio in Colorectal Liver Metastases. J Surg Res (2021) 258:414–21. 10.1016/j.jss.2020.08.059 [DOI] [PubMed] [Google Scholar]
- 31. Verter E, Berger Y, Perl G, Peretz I, Tovar A, Morgenstern S, et al. Neutrophil-to-lymphocyte Ratio Predicts Recurrence Pattern in Patients with Resectable Colorectal Liver Metastases. Ann Surg Oncol (2021) 28:4320–9. 10.1245/s10434-021-10000-6 [DOI] [PubMed] [Google Scholar]
- 32. Zeman M, Maciejewski A, Półtorak S, Kryj M. Evaluation of Outcomes and Treatment Safety of Patients with Metastatic Colorectal Cancer to the Liver with Estimation of Prognostic Factors. Pol Przegl Chir (2013) 85:333–9. 10.2478/pjs-2013-0050 [DOI] [PubMed] [Google Scholar]
- 33. Neal CP, Mann CD, Garcea G, Briggs CD, Dennison AR, Berry DP. Preoperative Systemic Inflammation and Infectious Complications after Resection of Colorectal Liver Metastases. Arch Surg (2011) 146:471–8. 10.1001/archsurg.2011.50 [DOI] [PubMed] [Google Scholar]
- 34. Nemoto T, Endo S, Isohata N, Takayanagi D, Nemoto D, Aizawa M, et al. Change in the Neutrophil-To-Lymphocyte Ratio during Chemotherapy May Predict Prognosis in Patients with Advanced or Metastatic Colorectal Cancer. Mol Clin Oncol (2021) 14:107. 10.3892/mco.2021.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eefsen RL, Engelholm L, Alpizar-Alpizar W, Van den Eynden GGE, Vermeulen PB, Christensen IJ, et al. Inflammation and uPAR-Expression in Colorectal Liver Metastases in Relation to Growth Pattern and Neo-Adjuvant Therapy. Cancer Microenvironment (2015) 8:93–100. 10.1007/s12307-015-0172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCoy MJ, Hemmings C, Miller TJ, Austin SJ, Bulsara MK, Zeps N, et al. Low Stromal Foxp3+ Regulatory T-Cell Density Is Associated with Complete Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Br J Cancer (2015) 113:1677–86. 10.1038/bjc.2015.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunnarsson U, Strigård K, Edin S, Gkekas I, Mustonen H, Kaprio T, et al. Association between Local Immune Cell Infiltration, Mismatch Repair Status and Systemic Inflammatory Response in Colorectal Cancer. J Transl Med (2020) 18:178. 10.1186/s12967-020-02336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kubo H, Murayama Y, Arita T, Kuriu Y, Nakanishi M, Otsuji E. The Prognostic Value of Preoperative Neutrophil-To-Lymphocyte Ratio in Colorectal Cancer. World J Surg (2016) 40:2796–802. 10.1007/s00268-016-3595-x [DOI] [PubMed] [Google Scholar]
- 39. Pine JK, Morris E, Hutchins GG, West NP, Jayne DG, Quirke P, et al. Systemic Neutrophil-To-Lymphocyte Ratio in Colorectal Cancer: the Relationship to Patient Survival, Tumour Biology and Local Lymphocytic Response to Tumour. Br J Cancer (2015) 113:204–11. 10.1038/bjc.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao B, Peng J, Wang Y, Deng Y, Ou Q, Wu X, et al. Prognostic Value of Tumor Infiltrating Lymphocytes Combined with PD-L1 Expression for Patients with Solitary Colorectal Cancer Liver Metastasis. Ann Transl Med (2020) 8:1221. 10.21037/atm-20-2762a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peng J, Wang Y, Zhang R, Deng Y, Xiao B, Ou Q, et al. Immune Cell Infiltration in the Microenvironment of Liver Oligometastasis from Colorectal Cancer: Intratumoural CD8/CD3 Ratio Is a Valuable Prognostic index for Patients Undergoing Liver Metastasectomy. Cancers (2019) 11:1922. 10.3390/cancers11121922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halama N, Spille A, Lerchl T, Brand K, Herpel E, Welte S, et al. Hepatic Metastases of Colorectal Cancer Are rather Homogeneous but Differ from Primary Lesions in Terms of Immune Cell Infiltration. Oncoimmunology (2013) 2:e24116. 10.4161/onci.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin H-C, Shao Q, Liang J-Y, Wang Y, Zhang H-Z, Yuan Y-F, et al. Primary Tumor Immune Score Fails to Predict the Prognosis of Colorectal Cancer Liver Metastases after Hepatectomy in Chinese Populations. Ann Transl Med (2021) 9:310. 10.21037/atm-20-4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahtiainen M, Elomaa H, Väyrynen JP, Wirta E-V, Kuopio T, Helminen O, et al. Immune Contexture of MMR-Proficient Primary Colorectal Cancer and Matched Liver and Lung Metastases. Cancers (2021) 13:1530. 10.3390/cancers13071530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang K, Xu D, Yan X-L, Poston G, Xing B-C. The Impact of Primary Tumour Location in Patients Undergoing Hepatic Resection for Colorectal Liver Metastasis. Eur J Surg Oncol (2018) 44:771–7. 10.1016/j.ejso.2018.02.210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available due to patient privacy. Requests to access the datasets should be directed to the corresponding author.