Abstract
A 53-year-old male Japanese patient with COVID-19 was admitted to our hospital after his respiratory condition worsened on day 9 of the disease. With the diagnosis of severe COVID-19, treatment with remdesivir, dexamethasone, and unfractionated heparin was started for the prevention of thrombosis. Although the patient's respiratory status data improved after treatment, severe respiratory failure persisted. Thrombocytopenia and D-dimer elevation were observed on day 8 after heparin therapy initiation. Heparin-induced thrombocytopenia (HIT) antibody measured by immunological assay was positive, and contrast computed tomography showed pulmonary artery thrombus. The patient was diagnosed with HIT because the pre-test probability score (4Ts score) for HIT was 7 points. Heparin was changed to apixaban, a direct oral anticoagulant, which resulted in a reduction of the pulmonary thrombus and improvement of the respiratory failure. In patients with COVID-19, anticoagulant therapy with heparin requires careful monitoring of thrombocytopenia and elevated D-dimer as possible complications related to HIT. (151/250 words).
Keywords: COVID-19, Heparin-induced thrombocytopenia, Anticoagulation therapy, SARS-CoV-2, HIT antibody, 4T's score
1. Introduction
Coronavirus disease 2019 (COVID-19) continues to present novel problems for health care practitioners. One serious complication of COVID-19 is thrombosis [1], and prophylactic anticoagulation with heparin to prevent thrombosis has been reported to reduce mortality [2]. For this reason, anticoagulation is recommended for severe COVID-19 patients in the guidelines of many countries [3].
Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse reaction of heparin treatment [4]. When exposed to heparin, some patients form platelet-activating antibodies against platelet factor 4/heparin complexes, and a subset of sensitized patients develops the severe and life-threating complications of thromboembolism. During the current COVID-19 pandemic, the increased use of heparin with a broader indication of anticoagulation therapy carries the risk of a rare but potentially fatal complication related to HIT in COVID-19 patients.
COVID-19 with HIT has been reported relatively frequently in Europe and the United States [5,6], but very few cases have been reported in Japan. Here, we report a case of COVID-19 in a Japanese patient with complications related to HIT during prophylactic anticoagulation therapy.
2. Case report
The patient was a 53-year-old Japanese male with a history of bilateral postoperative pneumothorax, postoperative tongue cancer, and a smoking history of 20 cigarettes/day for 17 years. He had no history of alcohol consumption or allergies. His colleague had developed COVID-19, and our patient presented with fever and general malaise around the same time. He was diagnosed with COVID-19, which was confirmed by a positive antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). During this period, the Alpha variant of SARS-CoV-2 was prevalent in Japan, and the patient was not vaccinated. Initially, he stayed at a facility for patients with mild symptoms, but he was transferred to our hospital on day 9 of the disease onset because of progressive hypoxemia. His blood oxygen saturation level measured at rest in ambient air with a pulse oximeter (SpO2) had dropped to 85%.
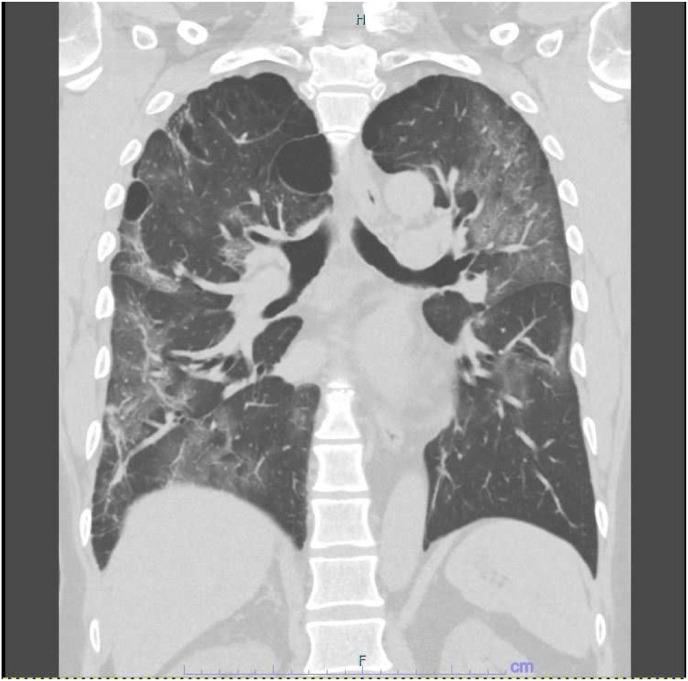
On admission, the patient was conscious with the following parameters: body mass index, 24.3 kg/m2; body temperature, 37.2 °C; blood pressure, 168/66 mmHg; pulse rate, 89 beats/min; respiratory rate, 20/min; and SpO2 93% under oxygen inhalation with a reservoir mask 6 L per minute (L/min). Fine crackles were heard in the bilateral lower lung fields on auscultation. Blood tests showed an elevated white blood cell count of 12,780/μL (90.9% neutrophils), elevated hepatobiliary enzymes of 66 IU/L aspartate transaminase, 44 IU/L alanine transaminase, and 228 IU/L alkaline phosphatase, 437 U/L lactate dehydrogenase, and 805 mg/dL fibrinogen. Platelet count was 139 × 103/μL. Both the fibrinogen/fibrin degradation products and D-dimer levels were within the normal range (2.7 μg/mL and <0.5 μg/mL, respectively), indicating no hypercoagulability at the time of admission. Serum C-reactive protein (CRP) was markedly elevated at 22.57 mg/dL. Under oxygen therapy with a fraction of inspired O2 (FiO2) of 0.6, arterial blood gas analysis showed pH 7.457, PaO2 77.7 Torr, and PaCO2 31.1 Torr. Chest X-ray showed ground-glass opacities in both lung fields, and plain computed tomography (CT) of the chest showed extensive non-zonal ground-glass opacities mainly in both subpleural areas (Fig. 1 ), with constrictive and band-like changes mainly in the lower lobes, consistent with COVID-19 pneumonia.
Fig. 1.
Plain computed tomography. Ground-glass opacities were observed in the subpleural predominance of both lung fields.
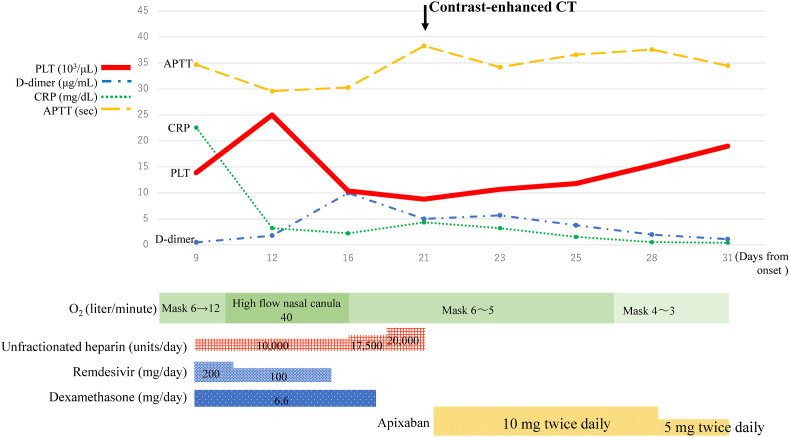
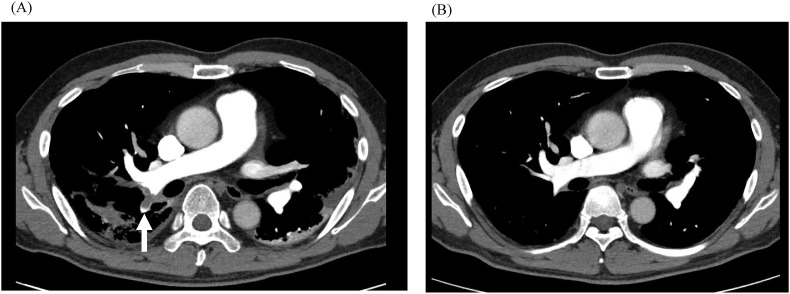
The clinical course of the patient is shown in Fig. 2 . The patient was diagnosed with severe COVID-19, and treatment with remdesivir and dexamethasone was started on admission (day 9 from onset). At the same time, 10,000 units per day of unfractionated heparin (UFH) was started by continuous intravenous infusion as a prophylactic anticoagulation therapy. Ceftriaxone and azithromycin were also administered for bacterial co-infection. The patient was changed to oxygen support with a high-flow nasal cannula because of the worsening of hypoxia within a few hours of admission. On day 4 of treatment, the respiratory status of the patient had improved, and CRP had decreased to 3.24 mg/dL. Hence, the patient was moved to an oxygen mask on day 8 of treatment. However, the D-dimer level had shown an increasing trend from day 6 of treatment (Fig. 2), and the dosage of UFH was increased sequentially. Nevertheless, the D-dimer level increased to 10 μg/mL on day 8 of treatment. The platelet count increased from 139 × 103/μL to 250 × 103/μL on day 4 of treatment but decreased to 104 × 103/μL on day 8 of treatment. Although the patient still needed 5 L/min of oxygen, his condition did not seem to worsen. Considering these conflicting findings, we suspected pulmonary thromboembolism or HIT. HIT antibody measured by latex immunoturbidimetric assay was positive at 2.9 U/mL (cut off value 1.0 U/mL), and a contrast CT from chest to leg showed a thrombus in the main trunk of the right pulmonary artery, while no solid-organ tumors causing thrombosis were observed (Fig. 3 A). Because the 4Ts clinical scoring of the pre-test for HIT [7] was 7 points, the patient was clinically diagnosed with HIT, and UFH was changed to apixaban on day 13 of treatment. The patient's respiratory status improved, and an increased platelet count and decreased D-dimer level were observed. D-dimer was confirmed to be negative on day 26 of treatment. The patient was discharged with home oxygen therapy (HOT) on day 33 of treatment. The patient continued to receive apixaban as an outpatient, and the disappearance of the pulmonary artery thrombus was confirmed by contrast-enhanced CT on day 74 of treatment (Fig. 3B). He did not need HOT on day 107 of treatment. Apixaban was administered for 95 days in total.
Fig. 2.
Clinical course. Abbreviations: PLT, platelet count; CRP, C-reactive protein; APTT, activated partial thromboplastin time.
Fig. 3.
Contrast-enhanced computed tomography. (A) A thrombus was identified in the right pulmonary artery from the central side to the periphery (white arrow). (B) Disappearance of the pulmonary artery thrombus was observed on day 82 from disease onset.
3. Discussion
In the current case, the patient's respiratory status improved steadily during the period in which heparin was used as a prophylactic anticoagulation therapy. As the D-dimer level increased and the platelet count decreased, the amount of heparin was gradually increased. However, improvements were no longer observed in the patient's respiratory status or D-dimer level. This indicated that the patient developed thromboembolism caused by HIT during this period.
Recent findings suggest that critically ill patients with COVID-19 are prone to complications with HIT [5,6]. A systemic review and meta-analysis on HIT in hospitalized COVID-19 patients reported that the incidence was higher in critically ill patients compared with non-critically ill patients (2.2% and 1.2%, respectively), although the incidence of 0.8% HIT in hospitalized patients with COVID-19 was comparable to that in patients without COVID-19 [6]. Clinical characteristics among the 19 confirmed cases of HIT in patients with COVID-19 in a systemic review [6] showed the following results: the median age was 62 years, and all but one patient were critically ill. The median time from heparin initiation to HIT diagnosis was 13.5 days, and 12 patients (63%) developed thrombosis after heparin administration. These findings were consistent with our case.
It is known that the incidence of HIT is higher when UFH is used instead of low molecular weight heparin (LMWH) or when therapeutic rather than prophylactic doses of heparin are used [8,9].Regarding the type of heparin used in the abovementioned 19 confirmed cases of HIT, 17 (63%) patients received UFH and 10 (37%) received LMWH. Of the 18 patients with available data on the heparin dose used, 13 (72%) received therapeutic doses of heparin, whereas 5 (28%) received a prophylactic dose. Furthermore, in patients with COVID-19, UFH or therapeutic doses of heparin is thought to increase the risk of HIT. Recent studies concerning the outcome of prophylactic anticoagulation therapy with heparin in patients with COVID-19 showed that a therapeutic dose of LMWH did not improve the clinical outcome of critically ill patients compared with prophylactic doses, while in non-critically ill patients, a therapeutic dose of LMWH improved clinical outcome compared with a prophylactic dose [10,11]. This suggests that the dose of heparin as an anticoagulant therapy needs to be considered according to the disease severity of COVID-19. However, HIT should be noted regardless of the type or dosage of heparin when anticoagulation therapy with heparin is undertaken in any case.
Autoimmune HIT (aHIT), independent of heparin administration, has been proposed as another mechanism for the development of HIT [12]. aHIT includes HIT associated with infections, and a small number of cases of aHIT caused by viral infections have been reported [13,14]. In fact, a case report [15] of a patient with COVID-19 who was positive for HIT antibodies and who developed thrombosis despite not using heparin has been published, although it is unclear at present if SARS-CoV-2 infection itself predisposes a person to HIT. It cannot be determined if aHIT was involved in our case because the thrombosis of our patient occurred after heparin administration.
The diagnosis of HIT based on antibody tests alone is difficult. Immunological tests such as latex immunoturbidimetry or enzyme-linked immunosorbent assay are generally used to test for HIT antibodies, but overdiagnosis because of false positives resulting from their low specificity is a problem [16]. For this reason, functional tests for HIT antibodies, such as serotonin release assay, are necessary as confirmatory tests, but these commercial-based tests are not usually performed in Japan. Consequently, the 4Ts pre-test clinical scoring system for HIT is used in clinical practice to make a semiquantitative diagnosis [17]. Reported confirmed cases of HIT in COVID-19 showed intermediate or high scores, with a median of 5 points in the 4Ts score [6,18], as in our case, which was as high as 7 points. It is important to promptly discontinue heparin and initiate non-heparin anticoagulation therapy when HIT is suspected with a 4Ts score of 4 points or greater.
In our case, the pulmonary thrombus was successfully treated by administering direct oral anticoagulants (DOACs), apixaban, for 3 months. According to the American Society of Hematology guidelines, discontinuation of heparin and initiation of non-heparin anticoagulants such as argatroban, bivalirudin, danaparoid, and fondaparinux or DOACs is recommended when HIT is suspected [19]. For the treatment of reported HIT cases in COVID-19, parenteral anticoagulants such as argatroban are frequently used, and most oral anticoagulants such as DOACs are used after bridging with the parenteral anticoagulants as necessary [6,18,20]. The use of DOACs as alternative anticoagulants in HIT has been increasingly reported during recent years [21]. Directly switching to DOACs for HIT appears to be effective in clinically stable patients, as in our case. Patients with thrombotic HIT are recommended to receive a non-heparin anticoagulant for a total of 3 months [22].
In terms of reducing the risk of HIT, LMWH is thought to be preferable to UFH, but it is difficult to use it in Japan because of its off-label status for this indication. Concerning the use of DOACs for the primary prevention of thrombosis, a recent study reported that rivaroxaban at therapeutic doses did not improve clinical outcomes and that it increased bleeding events compared with UFH or LMWH in hospitalized COVID-19 patients with elevated D-dimers. The authors concluded that therapeutic doses of DOACs should be avoided in the absence of an evidence-based indication [23]. As a practical matter, it is important to treat severe COVID-19 patients with UFH to prevent thrombosis while paying careful attention to the possibility of complications related to HIT by monitoring for thrombocytopenia and elevated D-dimer.
To our knowledge, only one case report of COVID-19 with HIT has been published in Japan, which detailed a case of HIT in a patient with severe COVID-19 during veno-arterial extracorporeal membrane oxygenation therapy [24]. A recent study suggested that the risk of thromboembolism in Japanese patients with COVID-19 might be lower than that in other countries [25]. It is not clear whether this feature may be related to the low complication rate of HIT, or whether it is underdiagnosed in Japan. Although there is concern about the increased risk of complications related to HIT caused by excessive anticoagulation with heparin thereafter, the epidemiological findings in this regard are currently unclear, and a future study is necessary to clarify this issue.
In conclusion, it is important that clinicians pay close attention to the possible development of HIT when patients with severe COVID-19 are treated with heparin as anticoagulant therapy.
Funding
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent for publication
Written informed consent was obtained from the patient in accordance with the Declaration of Helsinki for publication of this case report.
Authorship statement
All authors meet the ICMJE authorship criteria. All authors were involved in the diagnosis, management, and/or care of the patient. K.S. wrote manuscript draft. K.N., Y.M., and M.M. revised the manuscript for important intellectual content. M.M. and N.S. were responsible for patient care and supervised the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
None.
Acknowledgments
We thank H. Nikki March, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arepally G.M. Heparin-induced thrombocytopenia. Blood. 2017;129:2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daviet F., Guervilly C., Baldesi O., Bernard-Guervilly F., Pilarczyk E., Genin A., et al. Heparin-induced thrombocytopenia in severe COVID-19. Circulation. 2020;142:1875–1877. doi: 10.1161/CIRCULATIONAHA.120.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uaprasert N., Tangcheewinsirikul N., Rojnuckarin P., Patell R., Zwicker J.I., Chiasakul T. Heparin-induced thrombocytopenia in patients with COVID-19: a systematic review and meta-analysis. Blood Adv. 2021;5:4521–4534. doi: 10.1182/bloodadvances.2021005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo G.K., Juhl D., Warkentin T.E., Sigouin C.S., Eichler P., Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemostasis. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 8.Martel N., Lee J., Wells P.S. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 9.Smythe M.A., Koerber J.M., Mattson J.C. The incidence of recognized heparin-induced thrombocytopenia in a large, tertiary care teaching hospital. Chest. 2007;131:1644–1649. doi: 10.1378/chest.06-2109. [DOI] [PubMed] [Google Scholar]
- 10.Investigators A., Investigators AC-a, Investigators R.-C., Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Investigators R.-C., Investigators AC-a, Investigators A., Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warkentin T.E., Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40–51. doi: 10.1016/j.thromres.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Olah Z., Kerenyi A., Kappelmayer J., Schlammadinger A., Razso K., Boda Z. Rapid-onset heparin-induced thrombocytopenia without previous heparin exposure. Platelets. 2012;23:495–498. doi: 10.3109/09537104.2011.650245. [DOI] [PubMed] [Google Scholar]
- 14.Greinacher A. Me or not me? The danger of spontaneity. Blood. 2014;123:3536–3538. doi: 10.1182/blood-2014-04-566836. [DOI] [PubMed] [Google Scholar]
- 15.Julian K., Bucher D., Jain R. Autoimmune heparin-induced thrombocytopenia: a rare manifestation of COVID-19. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-243315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuker A., Cines D.B. How I treat heparin-induced thrombocytopenia. Blood. 2012;119:2209–2218. doi: 10.1182/blood-2011-11-376293. [DOI] [PubMed] [Google Scholar]
- 17.Nellen V., Sulzer I., Barizzi G., Lammle B., Alberio L. Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97:89–97. doi: 10.3324/haematol.2011.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favaloro E.J., Henry B.M., Lippi G. The complicated relationships of heparin-induced thrombocytopenia and platelet factor 4 antibodies with COVID-19. Int J Lab Hematol. 2021;43:547–558. doi: 10.1111/ijlh.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuker A., Arepally G.M., Chong B.H., Cines D.B., Greinacher A., Gruel Y., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulakshana S., Nayak S.S., Perumal S., Das B.P. Heparin-induced thrombocytopenia in COVID-19: a systematic review. Anesth Essays Res. 2021;15:341–347. doi: 10.4103/aer.aer_151_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farasatinasab M., Zarei B., Moghtadaei M., Nasiripour S., Ansarinejad N., Zarei M. Rivaroxaban as an alternative agent for heparin-induced thrombocytopenia. J Clin Pharmacol. 2020;60:1362–1366. doi: 10.1002/jcph.1635. [DOI] [PubMed] [Google Scholar]
- 22.Hvas A.M., Favaloro E.J., Hellfritzsch M. Heparin-induced thrombocytopenia: pathophysiology, diagnosis and treatment. Expet Rev Hematol. 2021;14:335–346. doi: 10.1080/17474086.2021.1905512. [DOI] [PubMed] [Google Scholar]
- 23.Lopes R.D., de Barros E.S.P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa Y., Nagata T., Akiyama T., Nishida K., Kumasawa J., Kohno M., et al. Argatroban therapy for heparin-induced thrombocytopenia in a patient with coronavirus disease 2019. J Thromb Thrombolysis. 2020;50:1012–1014. doi: 10.1007/s11239-020-02248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita Y., Yamada N., Mo M. The primary prevention of venous thromboembolism in patients with COVID-19 in Japan: current status and future perspective. Ann Vasc Dis. 2021;14:1–4. doi: 10.3400/avd.ra.20-00145. [DOI] [PMC free article] [PubMed] [Google Scholar]