Abstract
Cardiac calcified amorphous tumors are rare non-neoplastic intracavitary masses. Herein, we report a case of a 75-year-old woman who presented with dyspnea on exertion and multiple cerebral infarctions 3 months prior. Transthoracic echocardiography showed severe mitral regurgitation from the posterior mitral leaflet with valve perforation and severe mitral annular calcification. In addition, we observed a 13 mm mobile high echogenic mass, suggesting healed infective endocarditis. The mass was successfully resected, and the mitral valve was replaced with a bovine pericardial patch for the decalcified annulus. Histopathological examination confirmed cardiac calcified amorphous tumor; the postoperative course was uneventful. Mitral valve replacement and annulus patch repair effectively prevented postoperative recurrent systemic embolization.
<Learning objective: Calcified amorphous tumor (CAT) is a risk factor for systemic embolism. Cardiac CAT destroying the mitral valve tissue and causing mitral valvular disease have been scarcely reported. We present a case of cardiac CAT with mitral valve perforation and suspected systemic embolization, treated successfully through mitral valve replacement and calcified lesion coverage by surgical resection and patch repair.>
Keywords: Calcified amorphous tumor, Mitral valve perforation, Mitral valve replacement
Introduction
Cardiac calcified amorphous tumors (CCATs) are rare non-neoplastic cardiac tumors first described by Reynolds et al. in 1997 [1]. CCATs may be confused with primary cardiac neoplasms and infective endocarditis (IE) owing to commonalities in clinical features such as obstruction and embolization in both. Currently, surgical resection is the only treatment option available to mitigate symptoms, prevent embolization, and obtain a pathologic diagnosis. Several reports suggest that CCATs are commonly present on the mitral valve (MV) and on the right and left ventricles and atria [2]. Although calcified amorphous tumors (CAT) are a risk factor for systemic embolism, CCATs destroying the MV tissue and causing mitral valvular disease have been scarcely reported [3].
We present a case of CCAT with MV perforation and suspected systemic embolization, treated successfully through surgical resection and MV replacement.
Case report
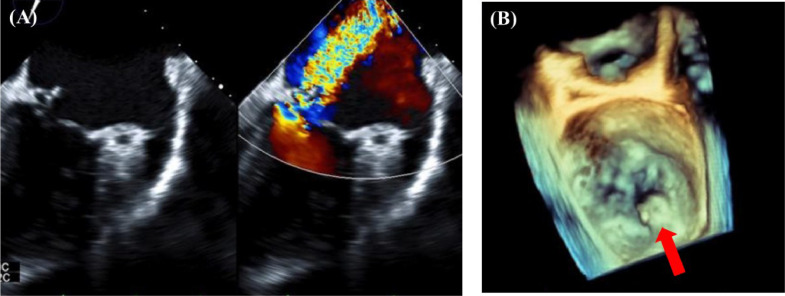
A 75-year-old woman with a history of multiple myeloma and dyslipidemia presented with dyspnea on exertion. She had experienced multiple cerebral infarctions 3 months prior, and dyspnea on exertion gradually appeared during the same period. Blood examination results revealed negative inflammatory findings, almost normal renal function (creatinine level, 0.63 mg/dl; glomerular filtration rate, 68.3 mL/min/1.73 m2), and an elevated B-type natriuretic peptide level. We suggested cardiac failure and systemic embolism. Transthoracic echocardiography and transesophageal echocardiography showed severe mitral regurgitation (MR) from the medial part base (P3) of the posterior mitral leaflet (PML), severe mitral annular calcification (MAC), and a 13-mm mobile high echogenic mass with valve aneurysm of P3, indicating healed IE attached to the same site. PML perforation with severe MR was detected directly below the mass (Fig. 1).
Fig. 1.
(A) Transesophageal echocardiogram (TEE) showing a calcified mitral valve tumor and severe mitral regurgitation. (B) Three-dimensional TEE showing a calcified mass emanating from P3 and mitral valve perforation (red arrow).
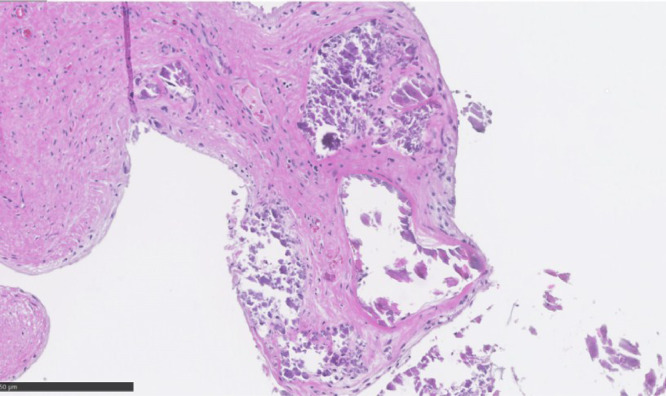
We resected the mass surgically. The MV was approached through a right-sided left atriotomy in median sternotomy using standard cardiopulmonary bypass and cardioplegic arrest. No lesion was detected in the anterior mitral leaflet (AML). We noted severe calcification in the PML and annulus, and perforation in PML P3 (Fig. 2A). The infection was absent in the both the MV leaflets and annulus. Fragile calcification was found from the perforation site on the left-ventricular side to the annulus and chordae tendineae (Fig. 2B). We speculated that the calcification had infiltrated the valve leaflets and caused perforation. Perforation repair seemed difficult owing to severe calcification widespread in the MV and posterior annulus along with the MR. Consequently, we decided to replace the MV. The PML was resected from the perforation, and the valve leaflet was excised with the chordae tendineae from P2 to P3. The calcification on the surface of the annulus was removed; the exposed calcification site was covered with a bovine pericardial patch to prevent embolization and atrioventricular groove disruption (Fig. 2C). Non-everted horizontal mattress sutures were placed from the left ventricle to the left atrium on the anterior half of the mitral annulus. Everted horizontal mattress sutures were placed on the left atrial wall close to the calcified posterior annulus to prevent needle protrusion. A 29 mm St. Jude biological valve was successfully placed at a supra-annular position. Finally, a donut-shaped bovine pericardial patch was sewn on the artificial valve annulus for reinforcement and on the left atrial wall to prevent perivalvular leakage (Fig. 2D). The patient was weaned from bypass and had an uneventful postoperative recovery. Histopathological examination of the resected mass did not show signs of infection. The findings of amorphous debris, fibrinous material, and calcified nodules (Fig. 3) were consistent with the diagnosis of CCAT. Valve microbiology examination did not show signs of infection. The patient recovered well without embolic events during the 18-month follow-up period. Written informed consent was obtained from the patient to publish this case report and accompanying images.
Fig. 2.
(A) Intraoperative presentation from the left atrium showing P3 perforation (black arrow) and calcified mass (asterisk). (B) Mitral valve perforation connected from the mitral annular calcification and papillary muscle calcification. (C) The entire exposed calcification site covered with a bovine pericardial patch. (D) Bioprosthetic valve placed at a supra-annular position (asterisk) and the pericardial patch sewn to the valve annulus was continuously sutured to the left atrium wall (black arrow).
Fig. 3.
Microscopic appearance of the mass. Histopathologic examinations revealed amorphous debris, fibrinous material, and calcified nodules (hematoxylin and eosin × 10).
Discussion
The histological features of CCATs include nodular calcium deposits in a background composed of fibrin or amorphous fibrillary materials. However, its origin and etiology remain unclear. Furthermore, studies on CCAT epidemiology, clinical imaging, treatment, and prognosis are limited. A review of CCAT cases from 1997 to 2019 suggests that CCATs most frequently arise from the MV and annulus (34/80 cases) [2,4,5]. In general, MV perforation occurs primarily owing to IE, and other rare causes include severe aortic regurgitation, connective tissue disease and iatrogenicity [6]. In our patient, histopathological findings and bacterial culture were negative for IE. Intraoperative findings showed that CCAT extending from the posterior annulus was connected to the valve perforation, and it was highly probable that CCAT itself physically caused MV perforation. Two previously reported cases showed CCAT-associated MV leaflet perforation that caused MR, requiring MV repair [3,4]. Among the various methods to treat MV perforation, MV repair using a patch should be the first choice. In our case, mitral valve regurgitation could also have been controlled through MV repair using a patch at the perforation site. Our patient carried the risk of preoperative cerebral infarction associated with CCAT valve destruction of the perforation site. Considering this risk, MV repair for the remaining exposed MV perforation and MAC was considered insufficient to prevent systemic embolization. Importantly, in CCATs, partial removal and non-reinforcement of the exposed site may cause recurrence or embolism. Recurrence has been previously reported in a patient who underwent incomplete right ventricular CCAT resection [7]. Additionally, a study reported that superimposed CCATs on MAC might represent one of the causes of MAC-induced stroke [8].
In conclusion, MV replacement and calcified lesion coverage through patch repair for CCAT with MV perforation should be considered an effective solution for preventing postoperative systemic embolization recurrence. We should also consider the possibility of CCAT in cases of heart failure with systemic embolism events.
Funding statement
None.
Declaration of Competing Interest
The authors have no conflict of interest.
Acknowledgments
None.
References
- 1.Reynolds C., Tazelaar H.D., Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT) Hum Pathol. 1997;28:601–606. doi: 10.1016/s0046-8177(97)90083-6. [DOI] [PubMed] [Google Scholar]
- 2.de Hemptinne Q., de Cannière D., Vandenbossche J.L., Unger P. Cardiac calcified amorphous tumor: a systematic review of the literature. Int J Cardiol Heart Vasc. 2015;7:1–5. doi: 10.1016/j.ijcha.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinugasa Y., Teshima H., Inoue Y., Tai R., Sato M., Ikebuchi M., Irie H. A calcified amorphous tumor causing mitral valve perforation and ruptured chordae tendineae. Jpn J Cardiovasc Surg. 2019;48:259–262. [Google Scholar]
- 4.Haruki N., Sumi N., Kobara S., Tsujimoto D., Inoue Y., Saito Y., Shirota K. Spontaneous mitral valve perforation associated with mitral annular calcification-related calcified amorphous tumor assessed by three-dimensional transesophageal echocardiography. J Med Ultrason. 2020;47:481–482. doi: 10.1007/s10396-020-01023-9. [DOI] [PubMed] [Google Scholar]
- 5.Torii Y., Yamada H., Matsukuma S., Nishio S., Kusunose K., Abe M., Sata M. Left ventricular lipomatous hamartoma mimicking a calcified amorphous tumor. Circulation. 2016;133:e408–e410. doi: 10.1161/CIRCULATIONAHA.115.019252. [DOI] [PubMed] [Google Scholar]
- 6.Mashicharan M., Cowburn P.J., Livesey S.A., Shah BN. Anterior mitral valve perforation in the absence of acute infection: diagnosis by two-dimensional and three-dimensional transesophageal echocardiography. Echocardiography. 2017;34:1953–1955. doi: 10.1111/echo.13734. [DOI] [PubMed] [Google Scholar]
- 7.Fealey M.E., Edwards W.D., Reynolds C.A., Pellikka P.A., Dearani JA. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol. 2007;16:115–118. doi: 10.1016/j.carpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Matsukuma S., Eishi K., Tanigawa K., Miura T., Matsumaru I., Hisatomi K., Tsuneto A. Swinging calcified amorphous tumors with related mitral annular calcification. Ann Thorac Surg. 2016;101:e103–e105. doi: 10.1016/j.athoracsur.2015.09.019. [DOI] [PubMed] [Google Scholar]