Abstract
Isolated coronary arteritis without systemic involvement in adults is exceedingly rare. A 60-year-old patient developed recurrent non–ST-segment elevation myocardial infarctions for 1 year. After an initial coronary angiogram that was normal, serial angiograms showed de novo aneurysm formation. The patient responded favorably to corticosteroids, supporting the diagnosis of isolated coronary arteritis. (Level of Difficulty: Intermediate.)
Key Words: coronary aneurysms, coronary vasculitis, recurrent myocardial infarction, myocardial infarction without obstructive coronary artery disease (MINOCA)
Abbreviations and Acronyms: CTA, computed tomographic angiography; DES, drug-eluting stent; LAD, left anterior descending artery; LM, left main; MINOCA, myocardial infarction without obstructive coronary artery disease; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention
Central Illustration
History of Presentation
A 60-year-old woman presented with typical angina at rest. She was diagnosed with myocardial infarction without obstructive coronary artery disease (MINOCA) 1 year earlier based on typical clinical presentation, biomarker elevation, and a normal coronary angiogram (Figure 1A and 1B, Videos 1 and 2). No further evaluation was undertaken.
Learning Objectives
-
•
To recognize that the scope of coronary artery disease reaches far beyond classic atherosclerosis.
-
•
To identify that MINOCA may herald future coronary events and/or uncommon disease processes.
-
•
To realize the importance of a multidisciplinary approach to a challenging case.
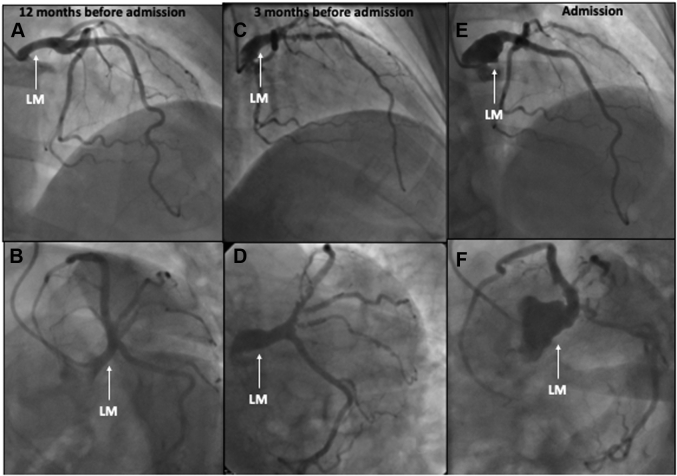
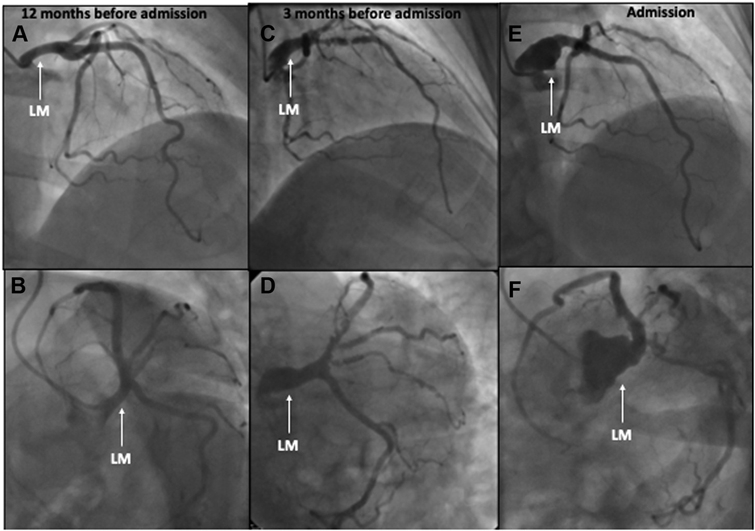
Figure 1.
Coronary Angiogram at 3 Different Times Since Admission
(A, B) Coronary angiography 12 months before admission showed no coronary lesions. (C, D) Significant de novo disease: left main (LM) artery aneurysm, long lesion in the proximal left anterior descending artery, and an ostial lesion of the intermediate ramus (3 months before admission). (E, F) A large LM aneurysm, demonstrating a rapid deterioration in a short time (current episode).
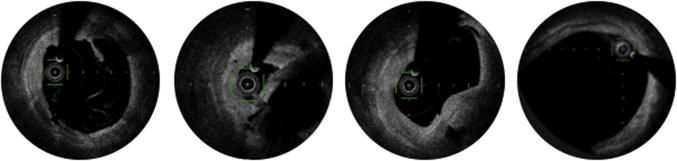
Nine months later (3 months before the current episode), she had another non-ST-segment elevation myocardial infarction (NSTEMI) (peak troponin I 1,123 ng/L [normal value <14 ng/L]). Her coronary angiogram showed significant de novo disease: a left main (LM) artery aneurysm, a 91% long lesion in the proximal segment of the left anterior descending artery (LAD), and an ostial lesion of the intermediate ramus (Videos 3 and 4). Optical coherence tomography showed a thin atheromatous plaque with multiple ulcerations and thrombi in the proximal LAD (Figure 2). Subsequently, she underwent percutaneous coronary intervention (PCI) with a sirolimus drug-eluting stent (DES) with a favorable final result.
Figure 2.
Optical Coherence Tomography
Optical coherence tomography showed a thin atheromatous plaque with multiple ulcerations and thrombi in the proximal left anterior descending artery.
The physical examination was unremarkable. Electrocardiography showed a left anterior fascicular block but was otherwise normal. Laboratory tests showed a peak high-sensitivity cardiac troponin I of 600 ng/L. Echocardiography was normal. She was diagnosed with NSTEMI and admitted to the cardiology department.
As part of the NSTEMI work-up, the patient underwent routine coronary angiography, which showed a large LM aneurysm (Figures 1C and 1D, Videos 5 and 6) and multiple aneurysms in the intermediate branch. The LAD showed no restenosis of a previously implanted DES. There was no de novo disease in the LAD or in the remaining arteries.
Past Medical History
The patient had a history of hypertension, dyslipidemia, hypothyroidism (secondary to Hashimoto thyroiditis), and obesity.
She had no history of other illnesses, recent infections, or travels.
Investigations
During hospitalization, computed tomographic angiography (CTA) was performed, and lesions in other major arteries outside the coronary circulation were excluded.
Given the angiography findings, the patient was assessed by the internal medicine autoimmune diseases team. She underwent extensive laboratory tests, which were suggestive of a nonspecific autoimmune phenomenon: positive antinuclear antibodies, positive erythrocyte sedimentation rate of 74 mm (normal value <20 mm), and mild normocytic and normochromic anemia. Tests to detect all specific markers for vasculitis returned negative results.
Management
After discussion within the heart team, the following conclusions were made: 1) the etiology of coronary disease did not seem primarily atheromatous but more like vasculitis; 2) the anatomic characteristics did not indicate that PCI should be performed; 3) there was no significant obstructive coronary disease; and 4) none of the aneurysms were large enough to indicate risk of rupture, which might warrant surgical resection. As a result, the final decision was to approach the case conservatively with medical therapy alone and focus primarily on secondary prevention measures. No empirical immunosuppressive therapy was undertaken initially, because no clear diagnosis was made.
The patient fared well during a 1-week hospital stay, with no recurrence of angina, electrocardiographic changes, or recurring troponin elevation. She was discharged on optimal medical therapy.
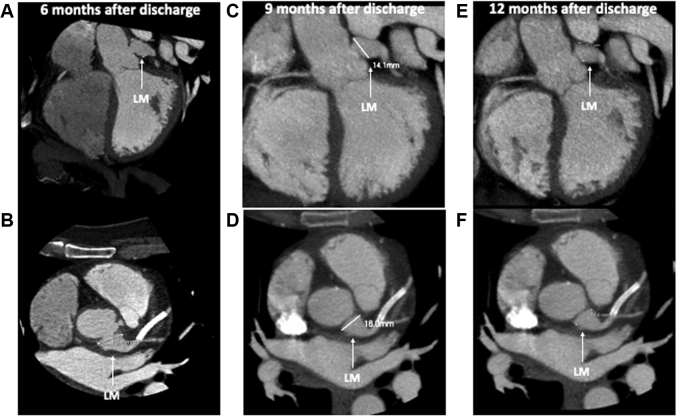
The patient was strictly followed in monthly intervals and remained asymptomatic. A scheduled coronary CTA (6 months after discharge) was undertaken: There was no coronary calcium, and de novo circumferential thickening of the aortic root involving the right coronary ostium with a resulting stenosis was noted. The LM aneurysm had also worsened and was now 18 × 17 mm wide (Figures 3A and 3B).
Figure 3.
Computerized Tomography Angiographies at 3 Different Times After Discharge
(A, B) Computerized tomographic angiography (CTA) 6 months after discharge showed a large left main (LM) artery aneurysm. (C, D) CTA was repeated 9 months after discharge (after 3 months of corticosteroid therapy), showing aneurysm regression. (E, F) A new CTA 12 months after discharge (after 6 months of corticosteroid therapy) showed a continuous regression of the aneurysm.
The disease progression, the aortic root thickening, and the laboratory findings were suggestive of ongoing inflammation and an autoimmune process. The internal medicine team reviewed the case again.
With no evidence of systemic disease, an organ-specific (coronary) idiopathic vasculitis was deemed to be the most likely diagnosis. Thus, the patient was started on prednisone at 25 mg/day.
At 9 and 12 months (with 3 and 6 months of prednisone therapy), the patient remained asymptomatic, and coronary CTAs were performed. Remarkably, significant improvement was noted: The aortic thickening was minimal, and the coronary aneurysms were smaller (Figures 3C to 3F). Since then, the patient has continued prednisone therapy with progressive reduction of the dosage to 10 mg/day), and no subsequent events have occurred.
Discussion
Despite the presence of multiple cardiovascular risk factors, the high recurrence of ischemic events and the atypical evolution of the coronary lesions raised other diagnostic hypotheses besides classic atheromatous disease.
Although there are no specific features on conventional coronary angiography that are diagnostic of coronary arteritis, certain features may be suggestive of a specific diagnosis. For example, in Takayasu arteritis, 3 main types of coronary lesions have been described from angiographic and histologic analysis: type 1, stenosis or occlusion of the ostia or proximal segments; type 2, diffuse or focal coronary arteritis that may extend diffusely to all epicardial branches or may involve focal segments (so-called skip lesions); and type 3, coronary aneurysms.1
Our patient’s case could fall into each of these 3 categories. She had coronary aneurysms, features of focal coronary arteritis, and an ostial lesion of the right coronary artery apparently due to aortic root thickening. However, there was no involvement of the main branches of the aorta, which is a hallmark of Takayasu arteritis. Therefore, the heart team ruled out that diagnosis.
The anatomic pattern of the coronary arteries could also fit into a polyarteritis nodosa picture, a small-to-medium vessel vasculitis. These lesions result in microaneurysm formation, aneurysmal rupture with hemorrhage, thrombosis, and, consequently, organ ischemia or infarction.2 However, the patient did not present any of the diagnostic criteria for polyarteritis nodosa according to American College of Rheumatology,3 or for any other type of systemic vasculitis.
Therefore, the most likely hypothesis was organ-specific vasculitis, in this case coronary vasculitis.
However, the other unusual aspect of this case is the fact that the aneurysms were not discovered incidentally in previously unknown coronary anatomy but rather on recently documented “normal” coronary arteries, which deteriorated very quickly in a only a few months.
Although coronary aneurysms are rare, they can result in fatal outcomes. The optimal treatment of coronary aneurysms or coronary ectasia in the absence of concomitant coronary stenosis or occlusion is uncertain, whether the aneurysms are accidentally found or diagnosed in the context of acute coronary syndrome.4 Controversies persist regarding the management of coronary aneurysms, and both percutaneous and surgical revascularization are associated with technical challenges. PCI of an aneurysmal/ectatic culprit vessel in the setting of acute myocardial infarction is associated with lower procedural success and a higher incidence of no-reflow and distal embolization.5,6 On the other hand, the ideal surgical approach has not yet been formally studied. Therefore, the management should be tailored to each patient based on a comprehensive clinical evaluation that encompasses the patient’s cardiovascular risk factors and comorbidities, and the nature and anatomy of the coronary aneurysm.7
Considering that no clear diagnosis was possible at presentation and that vasculitis restricted to the coronary territory is extremely rare, no immunosuppressive therapy was initiated. Indeed, we feared hampering the presentation of an underlying disease that might require specific therapy. Because the coronary disease rapidly progressed, we were left with no choice but to act.
After initiation of corticosteroid therapy, there was significant improvement of the coronary lesions. Because patients with vasculitis may have nonspecific symptoms that are attributed to other etiologies or go unrecognized for several months or years before diagnosis, this patient will need strict long-term surveillance.
This case also reveals that the MINOCA scenario really is like opening “Pandora’s box.” Indeed, these cases were often disregarded as clinically nonsignificant or only mildly relevant. However, increasing evidence suggests that MINOCA is a predictor of future events or the initial presentation of unapparent heart disease. These patients should be closely observed with follow-up, and further diagnostic investigation should be considered on a case-by-case basis.
Follow-Up
The patient remained asymptomatic with no recurrences of myocardial infarction or any other cardiovascular events.
Conclusions
Coronary vasculitis may occur in the setting of a systemic disease or in isolation. The latter is extremely rare. It may develop rapidly even in previously “normal” coronary arteries. Indeed, a MINOCA case should not be disregarded as a benign entity, because it may be a hallmark of future events. A multidisciplinary approach combined with strict clinical surveillance is paramount for a better outcome. Medical therapy may involve empirical immunosuppressive therapy, especially corticosteroids, in addition to classic secondary prevention measures.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Normal Coronary Angiogram—Right Anterior Oblique Cranial View.
Normal Coronary Angiogram—Spider View.
Coronary Angiography Showing Significant De Novo Disease. Left main (LM) artery aneurysm, a 91% long lesion in the proximal segment of the left anterior descending artery (LAD), and an ostial lesion of the intermediate ramus—right anterior oblique cranial view.
Coronary Angiography Showing Significant De Novo Disease—Spider View.
Coronary angiogram Showing Large Left Main Artery Aneurysm and Multiple Aneurysms in the Intermediate Branch—Right Anterior Oblique Cranial View.
Coronary Angiogram Showing Large Left Main Artery Aneurysm—Spider View
References
- 1.Matsubara O., Kuwata T., Nemoto T., Kasuga T., Numano F. Coronary artery lesions in Takayasu arteritis: pathological considerations. Heart Vessels. 1992;7(1):26–31. doi: 10.1007/BF01744540. [DOI] [PubMed] [Google Scholar]
- 2.Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017;13(6):381–386. doi: 10.1038/nrrheum.2017.68. [DOI] [PubMed] [Google Scholar]
- 3.Chung S., Gorelik M., Langford C., et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of polyarteritis nodosa. Arthritis Care Res. 2021;73(8):1061–1070. doi: 10.1002/acr.24633. [DOI] [PubMed] [Google Scholar]
- 4.Kawsara A., Núñez Gil I.J., Alqahtani F., Moreland J., Rihal C.S., Alkhouli M. Management of coronary artery aneurysms. J Am Coll Cardiol Intv. 2018;11(13):1211–1223. doi: 10.1016/j.jcin.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 5.Ipek G., Gungor B., Karatas M.B., et al. Risk factors and outcomes in patients with ectatic infarct-related artery who underwent primary percutaneous coronary intervention after ST elevated myocardial infarction. Catheter Cardiovasc Interv. 2016;88(5):748–753. doi: 10.1002/ccd.26553. [DOI] [PubMed] [Google Scholar]
- 6.Bogana Shanmugam V., Psaltis P.J., Wong D.T.L., Meredith I.T., Malaiapan Y., Ahmar W. Outcomes after primary percutaneous coronary intervention for ST-elevation myocardial infarction caused by ectatic infarct related arteries. Heart Lung Circ. 2017;26(10):1059–1068. doi: 10.1016/j.hlc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Abou Sherif S., Ozden Tok O., Taşköylü Ö., Goktekin O., Kilic I.D. Coronary artery aneurysms: a review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 2017;4:24. doi: 10.3389/fcvm.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normal Coronary Angiogram—Right Anterior Oblique Cranial View.
Normal Coronary Angiogram—Spider View.
Coronary Angiography Showing Significant De Novo Disease. Left main (LM) artery aneurysm, a 91% long lesion in the proximal segment of the left anterior descending artery (LAD), and an ostial lesion of the intermediate ramus—right anterior oblique cranial view.
Coronary Angiography Showing Significant De Novo Disease—Spider View.
Coronary angiogram Showing Large Left Main Artery Aneurysm and Multiple Aneurysms in the Intermediate Branch—Right Anterior Oblique Cranial View.
Coronary Angiogram Showing Large Left Main Artery Aneurysm—Spider View