Abstract
This case report describes a 64-year-old female with history of previous intravenous drug abuse on opioid substitution treatment with buprenorphine, who presented to the emergency department with angina and electrocardiographic findings suggestive of acute coronary syndrome. Echocardiography and left ventriculography were indicative of takotsubo cardiomyopathy, probably attributed to abrupt discontinuation of buprenorphine. Opioid withdrawal leads to sympathetic hyperactivity and increased catecholamine release, which in our case triggered takotsubo cardiomyopathy presentation.
<Learning objective: Buprenorphine withdrawal may precipitate takotsubo cardiomyopathy.>
Keywords: Stress cardiomyopathy, Takotsubo, Heart failure, Buprenorphine, Opioid withdrawal
Introduction
Takotsubo cardiomyopathy also known as broken heart syndrome is characterized by transient wall motion abnormality of the left ventricular apex resembling acute coronary syndrome in the absence of coronary artery disease. In Japanese, “tako-tsubo” means “fishing pot for trapping octopus,” due to the shape of the left ventricle at the end of systole. The syndrome usually affects postmenopausal women and although little is known about the pathogenesis of the disease, is triggered by emotional or physical stress. Here we describe the case of a 64-year-old woman who developed takotsubo cardiomyopathy following opioid use withdrawal.
Case report
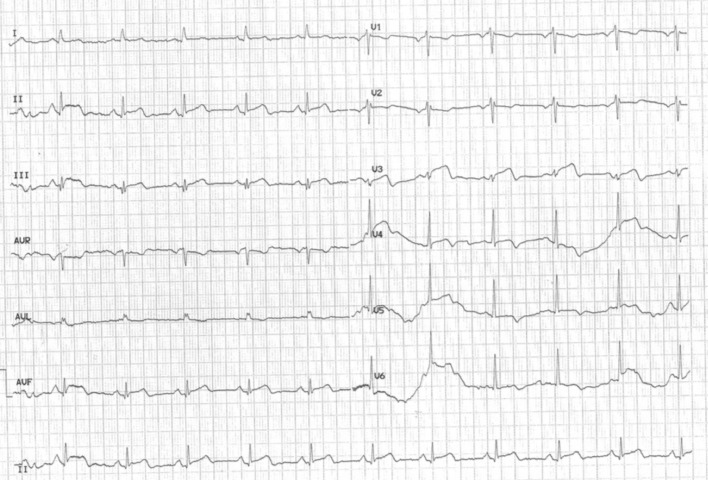
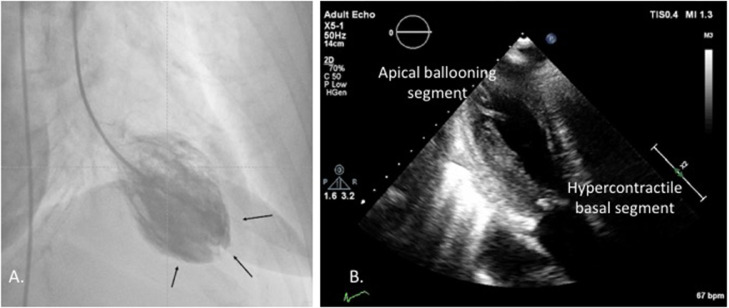
A 64-year-old woman with a history of previous intravenous drug abuse on opioid substitution treatment with buprenorphine, a partial opioid agonist, presented to the emergency department complaining of chest discomfort and dyspnea of sudden onset. She reported history of chronic hepatitis C, dyslipidemia, and smoking. She had started treatment with buprenorphine 3 years previously and was on levothyroxine sodium 125 μg od for hypothyroidism. However, she abstained from buprenorphine for 3 days. On arrival, she was hemodynamically stable but in distress with tachycardia, tachypnea, and bilateral rales. The patient's vital signs on admission were the following: blood pressure 105/62 mmHg, 80 beats per min, 96% SPO2 (FIO2 21%), and respiratory rate of 22 per minute. The electrocardiogram showed sinus rhythm with ST elevation in the inferior leads and T wave inversion in the anterolateral leads (Fig. 1). Admission laboratory work showed elevated troponin levels (Table 1). Our patient underwent urgent coronary angiography, revealing no obstructive coronary artery disease. Apical ballooning indicative of stress-induced or takotsubo cardiomyopathy was found in left ventriculography (Fig. 2A, Video 1A).
Fig. 1.
Electrocardiogram on admission. Twelve-lead electrocardiogram performed on admission showing sinus rhythm with ST elevation in the inferior leads and T wave inversion in the anterolateral leads.
Table 1.
Results of laboratory examinations
| Normal range | units | 1st day | 2nd day | 3rd day | |
|---|---|---|---|---|---|
| WBC | 5,2 - 12,4 | x10.e3 /uL | 7.04 | 7.01 | 5.98 |
| RBC | 4,2 - 6,1 | x10.e6 /uL | 4.65 | 4.64 | 4.88 |
| HGb | 12 - 18 | g/dL | 13 | 12.7 | 13,5 |
| HCT | 37 - 52 | % | 40 | 38.7 | 41.6 |
| PLT | 130 - 400 | x10.e3 /uL | 258 | 249 | 234 |
| Neut % | 40 - 74 | % | 74.3 | 72.7 | 68.1 |
| Lymph % | 19 - 48 | % | 15.5 | 19.3 | 22.7 |
| CRP | 0-5 | mg/L | 18.6 | 19.2 | |
| Glucose | 70-105 | mg/dL | 114 | 162 | 103 |
| Urea | 15-43 | mg/dL | 32 | 21 | 23 |
| Creatinine | 0.57-1.11 | mg/dL | 0.8 | 0.7 | 0.7 |
| K | 3.5-5.1 | mmol/L | 4.1 | 4.4 | 5.4 |
| Na | 136-145 | mmol/L | 140 | 133 | 135 |
| Ca | 8.4-10.2 | mg/dL | 8.9 | 8.4 | 9 |
| Mg | 1.6-2.6 | mg/dL | 1.7 | 2 | |
| P | 2.4-4.7 | mg/dL | 3.4 | ||
| ALT | 5-34 | U/L | 27 | 42 | 37 |
| AST | 0-55 | U/L | 12 | 12 | 13 |
| LDH | 125-220 | U/L | 255 | 425 | 631 |
| CPK total | 29-168 | U/L | 116 | 314 | 129 |
| γ GT | 9-36 | U/L | 11 | 9 | 10 |
| Total bilirubin | 0.2-1.2 | mg/dL | 0.2 | 0.4 | 0.5 |
| Total CHOL | 0-200 | mg/dL | 200 | ||
| HDL | >40 | mg/dL | 46 | ||
| LDL | <130 | mg/dL | 137 | ||
| Tg | 0-150 | mg/dL | 90 | ||
| TSH | 0.35-4.95 | μIU/ml | 0.24 | ||
| FT3 | 1.71-3.71 | pg/ml | 2.19 | ||
| FT4 | 0.7-1.48 | ng/dl | 1.24 | ||
| hs troponin I | <15.6 | pg/ml | 844.9 | 2989.2 | 2731.8 |
| INR | 1.2 |
Abbreviations: WBC, white blood cells; RBC, red blood cells; HGb, hemoglobin; HCT, hematocrit; PLT, platelets; Neut, neutrophils; Lymph, lymphocytes; CRP, C reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; γ GT, gamma-glutamyl transpeptidase; CHOL, cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; Tg, triglycerides; TSH, thyroid stimulating hormone; FT3, free triiodothyroinine; FT4, free thyroxine.
Fig. 2.
(A) Left ventriculography. Cardiac catheterization demonstrating left ventricular anterior-apical akinesis with compensatory inferior-posterior hypercontractility. (B) Transthoracic heart ultrasound. Echocardiography reveals left ventricular ejection fraction of 30% with apical ballooning pattern, i.e. apical akinesis with hypercontractility of the basal segments of the myocardium.
The patient was admitted hemodynamically stable in the coronary care unit with signs and symptoms of heart failure, attributed to takotsubo cardiomyopathy possibly induced by buprenorphine withdrawal, for close monitoring. Echocardiography on admission revealed apical akinesis with hypercontractility of the basal segments of the myocardium with reduced left ventricular ejection fraction ∼30% (Fig. 2B, Video 1). Electrocardiography on admission showed sinus rhythm with ST elevation in the inferior leads and T wave inversion in the anterolateral leads. Treatment with beta-blocker, angiotensin-converting enzyme inhibitor, and mineralcorticoid receptor antagonist was initiated. She remained hemodynamically stable with similar echocardiographic and electrocardiographic findings on the 2nd day of hospitalization (Online Fig. S1). However, on the 3rd day of hospitalization, the patient presented right-sided hemiplegia and motor aphasia. Echocardiography enhanced by contrast showed apical ballooning, without obvious intracardiac thrombus but with prominent spontaneous contrast (Video 1B). Therefore, low molecular weight heparin was initiated for thromboembolism prevention. However, on the fourth day our patient's course was complicated by a large ischemic stroke, presented as right-sided hemiplegia and motor aphasia (left middle cerebral artery territory). Carotid artery dissection was ruled out by means of contrast computed tomography, which revealed filling defect in the left distal middle cerebral artery (M1) segment, probably of thromboembolic origin. Unfortunately, our patient rapidly deteriorated, was intubated and mechanically ventilated but died several days later.
Discussion
Stress-induced cardiomyopathy or takotsubo syndrome is characterized by transient left ventricular dysfunction and electrocardiographic changes that mimic acute coronary syndrome in the absence of obstructive coronary heart disease [1, 2]. Takotsubo cardiomyopathy usually appears in postmenopausal women following mental or physical stress resulting in massive catecholamine release, inflammatory response, and cardiac stunning. Full recovery of left ventricular dysfunction is usually observed within days, however complications such as acute heart failure, mitral regurgitation, apical thrombus formation, and life-threatening arrhythmias or even cardiac arrest have been reported [3], [4], [5], [6].
Opioid withdrawal mediates sympathetic overdrive and may trigger takotsubo syndrome development, however it has been rarely reported in the literature [7], [8], [9]. Buprenorphine acts as a partial agonist of the mu opioid receptor and as a kappa opioid antagonist, and chronic administration produces dependence of the opioid type [10]. Abrupt discontinuation leads to withdrawal syndrome.
In our case we hypothesize that stress-induced cardiomyopathy was triggered by sympathetic hyperactivity and catecholamine surge following buprenorphine withdrawal, with symptoms presenting 3 days post discontinuation, which can be explained by the drug's long elimination half-life, estimated to be between 24 and 42 hours. Prophylactic administration of clonidine, a central sympatholytic drug which decreases presynaptic alpha-2 receptors in the locus coeruleus, has been suggested as a potential therapeutic option to reduce the risk of cardiotoxicity and mitigate opioid withdrawal symptoms. However, clonidine may reduce cardiac output due to its anti-adrenergic properties, thus its use in patients with heart failure should be carefully considered.
In conclusion, abrupt discontinuation of long-term opioids may induce malignant complications such as stress-induced cardiomyopathy. As substitution therapies are today more widely used, vigilance and awareness of treating physicians should be raised upon the potential for patients to develop cardiotoxicity following drug withdrawal, to ensure appropriate management and favorable outcome.
Conflict of interest
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.11.012.
Appendix. Supplementary materials
References
- 1.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–441. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 5.Lazaros G, Dimitriadis K, Xanthopoulou M, Latsios G, Antoniou C, Lazarou E, Tousoulis D. Takotsubo cardiomyopathy and Parkinson's disease: An exceptionally uncommon clinical duet. Hellenic J Cardiol. 2019;60:334–335. doi: 10.1016/j.hjc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Latsios G, Synetos A, Mastrokostopoulos A, Vogiatzi G, Bounas P, Nikitas G, Papanikolaou A, Parisis C, Kanakakis I, Goudevenos J. Cardiopulmonary resuscitation in patients with suspected or confirmed Covid-19. A consensus of the Working group on CardioPulmonary Resuscitation of the Hellenic Society of Cardiology. Hellenic J Cardiol. 2021;62:24–28. doi: 10.1016/j.hjc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemesle F, Lemesle F, Nicola W, Pierre Jonville-Béra A. First case of stress cardiomyopathy as a result of methadone withdrawal secondary to drug-drug interaction. Am J Emerg Med. 2010;28:387. doi: 10.1016/j.ajem.2009.07.007. .e5-6. [DOI] [PubMed] [Google Scholar]
- 8.Saiful FB, Lafferty J, Jun CH, Teli S, Duvvuri S, Khattri S, Bhat T. Tako-Tsubo cardiomyopathy due to iatrogenic methadone withdrawal. Rev Cardiovasc Med. 2011;12:164–167. [PubMed] [Google Scholar]
- 9.Spadotto V, Zorzi A, El Maghawry M, Meggiolaro M, Pittoni GM. Heart failure due to ‘stress cardiomyopathy’: a severe manifestation of the opioid withdrawal syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2:84–87. doi: 10.1177/2048872612474923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkader A, Sproule B. Buprenorphine: clinical pharmacokinetics in the treatment of opioid dependence. Clin Pharmacokinet. 2005;44:661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.