Abstract
Introduction
Prospective data on impact of educational attainment on prognosis in patients with chronic kidney disease (CKD) are scarce. We investigated the association between educational attainment and all-cause mortality, major adverse cardiovascular (CV) events (MACEs), kidney failure requiring dialysis, and CKD etiology.
Methods
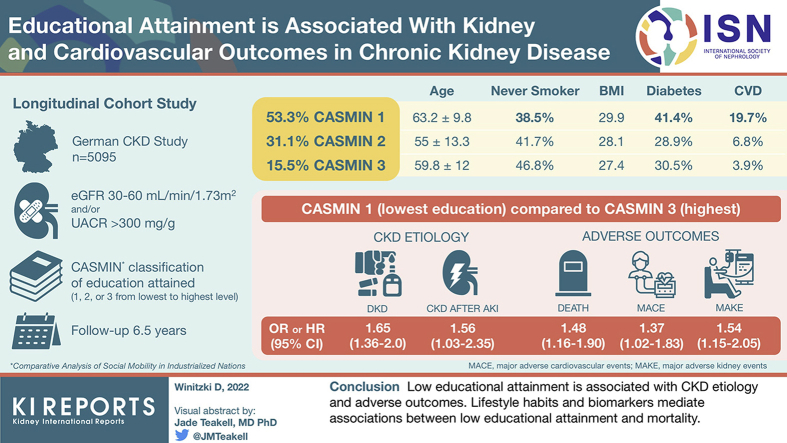
Participants (N = 5095, aged 18–74 years) of the ongoing multicenter German Chronic Kidney Disease (GCKD) cohort, enrolled on the basis of an estimated glomerular filtration rate (eGFR) of 30 to 60 ml/min (stages G3, A1–A3) or overt proteinuria (stages G1–G2, A3), were divided into 3 categories according to their educational attainment and were followed for 6.5 years.
Results
Participants with low educational attainment (vs. high) had a higher risk for mortality (hazard ratio [HR] 1.48, 95% CI: 1.16–1.90), MACE (HR 1.37, 95% CI: 1.02–1.83), and kidney failure (HR 1.54, 95% CI: 1.15–2.05). Mediators between low educational attainment and mortality were smoking, CV disease (CVD) at baseline, low income, higher body mass index, and higher serum levels of CRP, high-density lipoprotein cholesterol, uric acid, NGAL, BAP, NT-proBNP, OPN, H-FABP, and urea. Low educational attainment was positively associated with diabetic nephropathy (odds ratio [OR] 1.65, 95% CI: 1.36–2.0) and CKD subsequent to acute kidney injury (OR 1.56, 95% CI: 1.03–2.35), but negatively associated with IgA nephropathy (OR 0.68, 95% CI: 0.52–0.90).
Conclusion
Low educational attainment is associated with adverse outcomes and CKD etiology. Lifestyle habits and biomarkers mediate associations between low educational attainment and mortality. Recognition of the role of educational attainment and the associated health-relevant risk factors is important to optimize the care of patients with CKD and improve prognosis.
Keywords: biomarker, CASMIN, chronic kidney disease, CKD, educational attainment, socioeconomic status
Graphical abstract
CKD is a global health issue characterized by a high burden of comorbidities and mortality.1 The global prevalence of CKD has been estimated at 13.4% and is approximately 15% higher in low-income countries compared with high-income (industrialized) countries.2,3 One of the many factors that likely contributes to regional differences in CKD prevalence is educational attainment. Education is more often used than income to estimate socioeconomic status (SES).4 Persons with low educational attainment are more likely to develop risk factors for CKD, such as obesity, diabetes, and hypertension, than those with higher educational attainment,5, 6, 7 which may explain their higher incidence rates of CKD and kidney failure.8 Moreover, low education may favor the development of specific types of CKD, such as diabetic nephropathy, given the increased prevalence of diabetes mellitus in persons with low educational attainment. Until now, most observations on the association of educational attainment and all-cause CKD have been made in cross-sectional data.9,10 The association between educational attainment and CKD etiology has not yet been investigated in detail, although it would add to the overall understanding of the impact of educational attainment on CKD development and health-relevant outcomes. Studies on the association between educational attainment in patients with CKD and mortality or CVD in a longitudinal study setting are sparse. Besides 2 small studies in patients on dialysis,11,12 only Morton et al.4 found low educational attainment to be associated with increased risk of death among adults with CKD in the Study of Heart and Renal Protection. The authors, however, did not investigate systemically potential mediators of this association. In fact, they revealed that low educational attainment is associated with death even after adjustment for many demographic and clinical variables, suggesting mediation by other noninvestigated variables. Identification of such variables may result in the discovery of new targets for disease prevention and management.
The GCKD study is a well-characterized national prospective observational cohort study of >5000 patients to identify risk factors and biomarkers for the progression of CKD and CV complications.13 The aim of the current study is to evaluate the association between educational attainment and the adjudicated outcomes of death, CV events, kidney failure requiring kidney replacement therapy, and CKD etiology. Furthermore, the impact of educational attainment on these outcomes through lifestyle factors and novel CKD and CVD biomarkers was investigated.
Methods
Study Design and Population
Between 2010 and 2012, the GCKD study enrolled 5217 participants of European ancestry aged 18 to 74 years with an eGFR of 30 to 60 ml/min per 1.73 m2 or an eGFR ≥ 60 ml/min per 1.73 m2 in the presence of severely increased proteinuria/albuminuria (i.e., >300 mg/g creatinine).13,14 Main exclusion criteria were non-European ancestry, active malignancy in the previous 2 years, previous transplantations, or heart failure New York Heart Association class IV. Participants with missing information on their educational attainment (n = 122) were excluded from the current analysis. Thus, we analyzed a total of 5095 participants (Supplementary Figure S1). The follow-up time was 6.5 years.
Every participant in the study gave written informed consent. The ethics committees of all 9 German participating institutions approved the study. The study was carried out in accordance with approved guidelines and the Declaration of Helsinki. The reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology were followed. The study was registered at the German Clinical Trials Register (www.drks.de; ID DRKS00003971).
Baseline Variables
Information on sociodemographic factors, medical history, and medications was obtained by trained study teams through standardized questionnaires13 (Supplementary Methods). Information on the presumed leading cause of CKD was obtained by the participant’s treating nephrologists, who were asked to choose from a given list of etiology categories.14 For a subgroup analysis of the association between educational attainment and healthy dietary patterns, dietary information was evaluated using a self-administered food frequency questionnaire in the GCKD follow-up study visit in year 2 (2012–2014)15 (Supplementary Methods and Supplementary Figure S1). Participants with information on dietary intake (n = 3129) had similar demographic and clinical characteristics compared with the total GCKD study population (Supplementary Table S1). Observance of healthy dietary patterns was evaluated by the calculation of the Dietary Approaches to Stop Hypertension diet, Mediterranean diet, and CKD diet scores as described15,16 (Supplementary Methods). A set of nonfasting laboratory parameters and biomarkers was analyzed by a central laboratory13 (Supplementary Methods).
Exposure
The CASMIN (Comparative Analysis of Social Mobility in Industrial Nations) classification of education, which is one of the most widely used international comparable measurement instruments for SES, has been used to determine the individual educational attainment.17 The CASMIN classification distinguishes 3 main levels of educational attainment based on the reported highest level of schooling and professional training, which are as follows: low (CASMIN 1), medium (CASMIN 2), and high (CASMIN 3) (Supplementary Table S2). Educational attainment, and not other measures of SES, such as income, was used as the primary exposure because it is less likely to be influenced by CKD18 given that educational training is usually completed before incidence of CKD.
Outcomes
The outcomes included all-cause mortality; MACE defined as a composite of nonfatal myocardial infarction, stroke, and incident peripheral vascular disease; and incident of kidney failure requiring kidney replacement therapy (i.e., end-stage renal disease), a composite of incident dialysis or kidney transplantation. All events until December 2019 (data export) were taken into account for the analysis.
Statistical Analysis
The current study design is found in Supplementary Figure S2. Baseline characteristics were compared across different educational attainment levels using univariable ordinal regression analysis. We describe the baseline characteristics for the population overall and for the different educational attainment levels using mean values and SDs for normally distributed variables and median values and interquartile ranges for non-normally distributed variables. The values of categorical variables are presented as frequency distributions with percentages. Association between educational attainment and CKD etiology at baseline was analyzed by logistic regression analysis, adjusted for the nonmodifiable risk factors of age and gender. As some rare causes of CKD etiology were small in sample size, we did not adjust further the regression analysis. Mixed graphical models (MGMs)19, 20, 21 (Supplementary Methods) were used to identify baseline variables independently associated with educational attainment. An MGM is an unbiased and data-driven powerful tool to eliminate indirect associations discovered by routine univariate screening approaches and can reveal associations adjusted for all other variables in the data set.19, 20, 21 The identified variables were considered as mediator candidates between educational attainment and outcomes and were used in the Cox proportional hazard regression models (see model 2). A mediator is the variable that causes mediation between the exposure and the outcome variable (see subsequent discussion). MGM requires a complete data matrix. Participants, for whom at least 1 data point was missing (number of excluded participants = 726, 14.3%), were excluded before MGM analysis. Thus, the variables identified to be independently associated with educational attainment by MGMs were estimated based on data of 4369 study participants. Given that dietary information was obtained in follow-up visit year 2, but not at baseline, additional MGMs for each dietary pattern score (i.e., Dietary Approaches to Stop Hypertension diet, Mediterranean diet, and CKD diet score) based on data of 2147 study participants were estimated after exclusion of 982 participants (31%) owing to missing of at least 1 data point. Cox proportional hazard regression models were used to evaluate the association of educational attainment at baseline with all-cause mortality, MACE, and incident kidney failure during follow-up. If patients did not complete the 6.5-year follow-up period, censoring was done at the time of the last follow-up, that is, when participants left the study (did not want to participate in any more follow-up visits) or were lost to follow-up. The hazard estimates obtained from our models were cause specific in the sense that death from any cause, MACE, and kidney failure were considered as mutually exclusive competing events. Thus, in all Cox regression models, patients were censored when any of the competing events occurred before the event of interest. The resulting cause-specific HRs are presented with 95% CIs and visualized by forest plots. We evaluated a series of nested models for all outcomes: (i) unadjusted (univariate); (ii) adjusted for the confounders age and gender (model 1); and (iii) adjusted for age, gender, eGFR, urine albumin-to-creatinine ratio, and all mediator candidates identified by the estimated MGMs (model 2). Dietary pattern scores were not included in the Cox models as dietary information was obtained in follow-up visit year 2, but not at baseline. As we hypothesized that some of the total effect of exposure on outcome operates through mediators, we used the R package mediation to estimate mediation between educational attainment and all-cause mortality, MACE, and incident kidney failure by each potential mediator.8 Here, we simplified our statistical models by using one contrast for educational attainment (low vs. high, excluding those with medium educational attainment). Within a mediation analysis, the total effect is the sum of the direct effect (effect of exposure on outcome absent the mediator) and the indirect effect (effect of exposure on outcome that works through mediator).22 This is different to the epidemiologic concept of effect modification, which evaluates whether a variable changes direction or strength between exposure and outcome variable.23 We reported the proportion which is mediated by each mediator with respect to the total effect of low educational attainment on outcomes in percentages. The percentage is calculated by dividing the average casual mediation effects (indirect effect) by the total effect. Each mediator was analyzed separately and therefore estimates of proportion mediated do not take into account potential overlapping mediation effects. The 2-sided significance level was set to α = 0.05. Analyses were performed using IBM SPSS Statistics version 26, SAS, and R version 3.4.3.24
Results
Baseline Characteristics of the Study Participants
Detailed characteristics of the study participants (N = 5095) stratified by educational attainment are found in Table 1. Almost half of our cohort had low educational attainment, whereas 31.1% and 15.5% had medium and high educational attainment, respectively. In comparison to participants with higher educational attainment, individuals with low educational attainment were older, more frequently obese, had more often a smoking history, had lower income, had more frequently diabetes or CVD, had higher levels of several biomarkers known to be associated with chronic diseases, or used more frequently nonsteroidal anti-inflammatory drugs and other medications for chronic diseases (Table 1). Higher scores of dietary indices, that is, Dietary Approaches to Stop Hypertension, Mediterranean diet, and CKD diet, indicated higher observance of healthy dietary patterns in participants with higher educational attainment (Supplementary Table S3).
Table 1.
Baseline characteristics by educational attainment of GCKD cohort study participants included in the current analyses
| Characteristics | All patients, 5095 (100%) | Educational attainment |
||
|---|---|---|---|---|
| Low, 2718 (53.3%) | Medium, 1586 (31.1%) | High, 791 (15.5%) | ||
| Age, yr | 60.1 ± 11.9 | 63.2 ± 9.8 | 55 ± 13.3 | 59.8 ± 12 |
| Male, n (%) | 3057 (60) | 1648 (60.6) | 834 (52.6) | 575 (72.7) |
| BMI, (kg/m2) | 28.9 (7.5) | 29.9 (7.3) | 28.1 (8) | 27.4 (6.5) |
| Alcohol consumption, n (%) | ||||
| ≥3×/wk | 964 (18.9) | 471 (17.3) | 270 (17.0) | 223 (28.2) |
| <3×/wk | 4103 (80.5) | 2232 (82.1) | 1303 (82.2) | 568 (71.8) |
| Smoking status, n (%) | ||||
| (Former) smoker | 3003 (58.9) | 1663 (61.2) | 920 (58.2) | 420 (53.1) |
| Never smoker | 2079 (40.8) | 1047 (38.5) | 662 (41.7) | 370 (46.8) |
| Annual income, n (%) | ||||
| <25,000€ | 1742 (34.2) | 1144 (42.1) | 456 (28.8) | 142 (18.0) |
| ≥25,000€ | 1989 (39.1) | 784 (28.8) | 718 (45.3) | 487 (61.6) |
| Unknown | 1364 (26.8) | 790 (29.1) | 412 (26.0) | 162 (20.5) |
| Physical activity, n (%) | ||||
| <3×/wk | 2106 (41.3) | 1093 (40.2) | 678 (42.7) | 335 (42.3) |
| ≥3×/wk | 2918 (57.3) | 1580 (58.2) | 890 (56.1) | 448 (56.6) |
| Unknown | 66 (1.3) | 42 (1.5) | 17 (1.1) | 7 (0.9) |
| Private health insurance, n (%) | 353 (6.9) | 84 (3.1) | 105 (6.6) | 164 (20.7) |
| Medical history | ||||
| Diabetes mellitus, n (%) | 1824 (35.8) | 1125 (41.4) | 458 (28.9) | 241 (30.5) |
| Hypertension, n (%) | 4903 (96.2) | 2656 (97.7) | 1485 (94.3) | 752 (95.1) |
| SBP, mm Hg | 140 ± 20 | 141.1 ± 20.9 | 137.2 ± 19.5 | 138.8 ± 19.7 |
| DBP, mm Hg | 79 ± 12 | 78.5 ± 11.8 | 80.5 ± 11.8 | 79.6 ± 11.4 |
| CVD, n (%) | 1591 (30.5) | 1004 (19.7) | 348 (6.8) | 199 (3.9) |
| Gout, n (%) | 1255 (24.6) | 1918 (27.6) | 330 (20.8) | 176 (22.3) |
| Laboratory findings | ||||
| eGFR, ml/min per 1.73 m2 | 49.4 ± 18.2 | 45.7 ± 16.3 | 52.6 ± 20.2 | 51.3 ± 19.2 |
| UACR, mg/g | 50.9 (382.3) | 45.3 (306.7) | 59.6 (499.7) | 58.3 (488.8) |
| UACR categories, n (%) | ||||
| <30 mg/g | 2118 (41.6) | 1171 (43.1) | 630 (39.7) | 317 (40.1) |
| 30–300 mg/g | 1550 (30.4) | 847 (31.2) | 462 (29.1) | 241 (30.5) |
| >300 mg/g | 1408 (27.6) | 689 (25.3) | 489 (30.8) | 230 (29.1) |
| Cholesterol, mg/dl | 211.2 ± 53 | 208.2 ± 52 | 215.7 ± 53.4 | 212.6 ± 56.3 |
| HDL, mg/dl | 52 ± 18.1 | 50.5 ± 17.3 | 54.5 ± 19.4 | 52.5 ± 18 |
| LDL, mg/dl | 118.3 ± 43.5 | 116.1 ± 42.6 | 121 ± 44 | 120.2 ± 46.5 |
| Triglycerides, mg/dl | 199 ± 128 | 202.3 ± 131.6 | 194.9 ± 125.9 | 197.4 ± 119.5 |
| Uric acid, mg/dl | 7.2 ± 1.9 | 7.3 ± 1.9 | 7 ± 1.9 | 7.2 ± 1.9 |
| Hb, g/dl | 13.6 ± 1.7 | 13.6 ± 1.7 | 13.6 ± 1.7 | 13.8 ± 1.8 |
| CRP, mg/l | 2.3 (4) | 2.6 (4.5) | 2 (3.6) | 1.7 (2.8) |
| HbA1c, % | 6.0 (0.9) | 6.1 (1) | 5.9 (0.8) | 6 (0.8) |
| Calcium, mmol/l | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| Phosphorus, mmol/l | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Sodium, mmol/l | 139.7 ± 2.9 | 139.7 ± 3.0 | 139.7 ± 2.8 | 139.8 ± 2.9 |
| Serum NGAL, ng/ml | 82.1 (46) | 84.9 (46.9) | 79.6 (44.4) | 78.9 (41.4) |
| OPN, ng/ml | 29.2 (21.1) | 30.6 (21.9) | 27.3 (19.9) | 28 (20) |
| hs-TropT, mg/ml | 12 (11.3) | 13 (11.2) | 9.7 (9.8) | 12 (11.4) |
| NT-proBNP, pg/ml | 178 (327) | 214 (405.2) | 147 (245) | 146 (245.3) |
| H-FABP, ng/ml | 3.8 (2.6) | 4.1 (2.6) | 3.6 (2.5) | 3.6 (2.6) |
| BAP, μg/l | 16.4 (8.3) | 16.8 (8.8) | 16.7 (7.9) | 15.5 (7.6) |
| iPTH, pg/ml | 37.4 (33.8) | 39.3 (34.5) | 34.1 (33.4) | 35.6 (33.9) |
| Medication use | ||||
| Antihypertensive medication, n (%) | 4703 (92.3) | 2552 (93.9) | 1437 (90.6) | 714 (90.3) |
| Antidiabetic medication, n (%) | 1453 (28.5) | 915 (33.7) | 360 (22.7) | 178 (22.5) |
| Lipid-lowering medication, n (%) | 2602 (51.1) | 1495 (55.0) | 716 (45.1) | 391 (49.4) |
| Gout medication, n (%) | 1673 (32.8) | 988 (36.4) | 446 (28.1) | 239 (30.2) |
| NSAIDs, n (%) | 334 (6.6) | 195 (7.2) | 103 (6.5) | 36 (4.6) |
BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate based on Chronic Kidney Disease-Epidemiology Collaboration equation; GCKD, German Chronic Kidney Disease; Hb, hemoglobin; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; iPTH, intact plasma parathyroid hormone; LDL, low-density lipoprotein; NSAID, nonsteroidal anti-inflammatory drug; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Values are expressed as mean values ± SD, medians (interquartile range), or percentages where appropriate.
CKD Etiology Across Educational Attainment Strata
CKD etiology at baseline, stratified by educational attainment, is summarized in Supplementary Table S4. After adjusting for age and gender, low educational attainment (vs. high) remained positively associated with diabetic nephropathy (OR 1.65, 95% CI: 1.36–2.0) and CKD after acute kidney injury (OR 1.56, 95% CI: 1.03–2.35) (Table 2). In contrast, IgA nephropathy was less common in participants with low educational attainment (low vs. high educational attainment: OR 0.68, 95% CI: 0.52–0.90) (Table 2). In addition, individuals with a medium educational attainment less often developed membranous glomerulonephritis (GN) (medium vs. high educational attainment: OR 0.62, 95% CI: 0.39–1.00) compared with participants with high educational attainment (Table 2). Most cases of primary GN were biopsy proven (Supplementary Table S5).
Table 2.
Association between educational attainment and CKD etiology obtained by binary regression analysis, adjusted for age and gender, in GCKD cohort study participants included in the current analyses
| Outcome | Educational attainment |
||
|---|---|---|---|
| Low | Medium | High (reference) | |
| Diabetic nephropathy | 1.645 (1.355–1.998) | 1.289 (1.035–1.606) | 1.0 |
| Vascular nephropathy | 0.983 (0.830–1.164) | 0.984 (0.816–1.187) | 1.0 |
| Primary GN | 0.870 (0.716–1.058) | 0.800 (0.469–0.986) | 1.0 |
| MPGN | 1.017 (0.421–2.454) | 1.062 (0.438–2.575) | 1.0 |
| Postinfectious GN | 0.867 (0.308–2.440) | 0.765 (0.244–2.392) | 1.0 |
| IgA nephropathy | 0.681 (0.514–0.903) | 0.757 (0.566–1.011) | 1.0 |
| FSGS | 1.493 (0.970–2.297) | 1.209 (0.773–1.892) | 1.0 |
| Rapidly progressive pauci-immune GN | 0.928 (0.396–2.173) | 1.161 (0.468–2.878) | 1.0 |
| Minimal change GN | 1.928 (0.841–4.421) | 1.476 (0.636–3.424) | 1.0 |
| Rapidly progressive anti-GBM GN | 2.569 (0.291–22.645) | 0.284 (0.017–4.690) | 1.0 |
| Membranous GN | 0.951 (0.625–1.447) | 0.620 (0.385–0.999) | 1.0 |
| Other | 0.936 (0.652–1.345) | 0.829 (0.562–1.222) | 1.0 |
| Systemic disease | 1.096 (0.842–1.426) | 1.115 (0.846–1.469) | 1.0 |
| Granulomatosis with polyangiitis | 0.756 (0.434–1.315) | 1.476 (0.851–2.561) | 1.0 |
| Scleroderma | 0.197 (0.026–1.484) | 0.179 (0.016–2.036) | 1.0 |
| Microscopic polyangiitis | 0.753 (0.395–1.436) | 0.856 (0.428–1.712) | 1.0 |
| TTP | 0.359 (0.070–1.850) | 0.239 (0.039–1.477) | 1.0 |
| Amyloidosis | 1.055 (0.115–9.684) | 2.203 (0.260–18.676) | 1.0 |
| Lupus erythematosus | 0.953 (0.552–1.645) | 0.872 (0.514–1.478) | 1.0 |
| Sarcoidosis | 1.324 (0.437–4.010) | 1.300 (0.412–4.109) | 1.0 |
| Gout nephropathy | 1.622 (0.925–2.844) | 0.911 (0.463–1.794) | 1.0 |
| Interstitial nephropathy | 0.981 (0.727–1.325) | 1.206 (0.877–1.657) | 1.0 |
| Analgesic nephropathy | 1.701 (0.942–3.072) | 1.786 (0.952–3.352) | 1.0 |
| Hereditary disease | 0.829 (0.539–1.275) | 1.038 (0.673–1.601) | 1.0 |
| ADPKD | 0.831 (0.551–1.278) | 1.024 (0.664–1.581) | 1.0 |
| Acute kidney injury | 1.555 (1.027–2.354) | 1.158 (0.728–1.840) | 1.0 |
| Single kidney | 0.841 (0.605–1.170) | 1.205 (0.850–1.709) | 1.0 |
| Obstructive nephropathy | 1.015 (0.732–1.408) | 0.190 (0.844–1.678) | 1.0 |
| Miscellaneous | 1.075 (0.719–1.606) | 1.080 (0.701–1.662) | 1.0 |
| Undetermined | 0.879 (0.640–1.208) | 0.815 (0.573–1.160) | 1.0 |
ADPKD, autosomal dominant polycystic kidney disease; anti-GBM, antiglomerular basement membrane; CKD, chronic kidney disease; FSGS, focal segmental glomerulosclerosis; GCKD, German Chronic Kidney Disease; GN, glomerulonephritis; MPGN, membranoproliferative glomerulonephritis; TTP, thrombotic thrombocytopenic purpura.
Numbers represent odds ratio and 95% CI. Bold values indicate statistical significance.
Variables Independently Associated With Educational Attainment as Identified by MGM Analysis
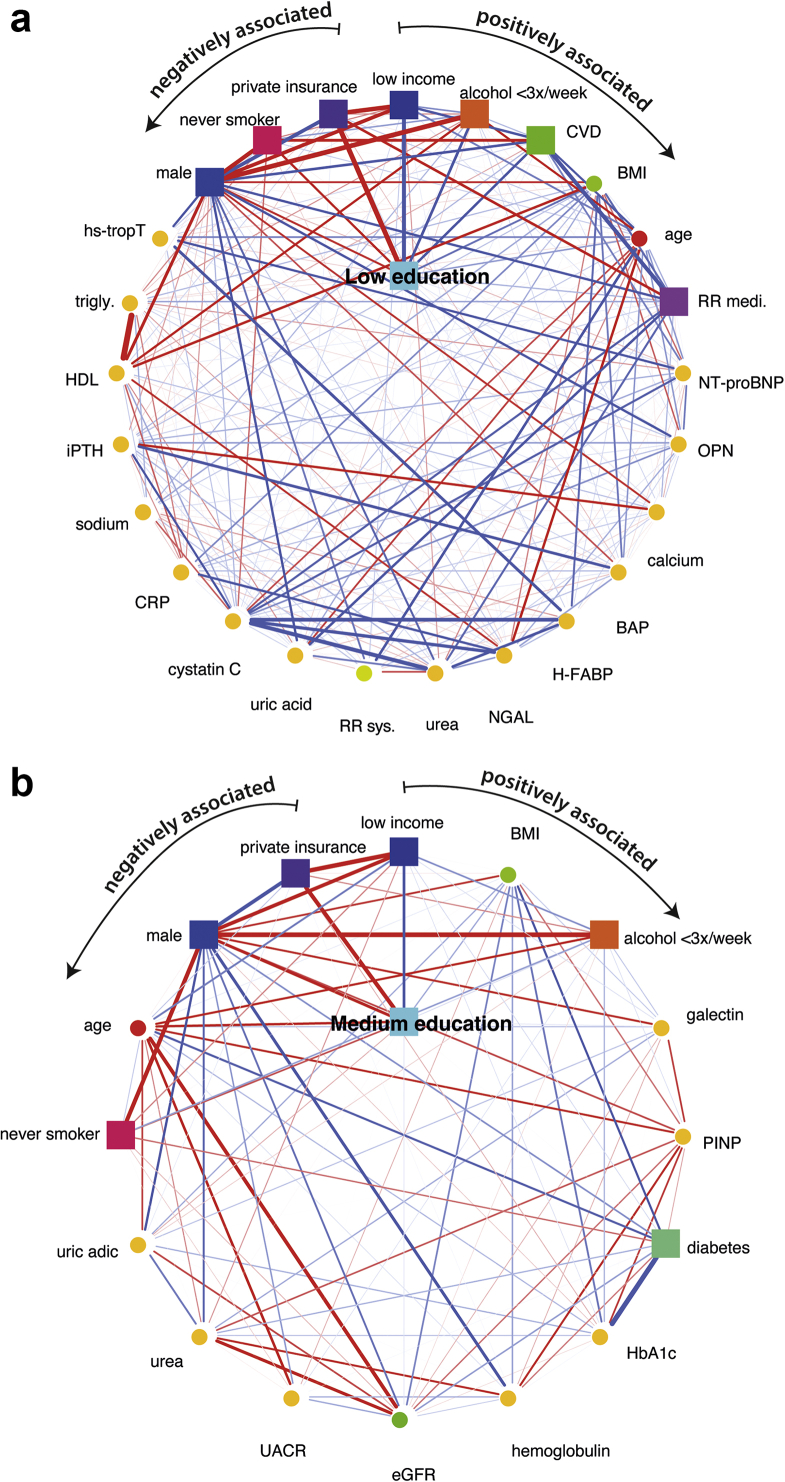
Compared with high educational attainment, low educational attainment was associated with lower income, lower alcohol consumption, prevalence of CVD, higher age and higher body mass index, intake of blood pressure medication, and higher serum levels of NT-proBNP and OPN (Figure 1a). Medium educational attainment (vs. high educational attainment) was associated with lower income, higher body mass index, lower alcohol consumption, and higher serum galectin levels (Figure 1b). The strengths of the associations with low or medium educational attainment, compared with high educational attainment, are presented in Supplementary Table S6. Separate MGM analysis including dietary information from the follow-up study visit in year 2 revealed that low versus high educational attainment was associated with poor observance of healthy dietary patterns (Supplementary Figure S3a, c, and e). No associations were found between dietary scores and medium level of educational attainment, compared with high educational attainment (Supplementary Figure S3b, d, and f).
Figure 1.
Variables independently associated with low or medium educational attainment compared with high educational attainment identified by the MGM algorithm in GCKD cohort study participants included in the current analyses. In the network representation, yellow nodes represent clinical chemistry parameters. Continuous variables are represented as circles and discrete variables as rectangles. Positive and negative associations are illustrated as blue and red edges, respectively. The strength of the association, that is, the weight of the corresponding coefficient, is encoded by the edge width. (a) First-order neighborhood of low versus high educational attainment. The first-order neighborhood as identified by the MGM comprises only nodes that are directly associated with low versus high educational attainment. The edges are ordered according to their strength in a clockwise manner for positive (from low income to CRP) and in an anticlockwise manner for negative associations (from private insurance to sodium), respectively. (b) First-order neighborhood of medium versus high educational attainment. The edges are ordered according to their strength in a clockwise manner for positive (from low income to HbA1c) and in an anticlockwise manner for negative associations (from private insurance to hemoglobulin), respectively. BMI, body mass index; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GCKD, German Chronic Kidney Disease; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; iPTH, intact plasma parathyroid hormone; MGM, mixed graphical model; trigly, triglyceride.
Association of Educational Attainment With All-Cause Death, MACE, and Kidney Failure
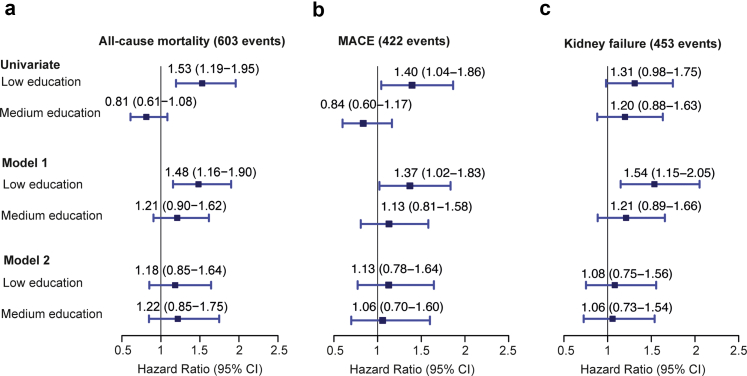
Compared with higher educational attainment, all-cause mortality was 48% higher among those with low educational attainment after adjustment for age and gender (model 1; HR 1.48, 95% CI: 1.16–1.90, 603 events) (Figure 2). Similarly, risk of MACE and risk for kidney failure were 37% and 54% higher among those with low educational attainment compared with high educational attainment (HR 1.37, 95% CI: 1.02–1.83, 422 events and HR 1.54, 95% CI: 1.15–2.05, 453 events, respectively) (Figure 2). After adjustment for eGFR, urine albumin-to-creatinine ratio, and potential mediators identified by our MGM approach (model 2), the association of educational attainment with mortality, MACE, and kidney failure was no longer significant (Figure 2).
Figure 2.
Association of educational attainment with all-cause mortality, MACE, and kidney failure in GCKD cohort study participants included in the current analyses. Results are presented as HRs with 95% CIs given in parentheses. The size of the square representing a relative risk is proportional to its inverse variance. High educational attainment served as the reference group. Model 1: Cox proportional hazard model adjusted for age and gender. Model 2: Cox proportional hazard model adjusted for age, gender, eGFR, urine albumin-to-creatinine ratio, and all potential mediators identified by mixed graphical model including BMI, smoking status (former + current vs. never smoker), alcohol consumption (≥3×/wk vs. <3×/wk), cardiovascular disease (yes vs. no), systolic blood pressure, antihypertensive medication (yes vs. no), HDL cholesterol, triglycerides, uric acid, calcium, urea, sodium, serum neutrophil gelatinase-associated lipocalin, bone-specific alkaline phosphatase, C-reactive protein, intact plasma parathyroid hormone, N-terminal pro–B-type natriuretic peptide, high-sensitive troponin T, OPN, H-FABP, income (<25,000€ vs. ≥25,000€), and health insurance (private vs. public). BMI, body mass index; eGFR, estimated glomerular filtration rate; GCKD, German Chronic Kidney Disease; HDL, high-density lipoprotein; HR, hazard ratio; MACE, major adverse cardiovascular event; UACR, urine albumin-to-creatinine ratio.
Mediators Between Educational Attainment and Outcomes
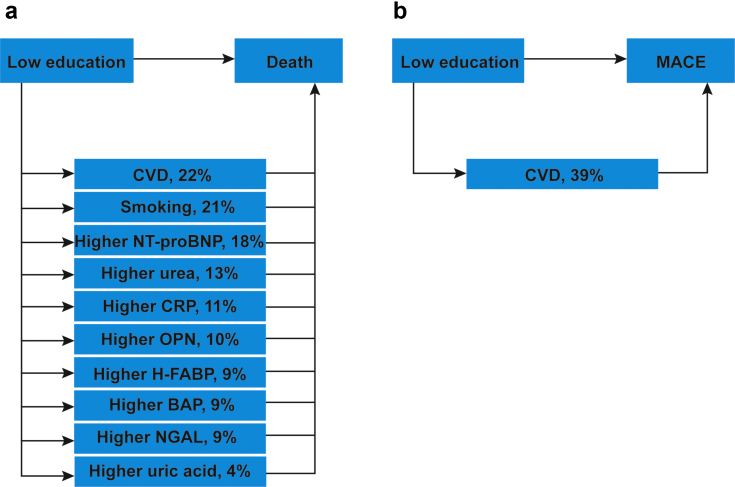
The mediation analysis found the association between low educational attainment and all-cause mortality to be mediated by smoking, prevalence of CVD, and higher serum CRP, uric acid, NGAL, BAP, NT-proBNP, OPN, H-FABP, and urea levels (Figure 3a and Supplementary Table S7). The association between low educational attainment and MACE was mediated by prevalence of CVD (Figure 3b and Supplementary Table S7). No mediators were identified between low educational attainment and kidney failure (Supplementary Table S7).
Figure 3.
Low educational attainment effect on outcomes by mediators in GCKD cohort study participants included in the current analyses. The mediation model aims to evaluate the mediators between low educational attainment and outcomes, after adjustment for age, sex, estimated glomerular filtration rate, and urine albumin-to-creatinine ratio. The percentages describe the proportion which is mediated by each mediator with respect to the total effect of low educational attainment on outcomes. The percentage is calculated by dividing the average casual mediation effects by the total effect (direct and indirect effects of low educational attainment on outcomes). For example, the mediated effect of low educational attainment on death by smoking status was 21%. Each mediator was analyzed separately, and therefore estimates of proportion mediated do not take into account potential overlapping mediation effects. (a) Mediated effect of low educational attainment on death. (b) Mediated effect of low educational attainment on MACE. CRP, C-reactive protein; CVD, cardiovascular disease; GCKD, German Chronic Kidney Disease; MACE, major adverse cardiovascular event.
Discussion
In this study, we observed higher incidence of mortality, MACE, and kidney failure among patients with CKD with low educational attainment in a large and well-defined CKD cohort. Mediators between educational attainment and outcomes were identified and evaluated in an unbiased manner, and smoking, prevalent CVD, and several biomarkers were found to be relevant in this context. We further revealed a greater burden of diabetic nephropathy and CKD subsequent to acute kidney injury in participants with low educational attainment. In contrast, IgA nephropathy and membranous GN were more prominent in participants with high educational attainment.
By using the MGM approach, we found low educational attainment to be independently associated with known health-relevant risk factors, such as older age, higher body mass index, low income, and smoking, including comorbidities such as prevalent CVD. Levels of alcohol consumption were higher in participants with higher educational attainment, an observation that has been also made in adults with CKD from the Study of Heart and Renal Protection.4 Lower levels of alcohol consumption among CKD participants with a low educational attainment may be due to abstinence as a result of decline in health conditions, underreporting of drinking amounts, or both.25 A healthy diet, as measured by various dietary pattern scores, was less prevalent among participants with low educational attainment. Several studies have revealed that higher adherence to these diets is associated with lower risk for several adverse outcomes.26,27 All these variables were likely to mediate the effects of educational attainment on adverse health outcomes.
We also found low educational attainment to be associated with all-cause mortality, MACE, and kidney failure. Similarly, Morton et al.4 described an increased risk for all-cause mortality in lower educated patients with CKD in the Study of Heart and Renal Protection, which persisted even after adjustment for several covariates. In contrast to their study, our Cox models were no longer significant after adjustment for potential mediators, suggesting we found potentially more potent mediators between educational attainment and outcomes. Morton et al.4 also observed an increased risk for vascular events, but they did not observe any association between educational level and CKD progression to kidney failure, although some other studies observed an inverse association between SES and kidney function.8,28,29 The Study of Heart and Renal Protection excluded patients with CKD with history of coronary heart disease.4 Because we did not exclude patients with prevalent CVD, which is common in CKD, our observational study may represent real-life conditions more closely.4
The mediation analysis revealed smoking history as a potential mediator between low educational attainment and mortality. This suggests that smoking is a significant cause of adverse outcomes in participants with lower educational attainment. This observation is of high relevance for intervention strategies and has high policy and practical implications. For example, health care providers and nephrologist should be trained to use plain language and techniques such as the teach-back method to deliver health risks by smoking and to increase health literacy among persons with lower educational attainment. There is a rising interest in new cardiac and kidney biomarkers for early detection and prognosis of heart and kidney disease. We had the unique opportunity to evaluate for the first time the gradient of several biomarkers and its impact as mediators on CV and kidney outcomes across educational attainment. We found serum levels of H-FABP, NT-proBNP, urea, OPN, BAP, uric acid, and CRP to be mediators between educational attainment and mortality. In addition, NGAL was identified as a mediator between low educational attainment and mortality. Although several studies indicated H-FABP,30,31 NT-proBNP,32,33 BAP,34,35 OPN,36 and NGAL37,38 as highly predictive for mortality, CV events, or kidney failure, only 2 studies addressed the association between educational attainment and biomarkers so far. Vart et al.39 revealed in the Atherosclerosis Risk in Communities cohort an inverse association between SES and NT-proBNP. Another study revealed a correlation between low income and higher BAP levels.40 Our analysis with several novel biomarkers helps to better understand the association between educational attainment and important outcomes and indicates that educational attainment should be acknowledged as a covariate in future studies when predictive values of biomarkers in CV and kidney disease are investigated.
Consistent with previous studies,41,42 participants with lower educational attainment had more frequently diabetes mellitus, which likely explains the higher prevalence of diabetic nephropathy among the participants with low educational attainment in our cohort. Wolf et al.6 also revealed an association between low SES and diabetic nephropathy, supporting our findings. All known risk factors for acute kidney failure such as older age, diabetes mellitus, hypertension, CVD, and intake of potential nephrotoxins such as nonsteroidal anti-inflammatory drugs43 were more prevalent in participants with low educational attainment in the current study. Thus, our study provides evidence that CKD among participants with low educational attainment may have been caused by modifiable risk factors, such as diabetes mellitus, hypertension, and high intake of analgesics.
Data on the association between autoimmune kidney disease and socioeconomic deprivation are sparse44,45 and contradictory.46,47 We observed an increased prevalence of IgA nephropathy and membranous GN in participants with high educational attainment. A retrospective study found no association between low income and IgA nephropathy, but between low income and higher incidence of lupus nephritis and antineutrophil cytoplasmic autoantibody-related GN.46 Another study observed greater social deprivation to be associated with higher incidence of IgA nephropathy.47 There are important differences between these studies and our analysis. First, we used educational attainment instead of income, because income may decrease owing to CKD,18 which in turn limits conclusions on the association between income and possible causes of CKD. Second, our well-characterized CKD cohort included participants from all over Germany, whereas the samples of the other studies were either primarily urban or only from 1 province and lacking data regarding specific comorbidities and lifestyle habits. Third, the latter study47 did not adjust for age and gender, which could have influenced the results.
The hygiene hypothesis was proposed as a mechanism to explain the marked increase of some types of GN in high-income countries.48 According to this hypothesis, early and frequent exposures to bacterial and other antigens cause a switch from T helper (Th)2-cell to Th1-cell phenotype, but improved control of infections and higher quality of life may cause a persistence of Th2-cell phenotype, and thus increase risk for allergies or autoimmune disease. Consistent with this hypothesis, GN with predominant Th1 immune response such as membranoproliferative GN is more prevalent in low-income countries, whereas IgA nephropathy or membranous GN as examples of Th2-dependent glomerular disease is more prevalent in high-income countries.48 In our study, the increased prevalence of IgA nephropathy and membranous GN in participants with high educational attainment is consistent with this theory. Membranoproliferative GN was too rare in our cohort (n = 45) to allow meaningful association studies with lower educational attainment.
Our study has several strengths and limitations. The strengths are the large CKD cohort size, the follow-up time of >6 years, the use of standardized questionnaires to evaluate participants’ characteristics, in-person study visits conducted by trained study nurses, and the centralized measurement of many laboratory parameters, including novel biomarkers. Outcomes were evaluated continuously by experienced physicians according to predefined criteria in a standardized fashion based on hospital discharge letters and death certificates. These strengths provided the unique opportunity to systematically and prospectively evaluate the association between educational attainment, CKD etiology, and important health outcomes. Our study was limited by missing information of the annual gross income in almost one-third of the participants. A possible explanation for the low response rate regarding income could be the reluctance to share this private information. Dietary intake may differ in participants who did not return the food frequency questionnaire, although the participants with dietary information had similar baseline characteristics compared with the total cohort. The participants were enrolled in Germany on the basis of prevalent nondialysis-dependent CKD, thus there is a selection bias of participants owing to this defined study cohort, which restricts the generalizability of the findings to other populations including more severe cases of CKD or populations in other countries or of different ancestry. More research is needed to understand the complex interplay between education, ethnicity, SES, and comorbidities on kidney and heart outcomes on the population level, for example, registry data. We defined kidney failure as initiation of maintenance dialysis therapy or kidney transplantation, but differences in practice patterns and patient preferences could influence the commencement of kidney replacement therapy, and thus the number of kidney outcome events. Therefore, future studies should also focus on GFR slope as a surrogate end point for kidney disease progression to evaluate the impact of educational attainment on CKD progression.
In conclusion, our study highlights the role of educational attainment on the development of CKD by specific causes and suggests an association of low educational attainment with increased mortality and poorer kidney and CV outcomes, even in a country with universal accessibility to health care. Health policy planners, physicians, and other health care workers should acknowledge the individual educational attainment and its associated health-relevant risk factors to optimize the care of patients with CKD and improve prognosis.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank all German Chronic Kidney Disease (GCKD) study participants for their time and important contributions, all participating nephrology practices and outpatient clinics for their continued support, and the GCKD study personnel for their enormous commitment. The authors are very grateful for the willingness and time of all study participants of the GCKD study. The enormous effort of the study personnel at the regional centers is highly appreciated. The authors also thank the large number of nephrologists for their support of the GCKD study (list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org). The GCKD study is supported by grants from the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung; BMBF; www.bmbf.de; FKZ 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, and 01ER 0821), the Foundation for Preventive Medicine of the KfH (Kuratorium für Heimdialyse und Nierentransplantation e.V.—Stiftung Präventivmedizin), and corporate sponsors. NGAL, H-FABP, and OPN measurements were supported by Bayer (Wuppertal, Germany), and NT-proBNP measurements were supported by Roche (Basel, Switzerland). This work was further supported by the German Research Foundation (SFB/TRR219 project C1). TS is supported by the START-Program of the Faculty of Medicine, RWTH Aachen (21/20) and clinician scientist program of the German Society of Internal Medicine (DGIM). HUZ is supported by the BMBF within the framework of the e:Med research and funding concept (grant 01ZX1912A).
Data Sharing Agreement
Public posting of individual-level participant data is not covered by the informed patient consent form. As stated in the patient consent form and approved by the Ethics Committees, a data set containing pseudonyms can be obtained by collaborating scientists on approval of a scientific project proposal (www.gckd.org).
Author Contributions
DW, HUZ, JN, and TS conceived this study. DW, TS, JN, HUZ, and MS analyzed the data. DW wrote the manuscript. DW, HUZ, JN, TS, and MS had full access to all data in the study and have verified the underlying data. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Figure S1. Flowchart of participants included in the analysis.
Figure S2. Design of the current study.
Figure S3. Variables independently associated with low or medium educational attainment compared with high educational attainment identified by the mixed graphical model (MGM) algorithm based on the follow-up visit in year 2 in the German Chronic Kidney Disease (GCKD) cohort. (A,B) Data included DASH diet score. (C,D) Data included Mediterranean diet score. (E,F) Data included CKD diet score.
Table S1. Baseline characteristics of participants with dietary information and all German Chronic Kidney Disease (GCKD) participants.
Table S2. Generation of the CASMIN (Comparative Analysis of Social Mobility in Industrial Nations) variable in the in the German Chronic Kidney Disease (GCKD) cohort study.
Table S3. Dietary intake by educational attainment in the German Chronic Kidney Disease (GCKD) cohort study (n = 3129).
Table S4. Distribution of diagnoses contributing to CKD across categories of educational attainment in the German Chronic Kidney Disease (GCKD) cohort study.
Table S5. Biopsies performed in patients with presumed leading cause of CKD in the German Chronic Kidney Disease (GCKD) cohort study.
Table S6. Coefficients obtained by mixed graphical model (MGM) in the German Chronic Kidney Disease (GCKD) cohort study.
Table S7. Mediators of the association between low educational attainment and all-cause mortality, MACE, and kidney failure in the German Chronic Kidney Disease (GCKD) cohort study.
STROBE Statement.
Supplementary Material
Figure S1. Flowchart of participants included in the analysis.
Figure S2. Design of the current study.
Figure S3. Variables independently associated with low or medium educational attainment compared with high educational attainment identified by the mixed graphical model (MGM) algorithm based on the follow-up visit in year 2 in the German Chronic Kidney Disease (GCKD) cohort. (A, B) Data included DASH diet score. (C, D) Data included Mediterranean diet score. (E, F) Data included CKD diet score.
Table S1. Baseline characteristics of participants with dietary information and all German Chronic Kidney Disease (GCKD) participants.
Table S2. Generation of the CASMIN (Comparative Analysis of Social Mobility in Industrial Nations) variable in the in the German Chronic Kidney Disease (GCKD) cohort study.
Table S3. Dietary intake by educational attainment in the German Chronic Kidney Disease (GCKD) cohort study (n = 3129).
Table S4. Distribution of diagnoses contributing to CKD across categories of educational attainment in the German Chronic Kidney Disease (GCKD) cohort study.
Table S5. Biopsies performed in patients with presumed leading cause of CKD in the German Chronic Kidney Disease (GCKD) cohort study.
Table S6. Coefficients obtained by mixed graphical model (MGM) in the German Chronic Kidney Disease (GCKD) cohort study.
Table S7. Mediators of the association between low educational attainment and all-cause mortality, MACE, and kidney failure in the German Chronic Kidney Disease (GCKD) cohort study.
STROBE Statement (PDF)
References
- 1.Thomas B., Matsushita K., Abate K.H., et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruck K., Stel V.S., Gambaro G., et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill N.R., Fatoba S.T., Oke J.L., et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton R.L., Schlackow I., Staplin N., et al. Impact of educational attainment on health outcomes in moderate to severe CKD. Am J Kidney Dis. 2016;67:31–39. doi: 10.1053/j.ajkd.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velde M., de Jong P.E., Gansevoort R.T. Comparison of the yield of different screening approaches to detect chronic kidney disease. Nephrol Dial Transplant. 2010;25:3222–3230. doi: 10.1093/ndt/gfq156. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G., Busch M., Müller N., Müller U.A. Association between socioeconomic status and renal function in a population of German patients with diabetic nephropathy treated at a tertiary centre. Nephrol Dial Transplant. 2011;26:4017–4023. doi: 10.1093/ndt/gfr185. [DOI] [PubMed] [Google Scholar]
- 7.Vart P., Grams M.E., Ballew S.H., Woodward M., Coresh J., Matsushita K. Socioeconomic status and risk of kidney dysfunction: the Atherosclerosis Risk in Communities study. Nephrol Dial Transplant. 2019;34:1361–1368. doi: 10.1093/ndt/gfy142. [DOI] [PubMed] [Google Scholar]
- 8.Thio C.H.L., Vart P., Kieneker L.M., et al. Educational level and risk of chronic kidney disease: longitudinal data from the PREVEND study. Nephrol Dial Transplant. 2020;35:1211–1218. doi: 10.1093/ndt/gfy361. [DOI] [PubMed] [Google Scholar]
- 9.Vart P., Gansevoort R.T., Joosten M.M., et al. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48:580–592. doi: 10.1016/j.amepre.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Zeng X., Liu J., Tao S., et al. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72:270–279. doi: 10.1136/jech-2017-209815. [DOI] [PubMed] [Google Scholar]
- 11.Cavanaugh K.L., Wingard R.L., Hakim R.M., et al. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol. 2010;21:1979–1985. doi: 10.1681/ASN.2009111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R., Han Q.F., Zhu T.Y., et al. Impact of individual and environmental socioeconomic status on peritoneal dialysis outcomes: a retrospective multicenter cohort study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckardt K.U., Bärthlein B., Baid-Agrawal S., et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2011;27:1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 14.Titze S., Schmid M., Köttgen A., et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30:441–451. doi: 10.1093/ndt/gfu294. [DOI] [PubMed] [Google Scholar]
- 15.Heindel J., Baid-Agrawal S., Rebholz C.M., et al. Association between dietary patterns and kidney function in patients with chronic kidney disease: a cross-sectional analysis of the German chronic kidney disease study. J Ren Nutr. 2020;30:296–304. doi: 10.1053/j.jrn.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaesler N., Baid-Agrawal S., Grams S., et al. Low adherence to CKD-specific dietary recommendations associates with impaired kidney function, dyslipidemia, and inflammation. Eur J Clin Nutr. 2021;75:1389–1397. doi: 10.1038/s41430-020-00849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wachtler B., Hoebel J., Lampert T. Trends in socioeconomic inequalities in self-rated health in Germany: a time-trend analysis of repeated cross-sectional health surveys between 2003 and 2012. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton R.L., Schlackow I., Gray A., et al. Impact of CKD on household income. Kidney Int Rep. 2018;3:610–618. doi: 10.1016/j.ekir.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauritzen S.L. Clarendon Press; 1996. Graph Models. [Google Scholar]
- 20.Altenbuchinger M., Zacharias H.U., Solbrig S., et al. A multi-source data integration approach reveals novel associations between metabolites and renal outcomes in the German Chronic Kidney Disease study. Sci Rep. 2019;9:13954. doi: 10.1038/s41598-019-50346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altenbuchinger M., Weihs A., Quackenbush J., Grabe H.J., Zacharias H.U. Gaussian and Mixed Graphical Models as (multi-)omics data analysis tools. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194418. doi: 10.1016/j.bbagrm.2019.194418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele T. Oxford University Press; 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. [Google Scholar]
- 23.Corraini P., Olsen M., Pedersen L., Dekkers O.M., Vandenbroucke J.P. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators [published correction appears in Clin Epidemiol. 2018;10:223] [published correction appears in Clin Epidemiol. 2019;11:245] Clin Epidemiol. 2017;9:331–338. doi: 10.2147/CLEP.S129728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/ Published 2013.
- 25.Roerecke M., Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 2014;12:182. doi: 10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanches Machado d’Almeida K., Ronchi Spillere S., Zuchinali P., Corrêa Souza G. Mediterranean diet and other dietary patterns in primary prevention of heart failure and changes in cardiac function markers: a systematic review. Nutrients. 2018;10:58. doi: 10.3390/nu10010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estruch R., Ros E., Salas-Salvadó J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 28.Crews D.C., Charles R.F., Evans M.K., Onderman A.B., Powe N.R. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkin S.S., Diez Roux A.V., Coresh J., Ried L.F., Jackson S.A., Powe N.R. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med. 2007;65:809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Otaki Y., Watanabe T., Takahashi H., et al. Association of heart-type fatty acid-binding protein with cardiovascular risk factors and all-cause mortality in the general population: the Takahata study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakabe A., Hata N., Kobayashi N., et al. Worsening renal failure in patients with acute heart failure: the importance of cardiac biomarkers. ESC Heart Fail. 2019;6:416–427. doi: 10.1002/ehf2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison T.G., Shukalek C.B., Hemmelgarn B.R., et al. Association of NT-proBNP and BNP with future clinical outcomes in patients with ESKD: a systematic review and meta-analysis. Am J Kidney Dis. 2020;76:233–247. doi: 10.1053/j.ajkd.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Gromadzinski L., Januszko-Giergielewicz B., Czarnacka K., Pruszczyk P. NT-proBNP in the prognosis of death or need for renal replacement therapy in patients with stage 3–5 chronic kidney disease. Cardiorenal Med. 2019;9:125–134. doi: 10.1159/000496238. [DOI] [PubMed] [Google Scholar]
- 34.Fahrleitner-Pammer A., Herberth J., Browning S.R., et al. Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res. 2008;23:1850–1858. doi: 10.1359/jbmr.080610. [DOI] [PubMed] [Google Scholar]
- 35.Drechsler C., Verduijn M., Pilz S., et al. Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1752–1759. doi: 10.2215/CJN.10091110. [DOI] [PubMed] [Google Scholar]
- 36.Lok Z.S.Y., Lyle A.N. Osteopontin in vascular disease. Arterioscler Thromb Vasc Biol. 2019;39:613–622. doi: 10.1161/ATVBAHA.118.311577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolignano D., Donato V., Coppolino G., et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 38.van Deursen V.M., Damman K., Voors A.A., et al. Prognostic value of plasma neutrophil gelatinase-associated lipocalin for mortality in patients with heart failure. Circ Heart Fail. 2014;7:35–42. doi: 10.1161/CIRCHEARTFAILURE.113.000242. [DOI] [PubMed] [Google Scholar]
- 39.Vart P., Matsushita K., Rawlings A.M., et al. SES, heart failure, and N-terminal Pro-b-type natriuretic peptide: the atherosclerosis risk in communities study. Am J Prev Med. 2018;54:229–236. doi: 10.1016/j.amepre.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crandall C.J., Miller-Martinez D., Greendale G.A., et al. Socioeconomic status, race, and bone turnover in the Midlife in the US Study. Osteoporos Int. 2012;23:1503–1512. doi: 10.1007/s00198-011-1736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkowitz S.A., Karter A.J., Lyles C.R., et al. Low socioeconomic status is associated with increased risk for hypoglycemia in diabetes patients: the Diabetes Study of Northern California (DISTANCE) J Health Care Poor Underserved. 2014;25:478–490. doi: 10.1353/hpu.2014.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas C., Nightingale C.M., Donin A.S., et al. Socio-economic position and type 2 diabetes risk factors: patterns in UK children of South Asian, Black African–Caribbean and White European origin. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellum J.A., Lameire N., Aspelin P., et al. Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 44.Byrne C., Nedelman J., Luke R.G. Race, socioeconomic status, and the development of end-stage renal disease. Am J Kidney Dis. 1994;23:16–22. doi: 10.1016/s0272-6386(12)80806-7. [DOI] [PubMed] [Google Scholar]
- 45.McQuarrie E.P., Mackinnon B., Bell S., et al. Multiple socioeconomic deprivation and impact on survival in patients with primary glomerulonephritis. Clin Kidney J. 2017;10:49–54. doi: 10.1093/ckj/sfw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canney M., Induruwage D., Sahota A., et al. Socioeconomic position and incidence of glomerular diseases. Clin J Am Soc Nephrol. 2020;15:367–374. doi: 10.2215/CJN.08060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McQuarrie E.P., Mackinnon B., McNeice V., Fox J.G., Geddes C.C. The incidence of biopsy-proven IgA nephropathy is associated with multiple socioeconomic deprivation. Kidney Int. 2014;85:198–203. doi: 10.1038/ki.2013.329. [DOI] [PubMed] [Google Scholar]
- 48.Hurtado A., Johnson R.J. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl. 2005;(97):S62–S67. doi: 10.1111/j.1523-1755.2005.09711.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.