Abstract
The term capillary leak syndrome (CLS) describes the manifestations associated with an increased capillary permeability to proteins leading to an escape of plasma from the blood circulatory system to surrounding tissues, muscle, organs, or body cavities. This results clinically in the typical triad of hypotension, edema, and elevated hematocrit. The more severe cases of CLS may present with cardiovascular collapse, shock, and death. The most classic form of this pathology is represented by the idiopathic systemic CLS (SCLS) also called Clarkson’s disease, but capillary leaks are also described as adverse drug reactions foremost among which are anticancer drugs. This review will focus on oncologic drugs such as gemcitabine, therapeutic growth factors or cytokines, and monoclonal antibodies (mAbs) that appear now as the strongest candidates for anticancer drug-induced CLS.
Keywords: anticancer drugs, capillary leak syndrome, cytokines, gemcitabine, immunotherapy, monoclonal antibodies
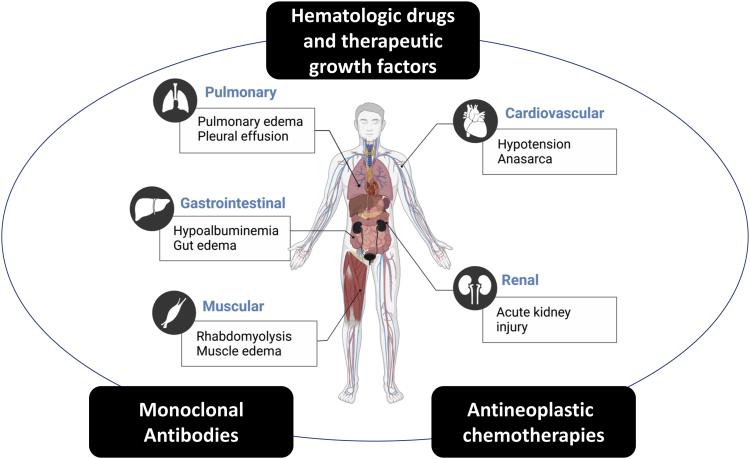
Capillary leak syndrome (CLS) has been used to describe a constellation of pathologic manifestations associated with an increased capillary permeability to proteins leading to hypoalbuminemia in the absence of albuminuria and an escape of plasma from the blood circulatory system to surrounding tissues, muscle, organs, or body cavities. This results clinically in hemoconcentration, hypotension, segmental or generalized edema, and anasarca and potentially causes damage to limb muscles and nerves, as well as to vital organs because of limited perfusion (Figure 1). The more severe cases of CLS may present with cardiovascular collapse, shock, and death.1
Figure 1.
The organ systems affected by capillary leak syndrome include cardiovascular, renal, pulmonary, gastrointestinal, and muscular systems. The boxes in black are anticancer drug classes that are associated with capillary leak syndrome.
Acute kidney injury (AKI) is a common complication of CLS, occurring in 28% to 62% of patients with CLS.1, 2, 3, 4, 5 Prerenal azotemia and acute tubular injury result from intravascular volume depletion and reduced kidney perfusion.2 Given that some patients with CLS develop rhabdomyolysis from muscle edema, they may experience myoglobin-related acute tubular injury.1,6 The most classic form of CLS is represented by primary CLS, also called SCLS, the idiopathic SCLS, or Clarkson’s disease. This rare and potentially life-threatening condition7 is characterized by the recurrence of acute self-reversing episodes of capillary leak. Episodes of capillary leaks in Clarkson’s disease typically occur in 2 phases, a hallmark early (1–3 days) phase of extravasation of fluid associated with syncope, dyspnea, and hypovolemia leading to circulatory collapse and organ damage and a second phase of reabsorption of fluid leading to polyuria and flash pulmonary edema.8 The pathogenesis of SCLS is unknown. It probably involves a defective and reversible cellular phenomenon in the capillaries leading to albumin leak. The role of monoclonal Ig, predominantly of the IgG-κ type, present in most patients with SCLS, is unknown. SCLS was usually diagnosed after a considerable delay from onset of symptoms.1
However, because the typical triad (acute profound arterial hypotension, hypoalbuminemia, and elevated hematocrit) is better known by clinicians, the prognosis of this severe condition seems to be improved by earlier diagnosis and preventive treatment.9
Capillary leaks, presenting in an acute form close to SCLS or in a more chronic form, are described in other conditions, such as sepsis, autoimmunity, hematologic diseases, after stem cell transplantation, differentiation syndrome following the treatment of acute promyelocytic leukemia, ovarian hyperstimulation syndrome, viral infections, snakebite envenomation, and secondary to treatments as adverse drug reactions.8,10, 11, 12, 13 Understanding the potential etiology can be crucial for diagnosis and successful treatment.
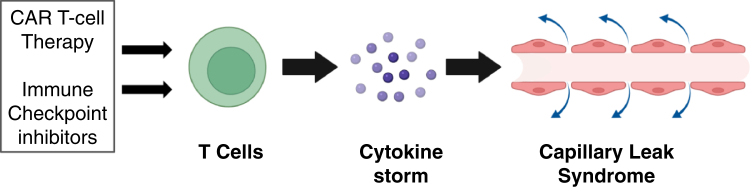
When CLS occurs in the context of hematologic malignancy treatment, it is often referred to as cytokine release syndrome (CRS) or cytokine storm. The shared pathophysiology of these disorders is a surge in systemic cytokines that causes increased capillary permeability (Figure 2). On the cellular level, endothelial cell injury appears to be a final common pathway of cytokine-induced injury. The role of mediators other than cytokines is unknown, and current therapies target the resulting cytokine storm.7
Figure 2.
CAR-T-cell therapy and immune checkpoint inhibitors activate T cells, leading to a cytokine storm. The excessive circulating cytokine levels lead to capillary leak syndrome. CAR-T, chimeric antigen receptor-T.
This review will focus on oncologic drugs and therapeutic growth factors, the first and foremost source of secondary CLS.14
Epidemiology of Anticancer Drug-Induced CLS
In cancer-treated patients, CLS is related to the cancer itself (43.6%), can occur after bone marrow transplantation (4.8%), but is mostly associated with anticancer agents (51.6%).4 CLS is often a fatal complication of cytotoxic chemotherapy agents with an estimated mortality of 24% at 5 years.1
Anticancer drug-induced CLS has a variable incidence depending on the diagnostic criteria used and the specific drug studied, varying from case reports under gemcitabine treatment10,15, 16, 17, 18, 19, 20, 21, 22 to a universal risk of severe capillary leak with rituximab in chronic lymphocytic leukemia when the lymphocyte count is >50 × 103/μl.10,15,23 In a similar way, as many as 16 of 25 (64%) of interleukin (IL)-2–treated patients with cancer experienced severe fluid retention.24 Capillary leak associated with engraftment syndrome following hematopoietic stem cell transplantation and differentiation syndrome following the treatment of acute promyelocytic leukemia with retinoic acid or arsenic trioxide occur in 7% to 55%10,25 and 25%,10,26 respectively. Granulocyte colony-stimulating factor (14.6%) followed by IL-2 (11.4%) was the most frequently associated drugs in systematic reviews.4,15
Recently, in an observational retrospective study, part of the MIMUTOX (Monitoring the IMmUological TOXicity of Drugs) research program, Mertz et al.14 used VigiBase (http://www.vigiaccess.org/), the World Health Organization global Individual Case Safety Report database, which contains reports of suspected adverse drug reactions collected by national drug authorities in >130 countries between 1967 and February 2018, to describe the main drugs associated with secondary CLS according to the term in the Medical Dictionary for Drug Regulatory Activities.14 in this study, which is the largest to date, the authors found 243 suspected drugs associated with at least 1 case of CLS. A significant pharmacovigilance signal was observed for 52 of these drugs responsible for 560 Individual Case Safety Reports. Of these 52 drugs, 45 (86.5%) were antineoplastic and immunomodulating agents (Table 1 adapted from Mertz et al.14). The drugs associated with the highest number of CLS cases were gemcitabine (n = 83 [14.8%]), clofarabine (n = 52 [9.3%]), and denileukin diftitox (n = 48 [8.6%]), some cases of CLS being associated with >1 suspected drug.14 The median delay between the start of the suspected inductor treatment and occurrence of the CLS was 8 days (interquartile range, 2.25–31.7).14 The clinical presentation of drug-induced CLS might be somewhat different from that of idiopathic CLS because pulmonary edema was reported in a substantial number of CLS cases from the VigiBase study, whereas this manifestation is uncommon in Clarkson’s disease. Death (directly linked to CLS or not) occurred in 27% of the cases of CLS.14
Table 1.
Antineoplastic agent-induced CLS using the World Health Organization VigiBase
| Substances | Molecules | Total no. of AEs | No. of CLS cases | Serious CLS, n (%) | Fatal CLS, n (%) |
|---|---|---|---|---|---|
| Antimetabolites | |||||
| Purine analogues | Clofarabine | 2086 | 52 | 51 (98) | 33 (63) |
| Fludarabine | 9772 | 10 | 9 (90) | 5 (50) | |
| Pyrimidine analogues | Gemcitabine | 40,688 | 83 | 72 (87) | 14 (17) |
| Cytarabine | 23,466 | 33 | 31 (94) | 12 (36) | |
| Folic acid analogues | Pemetrexed | 14,597 | 4 | 4 (100) | 1 (25) |
| Immunostimulants | |||||
| IL | IL-2 (Aldesleukin) | 1531 | 19 | 16 (84) | 6 (32) |
| IL-11 (Oprelvekin) | 509 | 11 | 6 (55) | 3 (27) | |
| Colony-stimulating factors | Pegfilgrastim | 20,409 | 20 | 20 (100) | 4 (20) |
| Filgrastim | 10,306 | 14 | 12 (86) | 5 (36) | |
| GM-CSF | 1920 | 6 | 3 (50) | 0 (0) | |
| G-CSF | 1222 | 4 | 4 (100) | 2 (50) | |
| Lenograstim | 1556 | 3 | 3 (100) | 0 (0) | |
| Plant alkaloids and other natural products | |||||
| Taxanes | Docetaxel | 69,525 | 46 | 40 (87) | 5 (11) |
| Paclitaxel | 71,543 | 12 | 9 (75) | 3 (25) | |
| Vinca alkaloids and analogues | Vincristine | 28,746 | 8 | 8 (100) | 3 (38) |
| Podophyllotoxin derivatives | Etoposide | 28,402 | 17 | 17 (100) | 10 (59) |
| Alkylating agents | |||||
| Nitrogen mustard analogues | Cyclophosphamide | 74,270 | 34 | 34 (100) | 14 (41) |
| Melphalan | 7559 | 6 | 5 (83) | 4 (67) | |
| Bendamustine | 7586 | 4 | 4 (100) | 2 (50) | |
| Alkyl sulfonates | Busulfan | 3880 | 6 | 6 (100) | 1 (17) |
| Treosulfan | 376 | 5 | 5 (100) | 3 (60) | |
| Ethylene imines | Thiotepa | 1688 | 5 | 5 (100) | 3 (60) |
| Cytotoxic antibiotics (anthracyclines) and related substances | Daunorubicin | 6122 | 10 | 10 (100) | 4 (40) |
| Doxorubicin | 60,792 | 8 | 8 (100) | 3 (38) | |
| Other antineoplastic agents | |||||
| Platinum compounds | Oxaliplatin | 57,505 | 10 | 10 (100) | 1 (10) |
| Carboplatin | 48,738 | 7 | 7 (100) | 2 (29) | |
| Monoclonal antibodies | Rituximab | 54,743 | 19 | 19 (100) | 5 (26) |
| Alemtuzumab | 8119 | 6 | 6 (100) | 3 (50) | |
| Trastuzumab | 23,502 | 11 | 11 (100) | 1 (9) | |
| Dinutuximab | 46 | 4 | 4 (100) | 0 (0) | |
| Bevacizumab | 51,962 | 11 | 11 (100) | 1 (9) | |
| Blinatumomab | 1671 | 3 | 3 (100) | 1 (33) | |
| Nivolumab | 17,578 | 4 | 4 (100) | 2 (50) | |
| Other agents | Denileukin diftitox | 348 | 48 | 36 (75) | 12 (25) |
| Bortezomib | 25,441 | 13 | 13 (100) | 4 (31) | |
| Pentostatin | 657 | 6 | 6 (100) | 1 (17) | |
| Bexarotene | 566 | 5 | 5 (100) | 0 (0) | |
| Pegaspargase | 3756 | 5 | 5 (100) | 2 (40) | |
AE, adverse event; CLS, capillary leak syndrome; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage CSF; IL, interleukin.
Adapted with permission from Pineton de Chambrun M, Gousseff M, Mauhin W, et al, EurêClark Study Group. Intravenous immunoglobulins improve survival in monoclonal gammopathy-associated systemic capillary-leak syndrome. Am J Med. 2017;130:1219.e19–1219.e27.
Characterization of the Anticancer Drug Inducers of CLS
In the past, cases of anticancer drug-induced CLS were described with interleukin (IL-2, -3, -4), interferon-α, immunotoxins constructed with ricin A chain, blocked ricin, saporin, poke-weed antiviral protein, and Pseudomonas exotoxin, granulocyte–macrophage colony-stimulating factors, antiganglioside antibodies, cyclosporin A, cyclophosphamide, mitomycin C, FK973, monocrotaline pyrrole, and cytosine arabinoside.10,15 With regard to IL-2 therapy, CLS has been observed when IL-2 is administered either alone or in combination with lymphokine-activated killer cells, tumor-infiltrating lymphocytes, or other cytokines or cyclophosphamide.10,15 The cytokines IL-11 and IL-12 have also been associated with CLS.10,15
More recently, several effective therapies for previously untreatable cancers are entering clinical practice at a rapid pace with the targeted and mAb therapies at the forefront of the oncological therapeutic arsenal. Accordingly, in their recent observational study, Mertz et al.14 found that denileukin diftitox, clofarabine, aldesleukin, oprelvekin, lenograstim, and nivolumab appear now as the strongest candidates for anticancer drug-induced CLS.
Hematologic Drugs and Therapeutic Growth Factors
Clofarabine
Clofarabine is a Food and Drug Administration-approved purine nucleoside analog, which acts by inhibiting DNA synthesis and ribonucleoside reductase.27 This drug is successfully used to treat relapsed or refractory pediatric acute lymphoblastic leukemia as well as acute myeloid leukemia in adults.27, 28, 29
Clofarabine has some adverse effects that can be fatal, such as CLS30,31 with a rate of 4%.32 Close monitoring, adequate hydration, and prophylactic steroid treatment were advised in chemotherapy protocols, including clofarabine to reduce the risk/effects of CLS. Kesik et al.30 reported that i.v. Ig at the dose of 400 mg/kg/d for following 2 days was effective for the treatment of a child with relapsed lymphoma who developed clofarabine-related CLS despite the pretreatment with methylprednisolone.
Colony-Stimulating Factors
Hemopoietic growth factors are polypeptides that interact with cell surface receptors on hematologic progenitor cells causing their activation, proliferation, and differentiation into mature circulating cells, such as red blood cells, white blood cells, neutrophils, monocytes, macrophages, and platelets. Among these, CSFs, such as the granulocyte colony-stimulating factor and the granulocyte–macrophage colony-stimulating factor, increase white blood cell counts, including neutrophils and macrophages/monocytes. Recombinant forms of granulocyte colony-stimulating factor (filgrastim, pegfilgrastim, lenograstim, and lipegfilgrastim) and granulocyte–macrophage colony-stimulating factor (sargramostim) have been developed and are approved for the use in patients with malignancies after chemotherapy-induced neutropenia and to support patients undergoing hematopoietic cell transplantation. Several cases of CLS have been reported with these treatments.14 In Europe, biosimilars of filgrastim (Neupogen), such as Tevagrastim, Ratiograstim, and Biograstim, have been approved since 2008. Furthermore, tbo-filgrastim has been approved in the United States by the Food and Drug Administration in 2012 as a biological product with 1 similar indication to filgrastim with the brand name Granix. CLS has not been observed with Tevagrastim, Ratiograstim, and Biograstim or tbo-filgrastim during the postmarket period.33
Bortezomib
Bortezomib was the first clinically approved 20S proteasome inhibitor and is now indicated for the treatment of multiple myeloma and relapsed mantle cell lymphoma. CLS as an unusual adverse effect of bortezomib was reported during treatment of antibody-mediated rejection in a kidney transplant recipient and was recurrent following reintroduction in a patient with relapsed myeloma.34,35
Monoclonal Antibodies
The mAbs can acutely activate large numbers of immune cells leading to abrupt increases in several cytokine secretions leading to the onset of symptoms minutes to hours after the mAb has been administered.10,36 CRS has been described with several therapeutic mAbs. CRS shares several characteristics with CLS.10,15 More particularly, the treatment of hematologic malignancies with rituximab and alemtuzumab has been associated in several cases with the occurrence of a CLS, by a mechanism similar to that involved in the CRS.10,14,36 In this respect, it is interesting to note that in CRS, rituximab has been shown to increase the secretion of tumor necrosis factor alpha and IL-6 in patients with B-cell leukemia,23 whereas alemtuzumab has been shown to increase the secretion of tumor necrosis factor alpha, interferon gamma, and IL-6.37
Other mAbs have been more recently associated with the occurrence of CLS in case reports:
Dinutuximab is a chimeric, anti-GD2 mAb approved in combination with granulocyte–macrophage colony-stimulating factor, aldesleukin, and isotretinoin for maintenance treatment of pediatric patients with high-risk neuroblastoma who achieve at least a partial response to first-line multiagent, multimodality therapy. This drug can induce CLS.38
Trastuzumab is a mAb targeting the epidermal growth factor receptor HER2 used as standard of care in patients with HER2-overexpressing tumors. It has been reported to induce CLS in a patient with a breast cancer39 and a strong CLS with respiratory insufficiency in a newborn infant of a pregnant treated-woman.40
Immunotherapy
There are 4 cases (nivolumab [2], pembrolizumab [1], unspecified [1]) of CLS induced by anti–programmed cell death protein 1 checkpoint inhibitor who responded to high-dose i.v. Ig therapy after a failure of high-dose steroids (3 cases) which have been reported.41, 42, 43, 44 Therefore, i.v. Ig might be proposed as the initial treatment in the management of immunotherapy-induced CLS.41, 42, 43
Antineoplastic Chemotherapies
Denileukin Diftitox
Denileukin diftitox is a recombinant fusion protein of human IL-2 attached to diphtheria toxin fragments A and B used as an antineoplastic agent to treat cutaneous T-cell lymphomas that express IL-2 receptors. Up to 25% of patients treated with this innovative drug develop CLS leading to peripheral edema, hypotension, and renal insufficiency that can cause shock, pulmonary edema, renal failure, and death.45
Gemcitabine
Gemcitabine is an S phase-specific pyrimidine antimetabolite that impairs DNA synthesis by competitively inhibiting DNA polymerase and gets incorporated in DNA leading to impaired chain elongation.46 This drug is typically well-tolerated and is used in a number of different malignancies, including the following: pancreatic adenocarcinoma,47 bladder, non–small cell lung, Hodgkin’s lymphoma, breast, ovarian, and testicular cancers. CLS is a rare18,19 but potentially lethal side effect of gemcitabine. It is the drug most often reported to be associated with CLS in the study by Mertz et al.14
The exact pathophysiology of gemcitabine-induced CLS is unclear. There are 2 different mechanisms postulated to take place in CLS secondary to this drug:
-
1.
First, it is hypothesized that this syndrome occurs along the spectrum with gemcitabine pulmonary toxicity, thought to be secondary to endothelial dysfunction subsequently causing capillary leakage.48, 49, 50 The incidence of gemcitabine-induced SCLS is unknown, but the overall incidence of gemcitabine pulmonary toxicity is <1%.40 Patients with gemcitabine-induced SCLS and/or gemcitabine pulmonary toxicity typically respond to systemic steroids.19,48,51
-
2.
Second, gemcitabine could induce cytotoxicity for capillary endothelial cells.19,20,22,52 There are 2 active metabolites of gemcitabine that could injure the mitochondria to cause massive generation and transmembrane transport of reactive oxygen species, which then trigger reactive oxygen species-mediated signaling pathways to induce the damage to cells in proliferative cycles.53 Wu et al.16 inferred that gemcitabine-induced dysfunction of the capillary endothelial barrier was primarily initiated by the downstream events in reactive oxygen species-mediated signal transduction pathways. Accordingly, regulation or inhibition of reactive oxygen species-mediated signaling pathways may potentially be the pivotal target and direction of the treatment to gemcitabine-induced CLS.16 Even now, there is no cure for this complication, and increasing peripheral edema along with decreasing serum albumin level in patients receiving gemcitabine should prompt the clinician to diagnose a SCLS and cessation of gemcitabine is mandatory.
Drug-induced SCLS has also been described after pemetrexed54 and oxaliplatin.55
Chimeric Antigen Receptor T Cell-Associated CLS in a Form of Cytokine Storm
Chimeric antigen receptor T-cell (CAR-T) therapy has emerged as a breakthrough therapy for hematologic malignancies, such as acute lymphoblastic leukemia, recurrent B-cell lymphoma, and mantle cell lymphoma, with newer generation CARs now being developed for solid tumors and multiple myeloma. CAR-T cells are manufactured by genetically engineering a patient’s T cells to target specific tumor antigens. These cells are expanded ex vivo into hundreds of millions of cells and reinfused in the patient, leading to the production of inflammatory cytokines. CRS, a form of CLS, is common after CAR-T infusion, manifesting as fever, tachycardia, and multiorgan dysfunction. AKI occurs due to cytokine-mediated capillary leak and intravascular volume depletion, with decreased kidney perfusion.56 Risk factors for AKI include history of prior autologous or allogeneic stem cell transplantation, requirement for intensive unit level care, and higher grade of CRS. Real-world studies suggest that AKI usually occurs in 19% to 30% of patients, and usually in the context of CRS; however, the incidence is lower (5%) in patients receiving a certain type of CAR, tisagenlecleucel, perhaps because of its attenuated inflammatory profile.57,58 Most cases of AKI present as prerenal azotemia reversible with hemodynamic support, though some patients can progress to ATN and the need for kidney replacement therapy.58,59 Tocilizumab, an IL-6 inhibitor, and dexamethasone, may reduce the risk of CRS and therefore AKI related to CAR-T therapy.58, 59, 60
Physiopathology and Therapeutic Approaches
The pathogenesis of secondary CLS because of anticancer treatment is not well-known, but there are several studies supporting the role of the same pathogenic molecules than in idiopathic CLS, including multiple cytokines, angiopoietin-2, C-X-C motif chemokine ligand 10, and vascular endothelial growth factor (VEGF).7,61, 62, 63 However, CLS by anticancer drugs could also develop because of a direct toxicity of the drug on the capillary system because these molecules are mostly related to increase vascular endothelial cell permeability leading to vascular leakage.15
Conservative Measures
Conservative measures involve balancing the need for intravascular volume expansion and diuresis of excess extracellular fluid volume.
There is a lack of evidence to guide the choice of fluid therapy. In patients with mild hypotension, blood pressure often responds to i.v. fluids in the form of crystalloids. In patients with severe shock, blood pressure may be only partially responsive or refractory to i.v. fluids.10
Albumin is an attractive choice for volume expansion in these patients, but its efficacy is attenuated because of albumin loss from the vascular space. High-molecular weight starches, such as pentastarch (molecular weight, 264 kilodalton) and hetastarch (molecular weight, 450 kilodalton), are theoretically attractive resuscitative fluids in patients with CLS.10 However, volume resuscitation with hydroxyethyl starch has been associated with increased mortality and AKI in critically ill patients compared with other resuscitative fluids.64
Amiloride, which is an epithelial sodium channel blocker with fewer antihypertensive effects, seems the more appropriate diuretic.43
Amiloride can be used in an attempt to balance the need for intravascular volume expansion and the maintenance of diuresis. Indeed, amiloride is a diuretic with a lesser hypotensive effect. Furthermore, there is evidence of the role of epithelial sodium channel blockers in the activation of antigen-presenting cells leading to T-cell activation and inflammatory cytokines, endothelial function, and vasculature.43,65 Patients who develop oliguric AKI may need kidney replacement therapy.2
Specific Measures
As in Clarkson’s disease, many agents have been tried sporadically in secondary CLS with varying degrees of effectiveness, including corticosteroids, β2-agonists (aminophylline, theophylline, or terbutaline), thalidomide, antihistamines, plasmapheresis, and i.v. Ig.1,61
Although one cohort study reported no survival benefit of i.v. Ig in patients requiring intensive care unit admission,35 several studies have suggested the use of high-dose i.v. Ig followed by monthly infusions.9,66,67
Ibata et al.68 describe a case of SCLS in which serum isoform D of VEGF was elevated. Isoform D of VEGF contributes to VEGF receptor-2 signaling, which is the major mediator of the permeability-enhancing effects of VEGF; hence, it may have been a causative factor of hypotensive crises.
Case reports suggested the use of anti-VEGF antibody (bevacizumab, 5 mg/kg/d for 5 days)69 or the anti-VEGF tyrosine kinase inhibitor axitinib in pembrolizumab-induced CLS.43 Dowden et al.70 also reported rapid improvement of a patient with classic acute SCLS who got infliximab.
In the case report of Ibata et al.,68 prevention of hypotensive crises was unsuccessfully attempted with theophylline, i.v. Ig, high-dose dexamethasone, bortezomib, melphalan, and prednisolone; however, the patient’s attacks dramatically disappeared after the introduction of thalidomide. The i.v. Igs, found to have possible efficacy in the idiopathic forms of CLS, have no documented efficacy in the forms associated with anticancer drugs.9,71
Thus, with failure of corticosteroids in the acute phase of the illness, one strategy could be to inhibit VEGF receptors, thereby blocking the angiogenesis implicated in the invasiveness and resistance of various tumors on top of high-dose i.v. Ig.43,72
For patients with severe cases of cytokine storm as seen with CAR-T therapy (typically manifesting as hypotension and/or hypoxia), anticytokine therapy with tocilizumab (human mAb against the IL-6 receptor) has been very effective in quickly reversing the cytokine storm in most patients.73 Tocilizumab is now considered a standard of care therapy for severe CRS and can be given with or without corticosteroids in patients with CAR-T treatment. Pretreatment with chemotherapy to reduce tumor burden and steroids is also considered to be important in the prevention of CRS.74
Conclusions
CLS is a known complication of anticancer drugs, rare but potentially fatal. Their physiopathologic mechanism is most often not known and could be different from that of idiopathic CLS with, in particular, the direct aggression of endothelial cells by the anticancer drug. There is no specific treatment of this drug side effect. As in idiopathic CLS, administration of saline infusions to restore intravascular volume should be limited as it may worsen the edema of the surrounding tissues and potentially cause damage to limb muscles and nerves. The data regarding the rechallenging with drugs and/or cross-reactivity among drugs of same class are not available for most of these agents. Timing of CLS after drug initiation is not well-studied in each class of anticancer agent. Data on specific risk factors that cause CLS are also sparse. We need a better understanding and treatment modalities for CLS associated with anticancer agents.
Disclosure
KDJ reports being a consultant for Astex Pharmaceuticals, Natera, GlaxoSmithKline, ChemoCentryx, Chinook, and Travere Therapeutics; being a paid contributor to UpToDate.com; and receiving honorarium from the International Society of Nephrology and the American Society of Nephrology. He is Editor-in-Chief for the ASN Kidney News; section editor for Onconephrology for Nephrology Dialysis Transplantation; and serves on the editorial board for Journal of Onconephrology, Kidney International, Clinical Journal of the American Society of Nephrology, American Journal of Kidney Diseases, and Clinical Kidney Journal. KDJ is the founder and co-president of the American Society of Onconephrology. All the other authors declared no competing interests.
References
- 1.Kapoor P., Greipp P.T., Schaefer E.W., et al. Idiopathic systemic capillary leak syndrome (Clarkson’s disease): the Mayo clinic experience. Mayo Clin Proc. 2010;85:905–912. doi: 10.4065/mcp.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aulagnon F., Lapidus N., Canet E., et al. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis. 2015;65:851–859. doi: 10.1053/j.ajkd.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Carreras E., Fernández-Avilés F., Silva L., et al. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant. 2010;45:1417–1422. doi: 10.1038/bmt.2009.363. [DOI] [PubMed] [Google Scholar]
- 4.Shin J.I., Lee K.H., Lee I.R., et al. Systemic capillary leak syndrome (Clarkson syndrome) in cancer patients: a systematic review. J Clin Med. 2018;7:418. doi: 10.3390/jcm7110418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suntharalingam G., Perry M.R., Ward S., et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 6.Dolberg-Stolik O.C., Putterman C., Rubinow A., et al. Idiopathic capillary leak syndrome complicated by massive rhabdomyolysis. Chest. 1993;104:123–126. doi: 10.1378/chest.104.1.123. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z., Ghosh C.C., Patel R., et al. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druey K.M., Greipp P.R. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–98. doi: 10.7326/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineton de Chambrun M., Gousseff M., Mauhin W., et al. EurêClark Study Group Intravenous immunoglobulins improve survival in monoclonal gammopathy-associated systemic capillary-leak syndrome. Am J Med. 2017;130:1219.e19–1219.e27. doi: 10.1016/j.amjmed.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Siddall E., Khatri M., Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 11.Eo T.S., Chun K.J., Hong S.J., et al. Clinical presentation, management, and prognostic factors of idiopathic systemic capillary leak syndrome: a systematic review. J Allergy Clin Immunol Pract. 2018;6:609–618. doi: 10.1016/j.jaip.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Druey K.M., Parikh S.M. Idiopathic systemic capillary leak syndrome (Clarkson disease) J Allergy Clin Immunoll. 2017;140:663–670. doi: 10.1016/j.jaci.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guffroy A., Dervieux B., Gravier S., et al. Systemic capillary leak syndrome and autoimmune diseases: a case series. Semin Arthritis Rheum. 2017;46:509–512. doi: 10.1016/j.semarthrit.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Mertz P., Lebrun-Vignes B., Salem J.E., Arnaud L. Characterizing drug-induced capillary leak syndromes using the World Health Organization VigiBase. J Allergy Clin Immunol. 2019;143:433–436. doi: 10.1016/j.jaci.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Jeong G.H., Lee K.H., Lee I.R., et al. Incidence of capillary leak syndrome as an adverse effect of drugs in cancer patients: a systematic review and meta-analysis. J Clin Med. 2019;8 doi: 10.3390/jcm8020143. pii: E143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu W., Xia Q., Luo R.J., et al. In vitro study of the antagonistic effect of low-dose liquiritigenin on gemcitabine-induced capillary leak syndrome in pancreatic adenocarcinoma via inhibiting ROS-mediated signalling pathways. Asian Pac J Cancer Prev. 2015;16:4369–4376. doi: 10.7314/apjcp.2015.16.10.4369. [DOI] [PubMed] [Google Scholar]
- 17.Gardini A.C., Aquilina M., Oboldi D., et al. Separate episodes of capillary leak syndrome and pulmonary hypertension after adjuvant gemcitabine and three years later after nab-paclitaxel for metastatic disease. BMC Cancer. 2013;13:542. doi: 10.1186/1471-2407-13-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron D., Mayo A., Kluger Y. Gemcitabine-induced chronic systemic capillary leak syndrome: a life-threatening disease. Clin Oncol (R Coll Radiol) 2006;18:90–91. doi: 10.1016/j.clon.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Biswas S., Nik S., Corrie P.G. Severe gemcitabine-induced capillary-leak syndrome mimicking cardiac failure in a patient with advanced pancreatic cancer and high-risk cardiovascular disease. Clin Oncol (R Coll Radiol) 2004;16:577–579. doi: 10.1016/j.clon.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Pulkkanen K., Kataja V., Johansson R. Systemic capillary leak syndrome resulting from gemcitabine treatment in renal cell carcinoma: a case report. J Chemother. 2003;15:287–289. doi: 10.1179/joc.2003.15.3.287. [DOI] [PubMed] [Google Scholar]
- 21.Bajwa R., Starr J., Daily K. Gemcitabine-induced chronic systemic capillary leak syndrome. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221068. bcr2017221068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Pas T., Curigliano G., Franceschelli L., et al. Gemcitabine-induced systemic capillary leak syndrome. Ann Oncol. 2001;12:1651–1652. doi: 10.1023/a:1013163831194. [DOI] [PubMed] [Google Scholar]
- 23.Winkler U., Jensen M., Manzke O., et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 24.Rosenberg S.A., Lotze M.T., Muul L.M., et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer T.R. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50:469–475. doi: 10.1038/bmt.2014.296. [DOI] [PubMed] [Google Scholar]
- 26.Luesink M., Jansen J.H. Advances in understanding the pulmonary infiltration in acute promyelocytic leukaemia. Br J Haematol. 2010;151:209–220. doi: 10.1111/j.1365-2141.2010.08325.x. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H., Gandhi V., Cortes J., et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 28.Nabhan C., Davis N., Bitran J.D., et al. Efficacy and safety of clofarabine in relapsed and/or refractory non-Hodgkin lymphoma, including rituximab-refractory patients. Cancer. 2011;117:1490–1497. doi: 10.1002/cncr.25603. [DOI] [PubMed] [Google Scholar]
- 29.Faderl S., Ravandi F., Huang X., et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesik V., Atas E., Korkmazer N., Babacan O. Clofarabine associated capillary leak syndrome in a child with lymphoma successfully treated with intravenous immunoglobulin. J Cancer Res Ther. 2015;11:653. doi: 10.4103/0973-1482.138028. [DOI] [PubMed] [Google Scholar]
- 31.Baytan B., Ozdemir O., Gunes A.M., Dönmez O. Clofarabine-induced capillary leak syndrome in a child with refractory acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:144–146. doi: 10.1097/MPH.0b013e3181bf298b. [DOI] [PubMed] [Google Scholar]
- 32.CLOLAR . Genzyme Corporation; 2013. Prescribing information. Warnings and Precautions; pp. 1–5. [Google Scholar]
- 33.Abboud C.N., Lang N., Fung H., et al. Real-world safety experience of tevagrastim/ratiograstim/biograstim and tbo-filgrastim, short-acting recombinant human granulocyte colony-stimulating factors. Support Care Cancer. 2019;27:2569–2577. doi: 10.1007/s00520-018-4522-5. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez-Sandoval J.C., Varela-Jimenez R., Morales-Buenrostro L.E. Capillary leak syndrome as a complication of antibody-mediated rejection treatment: a case report. CEN Case Rep. 2018;7:110–113. doi: 10.1007/s13730-018-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao S.C., Wang M.C., Chang H., Pei S.N. Recurrent capillary leak syndrome following bortezomib therapy in a patient with relapsed myeloma. Ann Pharmacother. 2010;44:587–589. doi: 10.1345/aph.1M585. [DOI] [PubMed] [Google Scholar]
- 36.Lee D.W., Gardner R., Porter D.L., et al. Current concepts in the diagnosis and management of cytokine release syndrome [published correction appears in Blood. 2015;126:1048] Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wing M.G., Moreau T., Greenwood J., et al. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996;98:2819–2826. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploessl C., Pan A., Maples K.T., Lowe D.K. Dinutuximab: an anti-GD2 monoclonal antibody for high-risk neuroblastoma. Ann Pharmacother. 2016;50:416–422. doi: 10.1177/1060028016632013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang F., Yang J., Li Z. Trastuzumab-induced systemic capillary leak syndrome in a breast cancer patient. Pathol Oncol Res. 2014;20:435–437. doi: 10.1007/s12253-013-9713-2. [DOI] [PubMed] [Google Scholar]
- 40.Witzel I.D., Müller V., Harps E., et al. Trastuzumab in pregnancy associated with poor fetal outcome. Ann Oncol. 2008;19:191–192. doi: 10.1093/annonc/mdm542. [DOI] [PubMed] [Google Scholar]
- 41.Lescure C., Lescoat A., Salé A., et al. Systemic capillary leak syndrome (Clarkson’s disease) as a complication of anti-programmed death 1 immunotherapy. J Thorac Oncol. 2019;14:e131–e132. doi: 10.1016/j.jtho.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Polishchuk I., Yakobson A., Zemel M., et al. Nivolumab-induced systemic capillary leak syndrome as an ultra rare life-threatening phenomenon of late toxicity and intravenous immunoglobulin efficacy. Immunotherapy. 2021;13:807–811. doi: 10.2217/imt-2020-0335. [DOI] [PubMed] [Google Scholar]
- 43.Qin H., Vlaminck B., Owoyemi I., et al. Successful treatment of pembrolizumab-induced severe capillary leak syndrome and lymphatic capillary dysfunction. Mayo Clin Proc Innov Qual Outcomes. 2021;5:670–674. doi: 10.1016/j.mayocpiqo.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umeda Y., Hayashi H., Sugiyama S., Aoyama Y. Systemic capillary leak syndrome triggered by anti-programmed death 1 checkpoint inhibitor in psoriasis. J Dermatol. 2020;47:1322–1325. doi: 10.1111/1346-8138.15541. [DOI] [PubMed] [Google Scholar]
- 45.Denileukin diftitox. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases;2012-. December 27, 2017.
- 46.Plunkett W., Huang P., Searcy C.E., Gandhi V. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996;23(suppl 10):3–15. [PubMed] [Google Scholar]
- 47.Lau P.C., Zheng S.F., Ng W.T., Yu S.C. Inoperable pancreatic adenocarcinoma rendered complete remission by high intensity focused ultrasound concurrent with gemcitabine/capecitabine chemotherapy: case report and topic review. J Dig Dis. 2012;13:60–64. doi: 10.1111/j.1751-2980.2011.00546.x. [DOI] [PubMed] [Google Scholar]
- 48.Pavlakis N., Bell D.R., Millward M.J., Levi J.A. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer. 1997;80:286–291. doi: 10.1002/(sici)1097-0142(19970715)80:2<286::aid-cncr17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Barlesi F., Villani P., Doddoli C., et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91. doi: 10.1046/j.0767-3981.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 50.Gupta N., Ahmed I., Steinberg H., et al. Gemcitabine-induced pulmonary toxicity: case report and review of the literature. Am J Clin Oncol. 2002;25:96–100. doi: 10.1097/00000421-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 51.Vander Els N.J., Miller V. Successful treatment of gemcitabine toxicity with a brief course of oral corticosteroid therapy. Chest. 1998;114:1779–1781. doi: 10.1378/chest.114.6.1779. [DOI] [PubMed] [Google Scholar]
- 52.Dahan L., Ressiot E., Cournede A., et al. Anasarca, a complication of chemotherapy with gemcitabine in two patients with pancreatic cancer. Gastroenterol Clin Biol. 2007;31:1143–1145. doi: 10.1016/s0399-8320(07)78353-7. [DOI] [PubMed] [Google Scholar]
- 53.Chen S.H., Li D.L., Yang F., et al. Gemcitabine-induced pancreatic cancer cell death is associated with MST1/cyclophilin D mitochondrial complexation. Biochimie. 2017;103:71–79. doi: 10.1016/j.biochi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Maroz N., Weiner I.D. Secondary capillary leak syndrome related to pemetrexed exposure. Am J Kidney Dis. 2012;59:582–583. doi: 10.1053/j.ajkd.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 55.Anderson B.J., Peterson L.L. Systemic capillary leak syndrome in a patient receiving adjuvant oxaliplatin for locally advanced colon cancer. J Oncol Pharm Pract. 2016;22:725–728. doi: 10.1177/1078155215591388. [DOI] [PubMed] [Google Scholar]
- 56.Kanduri S.R., Cheungpasitporn W., Thongprayoon C., et al. Systematic Review of Risk factors and Incidence of Acute Kidney Injury Among Patients Treated with CAR-T Cell Therapies. Kidney Int Rep. 2021;6:1416–1422. doi: 10.1016/j.ekir.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutgarts V., Jain T., Zheng J., et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant. 2020;26:1071–1076. doi: 10.1016/j.bbmt.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S., Seethapathy H., Strohbehn I.A., et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76:63–71. doi: 10.1053/j.ajkd.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee M.D., Strohbehn I.A., Seethapathy H.S., et al. Acute kidney injury after the CAR-T therapy tisagenlecleucel. Am J Kidney Dis. 2021;77:990–992. doi: 10.1053/j.ajkd.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caimi P.F., Pacheco Sanchez G., Sharma A., et al. Prophylactic tocilizumab prior to anti-CD19 CAR-T cell therapy for non-Hodgkin lymphoma. Front Immunol. 2021;12:745320. doi: 10.3389/fimmu.2021.745320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesterhuis W.J., Rennings A.J., Leenders W.P., et al. Vascular endothelial growth factor in systemic capillary leak syndrome. Am J Med. 2009;122:e5–e7. doi: 10.1016/j.amjmed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 62.Kinoshita Y., Kasaoka S., Fujita M., et al. Synchronized changes in serum vascular endothelial growth factor during the clinical course of chronic systemic capillary leak syndrome. Intern Med. 2010;49:791–794. doi: 10.2169/internalmedicine.49.2929. [DOI] [PubMed] [Google Scholar]
- 63.Xie Z., Chan E., Yin Y., et al. Inflammatory markers of the systemic capillary leak syndrome (Clarkson disease) J Clin Cell Immunol. 2014;5:1000213. doi: 10.4172/2155-9899.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarychanski R., Abou-Setta A.M., Turgeon A.F., et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis [published correction appears in JAMA. 2013;309:1229] JAMA. 2013;20:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 65.Mutchler S.M., Kleyman T.R. New insights regarding epithelial Na+ channel regulation and its role in the kidney, immune system and vasculature. Curr Opin Nephrol Hypertens. 2019;28:113–119. doi: 10.1097/MNH.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pineton de Chambrun M., Luyt C.E., Beloncle F., et al. EurêClark Study Group The clinical picture of severe systemic capillary leak syndrome episodes requiring ICU admission. Crit Care Med. 2017;45:1216–1223. doi: 10.1097/CCM.0000000000002496. [DOI] [PubMed] [Google Scholar]
- 67.Lambert M., Launay D., Hachulla E., et al. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit Care Med. 2008;36:2184–2187. doi: 10.1097/CCM.0b013e31817d7c71. [DOI] [PubMed] [Google Scholar]
- 68.Ibata S., Sato T., Takada K., et al. Isoform D of vascular endothelial growth factor in systemic capillary leak syndrome: a case report. J Med Case Rep. 2016;10:125. doi: 10.1186/s13256-016-0894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yabe H., Yabe M., Koike T., et al. Rapid improvement of life-threatening capillary leak syndrome after stem cell transplantation by bevacizumab. Blood. 2010;115:2723–2724. doi: 10.1182/blood-2009-11-247056. [DOI] [PubMed] [Google Scholar]
- 70.Dowden A.M., Rullo O.J., Aziz N., et al. Idiopathic systemic capillary leak syndrome: novel therapy for acute attacks. J Allergy Clin Immunol. 2009;124:1111–1113. doi: 10.1016/j.jaci.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 71.Gousseff M., Arnaud L., Lambert M., et al. The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med. 2011;154:464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 72.Lacal P.M., Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107. doi: 10.1016/j.phrs.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 73.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neelapu S.S., Tummala S., Kebriaei P., et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]