Abstract
Introduction
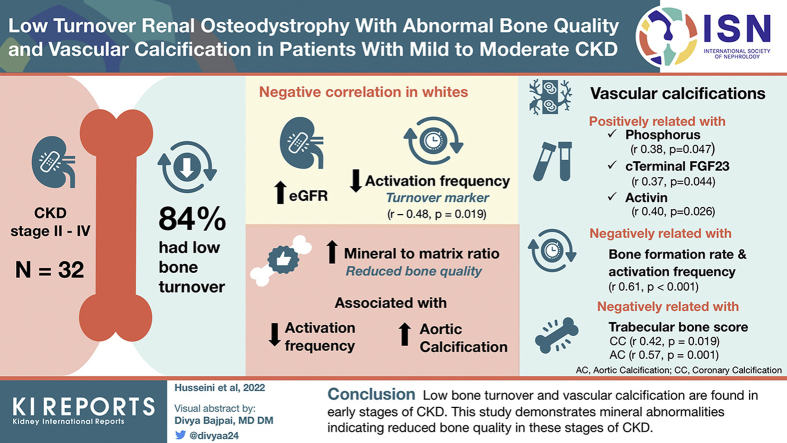
Limited information is available on renal osteodystrophy (ROD) and vascular calcification (VC) during early chronic kidney disease (CKD). This study was designed to evaluate ROD and VC in 32 patients with CKD stages II to IV.
Methods
Patients underwent dual-energy X-ray absorptiometry (DXA) for assessment of bone mineral density (BMD) and trabecular bone score (TBS), thoracic computed tomography for VC scoring using the Agatston method, and anterior iliac crest bone biopsy for mineralized bone histology, histomorphometry, and Fourier transform infrared spectroscopy (FTIR). Classical and novel bone markers were determined in the blood.
Results
Mean estimated glomerular filtration rate (eGFR) was 44 ± 16 ml/min per 1.73 m2. Of the patients, 84% had low bone turnover. In Whites, eGFR correlated negatively with the turnover parameter activation frequency (Ac.f) (r −0.48, P = 0.019) and with parameters of bone formation. Most patients had VC (>80%) which correlated positively with levels of phosphorus, c-terminal fibroblast growth factor-23, and activin. Aortic calcifications (ACs) correlated negatively with bone formation rate (BFR) and Ac.f (rho −0.62, −0.61, P < 0.001). TBS correlated negatively with coronary calcification (rho −0.42, P = 0.019) and AC (rho −0.57, P = 0.001). These relationships remained after adjustment of age. The mineral-to-matrix ratio, an FTIR metric reflecting bone quality, was negatively related to Ac.f and positively related to AC.
Conclusion
Low bone turnover and VC are predominant in early stages of CKD. This is the first study demonstrating mineral abnormalities indicating reduced bone quality in these stages of CKD.
Keywords: bone biopsy, bone quality, cardiovascular calcification, DXA, renal osteodystrophy, trabecular bone score
Graphical abstract
Approximately 37 million American adults (1 in 7) have CKD.1 CKD-mineral and bone disorder (CKD-MBD) is seen early during loss of kidney function and almost in all dialysis patients. ROD represents the bone manifestations of CKD-MBD; it is well described in advanced CKD stages,2 however, it develops early during the course of loss of kidney function.3 In 2006, ROD was described as abnormalities in bone turnover, mineralization, and bone balance/volume.4,5 It may present with high or low bone turnover which controls bone volume changes.6,7 If there is high turnover, there will be osteoporosis if resorption exceeds formation (negative balance), or there will be osteosclerosis if formation exceeds resorption (positive balance).8 Limited information is available on bone turnover and bone quality parameters in the early stages of CKD. Changes in bone quality have been observed with lower bone turnover and in elderly patients.6 FTIR is a very sensitive method of assessing material properties that have recently been used to study bone quality in patients with osteoporosis.9, 10, 11 We use it here for the first time in patients with CKD.
Determination of areal BMD by DXA is the most commonly used method for measuring bone loss in patients with CKD.12,13 A relatively new addition to the use of lumbar DXA images is measurement of the TBS.14,15 This score provides information on vertebral trabecular texture reflecting bone microarchitecture and correlates well with trabecular bone volume in patients with CKD.16 Moreover, an inverse relationship between TBS and AC was reported.17
ROD not only presents with abnormalities in turnover, mineralization, and volume but also is associated with increased risk of cardiovascular calcification18 and its related mortality.19, 20, 21, 22, 23 The correlations between cardiovascular calcifications and bone turnover abnormalities have been reported in patients with advanced CKD.23,24 However, cardiovascular calcifications may occur early in patients with CKD, and the relationship between ROD and cardiovascular calcification in patients with stages II to IV CKD is not well documented. The aim of the study was to comprehensively characterize the CKD-MBD syndrome in contemporary mild-to-moderate CKD. This includes examining bone quality in these patients.
Methods
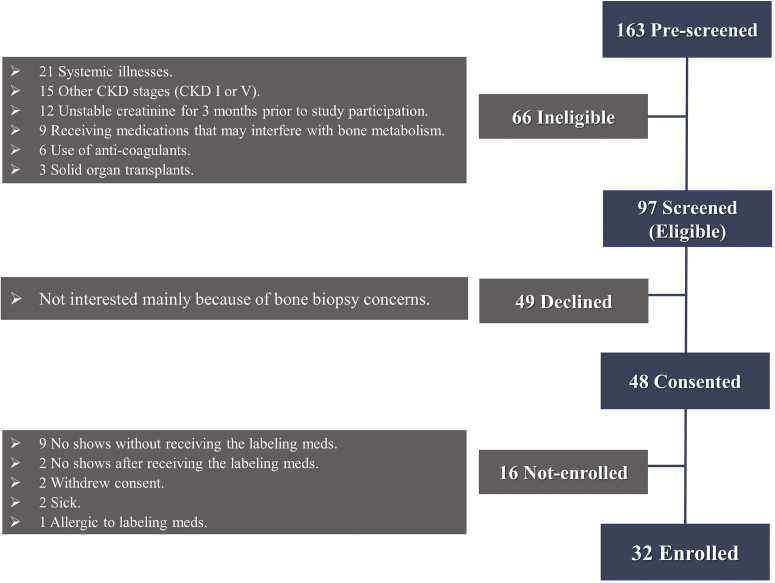
Patients were recruited from the CKD clinic at the University of Kentucky, from February 2016 to March 2018. Flowchart for recruitment, screening, and enrollment is shown in Figure 1.
Figure 1.
Flowchart for recruitment, screening, and enrollment. CKD, chronic kidney disease.
Patients agreed to undergo screening examinations including blood drawing, DXA, non-contrast thoracic computed tomography, and a bone biopsy for histomorphometry and FTIR. In our clinic, the patients are routinely treated following the Kidney Disease: Improving Global Outcomes guidelines for the management of CKD-MBD.25 The study was reviewed and approved by the Institutional Review Board at the University of Kentucky and was conducted according to the Declaration of Helsinki. Inclusion and exclusion criteria were applied to rule out patients with other causes of metabolic bone disease. Inclusion criteria were age ≥ 21 years, CKD stages II to IV, stable creatinine level for 3 months before study participation, willingness, and mental competence to participate in the study. Exclusion criteria were previous dialysis; organ transplantation; parathyroidectomy; pregnancy or lactation; allergy to tetracycline or demeclocycline; life-threatening comorbid conditions such as HIV, active infection, systemic illnesses, active or chronic liver disease; malabsorption; thyrotoxicosis; malignancy; chronic alcoholism; and/or drug addiction. Moreover, patients were ruled out if they were receiving medications that might affect bone metabolism (except oral calcium and parent vitamin D) such as vitamin D analogs, glucocorticoids, or any other immunosuppressants, bisphosphonates, calcimimetics, and systemic anticoagulants.
All patients underwent (i) a blood draw for determination of serum parameters; (ii) DXA of the femoral neck, total hip, and lumbar spine including measurement of the TBS; (iii) electrocardiogram-synchronized non-contrast thoracic computed tomography for coronary and aortic calcium measurement; and (iv) anterior iliac crest bone biopsy after double tetracycline labeling for mineralized bone histology, histomorphometry, and FTIR.
Determinations of Serum Parameters
Blood samples were withdrawn after an overnight fasting, on the day of the bone biopsy. Serum creatinine, calcium, and phosphorus levels were measured by automated techniques. eGFR was determined using the Modification of Diet in Renal Disease formula. Intact parathyroid hormone (iPTH) was measured by a radio-immunometric assay (Scantibodies, Santee, CA); normal range: 14 to 66 pg/ml; intra-assay coefficient of variation: <5%. Serum 25-hydroxy vitamin D concentrations were measured by electrochemiluminescence immunoassay on a Roche Elecsys 10100/201 system (Roche Diagnosis Elecsys, Mannheim, Germany). Vitamin D deficiency was defined as <10 ng/ml and insufficiency as <30 ng/ml. Activin A levels were measured using R&D Systems kits (Indianapolis, IN), sclerostin levels using Biomedica kits (Vienna, Austria), fibroblast growth factor-23 using Kainos kits (Tokyo, Japan), α-Klotho using IBL kits (Fujioka-Shi, Gunma, Japan), and bone-specific alkaline phosphatase and tartrate-resistant acid phosphatase 5b using Quidel kits (San Diego, CA). All measurements were performed in duplicate using enzyme-linked immunosorbent assay.
Measurements of BMD and TBS
DXA scans were performed by the same operator using the same machine for the duration of the study. An iDXA (GE Medical Systems Lunar, Madison, WI) was used. T scores for the DXA measurements are reported by the machine and the reference population used has been published previously.26 The coefficients of variation for DXA measurements were spine 1.35% and hip 0.52%. BMD absolute measurements were calculated as the average of the L1 through L4 values and as the average of bilateral femoral neck sites and total hip regions. The TBS of lumbar spines 1 to 4 was calculated from the same DXA scans using TBS iNsight software (version 2.2; Medimaps Group, Geneva, Switzerland). TBS results are classified as normal (TBS ≥ 1.35), partially degraded (1.21–1.34), or degraded (≤1.20) microarchitecture as previously reported.15 The coefficient of variation for TBS was 1.93%.
Computed Tomography for Cardiovascular Calcium Assessment
Prospective electrocardiogram-synchronized non-contrast computed tomographic scans of the thorax were obtained with a Siemens FORCE scanner (Siemens Healthineers, Erlangen, Germany). Contiguous 3-mm-thick axial images with a displayed field of view optimized for visualization of the heart and aorta were obtained within a z-axis range from the level of the proximal great vessels to the diaphragmatic hiatus. A kVp of 120 and automated mA modulation were utilized for all scans. Estimated effective radiation doses were normative for a seventh-generation scanner. Agatston calcium scoring and calcium volume measurements27 for both the aorta and the coronary arteries were performed by an experienced cardiovascular radiologist using a United States Food and Drug Administration–approved semiautomated algorithm (Aquarius Intuition, Intuition AI, Durham, NC). Agatston scores were stratified according to severity (risk for cardiovascular events); high severity (>400), moderate severity (100 < Agatston score ≤ 400), and low severity (1–100).28,29
Mineralized Bone Histology and Bone Histomorphometry
All bone samples were processed and analyzed at the Bone Diagnostic and Research Laboratory, University of Kentucky. This laboratory is accredited by the College of American Pathologists which conducts regular checkups for quality control and assurance. For double labeling of bone, patients were given oral demeclocycline hydrochloride 300 mg twice daily for 2 days followed by a 10-day free interval and then tetracycline hydrochloride 500 mg twice daily for 4 days. Anterior iliac crest bone biopsies were performed after an additional 4 days using a “J” 8 gauge needle (TRAPSYSTEM, Boca Raton, FL) with a vertical approach as previously described.2,7 Bone specimens were processed without removal of the mineral as described previously.7 Bone histomorphometry for static and dynamic parameters of bone structure, formation, and resorption was done at a magnification ×200 using the Osteoplan II image analysis system.30,31 All measured histomorphometric parameters are in compliance with the recommendations of the nomenclature committee of the American Society for Bone and Mineral Research.32,33 ROD was assessed by evaluation of its components “Turnover, Mineralization, and Volume.”4,5 Bone turnover was assessed by Ac.f, BFR/bone surface, and numbers of osteoblasts and osteoclast/bone perimeter based on published normal ranges2,7,34, 35, 36 (shown in results; Table 1). Mineralization was assessed by osteoid thickness and mineralization lag time. Osteomalacia was defined as osteoid thickness > 20 μm combined with mineralization lag time > 100 days.2 Bone volume was assessed by cancellous bone volume/tissue volume, trabecular thickness and separation, and cortical thickness and porosity
Table 1.
Bone histomorphometric results (N = 32)
| Median (range) | Normal values | |
|---|---|---|
| Turnover | ||
| Activation frequencya (number/yr) | 0.22 (0.001–0.80) | 0.49–0.72 |
| Bone formation rate/bone surfacea (mm3/cm2/yr) | 0.64 (0.001–1.94) | 1.80–3.80 |
| Number of osteoblasts/bone perimeter (number/100 mm) | 7.77 (4.23–159) | 10–200 |
| Number of osteoclast/bone perimeter (number/100 mm) | 9.35 (4.23–25.8) | 5–53 |
| Mineralization | ||
| Osteoid thickness (μm) | 6.42 (2.87–15.59) | <20 |
| Mineralization lag timea (d) | 19.89 (0.41–134) | <100 |
| Structure | ||
| Bone volume/tissue volume (%) | 18.22 (10.49–28.52) | 16.8–22.9 |
| Trabecular thickness (μm) | 107 (61.24–151) | 99–142 |
| Trabecular separation (μm) | 482 (304–717) | 280–658 |
| Cortical thickness (μm) | 252 (121–575) | 225–440 |
| Cortical porosity (%) | 6.11 (2.39–20.74) | <10 |
N = 31 patients; 1 patient had a single label only.
FTIR
FTIR is an established technique for analyzing various tissues in health and disease; it is especially useful for quantifying various bone mineral and matrix parameters reflecting bone quality and fracture resistance.37, 38, 39, 40 Bone mineral parameters included mean values of the mineral-to-matrix ratio, carbonate-to-phosphate ratio, and c-axis mineral crystal length (crystallinity). The bone matrix parameter was the ratio of mature to immature collagen crosslinks (crosslinking ratio).41 FTIR was performed on bone samples using our published methodology.11
Statistical Analyses
Results are given as mean ± SD or median and range when values were not normally distributed. Categorical variables are expressed as number and percentages. Spearman rho correlation is used to assess the correlation between variables. Mann-Whitney test and Kruskal-Wallis test are used to compare non-normally distributed continuous variables between groups. The one-way analysis of variance is used to determine whether there are any statistically significant differences between the means of ≥2 categories. Non-normally distributed parameters are log-transformed for multivariable regression analysis. Univariable and multivariable analyses of clinical, routine laboratory, bone, and cardiovascular calcification data are performed. We adjust for covariates that might impact the bone and cardiovascular calcification in a multivariable analysis. All statistical analyses are performed using SPSS version 24 (IBM Corp., Armonk, NY). A P ≤ 0.05 is considered statistically significant. RStudio version 1.4.1717 (RStudio Public-benefit Corp., Boston, MA) was used to create the violin plots.
Results
A total of 32 patients with CKD were included in the study with mean eGFR ± SD of 44.1 ± 15.9 ml/min per 1.73 m2, range: 16 to 85. Most of the patients were females (59%), White (72%), and nonsmokers (62%). Of the females, 3 were under 55 years of age, and none of them still had regular cycles. The most common comorbidities were hypertension, diabetes, dyslipidemia, and coronary artery disease. The demographic, clinical, medication usage, and laboratory characteristics are shown in Tables 2 and 3. No patient required treatment with bicarbonate.
Table 2.
Demographics and clinical data (N = 32 subjects)
| Age (yr) | 61.1 ± 11.3 | |
| Sex | Male | 13 (40.6) |
| Female | 19 (59.4) | |
| Race | White | 23 (71.9) |
| Black | 8 (25.0) | |
| Asian | 1 (3.1) | |
| BMI | 35.8 (23.8–51.4) | |
| CKD stages | Stage II | 3 (9.4) |
| Stage III | 24 (75) | |
| Stage IV | 5 (15.6) | |
| Smoking | Current smoker | 6 (18.8) |
| Ex-smoker | 6 (18.8) | |
| Nonsmoker | 20 (62.4) | |
| Hypertension | 31 (96.9) | |
| Diabetes | 19 (59.4) | |
| Dyslipidemia | 14 (43.8) | |
| Coronary artery disease | 6 (18.8) | |
| eGFR (ml/min per 1.73 m2) | 44.1 ± 15.9 | |
| Medication usage | ||
| Parent vitamin D | 12 (37.5) | |
| Calcium supplements | 2 (6.3) | |
| ACEI/ARBs | 18 (56.3) | |
| CCBs | 12 (37.5) | |
| BBs | 15 (46.9) | |
| Diuretics | 15 (46.9) | |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BB, β-blockers; BMI, body mass index; CCB, calcium channel blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Results presented as n (%) or mean ± SD.
Table 3.
Serum biochemical data
| Serum levels | Normal values | |
|---|---|---|
| Serum calcium (mg/dl) | 9.4 ± 0.4 | 8.5–10.3 |
| Serum phosphorus (mg/dl) | 3.5 ± 0.7 | 2.5–4.5 |
| Serum albumin (g/dl) | 3.7 ± 0.4 | 3.5–5.0 |
| Vitamin D level (ng/ml) | 31 (13–88) | >30 |
| iPTH (pg/ml) | 49 (16–221) | 14–66 |
| BSAP (U/l) | 22.12 (11.17–42.57) | 18–75 |
| TRAP-5b (U/l) | 2.27 (1.34–6.81) | 1.2–6.7 |
| Intact FGF23 (pg/ml) | 15.95 (3.20–45.40) | N/A |
| c-Terminal FGF23 (pg/ml) | 36.30 (12.20–914.6) | N/A |
| Klotho (pg/ml) | 329.0 (179.2–603.5) | 239–1266 |
| Sclerostin (pg/ml) | 291.6 (84.10–781.5) | 245–1182 |
| Activin (μg/ml) | 465.2 (219–896.5) | 142–753 |
BSAP, bone-specific alkaline phosphatase; FGF, fibroblast growth factor; iPTH, intact parathyroid hormone; max, maximum; min, minimum; N/A, not applicable; TRAP, tartrate-resistant acid phosphatase.
Results presented as mean ± SD or median (min–max).
Of the patients, 84% had low bone turnover. Low bone turnover was found as early as GFR of 85 and in the large majority of patients with eGFR of 20 to 60 ml/min per 1.73 m2. Median values for the bone turnover parameters Ac.f, BFR/bone surface, and number of osteoblasts/bone perimeter were clearly below normal ranges (Table 1). These low values were less pronounced with lower eGFR (r with Ac.f −0.48, P = 0.019). This was observed in non-Black patients only and remained significant after adjusting for age and diabetes. Patients with diabetes tended to have lower bone turnover than those without (Ac.f. 0.25 number/yr vs. 0.29 and BFR/bone surface 0.65 vs. 0.76 mm3/cm2/yr), but these differences did not reach significance. Similar relationships were observed in non-Black patients between eGFR and osteoid surface, osteoid volume, and BFR (Table 4). These relationships were not found in Black patients. Of the patients, 26% had cortical thickness below normal and 22% had increased cortical porosity. Mineralization defect was not found in any patient. No patient exhibited woven osteoid or peritrabecular fibrosis, and there was no stainable aluminum or any bone marrow abnormality. The bone histomorphometry results are shown in Table 1.
Table 4.
Linear regression analysis of histomorphometric parameters with eGFR in non-Blacks
| Dependent variable | r with eGFR | Linear regression adjusted for age and diabetes |
||
|---|---|---|---|---|
| β | 95% CI | P value | ||
| Osteoid surface/bone surface | −0.58 | −0.20 | −0.35 to −0.05 | 0.011 |
| Osteoid volume/bone volume | −0.48 | −0.03 | −0.06 to 0.00 | 0.064 |
| Bone formation rate/bone surface | −0.50 | −0.03 | −0.04 to −0.01 | 0.009 |
| Activation frequency | −0.47 | −0.01 | −0.02 to −0.003 | 0.009 |
CI, confidence interval; eGFR, estimated glomerular filtration rate.
The majority of the patients had coronary artery calcification (CAC) (72%; Table 5). CAC correlated positively with levels of serum phosphorus, c-terminal fibroblast growth factor-23, and activin (rho 0.378, P = 0.047; rho 0.370, P = 0.044; and rho 0.407, P = 0.026, respectively). Patients with CAC > 400 had median iPTH of 39 pg/ml (range 16–68) compared with 60 pg/ml (range 23–221) in patients with CAC < 100 (P = 0.007). There was no correlation between serum iPTH levels and AC scores.
Table 5.
Coronary and aortic calcification scores
| CAC score | 33.5 (0–3410) | |
| CAC severity category | Zero CAC | 28.1% |
| Low >0–100 | 28.1% | |
| Moderate 100–400 | 9.4% | |
| High >400 | 28.1% | |
| Coronary artery calcium volume score | 28.3 (0–2707) | |
| Aorta calcification score | 150 (0–5076) | |
| Aorta calcification volume | 144 (0–4073) | |
CAC, coronary artery calcification.
One patient had coronary artery graft so CAC measurements were not obtainable.
CAC and AC scores were positively correlated (rho 0.535, P = 0.002). AC scores correlated positively with age (rho 0.516, P = 0.003) as did CAC scores but only in females (rho 0.529, P = 0.024). AC scores were higher in patients with coronary artery disease (P = 0.018), dyslipidemia (P = 0.016), and smokers (P = 0.036). These relationships showed a trend with CAC as well, but limited number of patients and inability to measure CAC in patients with coronary stents limited the significance of the findings.
AC correlated negatively with bone turnover parameters (BFR [rho −0.621, P < 0.001] and Ac.f [rho −0.606, P < 0.001]). These relationships remained after adjusting for age and diabetes (adjusted B relative to log-transformed AC: for Ac.f −2.1, 95% CI −3.54 to −0.63, P = 0.007; for BFR/bone surface −1.04, 95% CI −1.70 to −0.37, P = 0.004). CAC scores had a negative correlation with erosion depth (μm) (rho −0.420, P = 0.021).
All patients had normal serum calcium and phosphorus levels. Serum iPTH and 25-vitamin D levels were within the normal range in the majority of patients (75% and 65%, respectively). In 10 patients with iPTH above normal, 8 had low bone turnover by histology. Serum phosphorus and 25-vitamin D levels did not correlate with any bone parameters, whereas serum calcium showed a weak positive correlation with bone volume (r 0.414, P = 0.019) and trabecular thickness (r 0.369, P = 0.038). There was no significant relationship found between serum sclerostin and the histomorphometrically measured bone parameters. Levels of serum iPTH, phosphorus, and c-terminal fibroblast growth factor-23 correlated negatively with eGFR (rho −0.43, P = 0.014; r −0.493, P = 0.006; rho −0.438, P = 0.012, respectively).
BMD measured by DXA was normal in the majority of patients; only 6.5% of the patients had osteoporosis at the femoral neck, whereas no patient had osteoporosis at the lumbar spine or total hip. Osteopenia was found in 44.8% of the patients at total hip, 35.5% at femoral neck, and 9.7% at lumbar spine. The only histomorphometric parameter that correlated with DXA-BMD measurements in all patients was trabecular separation (rho −0.438, P = 0.014, rho −0.394, P = 0.035 for lumbar and hip t scores, respectively). BMD t scores of the femoral neck, total hip, and lumbar spine correlated positively with α-klotho (rho 0.507, P 0.004; rho 0.390, P 0.033; and rho 0.425, P 0.015, respectively).
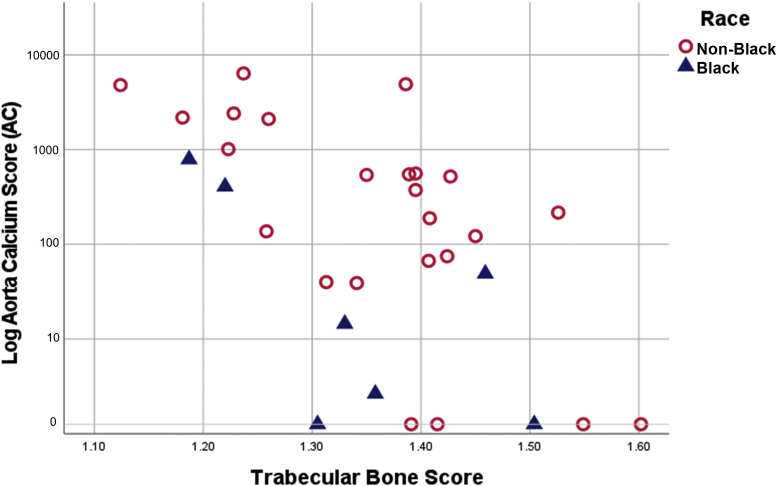
The mean TBS score was 1.35 ± 0.12. Of all the patients, 56% had normal TBS (≥1.35), 13% had TBS ≤ 1.20, and 31% had TBS between 1.21 and 1.34. TBS correlated negatively with age (r −0.393, P = 0.026) and positively with body mass index (r 0.394, P = 0.026). TBS was negatively correlated with CAC and AC scores (rho −0.42, P = 0.019 and −0.57, P = 0.001, respectively) (Figure 2). The inverse relationship between TBS and both CAC-score and AC-score remained significant after adjustment for age (β −4.11, P = 0.046 and β −3.03, P = 0.036, respectively).
Figure 2.
Correlation between trabecular bone score and aorta Agatston score log. AC, aortic calcification.
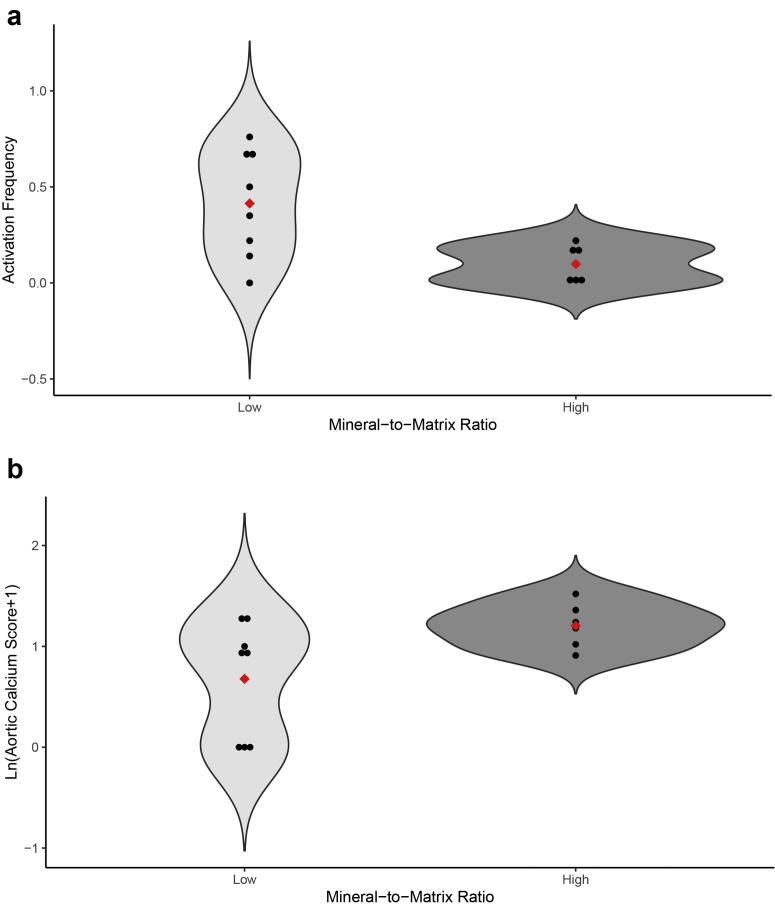
The mean FTIR values were within the normal range; however, some patients were below or above the normal range (Table 6). The mineral-to-matrix ratio was negatively related to Ac.f (r −0.421, P = 0.016) and positively related to AC (rho 0.379, P = 0.035) (Figure 3a and b). Of 27 patients with low turnover, 6 (22%) had abnormally high mineral-to-matrix ratio, and no patient with normal-high turnover had abnormally high mineral-to-matrix ratio. Additional significant correlations between FTIR results and age, race, histomorphometric parameters of bone turnover, AC, and serum biochemical parameters are shown in Table 7.
Table 6.
FTIR results
| Patients (mean ± SD) | Normal (mean ± SD) | % Below normal | % Above normal | |
|---|---|---|---|---|
| Mineral-to-matrix ratio | 4.55 ± 0.49 | 4.54 ± 0.36 | 25 | 18.8 |
| Carbonate-to-phosphate ratio | 0.95 ± 0.08 | 0.95 ± 0.05 | 18.8 | 25 |
| Collagen crosslinks | 3.50 ± 0.32 | 3.56 ± 0.21 | 28.1 | 21.9 |
| Crystal size | 1.18 ± 0.06 | 1.15 ± 0.04 | 6.3 | 34.4 |
FTIR, Fourier transform infrared spectroscopy.
Figure 3.
Relationship of mineral-to-matrix ratio with bone turnover and aortic calcification. Violin plots showing individual values and range of low and high mineral-to-matrix ratio groups and their relationship with activation frequency (a, upper panel) and aortic calcium score (b, lower panel). Red diamonds represent mean values.
Table 7.
Significant correlations with FTIR parameters
| Mineral-to-matrix ratio |
Collagen crosslinks |
Crystal size |
||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r/mean | P value | |
| Age | 0.456 | 0.009 | ||||
| Race (non-Black/Black) | (1.195/1.144) | 0.04 | ||||
| Osteoid volume/bone volume | −0.359 | 0.044 | ||||
| Osteoid thickness | −0.373 | 0.035 | ||||
| Bone formation rate/bone surface | −0.378 | 0.039 | ||||
| Activation frequency | −0.421 | 0.016 | ||||
| Aortic calcification score | 0.379 | 0.035 | 0.372 | 0.039 | ||
| Aortic calcification volume | 0.402 | 0.025 | 0.363 | 0.045 | ||
| BSAP | −0.428 | 0.016 | ||||
| Sclerostin | 0.464 | 0.007 | 0.381 | 0.032 | ||
BSAP, bone-specific alkaline phosphatase; FTIR, Fourier transform infrared spectroscopy.
Discussion
ROD is characterized as a disorder of bone turnover, mineralization, and balance or volume.4,5 Turnover is the most important component of ROD because it results in increased volume when balance is positive and decreased volume when balance is negative. In this study, low bone turnover, abnormal bone quality, and cardiovascular calcification were very prevalent in patients with CKD stages II to IV. In dialysis patients, both low (5%–58%) and high turnover (22%–24%) have been described.2,3,42, 43, 44 The onset and development of ROD during the course of CKD progression are not well known. There are limited bone histomorphometric data from patients with early CKD43,45,46 and no data on bone quality. More than 4 decades ago, Malluche et al.3 studied bone histomorphometry in 50 patients with CKD with an eGFR ranging from 6 to 80 ml/min per 1.73 m2. At that time, high turnover and mineralization defect were found in the majority of patients. This could be explained by the fact that vitamin D deficiency was common and aluminum intoxication was not uncommon at that time. More recently, Drüeke and Massy44 discussed the changing presentation of ROD with progression of CKD stages over the last several decades and described the transition from low to high turnover with progressive loss of kidney function. Brazilian investigators have also reported that low turnover ROD is common in patients with early stages of CKD.45, 46, 47 Conversely, Neto et al.48 published turnover data from patients with CKD stages III to IV and found a high percentage of patients with normal bone histology. These results could be explained by different patient characteristics, different histologic technique, different normal populations, or normal range definition, and/or other factors.
In the present study, a direct inverse relationship between eGFR and turnover was found. This relationship remained after adjustment for age and the presence of diabetes. Most of the patients with CKD stages II to IV (84%) had low bone turnover without use of vitamin D analogs, calcium-sensing receptors, or antiresorptive therapies. These results indicate that medications aimed at suppressing PTH activity and/or bone turnover in patients with mild-to-moderate CKD should not be routinely used. In our patients with moderately abnormal iPTH, 80% had low bone turnover by histology. Secondary hyperparathyroidism may be an adaptive mechanism to guard against low bone turnover, and a higher PTH level is required to overcome the apparent PTH resistance with the goal of maintaining normal BFR as CKD progresses.
In our cohort, eGFR correlated negatively with bone turnover parameters only in non-Black patients. However, it did not correlate with the bone histomorphometric values when we included Blacks. We previously reported that among dialysis patients, Whites exhibit predominantly low bone turnover, whereas in Blacks high bone turnover is the prominent ROD presentation. At any given plasma PTH level, bone turnover is lower in Blacks compared with Whites.2 Barreto et al.49 also reported that White dialysis patients tend to have lower PTH levels and lower turnover.
The association between Klotho and BMD requires further study. The available data are inconsistent; some studies show no relationship,50, 51, 52 whereas others show a negative correlation.53 Our findings in these 32 patients with CKD are worth reporting, but they do not allow us to make claims regarding pathogenetic relevance. The use of FTIR provided us with a very sensitive method for analyzing bone matrix and mineral quality which has not been previously used in patients with early CKD.6 The patients with the lowest turnover in our study had abnormal bone quality as evidenced by high mineral-to-matrix ratios. The mineral-to-matrix ratio also correlated with the bone markers bone-specific alkaline phosphatase and sclerostin, known to be related to turnover. The mineral-to-matrix ratio is a key parameter of bone composition which is related to bone stiffness and energy-to-fracture; high values are associated with reduced fracture toughness (fracture resistance),54 increased microdamage,55 and fractures.11,56 Further details on FTIR parameters and measurements of bone quality are described by Malluche et al.41
The TBS is a noninvasive tool to assess bone microarchitecture.57 In the present study, no significant relationship was found between TBS and bone histomorphometric parameters, including trabecular separation. Few studies have examined the relationship between histomorphometric bone parameters and TBS in patients with CKD before dialysis. Ramalho et al.16 found that TBS significantly correlated with trabecular bone volume and “width” in a multivariable analysis. However, they did not find any relationship between TBS and other histomorphometric parameters. Luckman et al.58 reported that in patients with CKD stages III and VD, TBS correlated with cortical and trabecular microarchitecture measured by high-resolution peripheral computed tomography before kidney transplantation.
The development of ROD in patients with CKD increases the risk of cardiovascular calcification and ultimately cardiovascular mortality. This association is well described in late stages of CKD, particularly dialysis patients; however, little is known about the bone-vascular axis in early stages of CKD. An interesting study by Mace et al.59 provided some experimental evidence for a vessel-bone axis documented by transplantation of aortic tissue from uremic rats into rats with normal kidney function. These rats showed induction of bone genes involved in formation and mineralization. Still, fundamental mechanisms that regulate the bone and vascular compartments in patients with CKD are not well known. It seems that the kidney-bone-cardiovascular crosstalk is important and may become even more relevant with CKD progression.60
In our cohort of patients, 72% had measurable CAC and 81% had AC. In agreement with these results, Tomiyama et al.46 reported CAC in 66% of Brazilian predialysis patients with CKD. Also, Ichii et al.61 reported that 79% of their predialysis patients with CKD had AC. In contrast, other studies reported lower prevalence of cardiovascular calcifications in predialysis patients with CKD.62,63 The differences in the results might be explained by interstudy variabilities in ethnicity, sex, age, smoking, alcohol abuse, body mass index, and methods of measurements.
Thoracic AC scores correlated negatively with bone turnover parameters in this study. Other studies have also shown association between histomorphometric bone abnormalities and cardiovascular calcifications in patients with CKD. London et al.64 reported an inverse correlation between osteoblastic surfaces and arterial calcification score and a positive correlation between bone histomorphometric parameters of low bone turnover and calcification scores in dialysis patients. Our group, in collaboration with Asci et al.,18 demonstrated a U-shaped relationship between CAC and bone turnover and suggested that the 2 extremes of bone turnover are associated with VC. Barreto et al.43 evaluated longitudinally the relationship between bone changes and CAC progression in dialysis patients over 1 year. They reported an association between improvement in bone turnover and lower CAC progression in patients with both high and low turnover bone disease. Yamamoto et al.65 evaluated the progression of abdominal AC over 3 years in asymptomatic patients with CKD before dialysis. The serum PTH levels significantly correlated with progression of AC among patients with advanced CKD (stages IV and V). There are also data describing a relationship between bone volume and calcification.23 Thus, interpretation of VC should consider both bone volume and bone turnover as potential cofactors. We have found that the presence of diabetes is not as strong a cofactor as the presence of CKD.
The relationship between abnormal bone quality and cardiovascular calcification in patients with CKD is an intriguing finding. Patients with high mineral-to-matrix ratio had higher AC, and we found an inverse relationship between TBS and AC. Aleksova et al.17 also found that TBS values correlated inversely with abdominal AC scores. Similar to our results, they did not find any correlation between abdominal AC scores and BMD measurements.
Limitations of the present study include its cross-sectional design, the relatively small sample size, and the higher number of patients with CKD stage III compared with stages II and IV. The strength of the study rests with the patients’ selection to represent routine CKD clinic patients with no specific bone biopsy indication and inclusion of only patients with CKD without other secondary disorders causing CKD-MBD or affecting the bone. This is the first study to assess bone quality by FTIR and its relation with cardiovascular calcification in patients with CKD before dialysis.
Conclusion
The obtained findings confirm that low bone turnover and cardiovascular calcification are seen in the vast majority of patients with early stages of CKD. Consideration should include control of risk factors for cardiovascular calcifications and low bone turnover. We also, for the first time, demonstrate abnormal bone quality as measured by FTIR in these patients. The link between abnormal bone quality and cardiovascular calcification is an interesting observation calling for longitudinal studies in a larger number of patients.
Disclosure
All authors declare no competing interests.
Acknowledgments
Support for the study was provided by the National Institutes of Health, grant 5RO1 DK 080770, and the Kentucky Nephrology Research Trust.
Footnotes
STROBE Statement.
Supplementary Material
STROBE statement
References
- 1.US Department of Health and Human Services, Center for Disease Control and Prevention Chronic Kidney Disease in the United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf Published 2019.
- 2.Malluche H.H., Mawad H.W., Monier-Faugere M.C. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–1376. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malluche H.H., Ritz E., Lange H.P., et al. Bone histology in incipient and advanced renal failure. Kidney Int. 1976;9:355–362. doi: 10.1038/ki.1976.42. [DOI] [PubMed] [Google Scholar]
- 4.Moe S., Drüeke T., Cunningham J., et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 5.Malluche H.H., Monier-Faugere M.C. Renal osteodystrophy: what’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol. 2006;65:235–242. doi: 10.5414/cnp65235. [DOI] [PubMed] [Google Scholar]
- 6.Malluche H.H., Porter D.S., Monier-Faugere M.C., Mawad H., Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol. 2012;23:525–532. doi: 10.1681/ASN.2010121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malluche H.H., Faugere M.C. Karger Publishers; 1986. Atlas of Mineralized Bone Histology. [Google Scholar]
- 8.Malluche H.H., Ritz E., Lange H.P., Arras D., Schoeppe W. Bone mass in maintenance haemodialysis. Prospective study with sequential biopsies. Eur J Clin Invest. 1976;6:265–271. doi: 10.1111/j.1365-2362.1976.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 9.Boskey A.L., Spevak L., Ma Y., et al. Insights into the bisphosphonate holiday: a preliminary FTIRI study. Osteoporos Int. 2018;29:699–705. doi: 10.1007/s00198-017-4324-5. [DOI] [PubMed] [Google Scholar]
- 10.Garcia I., Chiodo V., Ma Y., Boskey A. Evidence of altered matrix composition in iliac crest biopsies from patients with idiopathic juvenile osteoporosis. Connect Tissue Res. 2016;57:28–37. doi: 10.3109/03008207.2015.1088531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malluche H.H., Chen J., Lima F., Liu L.J., Monier-Faugere M.C., Pienkowski D. Bone quality and fractures in women with osteoporosis treated with bisphosphonates for 1 to 14 years. JBMR Plus. 2021;5 doi: 10.1002/jbm4.10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yenchek R.H., Ix J.H., Shlipak M.G., et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iimori S., Mori Y., Akita W., et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5-D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- 14.Schacter G.I., Leslie W.D., Majumdar S.R., Morin S.N., Lix L.M., Hans D. Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: the Manitoba BMD cohort. Osteoporos Int. 2017;28:3199–3203. doi: 10.1007/s00198-017-4166-1. [DOI] [PubMed] [Google Scholar]
- 15.Silva B.C., Leslie W.D., Resch H., et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29:518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 16.Ramalho J., Marques I.D.B., Hans D., et al. The trabecular bone score: relationships with trabecular and cortical microarchitecture measured by HR-pQCT and histomorphometry in patients with chronic kidney disease. Bone. 2018;116:215–220. doi: 10.1016/j.bone.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Aleksova J., Kurniawan S., Vucak-Dzumhur M., et al. Aortic vascular calcification is inversely associated with the trabecular bone score in patients receiving dialysis. Bone. 2018;113:118–123. doi: 10.1016/j.bone.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Asci G., Ok E., Savas R., et al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant. 2011;26:1010–1015. doi: 10.1093/ndt/gfq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anaya P., Blomquist G.A., Davenport D.L., Monier-Faugere M.C., Sorrell V.L., Malluche H.H. Coronary artery calcification in CKD-5D patients is tied to adverse cardiac function and increased mortality. Clin Nephrol. 2016;86:291–302. doi: 10.5414/CN108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamarche M.C., Hopman W.M., Garland J.S., White C.A., Holden R.M. Relationship of coronary artery calcification with renal function decline and mortality in predialysis chronic kidney disease patients. Nephrol Dial Transplant. 2019;34:1715–1722. doi: 10.1093/ndt/gfy183. [DOI] [PubMed] [Google Scholar]
- 21.Fahrleitner-Pammer A., Herberth J., Browning S.R., et al. Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res. 2008;23:1850–1858. doi: 10.1359/jbmr.080610. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Budoff M.J., Reilly M.P., et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adragao T., Herberth J., Monier-Faugere M.C., et al. Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:450–455. doi: 10.2215/CJN.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malluche H.H., Blomquist G., Monier-Faugere M.C., Cantor T.L., Davenport D.L. High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients on dialysis. J Am Soc Nephrol. 2015;26:2534–2544. doi: 10.1681/ASN.2014070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanis J.A. Vol. 4. World Health Organization; Geneva: 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO study group; pp. 368–381. [DOI] [PubMed] [Google Scholar]
- 27.McCollough C.H., Ulzheimer S., Halliburton S.S., Shanneik K., White R.D., Kalender W.A. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007;243:527–538. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 28.Raggi P., Shaw L.J., Berman D.S., Callister T.Q. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 29.Mahabadi A.A., Möhlenkamp S., Lehmann N., et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. J Am Coll Cardiol Imaging. 2017;10:143–153. doi: 10.1016/j.jcmg.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Malluche H.H., Sherman D., Meyer W., Massry S.G. A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int. 1982;34:439–448. doi: 10.1007/BF02411282. [DOI] [PubMed] [Google Scholar]
- 31.Manaka R.C., Malluche H.H. A program package for quantitative analysis of histologic structure and remodeling dynamics of bone. Comput Programs Biomed. 1981;13:191–201. doi: 10.1016/0010-468x(81)90098-2. [DOI] [PubMed] [Google Scholar]
- 32.Parfitt A.M., Drezner M.K., Glorieux F.H., et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 33.Dempster D.W., Compston J.E., Drezner M.K., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi Q., Monier-Faugere M.C., Geng Z., Malluche H.H. Predictive value of serum parathyroid hormone levels for bone turnover in patients on chronic maintenance dialysis. Am J Kidney Dis. 1995;26:622–631. doi: 10.1016/0272-6386(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 35.Sawaya B.P., Butros R., Naqvi S., et al. Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int. 2003;64:737–742. doi: 10.1046/j.1523-1755.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira A., Frazão J.M., Monier-Faugere M.C., et al. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19:405–412. doi: 10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boskey A.L., Donnelly E., Boskey E., et al. Examining the relationships between bone tissue composition, compositional heterogeneity, and fragility fracture: a matched case-controlled FTIRI study. J Bone Miner Res. 2016;31:1070–1081. doi: 10.1002/jbmr.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith T.E.L., Wooster M.J., Tattaris M., Griffith D.W.T. Absolute accuracy and sensitivity analysis of OP-FTIR retrievals of CO2, CH4, and CO over concentrations representative of “clean air” and “polluted plumes”. Atmos Meas Tech. 2011;4:97–116. doi: 10.5194/amt-4-97-2011. [DOI] [Google Scholar]
- 39.Boskey A., Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28:2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishimaru Y., Oshima Y., Imai Y., et al. Raman spectroscopic analysis to detect reduced bone quality after sciatic neurectomy in mice. Molecules. 2018;23:3081. doi: 10.3390/molecules23123081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malluche H.H., Porter D.S., Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9:671–680. doi: 10.1038/nrneph.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchison A.J., Whitehouse R.W., Boulton H.F., et al. Correlation of bone histology with parathyroid hormone, vitamin D3, and radiology in end-stage renal disease. Kidney Int. 1993;44:1071–1077. doi: 10.1038/ki.1993.350. [DOI] [PubMed] [Google Scholar]
- 43.Barreto D.V., Barreto Fde C., Carvalho A.B., et al. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52:1139–1150. doi: 10.1053/j.ajkd.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Drüeke T.B., Massy Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289–302. doi: 10.1016/j.kint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Barreto F.C., Barreto D.V., Canziani M.E., et al. Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J Bras Nephrol. 2014;36:289–296. doi: 10.5935/0101-2800.20140042. [DOI] [PubMed] [Google Scholar]
- 46.Tomiyama C., Carvalho A.B., Higa A., Jorgetti V., Draibe S.A., Canziani M.E. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. J Bone Miner Res. 2010;25:499–504. doi: 10.1359/jbmr.090735. [DOI] [PubMed] [Google Scholar]
- 47.Graciolli F.G., Neves K.R., Barreto F., et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017;91:1436–1446. doi: 10.1016/j.kint.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 48.Neto R., Pereira L., Magalhães J., et al. Sclerostin and DKK1 circulating levels associate with low bone turnover in patients with chronic kidney disease Stages 3 and 4. Clin Kidney J. 2021;14:2401–2408. doi: 10.1093/ckj/sfab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barreto F.C., Barreto D.V., Moyses R.M., et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- 50.Kužmová Z., Kužma M., Gažová A., et al. Fibroblast growth factor 23 and klotho are associated with trabecular bone score but not bone mineral density in the early stages of chronic kidney disease: results of the cross-sectional study. Physiol Res. 2021;70(suppl 1):S43–S51. doi: 10.33549/physiolres.934773. [DOI] [PubMed] [Google Scholar]
- 51.Marchelek-Mysliwiec M., Wisniewska M., Nowosiad-Magda M., et al. Association between plasma concentration of klotho protein, osteocalcin, leptin, adiponectin, and bone mineral density in patients with chronic kidney disease. Horm Metab Res. 2018;50:816–821. doi: 10.1055/a-0752-4615. [DOI] [PubMed] [Google Scholar]
- 52.Chalhoub D., Marques E., Meirelles O., et al. Association of serum klotho with loss of bone mineral density and fracture risk in older adults. J Am Geriatr Soc. 2016;64:e304–e308. doi: 10.1111/jgs.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamdy M., Shaheen I., Seif El Din H., et al. Klotho level as a marker of low bone mineral density in Egyptian sickle cell disease patients. J Pediatr Hematol Oncol. 2022;44:e40–e45. doi: 10.1097/MPH.0000000000002231. [DOI] [PubMed] [Google Scholar]
- 54.Alexander R.M. In: The Quarterly Review of Biology. Wainwright S.A., Biggs W.D., Currey J.D., Gosline J.M., editors. The university of Chicago Press; 1976. Mechanical design in organisms. [Google Scholar]
- 55.Allen M.R., Burr D.B. Mineralization, microdamage, and matrix: how bisphosphonates influence material properties of bone. IBMS BoneKEy. 2007;4:49–60. doi: 10.1138/20060248. [DOI] [Google Scholar]
- 56.Gourion-Arsiquaud S., Faibish D., Myers E., et al. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009;24:1565–1571. doi: 10.1359/jbmr.090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hans D., Goertzen A.L., Krieg M.A., Leslie W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26:2762–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 58.Luckman M., Hans D., Cortez N., et al. Spine trabecular bone score as an indicator of bone microarchitecture at the peripheral skeleton in kidney transplant recipients. Clin J Am Soc Nephrol. 2017;12:644–652. doi: 10.2215/CJN.09850916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mace M.L., Gravesen E., Nordholm A., et al. Chronic kidney disease-induced vascular calcification impairs bone metabolism. J Bone Miner Res. 2021;36:510–522. doi: 10.1002/jbmr.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson A.J., Raggi P., Wolf M., Gold A.M., Chertow G.M., Roe M.T. Targeting vascular calcification in chronic kidney disease. JACC Basic Transl Sci. 2020;5:398–412. doi: 10.1016/j.jacbts.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ichii M., Ishimura E., Shima H., et al. Quantitative analysis of abdominal aortic calcification in CKD patients without dialysis therapy by use of the Agatston score. Kidney Blood Press Res. 2013;38:196–204. doi: 10.1159/000355768. [DOI] [PubMed] [Google Scholar]
- 62.Suh-Chiou C., Moysés R.M., Bittencourt M.S., Bensenor I.M., Lotufo P.A. Chronic kidney disease and coronary artery calcification in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Clin Cardiol. 2017;40:1309–1315. doi: 10.1002/clc.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo D., Palmiero G., De Blasio A.P., Balletta M.M., Andreucci V.E. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 64.London G.M., Marty C., Marchais S.J., Guerin A.P., Metivier F., de Vernejoul M.C. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto D., Suzuki S., Ishii H., et al. Predictors of abdominal aortic calcification progression in patients with chronic kidney disease without hemodialysis. Atherosclerosis. 2016;253:15–21. doi: 10.1016/j.atherosclerosis.2016.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.