Antineutrophil cytoplasmic antibody-associated vasculitis (AAV) is a rare autoimmune disease,S1 with high mortality in the absence of treatment.S2 Induction therapy with glucocorticoids (GC) plus either cyclophosphamide or anti-CD20 followed by 2 years of maintenance treatment reduces AAV-related mortality from 80% to ∼5%.S3 However, there is still an unmet need to define treatment strategies to reduce drug side effects including GC toxicity,S1 to reverse rare cases of refractory AAV, and to improve long-term outcomes.
Recently, the role of the anaphylatoxin C5a in AAV has been demonstrated in mice.S4 C5a is a C5 cleavage product with chemoattracting activity on immune cells through the stimulation of the C5a receptor (C5aR1). Targeting C5aR may reduce AAV-induced lesions by inhibiting the recruitment of inflammatory cells to the active site of vasculitis.S5 The C5aR1 inhibitor avacopan (Vifor Pharma, Puteaux, France) has been developed for the treatment of AAV.1,S6,S7 In the ADVOCATE study, avacopan was noninferior to GC at week 24 and superior at week 52 in terms of reaching complete remission.2,3 The rate of relapse and the GC toxicity index were lower in the avacopan group, and no specific safety signal emerged. Real-life data are now only available in difficult-to-treat patients (refractory or GC dependent).4 Thus the first aim of this study was to assess the efficacy and tolerance of avacopan in standard patients, with a real GC sparing. As a second objective, we aimed to assess whether quantifying the urinary concentration of the soluble form of CD163 (usCD163), a marker of macrophages, may be a reliable marker of AAV. Indeed, in recent observational studies, usCD163 was described as a noninvasive and reliable marker of kidney inflammation in AAV, allowing early detection of relapses.5, 6, 7
Results
In this retrospective bicentric study, we included all the patients with AAV who received avacopan between March 2020 and September 2021 at the University Hospitals of Toulouse and Bordeaux (France), with a minimum of 12 months of follow-up. Two patients were excluded because of early withdrawal of avacopan related to swallowing disorders (n = 1) or too short follow-up (<3 months). Characteristics of the 9 patients are summarized in Table 1. Briefly, median age was 75 (34–86) years. A total of 5 patients (55%) had de novo AAV. Seven patients (78%) had anti-MPO antibodies, 1 (11%) had anti-PR3 antibodies, and 1 (11%) had both anti-MPO and anti-PR3 antibodies. All patients presented with rapidly progressive glomerulonephritis. Median estimated glomerular filtration rate (eGFR) was 30 ml/min per 1.73 m2 (ranges, 11–49). A kidney biopsy was performed in 7 patients and showed crescentic lesions. Extra-kidney involvements included the lungs (n = 3), peripheric nerves (n = 5), or ear-nose-throat (n = 2). Median Birmingham Vasculitis Activity Score (BVAS) was 18 (ranges, 12–29).
Table 1.
Main characteristics and outcomes in patients with AAV treated with avacopan
| Patient | Age (yr) | AAV |
Clinical characteristics at inclusion |
Kidney biopsy |
Immunosuppressive regimen |
Adverse events | Outcomes at mo 12 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De novo vs. relapse | ANCA type | BVAS | eGFR | Number of glomeruli with crescents, n (%) | Induction treatment | Cumulative dose for prednisone during first mo (total) in g | Overlap between Avacopan and GC (d) | BVAS | eGFR | ||||
| 1 | 54 | Relapse | MPO | 17 | 11 | 2 (25) | Rituximab | GC | 0.8 (0.8) | 0 | No | 0 | 23a |
| 2 | 86 | De novo | MPO | 12 | 26 | 7 (37) | Rituximab | GC | 0.78 (0.78) | 0 | No | 0 | 49 |
| 3 | 34 | De novo | Both | 29 | 49 | 8 (61) | Rituximab | GC + Pulseb | 4.02 (4.36) | 7 | No | 0 | 93 |
| 4 | 61 | De novo | MPO | 18 | 34 | 11 (50) | Rituximab | GC | 1.04 (1.2) | 21 | No | 0 | 28 |
| 5 | 83 | De novo | MPO | 23 | 15 | 1 (17) | Rituximab | GC | 1.14 (1.26) | 16 | Urinary infection | 0 | 11 |
| 6 | 76 | Relapse | PR3 | 24 | 30 | NA | Rituximab | GC | 1.56 (1.56) | 0 | No | 0 | 34 |
| 7 | 40 | Relapse | MPO | 12 | 40 | 6 (32) | Obinutuzumab | GC | 1.58 (1.73) | 21 | No | 0 | 50 |
| 8 | 85 | De novo | MPO | 21 | 35 | 1 (20) | Rituximab | — | — | NA | No | 1 | 45 |
| 9 | 75 | Relapse | MPO | 12 | 26 | NA | Rituximab | — | — | NA | No | 0 | 23 |
AAV, antineutrophil cytoplasmic antibody-associated vasculitis; BVAS, Birmingham Vasculitis Activity Score; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; NA, not applicable.
This patient required dialysis from month 1 to month 6.
1 g/d for 3 days.
The induction regimen before avacopan (used for all patients at 30 mg twice daily) relied on rituximab (375 mg/m2 on days 0–8–15–22), followed by maintenance with rituximab (500 mg at month 4, and every 6 months thereafter). One patient with an allergy to rituximab received obinutuzumab (1 g at day 0 and 15, repeated at month 6). None received plasma exchanges.
Reasons to prescribe avacopan were the following: neuropsychiatric disorders (n = 2), severe diabetes mellitus (n = 3), osteonecrosis of the femoral head (n = 1), and severe sleeping disorders (n = 3). The median delay between the diagnosis of AAV flare-up and the start of avacopan was 23 days (ranges, 17–45) (authorization and delivery process). Two of the 9 patients were treated without GCs, one because of osteonecrosis of the femoral head and the other one because of diabetes and metabolic syndrome. Seven patients received GCs (1 mg/kg/d of prednisone, except 1 patient [in Table 1, patient 3], who received methylprednisolone pulse 1 g/d for 3 days) in association with rituximab before avacopan, with 3 possible schemes:
-
1.
GCs were stopped 13 days before avacopan (patient 1).
-
2.
GCs were stopped on the day corresponding to the first dose of avacopan (patients 2 and 6).
-
3.
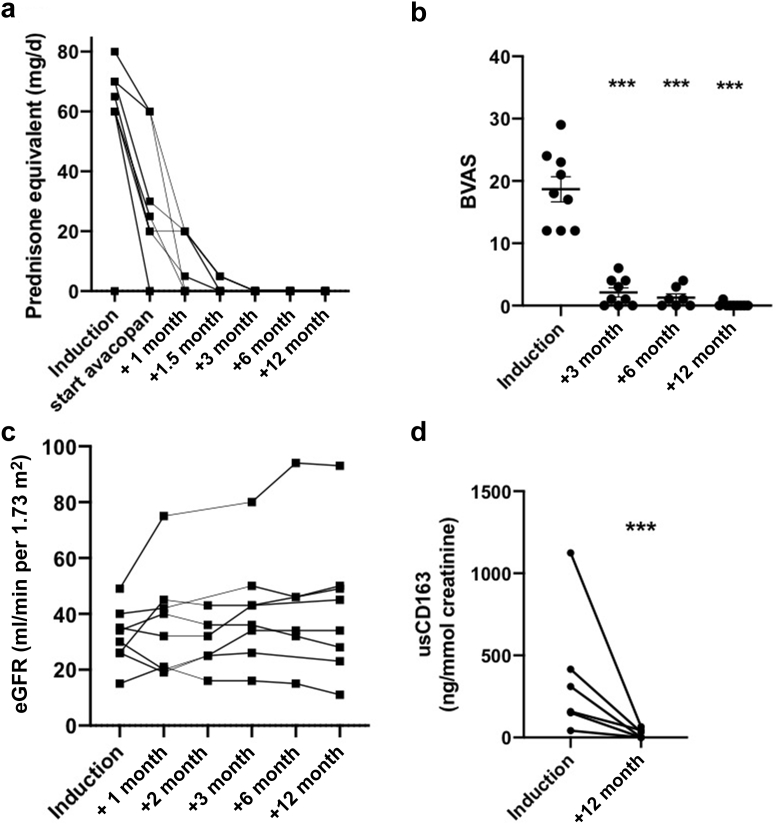
GCs were tapered, overlapping with avacopan for a median period of 19 days (ranges, 7–21) (patients 3, 4, 5, 7) (Figure 1a).
Figure 1.
Outcomes of 9 patients with ANCA-associated vasculitis who received avacopan. (a) Prednisone dosage in mg/d per patient at different time points. (b) BVAS at induction, month 3, and month 12. ∗∗∗ indicates P < 0.001 with paired t test. (c) eGFR at induction and months 1, 2, 3, 6, and 12. (d) usCD163 evolution. Urinary soluble CD163 concentration in urine normalized to urinary creatinine. Baseline corresponds to the start of avacopan, M12 to month 12. ∗∗∗P < 0.001 with paired t test. ANCA, Antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; eGFR, estimated glomerular filtration rate.
Median dose cumulative GC used was 1.14 g during the first month (ranges 0.8–4.02) and 1.26 (ranges 0.8–4.36) during the whole follow-up (Table 1). GCs were stopped after a median delay of 35 (ranges, 16–52) days after diagnosis. For all tested patients (n = 6), circulating CD19 cells count was <5/mm3 at month 12.
During the 12-month study period, no patient died. At month 2, BVAS was available only for 4 patients, with a median score of 4 (range, 0–6). At month 3, the median of BVAS (1 [ranges, 0–6]), significantly decreased compared with baseline (P < 0.001) and by month 12, 8 of the 9 patients had achieved complete remission (89%; median BVAS 0 [0–1]) (Figure 1b). No relapse occurred during the follow-up. One patient required kidney replacement therapy from months 1 to 6 and then recovered stable kidney function (eGFR 23 ml/min per 1.73 m2). In the 8 other patients, median eGFR was 34 ml/min per 1.73 m2 (ranges, 11–93) at month 12 (P = 0.14, compared with baseline) (Figure 1c). Overall, median VDI was 2 (0–4) at month 12.
For all patients with available urine samples during follow-up (n = 6), usCD163 was detected at diagnosis (median concentration 234 ng/mmol (ranges, 42–1124); Figure 1d). At month 12, median usCD163 was 17 ng/mmol (ranges, 0–66), including 3 with undetectable usCD163 (P = 0.009 compared with baseline). Individual values are available in Supplementary Figure S1. The patient requiring dialysis had the highest concentration of usCD163 (11–314 ng/mmol) at baseline. A total of 4 of 6 patients had usCD163 <250 ng/mmol7 before month 4.
During the 12-month study period, 1 patient developed a urinary infection. No hepatitis was reported. One patient with a history of age-related macular degeneration developed progressive visual acuity loss: given the fact that clinical remission of his AAV had been achieved, avacopan was stopped after 5.5 months.
Discussion
Avacopan is a new oral competitive inhibitor of C5aR. Recent data obtained in animal models and clinical studies confirmed its efficacy and safety in AAV, allowing GC-free regimen.8,9 Our work now adds new results in a real-life setting. First, the rate of remission at month 12 was much higher than in the ADVOCATE study (89% vs. 66%), despite similar severity at baseline according to the BVAS.2 No relapse occurred. The systematic use of rituximab as maintenance therapy may thus synergize with avacopan. Second, we confirmed that avacopan was associated with good kidney outcomes, including patient requiring dialysis. Together with data from ADVOCATE, our findings suggested that the beneficial effects of avacopan on kidney recovery could be prolonged beyond the first weeks of treatment. Third, no new safety signal was identified, and only 1 serious adverse event occurred (11%) compared with 37% in ADVOCATE. Fourth, our data reinforce the therapeutic potential of avacopan in real-life practice, as it was recently published only for difficult-to-treat patients.4 In our series, patients were diagnosed with either de novo or relapsing but not refractory AAV. This could explain why GC tapering was obtained much earlier and therefore why GC toxicity index was not calculated. Last, usCD163 emerged as a valuable early biomarker of AAV relapses. We show here that usCD163 concentration also follows the activity of AAV in patients receiving avacopan and that taking avacopan is followed by rapid control of kidney inflammation.
This study also has several limitations, as it included only 9 patients, but it is the first study with avacopan in a real-life setting for patients with de novo or relapsing AAV. Because of its retrospective nature, some urinary samples are lacking, and staining of CD163 on biopsy was not available. Furthermore, the proportion of MPO-positive patients is higher in our cohort than in ADVOCATE trial (89% vs. 56.6%). The consequence is that the proportion of PR3-positive patients, who are at highest risk to relapse, is lower. This should be taken into account in subsequent studies. Finally, all but 1 had eGFR equal or >15 ml/min. The prognosis for the only dialysis-dependent patient was good, with an increase in eGFR from 11 to 23 ml/min at the end of the cohort.
In conclusion, we confirmed that avacopan plus rituximab leads to a high rate of AAV remission and allows forgoing of GC. Avacopan may now belong to first-line treatments, not only in difficult-to-treat patients. usCD163 may help to individualize the length of avacopan therapy.
Disclosure
DR and DC received lecture fees from Vifor Pharma. SF received consulting fees from Abionyx Pharma (outside the scope of this study), congress travel support (Sanofi-Genzyme), and lecture fees (Vifor Pharma; Asahi). JB received lecture fee (Sanofi) outside the scope of this study. All the other authors declared no competing interests.
Footnotes
Supplementary Methods, Ethical Considerations, and Acknowledgements.
Supplementary References.
Figure S1. Kinetics of urinary soluble CD163 (usCD163) concentration in 6 patients with ANCA -associated vasculitis who received avacopan. sCD163 concentration in urine, normalized to urinary creatinine.
Supplementary Material
Supplementary Methods, Ethical Considerations, and Acknowledgements.
Supplementary References.
Figure S1. Kinetics of urinary soluble CD163 (usCD163) concentration in 6 patients with ANCA -associated vasculitis who received avacopan. sCD163 concentration in urine, normalized to urinary creatinine (PDF).
References
- 1.Jayne D.R.W., Bruchfeld A.N., Harper L., et al. Randomized trial of C5a receptor inhibitor Avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayne D.R.W., Merkel P.A., Schall T.J., Bekker P. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384:599–609. doi: 10.1056/NEJMOA2023386. [DOI] [PubMed] [Google Scholar]
- 3.Khan M.M., Molony D.A. In ANCA-associated vasculitis, avacopan was superior to prednisone taper for sustained remission. Ann Intern Med. 2021;174:JC79. doi: 10.7326/ACPJ202107200-079. [DOI] [PubMed] [Google Scholar]
- 4.Van Leeuwen J.R., Bredewold O.W., Van Dam L.S., et al. Compassionate use of Avacopan in difficult-to-treat ANCA-associated vasculitis. Kidney Int Rep. https://doi.org/10.1016/J.EKIR.2021.11.036 Published online December 8, 2021. [DOI] [PMC free article] [PubMed]
- 5.O’Reilly V.P., Wong L., Kennedy C., et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol. 2016;27:2906–2916. doi: 10.1681/ASN.2015050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villacorta J., Lucientes L., Goicoechea E., et al. Urinary soluble CD163 as a biomarker of disease activity and relapse in antineutrophil cytoplasm antibody-associated glomerulonephritis. Clin Kidney J. 2020;14:212–219. doi: 10.1093/CKJ/SFAA043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran S.M., Scott J., Clarkson M.R., et al. The clinical application of urine soluble CD163 in ANCA-associated vasculitis. J Am Soc Nephrol. 2021;32:2920–2932. doi: 10.1681/ASN.2021030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monti S., Brandolino F., Milanesi A., Xoxi B., Delvino P., Montecucco C. Novel therapies for ANCA-associated vasculitis. Curr Rheumatol Rep. 2021;23:38. doi: 10.1007/s11926-021-01010-0. [DOI] [PubMed] [Google Scholar]
- 9.Osman M., Cohen Tervaert J.W., Pagnoux C. Avacopan for the treatment of ANCA-associated vasculitis. Expert Rev Clin Immunol. 2021;17:717–726. doi: 10.1080/1744666X.2021.1932466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.