Abstract
Acute myocarditis is a rare but serious complication associated with mRNA-based coronavirus disease 2019 (COVID-19) vaccination. In this article, four COVID-19 mRNA vaccination induced myocarditis cases managed at our tertiary Medical Center have been discussed. Three patients had typical myocarditis. One patient suffered from atrioventricular block and heart failure, which required more intensive treatment, but eventually improved. Additionally, a review of cardiac magnetic resonance imaging (MRI) features related to the diagnosis of myocarditis showed that COVID-19 mRNA vaccine-associated myocarditis tend to have more late-gadolinium enhancement (LGE) accumulation in the inferior lateral wall direction. According to a report by the U.S. Centers for Disease Control and Prevention (CDC), the diagnosis of COVID-19 mRNA vaccine-associated myocarditis is based on clinical symptoms, altered myocardial enzymes, cardiac MRI finding, or histopathology. Cardiac MRI is relatively less invasive than myocardial biopsy and plays an important role in the diagnosis of myocarditis. This review may aid in the diagnosis of COVID-19 mRNA vaccine-associated myocarditis.
Keywords: cardiac MRI, COVID-19, mRNA vaccination, myocarditis, case series, review, case report
Introduction
The Ministry of Health, Labor and Welfare Japan approved a range of coronavirus disease 2019 (COVID-19) vaccines in February 2021, and vaccination has been since then widely promoted by the government through various health education campaigns and initiatives. By the end of November 2021, 79.2% of the Japanese population had received their first dose of the COVID-19 vaccine, and 77.3% had received their second dose (1).
As the younger population started receiving vaccines, adverse events different from those commonly seen in older adults began to occur, including myocarditis. In general, myopericarditis is a very rare adverse event associated with vaccination and has been reported particularly after administration of the smallpox vaccine (2, 3). To the best of our knowledge, in the case of COVID-19, as this is the first time that mRNA vaccines have been used clinically, the current occurrence of post-vaccination myocarditis is of particular concern.
In this study, several cases of myocarditis that were suspected to be associated with mRNA-based COVID-19 vaccination were reviewed, and a literature review has been presented regarding the efficacy and utility of late-gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (cMRI) in the diagnosis of myocarditis.
Case Presentation
From February to October 2021, four patients with COVID-19 mRNA vaccine-associated myocarditis were admitted to our hospital (Table 1). The diagnosis was based on the definition reported by the U.S. Centers for Disease Control and Prevention (CDC) (4). All patients fulfilled the Lake Louise Criteria (LLC) (5), which is considered as a diagnostic criterion for myocarditis on cMRI.
Table 1.
Patient demographic and medicals characteristics and associated health outcomes.
| Case 1 | Case 2 | Case 3 | Case 4 | ||
|---|---|---|---|---|---|
| Definition | Confirmed | Confirmed | Confirmed | Confirmed | |
| Age, y | 19 | 20 | 29 | 48 | |
| Sex | Male | Male | Male | Male | |
| Race/ethnicity | Caucasian | Again | Again | Again | |
| Vaccine type | |||||
| Types of mRNA vaccines | mRNA-1273-Moderna | mRNA-1273-Moderna | mRNA-1273-Moderna | BNT162b2 mRNA-Pfizer-BioNTech | |
| Number of vaccinations | 2 | 2 | 2 | 1 | |
| History of previous COVID-19 infection | Denied/ negative antigen | Denied/ negative antigen | Denied/ negative PCR | Denied/ negative PCR | |
| Symptoms | |||||
| Day 1 post-vaccination | Chest discomfort | Fever | Fever | No symptom | |
| Day 2 post-vaccination | Chest pain, pain with breathing, hospital admission | Chest pressure, nausea | Chest pain, hospital admission | Fever, tiredness, diarrhea | |
| Day 3 post-vaccination | Hospital admission | Tiredness | |||
| Day 4 post-vaccination | Tiredness | ||||
| Day 5 post-vaccination | Syncope, tiredness, hospital admission | ||||
| Vital signs at presentation | |||||
| Temperature, °C | 36.9 | 39.1 | 36.2 | 35.4 | |
| Heart rate, bpm | 100 | 106 | 73 | 80 | |
| Blood pressure, mm Hg | 109/58 | 120/57 | 117/69 | 85/57 | |
| Respirations, per min | 18 | 20 | 18 | 20 | |
| Chest x-ray findings | No acute pulmonary disease | No acute pulmonary disease | No acute pulmonary disease | enlarged cardiac shadow | |
| Cardiothoracic ratio (CTR) | 48.4% | 48.4% | 43.4% | 54.5% | |
| ECG findings | |||||
| ST changes | No | ST elevation in V3–6 | No | Negative T wave in V4–6 | |
| Rhythm | Normal sinus rhythm | Normal sinus rhythm | Normal sinus rhythm | Paroxysmal atrioventricular block | |
| Echocardiogram | |||||
| Number of days after vaccination | 3 days | 5 days | 3 days | 5 days | |
| LV ejection fraction | 52 | 62 | 58 | 30 | |
| LV end-diastolic internal dimension | 48 | 51 | 40 | 47 | |
| LV end-systolic internal dimension | 35 | 36 | 28 | 36 | |
| Intraventricular septal diastolic thickness | 9 | 10 | 9 | 14 | |
| LV posterior wall thickness | 12 | 10 | 12 | 15 | |
| E/A | 2.09 | 1.7 | 1.13 | 0.63 | |
| E/e' | 3.68 | 7.15 | 4.65 | 9.02 | |
| Regional wall motion abnor- malities | None | None | Non | Diffuse hypokinesis | |
| Diastolic function | Normal | Normal | Normal | ||
| Cardiac magnetic resonance imaging (cMRI) | |||||
| Number of days between last vaccination and cMRI | 5 days | 6 days (first time) | 45 days (second time) | 12 days | 12 days |
| LGE | Sub-epicardial wall of basal-mid infero-lateral LV | Na | mid wall of basal inferior LV | mid wall of anterior, and inferior LV | Mid-wall of basal inferior, Sub-epicardial wall of mid- and infero-septum LV |
| Definition | Confirmed | Confirmed | Confirmed | Confirmed | |
| T2WBB high signal | Sub-epicardial wall of basal-mid infero-lateral LV | mid wall of basal inferior LV | Na | mid wall of anterior, and inferior LV | Mid-wall of basal inferior, Sub-epicardial wall of mid- and infero-septum LV |
| Laboratory findings | |||||
| Cardiac troponin I pg/mL | |||||
| Presentation | 1,801.7 | 1,885.6 | 4,419.6 | 17,888.7 | |
| Peak | 5,321.9 | 5,749 | 4,419.6 | 17,888.7 | |
| Postdischarge | <10 | <10 | <10 | 15.5 | |
| CK U/L peak | 415 | 324 | 154 | 765 | |
| CK-MB U/L peak | 30.8 | 15.3 | 9 | 64 | |
| WBC | 6,500 | 5,900 | 6,100 | 5,000 | |
| BNP, pg/mL | 9.1 | 12.1 | 9 | 111 | |
| CRP, mg/dL | 5.63 | 8.78 | 1.16 | 11.32 | |
| Coronary angiography findings | ND | MRI negative | CCT negative | CAG no stenosis | |
| Clinical course | |||||
| Hospitalization duration | 5 | 8 | 5 | 11 | |
| Treatment(s) | Ibuprofen, ACE-I | Ibuprofen, ACE-I | Ibuprofen, ACE-I | dobutamine, Diuretic, Ibuprofen, ACE-I | |
BNP, B-type natriuretic peptide; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; E/A, transmitral Doppler early (E-wave) to late (A-wave) ventricular filling velocities; E/e', E-wave to tissue Doppler early diastolic mitral annular velocity; LGE, late gadolinium enhancement; LV, left ventricular; T2WBB, T2 Weighed black blood; WBC, white blood cell.
The study was approved by the institutional review board of the Japanese Red Cross Musashino Hospital and was conducted in accordance with the ethical principles of the Declaration of Helsinki as well as with the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. All participants provided their written informed consent for the anonymized publication of these findings and were provided information disclosure documents on our website. The participants were free to opt out of participation at any time without any adverse consequences or the loss of benefits to which they were otherwise entitled.
Case 1, Case 2, and Case 3
These three cases are relatively similar. Male patients under 30 years of age had developed myocarditis after their second dose of vaccination. They experienced some kind of chest symptoms such as chest pain and chest pressure within a few days after vaccine administration. Electrocardiography changes (ST elevation) were observed only in Case2. Particularly, negative T waves appeared after ST elevation; these negative T waves improved over time. High-sensitivity troponin-I levels were elevated in all cases, and creatine kinase-myocardial band (CK-MB) was also elevated to above the reference level in Case 1 and Case 2. There was no elevation of white blood cell (WBC) and B-type natriuretic peptide (BNP), but C-reactive protein (CRP) levels were elevated in all patients. Cardiac MRI was performed in all patients. LGE was observed in each case with varying localization, and was more common in the sub-epicardial wall. In Cases 1 and Case 3, T2 high signal intensity and LGE were observed simultaneously in the same segment. In Case 2, an examination performed 6 days after vaccination showed only T2 high signal intensity at the sub-epicardial wall of the basal inferior left ventricle. However, an examination performed 47 days later showed LGE in the same area and likewise demonstrated that the T2 high signal intensity had disappeared. Ibuprofen was administered to all patients due to its anti-inflammatory effects. An angiotensin-converting enzyme inhibitor (ACE-I) was administered as well to prevent remodeling.
Case 4
Case 4 had a relatively different course compared to the previous three cases. The patient developed fever the day after first dose vaccination, and was referred to the hospital 5 days after vaccination with syncope as the chief complaint. An electrocardiogram revealed paroxysmal atrioventricular block, which was thought to be the cause of syncope. Although a pacemaker lead had to be temporarily inserted for AV block, the paroxysmal AV block resolved 2 days after admission. On echocardiography, marked myocardial hypertrophy and decreased left ventricular contractility were observed. Improvements in hypertrophy and contraction were observed on subsequent echocardiography. In laboratory findings, compared to the previous three cases, BNP levels were elevated, and high-sensitivity troponin-I and CK-MB levels were relatively high. On cardiac MRI, T2 high signal intensity and LGE were observed simultaneously in the mid-wall of basal inferior and sub-epicardial wall of mid-septum and infero-septum left ventricular. Only this patient had an accumulation of LGE and high T2 signal on the left ventricular septum side (Figure 1). Catecholamines (i.e., dobutamine) and diuretics were administered during hospitalization as a treatment for heart failure. Diuretic, ibuprofen, and ACE-I were discontinued following confirmation of negative troponin levels in the outpatient clinic, with no apparent adverse events.
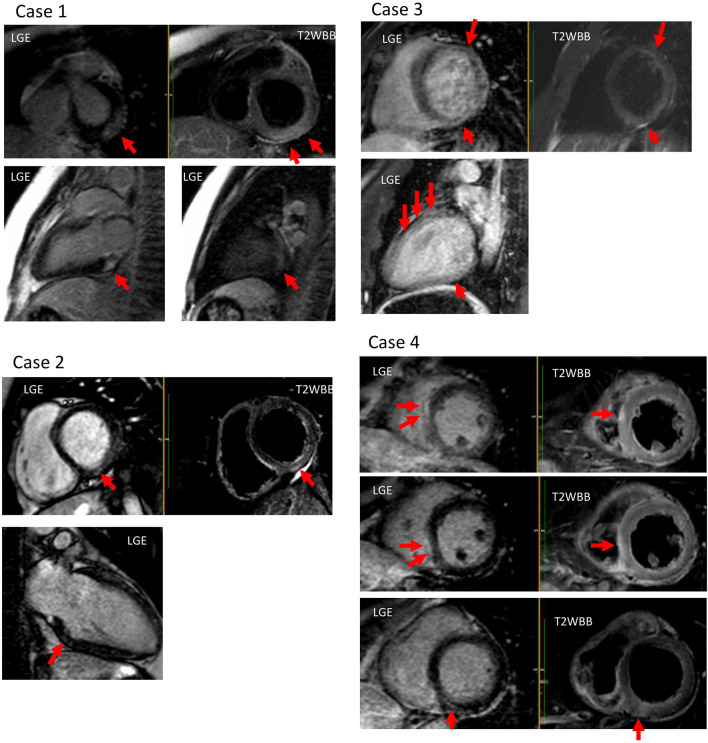
Figure 1.
Cardiac magnetic resonance imaging (MRI) of all profiled cases. Case 1: T2 high signal intensity and late-gadolinium enhancement (LGE) were observed at the sub-epicardial wall of the basal-mid inferolateral left ventricular (LV). Case 2: T2 high signal intensity and LGE were observed at the mid wall of the basal inferior LV. Case 3: T2 high signal intensity and LGE were observed at the mid wall of the anterior, and at the inferior LV. Case 4: T2 high signal intensity and LGE were observed at the mid-wall of basal inferior, and at the sub-epicardial wall of the mid- and inferoseptum LV.
Discussion
COVID-19 mRNA Vaccination-Associated Myocarditis
The CDC has recently reported diagnostic criteria for post-vaccination myocarditis (4). The criteria for diagnosis include specific clinical symptoms following vaccination, as well as cMRI findings consistent with myocarditis in the presence of troponin levels above the upper limit of normal and/or histopathologic confirmation of myocarditis. The diagnosis of myocarditis on cMRI is based on the implementation of either the original or the revised LLC (5, 6). We note that cMRI is less invasive than myocardial biopsy and is considered an important diagnostic tool for evaluating vaccine-associated myocarditis.
In this case series, cMRI was performed in all the cases. In Cases 1, 3, and 4, T2 high signals and LGE were observed simultaneously in the same segment, which was considered to fulfill the LLC. In Case 2, T2 high signal intensity was seen on initial examination. Later examination demonstrated the appearance of LGE in the same region. The initial examination showed inflammatory findings, and the LGE observed on the second examination was thought to be the result of fibrosis occurring due to these inflammatory findings. During the observation period, there were cases in which the MRI showed only LGE and no T2 findings, so the LLC could not be fulfilled, and the diagnosis could not be confirmed. More specifically, though these cases had clinical presentations consistent with myocarditis, there are two reasons they were not classified as confirmed cases. First, the quality of MRI was a limiting factor. Namely, the quality of cMRI at our hospital was unacceptable; specifically, the myocardial early gadolinium enhancement ratio could not be evaluated and used parametric mapping techniques with the currently available technology. Thus, findings of sufficient quality might not have been obtained; and may thus, have failed to confirm true myocarditis cases. The second limiting factor is the accuracy of the LLC. Though the LLC present a widely used diagnostic classification system for myocarditis, previous studies have reported that the sensitivity of diagnosing myocarditis when two out of the three main criteria were fulfilled was only ~78% (7). Thus, the accuracy of these criteria alone may not be sufficient to accurately diagnose myocarditis with acceptable sensitivity and specificity. However, studies to date indicate that the modified version of the LLC may provide more accuracy if parametric mapping techniques can be applied (6).
Cardiac MRI for Myocarditis
Numerous cases of COVID-19 mRNA vaccine-associated myocarditis have been reported till date. cMRI plays an important role in the diagnosis of any form of myocarditis. The American Heart Association (AHA) scientific statement on the management of myocarditis (8) as well as the current European Society of Cardiology (ESC) position statement (9) consider cMRI useful for the evaluation of suspected myocarditis. Japanese guidelines likewise suggest its usefulness (10). More specifically, cMRI provides a non-invasive, biopsy-like approach in order to verify the pathognomonic imaging features associated with and plays a role in the exclusion of myocardial inflammation. The current ESC guidelines on acute and chronic heart failure include a Class I indication for the efficacy of cMRI in the assessment of myocarditis (11). cMRI characteristics of myocardial inflammation may not only aid in the diagnosis of myocarditis but may also provide important and accurate information on prognoses. In acute cases, myocardial edema presenting without LGE on cMRI has been associated with improved recovery and outcomes (12). The relationship between the localization of LGE on cMRI and mortality in myocarditis has been reported as well (13). Thus, cMRI has evolved to become a key evaluation tool in patients with suspected myocardial inflammation.
Significance of LGE in Myocarditis
LGE is not an essential finding in the original or revised LLC for the diagnosis of myocarditis. However, LGE is the most established technique for detecting myocardial damage (14). The presence of LGE seems to be a good predictor of adverse outcomes in patients with biopsy-proven myocarditis, and has been shown to be superior to other variables in this regard (15). Some recent reports suggest that the location, pattern, extent, and distribution of LGE can stratify the risk for patients with suspected myocarditis. For example, Gräni et al. reported that septal and mid-wall LGE were most strongly associated with major cardiovascular events, including all-cause mortality, worsening heart failure, heart transplantation, and ventricular arrhythmias (16). Aquaro et al. showed that patients with anteroseptal LGE have a worse prognosis than those with LGE at other sites (17). Greulich et al. demonstrated that the presence of mid-wall LGE in the septal segments was associated with a higher long-term mortality rate as compared with the absence of LGE or other LGE patterns in patients with biopsy-proven viral myocarditis (13). One reported mechanism potentially mediating these effects is that the septal LGE might involve the conduction system, thus yielding the substrate for malignant arrhythmias (13). Among the cases presented in this report, only Case 4 revealed LGE in the septal segment. Specifically, Case 4 showed paroxysmal atrioventricular block as a disturbance of the conduction system; this case presentation was relatively severe as compared to the other three cases and thus necessitated intensive treatment. The importance of attaining a greater comprehension of LGE characteristics on cMRI of patients with myocarditis has been emphasized.
LGE in COVID-19 mRNA Vaccination-Associated Myocarditis
There have been numerous reports of COVID-19 mRNA vaccination-associated myocarditis in recent months; however, adverse reactions are relatively rare and comprehensive reports are limited. Shiyovich et al. reported 15 cases of vaccine-associated myocarditis; and, to the best of our knowledge, it is the largest case series published till date (18). In that report, LGE was observed in 13/15 patients with vaccine-associated myocarditis, and was more common in the inferolateral region.
“PubMed” was mainly accessed and reports were extracted using the keywords “myocarditis,” “mRNA vaccination,” and “COVID-19.” Cases, articles, and review articles without detailed MRI descriptions were excluded. Finally, numerous cases of COVID-19 mRNA vaccination-associated myocarditis presented within 24 publications were reviewed, all of which described the localization of LGE. A total of 62 cases (four cases evaluated by the study authors in the current report, and 58 cases evaluated within previously published case reports and case series) were reviewed in terms of diagnostic imaging, with a focus on LGE findings of cMRI (18–41). The localization was classified as anterior, anterolateral, lateral, inferolateral, inferior, inferoseptal, mid-septal, and anteroseptal. The layers of the myocardium were classified as epicardial or sub-epicardial, mid, and endocardial or sub-endocardial wall. Vertical localization (basal, mid, apical) was not described in many of the cases (i.e., only transverse localization was evaluated). In the present review, the examined cases comprised 61 males and one female with an average age of 29 (±12.4) years. Forty-one patients (66.1%) were under the age of 30 years. Forty-six patients (74.2%) had been vaccinated with the Pfizer mRNA-based vaccine and 16 patients (25.8%) had been vaccinated with Moderna mRNA-based vaccine. Table 2 and Figure 2 summarize the LGE features within cMRI. We found that LGE occurred more frequently on the free wall side (i.e., mainly in the inferolateral region) and occurred relatively less frequently on the septal side. LGE was mostly detected on the epicardia or sub-epicardia; no case with LGE on the left ventricular endocardia was identified.
Table 2.
Published case reports and case series regarding COVID-19 vaccine-associated myocarditis that describe LGE on cardiac magnetic resonance imaging (MRI).
| Age | Sex | Vaccine types | Number of vaccinations | Time from last vaccination to cardiac MRI | LGE: layer | LGE: segment | ||
|---|---|---|---|---|---|---|---|---|
| Marshall et al. | Case 1 | 16 | Male | Pfizer | 2nd | NA | Subepicardia | Apical and midchamber lateral wall |
| Case 2 | 19 | Male | Pfizer | 2nd | NA | Mid wall | Basal inferolateral wall | |
| Case 3 | 17 | Male | Pfizer | 2nd | NA | Subepicardial | Basal anterolateral segment, basal to midventricular inferolateral segments | |
| Case 5 | 17 | Male | Pfizer | 2nd | NA | Epicardia | Anterior and lateral LV | |
| Case 7 | 14 | Male | Pfizer | 2nd | 5 days | Subepicardial | Mid and apical left ventricle free wall | |
| Rosner et al. | Case 2 | 39 | Male | Pfizer | 2nd | 11 days | Subepicardial | Along the anterior and lateral walls |
| Case 3 | 39 | Male | Modelna | 2nd | 5 days | Subepicardial and midmyocardial | Anterior wall | |
| Case 4 | 24 | Male | Pfizer | 1st | 7 days | Midmyocardial | Septal and inferior walls | |
| Male | Subepicardial | Anterior, lateral, and inferior walls | ||||||
| Case 5 | 19 | Male | Pfizer | 2nd | 3 days | Multifocal patchy subepicardial and midmyocardial | Lateral and inferolateral walls | |
| Case 6 | 20 | Male | Pfizer | 2nd | 6 days | Subepicardial | Lateral, inferolateral, anterolateral walls, apex | |
| Case 7 | 23 | Male | Pfizer | 2nd | 3 days | Mid wall | Basal anteroseptal | |
| Mouch et al. | Case 1 | 24 | Male | Pfizer | 2nd | NA | Subepicardial | Basal septum |
| Mid myocardial | Inferolateral | |||||||
| Case 2 | 20 | Male | Pfizer | 2nd | NA | Subepicardial | Basal and middle anterolateral and inferolateral walls | |
| Case 3 | 29 | Male | Pfizer | 2nd | NA | Diffuse | Basal, inferolateral, anterolateral and anteroseptal walls | |
| Case 4 | 45 | Male | Pfizer | 1st | NA | Subepicardial | Middle anterolateral, inferolateral and apical anterior walls | |
| Case 5 | 16 | Male | Pfizer | 2nd | NA | Midmyocardial | Basal inferolateral | |
| Male | Subepicardial | Middle anterolateral | ||||||
| Case 6 | 17 | Male | Pfizer | 2nd | NA | Subepicardial | Basal inferolateral, middle inferolateral and infero-septal and apical lateral, anterior and inferior walls | |
| Mid-myocardial | Middle inferolateral and anterolateral and apical anterior and lateral walls | |||||||
| Kim et al. | Case 1 | 36 | Male | Moderna | 2nd | 3 days | Epicardial | Apical lateral |
| Case 4 | 24 | Male | Pfizer | 2nd | 3 days | Epicardial, patchy | Lateral | |
| Ammirati et al. | 56 | Male | Pfizer | 2nd | NA | Subepicardial-intramyocardial regions | Basal and apical segments of the infero-lateral wall | |
| Angelo et al. | 30 | Male | Pfizer | 2nd | 6 days | Subepicardial | Sparing of the basal and mid-septal segments | |
| Albert et al. | 24 | Male | Moderna | 2nd | 5 days | Mid-myocardial and epicardial | Lateral, anterolateral and inferolateral segments | |
| Muthukumar et al. | 52 | Male | Moderna | 2nd | 6 days | Midmyocardial and subepicardial | Infero-septal, inferolateral, anterolateral, and apical walls | |
| Subepicardial | Inferior basal and mesocardial midventricular region | |||||||
| Minocha et al. | 17 | Male | Pfizer | 2nd | NA | Subepicardial | Mid-lateral and apical | |
| Mansour et al. | Case 1 | 25 | Male | Moderna | 2nd | 6 days | Subepicardial | Anterolateral wall of the mid and apical left ventricle |
| Case 2 | 21 | Female | Moderna | 2nd | 4 days | Subepicardial | Inferolateral wall at the base | |
| Habib et al. | 37 | Male | Pfizer | 2nd | NA | Subepicardial | Basal lateral wall | |
| Cereda et al. | 21 | Male | Pfizer | 2nd | NA | Patchy epicardial | The posterior, anterior, inferior, and lateral walls | |
| Vidula et al. | Case 1 | 19 | Male | Pfizer | 2nd | NA | Subepicardial | Basal to mid lateral wall |
| Case 2 | 18 | Male | Moderna | 2nd | NA | Subepicardial | Mid lateral wall | |
| Williams et al. | 34 | Male | Moderna | 2nd | 7 days | Subepicardial | Anterolateral and inferolateral segments | |
| Isaak et al. | 15 | Male | Pfizer | 2nd | NA | Subepicardial | Inferolateral wall | |
| Hasnie et al. | 22 | Male | Moderna | 1st | NA | Subepicardial | Lateral wall and inferior segments at the midventricular and apical LV | |
| Patrignani et al. | 56 | Male | Pfizer | 1st | 11 days | Sub-epicardial | Basal and middle segments of the infero-lateral wall | |
| Kim et al. | 24 | Male | Pfizer | 2nd | NA | Sub-epicardial | Basal inferior and inferolateral segment | |
| Ehrlich et al. | 40 | Male | Pfizer | 1st | 12 days | Diffuse | Basal and mid anteroseptal and inferoseptal segments as well as in the apical septal segment | |
| Patel et al. | Case 1 | 22 | Male | Pfizer | 1st | NA | Subepicardial | Basal inferior, basal inferolateral, and apical lateral |
| Case 2 | 19 | Male | Pfizer | 2nd | NA | Subepicardial | Basal inferolateral | |
| Case 3 | 25 | Male | Moderna | 2nd | NA | Subepicardial | Lateral | |
| Case 4 | 37 | Male | Pfizer | 2nd | NA | Subepicardial | Basal anteroseptal segment | |
| Case 5 | 20 | Male | Pfizer | 2nd | NA | Subepicardial and mid-myocardial | Basal, mid, and apical lateral segments | |
| Tailor et al. | 44 | Male | Moderna | 2nd | 5 days | Mid-myocardial | Mid-septum, infero-septum, and inferior walls at the base to midventricle | |
| Sub-epicardial and mid-myocardial | Lateral wall at the mid-ventricle and apical lateral wall | |||||||
| Nguyen et al. | 20 | Male | Moderna | 1st | NA | Subepicardial | Mid and basal inferolateral segments | |
| Onderko et al. | Case 2 | 28 | Male | Pfizer | 2nd | NA | Epicardium | Apical lateral wall, midanterolateral segments |
| Case 3 | 36 | Male | Moderna | 2nd | NA | Epicardial | Mid- to distal inferolateral and lateral walls | |
| Shiyovich et al. | Case 1 | 41 | Male | Pfizer | 2nd | 107 days | Mid-wall | Inferolateral (basal) |
| Case 2 | 24 | Male | Pfizer | 2nd | 103 days | Mid-wall and epicardia | Inferolateral (basal) | |
| Case 3 | 17 | Male | Pfizer | 2nd | 7 days | Epicardial | Inferolateral, anterolateral, (basal to apical) | |
| Case 4 | 37 | Male | Pfizer | 1st | 48 days | Epicardial | Inferolateral (basal, mid) | |
| Case 5 | 39 | Male | Pfizer | 2nd | 8 days | Mid-wall and epicardia | Inferoseptal, anteroseptal (basal), inferolateral, anterolateral (basal), Inferolateral (med), septum, lateral (apical) | |
| Case 7 | 19 | Male | Pfizer | 2nd | 43 days | Mid-wall | Inferior (apicalbasal), Inferolateral (mid, basal), anterior (basal, mid), septum, lateral (apical) | |
| Case 8 | 28 | Male | Pfizer | 2nd | 139 days | Mid-wall | Inferolateral, anterolateral (basal) | |
| Case 10 | 17 | Male | Pfizer | 2nd | 17 days | Epicardial | Inferior, inferolateral (basal), inferior, inferoseptal, inferolateral (mid) | |
| Case 11 | 36 | Male | Pfizer | 1st | 63 days | Mid-wall | Lateral (apical) | |
| Case 12 | 27 | Male | Pfizer | 2nd | 105 days | Epicardial | Inferolateral (basal) | |
| Case 13 | 42 | Male | Pfizer | 1st | 53 days | Epicardial | Inferolateral (apical, basal), anterolateral (basal) | |
| Case 14 | 76 | Male | Pfizer | 2nd | 117 days | Mid-wall | Inferolateral (basal) | |
| Case 15 | 32 | Male | Pfizer | 2nd | 83 days | Mid-wall | Inferior (basal), inferolateral (basal) | |
| Our Cases | Case 1 | 19 | Male | Moderna | 2nd | 5 days | Sub-epicardial | Infero-lateral (basal-mid) |
| Case 2 | 20 | Male | Moderna | 2nd | 45 days | Mid-wall | Inferior (basal) | |
| Case 3 | 29 | Male | Moderna | 2nd | 12 days | mid-wall | Anterior, inferior wall | |
| Case 4 | 48 | Male | Pfizer | 1st | 12 days | Mid-wall | Inferior walls at the base to midventricle | |
| Subepicardial | Mid-septum, and infero-septum of left ventricular Wall |
LGE, late-gadolinium enhancement; LV, left ventricular.
Figure 2.
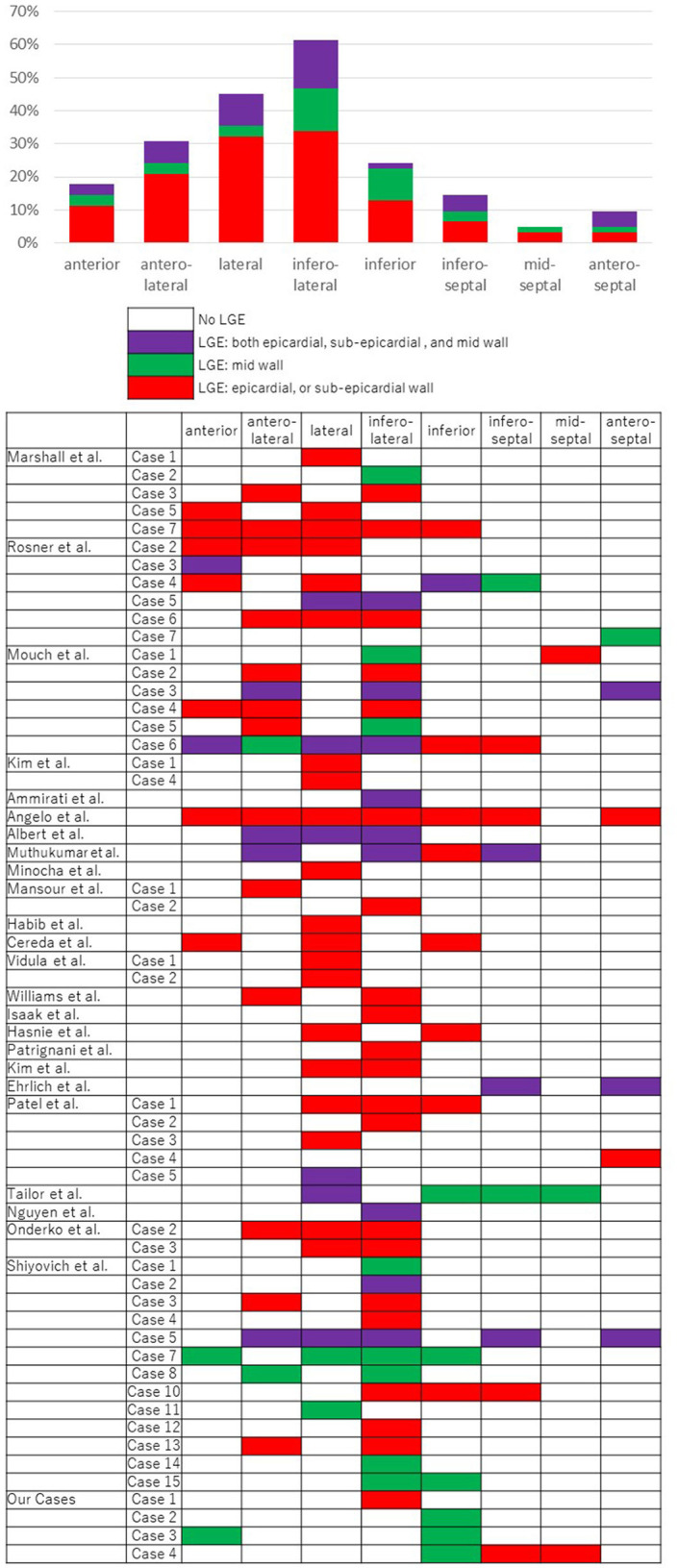
A summary of results regarding late-gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (MRI) for the diagnosis of myocarditis in published cases reports and case series. The localizations were classified as anterior, anterolateral, lateral, inferolateral, inferior, inferoseptal, mid-septal, and anteroseptal. The layers of the myocardium were classified as epicardial or sub-epicardial, mid, and endocardial or sub-endocardial wall.
Generally, myocarditic infiltrations due to viral infection occur in a peculiar pattern (i.e., predominantly in the lateral free wall, originating from the epicardial quartile of the ventricular wall in myocarditis patients) (42). The patterns of LGE occurring in general viral myocarditis as well as in COVID-19 mRNA vaccination-associated myocarditis appear to be similar.
Possible Mechanism of COVID-19 mRNA Vaccination-Associated Myocarditis
The mechanisms mediating COVID-19 mRNA vaccination-associated myocarditis have not been elucidated in detail till date. We suspect that the mechanisms mediating COVID-19 mRNA vaccination-associated myocarditis may be similar to those underlying viral myocarditis. Viral myocarditis is mainly due to direct viral damage and the subsequent IL-6-mediated immune response (43).
The mRNA vaccine mainly elicits a local immune response after being injected intramuscularly. However, this immune response is also present systematically, including in the liver, pancreas, and lymph nodes. In experiments among animal models, lipid nanoparticle-modified mRNA influenza vaccines were distributed mainly in the above mentioned organs, but were also detected to a lesser extent in the heart (44). mRNA vaccines do not cause COVID-19, as the mRNA breaks down rapidly in the cell and the vaccine encodes only a portion of the complete virion. Due to the structural design of the mRNA vaccine, it is uncertain whether distribution of vaccine components to the heart could cause direct damage.
The possibility of immunological mechanisms mediating the development of myocarditis following mRNA vaccination needs to be considered. For example, naive T lymphocytes may be primed by autologous proteins released from damaged cardiomyocytes via antigen-presenting cells. In rare cases, it has been reported that this can cause the migration of primed T lymphocytes into cardiovascular tissue, as well as cell-mediated cytotoxicity and lymphocytic myocarditis (45). Pro-inflammatory cytokines are released, increasing T lymphocyte activation and contributing to myocardial damage (46). In various published cases of vaccine-associated myocarditis, myocarditis was found to develop after the second vaccination. We suspect that T lymphocytes primed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins primed by the first vaccination may cause myocarditis. We note that the mRNA-based COVID-19 vaccine is a new vaccine that has not been used previously. More comprehensive elucidation of its pathogenesis is desirable to ensure its safety.
Report From the Ministry of Health, Labour and Welfare, Japan
In Japan, suspected myocarditis-associated events, including myocarditis and pericarditis, have been reported more frequently among males in the age groups of 10–19 and 20–29 years (3.69 and 9.62 cases per million, respectively, for combined first and second vaccinations for the Pfizer mRNA-based vaccine; and 28.83 and 25.65 cases per million, respectively, for combined first and second vaccinations for the Moderna mRNA- based vaccine) (47). Based on reports of suspected adverse drug reactions in Japan and overseas, the Japanese Ministry of Health, Labour and Welfare had decided to revise the medical package inserts for mRNA vaccines. More specifically, the Ministry considered issuing an alert in light of the high frequency of myocarditis-associated events in young males. The recommendation of the Pfizer-BioNTech mRNA-based vaccine is being considered for males in their teens and twenties, as the frequency of reports of suspected myocarditis-associated adverse events following vaccination with the Moderna mRNA-based vaccine were clearly higher than the frequency of events following vaccination with the Pfizer-BioNTech mRNA-based vaccine. Those who had received the Modena vaccine in the past would be able to choose the Pfizer-BioNTech vaccine later on.
Clinical Perspective
COVID-19 vaccine-associated cardiomyopathy is frequently reported to develop within 2–3 days following vaccination and presents as chest pain symptomology (44). Elevated myocardial devitalizing enzymes are found in all cases, whereas changes on electrocardiography as well as decreased contraction (left ventricular ejection fraction <50%) on echocardiography have been reported in only 87 and 15% of patients, respectively (44). If chest symptoms are observed following vaccination, it is advisable to consider the possibility of myocarditis and to perform appropriate blood tests and cMRI scans. In the aforementioned CDC report, myocardial biopsy is included as one of the diagnostic criteria for vaccine-associated myocarditis. However, there are few reports regarding relevant pathology findings, likely because the infiltration of inflammatory cells is reduced compared with that in ordinary acute myocarditis; hence, these findings may not lead directly to a definitive diagnosis. There have been many reports on the characteristics of cardiac MRI in evaluating COVID-19 myocarditis, including the current report, and these reports may be more useful and informative in guiding diagnostics and effective clinical decision-making.
In this review, LGE on cardiac MRI was found to be more common on the inferior lateral wall of the left ventricle and relatively less common on the septal side. This finding is similar to existing reports on viral acute myocarditis (14). In viral myocarditis, cases of LGE on the septal side are considered to have a poor prognosis because of its effect on the conduction system of stimulation to the myocardium. In the current review, Case 4, with LGE on the septal side, showed affected atrioventricular conduction and required relatively intensive treatment, and may have the same tendency in COVID-19 vaccine-associated cardiomyopathy. Hence, cardiac MRI may be useful not only for the diagnosis itself, but also with respect to risk stratification. Although most cases occur in young males and the severity of vaccine-associated myocarditis is relatively low in these age groups, cases of cardiac failure have been reported. Thus, based on existing reports, risk stratification should be performed, hospitalization should be considered in some cases, and careful follow-up is always necessary.
Conclusions
Herein, a report on the detailed features of COVID-19 vaccine-associated myocarditis has been presented. It was found that cMRI is minimally invasive and may aid in the diagnosis of myocarditis. LGE on cMRI tends to occur more frequently on the free wall side and relatively less frequently on the septal side, as in viral myocarditis. These findings can guide future epidemiologic research on this topic of immediate public health importance, directly inform medical guidelines, and help in effective clinical decision-making.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Japanese Red Cross Musashino Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KW, TA, and YM contributed principally to writing the manuscript. ST, MT, TK, MK, RN, SO, TL, TH, MN, GN, RM, SN, YN, TN, MG, and TS revised the manuscript. ST selected the cardiac MRI images and drafted an explanation for the images. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. (2021) 5:947–53. 10.1038/s41562-021-01122-8 [DOI] [PubMed] [Google Scholar]
- 2.Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. (2021) 39:839–45. 10.1016/j.vaccine.2020.12.046 [DOI] [PubMed] [Google Scholar]
- 3.Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. (2004) 43:1503–10. 10.1016/j.jacc.2003.11.053 [DOI] [PubMed] [Google Scholar]
- 4.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices — United States, June 2021. Morb Mortal Wkly Rep. (2021) 70:977–82. 10.15585/mmwr.mm7027e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. (2009) 53:1475–87. 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. (2018) 72:3158–76. 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 7.Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, et al. Comparison of original and 2018 lake louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging. (2019) 1:e190010. 10.1148/ryct.2019190010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation. (2016) 134:e579–646. 10.1161/CIR.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 9.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. (2013) 34:2636–48. 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 10.Group JJW. Guidelines for diagnosis and treatment of myocarditis (JCS 2009) – digest version. Circ J. (2011) 75:734–43. 10.1253/circj.CJ-88-0008 [DOI] [PubMed] [Google Scholar]
- 11.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 12.Vermes E, Childs H, Faris P, Friedrich MG. Predictive value of CMR criteria for LV functional improvement in patients with acute myocarditis. Eur Heart J Cardiovasc Imaging. (2014) 15:1140–4. 10.1093/ehjci/jeu099 [DOI] [PubMed] [Google Scholar]
- 13.Greulich S, Seitz A, Müller KAL, Grün S, Ong P, Ebadi N, et al. Predictors of mortality in patients with biopsy-proven viral myocarditis: 10-year outcome data. J Am Heart Assoc. (2020) 9:e015351. 10.1161/JAHA.119.015351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greulich S, Ferreira VM, Dall'Armellina E, Mahrholdt H. Myocardial inflammation-are we there yet? Curr Cardiovasc Imaging Rep. (2015) 8:6. 10.1007/s12410-015-9320-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grn S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. (2012) 59:1604–15. 10.1016/j.jacc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. (2017) 70:1964–76. 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol. (2017) 70:1977–87. 10.1016/j.jacc.2017.08.044 [DOI] [PubMed] [Google Scholar]
- 18.Shiyovich A, Witberg G, Aviv Y, Eisen A, Orvin K, Wiessman M, et al. Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur Hear J Cardiovasc Imaging. (2021) jeab230. 10.1093/ehjci/jeab230 [DOI] [PubMed] [Google Scholar]
- 19.Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in 7 adolescents after pfizer-biontech covid-19 vaccination. Pediatrics. (2021) 148:e2021052478. 10.1542/peds.2021-052478 [DOI] [PubMed] [Google Scholar]
- 20.Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. (2021) 144:502–5. 10.1161/CIRCULATIONAHA.121.055891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. (2021) 39:3790–3. 10.1016/j.vaccine.2021.05.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. (2021) 6:1196–201. 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. IJC Hear Vasc. (2021) 34:1–4. 10.1016/j.ijcha.2021.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Angelo T, Cattafi A, Carerj ML, Booz C, Ascenti G, Cicero G, et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol. (2021) 37:1665–7. 10.1016/j.cjca.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. (2021) 16:2142–5. 10.1016/j.radcr.2021.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F, et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. (2021) 144:487–98. 10.1161/CIRCULATIONAHA.121.056038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J Pediatr. (2021) 238:321–3. 10.1016/j.jpeds.2021.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour J, Short RG, Bhalla S, Woodard PK, Verma A, Robinson X, et al. Cardiothoracic imaging acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging. (2021) 78:247–9. 10.1016/j.clinimag.2021.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habib MB, Hamamyh T, Elyas A, Altermanini M, Elhassan M. Acute myocarditis following administration of BNT162b2 vaccine. IDCases. (2021) 25:e01197. 10.1016/j.idcr.2021.e01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cereda A, Conca C, Barbieri L, Ferrante G, Tumminello G, Lucreziotti S, et al. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: serendipity or atypical causal relationship? Anatol J Cardiol. (2021) 25:522–3. 10.5152/AnatolJCardiol.2021.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidula MK, Ambrose M, Glassberg H, Chokshi N, Chen T, Ferrari VA, et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus. (2021) 13:1–6. 10.7759/cureus.15576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams CB, Choi J, Hosseini F, Roberts J, Ramanathan K, Ong K. Acute myocarditis following mRNA-1273 SARS-CoV-2 vaccination. CJC Open. (2021) 3:1410–2. 10.1016/j.cjco.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaak A, Feisst A, Luetkens JA. Myocarditis following COVID-19 vaccination. Radiology. (2021) 301:E378–9. 10.1148/radiol.2021211766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasnie AA, Hasnie UA, Patel N, Aziz MU, Xie M, Lloyd SG, et al. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord. (2021) 21:1–6. 10.1186/s12872-021-02183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrignani A, Schicchi N, Calcagnoli F, Falchetti E, Ciampani N, Argalia G, et al. Acute myocarditis following comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol Case Rep. (2021) 16:3321–5. 10.1016/j.radcr.2021.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim IC, Kim H, Lee HJ, Kim JY, Kim JY. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J Korean Med Sci. (2021) 36:1–6. 10.3346/jkms.2021.36.e229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrlich P, Klingel K, Ohlmann-Knafo S, Hüttinger S, Sood N, Pickuth D, et al. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: case report. Clin Res Cardiol. (2021) 110:1855–9. 10.1007/s00392-021-01936-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel YR, Louis DW, Atalay M, Agarwal S, Shah NR. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series. J Cardiovasc Magn Reson. (2021) 23:1–8. 10.1186/s12968-021-00795-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tailor PD, Feighery AM, El-Sabawi B, Prasad A. Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine. Eur Heart J Case Reports. (2021) 5:1–6. 10.1093/ehjcr/ytab319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TD, Mall G, Westphal JG, Weingärtner O, Möbius-Winkler S, Schulze PC. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Hear Fail. (2021) 8:4710–4. 10.1002/ehf2.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onderko L, Starobin B, Riviere AE, Hohl PK, Phillips CT, Morgan RB, et al. Myocarditis in the setting of recent COVID-19 vaccination. Case Reports Cardiol. (2021) 2021:1–5. 10.1155/2021/6806500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. (2004) 109:1250–8. 10.1161/01.CIR.0000118493.13323.81 [DOI] [PubMed] [Google Scholar]
- 43.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol Mech Dis. (2008) 3:127–55. 10.1146/annurev.pathmechdis.3.121806.151534 [DOI] [PubMed] [Google Scholar]
- 44.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. (2017) 25:1316–27. 10.1016/j.ymthe.2017.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basso C, Leone O, Rizzo S, De Gaspari M, Van Der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. (2020) 41:3827–35. 10.1093/eurheartj/ehaa664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. (2020) 116:1097–100. 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ministry of Health Labour Welfare Japan . Status Report of Suspected Myocarditis-Related Events. Available from: https://www.mhlw.go.jp/content/10601000/000844075.pdf (accessed December 28, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.